Abstract
Background:
The formation of senile plaques and neurofibrillary tangles of the tau protein are the main pathological mechanism of Alzheimer’s disease (AD). Current therapies for AD offer discrete benefits to the clinical symptoms and do not prevent the continuing degeneration of neuronal cells. Therefore, novel therapeutic strategies have long been investigated, where curcumin (Curcuma longa) has shown some properties that can prevent the deleterious processes involved in neurodegenerative diseases.
Objective:
The aim of the present work is to review studies that addressed the effects of curcumin in experimental models (in vivo and in vitro) for AD.
Method:
This study is a systematic review conducted between January and June 2017, in which a consultation of scientific articles from indexed periodicals was carried out in Science Direct, United States National Library of Medicine (PubMed), Cochrane Library and Scielo databases, using the following descriptors: “Curcuma longa”, “Curcumin” and “Alzheimer’s disease”.
Results:
A total of 32 studies were analyzed, which indicated that curcumin supplementation reverses neurotoxic and behavioral damages in both in vivo and in vitro models of AD.
Conclusion:
The administration of curcumin in experimental models seems to be a promising approach in AD, even though it is suggested that additional studies must be conducted using distinct doses and through other routes of administration.
Keywords: Alzheimer’s disease, curcumin, Aβ aggregation, oxidative stress, therapeutics, brain
1. Introduction
Alzheimer’s disease (AD) is a degenerative and progressive clinical condition that results in damage to memory, thinking and behavior [1], described in 1906 by the German psychiatrist Alois Alzheimer [2]. Recent reports have shown that around 44 million people worldwide have AD or related dementia nowadays [3]. This disease usually results in speaking difficulty, inability to solve problems and alterations in other cognitive functions, which can directly affect the individual's ability to perform everyday activities [3], affecting the brain in a global manner [4].
AD is a neurological disorder related to damages on some nerve cells of the brain involved in cognitive functions [5]. In fact, the brain of a patient with AD shows a severe cortical atrophy in the temporal lobe, especially in the cortex and hippocampal formation. In addition, brain volume is widely reduced due to neuronal degeneration, which results in a substantial loss of synapses [6, 7].
The main pathological features of AD are the aggregations of amyloid-beta (Aβ) and tau proteins. Amyloid β is produced from the amyloid precursor protein (APP) by the sequential action of two β-proteins and secretases. The breakdown of the β-secretase product by the y-secretase produces the toxic Aβ42 and the non-toxic Aβ40. Tau is a protein that acts structuring and stabilizing the microtubules, whose ability to bind them is dependent on the number of phosphorylations present. The greater the extent of phosphorylation the more compromised its interaction with the microtubules becomes [8].
The production of reactive oxygen species (ROS) also seems to be involved in the AD’s degeneration cycle, thereby causing damage to mitochondrial DNA and to its electron transport chain [9]. The damage caused by free radicals occurs through an imbalance between their production and their neutralization by the cellular antioxidant systems [10, 11]. It has been suggested that ROS play an important role in the age-related neurodegeneration process and cognitive decline [12].
Recent advances in understanding the pathophysiological mechanisms of AD have led to new strategies in drug development. Animal models have contributed considerably to these advances as they play an important role in the evaluation of potential drug candidates that can alleviate the dementia and also delay the disease process [13].
The interest in natural products has increased significantly, resulting in the growing use of herbal medicines [14]. In a recent review, Izzo et al. (2016) report a 6.8% increase in sales of herbal products and dietary supplements in the United Sates in 2014, with an estimation of more than U$ 6.4 billion in total sales [15].
Some herbal medicines have shown neuroprotective effects, since plants have numerous bioactive molecules that can improve the body’s resistance to cell stress and prevent the cytotoxicity of various agents. Among them, polyphenols such as curcumin, resveratrol (non-flavonoids) and flavonoids, which receive much attention for their ability to reduce cell stress [16, 17], may be mentioned. These polyphenols inhibit toxin-mediated stress responses through their anti-inflammatory and antioxidant properties, as well as induce the expression of cytoprotective proteins [18-20].
Curcuma, for instance, contains high concentrations of polyphenols and flavonoids [21]. Its biosynthetic pathway begins with phenylalanine, which is a common precursor in the biosynthesis of flavonoids [22, 23]. Flavonoids are essential compounds for the development of clinically effective therapeutic agents in the treatment of neurodegenerative diseases, since their regular consumption has been associated with a reduced risk, since in addition to their antioxidant properties, these polyphenolic compounds exhibit neuroprotective properties through interaction with the cellular signaling pathways followed by transcription and translation that mediate cellular function under normal and pathological conditions [24].
Curcumin is the main polyphenol found in the turmeric curry (Curcuma longa), belonging to the Zingiberaceae family and being native to South Asia and grown in the tropics [25]. It has been reported that this compound has properties that can prevent or ameliorate pathological processes related to neurodegenerative diseases such as cognitive decline, mood disorders and dementia [26]. In addition, curcumin has been investigated in experimental models for Parkinson’s disease and has shown promising results [27].
Saffron components have been associated with biological properties such as anti-inflammatory, anti-proliferative, pro-apoptotic, antioxidant, antiviral, anti-amyloidogenic and anti-diabetic [28, 29]. Its most bioactive constituents are curcuminoids, including curcumin and derivatives such as demethoxycurcumin and bisdemethoxycurcumin [30, 31]. Saffron has shown potential to be used in the treatment of several chronic diseases [32].
The properties attributed to curcumin, such as inhibition of amyloid pathology, protection against oxidative stress and inflammation, inhibition of amyloid beta plaques aggregation and hyperphosphorylation of tau protein, suggest that this polyphenol may prevent or ameliorate pathological processes related to cognitive decline and age-related dementia, as occurs with AD patients [33, 34].
Current therapies for AD offer only limited benefits to its clinical symptoms and they do not prevent the degeneration of neuronal cells. Thus, many therapeutic strategies for delaying or preventing neurodegeneration have been the subject of research towards the optimal treatment for AD. Due to the aforementioned properties of curcumin, this current study is focused on the beneficial effects of curcumin on experimental models of AD. In light of this, the aim of this study is to systematically review the use of curcumin in in vivo and in vitro models for AD and thus understand its role in the behavioral and cellular protection activities.
2. Materials and Methods
2.1. Databases and Keywords
The present study comprises a systematic review of the literature, developed based on previously established stages of search, identification, selection and eligibility strategies. A review was performed through Science Direct, United States National Library of Medicine (PubMed), Cochrane Library and Scielo databases. The search in the databases was carried from 2006 and 2017 using the terminologies registered in the Descriptors in Sciences of Health (DeCS) created by the Virtual Health Library (VHL). In order to identify all articles that studied the effects of curcumin supplementation in experimental models of Alzheimer’s disease, the following descriptors were used: “Curcuma longa”, “Curcumin” and “Alzheimer’s disease”. Initial screening of the studies was based on the title and the abstract of the articles, and soon thereafter, the publication was reviewed in its entirety.
2.2. Eligibility/Exclusion Criteria
We included articles that evaluated the effects of curcumin in in vivo and in vitro Alzheimer’s disease models, where they studied the neuroprotective and symptoms relief properties of curcumin through behavioral and cell/tissue analyzes. The following exclusion criteria were considered: 1) Review articles; 2) Books; 3) Studies conducted with curcumin associated with other herbal supplementation; 4) Experimental models other than small rodents (rats and mice) or cell culture; 5) Absence of a control group.
The search for studies was carried out independently by two expert reviewers in the context discussed, through the titles, abstracts or both, solving discrepancies through a subsequent consensus meeting. Initially the terms were used separately, and then searched together. In the selection there was a restriction of languages, since only articles published in English were analyzed. Screening of the studies was done on the basis of the title and the abstract and soon after publication was reviewed in full and compared.
The eligibility criteria for inclusion in this systematic review were as follows: 1) All articles should be indexed and published in renowned journals with considerable impact factors; 2) All models used in the studies should present the clinical signs or symptoms related to AD; 3) Animal models should use amyloid beta or knockout animals models to induce neural injury; 4) Animals should be treated with curcumin; 5) In vivo studies should be performed for at least 7 days of treatment with curcumin and with a minimum of 3 experimental groups; 6) Control group had to be comparable to the group supplemented with curcumin; 7) The in vitro models should use either rat or mouse cells or human lineage cells.
Statistical grouping was not considered in this review due to methodological heterogeneities among the studies as there were lots of variation regarding the evaluation methods, the type of cell lines and the phytotherapeutic doses. Therefore, the meta-analyzes could not be performed on the data assessed.
3. Results
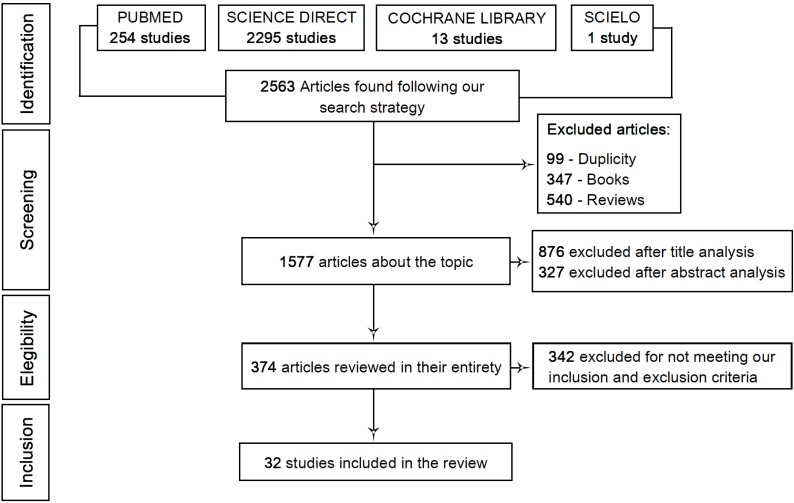
After the initial screening, a total of 2563 studies were found (254 in Pubmed, 2295 in Science Direct, 13 in Cochrane Library and 1 in Scielo). 99 of these studies were excluded due to duplicity between the databases, 347 because they were books and 540 because they were review articles. From the 1577 remaining studies, 876 were excluded after a title evaluation. Next step was to read the abstracts. After applying the aforementioned exclusion and inclusion criteria, a total of 327 articles were excluded. Therefore, 374 articles were reviewed in their entirety in which 341 were excluded because they failed to fit into our inclusion criteria as a great number of these studies used curcumin supplementation with other drugs and the experimental study was performed in models other than those proposed. Finally, a total of 32 articles were included in this review. The systematic procedure for selecting the articles is demonstrated through the flowchart depicted in Fig. (1).
Fig. (1).
Flowchart showing the selection process of the studies used in this review.
Regarding the main findings and general characteristics of these 32 studies, the oldest publication was from 2007 and all 32 articles were published in international journals. Different experimental designs were used in these articles, where 19 in vitro studies showed similarities in the methodology used. Seven of these studies were actually performed in vivo and most of them (6) performed in vitro and in vivo cell culture after conducting the behavioral tests. In addition, 7 studies evaluated the effect of curcumin administration on the production of ROS.
Cellular models varied among the studies, where human SH-SY5Y cells (9), PC12 cells (3), Wistar rats (5), Sprague-Dawley rats (6), as well as APPsWe/PS1 (6) and C57BL/6j mice (3) models were used. As for the number of hours or days of curcumin treatment, the in vivo studies varied from 5 days to 6 months, whereas the in vitro studies were performed for 24-48 hours and the ones that performed both in vivo and in vitro studies conducted for 7 days - 3 months. As for the number of groups, the in vivo studies used 3-12 groups, whereas the in vitro ones used 4-12 and the studies that performed both in vivo and in vitro used 3-8 groups.
The amounts of the curcumin used in the in vitro studies varied between 0.1 and 200 μL and between 7.5 and 400 mg for the in vivo studies, where they were all administered intraperitoneally.
3.1. Summary of the Studies
In brief, the main results of the articles can be summarized as follows:
A. In Vitro Studies
A total of 19 studies performed analysis of the mechanism of action of curcumin in vitro. Three of them show that curcumin attenuates beta-amyloid-induced PC12 cell neurotoxicity through inhibition of oxidative stress, intracellular calcium levels and hyperphosphorylation of tau protein [35], oxidative damage and inhibition of Aβ [36], thereby reducing the cytotoxicity caused by this protein, through mechanisms of suppression of apoptosis by inhibition of ROS and regulation of ERK cascade. In addition, curcumin pretreatment reversed deregulation of the two pathways MAPK and AKT, as well as improved the number and cell morphology [37].
Seven studies have analyzed the action of curcumin on SH-SY5Y cells, pointing out that curcumin reduces the Aβ insertion in the membrane by directly attenuating its toxic interactions and reducing its breakdown [38]. In addition, it was found that the protective action of curcumin on the decrease of Aβ production occurs through the inhibition of GSK-3-mediated activation of PS1 [39]. In agreement with these findings, a study by Huang et al. (2012) also found that curcumin regulates total expression of GSK-3β and phospho-Ser9, further inhibiting Aβ depolarization and suppressing proteins related to mitochondrial apoptosis (cytochrome c, caspase-3 and Bax), normalizing the action of the cellular antioxidant enzymes (SOD and catalase) [40].
In congruence with the findings of Park et al. (2008), Huang et al. (2015) showed that curcumin acts by reducing the level of intracellular calcium [41]. In addition, according to these authors curcumin is able to attenuate the elevation of the glutamate/GABA levels and reduce the activation of the NMDA receptor, and CREB and ATF-1 protein levels. Curcumin has also been shown to reduce mitochondrial dysfunction while maintaining biogenesis and synaptic activity [42]. The neuroprotective effect of curcumin is also possibly membrane-mediated, since its administration reduced the extent of membrane permeabilization induced by Aβ aggregates [43]. A study of Yin et al. (2012) found that the cytoprotection conferred by curcumin on SH-SY5Y cells transfected with APPswe is mediated by its ability to regulate the balance between heme oxygenates 1 and 2, besides to protecting cells against the cytotoxicity caused by H2O2 [44].
Two studies analyzed the mechanism of action of curcumin in SK-N-SH cells [45, 46]. After incubation with Aβ41-42, curcumin prevented the damage caused by Aβ by the regulation of the expression MAP-2 (Protein associated with microtubule 2), it acted on the cellular morphology, including the growth, extension and number of neurites, which were significantly modified after treatment [45], besides regulating the expression of hTERT [46].
B. In Vivo Studies
Three studies have analyzed the action of curcumin in cortical neurons in vivo [47-49], evidencing that it has a protective effect on pre-frontal cortical neurons against Aβ cytotoxicity. In addition, regulation of both AKT and caspase-3 are involved in the protective effects induced by curcumin [47]. Furthermore, it safeguarded neurons from the toxicity process, improved membrane potential and decreased ROS amount, inhibiting apoptotic cell death, as well as activated SIRT1 expression and decreased Bax expression. The study by Zhang et al. (2010) observed the action of curcumin on several cortical cell lines, acting on levels of Aβ 40 and Aβ 42, which decreased significantly in comparison to control in all cell models, besides attenuating maturation of APP [50]. Two studies have observed the action of curcumin on hippocampal cells, also demonstrating that it reduces ROS levels in cells of Sprague-Dawley rats [51], regulates the expression of caspase-3 and inhibits the levels of protein cyclin D1 with abnormal activation in Wistar rat cells [52]. Curcumin also prevented the development of microglial inflammatory response by abolishing interleukins (IL-1) and (IL-6) and TNF-α in cells of neonatal rats [53].
Three studies evaluated the action of curcumin in Sprague-Dawley rats, inhibiting the activation and expression of GFAP in the hippocampus and generating a significant improvement in the spatial memory capacity of the animals [54], as well as reduced learning and memory deficits in the AD model through a mechanism that may involve the suppression of CRMP-2, its hyperphosphorylation and/or its positive regulation mediated by axonal regeneration [54], acting on cognitive deficits related to AD and positively regulating ERK-BDNF signaling in the hippocampus [26]. Three studies performed in vivo analyzed transgenic mice transfected with the APPswe/PS1dE9 gene, showing that curcumin can prevent and reduce amyloid deposition and partially restores dendritic abnormalities [33], also decreasing InR and IRS-1 expression in the CA1 area of the hippocampus [55]. Furthermore, it has been observed that the reduction of Ab aggregation occurs through mechanisms that inhibit its PS-2 production and/or accelerate its clearance through the enhancement of degrading enzymes (insulin and neprilysin) [56]. A similar mechanism was observed in neonatal Wistar rats, pointing an action at the synaptic level [57].
C. In vitro and in vivo Studies
Six studies investigated the action of curcumin in both in vivo and in vitro models, which four were performed in experimental models with transgenic mice (three with models transfected with the APPswe/PS1dE9 gene and one resulting from a cross between 5 x transgenic male rats FAD cross-linked with female C57BL/6J mice), which observed that the action of curcumin resulted in improvements in the amount, structure and function of synapses, indicating that this occurs through the regulation of synaptic proteins PSD95 and Shank1 [58], significantly reducing the expression of Aβ40-42 and ADDL in the hippocampus of transgenic double mice [59]. One of these studies worked with a model of transgenic mice expressing the mutant AβPP695 and APPswe/PS1dE9, showing a reduction in the activation of microglia and astrocytes, as well as the production of cytokines, inhibiting the NF-κB pathway. In addition, it increased PPARy transcriptional activity, also attenuating memory deficits in behavioral tests [60]. Another transgenic mouse model was also established, resulting from cross-linking between 5 x FAD and C57BL/6J, in which Aβ production was reduced by decreasing BACE1 expression, preventing synaptic degradation and improving spatial learning and memory [61]. Moreover, two studies have observed the action of curcumin on nanoparticles acting on the protection, preservation and rescue of human neuronal cells against oxidative damage [62], thus reversing the limitations in memory and learning in an experimental model of AD type phenotypes [63]. A more detailed analysis of these results is depicted in Table 1.
In summary, the reviewed papers evidenced that curcumin reverses neurotoxic damage by preventing beta-amyloid aggregation and tau protein hyperphosphorylation, as well as oxidative stress, apoptosis and inflammation. In addition, the studies show that curcumin prevents behavioral disorders by improving memory capacity and cognitive deficits in animals with AD. Curcumin can also plays its protective role by decreasing ROS levels in neurons.
4. Discussion
AD has several implications for individuals and society, where the understanding of its neurochemical and behavioral mechanisms, as well as the performance of natural compounds that have been tested in in vivo and in vitro models, is detrimental. In this study, we reported the use of curcumin in 32 studies that investigated its use in in vitro and in vivo models for AD, which demonstrated promising results.
Extracellular deposits of the beta-amyloid peptide and the intracellular accumulation of hyperphosphorylated tau protein are the major hallmarks of AD [40]. As previously reported in the literature [64], experiments performed in vivo and in vitro with AD models (Table 1) show that Aβ plays a causal role in the pathogenesis of AD. Aβ accumulation leads to the formation of senile plaques, as well as to the activation of oxidative damage to the neuron, neuroinflammation, apoptosis and cognitive deficits [26, 40, 65].
In vitro studies have shown that curcumin protects neuronal cells from beta-amyloid damage by inhibiting oxidative damage. Evidence shows that neurotoxicity induced by intracellular Aβ is partially caused by the increased production of ROS, which leads to oxidative stress [66].
Oxidative stress might influence Aβ production through interaction with amyloid precursor protein [67]. Indirectly, oxidative stress can also influence Aβ production by modulating the activity and levels of both β- and γ-secretase [67, 68]. Previous reports have shown that curcumin treatment in in vivo and in vitro experimental models resulted in decreased production of Aβ42 protein by decreasing the production of β-secretase 1 [37] and by reducing the expression of γ-secretase and presenilin-2, as well as by increasing the expression of β-amyloid-degrading enzymes [35].
As shown in Table 1, several studies investigated the ability of curcumin in eliminating free radicals, where it significantly reduced the ROS and suppressed apoptosis after its supplementation [40, 44, 48, 51, 62, 69]. One of the mechanisms involves a decrease in the toxicity and formation of ROS due to the ability of curcumin in potentiating the cellular defense system, increasing the activity of superoxide dismutase and catalase [40]. In addition, it has been shown that curcumin induces cell protection by attenuating the cytotoxicity caused by H2O2 [70].
The generation of free radicals by Aβ increases lipid peroxidation, protein oxidation [71] and DNA damage [72], but decreases the activity of antioxidant enzymes such as superoxide oxidase and catalase [73]. In addition, tissues obtained post-mortem from AD patients confirmed oxidative damage induced by Aβ [74].
These results corroborate the findings of Park et al. (2008) as they hypothesize that curcumin treatment may play a protective role through the reduction of ROS levels in cultured neurons by a significant regulation of superoxide oxidase and catalase activities, with consequent inhibition of oxidative damage, as well as a decrease of DNA damage [35]. This study also evaluated the elevation of intracellular calcium, as it is introduced by extracellular sources into primary neurons, such as cortical and hippocampal neurons, which seems to mediate Aβ-induced neurotoxicity [55, 75]. Other mechanisms are also involved in the protective effect of curcumin as the group treated with Aβ and curcumin showed considerably lower levels of extracellular H2O2 and intracellular ROS in comparison with the group treated with Aβ alone [40]. In addition, this study showed that curcumin had much stronger capacity for removal of O2 anion than that of trolox in a free cell solution.
ROS produced from the cellular energy machinery may in turn act at different levels and impair mitochondrial function, activating the caspase family proteins and causing loss of mitochondrial membrane potential as well as deregulation of the MAPKs and AKT pathways [47]. As previously reported, exposure of cells to 10μl of Aβ for 24 hours significantly activated caspase-3, 8 and 9, whereas pre-treatment with curcumin attenuated its activations and regulation of p38, MAPK and AKT pathways [62]. Similar results were also observed regarding the protective effect of curcumin in regulating the activity of these pathways [18, 70]. Corroborating with these studies, Qin and co-workers (2010) also showed a protective effect of curcumin resulting from the neutralization of caspase-3 and AKT [47].
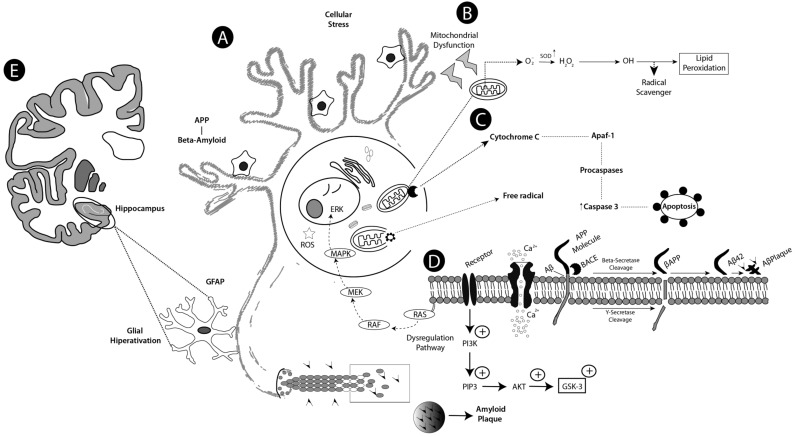
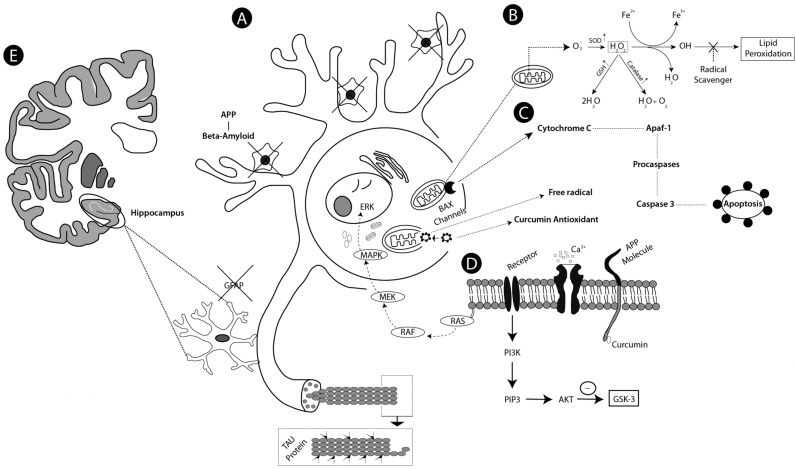
A wide variety of TNF-activating cell signaling pathways, including JNK, MAPK, and PI3K AKT, have shown to be regulated by curcumin. Thus, curcumin can bind or directly block TNF production and be involved in pro-inflammatory pathways related to most chronic diseases [76]. The proposed mechanisms for above cited studies, concerning the pathophysiology of the AD and neuroprotective action of the curcumin, are depicted in Figs. (2 and 3), respectively.
Fig. (2).
Schematic drawing showing proposed mechanisms of action on Alzheimer’s disease (AD). Stage A shows a neuronal cell with pathological characteristics of AD, with formation and aggregation of β-amyloid protein and hispanphosphorylation of tau protein. A sequential action between the activity of these proteins, potentializing the toxicity and cell stress, make dendrites become less branched as a result of deregulation with increased ROS and free radicals by the mismatching of at least one electron in its outer orbitals. Step B represents the mitochondrial dysfunction generating a decrease of the enzymes of the cellular antioxidant defense system with the exception of SOD (superoxide dismutase) which is high. Then the superoxide anion (O2-) is converted to hydrogen peroxide, which can be converted into hydroxyl ion that activates lipid peroxidation. Stage C shows the cytochrome C enzyme activating Apaf-1 (Activation factor of apoptosis 1) that in turn activates the procaspases, which, in turn, later activate effector caspase 3, resulting in apoptosis. In stage D, cellular hyperactivation occurs due to excessive Ca+2 influx causing excitotoxicity. Beta amyloid is formed by the action of PPA (amyloid precursor protein) and by the breakdown of its molecule by BACE (Beta secretase), then the BACE product is also cleaved by y-secretase resulting in formation of AB42 (Amyloid toxic) which culminates in senile plaques, deregulation of the MAPK (Mitogen Activated Protein Kinase) and AKT (Family of Protein Kinase) pathways also occurs. In step E, glial hyperactivation occurs in the hippocampus, indicating neuroinflammation.
Fig. (3).
Schematic drawing showing proposed mechanisms of action of Curcuma longa (curcumin). Curcumin action reversing mechanisms in AD. In stage A curcumin binds beta amyloid plaques significantly reducing their formation and avoiding the hyperphosphorylation of the tau protein, avoiding the toxicity and cellular stress, then the dendrites of the neurons remain branched and there is a decrease in the production of ROS and free radicals by electron mismatching. In step B, curcumin prevents mitochondrial dysfunction as it acts by enhancing the action of enzymes of the antioxidant defense system SOD, catalase and GSH that converts the hydrogen peroxide to H2O and O2 avoiding lipid peroxidation. In step C, curcumin works by inhibiting the apoptotic pathway, represented succinctly as these were evaluated in the reviewed studies, so curcumin is seen to reduce BAX channel activation, which accelerates programmed cell death and increases Bcl-2 regulation, and is repressive of apoptosis. In addition curcumin prevents hyperactivation of Apaf-1 and subsequent activation of procaspases that activate effector caspase 3. In D curcumin prevented hyperexcitation caused by excessive intracellular Ca+2 influx, inhibiting the formation of B-amyloid by APP and avoiding the deregulation of the MAPK and AKT pathways, reducing hyperexcitation. Stage E shows significantly reduced glial hyperactivation in the hippocampus, reducing neuroinflammation.
Oxidative stress may also influence the production of Aβ in which interacts with amyloid precursor protein [77]. Genetic studies of AD revealed a complex and strong genetic etiology. Four genes were identified as the cause of AD, which begins early with complete penetrance of amyloid precursor protein (APP), presenilin 1 (PSEN1), and presenilin 2 (PSEN2) or through an increase in the susceptibility to late onset of AD with partial penetrance (APOE) [49].
These studies corroborate with Wang et al. (2014), when the performance of curcumin in an AD model was evaluated.
The authors observed that curcumin decreased the ratio of mature APP to immature APP when comparing with the control group [56]. They also investigated whether curcumin was able to alter plasma membrane levels of APLP2, which is a homolog of the APP protein. However, no differences were found in the levels of APLP2 (amyloid precursor protein type 2) among cells treated with curcumin when compared to those of the control [56].
In vivo studies have also shown the protective effect of curcumin in tests that evaluated behavioral activities in mice, where most of these studies used Morris aquatic labyrinth test, which is a well-established behavioral test for assessing memory and learning [78]. The study conducted by Wang et al. (2014) using the Morris water maze test found that there is an obvious difference in the rat search strategies, where the time taken to find the platform decreased in response to training. Three months after treatment with curcumin, the animals spent much less time finding the platform at day 5 in comparison with the mice in the control group [56].
In addition, Wang et al. (2013) reported that although the spatial memory capacity of the curcumin treated group was lower than that of the simulated control group, it was significantly higher than that in AD [54]. While Zhang et al. (2015) demonstrated that spatial learning acquisition was obviously impaired in Aβ1-42-treated rats compared to the treated and control groups after 10 days of training. They reported that chronic curcumin treatment improves cognitive function and promotes the expression of BDNF and phospho-ERK in the hippocampus [26].
Neurotrophin BDNF plays the role of regulating diversified neuronal structure and function in the development of the adult CNS and has emerged as one of the most important signaling molecules for the development of the nervous system, impaired nervous system and multiple diseases including AD [79, 80]. In fact, it has been reported that BDNF plays a critical role in neuronal survival, synaptic plasticity and memory [80].
Although the aforementioned properties of curcumin indicate its potential use as an adjuvant treatment for AD, a factor that limits curcumin therapy is its poor bioavailability, which is due to intestinal and hepatic glucuronidation. Shoba et al. (1998) demonstrated the effect of curcumin (2g) when administered associated with piperine (20mg), where the latter significantly increased the serum levels of curcumin, improving its bioavailability by 2000% [81]. Moreover, no toxicity was reported within the subjects (n=10) who participated in this study. Similarly, Suresh and Srinivasan (2010) used a concentration of 500mg/kg of curcumin, co-administered orally with 20mg/kg of piperine, resulting in higher amounts of curcumin in the brain [82].
Other strategies to improve the release of curcumin have been investigated such as the use of nanocapsules. As shown in Table 1, NanoCurc™ consists of curcumin-loaded nanoparticles with improved ability to cross the BBB and enhance curcumin’s activity against ROS-mediated neuronal damage in both human cell culture and animal models [51]. Another study reported that the use of curcumin loaded into lipidic nanostructures (NLC-Cur) resulted in significantly higher bioavailability in the brain in both in vitro and in vivo models. This study was performed with the purpose of treating brain cancer, where the results showed that by loading curcumin into lipidic nanostructures an increasing in drug’s efficiency from 19.5% to 82.3% was achieved [83].
Clinical studies were conducted to evaluate the cognitive potential of curcumin in both healthy elderly and AD patients. A randomized, double-blind, placebo-controlled study was conducted with 60 elderly individuals aged between 60 to 85, using 400 mg of curcumin per day for one month, where the mood, cognition and blood markers were evaluated. The results showed that curcumin administration improved attention and working memory after acute administration while it improved working memory and mood after chronic administration [84].
However, some studies that investigated the supplementation of curcumin to AD models showed no significant effects [85, 86]. One of the reasons that may explain the unsatisfactory results is the curcumin’s low bioavailability and its form of delivery [87], which impair curcumin’s ability to be delivered to the brain at concentrations sufficient to provide the expected benefits.
A study conducted by Hishikawa et al. (2001) with three patients with advanced-stage AD, investigated the use of 100 mg of curcumin co-administered orally for 12 weeks with an acetylcholinesterase inhibitor [87]. The three elderly patients presented reduction of anxiety, agitation and irritability. One of the patients also improved the MMSE score. Thus, the study suggests that curcumin acts as an adjuvant in the treatment of AD and that it can provide beneficial effects even in the more advanced stages of AD. In addition, epidemiological evidence shows that the daily intake of 80 to 200 mg of curcumin by the Indian population might be responsible for the lower incidence and prevalence of AD in this country [88].
Curcumin also presented a benefic effect against glutamate excitotoxicity [89], especially on the expression of Brain-Derived Neurotrophic Factor (BDNF), since pretreatment of neurons with this substance reversed the BDNF expression and cell viability in a dose- and time-dependent manner [90].
Transgenic mice (Tg) models are well documented in the literature [91]. The main studies with transgenic animals involving curcumin are focused on the expression of the APPswe/PS1dE9 genes and crossing between 5 x FAD transgenic male animals and C57BL/6J females. The animal model used in these studies has a higher Ab42/40 ratio possibly due to the PS1 mutation [92, 93], which is a gene established to induce AD and accelerate the deposition of Aβ [76, 77]. In the 5x FAD transgenic model, Zheng et al. (2017) co-express five familial AD mutations (APPK670N / M671L + V717I + I716V and PS1 L286V + M146L) [61]. This study demonstrated that at 4 months, the mice developed cerebral amyloid plaques and at 6 months of age they began to exhibit memory deficits due to changes in hippocampal formation. In order to observe the action of curcumin on cognitive impairment, it was administered intragastrically for 2 months, with the findings indicating that its mechanisms of action attenuate impairment of learning and memory, by preventing structural damage to synapses and inhibition of Aβ accumulation and deposition, especially Aβ1-42 in the brain by regulating the expression of BACE1 [61].
Conclusion
The administration of curcumin demonstrates to be effective for the treatment of AD, as the in vivo and in vitro studies show. These studies help to elucidate the mechanism of action of curcumin, where they suggest that it partially reverses the neurotoxic and behavioral damages in animals with AD and other cell lines. However, there are still limitations in the response to treatment in humans which seems to be due to the poor bioavailability of curcumin and to its limited ability to cross the BBB and reach the central nervous system. Finally, curcumin therapy may lead to more effective clinical studies for the prevention of beta-amyloid aggregation, oxidative stress inflammation and neurotoxicity associated with AD. Incorporation of curcumin into nanoparticulate systems or its association with piperine are important alternatives to improve drug’s therapy. It is suggested that further long-term clinical studies with new doses and elaborated strategies to improve bioavailability and permeability towards the CNS need to be conducted.
Table 1.
Studies that investigated the use of Curcuma longa in both in vivo and in vitro models for Alzheimer’s disease.
| Refs. | Experimental Model | Experimental Groups and Curcumin's Dose | Main Findings | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [35] | Rat pheochromocytoma cells - PC12 In vitro |
For each treated group, two slides were prepared and then 50-100 cells were randomly selected. For each analysis, different groups were used such as control, curcumin administered with or without Aβ (0.1, 1, 10, 50μl) and Aβ alone (10, 25 and 50μl). α-tocopherol was used as positive control (4, 20 and 100μl). PC12 cells were pretreated with different concentrations of curcumin for 1 h and then incubated with or without Aβ for 24 h. | Pre-treatment with 5 μg/mL and 10 μg/mL of curcumin increased the viability of PC12 cells against cytotoxicity. This study indicated that curcumin attenuates Aβ-induced PC12 cell neurotoxicity through inhibition of oxidative stress, intracellular calcium levels and hyperphosphorylation of tau protein. | ||||||||||||
| [47] | Cortical neurons of Sprague-Dawley rats In vitro |
This study was designed using six groups: Control group, Aβ induced group and groups treated with 1, 10, 20 and 50 μg/mL of curcumin, over 7 days. | Curcumin may play a protective effect on pre-frontal cortical neurons against induced Aβ cytotoxicity. In addition, both AKT and caspase-3 are involved in the protective effects induced by curcumin. |
||||||||||||
| [49] | Different lineages and cortical neurons/APP: Human neuroglioma cells, rat neuroblastoma and Chinese hamster ovary cells In vitro |
Different concentrations of curcumin (1, 2.5, 5, 7.5, 10 and 20 μL) were investigated in this study after 24h, where several cell models were used. | Treatment of primary cortical neurons with different concentrations of curcumin demonstrated that the levels of both Aβ40 and Aβ42 significantly decreased when compared to control in all cell models. In addition, curcumin administration was able to reduce APP maturation. |
||||||||||||
| [45] | SK-N-SH cells In vitro |
This study used a control as well as 10 μg/mL of 6 curcumin derivatives (Cur1, Cur2, Cur3, Cur4, Cur5, Cur6). Aβ1-42 (10 μg/mL) was further added as a damaged cellular model. The cells were incubated for 24 hours with Aβ41-42. | The neuroprotective effect of curcumin and curcumin derivatives does not only depend directly on their particular chemical properties, but they can withstand Aβ damage by the regulation of MAP-2 expression. It is suggested that curcumin and Curl may be considered as an ideal therapeutic association for the treatment of AD. |
||||||||||||
| [39] | Human SH-SY5Y neuroblastoma cells transfected with APP In vitro |
Several groups were tested: Control; Curcumin at 0.1, 2.5, 5.0 and 20.0 μM for 24 hours or at 5.0 μM for 0, 12, 24 and 48 hours. AD models were obtained through lesions induced by Aβ40 and Aβ41 | Treatment with curcumin decreased both Presenilin 1 (PS1) and GSK-3 mRNA levels. In addition, curcumin increased the inhibitory phosphorylation of GSK-3 protein on Ser 9. Therefore, this study suggests that curcumin decreases AB production through inhibition of GSK-3-mediated activation of PS1. |
||||||||||||
| [40] | PC12 cell line dervided from rat pheochromocytoma In vitro |
For the group lesioned with Aβ, cortical neurons were cultured for 24 or 48 hours in culture medium at 5μL of Aβ. For the treated groups, neurons were incubated at 5, 10 and 20μM of curcumin for 24 hours with or without Aβ, where the same concentration of DMSO was used as vehicle control and Trolox, a vitamin E analogue, was used as positive control. |
Curcumin showed a protective effect on neuronal oxidative damage when added to Aβ cultured neurons. The ability of curcumin in eliminating free radicals and in inhibiting the formation and aggregation of Aβ, prevented the oxidative damage caused by the latter. |
||||||||||||
| [36] | Human SH-SY5Y neuroblastoma cells In vitro |
Four groups were tested: 1: Control group; 2: Aβ40 treated group; 3: Curcumin (5μM) pre-treatment prior to treatment with Aβ; 4: Curcumin (10μM) pretreatment prior to treatment with Aβ. The experiment protocol followed the treatment with Aβ for 24 hours and curcumin for 4 hours. | Curcumin protected cells against damage to mitochondrial energy metabolism induced by Aβ through inhibition of its depolarization and suppression of proteins related to mitochondrial apoptosis (cytochrome c, caspase-3 and Bax). Curcumin also normalized cellular antioxidant enzymes (SOD and catalase) in both expression and activity, which lead to a decrease in the levels of oxidative stress. In addition, it also regulated total expression of GSK-3β and phospho-Ser9. |
||||||||||||
| Refs. | Experimental Model | Experimental Groups and Curcumin's Dose | Main Findings | ||||||||||||
| [44] | The APPswe plasmid was transfected into the SH-SY5Y neuroblastoma cell line In vitro |
Cells were treated in group 1 with curcumin at 0, 1, 2.5, 5.0 and 20.0 μM (for 24 hours) or with curcumin at 5.0 μM for 0, 12, 24 and 48 hours. Group 2: Cells transfected with APPswe; Group 3: Cells transfected with APPswe and treated with curcumin at 5μM. Group 4: cells transfected with APPswe and treated with 5μM curcumin and LY294002; Group 5: Cells transfected with APPswe and treated with 5μM curcumin, LY294002 and Nrf2siRNA; Group 6: cells transfected with APPswe and treated with LY294002; Group 7: cells transfected with APPswe and treated with LY294002 and Nrf2siRNA. |
The results indicated that the cytoprotection induced by curcumin on SH-SY5Y cells transfected with APPswe was due to its ability to regulate the balance between heme oxygenase 1 and 2 via the intracellular PI3K/Akt/Nrf2 signaling pathway. In addition, curcumin showed to protect cells from cytotoxicity caused by H2O2. | ||||||||||||
| [51] | Primary neurons of the hippocampus of Sprague-Dawley rats In vitro |
The study groups were control, beta-amyloid group and groups treated with different concentrations of curcumin: 1, 5, 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 μL. Cells were treated for 7 days in culture. | The findings suggest that ROS mediate the toxicity of intracellular beta-amyloid and that curcumin may play a protective role by lowering the levels of ROS in neurons. The study indicated that curcumin may be beneficial for the treatment of AD. | ||||||||||||
| [52] | Wistar rats hippocampal cells In vitro |
6-8 days old animals were used in this study, which was divided into the following groups: Aβ25-35 control group, curcumin 1μM (Aβ + Cur 1μM), curcumin 5μM (Aβ + Cur 5μM) and curcumin 10μM (Aβ + Cur 10μM). Neurons were treated with Aβ at a concentration of 40 μg/mL. |
The results suggest that curcumin has a protective effect against the toxicity of Aβ25-35, regulating caspase-3 expression and inhibiting cyclin D1 protein with abnormal activation. | ||||||||||||
| [38] | Cultured human neuroblastoma cells SH-SY5Y In vitro |
A 40 μM aliquot of Aβ40 in the presence of 0, 0.5, 2.5 and 5 μM of curcumin (for DMPG spreading) or 40 μM of Aβ40 alone (for DMPG/DMPC or DMPG blends/curcumin spreading solutions) were used in this study. Cultivation ocurred between 7 and 10 days. |
Curcumin reduced Aβ-induced toxicity through a novel mechanism of neuroprotection by decreasing Aβ insertion in the membrane, which directly attenuates its toxic interactions, further reducing membrane rupture and decreasing the extent of insertion of Aβ in anionic lipid monolayers. | ||||||||||||
| [57] | Adult Wistar rats In vitro |
The animals were divided into 8 groups: (1) Control, (2) treated with free curcumin (Cur group) and (3) treated with curcumin-loaded lipid core nanocapsules (Cur-LNC group). Aβ-treated animals (1-42) were randomly divided into the following groups: (4) untreated (Group Aβ), (5) treated with free curcumin vehicle (Aβ Veh group), (6) treated with blank lipid core nanocapsules (Aβ B-LNC group), (7) treated with free curcumin (group Aβ Cur) and (8) treated with curcumin-loaded lipid core nanocapsules (Aβ-Cur-LNC group). | Curcumin administration was effective in preventing behavioral deficiencies, neuroinflammation and tau hyperphosphorylation, as well as cellular signaling disorders. Cur-LNC showed similar neuroprotective results in a 20-fold lower dose compared to the effective dose of free curcumin. The data suggests that curcumin is a potential therapeutic agent for neurocognition. | ||||||||||||
| [48] | Cortical neurons of Sprague-Dawley rats In vitro |
After 7 days of culture, the neurons were treated with curcumin (1, 5, 10, 25 and 50 μM) and compared to the control groups and to the ones treated with different concentrations of Aβ25-35 (1,5, 10, 25, 50μM) for 24 hours. Another group was pre-treated with curcumin (5-50μM) for 2 hours and then incubated in the presence or absence of Aβ25-35 (25μM) for 24 hours. |
The pre-treatment with curcumin protected neurons from toxicity, improved membrane potential and decreased ROS, resulting in inhibition of apoptotic cell death. In addition, curcumin was found to activate SIRT1 expression and decrease Bax expression. The protective effect of curcumin was blocked by SIRT1 siRNA. However, the results suggest that activation of SIRT1 is involved in the neuroprotective activity of curcumin. | ||||||||||||
| [46] | SK-N-SH cells In vitro |
Groups: Control group comprised SK-N-SH cells cultured in normal medium (DMEM/F12 containing 10% FBS); Lesioned group: 10 mg/mL of curcumin and Cur1 were used for 3 days and before the administration of Aβ1-42 (10 mg/mL), followed by incubation for 24 hours. | Curcumin and Cur1 protected Aβ1-42 cells and regulated the expression of hTERT. In addition, Curl has shown to have stronger protective effects than curcumin. However, when telomerase was inhibited by TERT siRNA, the neuroprotection caused by curcumin and Cur1 were ceased, as the effect depended on both curcumin and Cur1. |
||||||||||||
| Refs. | Experimental Model | Experimental Groups and Curcumin's Dose | Main Findings | ||||||||||||
| [41] | SH-SY5Y cells In vitro |
An injured group comprised Aβ treated cells was cultured in 10 mM Aβ-40 culture medium. For the curcumin treated group, the cells were incubated with curcumin for 4 hours and then incubated with 10mM Aβ-40, where the same concentration of DMSO was used as control. Cells were pretreated with curcumin (0.1mM, 5mM, and 10mM) for 4 hours followed by incubation with 10mM Aβ1-40 for 24 hours. | Curcumin induced cell growth and decreased Aβ-induced damage, attenuating the elevation of the cellular glutamate/GABA ratio. It also inhibited the increase of cellular Ca2+ and decreased the phosphorylation of both NMDA receptor and the cyclic AMP (CREB). Besides, curcumin decreased the activating transcription factor 1 (ATF-1). | ||||||||||||
| [53] | Neonate rat cells In vitro |
Groups: a) Control; b) Curcumin treated (microglia were treated with increasing concentrations of curcumin - 0.01, 0.1, 1, 5, 10 μm); c) Aβ42 (10 μm) alone; d) Aβ42 + curcumin (10 μm). Cells were evaluated over 0, 4, 24 and 48 hours. | Curcumin suppressed the inflammatory responses of brain microglia, abolishing interleukin (IL-1) and (IL-6), tumor necrosis factor alpha (TNF-α). In addition, it showed an inhibitory effect on the phosphorylation of ERK1/2 and p38 in microglia. | ||||||||||||
| [37] | PC12 cells In vitro |
PC12 cells (104 cells) seeded in a 96-well plate cultured for 3 days and pre-treated with Curcumin (12.5-200μl) for 0, 1, 3, 6, or 12 hours and/or associated with 2-40 μL of Aβ for an additional 24 hours. | The results suggest that curcumin suppresses apoptosis by inhibiting ROS-mediated oxidative damage and regulating ERK cascade. In addition, it improves the morphology and number of cells. Curcumin pre-treatment reversed the deregulation of the two pathways (MAPK and AKT). These results indicate that curcumin supplementation has the potential to reduce Aβ-induced cytotoxicity in PC12 cells. |
||||||||||||
| [42] | SHSY5Y cells In vitro |
Five different groups of cells were used: 1. Untreated SHSY5Y cells; 2. SHSY5Y cells incubated with Aβ 25-35 (30μM) for 4 hours; 3. SHSY5Y treated with curcumin (66.3μM) for 24 hours; 4. Aβ-treated SHSY5Y cells for 4 hours + curcumin for 24 hours; 5. SHSY5Y cells treated with curcumin for 24 hours and Aβ for 4 hours. |
The findings suggest that Aβ-incubated cells pre- and post-treated with curcumin reduced mitochondrial dysfunction and maintained viability, biogenesis and synaptic activity. Such protective effects were stronger in pre-treated cells, indicating that curcumin works best as a prophylactic agent in neurons with AD. | ||||||||||||
| [43] | Human neuroblastoma SH-SY5Y cells In vitro |
The groups used in this study were: Control (cultured cells); Cells + Aβ (100μM) and a group co-incubated with curcumin (50μM) + Aβ for 1, 4 or 10 days. | Curcumin showed a protective effect against Aβ-induced toxicity by modifying the Aβ aggregation pathway to form non-toxic aggregates. The curcumin’s neuroprotective effect is possibly mediated by the membrane as curcumin reduced the extent of cell membrane permeation induced by Aβ aggregates. | ||||||||||||
| [33] | Ratos APPswe / PS1dE9 In vivo |
Adult male and female APPswe rats/PS1dE9 (7.5-8.5 weeks old) were used in this study. 3 rats were treated with curcumin (7.5mg / kg / day) i.v. for 7 days. 6 groups treated with soluble Aβ (Aβ40, Aβ42, Aβ40/42) alone or in association with curcumin and 6 groups treated with insoluble Aβ (Aβ40, Aβ42, Aβ40/42) alone or in association with curcumin. | It was found that after daily administration of curcumin, senile and CAA plaques indicated that curcumin crosses the BBB and accumulates near the plaques. These data show that curcumin can reduce amyloid deposition in vivo and partially restores dendritic abnormalities, suggesting that such effects may reverse pathological AD symptoms. | ||||||||||||
| [57] | Neonates Wistar rats In vivo |
Animals with 6 to 8 days were used. 5 groups: Control, curcumin alone (10μm) for 24 hours and 48 hours, Aβ24, Aβ48 and Aβ48 + curcumin. The animals received a single intracerebroventricular injection of Aβ (1-42) and curcumin alone or as curcumin-loaded-lipid-core nanoparticles (Cur-LNC), given intraperitoneally for 10 days. | The results show the neuroprotective role of curcumin at a synaptic level. The identification of these mechanisms underlying the effects of curcumin may lead to new targets for future therapies toward AD. | ||||||||||||
| [54] | Sprague-Dawley rats In vivo |
53 male rats were randomly divided into three groups: a control group, an AD control group and an experimental group treated with curcumin (n = 16 per group). Control group received injection of 10μL of saline solution in the hippocampus. The AD control group received Aβ1-40 injection (10μL) in the hippocampus, whereas the experimental group was injected intraperitoneally with 300mg/kg of curcumin daily for 7 days. |
This study showed that curcumin inhibits the activation and expression of GFAP in the hippocampus. In addition, it significantly improves the spatial memory capacity of rats with AD. The authors concluded that curcumin, based on these pharmacological evidences, may present a new perspective for the treatment of AD. | ||||||||||||
| Refs. | Experimental Model | Experimental Groups and Curcumin's Dose | Main Findings | ||||||||||||
| [94] | Sprague-Dawley rats In vivo |
52 male rats were randomly divided into 3 groups: A simulated control group, an AD model group and a group treated with curcumin. 10 μL of saline solution was injected into the hippocampus, where the same volume of Aβ1-40 solution was also injected. The AD model rats were treated with 300mg/kg of curcumin or vehicle (DMSO) through intraperitoneal injection once daily for 7 days |
Curcumin improved learning and memory deficits in the AD model through a mechanism that might involve the suppression of CRMP-2 hyperphosphorylation and upregulation of CRMP-2 mediated by axonal regeneration. These findings, together with the proved safety of this compound, suggest that curcumin has great potential for treating AD. |
||||||||||||
| [56] | APPswe/PS1dE9 transgenic mice bred from C57/BL6J wild-type mice In vivo |
3 months old rats were randomly divided into 6 groups. C57/BL6J wild-type mice were used in the normal control group. In the other 5 groups, APPswe double knockout rats were used. The other 5 groups comprise the normal control group (vehicle), a positive control (10 mg/kg/day of rosiglitazone maleate) and 3 groups treated with different doses of curcumin: A low dose (100 mg/kg/day), a median dose (200 mg/kg/day) and a high dose (400 mg/kg/day). The study was conducted through 3 months. | Curcumin reduced Aβ aggregation through multiple mechanisms. This is the first study to demonstrate that the administration of curcumin reduces the expression of y-secretase specifically in the PS2 subunit, which has been associated with the decrease in the Aβ42 levels. |
||||||||||||
| [26] | Sprague Dawley rats In vivo |
Male rats were treated with different doses of curcumin (50, 100, and 200 mg/kg, i.p., once daily) through a chronic (seven consecutive days, once daily) or acute (single) administration. Saline solution was used as control. |
The findings suggest that the chronic administration of curcumin improves AD-related cognitive deficits as it upregulates ERK-BDNF signaling in the hippocampus. This study might elucidate the mechanism involved in the protective effect of curcumin against Aβ-induced cognitive dysfunction. |
||||||||||||
| [55] | APPSwe/PS1dE9 transgenic rats In vivo |
Rosiglitazone maleate 10mg/kg/day was used as positive control, whereas curcumin was used at three different concentrations: 400, 200 and 100 mg/kg/days during 6 months. |
The treatment with curcumin induced a decrease in the expression of InR and IRS-1 in the CA1 hippocampal area, while the expression of phosphorylated PI3K and AKT increased. The results showed that the curcumin dose of 200 mg/kg/day was the most effective. | ||||||||||||
| [59] | APPswe/PS1dE9 double transgenic mice In vivo and in vitro |
Animals with 3 months of age were used after 6 months of intervention with curcumin. Rats were randomly assigned to rosiglitazone maleate (10 mg x kg(-1) x d(-1) and curcumin at high (400 mg x kg(-1) x d(-1) 200 mg x kg(-1) x d(-1)) and low dose groups (100 mg x kg(-1) x d(-1)). | Curcumin significantly reduced Aβ40-42 and ADDL expressions in the hippocampus of transgenic mice. In addition, curcumin affected the Aβ cascade reaction by a mechanism of negative regulation expression. | ||||||||||||
| [58] | Hippocampal cells from APPswe / PS1dE9 transgenic mice and wild-type C57/ BL6J mice In vivo and in vitro |
Rats were randomly divided into 6 groups: Wild-type BL6J were used as the control group. APPswe / PS1dE9 double knockout mice were used in the other 5 groups. Only vehicle was used in the model group and rosiglitazone maleate (10 mg/kg/day) was used as positive control. The following doses were used in the treatment with curcumin: low dose curcumin group (LDC) received 100 mg/kg/day, the medium dose of curcumin (MDC) received 200 mg/kg/day and the high dose curcumin (HDC) group received 400 mg/kg/day. | Treatment with curcumin resulted in improvements in the amount, structure, and function of the synapses. The findings suggest that these positive results must have occurred through the regulation of the synaptic proteins PSD95 and Shank1. |
||||||||||||
| [61] | 5x FAD transgenic mice In vivo and in vitro |
Rats were randomly assigned to 3 groups: 1. Treated with 5x FAD vehicle; 2. Curcumin treated (5x FAD + curcumin at 150 mg/kg/day); 3. Curcumin treated at 300 mg/kg/day). These agents were administered by intragastric infusion over 60 days. |
The results showed that administration of curcumin (150 or 300 mg/kg/day) dramatically reduced Aβ production by decreasing BACE1 expression, which prevented synaptic degradation and improved spatial learning and memory impairment. |
||||||||||||
| [62] | Athymic rats and SK-N-SH cells In vivo and in vitro |
Animals with 6 weeks of age were used in this study. NanoCurc™ (equivalent to a dose of 25 mg/kg of curcumin) were administered to two groups (n = 12), whereas another group received the nanoparticles without curcumin (n = 6). These formulations were administered twice daily intraperitoneally for 4 weeks. Curcumin doses for the in vitro study were 1 nM, 10 nM, 50 nM, 100 nM, 500 nM, 1 pM, and 5 uM). |
The results suggest that NanoCurc™ administration protected human neuronal cells from oxidative damage. This nanoparticle formulation was able to cross the BBB and release significant amounts of curcumin into the rats’ brain. Moreover, it has the ability to reduce ROS-mediated damage, both in human cell culture and in animal models. |
||||||||||||
| Refs. | Experimental Model | Experimental Groups and Curcumin's Dose | Main Findings | ||||||||||||
| [63] | Wistar rats and cultured cells In vivo and in vitro |
Free curcumin as well as encapsulated in PLGA nanoparticles (CurPLGA) were investigated in this study at doses of 0.001, 0.01, 0.1, 0.2, 0.5, 5 and 50μM. Groups used in this study: Control; Aβ + β-catenin (0.5 mg/kg); Aβ + β-catenin (20 mg/kg); Aβ; Aβ + Cur-PLGANPS (0.5 mg/kg); Aβ + Cur-PLGANPS (20 mg/kg). |
The curcumin-loaded PLGA nanoparticles reversed memory and learning impairments in an experimental model of AD-like phenotypes. It is suggested that curcumin nanoparticles induce neurogenesis by activating the canonical pathway of Wnt/β-catenin and may offer a therapeutic approach for the treatment of neurodegenerative diseases such as AD. |
||||||||||||
| [60] | APP/PS1 transgenic mice and beta-amyloid-induced neuroinflammation in mixed neuronal/glial cultures In vivo and in vitro |
Groups: Control, APP/PS1; Curcumin (150 mg/kg) + APP/PS1; Curcumin + APP/PS1 + PPARγ antagonist GW9662. | Curcumin administration reduced the activation of microglia and astrocytes as well as the production of cytokines. It also inhibited the NF-κB pathway, suggesting that its beneficial effects might be due to the suppression of neuroinflammation. In addition, curcumin binds directly to PPARγ and increased its transcriptional activity. These findings resulted in significant improvement in memory deficits, where it seems that PPARγ is a potential target for curcumin. | ||||||||||||
Acknowledgements
Thanks go to Dr. Daniela Mendes dos Reis Riccardi, Post-graduation in Prescription of Herbal Medicines and Nutritional Supplementation Clinical and Sports, Estácio de Sá University, São Paulo, Brazil. All authors have made substantial, direct and intellectual contribution to the study, and gave final approval for its publication. This study was part of the requirements for the Master’s degree obtained by IMC (PPGSS/UERN). IMC was a recipient of a CAPES fellowship.
List of Abbreviations
- Ab 42
Amyloid beta 42
- AD
Alzheimer’s disease
- AKT
Protein kinase
- APP
Amyloid precursor protein
- ATF-1
Transcription factor 1
- Aβ
Amyloid-β
- BACE1
Beta-secretase 1
- BDNF
Brain Derived Neurotrophic Factor
- BHE
Hematoencephalic barrier
- CA1
Cornu ammonis 1
- CAA
Vascular amyloid
- CREB
Cellular transcription factor
- ERK
Extracellular signal-regulated kinase
- GABA
γ- aminobutyric acid
- GFAP
Glial fibrillary acid protein
- GSK3
Glycogen synthase kinase 3
- InR
Insulin receptor
- IRS-1
Insulin receptor substrate
- LDH
Lactate dehydrogenase
- MAPK
Mitogen-activated protein kinase
- Microliter
μl
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- Nanomolar
nM
- NFκB
Nuclear factor kappa B
- PC12
Cell line derived from pheochromocytoma of rat adrenal medulla
- PI3K
Phosphatidylinositol-3-kinase
- PPAR-γ
Peroxisome proliferator-activated receptor-gamma
- PS1
Presenilin 1
- PS2
Presenilin 2
- PSD95
Post-synaptic density protein 95
- ROS
Reactive oxygen species
- Shank 1
Multiple ankyrin repeat domains protein 1
- SHSY5Y
Human neuroblastoma cell line
- SIRT1
Sirtuin 1
- TNF-α
Tumor necrosis factor alpha
Consent for Publication
Not applicable.
Conflict of Interest
The authors certify that they have no affiliation with or financial involvement in any organization with a direct financial interest in the subject matter or materials discussed in the manuscript. This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Cole G.M., Frautschy S.A. Docosahexaenoic acid protects from amyloid and dendritic pathology in an Alzheimer’s disease mouse model. Nutr. Health. 2006;18(3):249–259. doi: 10.1177/026010600601800307. [http://dx.doi.org/10.1177/026010600601800307]. [PMID: 17180870]. [DOI] [PubMed] [Google Scholar]
- 2.Small D.H., Cappai R. Alois Alzheimer and Alzheimer’s disease: a centennial perspective. J. Neurochem. 2006;99(3):708–710. doi: 10.1111/j.1471-4159.2006.04212.x. [http://dx.doi.org/10.1111/j.1471-4159.2006.04212.x]. [PMID: 17076655]. [DOI] [PubMed] [Google Scholar]
- 3.2016 Alzheimer’s disease facts and figures. Alzheimers Dement. 2016;12(4):459–509. doi: 10.1016/j.jalz.2016.03.001. [http://dx.doi.org/10.1016/j.jalz.2016.03.001]. [PMID: 27570871]. [DOI] [PubMed] [Google Scholar]
- 4.Norrara B., Doerl J.G., Guzen F.P., Cavalcanti J.R.L.P., Freire M.A.M. Commentary: Localized vs. systematic neurodegeneration: A paradigm shift in understanding neurodegenerative diseases. Front. Syst. Neurosci. 2017;11:91. doi: 10.3389/fnsys.2017.00091. [http://dx.doi.org/10.3389/fnsys.2017.00091]. [PMID: 29270113]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santos J.R., Gois A.M., Mendonça D.M., Freire M.A.M. Nutritional status, oxidative stress and dementia: the role of selenium in Alzheimer’s disease. Front. Aging Neurosci. 2014;6:206. doi: 10.3389/fnagi.2014.00206. [http://dx.doi.org/10.3389/fnagi.2014.00206]. [PMID: 25221506]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heneka M.T., O’Banion M.K. Inflammatory processes in Alzheimer’s disease. J. Neuroimmunol. 2007;184(1-2):69–91. doi: 10.1016/j.jneuroim.2006.11.017. [http://dx.doi.org/10.1016/j.jneuroim.2006.11.017]. [PMID: 17222916]. [DOI] [PubMed] [Google Scholar]
- 7.Mattson M.P. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–639. doi: 10.1038/nature02621. [http://dx.doi.org/10.1038/nature02621]. [PMID: 15295589]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kasper D., Fauci A., Hauser S., Longo D., Jameson J.L., Loscalzo J. Harrison’s Principles of Internal Medicine. 19th ed. 2015. [Google Scholar]
- 9.Patten D.A., Germain M., Kelly M.A., Slack R.S. Reactive oxygen species: stuck in the middle of neurodegeneration. J. Alzheimers Dis. 2010;20(Suppl. 2):S357–S367. doi: 10.3233/JAD-2010-100498. [http://dx.doi.org/10.3233/JAD-2010-100498]. [PMID: 20421690]. [DOI] [PubMed] [Google Scholar]
- 10.Freire M.A.M. Pathophysiology of neurodegeneration following traumatic brain injury. West Indian Med. J. 2012;61(7):751–755. [PMID: 23620976]. [PubMed] [Google Scholar]
- 11.Houstis N., Rosen E.D., Lander E.S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [http://dx.doi.org/10.1038/nature04634]. [PMID: 16612386]. [DOI] [PubMed] [Google Scholar]
- 12.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7(1):65–74. doi: 10.2174/157015909787602823. [http://dx.doi.org/10.2174/157015909787602823]. [PMID: 19721819]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dam D., De Deyn P.P. Drug discovery in dementia: the role of rodent models. Nat. Rev. Drug Discov. 2006;5(11):956–970. doi: 10.1038/nrd2075. [http://dx.doi.org/10.1038/nrd2075]. [PMID: 17080031]. [DOI] [PubMed] [Google Scholar]
- 14.Sachan A., Singh S., Singh H.K., Shankar P., Kumar D., Sachan A.K., Nath R., Dixit R. An experimental study to evaluate the effect of mucuna pruriens on learning and memory in mice. Int J Innov Sci Res. 2015;4:144–148. [Google Scholar]
- 15.Izzo A.A., Hoon-Kim S., Radhakrishnan R., Williamson E.M. A critical approach to evaluating clinical efficacy, adverse events and drug interactions of herbal remedies. Phytother. Res. 2016;30(5):691–700. doi: 10.1002/ptr.5591. [http://dx.doi.org/10.1002/ptr.5591]. [PMID: 26887532]. [DOI] [PubMed] [Google Scholar]
- 16.Bisht K., Wagner K.H., Bulmer A.C. Curcumin, resveratrol and flavonoids as anti-inflammatory, cyto- and DNA-protective dietary compounds. Toxicology. 2010;278(1):88–100. doi: 10.1016/j.tox.2009.11.008. [http://dx.doi.org/10.1016/j.tox.2009.11.008]. [PMID: 19903510]. [DOI] [PubMed] [Google Scholar]
- 17.Mattson M.P., Son T.G., Camandola S. Viewpoint: mechanisms of action and therapeutic potential of neurohormetic phytochemicals. Dose Response. 2007;5(3):174–186. doi: 10.2203/dose-response.07-004.Mattson. [http://dx.doi.org/10.2203/dose-response.07-004.Mattson]. [PMID: 18648607]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal B.B., Gupta S.C., Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013;169(8):1672–1692. doi: 10.1111/bph.12131. [http://dx.doi.org/10.1111/bph.12131]. [PMID: 23425071]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Carlo G., Mascolo N., Izzo A.A., Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999;65(4):337–353. doi: 10.1016/s0024-3205(99)00120-4. [http://dx.doi.org/10.1016/S0024-3205(99)00120-4]. [PMID: 10421421]. [DOI] [PubMed] [Google Scholar]
- 20.Shakibaei M., Harikumar K.B., Aggarwal B.B. Resveratrol addiction: to die or not to die. Mol. Nutr. Food Res. 2009;53(1):115–128. doi: 10.1002/mnfr.200800148. [http://dx.doi.org/10.1002/mnfr.200800148]. [PMID: 19072742]. [DOI] [PubMed] [Google Scholar]
- 21.Hossen M.S., Tanvir E.M., Prince M.B., Paul S., Saha M., Ali M.Y., Gan S.H., Khalil M.I., Karim N. Protective mechanism of turmeric (Curcuma longa) on carbofuran-induced hematological and hepatic toxicities in a rat model. Pharm. Biol. 2017;55(1):1937–1945. doi: 10.1080/13880209.2017.1345951. [http://dx.doi.org/10.1080/13880209.2017.1345951]. [PMID: 28675957]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kita T., Imai S., Sawada H., Kumagai H., Seto H. The biosynthetic pathway of curcuminoid in turmeric (Curcuma longa) as revealed by 13C-labeled precursors. Biosci. Biotechnol. Biochem. 2008;72(7):1789–1798. doi: 10.1271/bbb.80075. [http://dx.doi.org/10.1271/bbb.80075]. [PMID: 18603793]. [DOI] [PubMed] [Google Scholar]
- 23.Sandhu A.K., Gray D.J., Lu J., Gu L. Effects of exogenous abscisic acid on antioxidant capacities, anthocyanins, and flavonol contents of muscadine grape (Vitis rotundifolia) skins. Food Chem. 2011;126:982–988. [http://dx.doi.org/10.1016/j.foodchem.2010.11.105]. [Google Scholar]
- 24.Solanki I., Parihar P., Mansuri M.L., Parihar M.S. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 2015;6(1):64–72. doi: 10.3945/an.114.007500. [http://dx.doi.org/10.3945/an.114.007500]. [PMID: 25593144]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lorenzi H., Matos F.J.A. Plantas medicinais no Brasil: nativas e exóticas cultivadas. 2002.
- 26.Zhang L., Fang Y., Xu Y., Lian Y., Xie N., Wu T., Zhang H., Sun L., Zhang R., Wang Z. Curcumin improves amyloid β-Peptide (1-42) induced spatial memory deficits through BDNF-ERK signaling pathway. PLoS One. 2015;10(6):e0131525. doi: 10.1371/journal.pone.0131525. [http://dx.doi.org/10.1371/journal.pone.0131525]. [PMID: 26114940]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Costa I.M., Cavalcanti J.R.L.P., de Queiroz D.B., de Azevedo E.P., do Rêgo A.C.M., Araújo Filho I., Parente P., Botelho M.A., Guzen F.P. Supplementation with herbal Extracts to promote behavioral and neuroprotective effects in experimental models of Parkinson’s Disease: A systematic review. Phytother. Res. 2017;31(7):959–970. doi: 10.1002/ptr.5813. [http://dx.doi.org/10.1002/ptr.5813]. [PMID: 28544038]. [DOI] [PubMed] [Google Scholar]
- 28.Darvesh A.S., Carroll R.T., Bishayee A., Novotny N.A., Geldenhuys W.J., Van der Schyf C.J. Curcumin and neurodegenerative diseases: a perspective. Expert Opin. Investig. Drugs. 2012;21(8):1123–1140. doi: 10.1517/13543784.2012.693479. [http://dx.doi.org/10.1517/13543784.2012.693479]. [PMID: 22668065]. [DOI] [PubMed] [Google Scholar]
- 29.Strimpakos A.S., Sharma R.A. Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid. Redox Signal. 2008;10(3):511–545. doi: 10.1089/ars.2007.1769. [http://dx.doi.org/10.1089/ars.2007.1769]. [PMID: 18370854]. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed T., Gilani A.H. Therapeutic potential of turmeric in Alzheimer’s disease: curcumin or curcuminoids? Phytother. Res. 2014;28(4):517–525. doi: 10.1002/ptr.5030. [http://dx.doi.org/10.1002/ptr.5030]. [PMID: 23873854]. [DOI] [PubMed] [Google Scholar]
- 31.Wright L.E., Frye J.B., Gorti B., Timmermann B.N., Funk J.L. Bioactivity of turmeric-derived curcuminoids and related metabolites in breast cancer. Curr. Pharm. Des. 2013;19(34):6218–6225. doi: 10.2174/1381612811319340013. [http://dx.doi.org/10.2174/1381612811319340013]. [PMID: 23448448]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunnumakkara A.B., Bordoloi D., Padmavathi G., Monisha J., Roy N.K., Prasad S., Aggarwal B.B. Curcumin, the golden nutraceutical: multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017;174(11):1325–1348. doi: 10.1111/bph.13621. [http://dx.doi.org/10.1111/bph.13621]. [PMID: 27638428]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Alloza M., Borrelli L.A., Rozkalne A., Hyman B.T., Bacskai B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007;102(4):1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [http://dx.doi.org/10.1111/j.1471-4159.2007.04613.x]. [PMID: 17472706]. [DOI] [PubMed] [Google Scholar]
- 34.Lim G.P., Chu T., Yang F., Beech W., Frautschy S.A., Cole G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21(21):8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [http://dx.doi.org/10.1523/JNEUROSCI.21-21-08370.2001]. [PMID: 11606625]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park S.Y., Kim H.S., Cho E.K., Kwon B.Y., Phark S., Hwang K.W., Sul D. Curcumin protected PC12 cells against beta-amyloid-induced toxicity through the inhibition of oxidative damage and tau hyperphosphorylation. Food Chem. Toxicol. 2008;46(8):2881–2887. doi: 10.1016/j.fct.2008.05.030. [http://dx.doi.org/10.1016/j.fct.2008.05.030]. [PMID: 18573304]. [DOI] [PubMed] [Google Scholar]
- 36.Huang H.C., Chang P., Dai X.L., Jiang Z.F. Protective effects of curcumin on amyloid-β-induced neuronal oxidative damage. Neurochem. Res. 2012;37(7):1584–1597. doi: 10.1007/s11064-012-0754-9. [http://dx.doi.org/10.1007/s11064-012-0754-9]. [PMID: 22476982]. [DOI] [PubMed] [Google Scholar]
- 37.Fan C.D., Li Y., Fu X.T., Wu Q.J., Hou Y.J., Yang M.F., Sun J.Y., Fu X.Y., Zheng Z.C., Sun B.L. Reversal of beta-amyloid-induced neurotoxicity in PC12 cells by curcumin, the important role of ROS-mediated signaling and ERK pathway. Cell. Mol. Neurobiol. 2017;37(2):211–222. doi: 10.1007/s10571-016-0362-3. [http://dx.doi.org/10.1007/s10571-016-0362-3]. [PMID: 26971524]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thapa A., Vernon B.C., De la Peña K., Soliz G., Moreno H.A., López G.P., Chi E.Y. Membrane-mediated neuroprotection by curcumin from amyloid-β-peptide-induced toxicity. Langmuir. 2013;29(37):11713–11723. doi: 10.1021/la4020459. [http://dx.doi.org/10.1021/la4020459]. [PMID: 24004419]. [DOI] [PubMed] [Google Scholar]
- 39.Xiong Z., Hongmei Z., Lu S., Yu L. Curcumin mediates presenilin-1 activity to reduce β-amyloid production in a model of Alzheimer’s disease. Pharmacol. Rep. 2011;63(5):1101–1108. doi: 10.1016/s1734-1140(11)70629-6. [http://dx.doi.org/10.1016/S1734-1140(11)70629-6]. [PMID: 22180352]. [DOI] [PubMed] [Google Scholar]
- 40.Huang H.C., Xu K., Jiang Z.F. Curcumin-mediated neuroprotection against amyloid-β-induced mitochondrial dysfunction involves the inhibition of GSK-3β. J. Alzheimers Dis. 2012;32(4):981–996. doi: 10.3233/JAD-2012-120688. [http://dx.doi.org/10.3233/JAD-2012-120688]. [PMID: 22886017]. [DOI] [PubMed] [Google Scholar]
- 41.Huang H.C., Chang P., Lu S.Y., Zheng B.W., Jiang Z.F. Protection of curcumin against amyloid-β-induced cell damage and death involves the prevention from NMDA receptor-mediated intracellular Ca2+ elevation. J. Recept. Signal Transduct. Res. 2015;35(5):450–457. doi: 10.3109/10799893.2015.1006331. [http://dx.doi.org/10.3109/10799893.2015.1006331]. [PMID: 26053510]. [DOI] [PubMed] [Google Scholar]
- 42.Reddy P.H., Manczak M., Yin X., Grady M.C., Mitchell A., Kandimalla R., Kuruva C.S. Protective effects of a natural product, curcumin, against amyloid β induced mitochondrial and synaptic toxicities in Alzheimer’s disease. J. Investig. Med. 2016;64(8):1220–1234. doi: 10.1136/jim-2016-000240. [http://dx.doi.org/10.1136/jim-2016-000240]. [PMID: 27521081]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thapa A., Jett S.D., Chi E.Y. Curcumin attenuates Amyloid-β aggregate Ttoxicity and modulates amyloid-β aggregation pathway. ACS Chem. Neurosci. 2016;7(1):56–68. doi: 10.1021/acschemneuro.5b00214. [http://dx.doi.org/10.1021/acschemneuro.5b00214]. [PMID: 26529184]. [DOI] [PubMed] [Google Scholar]
- 44.Yin W., Zhang X., Shi X., Li Y. Curcumin protects SH-SY5Y cells from oxidative stress by up-regulating HO-1 via Phosphatidylinositol 3 Kinase/Akt/Nrf-2 and down-regulating HO-2. Mol. Neurodegener. 2012;7(Suppl. 1):S14. [http://dx.doi.org/10.1186/1750-1326-7-S1-S14]. [Google Scholar]
- 45.Xiao Z., Lin L., Liu Z., Ji F., Shao W., Wang M., Liu L., Li S., Li F., Bu X. Potential therapeutic effects of curcumin: relationship to microtubule-associated proteins 2 in Aβ1-42 insult. Brain Res. 2010;1361:115–123. doi: 10.1016/j.brainres.2010.09.019. [http://dx.doi.org/10.1016/j.brainres.2010.09.019]. [PMID: 20840842]. [DOI] [PubMed] [Google Scholar]
- 46.Xiao Z., Zhang A., Lin J., Zheng Z., Shi X., Di W., Qi W., Zhu Y., Zhou G., Fang Y. Telomerase: a target for therapeutic effects of curcumin and a curcumin derivative in Aβ1-42 insult in vitro. PLoS One. 2014;9(7):e101251. doi: 10.1371/journal.pone.0101251. [http://dx.doi.org/10.1371/journal.pone.0101251]. [PMID: 24983737]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin X.Y., Cheng Y., Yu L.C. Potential protection of curcumin against intracellular amyloid beta-induced toxicity in cultured rat prefrontal cortical neurons. Neurosci. Lett. 2010;480(1):21–24. doi: 10.1016/j.neulet.2010.05.062. [http://dx.doi.org/10.1016/j.neulet.2010.05.062]. [PMID: 20638958]. [DOI] [PubMed] [Google Scholar]
- 48.Sun Q., Jia N., Wang W., Jin H., Xu J., Hu H. Protective effects of astragaloside IV against amyloid beta1-42 neurotoxicity by inhibiting the mitochondrial permeability transition pore opening. PLoS One. 2014;9(6):e98866. doi: 10.1371/journal.pone.0098866. [http://dx.doi.org/10.1371/journal.pone.0098866]. [PMID: 24905226]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C., Browne A., Child D., Tanzi R.E. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J. Biol. Chem. 2010;285(37):28472–28480. doi: 10.1074/jbc.M110.133520. [http://dx.doi.org/10.1074/jbc.M110.133520]. [PMID: 20622013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang C., Browne A., Divito J.R., Stevenson J.A., Romano D., Dong Y., Xie Z., Tanzi R.E. Amyloid-β production via cleavage of amyloid-β protein precursor is modulated by cell density. J. Alzheimers Dis. 2010;22(2):683–984. doi: 10.3233/JAD-2010-100816. [http://dx.doi.org/10.3233/JAD-2010-100816]. [PMID: 20847415]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye M.X., Li Y., Yin H., Zhang J. Curcumin: updated molecular mechanisms and intervention targets in human lung cancer. Int. J. Mol. Sci. 2012;13(3):3959–3978. doi: 10.3390/ijms13033959. [http://dx.doi.org/10.3390/ijms13033959]. [PMID: 22489192]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J., Zhang Y.J., Du S. The protective effect of curcumin on Aβ induced aberrant cell cycle reentry on primary cultured rat cortical neurons. Eur. Rev. Med. Pharmacol. Sci. 2012;16(4):445–454. [PMID: 22696871]. [PubMed] [Google Scholar]
- 53.Shi X., Zheng Z., Li J., Xiao Z., Qi W., Zhang A., Wu Q., Fang Y. Curcumin inhibits Aβ-induced microglial inflammatory responses in vitro: Involvement of ERK1/2 and p38 signaling pathways. Neurosci. Lett. 2015;594:105–110. doi: 10.1016/j.neulet.2015.03.045. [http://dx.doi.org/10.1016/j.neulet.2015.03.045]. [PMID: 25818332]. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y., Yin H., Wang L., Shuboy A., Lou J., Han B., Zhang X., Li J. Curcumin as a potential treatment for Alzheimer’s disease: a study of the effects of curcumin on hippocampal expression of glial fibrillary acidic protein. Am. J. Chin. Med. 2013;41(1):59–70. doi: 10.1142/S0192415X13500055. [http://dx.doi.org/10.1142/S0192415X13500055]. [PMID: 23336507]. [DOI] [PubMed] [Google Scholar]
- 55.Feng H.L., Dang H.Z., Fan H., Chen X.P., Rao Y.X., Ren Y., Yang J.D., Shi J., Wang P.W., Tian J.Z. Curcumin ameliorates insulin signalling pathway in brain of Alzheimer’s disease transgenic mice. Int. J. Immunopathol. Pharmacol. 2016;29(4):734–741. doi: 10.1177/0394632016659494. [http://dx.doi.org/10.1177/0394632016659494]. [PMID: 27466310]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang P., Su C., Li R., Wang H., Ren Y., Sun H., Yang J., Sun J., Shi J., Tian J., Jiang S. Mechanisms and effects of curcumin on spatial learning and memory improvement in APPswe/PS1dE9 mice. J. Neurosci. Res. 2014;92(2):218–231. doi: 10.1002/jnr.23322. [http://dx.doi.org/10.1002/jnr.23322]. [PMID: 24273069]. [DOI] [PubMed] [Google Scholar]
- 57.Hoppe J.B., Coradini K., Frozza R.L., Oliveira C.M., Meneghetti A.B., Bernardi A., Pires E.S., Beck R.C., Salbego C.G. Free and nanoencapsulated curcumin suppress β-amyloid-induced cognitive impairments in rats: involvement of BDNF and Akt/GSK-3β signaling pathway. Neurobiol. Learn. Mem. 2013;106:134–144. doi: 10.1016/j.nlm.2013.08.001. [http://dx.doi.org/10.1016/j.nlm.2013.08.001]. [PMID: 23954730]. [DOI] [PubMed] [Google Scholar]
- 58.He Y., Wang P., Wei P., Feng H., Ren Y., Yang J., Rao Y., Shi J., Tian J. Effects of curcumin on synapses in APPswe/PS1dE9 mice. Int. J. Immunopathol. Pharmacol. 2016;29(2):217–225. doi: 10.1177/0394632016638099. [http://dx.doi.org/10.1177/0394632016638099]. [PMID: 26957323]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng H.L., Fan H., Dang H.Z., Chen X.P., Ren Y., Yang J.D., Wang P.W. Zhongguo Zhongyao Zazhi. 2014;39(19):3846–3849. [Neuroprotective effect of curcumin to Aβ of double transgenic mice with Alzheimer’s disease]. [PMID: 25612452]. [PubMed] [Google Scholar]
- 60.Liu Z.J., Li Z.H., Liu L., Tang W.X., Wang Y., Dong M.R., Xiao C. Curcumin attenuates beta-Amyloid-induced neuroinflammation via activation of peroxisome proliferator-activated receptor-gamma function in a rat model of Alzheimer’s disease. Front. Pharmacol. 2016;7:261. doi: 10.3389/fphar.2016.00261. [http://dx.doi.org/10.3389/fphar.2016.00261]. [PMID: 27594837]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng K., Dai X., Xiao N., Wu X., Wei Z., Fang W., Zhu Y., Zhang J., Chen X. Curcumin ameliorates memory decline via inhibiting BACE1 expression and β-Amyloid Pathology in 5×FAD transgenic mice. Mol. Neurobiol. 2017;54(3):1967–1977. doi: 10.1007/s12035-016-9802-9. [http://dx.doi.org/10.1007/s12035-016-9802-9]. [PMID: 26910813]. [DOI] [PubMed] [Google Scholar]
- 62.Ray B., Bisht S., Maitra A., Maitra A., Lahiri D.K. Neuroprotective and neurorescue effects of a novel polymeric nanoparticle formulation of curcumin (NanoCurc™) in the neuronal cell culture and animal model: implications for Alzheimer’s disease. J. Alzheimers Dis. 2011;23(1):61–77. doi: 10.3233/JAD-2010-101374. [http://dx.doi.org/10.3233/JAD-2010-101374]. [PMID: 20930270]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tiwari S.K., Agarwal S., Seth B., Yadav A., Nair S., Bhatnagar P., Karmakar M., Kumari M., Chauhan L.K., Patel D.K., Srivastava V., Singh D., Gupta S.K., Tripathi A., Chaturvedi R.K., Gupta K.C. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano. 2014;8(1):76–103. doi: 10.1021/nn405077y. [http://dx.doi.org/10.1021/nn405077y]. [PMID: 24467380]. [DOI] [PubMed] [Google Scholar]
- 64.Masters C.L., Selkoe D.J. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2(6):a006262. doi: 10.1101/cshperspect.a006262. [http://dx.doi.org/10.1101/cshperspect.a006262]. [PMID: 22675658]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng X.Y. Pharmacopoeia of the Peoples Republic of China. (Chinese edn.) 2005;Vol. 1 [Google Scholar]
- 66.Praticò D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: lights and shadows. Ann. N. Y. Acad. Sci. 2008;1147:70–78. doi: 10.1196/annals.1427.010. [http://dx.doi.org/10.1196/annals.1427.010]. [PMID: 19076432]. [DOI] [PubMed] [Google Scholar]
- 67.Praticò D. Oxidative stress hypothesis in Alzheimer’s disease: a reappraisal. Trends Pharmacol. Sci. 2008;29(12):609–615. doi: 10.1016/j.tips.2008.09.001. [http://dx.doi.org/10.1016/j.tips.2008.09.001]. [PMID: 18838179]. [DOI] [PubMed] [Google Scholar]
- 68.Fukui K., Takatsu H., Shinkai T., Suzuki S., Abe K., Urano S. Appearance of amyloid beta-like substances and delayed-type apoptosis in rat hippocampus CA1 region through aging and oxidative stress. J. Alzheimers Dis. 2005;8(3):299–309. doi: 10.3233/jad-2005-8309. [http://dx.doi.org/10.3233/JAD-2005-8309]. [PMID: 16340088]. [DOI] [PubMed] [Google Scholar]
- 69.Butterfield D.A., Lauderback C.M. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002;32(11):1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [http://dx.doi.org/10.1016/S0891-5849(02)00794-3]. [PMID: 12031889]. [DOI] [PubMed] [Google Scholar]
- 70.Abdul H.M., Calabrese V., Calvani M., Butterfield D.A. Acetyl-L-carnitine-induced up-regulation of heat shock proteins protects cortical neurons against amyloid-beta peptide 1-42-mediated oxidative stress and neurotoxicity: implications for Alzheimer’s disease. J. Neurosci. Res. 2006;84(2):398–408. doi: 10.1002/jnr.20877. [http://dx.doi.org/10.1002/jnr.20877]. [PMID: 16634066]. [DOI] [PubMed] [Google Scholar]
- 71.Xiao X.Q., Wang R., Tang X.C. Huperzine A and tacrine attenuate beta-amyloid peptide-induced oxidative injury. J. Neurosci. Res. 2000;61(5):564–569. doi: 10.1002/1097-4547(20000901)61:5<564::AID-JNR11>3.0.CO;2-X. [http://dx.doi.org/10.1002/1097-4547(20000901)61:5<564:AID-JNR11>3.0.CO;2-X]. [PMID: 10956426]. [DOI] [PubMed] [Google Scholar]
- 72.Canevari L., Abramov A.Y., Duchen M.R. Toxicity of amyloid beta peptide: tales of calcium, mitochondria, and oxidative stress. Neurochem. Res. 2004;29(3):637–650. doi: 10.1023/b:nere.0000014834.06405.af. [http://dx.doi.org/10.1023/B:NERE.0000014834.06405.af]. [PMID: 15038611]. [DOI] [PubMed] [Google Scholar]
- 73.Ban J.Y., Jeon S.Y., Bae K., Song K.S., Seong Y.H. Catechin and epicatechin from Smilacis chinae rhizome protect cultured rat cortical neurons against amyloid beta protein (25-35)-induced neurotoxicity through inhibition of cytosolic calcium elevation. Life Sci. 2006;79(24):2251–2259. doi: 10.1016/j.lfs.2006.07.021. [http://dx.doi.org/10.1016/j.lfs.2006.07.021]. [PMID: 16978655]. [DOI] [PubMed] [Google Scholar]
- 74.Fu H., Li W., Lao Y., Luo J., Lee N.T., Kan K.K., Tsang H.W., Tsim K.W., Pang Y., Li Z., Chang D.C., Li M., Han Y. Bis(7)-tacrine attenuates beta amyloid-induced neuronal apoptosis by regulating L-type calcium channels. J. Neurochem. 2006;98(5):1400–1410. doi: 10.1111/j.1471-4159.2006.03960.x. [http://dx.doi.org/10.1111/j.1471-4159.2006.03960.x]. [PMID: 16771827]. [DOI] [PubMed] [Google Scholar]
- 75.Swerdlow R.H. Mitochondria in cybrids containing mtDNA from persons with mitochondriopathies. J. Neurosci. Res. 2007;85(15):3416–3428. doi: 10.1002/jnr.21167. [http://dx.doi.org/10.1002/jnr.21167]. [PMID: 17243174]. [DOI] [PubMed] [Google Scholar]
- 76.Bertram L., Tanzi R.E. Thirty years of Alzheimer’s disease genetics: the implications of systematic meta-analyses. Nat. Rev. Neurosci. 2008;9(10):768–778. doi: 10.1038/nrn2494. [http://dx.doi.org/10.1038/nrn2494]. [PMID: 18802446]. [DOI] [PubMed] [Google Scholar]
- 77.Tanzi R.E., Gusella J.F., Watkins P.C., Bruns G.A., St George-Hyslop P., Van Keuren M.L., Patterson D., Pagan S., Kurnit D.M., Neve R.L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987;235(4791):880–884. doi: 10.1126/science.2949367. [http://dx.doi.org/10.1126/science.2949367]. [PMID: 2949367]. [DOI] [PubMed] [Google Scholar]
- 78.Morris R.G., Garrud P., Rawlins J.N., O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [http://dx.doi.org/10.1038/297681a0]. [PMID: 7088155]. [DOI] [PubMed] [Google Scholar]
- 79.Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y.A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341(6238):149–152. doi: 10.1038/341149a0. [http://dx.doi.org/10.1038/341149a0]. [PMID: 2779653]. [DOI] [PubMed] [Google Scholar]
- 80.Lu B., Nagappan G., Lu Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [http://dx.doi.org/10.1007/978-3-642-45106-5_9]. [PMID: 24668475]. [DOI] [PubMed] [Google Scholar]
- 81.Shoba G., Joy D., Joseph T., Majeed M., Rajendran R., Srinivas P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–356. doi: 10.1055/s-2006-957450. [http://dx.doi.org/10.1055/s-2006-957450]. [PMID: 9619120]. [DOI] [PubMed] [Google Scholar]
- 82.Suresh D., Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J. Med. Res. 2010;131:682–691. [PMID: 20516541]. [PubMed] [Google Scholar]
- 83.Cox K.H., Pipingas A., Scholey A.B. Investigation of the effects of solid lipid curcumin on cognition and mood in a healthy older population. J. Psychopharmacol. (Oxford) 2015;29(5):642–651. doi: 10.1177/0269881114552744. [http://dx.doi.org/10.1177/0269881114552744]. [PMID: 25277322]. [DOI] [PubMed] [Google Scholar]
- 84.Baum L., Lam C.W., Cheung S.K., Kwok T., Lui V., Tsoh J., Lam L., Leung V., Hui E., Ng C., Woo J., Chiu H.F., Goggins W.B., Zee B.C., Cheng K.F., Fong C.Y., Wong A., Mok H., Chow M.S., Ho P.C., Ip S.P., Ho C.S., Yu X.W., Lai C.Y., Chan M.H., Szeto S., Chan I.H., Mok V. Six-month randomized, placebo-controlled, double-blind, pilot clinical trial of curcumin in patients with Alzheimer disease. J. Clin. Psychopharmacol. 2008;28(1):110–113. doi: 10.1097/jcp.0b013e318160862c. [http://dx.doi.org/10.1097/jcp.0b013e318160862c]. [PMID: 18204357]. [DOI] [PubMed] [Google Scholar]
- 85.Begum A.N., Jones M.R., Lim G.P., Morihara T., Kim P., Heath D.D., Rock C.L., Pruitt M.A., Yang F., Hudspeth B., Hu S., Faull K.F., Teter B., Cole G.M., Frautschy S.A. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008;326(1):196–208. doi: 10.1124/jpet.108.137455. [http://dx.doi.org/10.1124/jpet.108.137455]. [PMID: 18417733]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ringman J.M., Frautschy S.A., Teng E., Begum A.N., Bardens J., Beigi M., Gylys K.H., Badmaev V., Heath D.D., Apostolova L.G., Porter V., Vanek Z., Marshall G.A., Hellemann G., Sugar C., Masterman D.L., Montine T.J., Cummings J.L., Cole G.M. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimers Res. Ther. 2012;4(5):43. doi: 10.1186/alzrt146. [http://dx.doi.org/10.1186/alzrt146]. [PMID: 23107780]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hishikawa N., Takahashi Y., Amakusa Y., Tanno Y., Tuji Y., Niwa H., Murakami N., Krishna U.K. Effects of turmeric on Alzheimer’s disease with behavioral and psychological symptoms of dementia. Ayu. 2012;33(4):499–504. doi: 10.4103/0974-8520.110524. [http://dx.doi.org/10.4103/0974-8520.110524]. [PMID: 23723666]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chandra V., Pandav R., Dodge H.H., Johnston J.M., Belle S.H., DeKosky S.T., Ganguli M. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology. 2001;57(6):985–989. doi: 10.1212/wnl.57.6.985. [http://dx.doi.org/10.1212/WNL.57.6.985]. [PMID: 11571321]. [DOI] [PubMed] [Google Scholar]
- 89.Chang C.H., Chen H.X., Yü G., Peng C.C., Peng R.Y. Curcumin-Protected PC12 Cells Against Glutamate-Induced Oxidative Toxicity. Food Technol. Biotechnol. 2014;52(4):468–478. doi: 10.17113/ftb.52.04.14.3622. [http://dx.doi.org/10.17113/ftb.52.04.14.3622]. [PMID: 27904320]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang R., Li Y.B., Li Y.H., Xu Y., Wu H.L., Li X.J. Curcumin protects against glutamate excitotoxicity in rat cerebral cortical neurons by increasing brain-derived neurotrophic factor level and activating TrkB. Brain Res. 2008;1210:84–91. doi: 10.1016/j.brainres.2008.01.104. [http://dx.doi.org/10.1016/j.brainres.2008.01.104]. [PMID: 18420184]. [DOI] [PubMed] [Google Scholar]
- 91.Yang F., Lim G.P., Begum A.N., Ubeda O.J., Simmons M.R., Ambegaokar S.S., Chen P.P., Kayed R., Glabe C.G., Frautschy S.A., Cole G.M. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [http://dx.doi.org/10.1074/jbc.M404751200]. [PMID: 15590663]. [DOI] [PubMed] [Google Scholar]
- 92.Borchelt D.R., Lee M.K., Gonzales V., Slunt H.H., Ratovitski T., Jenkins N.A., Copeland N.G., Price D.L., Sisodia S.S. Accumulation of proteolytic fragments of mutant presenilin 1 and accelerated amyloid deposition are co-regulated in transgenic mice. Neurobiol. Aging. 2002;23(2):171–177. doi: 10.1016/s0197-4580(01)00280-9. [http://dx.doi.org/10.1016/S0197-4580(01)00280-9]. [PMID: 11804700]. [DOI] [PubMed] [Google Scholar]
- 93.Garcia-Alloza M., Robbins E.M., Zhang-Nunes S.X., Purcell S.M., Betensky R.A., Raju S., Prada C., Greenberg S.M., Bacskai B.J., Frosch M.P. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis. 2006;24(3):516–524. doi: 10.1016/j.nbd.2006.08.017. [http://dx.doi.org/10.1016/j.nbd.2006.08.017]. [PMID: 17029828]. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y., Yin H., Li J., Zhang Y., Han B., Zeng Z., Qiao N., Cui X., Lou J., Li J. Amelioration of beta-amyloid-induced cognitive dysfunction and hippocampal axon degeneration by curcumin is associat-ed with suppression of CRMP-2 hyperphosphorylation. 2013. [DOI] [PubMed]