Abstract
Specific memory processes and neurological disorders can be ascribed to different dorsoventral regions of the hippocampus. Recently, differences in the anatomical and physiological properties between dorsal and ventral hippocampal CA1 neurons were described for both the rat and mouse hippocampus and have greatly contributed to our understanding of these processes. While differences in the subthreshold properties were similar between rat and mouse neurons, differences in action potential output between dorsal and ventral neurons were strikingly less divergent in mouse compared with rat CA1 neurons. Here, we investigate the mechanism underlying the lack of difference in action potential firing between dorsal and ventral CA1 pyramidal neurons in mouse hippocampus. Consistent with rat, we found that ventral CA1 neurons had a more depolarized resting membrane potential and higher input resistance than dorsal CA1 neurons in the mouse hippocampus. Despite these differences, action potential output in response to current injection was not significantly different. We found that ventral neurons have a more depolarized action potential threshold compared with dorsal neurons and that threshold in ventral neurons was more sensitive to block of KV1 channels compared with dorsal neurons. Outside-out voltage-clamp recordings found that slowly inactivating K+ currents were larger in ventral CA1 neurons. These results suggest that, despite differences in subthreshold properties between dorsal and ventral CA1 neurons, action potential output is normalized by the differential functional expression of D-type K+ channels.
NEW & NOTEWORTHY Understanding differences in neurons within a brain region is integral in the reliable interpretation of comparative studies. Our findings identify a novel mechanism by which D-type potassium channels normalize action potential firing between dorsal and ventral CA1 neurons of mouse hippocampus despite differences in subthreshold intrinsic properties. Action potential threshold in ventral neurons is influenced by a greater functional expression of D-type potassium channels resulting in a depolarized action potential threshold compared with dorsal hippocampus.
Keywords: 4-AP, IK-SLOW, intrinsic excitability, morphology, outside-out patch clamp
INTRODUCTION
The hippocampus has long been associated with learning and memory (Jarrard 1983; Kim and Frank 2009; Savage et al. 2004; Scoville and Milner 1957; Squire 1992) and as such the circuitry of the hippocampus has been particularly well characterized (Amaral 1978; Blackstad 1956; Chevaleyre and Siegelbaum 2010; Hjorth-Simonsen 1971; Ishizuka et al. 1990; Roy et al. 2017). This thorough understanding of hippocampal organization and function makes the hippocampus an attractive model system for studying the cellular nature of learning and memory. Recent studies suggest, however, that the intrinsic and synaptic properties are not uniform along the dorsal-ventral axis of the hippocampus (Dougherty et al. 2012, 2013; Kim and Johnston 2015; Maggio and Segal 2007; Malik et al. 2016; Malik and Johnston 2017; Papatheodoropoulos and Kostopoulos 2000; Sotiriou et al. 2005). These differences may be critical to understanding how the functionally distinct regions of the hippocampus are pivotal to the mechanisms of learning and memory specific to the dorsal and ventral regions of the hippocampus.
The dorsal and ventral poles of hippocampus are functionally distinct (Dong et al. 2009; Fanselow and Dong 2010; Swanson and Cowan 1977). The dorsal hippocampus shows greater involvement in spatial memory tasks (Moser et al. 1993, 1995), with a greater density of place fields compared with ventral hippocampus (Jung et al. 1994). The ventral hippocampus is associated with memory tasks involving an emotional component that relates to greater connectivity with the amygdala (Henke 1990; Jay et al. 1989). These regional differences are accompanied by differences in neuron morphology, excitability, and ion channel expression along the dorsal-ventral axis of the hippocampus (Dougherty et al. 2012, 2013; Hönigsperger et al. 2015; Kim and Johnston 2015; Malik et al. 2016; Marcelin et al. 2012; Milior et al. 2016).
The majority of the physiological investigations comparing dorsal and ventral hippocampus were performed using rats as a model organism. However, many models of neurological disease rely on mice, and limitations in our knowledge of the dorsoventral differences in the cellular properties across the mouse hippocampus make comparative interpretation of previous studies difficult to parse. In the rat hippocampus, ventral hippocampal CA1 neurons have a more depolarized resting membrane potential (Vm) and higher input resistance (RN) compared with dorsal neurons due to morphological differences and the differential contributions of h-channels and G protein-coupled inwardly rectifying K+ (GIRK) channels (Dougherty et al. 2012; Kim and Johnston 2015; Malik et al. 2016). As a result, ventral neurons fire more action potentials on average than dorsal neurons. While the comparison of hippocampal properties across the dorsal-ventral axis in rat has greatly increased our understanding of hippocampal structure and function, it remains unclear whether the differences identified in rat hippocampus translate to the mouse hippocampus. A recent study of dorsal and ventral properties of CA1 neurons in mouse hippocampus found that subthreshold intrinsic properties showed differences that align with data from rat hippocampus. However, action potential firing, while different at lower amplitude current injections, were much more similar between dorsal and ventral neurons of mouse compared with rat hippocampus (Milior et al. 2016).
In this study, we use a combination of histology, current clamp, and outside-out patch clamp to systematically investigate the mechanisms resulting in normalized action potential firing between dorsal and ventral CA1 neurons of the mouse hippocampus. In agreement with studies from rat, we found that dorsal CA1 neurons had a more hyperpolarized VM and lower RN compared with ventral CA1 neurons. Also consistent with rat, we found that dorsal CA1 neurons have greater dendritic branching stratum radiatum compared with ventral neurons. However, our findings, contrary to those in rat hippocampus, show no difference in the functional expression of h-channels and GIRK/IRK channels. We found that a more depolarized action potential threshold in ventral neurons was consistent between rat and mouse. Our results show that higher functional expression of D-type (KV1-like) K+ channels is responsible for this difference in excitability between dorsal and ventral CA1 neurons of mouse hippocampus.
METHODS
Animals.
The University of Texas at Austin Institutional Animal Care and Use Committee approved all animal procedures. Male C57/B6 mice 8–16 wk old from either The Jackson Laboratory or a colony of mice bred from those shipped from The Jackson Laboratory were used for all experiments. Mice bred in house were weaned at postnatal day 20. Mice shipped from The Jackson Laboratory were between 8 and 10 wk old at the time of shipment and were habituated to the facility for 3 days before experimental use. Animals were housed in single-sex groups at room temperature with ad libitum access to food and water and set on a reverse 12-h light cycle in the University of Texas at Austin vivarium located in the Norman Hackerman Building. Mice were anesthetized with acute isoflurane exposure followed by injection of ketamine/xylazine cocktail (100/10 mg/kg ip). Mice were then perfused through the heart with ice-cold saline consisting of (in mM): 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 7 dextrose, 205 sucrose, 1.3 ascorbate, and 3 sodium pyruvate (bubbled with 95% O2-5% CO2 to maintain pH at ~7.4). The brain was removed, trimmed, and a vibrating tissue slicer (Vibratome 3000, Vibratome) was used to make 300-μm-thick transverse sections of either dorsal or ventral hippocampus (Dougherty et al. 2012). Slices were held for 30 min at 35°C in a chamber filled with artificial cerebral spinal fluid (aCSF) consisting of (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 2 MgCl2, 10 dextrose, and 3 sodium pyruvate (bubbled with 95% O2-5% CO2) and then at room temperature until the time of recording.
Electrophysiology
Slices were submerged in a heated (32–34°C) recording chamber and continually perfused (1−2 ml/min) with bubbled aCSF containing (in mM) 125 NaCl, 3.0 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2, 10 dextrose, 3 sodium pyruvate, 0.025 AP5, 0.02 DNQX, and 0.002 gabazine. In some cases, 50 μM 4-AP was bath applied as previously described (Kalmbach et al. 2015; Sosanya et al. 2015). Recordings were made from visually identified CA1 pyramidal neurons using DIC or Dodt contrast in dorsal or ventral hippocampal slices. In experiments measuring effects of GIRK/IRK channel activity, 25 μM BaCl2 was applied to the extracellular saline to block this current. All drugs were prepared from concentrated stock solutions in water and were obtained from Abcam or Tocris. For current-clamp recordings, patch pipettes (4−8 MΩ) were pulled from borosilicate glass and filled with (in mM) 120 K-gluconate, 16 KCl, 10 HEPES, 8 NaCl, 7 K2 phosphocreatine, 0.3 Na-GTP, 4 Mg-ATP (pH 7.3 with KOH). Neurobiotin (Vector Laboratories; 2%) was included in the internal recording solution to determine the recording location during post hoc morphological reconstruction. Upon processing and examining filled neurons, those that had a significant portion of oblique dendrites cut or any portion of the apical dendrite were excluded from analysis.
Data were acquired using a Dagan BVC-700 amplifier and Axograph (Axograph X) or custom data acquisition software written using Igor Pro (Wavemetrics). Data were sampled at 10−50 kHz, filtered at 3–5 kHz, and digitized using an ITC-18 interface (InstruTech). Pipette capacitance was compensated and the bridge was balanced before each recording. Series resistance was monitored throughout each experiment. If series resistance exceeded 30 MΩ during an experiment, those data were excluded from analysis. Liquid junction potential was estimated using the Patcher’s Power Tools add-on in Igor Pro. Liquid junction potential was estimated to be 14.3 mV and was not corrected for.
Analysis of current-clamp data
Data were analyzed using custom analysis software written in Igor Pro or Axograph. RN was calculated from the linear portion of the current−voltage relationship generated in response to a family of current injections (−50 to +50 pA, 10-pA steps). Voltage sag was measured by calculating the ratio of the maximum RN to the steady-state RN. Rebound slope was measured by plotting the amplitude of the voltage rebound at the offset of a current step as a function of steady-state voltage during the current step and calculated by fitting a linear regression to the data points. Resonant frequency (fR) was determined as the frequency where the peak of the impedance amplitude profile occurred in response to a constant amplitude sinusoidal current injection that linearly increased in frequency from 1−15 Hz over 15 s (Narayanan and Johnston 2007). Simulated excitatory postsynaptic currents were generated by using alpha current injections, where I = Imax(t/α)e−αt. Imax and α were adjusted to produce excitatory postsynaptic potential (EPSP)-like waveforms. Summation was recorded by giving a series of five α-current injections at 20 Hz and quantified as the ratio of the amplitude of the fifth EPSP to the amplitude of the first EPSP. The membrane time constant was measured by delivering 1-ms current injections (±100 pA) and fitting a double exponential to the decay of the voltage, the slower tau value was used to estimate membrane time constant (Routh et al. 2009). Action potential firing was measured by delivering 500 (Fig. 1) or 1,000 (Fig. 7)-ms current steps of increasing amplitude beginning with 50 pA in 50-pA intervals. Threshold was calculated as the membrane voltage at which dV/dt reached 20 mV/ms. Threshold was compared between dorsal and ventral CA1 neurons using two methods. The first was determined as in Dougherty et al. (2012), in which threshold of the first spike in a train that occurred ~50 ms after the onset of the current step was compared between dorsal and ventral neurons. The second method compared threshold using current steps of varying duration (1.5, 3, 6, 12, 24, 50, and 100 ms) adjusting the current amplitude to elicit a single action potential at the end of the current step (Higgs and Spain 2011). Action potential amplitude was measured from baseline Vm to the peak of the action potential. Action potential duration was measured as the width at the half maximal amplitude.
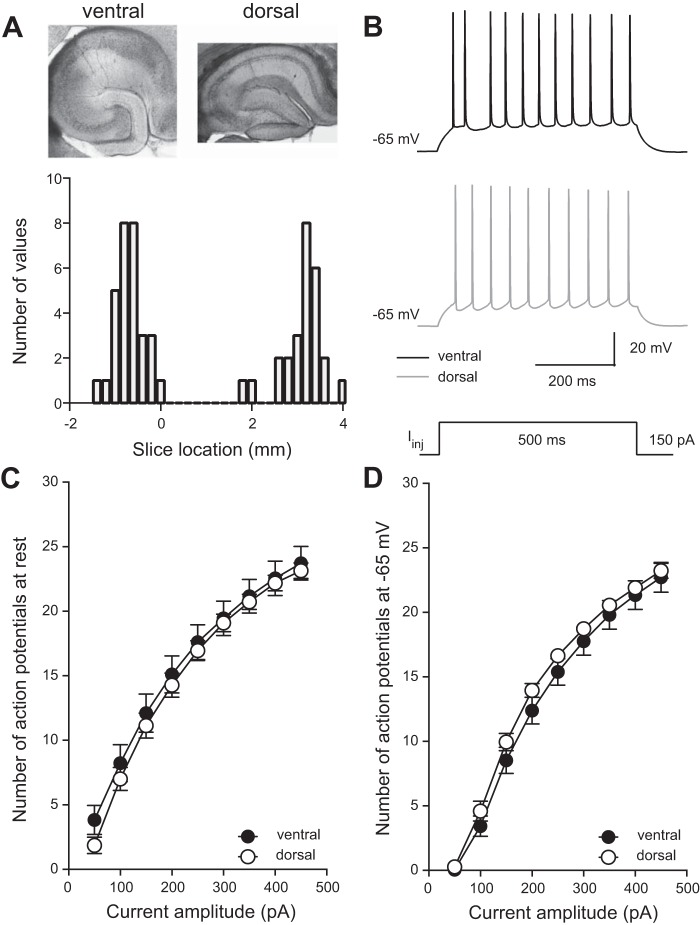
Fig. 1.
Mapping of slice location along the dorsal-ventral axis of hippocampus. Action potential firing is not different between dorsal and ventral neurons of mouse hippocampus. A: representative slices from ventral (top left) and dorsal (top right) hippocampus. Histogram of estimated slice location along the dorsal-ventral axis of hippocampus (bottom). Ventral is represented by −2 to 0 mm and dorsal by 2 to 4 mm (n = 26 ventral cells and 21 dorsal cells from 33 mice). B: voltage responses showing representative action potential firing in a ventral (top) and dorsal (middle) CA1 neuron during a 150-pA current injection (bottom). C and D: the mean number of action potentials fired during varying current injections was not significantly different between dorsal and ventral CA1 neurons when at resting membrane potential (Vm) or when held at the common Vm of −65 mV. Analyzed using 2-way ANOVA (n = 18 ventral cells and 22 dorsal cells from 33 mice). Data are presented as means ± SE. See Supplemental Table S1 for details.
Fig. 7.
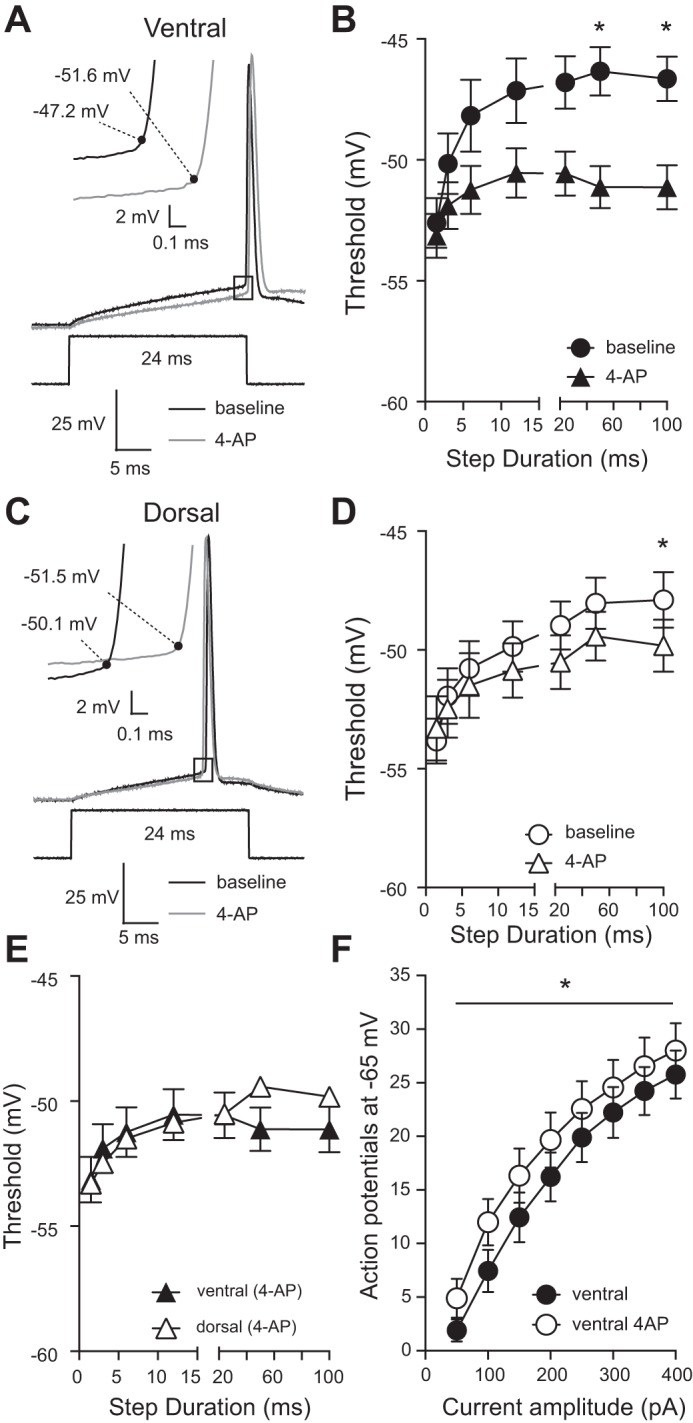
Application of 4-aminopyridine (4-AP) abolishes the difference in action potential threshold between ventral and dorsal CA1 neurons. A: representative voltage responses showing action potentials elicited from a ventral CA1 neuron before and after application of 50 µM 4-AP. Inset: expanded area indicated by the black box showing action potential threshold (black dot). B: application of 4-AP significantly hyperpolarized action potential threshold in ventral CA1 neurons (*P < 0.05) (n = 10 ventral cells from 9 mice). C: representative voltage responses showing action potentials elicited from a dorsal CA1 neuron before and after application of 50 µM 4-AP. Inset: expanded area indicated by the black box showing action potential threshold (black dot). D: application of 4-AP had no significant effect on action potential threshold in dorsal CA1 neurons (n = 7 dorsal cells from 9 mice). E: in the presence of 4-AP action potential threshold is not significantly different between dorsal and ventral CA1 neurons (n = 10 ventral cells and 7 dorsal cells from 9 mice). F: the mean number of action potentials fired during 1-s current injections of varying amplitudes differed at all current amplitudes in the presence of 4-AP in ventral CA1 neurons. Ventral: n = 9; dorsal: n = 8; n = 9 animals. Data presented as means ± SE and analyzed using 2-way ANOVA with Sidak’s multiple-comparison test. See Supplemental Table S1 for details.
Voltage-clamp recordings of K+ current
Outside-out voltage-clamp recordings were performed using an Axopatch 200B amplifier (Molecular Devices). Data were acquired at 10 kHz and filtered at 2 kHz and then digitized using an ITC-18 interface (InstruTech) and recorded using Axograph X software (Axograph). Separation of fast-inactivating, slow-inactivating, and sustained K+ current was performed as in (Kalmbach et al. 2015). Total potassium current IK was measured by applying 800-ms voltage steps ranging to −70 to 50 mV at 20 mV intervals from a holding potential of −90 mV. A 100-ms prestep to −20 mV was used to remove the rapidly inactivating component (Kalmbach et al. 2015). Isolation of the sustained component was achieved by using the same range of voltage commands but from a holding potential of −20 mV. Offline subtraction of isolated components from IK allowed for the separation of the fast, slow and sustained K+ currents (Kalmbach et al. 2015; Routh et al. 2017). Activation curves were fit with a single Boltzmann function using a least squares program (Kalmbach et al. 2015). Linear leakage and capacitive currents were digitally subtracted by scaling traces at smaller voltages in which no voltage-dependent current was activated.
DAB reaction and cellular reconstruction
Cells were filled with Neurobiotin (Vector Laboratories, Burlingame, CA) during current-clamp experiments and at the end of the recording, slices were fixed in 3% glutaraldehyde for a minimum of 24 h. Slices were washed in 0.1 M phosphate buffer and incubated in 0.5% H2O2 for 30 min. Slices were then washed in phosphate buffer and incubated in ABC reagent (Vector Laboratories) containing Avidin DH and biotinylated horseradish peroxidase H for 24–48 h at 4°C. Slices were then incubated in DAB solution (Vector Laboratories) in the presence of H2O2, causing a visible color change to the slices and Neurobiotin-filled cell. Slices were dehydrated in glycerol and mounted on glass slides for imaging. Identifiable neurons from both dorsal and ventral slices were reconstructed at ×40 magnification using a Leitz Diaplan microscope with Neurolucida 6.0 software (MicroBrightField, Williston, VT). Distance from soma was scaled so that there were 10 and 25 concentric circles measuring the basal dendrites and apical dendrites respectively.
Dorsal-ventral mapping
We used a predictive algorithm, developed in (Malik et al. 2016), to predict the location of slices used for electrophysiological recordings along the dorsal-ventral axis of the hippocampus. Anatomical markers of the hippocampal formation in an individual slice are compared with normal hippocampal measurements to ensure that recorded cells were from slices in either the dorsal or ventral pole of the hippocampus. Anatomical indicators were the ratio of the transverse length of area CA1 to the radial length of area stratum lacunosum-moleculare, the ratio of the transverse length of area CA3 to the radial length of area CA3, and the transverse length of dentate gyrus to the distance between the horns of dentate gyrus. By using coefficients determined from the measurement of 100 hippocampal slices across the dorsal-ventral axis and anatomical measurement ratios taken from each slice, we estimated the location of slices that were recorded from within the mouse hippocampus.
Statistics
The Kolmogorov-Smirnov test was used to assess whether data were normally distributed. Data sets that were shown to be normally distributed were further assessed using the F-test to determine whether groups exhibited similar variance. Parametric data were analyzed using Student’s t-test and nonparametric data were analyzed using the Mann-Whitney rank sum test. Two-way ANOVA was applied to experiments in which multiple observations were recorded while manipulating a single independent variable. Sidak’s multiple-comparison test was used to compare row means between groups. Data are presented as mean ± standard error. Alpha was set to P = 0.05 for all experiments. Group sizes were determined based on previous publications investigating these same electrophysiological parameters of dorsal and ventral CA1 neurons in the rodent hippocampus (Dougherty et al. 2012, 2013; Kim and Johnston 2015; Malik et al. 2016). Detailed information on statistical analyses are presented in Supplemental Table S1. (Supplemental Material for this article is available online at the Journal website.)
RESULTS
Active properties across the dorsal-ventral axis
To determine the location of our acute slices within the mouse hippocampus, we used a mapping system based on anatomical markers within the hippocampal formation to estimate the position of acute slices within either the dorsal or ventral poles of the hippocampus (Malik et al. 2016). At the end of electrophysiological recording, measurements were made of the dentate gyrus, CA3, and CA1 regions of dorsal and ventral hippocampal slices (Fig. 1A, top; see methods for details). When slices were mapped back into the mouse hippocampal formation, the ventral pole is represented by negative numbers and the dorsal pole more positive numbers; we found that the acute slices used for this study were from either the dorsal or ventral poles of the mouse hippocampus (Fig. 1A, bottom).
Previous electrophysiological data from rat hippocampus shows higher spike firing in ventral compared with dorsal CA1 hippocampal neurons (Dougherty et al. 2012; Malik et al. 2016). To test whether this was consistent in mouse, we recorded the number of action potentials fired in response to a 500-ms current injection of varying amplitudes (50–450 pA at 50-pA intervals) (Fig. 1B). In contrast to rat, we found that the average numbers of spikes fired in dorsal and ventral CA1 neurons were not different when cells were held at resting Vm (Fig. 1C) or at the common potential of −65 mV (Fig. 1D). Refer to Supplemental Table S1 for detailed information on statistical tests. In rat, a combination of differences in anatomy as well as subthreshold and suprathreshold properties contribute to the higher action potential output in ventral CA1 neurons. The lack of difference between dorsal and ventral mouse CA1 neurons suggests that the distribution of ion channels and/or anatomical differences between the dorsal and ventral regions of the mouse hippocampus may not align with observations in rat.
Subthreshold properties of dorsal-ventral mouse CA1 pyramidal neurons
We performed whole cell current-clamp recordings from the soma of either dorsal or ventral CA1 pyramidal neurons. We found that the resting Vm was ~2 mV more depolarized in ventral neurons compared with dorsal neurons (Fig. 2A). Although small, this difference is in agreement with previous findings in rat hippocampus (Dougherty et al. 2012; Malik et al. 2016) as well as in mouse hippocampus (Milior et al. 2016). RN was measured at both the resting Vm and at a common Vm (−65 mV) (Fig. 2, B–D). In both cases, and consistent with findings in rat hippocampus, RN was significantly higher in ventral CA1 neurons compared with dorsal CA1 neurons (Fig. 2, C and D).
Fig. 2.
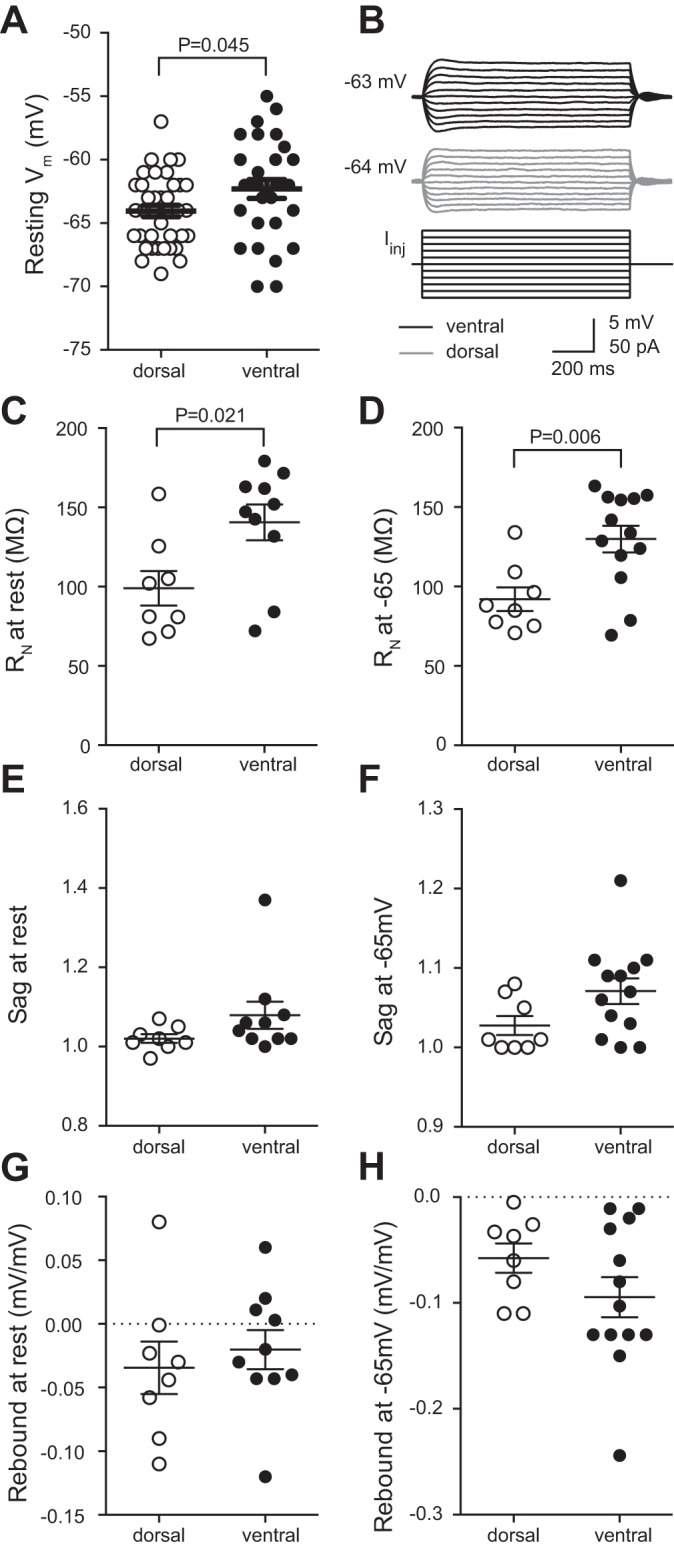
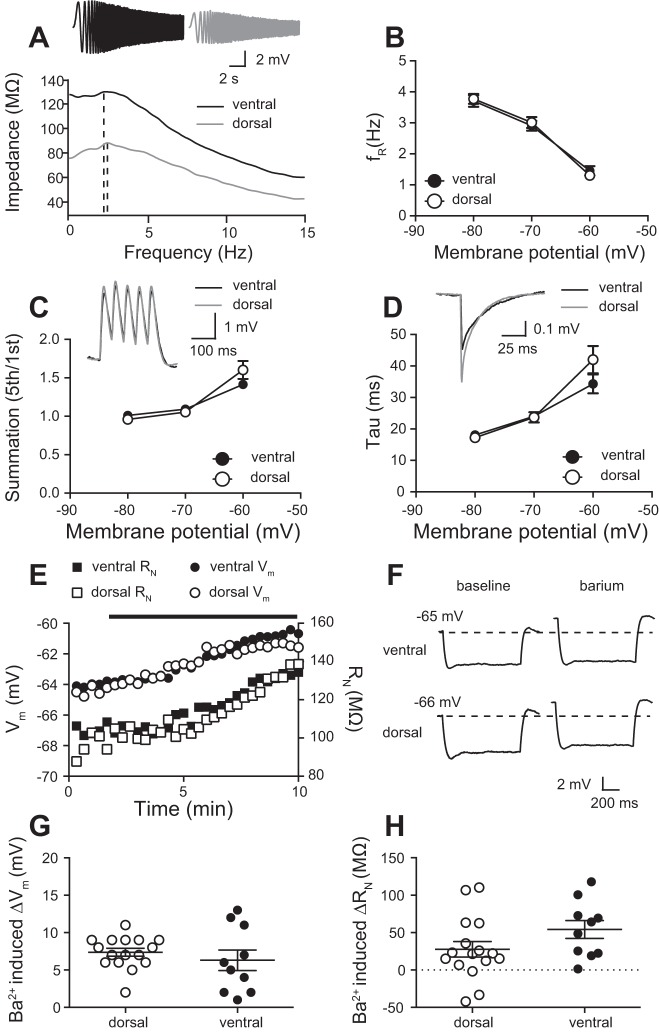
Comparison of subthreshold intrinsic membrane properties between dorsal and ventral CA1 neurons. A: ventral neurons have a more depolarized resting membrane potential (Vm) compared with dorsal CA1 neurons; n = 29 ventral cells and 38 dorsal cells from 43 mice). B: representative traces voltage responses from ventral (top) and dorsal (middle) CA1 neurons in response to a series of current injections (bottom). Resting Vm is shown at the right. C and D: ventral neurons have a higher input resistance (RN) than dorsal CA1 neurons measured from either rest (C; n = 10 ventral cells and 8 dorsal cells from 10 mice) or −65 mV (D; n = 13 ventral cells and 8 dorsal cells from 10 mice). E and F: there was no significant difference in voltage sag between ventral and dorsal CA1 neurons measured from either rest (E; n = 10 ventral cells and 8 dorsal cells from 10 mice) or −65 mV (F; n = 13 ventral cells and 8 dorsal cells from 10 mice). G and H: there was no significant difference in rebound slope between ventral and dorsal CA1 neurons measured from either rest (G; n = 10 ventral cells and 8 dorsal cells from 10 mice) or −65 mV (H; n = 13 ventral cells and 8 dorsal cells from 10 mice). Data are presented as means ± SE. A, E, and F analyzed using Mann-Whitney test. C, D, G, and H analyzed using t-test. See Supplemental Table S1 for details.
In rat, the difference in Vm and RN is due to the difference in both h-channels (Ih) and GIRK channels (Dougherty et al. 2012, 2013; Kim and Johnston 2015). Subthreshold differences in intrinsic properties contribute to the greater firing of action potentials in ventral compared with dorsal neurons in rat hippocampus. Therefore, we investigated the possibility that differences in the functional expression in h- and GIRK/IRK-channels could underlie the normalized action potential firing between dorsal and ventral CA1 neurons. To estimate difference in Ih between dorsal and ventral CA1 neurons, we measured voltage sag and rebound, two properties that are reflective of h-channel function owing to the slow kinetics of Ih (Brager and Johnston 2007; Brager et al. 2012; Dougherty et al. 2013; Magee 1998; Malik et al. 2016). There was no significant difference in either sag or rebound between dorsal and ventral CA1 neurons at either the resting Vm or −65 mV (Fig. 2, E–H). As these results disagreed with previous results in rat, we measured three additional h-channel-sensitive properties: resonance frequency (fR), summation, and membrane time constant (τM). In rat, ventral CA1 neurons have a higher fR, indicative of the higher expression of functional h-channels compared with dorsal CA1 neurons (Dougherty et al. 2013). In contrast, we found that there was no significant difference in fR between dorsal and ventral CA1 neurons in mouse (Fig. 3, A and B). Additionally, summation (Fig. 3C) and τM (Fig. 3D) were also not shown to be different. These results suggest that, unlike in rat, differences in Vm and RN cannot be explained by a difference in Ih between dorsal and ventral CA1 neurons of the mouse hippocampus.
Fig. 3.
The h-channel and inwardly rectifying K+ channel activity does not differ between dorsal and ventral CA1 neurons. A: impedance amplitude profiles that show the resonant frequency, fR, of representative ventral (3.4 Hz) and dorsal (3.2 Hz) CA1 neurons. Inset: voltage traces showing response to a chirp current injection. B: fR measured while varying membrane potential (Vm) is not significantly different between dorsal and ventral CA1 neurons. C: summation measured while varying Vm is not significantly different between dorsal and ventral CA1 neurons. Inset: representative voltage responses to injection of 5 alpha waveforms at 20 Hz. D: membrane time constant (τM) measured while varying Vm is not significantly different between dorsal and ventral CA1 neurons. Inset: representative voltage responses to −100-pA current injection used to determine τM. B–D: n = 19 ventral cells and 18 dorsal cells from 23 mice. E: time-dependent effect of extracellular 25 µM Ba2+ on resting Vm and input resistance (RN) dorsal and ventral CA1 neurons. Black bar represents the application of Ba2+. F: voltage response to −50-pA current injection before and after Ba2+ wash-on. Note the depolarization of Vm and increase in RN. G and H: the effect on extracellular Ba2+ application on resting Vm (G) and RN (H) is not significantly different between dorsal and ventral CA1 neurons (n = 10 ventral cells and 16 dorsal cells from 19 mice). Data presented as means ± SE. B, C, and D analyzed using 2-way ANOVA. G was analyzed using a two-tailed Mann-Whitney test and H was analyzed using a 2-way unpaired t-test. See Supplemental Table S1 for details.
In rat, there is a greater functional expression of GIRK channels in dorsal compared with ventral rat hippocampus (Kim and Johnston 2015). We hypothesized that the depolarized Vm and higher RN in mouse ventral CA1 neurons could be due to lower functional expression of GIRK channels. Low concentrations of extracellular Ba2+ can be used to block inwardly rectifying K+ channels (IRK) including GIRK channels (Kim and Johnston 2015; Malik and Johnston 2017). In rat hippocampus, Ba2+ has greater effect on Vm and RN in dorsal compared with ventral neurons (Kim and Johnston 2015). Bath application of 25 μM Ba2+ depolarized Vm and increased RN in both dorsal and ventral mouse hippocampal neurons (Fig. 3, E and F). In contrast to rat, however, we found that the effect of Ba2+ on Vm (Fig. 3G) and RN (Fig. 3H) was not significantly different between dorsal and ventral CA1 pyramidal neurons. These results suggest that, unlike in rat, difference in resting Vm and RN between dorsal and ventral mouse CA1 neurons does not result from differences in h- or GIRK/IRK channel expression.
Neuronal reconstructions
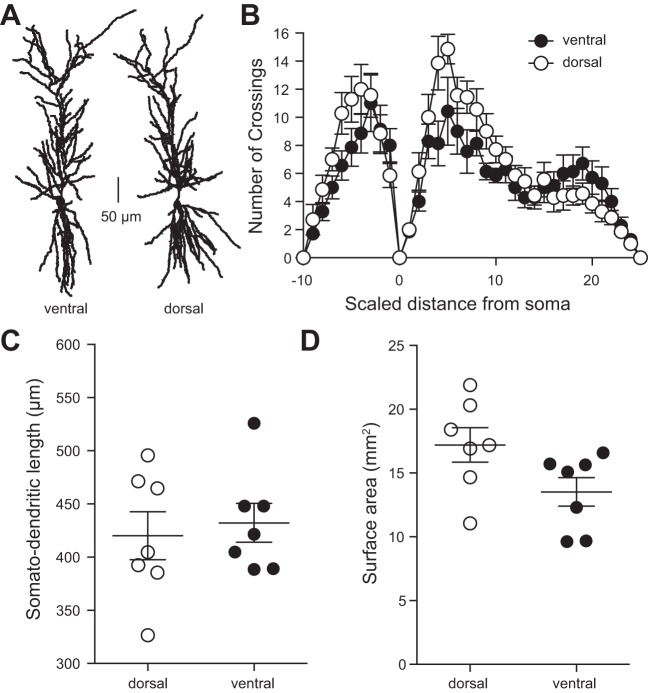
In the rat hippocampus, dorsal CA1 neurons have a shorter somato-dendritic axis, greater number of branches, and greater surface area compared with ventral CA1 neurons (Dougherty et al. 2012; Malik et al. 2016). We used Neurolucida software to reconstruct dorsal and ventral mouse CA1 neurons and asked whether these same differences were present (Fig. 4A). Sholl analysis revealed that dendritic branching was significantly greater in dorsal compared with ventral neurons of the mouse hippocampus, specifically in the stratum radiatum region of CA1 (Fig. 4B). In contrast with rat data, however, somato-dendritic length (Fig. 4C) and surface area (Fig. 4D) were not different between dorsal and ventral CA1 neurons.
Fig. 4.
A: representative cellular reconstructions of ventral (left) and dorsal (right) CA1 neurons of the hippocampus, scale bar represents 50 μm. B: dendritic branching as measured by Sholl analysis (see methods) is not significantly different between dorsal and ventral CA1 neurons. C: somato-dendritic distance is not significantly different between dorsal and ventral CA1 neurons. D: total dendritic surface area is not significantly different between dorsal and ventral CA1 neurons. B–D: n = 7 ventral cells and 7 dorsal cells from 10 mice. Data presented as means ± SE. B analyzed using 2-way ANOVA. C and D analyzed using t-test. See Supplemental Table S1 for details.
Action potential threshold
One possible explanation for the normalized action potential firing between dorsal and ventral neurons in mouse hippocampus is a difference in action potential threshold. It was previously reported that ventral CA1 neurons in rat have a significantly more depolarized action potential threshold compared with dorsal CA1 neurons (Dougherty et al. 2012; Malik et al. 2016). However, the underlying cause of the difference in threshold in rat hippocampus remains uninvestigated. To compare with studies in rat hippocampus, we measured the threshold of the first action potential in a train where the first action potential occurred with a latency of ~50 ms (Dougherty et al. 2012) (see methods for details). Consistent with previous reports in rat, we found that action potential threshold in ventral neurons was significantly more depolarized than in dorsal CA1 neurons in mouse hippocampus (Fig. 5A).
Fig. 5.
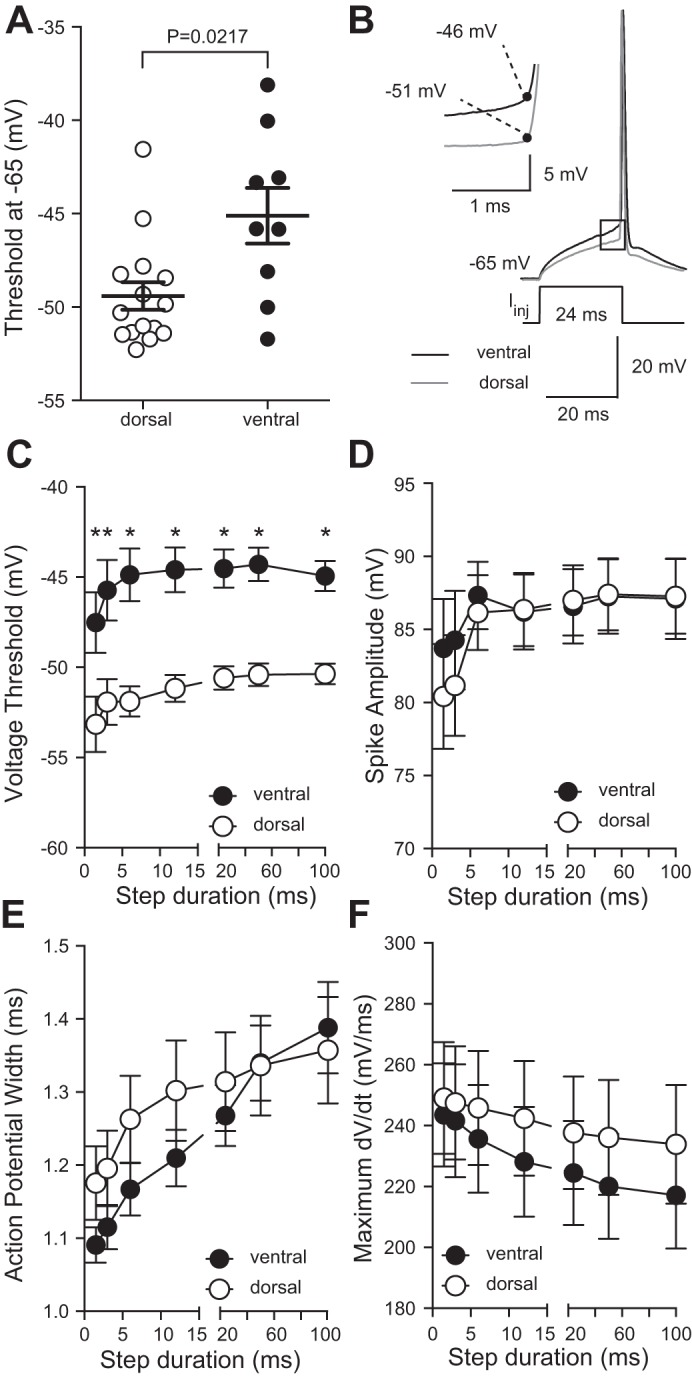
Action potential threshold is more depolarized for long, but not short, current injections in ventral compared with dorsal CA1 neurons. A: action potential threshold was more depolarized in ventral CA1 neurons compared with dorsal CA1 neurons when measured as in (Dougherty et al. 2012) (n = 9 ventral cells and 15 dorsal cells from 23 mice). B: representative voltage responses showing action potentials elicited near the end of a 24-ms current injection. Inset: expanded area indicated by the black box showing action potential threshold (black dot). C: action potential threshold is significantly depolarized for current injections longer than 6 ms in ventral CA1 neurons compared with dorsal CA1 neurons. (*P < 0.05; n = 16 ventral cells and 13 dorsal cells from 18 mice). D–F: action potential amplitude (D), duration (E), and maximum dV/dt (F) is not significantly different between dorsal and ventral CA1 neurons (n = 16 ventral cells and 15 dorsal cells from 16 mice). Data presented as means ± SE and analyzed using 2-way ANOVA with Bonferroni’s multiple-comparison test. See Supplemental Table S1 for details.
To further investigate the difference in action potential threshold between dorsal and ventral CA1 neurons, we utilized a more systematic approach for measuring action potential threshold (Higgs and Spain 2011; Kalmbach et al. 2015). Current injections of varying durations (1.5–100 ms) were delivered to dorsal and ventral CA1 neurons from a common Vm of −65 mV. The current amplitude was adjusted to elicit a single action potential at the end of the current step (Fig. 5B). We found that action potential threshold was significantly more depolarized in ventral neurons at all measured current durations (Fig. 5C). Action potential amplitude, duration, and maximum rate of rise were not significantly different between dorsal and ventral CA1 pyramidal neurons (Fig. 5, D–F).
K+ channel voltage-clamp measurements
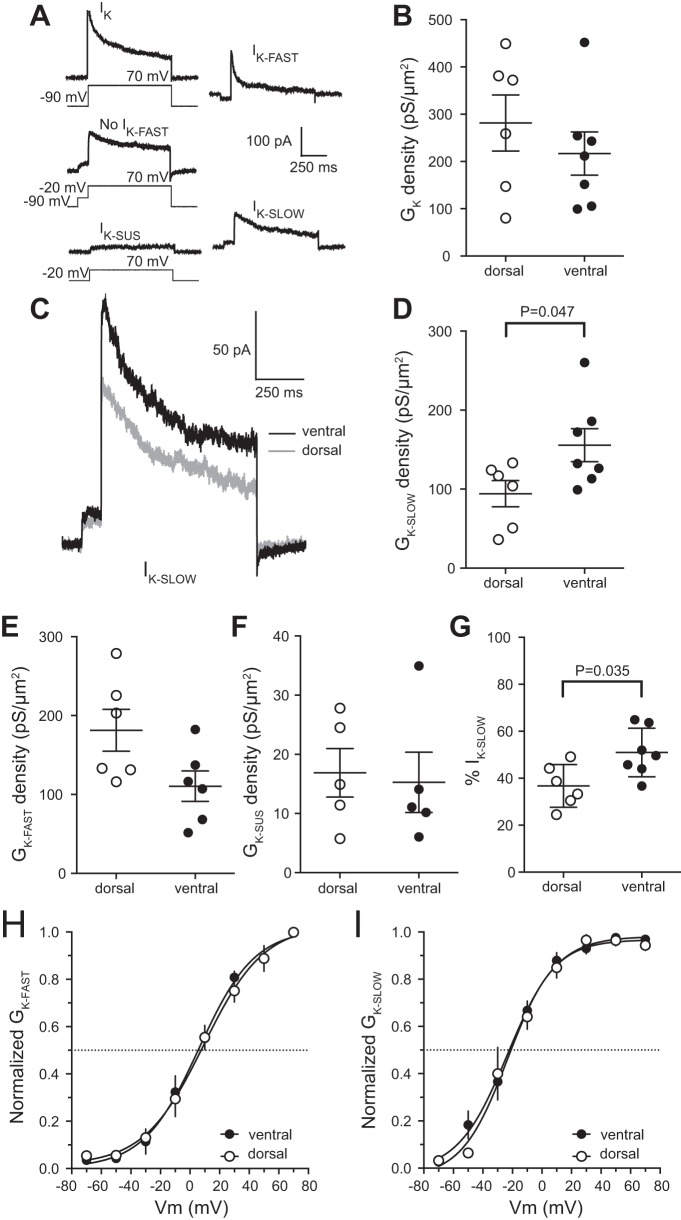
Slowly inactivating D-type K+ channels affect action potential threshold during longer current steps while having few effects on other physiological properties (Bekkers and Delaney 2001; Guan et al. 2007; Higgs and Spain 2011; Kalmbach et al. 2015; Sosanya et al. 2015; Yu et al. 2008). The change in threshold, but not in other action potential properties, led us to hypothesize that differences in the functional expression of D-type K+ channels contributed to the difference in threshold between dorsal and ventral mouse CA1 neurons. To test for differences in voltage-gated K+ channel function we used outside-out voltage clamp to measure K+ current from somatic patches from dorsal and ventral CA1 neurons. We used a series of voltage commands to separate IK into three components: a rapidly inactivating IK-FAST, a slowly inactivating IK-SLOW, and noninactivating IK-SUS (Fig. 6A) (Kalmbach et al. 2015; Routh et al. 2017). There was no significant difference in IK between dorsal and ventral CA1 neurons (Fig. 6B). We found that IK-SLOW conductance density was greater in patches from ventral CA1 neurons compared with dorsal CA1 neurons (Fig. 6, C and D). There was no significant difference in either the rapidly inactivating or sustained current (Fig. 6, E and F). Accordingly, the slowly inactivating current makes up a greater percentage of IK in ventral than in dorsal neurons (Fig. 6G). There was no significant difference in the activation of either IK-FAST or IK-SLOW between dorsal and ventral CA1 neurons (Fig. 6, H and I). These findings support the hypothesis that the more depolarized action potential threshold in ventral CA1 neurons is due to higher functional expression of slowly inactivating D-type K+ current.
Fig. 6.
D-type K+ channel conductance density is higher in ventral compared with dorsal CA1 neurons. A: IK recorded from an outside-out patch (left) was separated in 3 K+ current components based on voltage dependence (IK-FAST, top right; IK-SLOW, bottom right; and IK-SUS, bottom left). Total IK was measured by using 800-ms voltage steps ranging from −70 to 50 mV at 20-mV intervals from a holding potential of −90 mV (top left). A 100-ms prestep to −20 mV was used to remove the rapidly inactivating component (middle left). Sustained IK was isolated by using 800-ms voltage steps ranging from −70 to 50 mV at 20 mV intervals from a holding potential of −20 mV (bottom left). Offline subtraction of isolated IK components provided individual fast, slow and sustained K+ currents (Kalmbach et al. 2015; Routh et al. 2017). B: the K+ conductance density is not significantly different between dorsal and ventral CA1 neurons (n = 7 ventral cells and 6 dorsal cells from 9 mice). C: representative traces showing the maximum IK-SLOW from ventral (black) and dorsal (gray) CA1 pyramidal neurons. D: the conductance density for IK-SLOW channels is significantly higher in ventral CA1 neurons (n = 7 ventral cells and 6 dorsal cells from 9 mice). E: the conductance density for IK-FAST channels was not significantly different between dorsal and ventral CA1 neurons (n = 7 ventral cells and 6 dorsal cells from 9 mice). F: the conductance density for IK-SUS channels was not significantly different between dorsal and ventral CA1 neurons (n = 5 ventral cells and 5 dorsal cells from 9 mice). G: the percentage of IK made up by IK-SLOW is significantly higher for ventral compared with dorsal CA1 neurons (n = 7 ventral cells and 6 dorsal cells from 9 mice). H and I: there was no significant difference in the activation curves for either IK-FAST (H) or IK-SLOW channels (I) between dorsal and ventral CA1 neurons (n = 7 ventral cells and 6 dorsal cells from 9 mice). Data presented as means ± SE and analyzed using t-test. See Supplemental Table S1 for details. IK, total K+ current; IK-fast, rapidly inactivating K+ current; IK-slow, slowly inactivating K+ current; IK-sus, noninactivating K+ current; GK, total K+ conductance; GK-fast, rapidly inactivating K+ conductance; GK-slow, slowly inactivating K+ conductance; GK-sus, noninactivating K+ conductance.
4-AP experiments
D-type K+ channels are sensitive to low concentrations of the K+ channel blocker 4-aminopyridine (4-AP). Low concentrations of 4-AP can be used to test for the role of D-type K+ channels on action potential threshold (Castle et al. 1994; Higgs and Spain 2011; Kalmbach et al. 2015; Russell et al. 1994; Sosanya et al. 2015). We previously showed that the slowly inactivating component of IK (IK-SLOW) is blocked by low concentrations of 4-AP or α-dendrotoxin (Kalmbach et al. 2015), suggesting that IK-SLOW is mediated by KV1-containing D-type K+ channels. Based on the greater IK-SLOW in ventral neurons, we hypothesized that action potential threshold in ventral CA1 neurons will be more sensitive to low 4-AP compared with dorsal CA1 neurons. We measured action potential threshold before and after bath application of 50 µM 4-AP to both dorsal and ventral CA1 neurons. 4-AP hyperpolarized action potential threshold in dorsal neurons at varying current step durations by between 0 and −2.3 mV and in ventral neurons by between −1 and −4.5 mV. The effect of 4-AP was greater in ventral compared with dorsal CA1 neurons of mouse hippocampus with a mean change in threshold due to 4-AP of 3.11 mV in ventral and 0.94 mV in dorsal hippocampus (Fig. 7, A–D). Application of 4-AP significantly increased the number of action potentials fired in both dorsal and ventral CA1 neurons, however, in ventral neurons the difference in firing was significant at all current amplitudes with an average increase in firing of 3.05 Hz, while in dorsal neurons only the 100-pA current step differed significantly due to the application of 4-AP with an average increase in firing of 2.02 Hz (Fig. 7F). These data support the hypothesis that higher functional expression of D-type K+ channels in ventral CA1 pyramidal neurons contributes to the more depolarized threshold relative to dorsal CA1 pyramidal neurons and thus to a normalization of action potential firing between dorsal and ventral CA1 neurons.
DISCUSSION
We investigated the mechanism behind the normalized action potential firing between dorsal and ventral CA1 pyramidal neurons of mouse hippocampus. We found that Vm was more depolarized and RN higher in ventral CA1 neurons compared with dorsal CA1 neurons consistent with rat (Dougherty et al. 2012; Kim and Johnston 2015; Malik et al. 2016). However, unlike rat, these differences do not depend on differential expression of h- and/or GIRK/IRK channels. Furthermore, although RN was higher in ventral CA1 neurons there was no significant difference in action potential firing between dorsal and ventral CA1 neurons. We found that ventral CA1 neurons have a higher expression of D-type K+ channels, which contributes to a more depolarized action potential threshold in ventral compared with dorsal CA1 neurons. These findings suggest that differences in subthreshold properties between dorsal and ventral CA1 neurons in mouse are largely ameliorated by differential expression of D-type K+ channels.
Dorsal-ventral subthreshold differences are smaller in mouse compared with rat
Research examining dorsal-ventral differences in subthreshold properties has largely been performed in the rat hippocampus. Ventral CA1 neurons consistently have a more depolarized resting Vm (by 4–8 mV) and higher RN (~200%) compared with dorsal CA1 neurons (Dougherty et al. 2012; Kim and Johnston 2015; Malik et al. 2016). We found that CA1 neurons in the mouse hippocampus express similar differences but at a lesser magnitude, with ventral neurons resting 2 mV depolarized and with a 60% greater RN compared with dorsal CA1 neurons (Fig. 2). In rat, the difference in subthreshold properties between dorsal and ventral CA1 neurons is due to a combination of h-channel activity, greater in ventral CA1 neurons; GIRK channel activity, greater in dorsal neurons; and dendritic morphology, more branching in dorsal CA1 neurons (Dougherty et al. 2012, 2013; Kim and Johnston 2015; Malik et al. 2016; Malik and Johnston 2017).
We used a variety of h-channel-sensitive electrophysiological measurements to test whether differences in h-channel function contribute to the differences in Vm and RN in mouse CA1 neurons. Unlike in rat, we found no significant differences in sag, rebound slope, fR, or membrane time constant. Our results suggest that the functional expression of h-channels does not contribute to differences in somatic function between dorsal and ventral CA1 neurons in the mouse hippocampus. A comparison of middle hippocampus between two mouse strains (C57BL6 and 129/SvEv) and one rat strain (Sprague-Dawley) found that there is a lower functional expression of h-channels in mouse compared with rat hippocampus (Routh et al. 2009). These findings raise the possibility that, despite using multiple measures of h-channel function, decreased h-channel functional expression in mouse hippocampus compared with rat hippocampus made it difficult to resolve differences in h-channel function in the soma of dorsal and ventral CA1 neurons in this study. A recent comparison of place cell function between rats and mice found that mice have similar theta oscillations but smaller, less spatially specific place fields (Mou et al. 2018), suggesting relatively conserved function between mouse and rat hippocampal CA1 neurons. These studies suggest that, despite subtle differences in physiology and function between rats and mice, the functional output of the hippocampus remains largely the same.
We used a low concentration of extracellular Ba2+ to test whether higher functional expression of GIRK/IRK channels in dorsal CA1 neurons contributed to the difference in Vm and RN between dorsal and ventral CA1 neurons in the mouse hippocampus (Kim and Johnston 2015). Ba2+ depolarized and increased the RN of both dorsal and ventral CA1 neurons. However, in contrast to rat, we found that the effect of Ba2+ on both Vm and RN was not significantly different between dorsal and ventral CA1 neurons in mouse. Although both dorsal and ventral CA1 neurons express GIRK/IRK channels, as described in rat, there does not appear to be a differential contribution of GIRK/IRK channels to the subthreshold properties of dorsal and ventral CA1 neurons in mouse. A nonsignificant trend was observed in the change in RN indicating slightly greater increase in ventral hippocampal RN compared with dorsal. However, this Ba2+ induced change is contrary to findings in rat hippocampus (Kim and Johnston 2015) and fails to explain the lower resting RN in dorsal compared with ventral neurons of the mouse hippocampus.
Prior to this study, a morphological comparison of dorsal and ventral CA1 neurons in the mouse hippocampus was lacking. In agreement with data from rat hippocampus, our results show that dorsal CA1 neurons show greater branching in the stratum radiatum region of the hippocampus compared with ventral CA1 neurons. It is likely that the increased branching underlies the lower RN in dorsal CA1 neurons compared with ventral CA1 neurons of mouse hippocampus. While the difference in dendritic morphology suggests a role for dendritic branching in the difference in RN between dorsal and ventral neurons, our findings do not explain the observed depolarized ventral resting Vm compared with dorsal CA1 neurons.
Somatic excitability is normalized due to dorsal-ventral differences in D-type K+ channel function
Dorsal-ventral comparisons of CA1 rat neurons show that ventral hippocampus is significantly more excitable than dorsal hippocampus. The higher excitability of ventral CA1 neurons arises from the depolarized resting Vm and higher RN (Dougherty et al. 2012, 2013; Kim and Johnston 2015; Malik et al. 2016). In rat, the consequence of these subthreshold differences is that ventral CA1 neurons fire more action potentials compared with dorsal CA1 neurons. Our findings mostly agree with a previous mouse study that found no significant difference in action potential firing except with small current injections (Milior et al. 2016). This difference at lower amplitude current steps but not with increasing amplitude current injections differs from data in the rat hippocampus in which the divergence in action potential firing increases with increasing current step amplitudes (Dougherty et al. 2012; Malik et al. 2016). Previous studies in rat found that the action potential threshold for ventral CA1 neurons is more depolarized compared with dorsal CA1 neurons (Dougherty et al. 2012; Malik et al. 2016). A more depolarized threshold should make ventral CA1 neurons less excitable than dorsal CA1 neurons; however, the significantly higher RN and more depolarized Vm of ventral neurons in rat that overcomes this difference in action potential threshold. In contrast, while our findings, concurrent with others, found a depolarized resting Vm and higher RN in ventral CA1 neurons (Milior et al. 2016), the action potential output is not significantly different between dorsal and ventral CA1 neurons. These results suggest a fundamental difference in input-output between CA1 neurons of mouse and rat hippocampus.
Action potential threshold is dictated by the complement of voltage-gated ion channels expressed by a neuron. It is commonly thought that threshold is dictated by the interplay between Na+ channels and K+ channels (Hodgkin and Huxley 1952a, 1952b; Stafstrom et al. 1984). We found no difference in maximum dV/dt, often an indicator of Na+ channel function, suggesting that a difference in Na+ channel functional expression did not contribute to the difference in threshold. While we cannot directly rule out a difference in the voltage dependence of Na+ channel activation contributing to the difference in action potential threshold, the normalization of threshold between dorsal and ventral neurons by the application of 4-AP strongly suggests that the difference in threshold is primarily mediated by D-type K+ channels. Pharmacological evidence shows that action potential threshold is influenced by dendrotoxin and low 4-AP-sensitive (D-type) K+ channels from the KV1 family (Bekkers and Delaney 2001; Guan et al. 2007; Higgs and Spain 2011; Storm 1988, 1993; Yu et al. 2008). Our measurements of rapidly inactivating K+ channels show a small, yet not significant, difference in current with a trend toward dorsal hippocampus having greater IK-FAST compared with ventral. This finding is in support of previous research that shows greater Kv4.2, a rapidly inactivating K+ channel, in dorsal compared with ventral CA1 neuron dendrites (Marcelin et al. 2012). Because our measurements were taken from neuron somata instead of dendrites, it is possible that the functional expression of rapidly inactivating K+ channels is greater in the dendrites of dorsal compared with ventral neurons of mouse hippocampus. However, this difference cannot explain the difference in threshold between dorsal and ventral neurons of mouse hippocampus. Cells analyzed in this study had intact apical and basal dendritic arbors based upon Neurobiotin fills. However, the axon was not visible in the majority of cells, preventing the determination of whether the axon was cut during the preparation of acute slices. While we cannot definitively say that axon was severed near the soma, which would influence action potential threshold, the majority of our reconstructions had intact basal dendritic arbors. It is thus likely that any severing of the axon that may occur was beyond the extent of the basal dendrites. Together with the observation that the application of 4-AP normalizes action potential threshold it is unlikely that artifacts due to the preparation of dorsal versus ventral hippocampal slices influenced our conclusions.
In agreement with studies in rat hippocampus, we found that ventral CA1 neurons have a depolarized action potential threshold in the mouse hippocampus. While the underlying cause of the dorsal-ventral difference in threshold in rat hippocampus has yet to be identified, we demonstrate here that the functional expression of D-type K+ channels of the KV1 family was higher in ventral CA1 neurons resulting in the dorsoventral difference in action potential threshold in mouse hippocampus. Our findings indicate that the primary current underlying the dorsoventral difference in action potential threshold by showing that when D-type K+ channels were blocked using 4-AP, action potential threshold was no longer significantly different between ventral and dorsal CA1 neurons (Fig. 7).
Functional consequences
The ventral hippocampus is more susceptible to insults that result in the development of epilepsy and temporal lobe seizures are more likely to originate within the ventral pole of the hippocampus (Ekstrand et al. 2011; Lothman and Collins 1981; Papatheodoropoulos et al. 2005; Racine et al. 1977; Toyoda et al. 2013). It was previously shown that a downregulation of KV1.1 and concomitant hyperpolarization of action potential threshold occurs in a rodent model of TLE (Sosanya et al. 2015). This change was also accompanied by a reduction in the sensitivity of action potential threshold to low concentrations of 4-AP. Our results suggest that the vulnerability of the ventral hippocampus to epileptogenesis may be due in part to the higher functional expression of 4-AP-sensitive (putative KV1, D-type) K+ channels in ventral CA1 neurons. Given that ventral CA1 neurons have a more depolarized resting Vm and higher RN, any loss in functional expression of D-type K+ channels would result in an increase in action potential firing and may contribute to temporal lobe seizure activity.
Our results identify the mechanism underlying the more depolarized action potential threshold in ventral as compared with dorsal neurons and the resulting normalization of action potential firing between dorsal and ventral CA1 hippocampal neurons. Application of low concentrations of 4-AP hyperpolarized the action potential threshold in ventral neurons to values comparable to that of dorsal neurons. Furthermore, we identified greater functional expression of a slowly inactivating potassium current in ventral compared with dorsal CA1 neurons. Taken together, these results support the conclusion that there is an increase in a KV1-type potassium channel in ventral neurons that contributes to a more depolarized action potential threshold. Interestingly, the more depolarized threshold, in combination with higher RN and depolarized resting Vm in ventral CA1 neurons, is a firing rate that is equivalent between dorsal and ventral CA1 neurons of the mouse hippocampus. The incongruent findings between our study in mice and research performed in the CA1 region of rat hippocampus across the dorsal-ventral axis highlights the importance of characterizing and understanding neural variability. Despite reported differences in signal processing and output, mouse and rat hippocampi are successful in their intended function. Understanding how the same neural computation can occur through distinct mechanistic pathways is pivotal to understanding how basic physiological functions result in complex animal behavior.
GRANTS
This work was supported by National Institutes of Health grant R01 MH100510 (DHB) and an Institutional Training Grant from National Institutes of Health 5T32DA018926.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.J.O., C.J.A., and D.H.B. performed experiments; G.J.O. and D.H.B. analyzed data; G.J.O. and D.H.B. interpreted results of experiments; G.J.O. and D.H.B. prepared figures; G.J.O. and D.H.B. drafted manuscript; G.J.O., C.J.A., and D.H.B. edited and revised manuscript; G.J.O. and D.H.B. approved final version of manuscript; D.H.B. conceived and designed research.
Supplemental Table
ACKNOWLEDGMENTS
We thank Drs. Rick Gray, Chung-Sub Kim, and Rahul Rathour for comments on the manuscript and Meagan Volquardsen for histological processing.
REFERENCES
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol 182: 851–914, 1978. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Delaney AJ. Modulation of excitability by alpha-dendrotoxin-sensitive potassium channels in neocortical pyramidal neurons. J Neurosci 21: 6553–6560, 2001. doi: 10.1523/JNEUROSCI.21-17-06553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstad TW. Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol 105: 417–537, 1956. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- Brager DH, Akhavan AR, Johnston D. Impaired dendritic expression and plasticity of h-channels in the fmr1(−/y) mouse model of fragile X syndrome. Cell Reports 1: 225–233, 2012. doi: 10.1016/j.celrep.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in Ih in hippocampal CA1 pyramidal neurons. J Neurosci 27: 13926–13937, 2007. doi: 10.1523/JNEUROSCI.3520-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle NA, Fadous S, Logothetis DE, Wang GK. Aminopyridine block of Kv1.1 potassium channels expressed in mammalian cells and Xenopus oocytes. Mol Pharmacol 45: 1242–1252, 1994. [PubMed] [Google Scholar]
- Chevaleyre V, Siegelbaum SA. Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron 66: 560–572, 2010. doi: 10.1016/j.neuron.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H-W, Swanson LW, Chen L, Fanselow MS, Toga AW. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc Natl Acad Sci USA 106: 11794–11799, 2009. doi: 10.1073/pnas.0812608106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KA, Islam T, Johnston D. Intrinsic excitability of CA1 pyramidal neurones from the rat dorsal and ventral hippocampus. J Physiol 590: 5707–5722, 2012. doi: 10.1113/jphysiol.2012.242693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty KA, Nicholson DA, Diaz L, Buss EW, Neuman KM, Chetkovich DM, Johnston D. Differential expression of HCN subunits alters voltage-dependent gating of h-channels in CA1 pyramidal neurons from dorsal and ventral hippocampus. J Neurophysiol 109: 1940–1953, 2013. doi: 10.1152/jn.00010.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrand JJ, Pouliot W, Scheerlinck P, Dudek FE. Lithium pilocarpine-induced status epilepticus in postnatal day 20 rats results in greater neuronal injury in ventral versus dorsal hippocampus. Neuroscience 192: 699–707, 2011. doi: 10.1016/j.neuroscience.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65: 7–19, 2010. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D, Lee JCF, Higgs MH, Spain WJ, Foehring RC. Functional roles of Kv1 channels in neocortical pyramidal neurons. J Neurophysiol 97: 1931–1940, 2007. doi: 10.1152/jn.00933.2006. [DOI] [PubMed] [Google Scholar]
- Henke PG. Hippocampal pathway to the amygdala and stress ulcer development. Brain Res Bull 25: 691–695, 1990. doi: 10.1016/0361-9230(90)90044-Z. [DOI] [PubMed] [Google Scholar]
- Higgs MH, Spain WJ. Kv1 channels control spike threshold dynamics and spike timing in cortical pyramidal neurones. J Physiol 589: 5125–5142, 2011. doi: 10.1113/jphysiol.2011.216721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth-Simonsen A. Hippocampal efferents to the ipsilateral entorhinal area: an experimental study in the rat. J Comp Neurol 142: 417–437, 1971. doi: 10.1002/cne.901420403. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol 116: 449–472, 1952a. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117: 500–544, 1952b. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hönigsperger C, Marosi M, Murphy R, Storm JF. Dorsoventral differences in Kv7/M-current and its impact on resonance, temporal summation and excitability in rat hippocampal pyramidal cells. J Physiol 593: 1551–1580, 2015. doi: 10.1113/jphysiol.2014.280826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol 295: 580–623, 1990. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jarrard LE. Selective hippocampal lesions and behavior: effects of kainic acid lesions on performance of place and cue tasks. Behav Neurosci 97: 873–889, 1983. doi: 10.1037/0735-7044.97.6.873. [DOI] [PubMed] [Google Scholar]
- Jay TM, Glowinski J, Thierry AM. Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Res 505: 337–340, 1989. doi: 10.1016/0006-8993(89)91464-9. [DOI] [PubMed] [Google Scholar]
- Jung MW, Wiener SI, McNaughton BL. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci 14: 7347–7356, 1994. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach BE, Johnston D, Brager DH. Cell-type specific channelopathies in the prefrontal cortex of the fmr1-/y mouse model of fragile X syndrome. eNeuro 2: ENEURO.0114-15.2015, 2015. doi: 10.1523/ENEURO.0114-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CS, Johnston D. A1 adenosine receptor-mediated GIRK channels contribute to the resting conductance of CA1 neurons in the dorsal hippocampus. J Neurophysiol 113: 2511–2523, 2015. doi: 10.1152/jn.00951.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Frank LM. Hippocampal lesions impair rapid learning of a continuous spatial alternation task. PLoS One 4: e5494, 2009. doi: 10.1371/journal.pone.0005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lothman EW, Collins RC. Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res 218: 299–318, 1981. doi: 10.1016/0006-8993(81)91308-1. [DOI] [PubMed] [Google Scholar]
- Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613–7624, 1998. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio N, Segal M. Unique regulation of long term potentiation in the rat ventral hippocampus. Hippocampus 17: 10–25, 2007. doi: 10.1002/hipo.20237. [DOI] [PubMed] [Google Scholar]
- Malik R, Dougherty KA, Parikh K, Byrne C, Johnston D. Mapping the electrophysiological and morphological properties of CA1 pyramidal neurons along the longitudinal hippocampal axis. Hippocampus 26: 341–361, 2016. doi: 10.1002/hipo.22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Johnston D. Dendritic GIRK channels gate the integration window, plateau potentials, and induction of synaptic plasticity in dorsal but not ventral CA1 neurons. J Neurosci 37: 3940–3955, 2017. doi: 10.1523/JNEUROSCI.2784-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelin B, Lugo JN, Brewster AL, Liu Z, Lewis AS, McClelland S, Chetkovich DM, Baram TZ, Anderson AE, Becker A, Esclapez M, Bernard C. Differential dorso-ventral distributions of Kv4.2 and HCN proteins confer distinct integrative properties to hippocampal CA1 pyramidal cell distal dendrites. J Biol Chem 287: 17656–17661, 2012. doi: 10.1074/jbc.C112.367110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milior G, Di Castro MA, Sciarria LP, Garofalo S, Branchi I, Ragozzino D, Limatola C, Maggi L. Electrophysiological properties of CA1 pyramidal neurons along the longitudinal axis of the mouse hippocampus. Sci Rep 6: 38242, 2016. doi: 10.1038/srep38242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci 13: 3916–3925, 1993. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA 92: 9697–9701, 1995. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou X, Cheng J, Yu YSW, Kee SE, Ji D. Comparing mouse and rat hippocampal place cell activities and firing sequences in the same environments. Front Cell Neurosci 12: 332, 2018. doi: 10.3389/fncel.2018.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan R, Johnston D. Long-term potentiation in rat hippocampal neurons is accompanied by spatially widespread changes in intrinsic oscillatory dynamics and excitability. Neuron 56: 1061–1075, 2007. doi: 10.1016/j.neuron.2007.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Kostopoulos G. Dorsal-ventral differentiation of short-term synaptic plasticity in rat CA1 hippocampal region. Neurosci Lett 286: 57–60, 2000. doi: 10.1016/S0304-3940(00)01084-3. [DOI] [PubMed] [Google Scholar]
- Papatheodoropoulos C, Moschovos C, Kostopoulos G. Greater contribution of N-methyl-D-aspartic acid receptors in ventral compared to dorsal hippocampal slices in the expression and long-term maintenance of epileptiform activity. Neuroscience 135: 765–779, 2005. doi: 10.1016/j.neuroscience.2005.06.024. [DOI] [PubMed] [Google Scholar]
- Racine R, Rose PA, Burnham WM. Afterdischarge thresholds and kindling rates in dorsal and ventral hippocampus and dentate gyrus. Can J Neurol Sci 4: 273–278, 1977. doi: 10.1017/S0317167100025117. [DOI] [PubMed] [Google Scholar]
- Routh BN, Johnston D, Harris K, Chitwood RA. Anatomical and electrophysiological comparison of CA1 pyramidal neurons of the rat and mouse. J Neurophysiol 102: 2288–2302, 2009. doi: 10.1152/jn.00082.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routh BN, Rathour RK, Baumgardner ME, Kalmbach BE, Johnston D, Brager DH. Increased transient Na+ conductance and action potential output in layer 2/3 prefrontal cortex neurons of the fmr1-/y mouse. J Physiol 595: 4431–4448, 2017. doi: 10.1113/JP274258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Svensson FP, Mazeh A, Kocsis B. Prefrontal-hippocampal coupling by theta rhythm and by 2-5 Hz oscillation in the delta band: the role of the nucleus reuniens of the thalamus. Brain Struct Funct 222: 2819–2830, 2017. doi: 10.1007/s00429-017-1374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SN, Publicover NG, Hart PJ, Carl A, Hume JR, Sanders KM, Horowitz B. Block by 4-aminopyridine of a Kv1.2 delayed rectifier K+ current expressed in Xenopus oocytes. J Physiol 481: 571–584, 1994. doi: 10.1113/jphysiol.1994.sp020464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage LM, Buzzetti RA, Ramirez DR. The effects of hippocampal lesions on learning, memory, and reward expectancies. Neurobiol Learn Mem 82: 109–119, 2004. doi: 10.1016/j.nlm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20: 11–21, 1957. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosanya NM, Brager DH, Wolfe S, Niere F, Raab-Graham KF. Rapamycin reveals an mTOR-independent repression of Kv1.1 expression during epileptogenesis. Neurobiol Dis 73: 96–105, 2015. doi: 10.1016/j.nbd.2014.09.011. [DOI] [PubMed] [Google Scholar]
- Sotiriou E, Papatheodoropoulos C, Angelatou F. Differential expression of gamma-aminobutyric acid—a receptor subunits in rat dorsal and ventral hippocampus. J Neurosci Res 82: 690–700, 2005. doi: 10.1002/jnr.20670. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev 99: 195–231, 1992. doi: 10.1037/0033-295X.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Schwindt PC, Crill WE. Repetitive firing in layer V neurons from cat neocortex in vitro. J Neurophysiol 52: 264–277, 1984. doi: 10.1152/jn.1984.52.2.264. [DOI] [PubMed] [Google Scholar]
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature 336: 379–381, 1988. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Storm JF. Functional diversity of K+ currents in hippocampal pyramidal neurons. Semin Neurosci 5: 79–92, 1993. doi: 10.1016/S1044-5765(05)80002-8. [DOI] [Google Scholar]
- Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol 172: 49–84, 1977. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- Toyoda I, Bower MR, Leyva F, Buckmaster PS. Early activation of ventral hippocampus and subiculum during spontaneous seizures in a rat model of temporal lobe epilepsy. J Neurosci 33: 11100–11115, 2013. doi: 10.1523/JNEUROSCI.0472-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Shu Y, McCormick DA. Cortical action potential backpropagation explains spike threshold variability and rapid-onset kinetics. J Neurosci 28: 7260–7272, 2008. doi: 10.1523/JNEUROSCI.1613-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.