Abstract
Background
Community-based efforts to promote physical activity (PA) in adults have been found to be cost-effective in general, but it is unknown if this is true in middle-age specifically. Age group-specific economic evaluations could help inform the design and delivery of better and more tailored PA promotion.
Methods
A Markov model was developed to estimate the cost-effectiveness (CE) of 7 exemplar community-level interventions to promote PA recommended by the Guide to Community Preventive Services, over a 20-year horizon. The CE of these interventions in 25- to 64-year-old adults was compared with their CE in middle-aged adults, aged 50 to 64 years. The robustness of the results was examined through sensitivity analyses.
Results
Cost/QALY (quality-adjusted life year) of the evaluated interventions in 25- to 64-year-olds ranged from $42,456/QALY to $145,868/QALY. Interventions were more cost-effective in middle-aged adults, with CE ratios 38% to 47% lower than in 25- to 64-year-old adults. Sensitivity analyses showed greater than a 90% probability that the true CE of 4 of the 7 interventions was below $125,000/QALY in adults aged 50 to 64 years.
Conclusion
The exemplar PA promotion interventions evaluated appeared to be especially cost-effective for middle-aged adults. Prioritizing such efforts to this age group is a good use of societal resources.
Keywords: exercise, health promotion, middle-age, economics
Lack of regular physical activity (PA) is a major preventable cause of chronic disease and premature mortality.1 Based on systematic reviews of research, the Guide to Community Preventive Services (or the Community Guide) strongly recommends 5 community-level strategies to promote PA.2,3 These strategies include community-wide campaigns, individually-adapted behavior change programs, and enhanced access to recreational facilities and other places for PA.
While many factors influence whether recommended strategies are implemented in a community, the cost-effectiveness of a particular intervention within a given strategy is an important consideration. Information on cost-effectiveness of Community Guide interventions is limited,4 including information on how cost-effectiveness varies by population subgroup. If funds for a programmatic approach (eg, the diabetes prevention program5) or community campaign were limited, one could consider tailoring interventions to age groups where they are most cost-effective. This would reduce the development and implementation costs of a particular approach, and could help target scarce resources toward the most appropriate PA promotion effort for the age group that stands to benefit most (eg, simple walking trails for middle-aged adults versus multiuse sporting complexes which must accommodate a broad range of physical activity opportunities across the age spectrum).
One population subgroup of interest is the “baby boomer” generation, which was born between 1945 and 1964, and is now entering late middle and old age in large numbers. In the largest group ever to become older adults, to what extent can prevention efforts reduce the burden on the health care system and improve quality of life?
Recently, a Markov simulation model, the Centers for Disease Control (CDC) Measurement of the Value of Exercise (MOVE) model, was developed to measure the cost-effectiveness of 7 exemplar PA interventions, representative of 4 of the 5 “strongly recommended” Community Guide strategies for community-based promotion of PA in adults (ie, community-wide campaigns, individually-adapted health behavior change, social support interventions in community settings, and creation of or enhanced access to places for PA combined with informational outreach activities).5–12 That cost-effectiveness analysis estimated the societal costs, health gains, and cost-effectiveness of these interventions among adults aged 25 to 64 years, across a 40-year time-horizon.12
Using the MOVE model, this current study aimed to: (1) estimate cost-effectiveness of these same exemplars over a shorter, 20-year time horizon across 2 age cohorts, middle-aged adults (adults age 50 to 64) and all adults aged 25 to 64 years; and (2) to determine the sensitivity of the model’s cost-effectiveness calculations to the parameter estimates under evaluation. By comparing cost-effectiveness of public health interventions to promote PA in adults aged 50 to 64 years to that of adults aged 25 to 64 years, we sought to provide insight on how cost-effectiveness might vary across these age cohorts.
Methods
In this study, the MOVE model,12 which is visually represented in Figure 1, was used to estimate the cost-effectiveness of the aforementioned 7 exemplar interventions over a 20-year time horizon, under 2 different scenarios. In the first scenario, we applied the interventions to a closed cohort, the size of the US adult population, aged 25 to 64 years, in 2004. In the second scenario, we applied the interventions only to the older members of this cohort, aged 50 to 64 years. A 20-year time horizon was chosen for this study to more accurately reflect health issues over the expected life span of this older population subset. Below we summarize the model simulations, the data upon which the model relies, and the methods used to calculate cost-effectiveness. A more detailed description of the model is provided in the previous study,12 and a methodological technical appendix is available upon request.
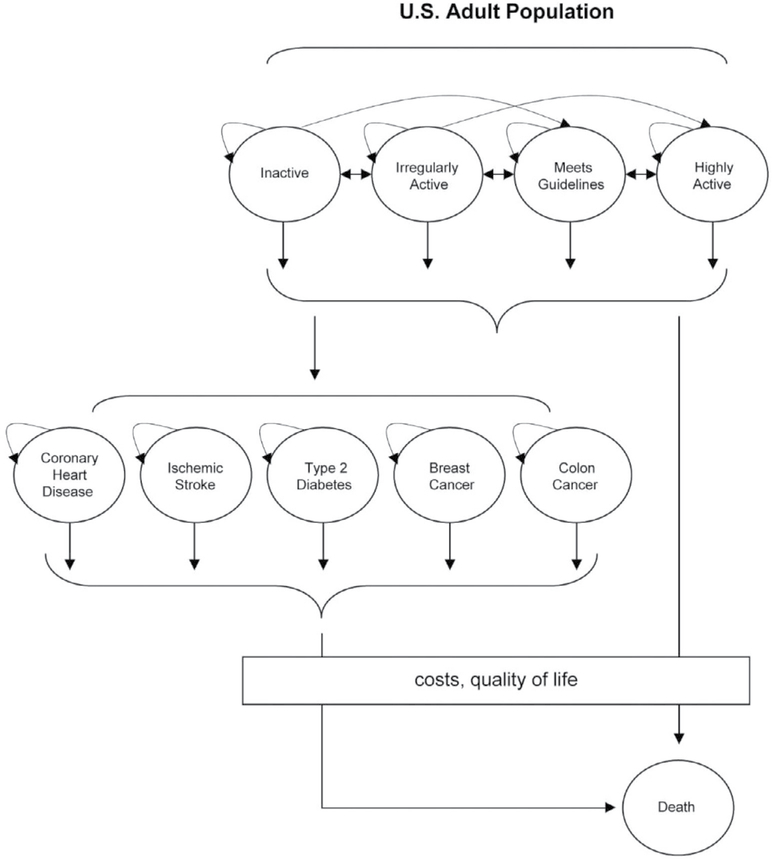
Figure 1 —
Conceptual overview of the Center for Disease Control Measurement of the Value of Exercise (MOVE) model. The illustration of our 10-state Markov process is represented as a state-transition diagram. In this process, circles represent possible health states, and arrows represent allowed transitions between these discrete health states. In each cycle of the Markov model, transition probabilities denote the likelihood with which people within a particular health state will stay in that state (represented by the tight curvilinear arrows to and from a single circle), transition to a new health state, or die. Death is an absorbing state from which no future transitions are possible. The output from the Markov process is depicted by the box, a running tally of the total costs and quality of life benefits generated during each cycle as a result of being in a series of health states over time.
Center for Disease Control Measurement of the Value of Exercise Model
All simulations were based on a cohort the size of the US population aged 25 to 64 years of age in 2004. At the start of the simulation, we assumed that all cohort members were healthy, which we defined as the absence of 5 modeled diseases: type 2 diabetes, coronary heart disease, ischemic stroke, breast cancer, and colorectal cancer. This approach is conservative, because it considers only the preventive benefits of activity. In practice, community-level interventions reach adults with chronic diseases such as diabetes and heart disease. There are well-documented therapeutic effects of PA in many chronic diseases,13 and ignoring such benefits reduces the estimated cost-effectiveness estimates. The cohort, the size of the adult US population in 2004, was stratified by age, sex, and level of physical activity, using US Census Bureau and 2003 BRFSS data.14,15 At the start of the simulation, all cohort members were assumed healthy.
The model then simulated how physical activity levels changed over the course of 20 years, and simulated the effects of these changes on incidence of the 5 diseases, quality of life, and mortality. The MOVE model incorporated data on how interventions moved cohort members across physical activity levels, and also incorporated data that specified how PA levels changed over time, in the absence of intervention (the “natural history model”).
In the first scenario, all cohort members, aged 25 to 64 years, were simulated to receive the exemplar interventions for 1 year, immediately upon entry into the model. Each cohort member had an intervention-specific probability of improving their level of PA. The effect of no intervention was also simulated. In the second scenario, the exemplar interventions were only applied to those adults 50 to 64 years of age, while the change in PA levels at 1 year for adults aged 25 to 49 years was determined using the natural history model.
The CDC MOVE model in this study retained parameter estimates used in the original study.12 After the first year (ie, for the next 19 years), in each scenario, cohort members had an annual probability of either remaining at the same physical activity level or moving to a lower activity level, as it was conservatively assumed that the impact of an intervention would decline after the intervention had ended. For all of the interventions, with the exception of the enhanced access intervention, a 50% decline in physical activity in year 2 was modeled. For the enhanced access study, a 33% decline was modeled in this second year, because the environmental enhancements persisted long after the intervention had ended.
Following this assumed substantial decline (33% to 50%) in physical activity postintervention in year 2, cohort members were transitioned into a natural-history model, which modeled the general decline in physical activity that occurs with age. Thus each year participants faced sex- and age-specific probabilities of moving to a lower level or remaining at the same physical activity level. Among the healthy, the risk of developing one of the 5 diseases depended on activity level, age, and sex. The risk of death depended on age, sex, and disease status.
Data Sources
Population Demographics and Physical Activity Levels
We obtained data on age and sex distributions of the US population from the US Census Bureau.15 Based upon public health PA recommendations2 in place at the time of the study, CDC classifies adults into 1 of 3 levels of PA (inactive, irregularly active, meets public health recommendations). This study used 4 levels of PA by dividing the “meets public health recommendations” group into “sufficiently active” and “highly active”. We obtained data on the distribution of physical activity levels by sex and age from the 2003 BRFSS (Behavioral Risk Factor Surveillance System).14 While there are more recent BRFSS data on PA levels, the 2003 data are used for comparability with the previous study’s methods, and because despite modest improvements in some PA population indicators like walking,16 there have been no major shifts in PA levels of the US population.
Disease Risk
To estimate the annual probability of developing each disease, we combined population-based disease-specific incidence data17–22 with relative risks derived from epidemiologic studies, specific for PA level and disease.23–25
Mortality Risk
The annual probability of death was estimated for both healthy individuals, and for individuals in the simulation who developed one of the 5 diseases. The probability of death was estimated for each age (5-year age group) and sex subgroup. In addition to disease-specific prevalence data,26–30 data from 2002 National Vital Statistics Reports were used to estimate the annual probability of death in people with coronary heart disease (CHD), ischemic stroke, or type 2 diabetes, while the SEER database was used to estimate annual probability of death from breast or colon cancers.31–34
To estimate the annual probability of death for healthy individuals, available mortality data35 excluding disease-specific death rates for the 5 modeled diseases, were adjusted for age group and sex.
Quality of Life
Quality of life (QOL) data were obtained for all disease and activity states from new analyses of the 2001 National Health Interview Survey, using previously validated scales for Quality of Well Being (QWB) widely used for assessing health-related quality of life.36–39 We performed multiple regressions to estimate QOL as a function of age, sex, disease, and PA level.
Intervention Effectiveness and Costs
Data on the effectiveness of the 7 exemplar interventions was ascertained from published reports.5–11 To determine all associated costs, the published protocols of each study were thoroughly reviewed to identify the components of each intervention. Tallied costs included material and intervention delivery costs, out-of-pocket expenses, participant time costs, and where applicable, costs associated with developing and maintaining the infrastructural components of an intervention. When possible, cost data for intervention components was obtained through direct communication with authors of the published reports.
To derive direct medical cost estimates, we used a longitudinal medical claims database40 and analyzed claims for the 5 disease states by ICD-9 codes. An annual medical inflation factor of 8% was applied,41 and the costs were discounted back to the present at 3% per year.42 It should be noted that this discounting strategy may tend to subtly favor PA promotion and disease prevention in older cohorts, because of the longer period of discounting of medical costs in younger cohorts. However, health benefits were also discounted to balance this discounting phenomenon. To improve their national representativeness, medical claims data were then adjusted using Medical Expenditure Panel Survey data.43 From these costs, we calculated the effective annual cost for each of the diseases over 20 years.
Modeling the Effect of Interventions
We characterized the 4 PA levels (inactive, irregularly active, sufficiently active, and highly active) in terms of a range of MET-minutes per week using BRFSS data (MET-minutes estimate the energy expended on PA, based upon the intensity, duration, and frequency of PA, and are used as a measure of total PA per week).14 The effect size of each intervention was converted into an increase in MET-minutes per week. To estimate the probability of moving to a higher PA level after intervention, we added intervention-specific MET-minutes per week to the current level, and noted the proportion of the cohort that moved from one level of PA to another as a result of an intervention. This proportion was then used as our transition probability. For example, if adding a certain number of MET-minutes per week to all persons in the inactive group caused 25% of them to move up to the irregularly active group, we estimated the probability of moving from inactive to irregularly active as 0.25 in the first year following the intervention. For the analysis focusing on the middle-aged cohort, we applied the increase in MET-minutes and intervention costs only to cohort members 50 to 64 years of age. The intervention effect size was assumed constant across age groups, but sex-specific.
We modeled a substantial decline (33% to 50%) in intervention effect following the 1-year intervention, based on limited data available on long-term maintenance of PA resulting from interventions.44–46 Following this decline, further changes in activity levels over time were determined by the natural history model.47–49 That is, for each year except the first, individuals were assigned sex- and age-specific probabilities of either moving to a lower PA level or remaining at the same level.
Estimating Cost-Effectiveness
In both groups of simulations, we estimated the cost-effectiveness of each intervention as cost divided by quality-adjusted life year (cost/QALY), using methods consistent with the guidelines established by the Panel on Cost-Effectiveness in Health and Medicine.42 Over a 20-year time horizon, the MOVE model was used to project costs, gains in life-years (survival), and gains in QALYs associated with each intervention, as well as with no intervention (natural history). Consistent with the panel’s recommendations, the societal perspective was adopted, and future costs and benefits were discounted to the present at an annual rate of 3%.42 Under each scenario, the performance of each intervention compared with no intervention was assessed using a ratio of the additional expected cost of each program divided by additional expected QALYs gained relative to the no intervention alternative. In addition, the number of cases of disease prevented was also estimated.
To determine the robustness of the final results, we conducted probabilistic sensitivity analyses, with particular emphasis placed on intervention effect size and cost estimates.
Sensitivity Analyses
To assess the impact of uncertain intervention cost and effect size parameter estimates on uncertainty in cost-effectiveness, we performed a probabilistic sensitivity analysis. When running such an analysis, we obtained not just a single cost-effectiveness ratio for each intervention, but a distribution of ratios reflecting joint parameter uncertainty of its cost and effectiveness.
Using the distributions from this analysis, we assessed the probability that the cost-effectiveness of each intervention was below various thresholds that are commonly used to determine whether interventions provide good value for money.
Results
Average Cost-Effectiveness
Tables 1 and 2 summarize the average costs, effectiveness, and cost-effectiveness ratios associated with a one-time application of each PA promotion intervention, relative to no intervention. Results are cumulative over a 20-year time horizon, but average per person values are reported in both tables.
Table 1.
Cost-Effectiveness of Each Intervention Compared With No Intervention in All Adults (25–64 Years) at 20 Years
| Exemplar Study | Intervention Strategy | Total cost, 2003 $US | Total life years | Total QALY | Incremental cost ($) | Incremental life years | Incremental QALY | Cost/LY ($/LY) | Cost/QALY ($/QALY) |
|---|---|---|---|---|---|---|---|---|---|
| No intervention | 61,092 | 14,389 | 11.182 | — | — | — | — | — | |
| Reger et al10 | Community-wide campaign | 62,292 | 14,397 | 11.210 | 1,200 | 0.009 | 0.028 | 136,341 | 42,546 |
| Lombard et al9 | Social support | 64,794 | 14,408 | 11.244 | 3,702 | 0.020 | 0.063 | 186,980 | 59,235 |
| Linenger et al8 | Enhanced access | 62,087 | 14,393 | 11.197 | 995 | 0.005 | 0.015 | 211,787 | 65,921 |
| Jeffery et al6 | Individually-adapted health behavior | 63,695 | 14,400 | 11.218 | 2,603 | 0.011 | 0.037 | 232,393 | 71,115 |
| Kriska et al7 | Social support | 62,671 | 14,394 | 11.199 | 1,580 | 0.005 | 0.018 | 298,019 | 89,744 |
| Knowler et al5 (DPP) | Individually-adapted health behavior | 64,395 | 14,399 | 11.215 | 3,303 | 0.010 | 0.033 | 317,596 | 99,789 |
| Young et al11 | Community-wide campaign | 62,200 | 14,391 | 11.189 | 1,109 | 0.002 | 0.008 | 461,917 | 145,868 |
Note. QALY = quality-adjusted life year; LY = life year; DPP = diabetes prevention program. Values in Table 1 are rounded to no more than 3 decimal places Future cumulative costs and benefits over a 20-year time horizon are reported in table as discounted, average, per person costs, and QALYs.
Table 2.
Cost-Effectiveness of Each Intervention Compared With No Intervention in Middle-Aged Adults (50–64 Years) at 20 Years
| Exemplar Study | Intervention Strategy | Total cost, 2003 $US | Total life years | Total QALY | Incremental cost ($) | Incremental life years | Incremental QALY | Cost/LY ($/LY) | Cost/QALY ($/QALY) |
|---|---|---|---|---|---|---|---|---|---|
| No intervention | 61,092 | 14.389 | 11.182 | — | — | — | — | — | |
| Reger et al10 | Community-wide campaign | 61,455 | 14.394 | 11.192 | 363 | 0.006 | 0.011 | 63,737 | 33,639 |
| Lombard et al9 | Social support | 61,410 | 14.392 | 11.187 | 318 | 0.003 | 0.006 | 105,967 | 56,768 |
| Linenger et al8 | Enhanced access | 62,243 | 14.401 | 11.206 | 1,151 | 0.013 | 0.024 | 89,217 | 47,954 |
| Jeffery et al6 | Individually adapted health behavior | 61,916 | 14.396 | 11.195 | 825 | 0.007 | 0.014 | 112,973 | 59,331 |
| Kriska et al7 | Social support | 61,607 | 14.392 | 11.188 | 515 | 0.003 | 0.007 | 151,500 | 79,246 |
| Knowler et al5 (DPP) | Individually-adapted health behavior | 62,141 | 14.395 | 11.194 | 1,050 | 0.007 | 0.013 | 156,657 | 82,646 |
| Young et al11 | Community-wide campaign | 61,449 | 14.390 | 11.184 | 357 | 0.002 | 0.003 | 237,933 | 127,464 |
Note. QALY = quality-adjusted life year; LY = life year; DPP = diabetes prevention program. Values in Table 2 are rounded to no more than 3 decimal places. Future cumulative costs and benefits over a 20-year time horizon are reported in table as discounted, average, per person costs, and QALYs.
In the first scenario (Table 1), interventions were applied to the entire cohort of adults aged 25 to 64 years, and compared with no intervention. With no intervention, the average discounted quality-adjusted life expectancy (total QALY) was predicted as 11.18 years, and 20-year cumulative costs were estimated at about $61,100. Intervention participation increased average total QALY by 0.008 to 0.063 QALYs (Table 1), or equivalently, intervention participation improved average healthy life expectancy by 0.42 to 3.28 weeks. Cost-effectiveness ratios ranged between ~$42,000 and ~$146,000 per QALY gained. The Lombard social support intervention9 was estimated as the most effective, with the largest gain in QALYs (0.063), compared with no intervention. The Reger community-wide campaign10 was estimated as the most cost-effective (about $42,500/QALY).
In the second scenario (Table 2), interventions were applied only to persons aged 50 to 64 years, and compared with no intervention. Intervention participation improved average QALYs by 0.003 to 0.024, which is equivalent to 0.16 to 1.25 weeks. Cost/QALY in middle-aged adults (age 50 to 64 years) were 38% to 47% lower depending upon the intervention, and ranged from ~$34,000 and ~$127,000 per QALY gained. That is, for each intervention, the cost-effectiveness was better when it was applied only to adults age 50 to 64.
All interventions reduced disease incidence in the simulations. The reductions ranged from 3 to 20 cases per 100,000 for colon cancer and from 55 to 420 cases per 100,000 for CHD.
Sensitivity Analyses
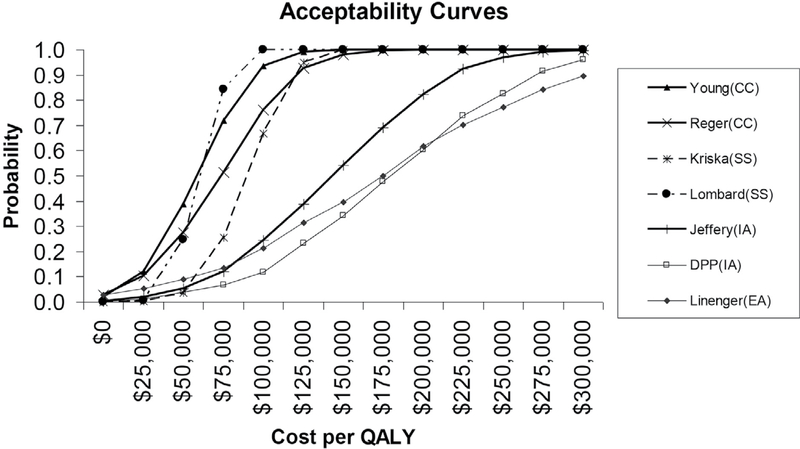
Results from the probabilistic sensitivity analysis for the middle-aged cohort are shown in the acceptability curves in Figure 2. For example, there is about a 40% chance that the cost/QALY of the young intervention11 is less than $50,000 per QALY (a traditionally used benchmark of cost-effectiveness), and virtually a 100% chance that it is below the more contemporary threshold of $200,000/QALY.50 A review of several studies found that the median estimate of willingness to pay for gains in quality of life was $265,345 per QALY.50 That is, despite model parameter and resultant cost/QALY ratio uncertainties, it is almost certain that this intervention is an acceptable use of societal resources.
Figure 2 —
Results from probabilistic sensitivity analyses for the middle-aged cohort (50–64 years). Acceptability curves represent variation of intervention costs and effect sizes. The schematic of curves depicts the probability with which an intervention (despite uncertainties in its associated cost and effect estimates) is deemed an acceptable use of societal resources, on the basis of its cost per quality-adjusted life year (QALY) ratio being less than a given dollar amount. Studies: Young et al11, Reger et al10, Kriska et al7, Lombard et al9, Jeffery et al6, Knowler et al5 (Diabetes prevention program [DPP]), Linenger et al8. CC = community-wide campaign; SS = social support; IA = individually-adapted; EA = enhanced access.
Discussion
The results of the study suggest that 7 exemplar interventions have acceptable cost-effectiveness when applied to middle-aged adults (age 50 to 64 years). The MOVE model estimated cost-effectiveness ratios between ~$33,600/QALY and ~$127,400/QALY for the 7 interventions. Cost-effectiveness ratios in this range imply good value for money, or an acceptable use of societal resources and are typical of many well-accepted and widely-implemented health interventions.51–54 The conclusion of acceptable cost-effectiveness was supported by the sensitivity analysis, especially for the 4 interventions with the lowest cost/QALY ratios in middle-aged adults. The cost-effectiveness estimates were for a 20-year time horizon, and so they differ from the estimates in the previous study, which used a 40-year time horizon.12 Together, the 2 studies suggest that community-level interventions to promote PA have acceptable cost-effectiveness over both a 20-year period and a 40-year period.
The exemplar interventions were more cost-effective when applied only to middle-aged adults than when applied to the larger population of adults aged 25 to 64 years. Depending upon the intervention, the cost-effectiveness ratios were 38% to 47% lower in middle-aged adults. As the Markov model does not provide standard errors for the cost-effectiveness estimates, we did not use statistical tests to determine if the cost-effectiveness ratios were significantly lower in middle-aged adults. Rather, to establish the robustness of our results, we conducted sensitivity analyses (Figure 2) that essentially showed that the lower the cost/QALY point estimates from the Markov model, the higher the probabilities that the true cost-effectiveness ratios were in the acceptable range. That is, over a 20-year time horizon, the results of this study indicate that although all interventions were cost-effective in both scenarios, there was a higher probability that they were cost-effective in the middle-aged subgroup.
What might account for better cost-effectiveness ratios in middle-aged adults? The cost-effectiveness estimates from the Markov model are most sensitive to intervention cost and to intervention effect size, but the model used the same cost and effect size for all age groups. However, with population-wide interventions, the absolute risk of a disease affects the cost-effectiveness of an intervention which prevents the disease; the higher the disease risk, the better the cost-effectiveness. Middle-aged adults have a higher risk of the 5 diseases in the MOVE model, so for this reason it is not surprising that the cost-effectiveness is better in middle-aged adults.
Several community interventions have successfully targeted specific age groups. AARP conducted a community-wide campaign, which targeted older adults.55 The Wheeling Walks community-wide campaign targeted middle-aged adults, as well as older adults—an age group for which walking is the main form of activity.10 The Active for Life program successfully translated individually-adapted behavior change interventions just for older adults.56 The Environmental Protection Agency offers an award program for community design which promotes physical activity in older adults.57 Of course, with some interventions, it is more difficult to target a specific age group. If a community created a new park, there would be many societal benefits for all citizens to enjoy. However, facilities within a park might target a specific age group. For example, while playgrounds generally would attract children, easy walking trails would likely attract more middle-aged and older adults.
The previous report on the MOVE model discussed several of its limitations,12 such as the model having made several assumptions because the data necessary to include a proposed component in the model were not always available. Additional issues arise when the model is used to compare cost-effectiveness between 2 different scenarios. First, the model does not take into account a person’s PA history, because of insufficient information on how lifetime PA affects disease risk. A person who is sedentary until 50 years of age and then becomes regularly active is assigned the same health benefit due to activity as a person who is sedentary until 25 years of age and is regularly active thereafter. This feature of the model may make interventions in younger adults appear less cost-effective than they actually are. Second, the MOVE model does not currently take into account therapeutic benefits of activity in persons with chronic disease, which would tend to increase the benefits of PA in older populations with more chronic disease. Third, as noted above, the study presumes that the 7 interventions can be implemented in a manner so as to reach only certain age groups, which may lead to more conservative estimates of cost-effectiveness. Fourth, due to incomplete data on age-specific response rates to PA promotion, these rates are considered to be constant across age and initial PA level-specific groups. More research is required to determine how middle-aged adults respond to PA promotion efforts, and how durable these responses are. We attempt to account for the present uncertainty about these age-specific PA behaviors in sensitivity analyses, which demonstrate cost-effectiveness across a broad range of response rates. Fifth, due to limited data on ethnicity-specific disease outcomes, PA, and intervention effects, it is not possible to extend the model to assess the cost-effectiveness of interventions in subpopulations by ethnicity. It remains a priority for future PA promotion research to evaluate and address specific societal determinants of health to achieve the greatest benefits in vulnerable populations. Finally, the model focuses only on health benefits and does not include potential corollary benefits of increasing physical activity among older adults such as enhanced social interaction, cognitive function, greater mobility, and independence, not to mention the positive ripple effects extending to communities with some targeted enhanced-access opportunities. Thus, we believe that this analysis underestimates the overall benefits and cost-effectiveness of interventions to increase physical activity in older adults.
As health care expenditures continue to increase faster than inflation, policymakers are increasingly interested in prevention and in promoting overall health of communities. We believe the MOVE model is of importance to stakeholders in community health. The simulations suggest community-level, population-based approaches to promoting PA are cost-effective. The simulations quantify how much cost-effectiveness is improved by focusing interventions on middle-aged adults at higher risk for disease. It is important and justifiable to promote PA in all age groups. As the science of PA promotion advances, and newer and more effective interventions emerge along with new strategies to combine the best PA promotion strategies, cost-effectiveness of PA promotion will undoubtedly continue to evolve. However, given the current PA recommendations and finite resources, if the objective is to cost-effectively reduce relatively short-term disease risks, it may be appropriate to emphasize promotion of PA in older age groups. In particular, the middle-aged “baby boomer” generation will soon become the older adults group (aged 65 years and older) in great numbers. Preventive interventions in this age group could substantially delay health care costs due to age-related diseases.
Acknowledgments
This study stems from our original work on Project MOVE, which integrated the expertise and dedication of a large multidisciplinary team of accomplished investigators and policy leaders from academic centers across the United States and from our colleagues in the Physical Activity and Health Branch at the CDC (Chantelle Avery, Laura Biazzo, Mario Bracco, David Casey Hannan, Gregory Heath, Carrie Heitzler, Harold W. Kohl III, Diana C. Parra, Candace Rutt, Guijing Wang, Linda West, Teri L. Yanagawa, and Michelle M. Yore), the CDC Foundation (C. Adam Brush, Connie L. Carmack, and John R. Moore), Milliman Inc. (Jill Van Den Bos), the Robert Wood Johnson Foundation (Lori Melichar, Pamela G. Russo, and Kathryn A. Thomas), and on the Project MOVE Advisory Committee (Ross C. Brownson, John Cawley, Brian Cole, Jonathan E. Fielding, Eric Finkelstein, William L. Haskell, Robert M. Kaplan, David Meltzer, Kenneth E. Powell, Tammy O. Tengs, and Steven Teutsch).
This scale of collaboration was made possible by the commitment and financial support of the Robert Wood Johnson Foundation and the CDC Foundation and their project officers.
The principal investigator of this study, Larissa Roux, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This work was supported by a generous grant from the Robert Wood Johnson Foundation, through the CDC Foundation (Project # 124432-0100-03). The overall project was entitled, Project MOVE: Measurement of the Value of Exercise: Economic analysis of community interventions to increase physical activity (Physical Inactivity and Sedentary Lifestyles). The project sought to improve public health public policy decision making by developing methods and tools that allowed for measuring, valuing, and comparing the health and economic impacts of physical activity promotion. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor Information
Larissa Roux, LifeMark Sport Medicine, Richmond Olympic Oval, Vancouver, Canada..
Mike Pratt, WHO Collaborating Center for Physical Activity and Health Promotion, CDC, Atlanta, GA..
I-Min Lee, Dept of Epidemiology, Harvard Medical School, Boston, MA..
Terry Bazzarre, Robert Wood Johnson Foundation, Princeton, NJ..
David Buchner, Dept of Kinesiology and Community Health, University of Illinois at Urbana-Champaign..
References
- 1.U.S. Department of Health & Human Services. 2008. Physical Activity Guidelines for Americans: Advisory Committee Report. http://www.health.gov/paguidelines/report/. Last accessed 8 October 2009.
- 2.Kahn EB, Ramsey LT, Brownson RC, et al. The effectiveness of interventions to increase physical activity: a systematic review. Am J Prev Med. 2002;22(4 Suppl):73–107. doi: 10.1016/S0749-3797(02)00434-8 [DOI] [PubMed] [Google Scholar]
- 3.Haskell WL, Lee I, Pate RR, et al. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27 [DOI] [PubMed] [Google Scholar]
- 4.Truman BI, Smith-Akin CK, Hinman AR, et al. Developing the Guide to Community Preventive Services–overview and rationale. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18(1, Suppl):18–26. doi: 10.1016/S0749-3797(99)00124-5 [DOI] [PubMed] [Google Scholar]
- 5.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeffery RW, Wing RR, Thorson C, Burton LR. Use of personal trainers and financial incentives to increase exercise in a behavioral weight-loss program. J Consult Clin Psychol. 1998;66(5):777–783. doi: 10.1037/0022-006X.66.5.777 [DOI] [PubMed] [Google Scholar]
- 7.Kriska AM, Bayles C, Cauley JA, LaPorte RE, Sandler RB, Pambianco G. A randomized exercise trial in older women: increased activity over two years and the factors associated with compliance. Med Sci Sports Exerc. 1986;18(5):557–562. doi: 10.1249/00005768-198610000-00011 [DOI] [PubMed] [Google Scholar]
- 8.Linenger JM, Chesson CV 2nd, Nice DS. Physical fitness gains following simple environmental change. Am J Prev Med. 1991;7(5):298–310. [PubMed] [Google Scholar]
- 9.Lombard DN, Lombard TN, Winett RA. Walking to meet health guidelines: the effect of prompting frequency and prompt structure. Health Psychol. 1995;14(2):164–170. doi: 10.1037/0278-6133.14.2.164 [DOI] [PubMed] [Google Scholar]
- 10.Reger B, Cooper L, Booth-Butterfield S, et al. Wheeling Walks: a community campaign using paid media to encourage walking among sedentary older adults. Prev Med. 2002;35(3):285–292. doi: 10.1006/pmed.2002.1074 [DOI] [PubMed] [Google Scholar]
- 11.Young DR, Haskell WL, Taylor CB, Fortmann SP. Effect of com-munity health education on physical activity knowledge, attitudes, and behavior. The Stanford Five-City Project. Am J Epidemiol. 1996;144(3):264–274. doi: 10.1093/oxfordjournals.aje.a008921 [DOI] [PubMed] [Google Scholar]
- 12.Roux L, Pratt M, Tengs TO, et al. Cost-effectiveness of community-based physical activity interventions. Am J Prev Med. 2008;35(6):578–588. doi: 10.1016/j.amepre.2008.06.040 [DOI] [PubMed] [Google Scholar]
- 13.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: Recommendations from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–1445. doi: 10.1249/mss.0b013e3180616aa2 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System. Available at http://www.cdc.gov/brfss/. (Accessed January 6, 2009).
- 15.US Census Bureau. Population projections of the resident popula-tion by age, sex, race, and hispanic origin. Available at: https://www.census.gov/population/projections/data/national/2008.html (Accessed January 6, 2009).
- 16.Centers for Disease Control and Prevention. Morbidity and Mortal-ity Weekly Report (MMWR). Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6131a4.htm
- 17.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.CIR.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 18.Wolfe CD, Rudd AG, Howard R, et al. Incidence and case fatality rates of stroke subtypes in a multiethnic population: the South London Stroke Register. J Neurol Neurosurg Psychiatry. 2002;72(2):211–216. doi: 10.1136/jnnp.72.2.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27(3):373–380. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. National diabetes fact sheet. Available at http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. (Accessed January 14, 2013).
- 21.Centers for Disease Control and Prevention. Diabetes Surveillance System: Incidence of diabetes. Available at http://www.cdc.gov/diabetes/data/. (Accessed August 23, 2004).
- 22.National Cancer Institute. Surveillance, epidemiology, and end results. Available at: http://seer.cancer.gov/. (Accessed January 8, 2009).
- 23.Finkelstein EA, Wang G, Lee IM, et al. National and state-specific inactivity-attributable medical expenditures for six diseases Final report prepared for the Centers for Disease Control and Prevention by the Research Triangle Institute. Centers for Disease Control and Prevention and Research Triangle Institute; November 2004. [Google Scholar]
- 24.Hu FB, Stampfer MJ, Colditz GA, et al. Physical activity and risk of stroke in women. JAMA. 2000;283(22):2961–2967. doi: 10.1001/jama.283.22.2961 [DOI] [PubMed] [Google Scholar]
- 25.Katzmarzyk PT, Janssen I. The economic costs associated with physi-cal inactivity and obesity in Canada: an update. Can J Appl Physiol. 2004;29(1):90–115. doi: 10.1139/h04-008 [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Self-reported heart disease and stroke among adults with and without diabetes–United States, 1999–2001. MMWR Morb Mortal Wkly Rep. 2003;52:1065–1070. [PubMed] [Google Scholar]
- 27.National Cancer Institute. Surveillance, Epidemiology and End Results. Available at http://seer.cancer.gov/. (Accessed January 8, 2009).
- 28.Centers for Disease Control. Diabetes Surveillance System: Prevalence of Diabetes. Available at http://www.cdc.gov/diabetes/data/. (Accessed January 8, 2009).
- 29.Brown RD, Whisnat JP, Sicks JD, O’Fallon EM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–380. [PubMed] [Google Scholar]
- 30.Centers for Disease Control. National Diabetes Fact Sheet. Available at http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. (Accessed January 14, 2013).
- 31.Lee IM, Skerrett PJ. Physical activity and all-cause mortality: what is the dose-response relation? Med Sci Sports Exerc. 2001;33(6 Suppl):S459–S471; discussion S493–S454. [DOI] [PubMed] [Google Scholar]
- 32.Arias E United States life tables, 2000. Natl Vital Stat Rep. 2002;51(3):1–38. [PubMed] [Google Scholar]
- 33.National Cancer Institute. Surveillance, epidemiology, and end results. Available at http://seer.cancer.gov/statfacts/html/colorect.html. (Accessed January 8, 2009).
- 34.National Cancer Institute. Surveillance, epidemiology, and end results. Available at http://seer.cancer.gov/statfacts/html/breast.html. (Accessed January 8, 2009).
- 35.Arias E, Anderson RN, Kung HC, Murphy SL, Kochanek KD. Deaths: final data for 2001. Natl Vital Stat Rep. 2003;52(3)1–115. [PubMed] [Google Scholar]
- 36.Tengs TO, Lin TH. A meta-analysis of quality of life estimates for stroke. Pharmacoeconomics. 2003;21(3):191–200. doi: 10.2165/00019053-200321030-00004 [DOI] [PubMed] [Google Scholar]
- 37.Kaplan RM, Anderson JP, Ake CF. Gender differences in quality-adjusted life expectancy: Results from the National Health Interview Survey. Clin J Womens Health. 2001;1(4):191–197. doi: 10.1053/cjwh.2001.28299 [DOI] [Google Scholar]
- 38.Kaplan R, Anderson J. The general health policy model: An integrated approach In: Spilker B, ed. Quality of Life and Pharmacoeconomics in Clinical Trials. New York, NY: Raven; 1996:309–322. [Google Scholar]
- 39.Kaplan RM, Anderson JP, Patterson TL, et al. Validity of the Quality of Well-Being Scale for persons with human immunodeficiency virus infection. HNRC Group. HIV Neurobehavioral Research Center. Psychosom Med. 1995;57(2):138–147. doi: 10.1097/00006842-199503000-00006 [DOI] [PubMed] [Google Scholar]
- 40.MarketScan Research Databases User Guide and Database Dictionary. Ann Arbor, MI: Thomson Medstat; 2004. [Google Scholar]
- 41.Centers for Medicare & Medicaid Services. National Health Expen-diture Data. Available https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpend-Data/index.html?redirect=/NationalHealthexpendData/
- 42.Gold M, Siegel J, Russell L, Weinstein M. Cost-effectiveness in health and medicine. New York: Oxford University Press, Inc.; 1996. [Google Scholar]
- 43.Agency for Healthcare Research and Quality. Medical expenditure panel survey. Available: http://meps.ahrq.gov/mepsweb/; 2001. [PubMed] [Google Scholar]
- 44.King AC, Kiernan M, Oman RF, Kraemer HC, Hull M, Ahn D. Can we identify who will adhere to long-term physical activity? Signal detection methodology as a potential aid to clinical decision making. Health Psychol. 1997;16(4):380–389. doi: 10.1037/0278-6133.16.4.380 [DOI] [PubMed] [Google Scholar]
- 45.Marcus BH, Dubbert PM, Forsyth LH, et al. Physical activity behavior change: issues in adoption and maintenance. Health Psychol. 2000;19(1, Suppl):32–41. doi: 10.1037/0278-6133.19.Suppl1.32 [DOI] [PubMed] [Google Scholar]
- 46.Linenger JM, Chesson CV 2nd, Nice DS. Physical fitness gains follow-ing simple environmental change. Am J Prev Med. 1991;7(5):298–310. [PubMed] [Google Scholar]
- 47.US Department of Health and Human Services. Physical activity and health: A report of the Surgeon General. Atlanta: Centers for Disease Control and Prevention; 1996. [Google Scholar]
- 48.Pratt M, Macera CA, Blanton C. Levels of physical activity and inac-tivity in children and adults in the United States: current evidence and research issues. Med Sci Sports Exerc. 1999;31(11, Suppl):S526–S533. doi: 10.1097/00005768-199911001-00007 [DOI] [PubMed] [Google Scholar]
- 49.Sapkota S, Bowles HR, Ham SA, Kohl HW III. Adult participation in recommended levels of physical activity—United States, 2001 and 2003. MMWR Morb Mortal Wkly Rep. 2005;54:1208–1212. [PubMed] [Google Scholar]
- 50.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20(3):332–342. doi: 10.1177/0272989X0002000310 [DOI] [PubMed] [Google Scholar]
- 51.Salkeld G, Phongsavan P, Oldenburg B, et al. The cost-effectiveness of a cardiovascular risk reduction program in general practice. Health Policy. 1997;41(2):105–119. doi: 10.1016/S0168-8510(97)00015-8 [DOI] [PubMed] [Google Scholar]
- 52.Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost-effectiveness analysis. Ann Intern Med. 2004;140(9):689–699. doi: 10.7326/0003-4819-140-9-200405040-00008 [DOI] [PubMed] [Google Scholar]
- 53.Neumann PJ, Rosen AB, Greenberg D, et al. Can we better pri-oritize resources for cost-utility research? Med Decis Making. 2005;25(4):429–436. doi: 10.1177/0272989X05276853 [DOI] [PubMed] [Google Scholar]
- 54.Stone PW, Teutsch S, Chapman RH, Bell C, Goldie SJ, Neumann PJ. Cost-utility analyses of clinical preventive services: published ratios, 1976–1997. Am J Prev Med. 2000;19(1):15–23. doi: 10.1016/S0749-3797(00)00151-3 [DOI] [PubMed] [Google Scholar]
- 55.AARP. Promoting Physical Activity Among Those 50+. Available http://www.aarp.org/health/fitness/info-06-2010/promoting_physicalactivityamongthose50.html (Last accessed 8 October 2009).
- 56.Wilcox S, Dowda M, Leviton LC, et al. Final results from the transla-tion of two physical activity programs. Am J Prev Med. 2008;35:340–351. doi: 10.1016/j.amepre.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 57.US Environmental Protection Agency. Building Healthy Communities for Activity Aging Awards, 2008. Available http://www.epa.gov/aging/bhc/2008-awards.htm. Last accessed 8 February 2013.