Abstract
Background
Benfotiamine (BFT) is a synthetic thiamine precursor with high bioavailability. It is efficient in treating complications of type 2 diabetes and has beneficial effects in mouse models of neurodegenerative diseases. The mechanism of action of BFT remains unknown, though it is sometimes suggested that it may be linked to increased thiamine diphosphate (ThDP) coenzyme function.
Methods
We used a mouse neuroblastoma cell line (Neuro2a) grown in thiamine-restricted medium. The cells were stressed by exposure to paraquat (PQ) or amyloid β1-42 peptide in the presence or absence of BFT and the cell survival was measured using the MTT method. In each case, BFT was compared with sulbutiamine (SuBT), an unrelated thiamine precursor, and thiamine. Metabolites of BFT were determined by HPLC and mass spectrometry.
Results
At 50 μM, BFT protects the cells against PQ and amyloid β1-42 peptide-induced toxicity with the same efficacy. Protective effects were also observed with SuBT and with higher concentrations of thiamine. The main metabolites of BFT were thiamine and S-benzoylthiamine (S-BT). Treatment with both precursors induces a strong increase in intracellular content of thiamine. Protective effects of BFT and SuBT are directly related to thiamine (but not ThDP) levels in Neuro2a cells.
Conclusions
BFT, SuBT and thiamine all protect the cells against oxidative stress, suggesting an antioxidant effect of thiamine. Our results are not in favor of a direct ROS scavenging effect of thiamine but rather an indirect effect possibly mediated by some antioxidant signaling pathway. It is however not clear whether this effect is due to thiamine itself, its thiol form or an unknown metabolite.
General significance
Our results suggest a role of thiamine in protection against oxidative stress, independent of the coenzyme function of thiamine diphosphate.
Keywords: Cell biology, Neuroscience
Abbreviations: ARE, antioxidant response element; BFT, benfotiamine; FBS, fetal bovine serum; O-BT, O-benzoylthiamine; S-BT, S-benzoylthiamine; PQ, paraquat; ROS, reactive oxygen species; SuBT, sulbutiamine; ThDP, thiamine diphosphate; ThMP, thiamine monophosphate; TPK, thiamine pyrophosphokinase
1. Introduction
Thiamine (vitamin B1) is essential for the nervous system. It is the precursor of thiamine diphosphate (ThDP), an important coenzyme for transketolase, pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase [1]. It is thus essential for brain energy metabolism. ThDP is formed by pyrophosphorylation of thiamine, a reaction catalyzed by thiamine pyrophosphokinase (TPK). Thiamine monophosphate (ThMP) is also found in the brain as a minor thiamine compound. It is formed by dephosporylation of ThDP, but it has no known specific role. Non-coenzyme roles of thiamine have also been suggested [2, 3].
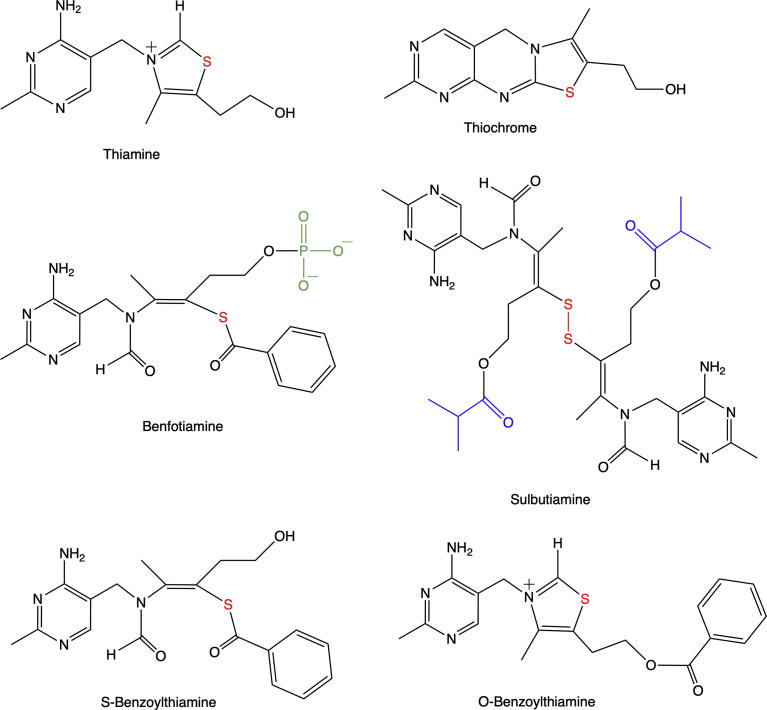
Thiamine deficiency leads to severe lesions of the nervous system. Thiamine deficiency disorders are complex and can adopt various forms [1]. While nutritional thiamine deficiency often leads to polyneuritic syndromes such as beriberi, alcohol-induced thiamine deficiency leads to Wernicke's encephalopathy, with potential fatal outcome, and Korsakoff's syndrome, a memory disorder [4]. When no irreversible brain lesions are present, thiamine deficiency responds well to treatment with orally administered thiamine. However, the intestinal absorption of thiamine, which requires specific transporters, is rate-limiting [5]. For this reason, thiamine precursors with higher bioavailability were developed (Fig. 1). Presently, the most widely used is benfotiamine (BFT). Initially, BFT was used to increase blood thiamine levels in patients suffering from thiamine deficiency. It was later shown to prevent experimental diabetic retinopathy [6]. More recently, BFT was shown to have powerful beneficial effects in mouse models of neurodegenerative diseases [7, 8]. An interesting point is that in both studies, BFT did not lead to increased levels of ThDP in the brain: only thiamine slightly increased. This observation is in agreement with at least two other studies in which BFT treatment did not lead to increased brain ThDP content, while it was increased in blood and liver [9, 10]. In the latter study [10], thiamine and BFT prevented predator stress-induced suppression of neurogenesis. Hence, it is now well documented that BFT can have powerful neuroprotective and anti-stress effects in the rodent brain, but they do not seem to be mediated by stimulation of ThDP-dependent enzymes.
Fig. 1.
Chemical structures of thiamine, thiochrome, benfotiamine, sulbutiamine, S-benzoylthiamine and O-benzoylthiamine. The thiamine sulfur is indicated in red, the isobutyrate group (substituted by a H in thiamine disulfide) of SuBT is in blue, and the phosphoryl group of benfotiamine is in green.
In a mouse model of tauopathy [8], long-term treatment with BFT not only reduced hyperphosphorylated tau in brain but also increased the life-span of the animals, prevented death of spinal cord motor neurons and decreased oxidative stress and inflammation. However, brain transketolase activity was only slightly increased and α-ketoglutarate dehydrogenase activity was not significantly changed, in agreement with unaffected ThDP levels. The authors suggested a model in which products of BFT metabolism such as S-benzoylthiamine (S-BT) or O-benzoylthiamine (O-BT) (Fig. 1) would activate the Nrf2-antioxidant response element (ARE) signaling pathway, critical for the activation of genes involved in the elimination of reactive oxygen species (ROS) [11].
This hypothesis was based on three findings:
-
1)
In transgenic mice, BFT treatment increases the expression of some oxidative stress-protective enzymes known to be under the control of Nrf2.
-
2)
BFT, S-BT and O-BT activate the Nrf2 transcription pathway in cultured cells, but concentrations as high as 100 μM were required for full activation.
-
3)
Docking studies with Keap-1, a protein sequestering Nrf-2, suggest that BFT, S-BT and O-BT could bind to Keap-1 with high affinity, thereby releasing Nrf2 and allowing its transfer to the nucleus, promoting its transcription factor activity.
Though these data suggest that BFT, S-BT and O-BT can indeed activate the Nrf2/ARE pathway in vitro, there is so far no evidence that significant amounts of these compounds can reach the brain parenchyma, even when the animals are treated with high doses of BFT.
It is generally agreed upon that BFT, when taken orally, is dephosphorylated to S-BT by the alkaline phosphatases of the intestinal epithelium. This is why oral administration of BFT is more efficient than parenteral routes. The lipophilic S-BT can then diffuse through the epithelium and reach the blood stream. It is not known, however, what concentrations of S-BT could be present in the blood and what could be its half-life in this compartment. What is clearly demonstrated is that oral treatment with BFT strongly increases blood thiamine levels in mice, the maximum being reached two hours after gavage [9]. This suggests that most of the S-BT that reaches the blood stream is converted to free thiamine after a few hours. In the brain, there is a slight increase in thiamine content but ThDP does not increase significantly, in line with data obtained in mouse models of stress and neurodegenerative diseases. Taken together, those data strongly suggest that the effects of BFT treatment are not linked to an activation of ThDP-dependent enzymes and subsequent boosting of brain energy metabolism. As the most conspicuous effect of BFT treatment is a strong increase in blood thiamine levels, it is tempting to assume that the neuroprotective effects of BFT involve free thiamine rather than the coenzyme ThDP. In the above-mentioned study [8], treatment with free thiamine was not found to be neuroprotective, but other studies [12, 13] demonstrated anti-stress and anti-depressive effects of BFT and thiamine in mice and an involvement of glycogen synthase kinase-3β was suggested.
In any event, the mechanisms underlying the neuroprotective effects of thiamine and its precursors remain largely enigmatic, both concerning the derivative(s) responsible for these effects and the targets involved. In the present study, we tackle the problem by studying the metabolization of BFT in vitro, using cultures of neuroblastoma (Neuro2a) cells. We developed a UHPLC-ESI mass spectrometry method to identify the major metabolites of BFT. We then investigated the protective effects of BFT on paraquat (PQ)-induced cell death, a model of oxidative stress.
To clarify the possible roles of BFT metabolites in cell protection, we systematically compared the effects of BFT with those of thiamine and sulbutiamine (SuBT, Fig. 1), a disulfide derivative with a different pharmacological profile [9]. SuBT is a dimer with both thiamine moieties linked by a disulfide bridge. An isobutyryl group, increasing the hydrophobic character of the molecule, esterifies both moieties. SuBT freely diffuses through biological membranes and is rapidly converted to thiamine in the cytoplasm. It is highly effective in increasing blood and tissue thiamine levels when administered to rats [14, 15]. In humans, SuBT was reported to be effective against asthenia and other fatigue syndromes [16, 17].
Our results suggest that BFT exerts cell-protective effects against oxidative stress and that these effects are mediated by thiamine or a presently unknown thiamine metabolite, independently of the coenzyme function of ThDP. Our results are not in favor of a specific BFT metabolite being responsible for these effects.
2. Results
As cell culture media generally contain a high thiamine concentration (approximately 10 μM) we first adapted our cells for 7 days to a low-thiamine medium as previously described [18, 19]. The culture medium is devoid of the vitamin and the only source of thiamine is fetal bovine serum (FBS) with a concentration of about 100 nM. The final thiamine concentration in the growth medium was thus 10 nM. This amount of thiamine is sufficient for normal growth of Neuro2a cells. Thus our “thiamine-restricted” medium does not cause any deficiency of the vitamin, though the total thiamine content of the cells was about 10-fold lower than when the cells were grown in thiamine-rich (10 μM) medium [18, 19].
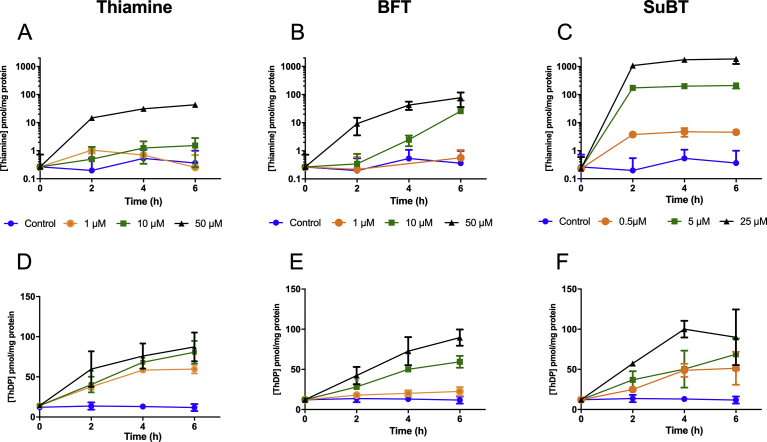
Neuro2a cells grown for 7 days in thiamine-restricted medium were exposed to different concentrations of thiamine, SuBT and BFT for up to 6 h in the same medium but without FBS, and the intracellular thiamine and ThDP contents were estimated (Fig. 2). For simplification the content in the minor ThMP derivative is not indicated here. The uptake of thiamine, which can only enter the cells through specific transporters, is limited by the low catalytic turnover of these transporters.
Fig. 2.
Intracellular content (pmol/mg of protein) of thiamine (A, B, C) and ThDP (D, E, F) as a function of time after addition of 0 (control), 1, 10 and 50 μM thiamine (A, D), BFT (B, E) or 0 (control), 0.5, 5 and 25 μM SuBT (C, F). As the medium contained no serum, a small amount of thiamine (10 nM) was added to the thiamine-free DMEM medium at t = 0 in all samples. Please note the logarithmic scales in A, B and C. (Mean ± SD, n-3).
BFT is a particular case as it is negatively charged because of the phosphate group. It is thought that it must be dephosphorylated to S-BT by extracellular phosphatases; the lipophilic S-BT may then diffuse into the cells. The slow hydrolysis of BFT by exogenous phosphatases (presumably membrane-bound ectophosphatases) explains that it takes more than two hours of exposure before the cell content of thiamine increases significantly.
In contrast, when the lipophilic SuBT is used, the maximum intracellular concentration of thiamine is reached after two hours and little increase is observed after longer times. Note that SuBT is always used at half the molar concentrations of the other derivatives as it yields two molecules of thiamine (Fig. 1). It is obvious that the lipophilic SuBT is by far the most efficient in increasing cell thiamine content as it can directly diffuse through the cell membranes.
Concerning intracellular ThDP content, a slow but substantial increase is observed (from 15 - 20 to nearly 100 pmol/mg protein), but the results are not very different when free thiamine or the two precursors are used. This would be in line with the assumption that the rate-limiting factor for ThDP formation is the activity of the enzyme TPK, which has a low Km (10−7-10−6 M) for thiamine. Therefore, the rate of ThDP synthesis should not increase substantially when the intracellular concentration of free thiamine increases.
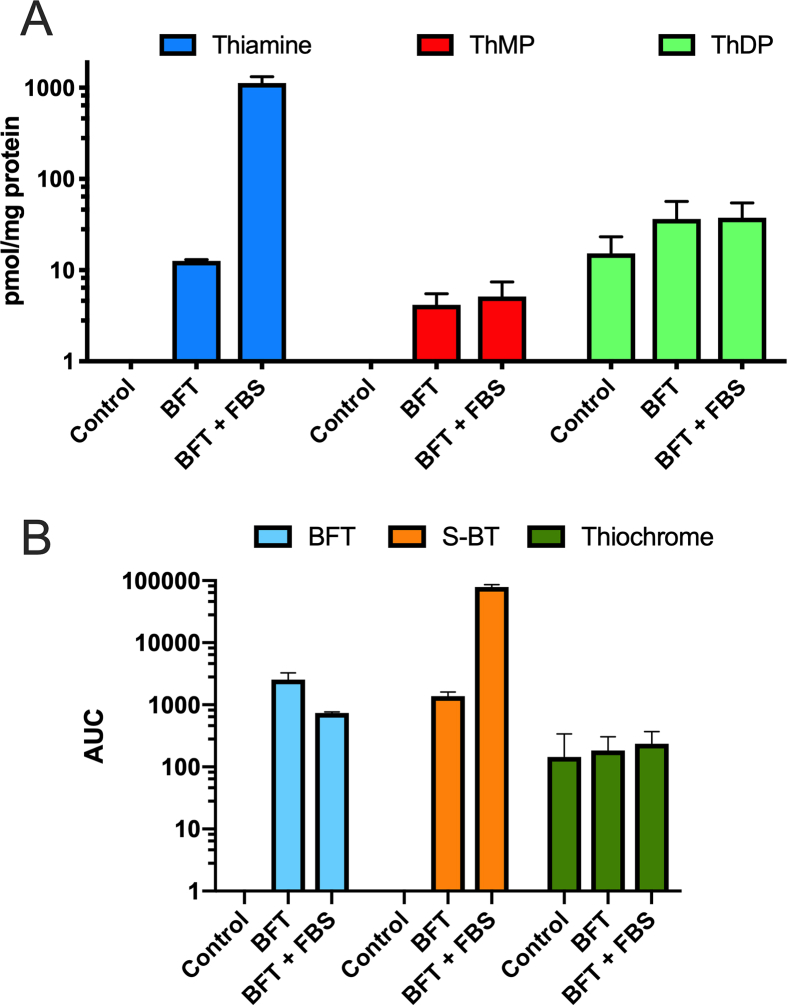
Data from Fig. 2B can be interpreted if we assume that, in serum-free medium, BFT is only slowly hydrolyzed to S-BT by phosphatases bound to cell membranes. We suspected that BFT hydrolysis might become faster in the presence of serum, which might also contain phosphatases. Thus, we incubated the cells with 50 μM BFT in the presence or in the absence of 10% FBS (Fig. 3 and Fig. 4). As shown in Fig. 3A, there is a very strong accumulation of thiamine in the presence of FBS after 1 h, while in the absence of serum, the accumulation of intracellular thiamine was much smaller, even after several hours (Fig. 2B). However, this accumulation does not lead to a significant increase in the coenzyme ThDP or in ThMP.
Fig. 3.
Effect of FBS on the intracellular content of thiamine, BFT and thiochrome after incubation of Neuro2a cells with BFT for one hour in the absence or the presence of 10% FBS. (A) Intracellular content of thiamine, ThMP and ThDP determined by HPLC (mean ± SD, n = 3). (B) Intracellular content of BFT, S-BT and thiochrome determined by mass spectrometry (Mean ± SD, n = 3; AUC, area under curve).
Fig. 4.
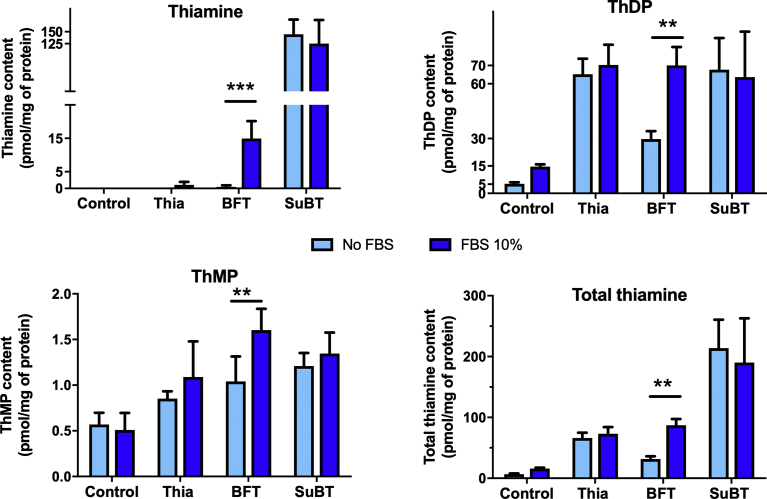
Effect of FBS on the intracellular content of thiamine derivatives after incubation of Neuro2a cells in the presence of thiamine (10 nM), thiamine (10 μM), BFT (10 μM) or SuBT (5 μM) for 2 h. Statistical analysis by two-way ANOVA followed by Bonferroni's post-hoc comparisons test: **p < 0.01, ***p < 0.001 (Mean ± SD, n = 4).
This effect of FBS is specific for BFT. Indeed, in the presence of thiamine (10 μM) or SuBT (5 μM) no effect of FBS on intracellular thiamine content is observed (Fig. 4).
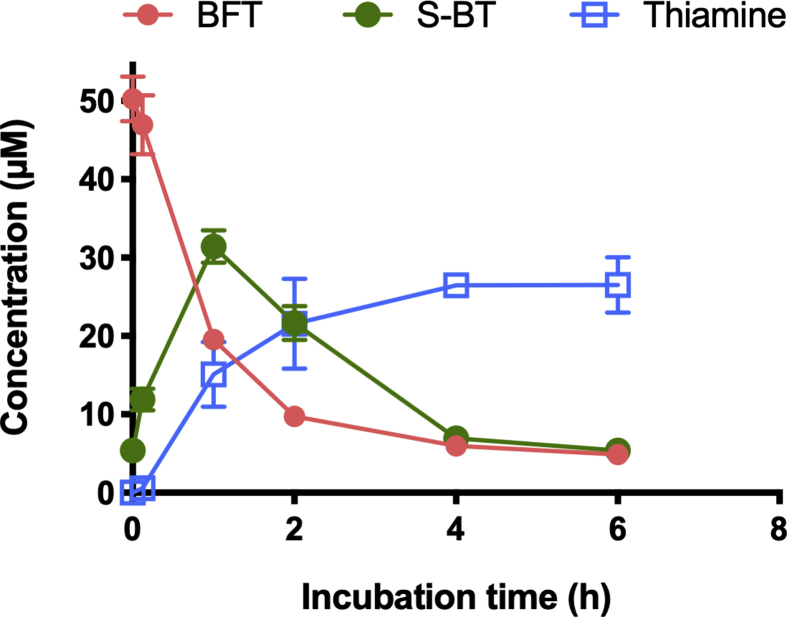
With our HPLC method, which is based on the fluorescent detection of thiochrome derivatives, we can detect only compounds that can be directly oxidized into fluorescent thiochromes. This is not the case of BFT, S-BT or other potential metabolites of these compounds with an open thiazolium ring. Therefore, we used mass spectrometry to detect such metabolites in the cells after treatment with BFT (Fig. 3B). After incubation with BFT (50 μM) for 1 h, the major metabolite detected in the cells (in addition to thiamine) was S-BT. Only minor amounts of thiochrome were detected. Moreover, the amount of thiochrome was not significantly higher than in controls. This suggests that thiochrome may be formed intracellularly in small amounts, but this is not dependent on treatment with BFT. Even if we did not quantify each metabolite using a calibration curve in those experiments, it seems that the most abundant metabolite of BFT is S-BT and that the presence of FBS strongly increases the formation and entry of S-BT into the cells, where thiamine is formed rapidly. This effect can be due either to the presence of phosphatases in the FBS or the induction of ectophosphatases by the cells. In order to distinguish between these two possibilities, BFT was incubated in the presence of FBS (without cells) and we quantified its metabolites as a function of time (Fig. 5). It can be seen that BFT rapidly disappears while a simultaneous appearance of S-BT is observed after 60 min. The amount of BFT drops from 50 to 20 μM, while 30 μM S-BT is formed, suggesting that within 1 h about 60% of the BFT is hydrolyzed by phosphatases present in the serum. However, when the incubation is prolonged from 1 to 6 h, the concentration of S-BT slowly decreases while free thiamine is formed.
Fig. 5.
Time-dependence of the concentrations of BFT, S-BT and thiamine in a culture medium supplemented with 50 μM BFT (without thiamine) and 10 % FBS. (Mean ± SD, n = 3).
Two main conclusions can be drawn from these data:
-
1)
When BFT is added to Neuro2a cells cultured in thiamine-restricted medium, the first metabolite detected in the cells is S-BT. The appearance of S-BT is faster when the medium contains 10% FBS. Under these conditions, 50% of BFT is hydrolyzed in less than 60 min. Thus, BFT appears to be a good substrate for phosphatases present in FBS. It must be emphasized that FBS is at a concentration of 10% in the culture medium. We can thus expect that in the blood these transformations will be even much more rapid and it is doubtful that significant concentrations of BFT are reached in blood after BFT administration.
-
2)
A second metabolite of BFT is thiamine. In Neuro2a cells, it is plausible that, after freely diffusing into the cytoplasm, S-BT is converted to the thiol form of thiamine through the action of endogenous thioesterases. At an intracellular pH close to neutral, the thiol form is quickly converted to thiamine. Unexpectedly, we found that in the absence of cells, S-BT is also unstable and is slowly converted to thiamine (Fig. 5), suggesting that thioesterases are also present in the serum. After oral administration, BFT will be hydrolyzed by intestinal alkaline phosphatases to S-BT, which will be absorbed by the intestinal epithelium. It is probable that a large proportion of S-BT will be transformed to thiamine already within the epithelial cells and transported into the blood. Even if relatively large amounts of S-BT would appear in the blood, they should be converted to thiamine by thioesterases. S-BT may also be transformed in the liver. It is therefore not likely that large amounts of S-BT will be present in the blood. If significant amounts were present shortly after BFT administration, they would probably disappear within a short time. Note, that is was reported [20] that S-BT is unstable and that it can be spontaneously converted to O-BT at pH ≥ 7.
In a second part of this study, we wanted to compare the protective effects of thiamine and its precursors (BFT and SuBT) against PQ toxicity in Neuro2a cells. The herbicide PQ is known to be toxic to many cell types, including neuronal cells, through production of ROS [21, 22].
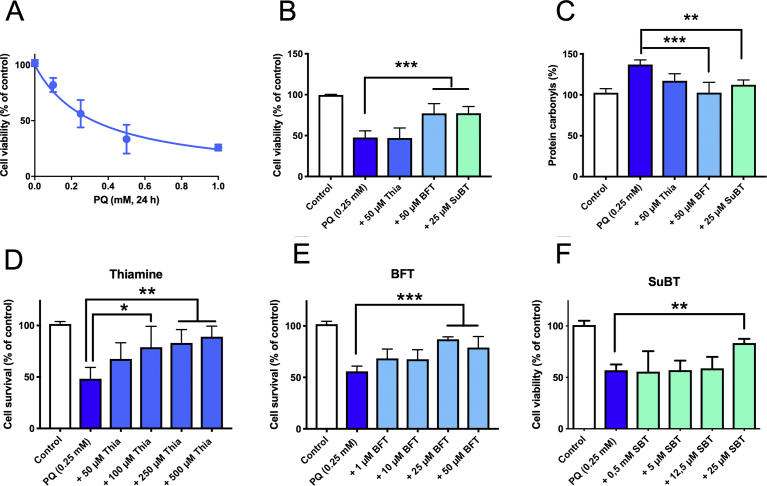
When Neuro2a cells were grown in thiamine-restricted medium containing 10% FBS, a 24 h exposure to PQ in the same medium caused a dose-dependent decrease of cell viability with an IC50 of 0.32 ± 0.04 mM (Fig. 6A). To study the possible protective effects of thiamine and its precursors, the cells were first exposed to 50 μM thiamine, BFT or SuBT for 1 h prior to addition of 0.25 mM PQ and incubation for 24 h. As shown in Fig. 6B, the cell survival rate was 50–60% after PQ exposure. The addition of thiamine (50 μM) to the medium had no significant effect on cell survival. In contrast, addition of BFT (50 μM) significantly improved survival. With SuBT (25 μM), a significant protective effect was also observed.
Fig. 6.
Protection of thiamine, BFT and SuBT against PQ-induced cell death. A) PQ dose-survival curve (mean ± SD, n = 3). B) Effect of thiamine (50 μM), BFT (50 μM) and SuBT (25 μM) on cell survival after exposure (24 h) to 0.25 mM PQ (mean ± SD, n = 3). C) Protein carbonylation under the same conditions (mean ± SD, n = 4). D-F) Effect of various concentrations of thiamine, BFT and SuBT on the survival of Neuro2a cell after exposure to PQ. (Mean ± SD, n = 3).
As PQ is known to be an inducer of oxidative stress, it seemed plausible that the protective effects of thiamine precursors could be linked to an antioxidant effect. We therefore measured the level of protein carbonylation, a well-known indicator of oxidative damage, in Neuro2a cells in our experimental conditions (Fig. 6C). As expected, exposure of the cells to PQ increased protein carbonylation and this was relieved by either BFT (50 μM) or SuBT (25 μM). Though there seemed to be an effect of thiamine (50 μM), it was not significant. This suggests that the two precursors exert antioxidant effects in Neuro2a cells exposed to PQ.
We then studied the effects of increasing concentrations of thiamine, BFT and SuBT on cell survival in the presence of PQ. As shown in Fig. 6D, thiamine exerted little protection at low concentrations but a significant protective effect was observed at 100 μM or higher. This is probably linked to thiamine uptake through a low-affinity thiamine transporter that was previously shown to be present in Neuro2a cells [18]. With BFT (Fig. 6E), maximal protection was observed at 25 μM, and a similar protective effect was observed with the same concentration of SuBT (Fig. 6F), but remember it is a dimer. This is an important result, as metabolites such as S-BT or O-BT can only be formed from BFT, but not from the disulfide SuBT or from thiamine. The only common metabolite of BFT and SuBT is thiamine, suggesting that the pharmacologically efficient compound is thiamine or possibly an unknown metabolite of thiamine. We considered the possibility that part of the intracellular thiamine might be oxidized to thiochrome in the presence of PQ. Treatment with PQ or the combination of PQ and thiamine compounds had no effect on intracellular thiochrome content (data not shown), suggesting that this compound is not involved in the cell-protective effects.
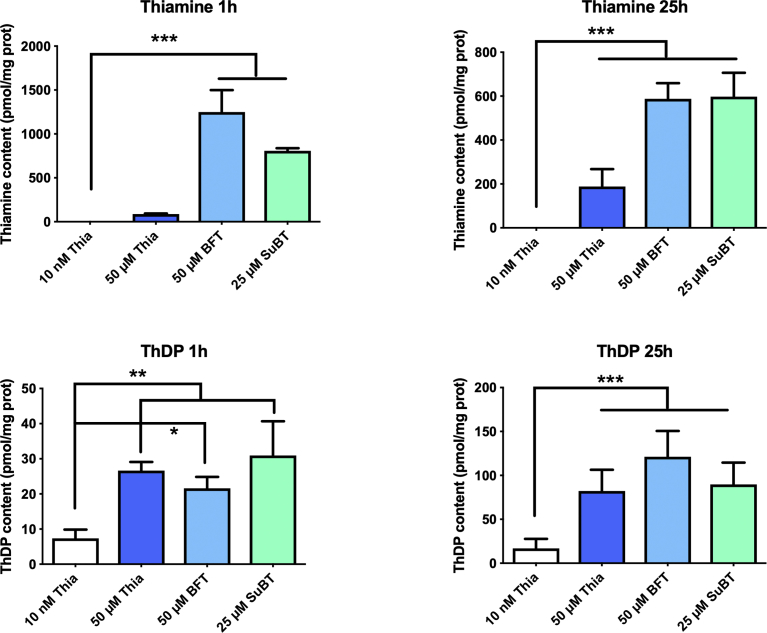
The results presented in Fig. 2 show that when Neuro2a cells grown in thiamine-restricted medium were exposed to thiamine, BFT or SuBT for 6 h, the intracellular concentrations of both thiamine and ThDP increased. This raised the possibility that the protective effects of BFT or SuBT could be linked to an increase in either thiamine or ThDP contents of the cells. We therefore measured the intracellular contents of thiamine and ThDP after incubation in a medium supplemented with 10% FBS after 1 and 25 h (corresponding to the time points of the experiments in Fig. 6). As shown in Fig. 7, the two precursors were much more efficient than thiamine in raising intracellular thiamine content, both after 1 h and 25 h. Note that the strong increase observed after 1 h in the presence of 50 μM BFT is linked to the presence of serum in the medium: serum phosphatases hydrolyze BFT, releasing the lipophilic S-BT that enters the cells where it is converted to thiamine (see above, Fig. 4A).
Fig. 7.
Effect of thiamine, BFT and SuBT on intracellular thiamine and ThDP after 1 and 25 h. One-way ANOVA followed by Sidak's multiple comparisons test (n = 3): *, p < 0.05; **, p < 0.01; ***, p < 0.001.
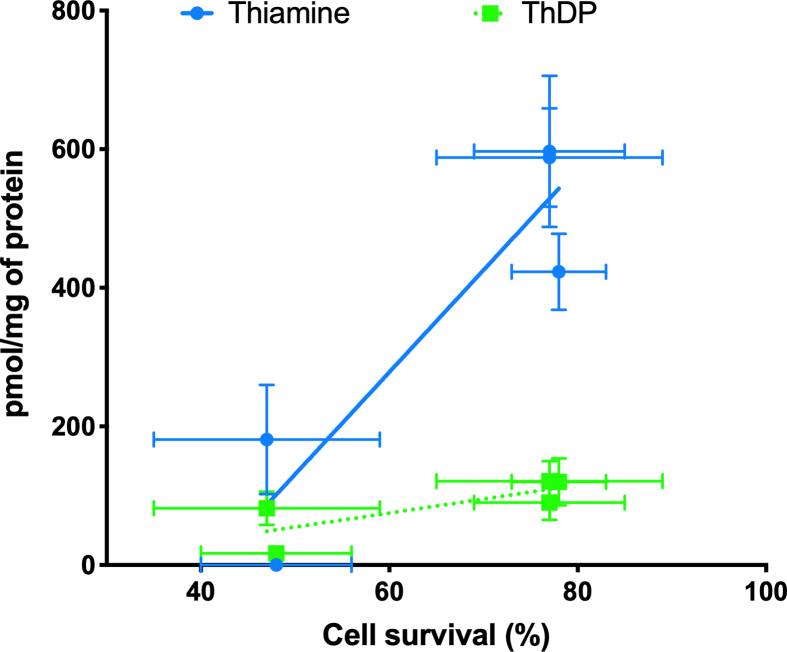
However, the increase of intracellular ThDP in the presence of BFT or SuBT was not significantly higher than when the cells were exposed to free thiamine. This suggests that the protective effects of BFT and SuBT are not linked to increased contents of the coenzyme ThDP in the cells. In order to test this hypothesis, we expressed the cell survival as a function of the intracellular thiamine and ThDP concentrations obtained with various precursors (Fig. 8). A significant correlation was obtained between cell survival and intracellular thiamine concentration but not with intracellular ThDP concentration. This is in agreement with many observations showing that treatment of mice with BFT has many beneficial effects in the central nervous system, but does not cause any increase in brain ThDP levels [7, 8, 9, 10].
Fig. 8.
Correlation between cell survival (%) and intracellular thiamine and ThDP concentrations after 25 h in the absence or presence of various thiamine precursors and in the presence of PQ (0.25 mM) as described in legend to Fig. 5. Pearson's correlation regression analysis revealed a significant correlation with thiamine (p = 0.0267, r = 0.9204, n = 5) but not with ThDP (p = 0.1212, r = 0.7781, n = 5). Both slopes were significantly different (p = 0.0144).
It should be emphasized that ThDP concentrations increase with thiamine concentrations as the latter is the precursor of the former through the reaction Thiamine + ATP ⇆ ThDP + AMP, catalyzed by TPK.
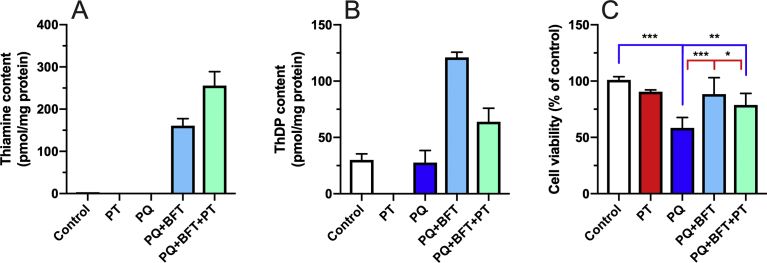
In order to further investigate the possibility that the protective effects of BFT are due to increased intracellular thiamine rather than ThDP, we measured the effect of pyrithiamine on the protection of BFT against PQ-induced cell death (Fig. 9). Pyrithiamine is a potent inhibitor of thiamine transport [18], but it also competitively inhibits TPK [23] while also serving as a substrate for the enzyme. However, pyrithiamine diphosphate cannot act a coenzyme for ThDP-dependent enzymes. It is therefore a very powerful thiamine antimetabolite [24]. When the cells were exposed to pyrithiamine, intracellular ThDP levels dramatically decreased, becoming undetectable after 24 h (Fig. 9B). We have previously shown (using the thiamine transport inhibitor amprolium instead of pyrithiamine) that under such harsh condition Neuro2a cell start to die after 48 h [19]. In the presence of PQ and BFT, pyrithiamine strongly decreased intracellular ThDP levels (Fig. 9B), while thiamine levels were increased (Fig. 9A), in agreement with an inhibition of TPK leading to an accumulation of free thiamine. The fact that ThDP levels are higher in the presence of BFT and pyrithiamine than in controls is probably due to a competition between intracellular thiamine and pyrithiamine for TPK. However, it can be seen that BFT was nearly as protective in the presence than in the absence of pyrithiamine (Fig. 9C). Though this experiment does not allow us to completely exclude the possibility that the protective effects of BFT are due to the coenzyme ThDP, this appears to be unlikely.
Fig. 9.
Effect of pyrithiamine on the intracellular content of thiamine (A) and ThDP (B) and cell survival (C) in the presence of PQ in Neuro2a cells. The cells were incubated in the presence of BFT and or pyrithiamine (PT) at 25 μM for 25 h at 37 °C in 6-well plates. PQ (0.25 mM) was added 1 h after addition of pyrithiamine or BFT. Statistical analysis were made by one-way ANOVA (p < 0.0001) followed by Sidak's multiple comparisons test (n = 6): *, p < 0.05; **, p < 0.01; ***, p < 0.001 (Blue, comparison with control; red, comparison with PQ).
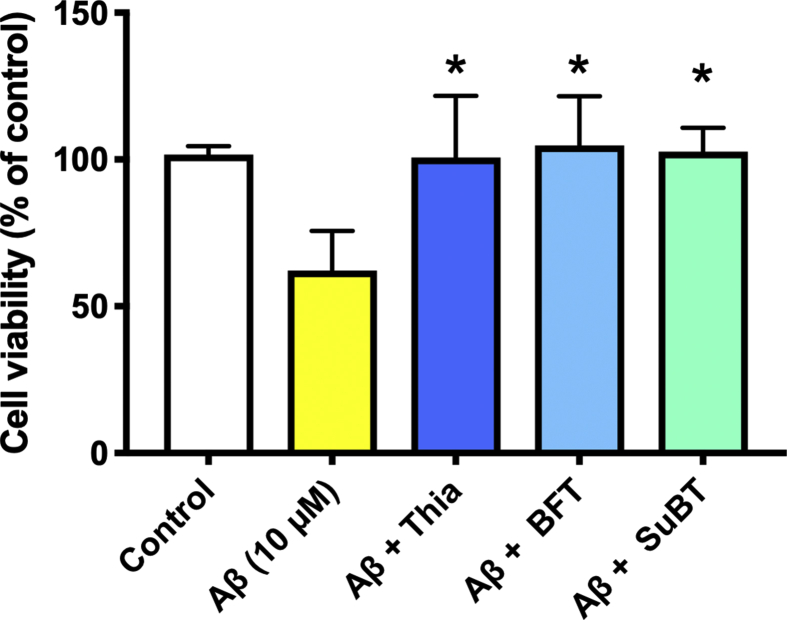
We wanted to test another kind of stress, and more relevant to Alzheimer's disease than PQ. It is indeed well known that soluble Aβ, and in particular the Aβ1-42 peptide is toxic for cells. Indeed, at a concentration >1 μM it decreases cell survival in our Neuro2a cell line (Fig. 10A). These concentrations are in agreement with published data [25, 26, 27]. The Aβ1-42-induced decrease in cell survival was antagonized by thiamine, BFT and SuBT (Fig. 10B). Thiamine was already active at 50 μM, while in PQ-induced decreased cell survival a concentration higher than 50 μM thiamine was required (Fig. 6D).
Fig. 10.
Protection of Neuro2a cells by thiamine, BFT and SuBT against Aβ1-42-induced cell death. A) Effect of increasing concentrations of Aβ1-42 on the survival rate of Neuro2a cells for 24h in thiamine-restricted medium (***, p < 0,001; mean ± SD; n = 3). B) Effect of thiamine (50 μM), BFT (50 μM) and SuBT (25 μM) on the survival rate of Neuro2a cells exposed to 10 μM Aβ1-42 for 24h in thiamine-restricted medium (*, p < 0.05). Each column represents the mean ± SD (n = 3 with 3 replicates each time).
A plausible interpretation of the above results is that the protective effects of BFT and SuBT against PQ or Aβ1-42 toxicity are linked to the important intracellular accumulation of thiamine. How could thiamine relieve the damaging effects of ROS ? It could have either direct or indirect antioxidant effects.
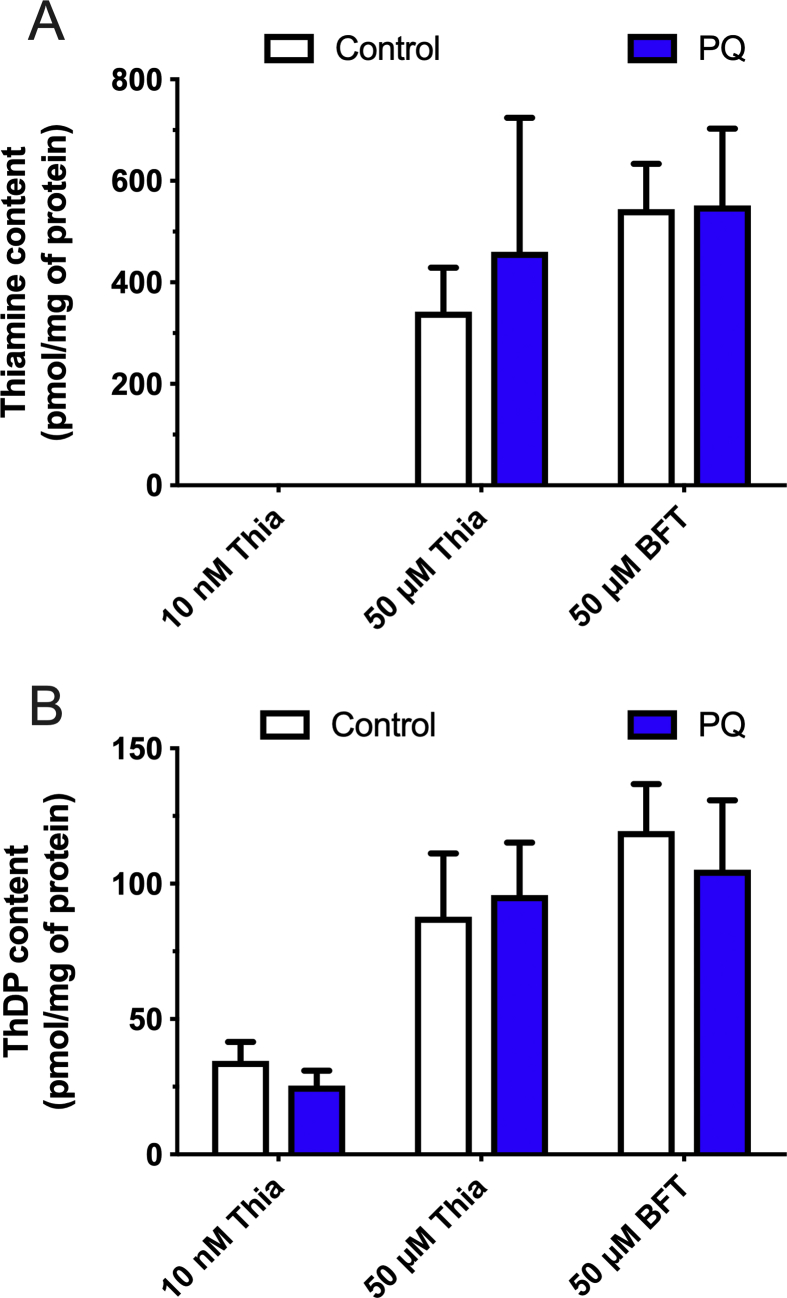
Direct antioxidant effects should be considered, as it has been reported [28, 29] that thiamine and ThDP have antioxidant or radical scavenging activities in vitro. Using a superoxide-generating system, Okai et al. [29], showed that thiamine and ThDP exerted suppressive effects on superoxide production, with IC50 values around 158 μM for thiamine and 56 μM for ThDP. If intracellular thiamine or ThDP concentrations of this order could be reached in our cells after treatment with thiamine precursors, the possibility of a protection against PQ by a direct reaction between thiamine and superoxide should be considered. As shown in Fig. 11A, the amount of intracellular thiamine after treatment with 50 μM BFT can reach approximately 550 pmol/mg of protein. If the cell volume is 4.5 μl per mg of protein [18], the intracellular concentration of thiamine would be 120 μM, which might significantly reduce the amount of superoxide in Neuro2a cells. However, we find that intracellular thiamine is not decreased by exposure to PQ (Fig. 11A), suggesting that no appreciable amounts of thiamine have reacted with ROS produced in the presence of PQ. On the other hand, it was suggested [28] that the oxidation of thiamine by ROS mainly yields thiochrome. We detected no increase in thiochrome production by our cells after exposure to PQ (data not shown).
Fig. 11.
Effect of PQ on thiamine and ThDP content in Neuro2a cells. Neuro2a cells were incubated with or without PQ (0.25 mM) for 24 h at 37 °C in 6-well plates in the absence of added thiamine, or in the presence of 50 μM thiamine or BFT. None of the differences between control and PQ were significant.
Concerning ThDP, Okai et al. [29], reported that it was more efficient than thiamine as a superoxide-scavenging agent, with an IC50 around 56 μM. According to data shown in Fig. 11B, the intracellular ThDP concentration after BFT treatment would reach 25 μM. However, the major part of it is bound to apoenzymes [30] and, again, exposure to PQ had no significant effect on intracellular ThDP content (Fig. 11B). We conclude that the direct antixoxidant effects of thiamine and ThDP contribute at best to a small extent to the protection exerted by thiamine precursors on PQ toxicity.
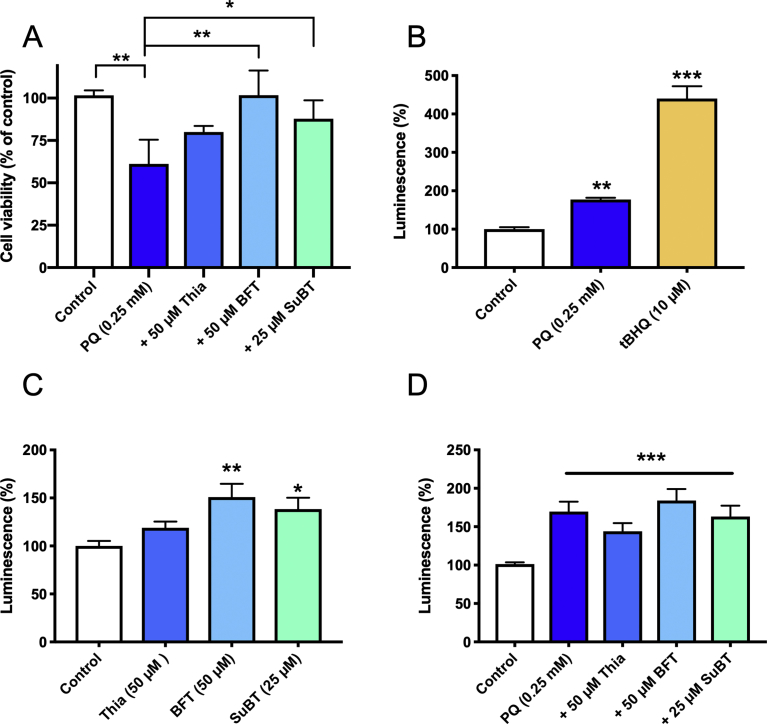
We therefore considered that the protective effects of BFT and SuBT were mainly linked to indirect mechanisms. Activation of the Nrf2/ARE pathway has been extensively studied as a mechanism involved in neuroprotection [31]. Nrf2 is a transcription factor that can be transferred from the cytosol to the nucleus when ROS or electrophilic compounds are present in the cells. In the nucleus, Nrf2 binds to a promotor element called the antioxidant response element (ARE). This activates the expression of a battery of genes involved in protection against oxidative stress. Here we used a luciferase reporter stable cell line (3T3 cells) to test the effects of thiamine and its precursors on Nrf2 activation. In the presence of luciferase substrates, luminescence is induced in the cells when Nrf2 binds to ARE. We first demonstrated that, in 3T3 cells as in Neuro2a cells, thiamine precursors protect against PQ toxicity. As shown in Fig. 12A, 24h exposure to 0.25 mM PQ decreased 3T3 cell viability by 40%, and this was relieved in the presence of 50 μM BFT or 25 μM SuBT. We then measured the luminescence in the presence of PQ, thiamine precursors and, as a positive control, tBHQ. The latter is an electrophilic compound which is a prototypical Nrf2 stabilizer [32]. As expected, tBHQ strongly increased the luminescence of the cells (400%) (Fig. 12B). tBHQ also protected against PQ toxicity in both 3T3 and Neuro2a cells (not shown), indicating that the Nrf2/ARE pathway can be activated to protect these cells against oxidative stress. Exposure to 0.25 mM PQ induced a modest (70–80%) increase in luminescence, in line with the known ability of ROS production to activate the Nrf2/ARE pathway [31]. However, PQ was much less effective than tBHQ, suggesting that the Nrf2/ARE pathway is not fully activated by exposure to PQ. As shown in Fig. 12C, incubation of 3T3 cells with 50 μM BFT or 25 μΜ SuBT for 24 h also caused a modest but significant increase in luminescence (about 50%). This is in agreement with a recent study [8] showing a modest activation of the Nrf2/ARE pathway by 50 μM BFT in a human neuroblastoma reporter cell line; full activation required a concentration of 100 μM.
Fig. 12.
Effects of tBHQ, PQ, thiamine BFT and SuBT on viability and luminescence of a Nrf2/ARE luciferase reporter 3T3 stable cell line. A) Protective effects of thiamine, BFT and SuBT against PQ-induced cell death. B) Effects of PQ and tBHQ on Nrf2/ARE activation (24-h incubation). C) Effects of thiamine, BFT and SuBT on Nrf2/ARE activation (24 h incubation). D) Combination of PQ and thiamine, BFT and SuBT on Nrf2/ARE activation. Thiamine and precursors were added 1 h prior to PQ. One-way ANOVA followed by Dunnett's multiple comparisons test (B and C). One-way ANOVA followed by Tukey's multiple comparisons test (A and D).
We can conclude from these data that in 3T3 cells, exposure to either PQ (0.25 mM) of BFT (50 μM) can activate the Nrf2/ARE pathway to some extent, but this is only a weak stimulation compared to the effect of tBHQ. Moreover, data from Fig. 12D show that when 3T3 cells are exposed to PQ, the simultaneous presence of thiamine, BFT or SuBT fails to induce a further increase in luminescence. This suggests that, under our conditions, the protective effects of thiamine and its precursors against PQ toxicity are not mainly linked to the activation of the Nrf2/ARE pathway. This is in line with the fact that the main effect of BFT and SuBT is to increase the intracellular concentration of thiamine. In contrast to tBHQ, thiamine has no marked electrophilic properties and is thus not expected to cause an important stabilization of Nrf2.
3. Discussion
Several animal and human studies have demonstrated that treatment with thiamine and precursors with high bioavailability has beneficial effects in various pathological conditions such as complications of type II diabetes [6], mouse models of depression related to chronic stress [10, 12] and models of Alzheimer's disease [7] and tauopathies [8]. Most of these investigations were performed using BFT as the thiamine precursor, and its effectiveness has been demonstrated both in vivo and in cellular models. However, the molecular mechanisms underlying the neuroprotective effects of thiamine and BFT remain unclear. A first possible explanation is that the increase in thiamine content of tissues after treatment with high doses of thiamine or BFT increases the production of the coenzyme ThDP. Up to now, most workers in the field have indeed considered that the only biologically active form of thiamine is ThDP. The harmful effects of thiamine deficiency in the brain are generally ascribed to reduced activity of ThDP-dependent enzymes such as 2-oxoglutarate dehydrogenase complex. This causes an impairment of the citric acid cycle and respiratory chain activity, with increased production of ROS and impairment of oxidative metabolism [33]. This is obviously harmful for neurons, as their activity and survival is heavily dependent on oxidative energy metabolism.
Although pathological features of thiamine deficiency may be related — at least in part — to reduced activity of ThDP-dependent enzymes, activation of these enzymes does not appear to be a satisfactory explanation for the beneficial effects of BFT treatment in mouse models of neurodegeneration and other pathological syndromes. Indeed, such effects have been demonstrated in animals that were not deficient in thiamine and had normal brain levels of ThDP. Many different studies from several laboratories have shown that administration of even high doses (200 mg/kg) of thiamine or BFT in mice does not lead to increased ThDP levels; only thiamine is increased by 50–100% [7, 8, 9, 10]. This suggests that the co-enzyme function of ThDP is not substantially enhanced in the brain after BFT treatment.
So far, no alternative mechanisms to explain the neuroprotective effects of thiamine and its precursors have been proposed. It is well established that oral administration of BFT in mice results in a strong increase in blood concentrations of thiamine after a few hours. In the brain, however, the content of free thiamine increases by at best 100% [9] and it seems unlikely that the low amount of unphosphorylated thiamine present in the brain parenchyma could exert marked protective effects. This raises the possibility that unidentified metabolites of thiamine or BFT and even SuBT might be the active neuroprotective agents.
We therefore decided to investigate BFT metabolism in a simpler in vitro system, using cultured Neuro2a cells, that we have previously characterized as to their thiamine metabolism, thiamine transport system and resistance to thiamine deficiency [18, 19, 30]. Several authors reported protective effects of BFT in various cell types, but none of these studies tried to identify metabolites appearing in the cells after exposure to BFT. Antioxidant effects have been reported [34] but high concentrations of BFT (300 μM) were used, and the cells were grown in a thiamine-rich medium. In this case, we are probably dealing with direct antioxidant effects of BFT that are not related to thiamine and are not relevant to in vivo effects: there is indeed no evidence that BFT can be found in the blood, let alone in the brain. So far, no BFT metabolites other than free thiamine have been found in the blood or in the brain.
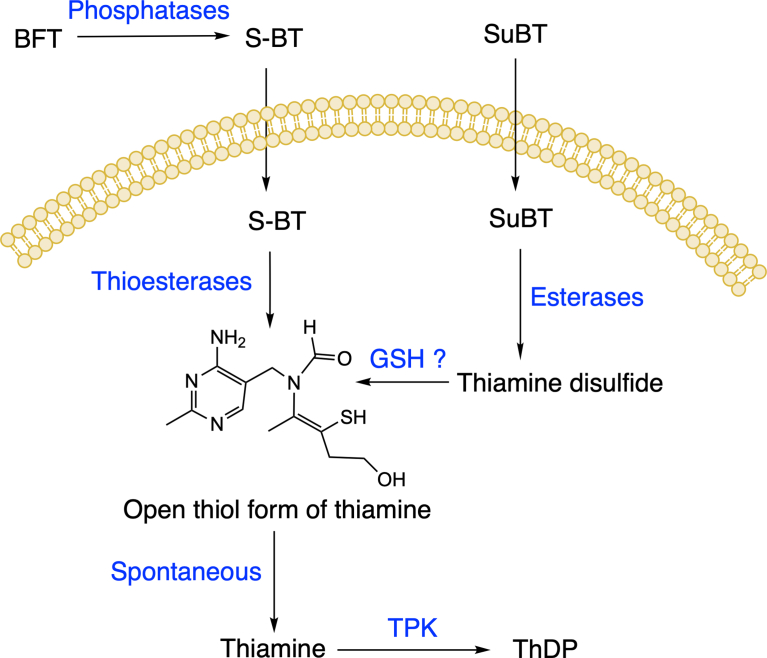
The present study is the first to analyze the possible role of intracellular metabolites of BFT and SuBT in the protection of neuroblastoma cells against oxidative stress. A major advantage of our study above all previous studies is that we compare the effects of thiamine, BFT (a thioester) and SuBT (a disulfide). Our results show that BFT, a polar compound, is unable to cross cell membranes and must be dephosphorylated by membrane-bound phosphatase (ectoenzymes) and phosphatases present in the serum. This reaction yields S-BT, a lipophilic compound that easily diffuses through the membranes and accumulates in the cytoplasm. Within a few hours, however, it is hydrolyzed to the open thiol form of thiamine by intracellular thioesterases. This thiol form is unstable at physiological pH and is spontaneously converted to thiamine. As to SuBT, it is a lipophilic compound that diffuses through the membranes and is first converted to thiamine disulfide by intracellular esterases. The disulfide is then reduced (presumably by reduced glutathione [35]) to the thiol form of thiamine. The pathways leading to thiamine accumulation when Neuro2a cells are treated with either BFT or SuBT are displayed in Fig. 13. Note that, except for thiamine, there is only one common metabolite for BFT and SuBT, i.e. the thiol form of thiamine. As it is quickly converted to thiamine, it is tempting to consider that the protective effects of the precursors are due essentially to the intracellular accumulation of thiamine. The opening of the thiamine thiazolium ring to form the open thiol form requires alkaline condition [35] which is not likely under physiological conditions.
Fig. 13.
Proposed mechanism of action of BFT and SuBT. (GSH, reduced glutathione; TPK, thiamine pyrophosphokinase).
Thiamine has indeed been shown to have radical-scavenging properties in vitro, but relatively high concentrations (100–200 μM) are required [29]. However, our results suggest that the direct antioxidant effects of thiamine (and ThDP) contribute only to a small extent to the protective effects of BFT. We therefore considered the possibility that thiamine protects against oxidative stress through indirect mechanisms. An obvious possibility is the activation of the Nrf2/ARE pathway, which is the master regulator of the expression of many genes involved in cell protection against oxidative stress [31]. Tapias et al. [8] indeed reported that this pathway was activated when cells were treated with relatively high concentrations (>100 μM) of BFT or its putative metabolites S-BT and O-BT. However, thiamine was ineffective. Our results show that thiamine and its precursors only weakly activate the Nrf2/ARE pathway and this is not sufficient to explain the protective effects of BFT and SuBT.
Yet, our results strongly suggest that the cell-protective effects of thiamine precursors are linked to indirect antioxidant effects of intracellularly accumulated thiamine and possibly unknown metabolites of thiamine or its thiol form. Identification of the neuroprotective pathways (other than Nrf2/ARE) activated by thiamine compounds will require further investigations.
In conclusion, the present study shows that the widely used thiamine precursor BFT exerts potent antioxidant and protective effects in cultured Neuro2a cells. As similar effects are obtained with SuBT (that yields different metabolites), it is unlikely that specific effects of BFT metabolites are involved in the protective effects of BFT. Our results suggest that cell-protective effects of thiamine precursors are most probably mediated by thiamine or presently unidentified thiamine metabolites, independently of the coenzyme function of ThDP.
Considering its high bioavailability in vivo, its beneficial effects in animal models of neurodegenerative diseases and its absence of toxicity, BFT could be considered for therapeutic purposes in humans.
4. Materials and methods
4.1. Materials and reagents
Paraquat dichloride (Methyl viologen dichloride hydrate, PQ), amyloid-ß protein fragment 1–42 (Aβ1-42), tert-butylhydroquinone 97% (tBHQ), benfotiamine (S-benzoylthiamine O-monophosphate, BFT), pyrithiamine hydrobromide and thiochrome were from Sigma-Aldrich BVBA (Overijse, Belgium). Luciferase Assay System was from Promega Benelux BV (Leiden, The Netherlands) and luminescence was detected in 96-well plates using a Promega GloMax-Multi Detection plate reader. Sulbutiamine (SuBT) was from Mind Nutrition (https://mindnutrition.com).
4.2. Cells and cell culture
After thawing, mouse neuroblastoma (Neuro2a) cells were first grown in DMEM medium (Biowest, Riverside, MO, US) supplemented with 10% fetal bovine serum (FBS) (Gibco, Life Technologies Europe BV, Merelbeke, Belgium) in a 5% CO2 humidified atmosphere at 37 °C. Then the cells were adapted to a custom-made DMEM medium dry-packed without thiamine (Life Technologies Limited, Paisley, Scotland, UK) and 10% fetal bovine serum (FBS) as previously described [19]. Under these conditions, the total thiamine concentration in the medium is around 10 nM (roughly equivalent to the one in human plasma or serum [36], solely coming from the FBS (100 nM). This concentration is enough to keep the cells growing normally without signs of thiamine deficiency.
The Nrf2/ARE luciferase reporter NIH3T3 cell line (Signosis, Gentauer Molecular Products, Kampenhout, Belgium) is stably transfected with a pTA-ARE-luciferase reporter vector, which contains 4 repeats of ARE, upstream of a firefly luciferase-coding region. When Nrf2 is stabilized, it goes into the nucleus where it binds to the ARE and initiates the transcription of luciferase gene. The cells were grown in DMEM medium and then adapted to the DMEM medium without thiamine in the presence of 10% FBS as for Neuro2a cells.
4.3. Determination of thiamine derivatives by HPLC
Briefly, the cells were grown in 6-well plates under various experimental conditions. For the determination of thiamine derivatives, the cells were washed three times with PBS. Then the cells were scraped in Tris base solution (20 mM, pH 8–8.5), transferred to Eppendorf tubes and sonicated. Cells were treated with trichloroacetic acid (TCA, 12%) to precipitate proteins and then centrifuged at 5000 ×g for 15 min. The pellet containing the proteins was dissolved in NaOH 0.8 N solution. The amount of protein was estimated by the method of Peterson [37]. The supernatant containing thiamine and its phosphate esters was used for analysis by a HPLC method using a PRP-1 column (5 μm, 4.1 × 150 mm) protected by a PRP-1 10 μm guard column cartridge (Sigma-Aldrich) after extraction of TCA by 3 × 1.5 ml diethylether as previously described [38]. In addition to mass spectrometry (see 4.9), thiochrome can also be detected by this HPLC methods by injecting the samples without prior derivatization.
4.4. Preparation of S-benzoylthiamine
S-BT was obtained from BFT through the action of alkaline phosphatase (Sigma-Aldrich). BFT (1 ml, 1 mM) was incubated for 1 h at 37 °C with alkaline phosphatase (100 DEA units) from bovine intestinal mucosa (Sigma-Aldrich). The hydrolysis of BFT was checked by measuring the concentration of inorganic phosphate released by a colorimetric method [39]. The conversion yield was 75%.
4.5. Treatment of Neuro2a cells with neurotoxic agents
Neuro2a cells were cultured in thiamine-restricted DMEM medium containing 10% FBS. To assess cell viability, they were incubated 24 h at 37 °C in 96-well plates in the same medium containing various concentrations of either PQ or Aβ1-42. Aβ1-42 was dissolved in dimethylsulfoxide to prepare stock solutions. Final concentrations of dimethylsulfoxide in assay media were 0.2%. When protective effects of thiamine or its precursors were investigated, the cells were exposed to various concentrations of thiamine BFT or SuBT for 1 h prior to addition of PQ or Aβ1-42.
To evaluate the influence of PQ on intracellular content of thiamine and its phosphorylated precursors, the cells were incubated for 24 h at 37 °C in 6-well plates. The cells were exposed to thiamine and its precursors starting 1 h prior to addition of PQ.
4.6. MTT cell survival test
To assess the viability of cultured cells, we used the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich) assay. MTT tetrazolium salt was dissolved in serum-free culture medium. Neuro2a cells were incubated with or without PQ or thiamine precursors overnight in 96-well plates. Then, the cells were incubated in presence of MTT tetrazolium salt (0.15 mg/ml) for 3 h at 37 °C. Finally, the medium was removed and the cells were dissolved in a solution of isopropanol and HCl (0.22 N). The absorbance was measured at 580 nm with a Thermo Labsystem plate reader. Results were expressed in percentage compared to the control conditions.
4.7. Monitoring of the activation of the antioxidant response pathway in a Nrf2/ARE luciferase reporter NIH3T3 stable cell line
Nrf2/ARE luciferase reporter NIH3T3 cells were grown into 96-well white-wall plates overnight in an incubator at 37 °C with 5% CO2. After treatment with PQ and thiamine precursors, the cells were washed with 100 μl of PBS and lysed by lysis buffer (Luciferase Cell Culture Lysis, Promega) for 15 min at room temperature. Luciferase substrate (Luciferase Assay System, Promega) was added in each well and after mixing, the luminescence was measured by a luminometer (GloMax®-Multi Detection System, Promega).
4.8. Protein carbonyl assay
Protein carbonyls were detected using the OxiSelect protein fluorometric assay (Cell Biolabs, Bio-Connect Life Sciences, TE Huissen, The Netherlands).
4.9. Mass spectrometry (UHPLC-ESI-MS/MS)
UHPLC was performed on a 1290 Infinity LC system coupled to a 6495 triple quadrupole mass spectrometer equipped with the iFunnel technology (Agilent Technologies, Waldbronn, Germany). Chromatographic separation was performed on a reverse-phase Kinetex F5 column (2.6 μm, 100 × 2.1 mm ID) protected with a Security Guard Ultra F5 precolumn (both from Phenomenex, Torrance, CA, USA). The column compartment was thermostated at 40 °C. The separation was carried out in gradient mode with mobile phase A (H2O + 0.1 % formic acid) and B (ACN + 0.1% formic acid) at 0.5 ml/min. The gradient started at 2% B and ramped to 40% B in 3 minutes. It was set at 95% B from 3 to 6 minutes before being decreased to 2% for 2.5 minutes. 2 μl of the samples were injected.
The electrospray source was operated in positive ionization mode (ESI+). The source conditions were optimized using the Source Optimizer software included in the MassHunter software package (Agilent Technologies, Waldbronn, Germany). The capillary voltage was set at 1500 V. Nitrogen was used as dry gas and sheath gas heated at 210 °C with a flow rate of 17 L/min and 400 °C at 12 L/min, respectively. The nebulizer pressure was settled at 55 psi. The high pressure and low-pressure funnels were operated at 150 and 100 V. Fragmentation and collision energies were optimized for each analyte using the Optimizer software. Unit mass resolution was set in both mass-resolving quadrupoles Q1 and Q3. Cell accelerator voltage was kept at 4 V. Analyses were conducted in dynamic multiple reaction monitoring (dMRM) mode. Two transitions were followed for thiochrome, BFT and S-BT. The monitored transitions were m/z 263 → 231 (quantifier) and m/z 263 → 198 (qualifier) for thiochrome, m/z 467 → 122 (quantifier) and m/z 467 → 105 (qualifier) for BFT and m/z 387 → 122 (quantifier) and m/z 387 → 104 (qualifier) for S-BT.
Samples were prepared as described in paragraph 4.3. Calibration curves were done using thiochrome, BFT, S-BT standards at 1 μM, 5 μM, 10 μM, 50 μM and 100 μM prepared in thiamine-free medium with 10 % of FBS. 20 μl of TCA was added to 100 μl of the standard mix. It was vortexed for 20 s and centrifuged for 15 min. The supernatant was collected and washed with ether three times to remove TCA.
Both samples and standards for calibration curves were diluted 1000 times with H2O + 0.1% formic acid. 50 μL of sample were loaded on Ostro 96 well plates (Waters, Dublin, Ireland) and mixed with 150 μl ACN + 1% formic acid. Samples were passed through the plate and extracts were vacuum-dried at 30 °C for 90 minutes using a CentriVap Concentrator (LabConco, Kansas-City, MO, USA). Samples were eventually resuspended in 200 μL of a mixture H2O/MeOH/Formic acid 50:50:0.1 v/v/v.
Declarations
Author contribution statement
Margaux Sambon: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Aurore Napp, Alice Demelenne, Julie Vignisse: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Pierre Wins: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Marianne Fillet: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Lucien Bettendorff: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Margaux Sambon was supported by the Fonds Léon Fredericq, Liège, Belgium.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
L. Bettendorff is Research Director of the Funds for Scientific Research (F.R.S.-FNRS, Belgium). M. Sambon and J. Vignisse were Research Fellows of the “Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture” (F.R.I.A.).
References
- 1.Bettendorff L. Thiamine. In: Zempleni J., Suttie J., 3rd Gregory J.F., Stover P., editors. Handb. Vitam. fifth ed. CRC Press; Boca Raton: 2013. pp. 268–323. [Google Scholar]
- 2.Bettendorff L., Wins P. Thiamin diphosphate in biological chemistry: new aspects of thiamin metabolism, especially triphosphate derivatives acting other than as cofactors. FEBS J. 2009;276:2917–2925. doi: 10.1111/j.1742-4658.2009.07019.x. [DOI] [PubMed] [Google Scholar]
- 3.Mkrtchyan G., Aleshin V., Parkhomenko Y., Kaehne T., Luigi Di Salvo M., Parroni A., Contestabile R., Vovk A., Bettendorff L., Bunik V. Molecular mechanisms of the non-coenzyme action of thiamin in brain: biochemical, structural and pathway analysis. Sci. Rep. 2015;5:12583. doi: 10.1038/srep12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moretti R., Caruso P., Ben M.D., Gazzin S., Tiribelli C. Thiamine and alcohol for brain pathology: super-imposing or different causative factors for brain damage? Curr. Drug Abuse Rev. 2017;10:44–51. doi: 10.2174/1874473711666180402142012. [DOI] [PubMed] [Google Scholar]
- 5.Nozaki S., Mawatari A., Nakatani Y., Hayashinaka E., Wada Y., Nomura Y., Kitayoshi T., Akimoto K., Ninomiya S., Doi H., Watanabe Y. PET imaging analysis of vitamin B1 kinetics with [11C]thiamine and its derivative [11C]thiamine tetrahydrofurfuryl disulfide in rats. Mol. Imaging Biol. 2018;20:1001–1007. doi: 10.1007/s11307-018-1186-y. [DOI] [PubMed] [Google Scholar]
- 6.Hammes H.P., Du X., Edelstein D., Taguchi T., Matsumura T., Ju Q., Lin J., Bierhaus A., Nawroth P., Hannak D., Neumaier M., Bergfeld R., Giardino I., Brownlee M. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat. Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 7.Pan X., Gong N., Zhao J., Yu Z., Gu F., Chen J., Sun X., Zhao L., Yu M., Xu Z., Dong W., Qin Y., Fei G., Zhong C., Xu T.L. Powerful beneficial effects of benfotiamine on cognitive impairment and beta-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain. 2010;133:1342–1351. doi: 10.1093/brain/awq069. [DOI] [PubMed] [Google Scholar]
- 8.Tapias V., Jainuddin S., Ahuja M., Stack C., Elipenahli C., Vignisse J., Gerges M., Starkova N., Xu H., Starkov A.A., Bettendorff L., Hushpulian D.M., Smirnova N.A., Gazaryan I.G., Kaidery N.A., Wakade S., Calingasan N.Y., Thomas B., Gibson G.E., Dumont M., Beal M.F. Benfotiamine treatment activates the Nrf2/ARE pathway and is neuroprotective in a transgenic mouse model of tauopathy. Hum. Mol. Genet. 2018;27:2874–2892. doi: 10.1093/hmg/ddy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volvert M.L., Seyen S., Piette M., Evrard B., Gangolf M., Plumier J.C., Bettendorff L. Benfotiamine, a synthetic S-acyl thiamine derivative, has different mechanisms of action and a different pharmacological profile than lipid-soluble thiamine disulfide derivatives. BMC Pharmacol. 2008;8:10. doi: 10.1186/1471-2210-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vignisse J., Sambon M., Gorlova A., Pavlov D., Caron N., Malgrange B., Shevtsova E., Svistunov A., Anthony D.C., Markova N., Bazhenova N., Coumans B., Lakaye B., Wins P., Strekalova T., Bettendorff L. Thiamine and benfotiamine prevent stress-induced suppression of hippocampal neurogenesis in mice exposed to predation without affecting brain thiamine diphosphate levels. Mol. Cell. Neurosci. 2017;82:126–136. doi: 10.1016/j.mcn.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Raghunath A., Sundarraj K., Nagarajan R., Arfuso F., Bian J., Kumar A.P., Sethi G., Perumal E. Antioxidant response elements: discovery, classes, regulation and potential applications. Redox Biol. 2018;17:297–314. doi: 10.1016/j.redox.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markova N., Bazhenova N., Anthony D.C., Vignisse J., Svistunov A., Lesch K.-P., Bettendorff L., Strekalova T. Thiamine and benfotiamine improve cognition and ameliorate GSK-3β-associated stress-induced behaviours in mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2017;75:148–156. doi: 10.1016/j.pnpbp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Pavlov D., Markova N., Bettendorff L., Chekhonin V., Pomytkin I., Lioudyno V., Svistunov A., Ponomarev E., Lesch K.-P., Strekalova T. Elucidating the functions of brain GSK3α: possible synergy with GSK3β upregulation and reversal by antidepressant treatment in a mouse model of depressive-like behaviour. Behav. Brain Res. 2017;335:122–127. doi: 10.1016/j.bbr.2017.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Bettendorff L., Weekers L., Wins P., Schoffeniels E. Injection of sulbutiamine induces an increase in thiamine triphosphate in rat tissues. Biochem. Pharmacol. 1990;40:2557–2560. doi: 10.1016/0006-2952(90)90099-7. [DOI] [PubMed] [Google Scholar]
- 15.Bettendorff L., Wins P., Lesourd M. Subcellular localization and compartmentation of thiamine derivatives in rat brain. Biochim. Biophys. Acta. 1994;1222:1–6. doi: 10.1016/0167-4889(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 16.Van Reeth O. Pharmacologic and therapeutic features of sulbutiamine. Drugs Today. 1999;35:187–192. doi: 10.1358/dot.1999.35.3.533848. [DOI] [PubMed] [Google Scholar]
- 17.Sevim S., Kaleağası H., Taşdelen B. Sulbutiamine shows promising results in reducing fatigue in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2017;16:40–43. doi: 10.1016/j.msard.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Bettendorff L., Wins P. Mechanism of thiamine transport in neuroblastoma cells. Inhibition of a high affinity carrier by sodium channel activators and dependence of thiamine uptake on membrane potential and intracellular ATP. J. Biol. Chem. 1994;269:14379–14385. [PubMed] [Google Scholar]
- 19.Bettendorff L., Goessens G., Sluse F., Wins P., Bureau M., Laschet J., Grisar T. Thiamine deficiency in cultured neuroblastoma cells: effect on mitochondrial function and peripheral benzodiazepine receptors. J. Neurochem. 1995;64:2013–2021. doi: 10.1046/j.1471-4159.1995.64052013.x. [DOI] [PubMed] [Google Scholar]
- 20.Hurt J.K., Coleman J.L., Fitzpatrick B.J., Taylor-Blake B., Bridges A.S., Vihko P., Zylka M.J. Prostatic Acid phosphatase is required for the antinociceptive effects of thiamine and benfotiamine. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bus J.S., Aust S.D., Gibson J.E. Superoxide- and singlet oxygen-catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem. Biophys. Res. Commun. 1974;58:749–755. doi: 10.1016/s0006-291x(74)80481-x. [DOI] [PubMed] [Google Scholar]
- 22.Dou T., Yan M., Wang X., Lu W., Zhao L., Lou D., Wu C., Chang X., Zhou Z. Nrf2/ARE pathway involved in oxidative stress induced by paraquat in human neural progenitor cells. Oxid. Med. Cell. Longev. 2016;2016:8923860. doi: 10.1155/2016/8923860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J.Y., Timm D.E., Hurley T.D. Pyrithiamine as a substrate for thiamine pyrophosphokinase. J. Biol. Chem. 2006;281:6601–6607. doi: 10.1074/jbc.M510951200. [DOI] [PubMed] [Google Scholar]
- 24.Hazell A.S., Wang D., Oanea R., Sun S., Aghourian M., Yong J.J. Pyrithiamine-induced thiamine deficiency alters proliferation and neurogenesis in both neurogenic and vulnerable areas of the rat brain. Metab. Brain Dis. 2014;29:145–152. doi: 10.1007/s11011-013-9436-9. [DOI] [PubMed] [Google Scholar]
- 25.Amiri M., Braidy N., Aminzadeh M. Protective effects of fibroblast growth factor 21 against amyloid-beta1-42-induced toxicity in SH-SY5Y cells. Neurotox. Res. 2018;34:574–583. doi: 10.1007/s12640-018-9914-2. [DOI] [PubMed] [Google Scholar]
- 26.Seino S., Kimoto T., Yoshida H., Tanji K., Matsumiya T., Hayakari R., Seya K., Kawaguchi S., Tsuruga K., Tanaka H., Imaizumi T., Gnetin C. A resveratrol dimer, reduces amyloid-β 1-42 (Aβ42) production and ameliorates Aβ42-lowered cell viability in cultured SH-SY5Y human neuroblastoma cells. Biomed. Res. Tokyo Jpn. 2018;39:105–115. doi: 10.2220/biomedres.39.105. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q., Na Z., Cheng Y., Wang F. Low-molecular-weight chondroitin sulfate attenuated injury by inhibiting oxidative stress in amyloid β-treated SH-SY5Y cells. Neuroreport. 2018;29:1174–1179. doi: 10.1097/WNR.0000000000001092. [DOI] [PubMed] [Google Scholar]
- 28.Lukienko P.I., Mel’nichenko N.G., Zverinskii I.V., Zabrodskaya S.V. Antioxidant properties of thiamine. Bull. Exp. Biol. Med. 2000;130:874–876. [PubMed] [Google Scholar]
- 29.Okai Y., Higashi-Okai K., F Sato E., Konaka R., Inoue M. Potent radical-scavenging activities of thiamin and thiamin diphosphate. J. Clin. Biochem. Nutr. 2007;40:42–48. doi: 10.3164/jcbn.40.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bettendorff L. The compartmentation of phosphorylated thiamine derivatives in cultured neuroblastoma cells. Biochim. Biophys. Acta. 1994;1222:7–14. doi: 10.1016/0167-4889(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 31.Tebay L.E., Robertson H., Durant S.T., Vitale S.R., Penning T.M., Dinkova-Kostova A.T., Hayes J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015;88:108–146. doi: 10.1016/j.freeradbiomed.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., Johnson D., Calkins M., Wright L., Svendsen C., Johnson J. Stabilization of Nrf2 by tBHQ confers protection against oxidative stress-induced cell death in human neural stem cells. Toxicol. Sci. 2005;83:313–328. doi: 10.1093/toxsci/kfi027. [DOI] [PubMed] [Google Scholar]
- 33.Hazell A.S., Faim S., Wertheimer G., Silva V.R., Marques C.S. The impact of oxidative stress in thiamine deficiency: A multifactorial targeting issue. Neurochem. Int. 2013;62:796–802. doi: 10.1016/j.neuint.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Schmid U., Stopper H., Heidland A., Schupp N. Benfotiamine exhibits direct antioxidative capacity and prevents induction of DNA damage in vitro. Diabetes Metab Res Rev. 2008;24:371–377. doi: 10.1002/dmrr.860. [DOI] [PubMed] [Google Scholar]
- 35.Stepuro A.I., Piletskaya T.P., Stepuro I.I. Role of thiamine thiol form in nitric oxide metabolism. Biokhimiya Mosc. 2005;70:339–349. doi: 10.1007/s10541-005-0120-5. [DOI] [PubMed] [Google Scholar]
- 36.Bettendorff L., Grandfils C., De Rycker C., Schoffeniels E. Determination of thiamine and its phosphate esters in human blood serum at femtomole levels. J. Chromatogr. 1986;382:297–302. doi: 10.1016/s0378-4347(00)83533-1. [DOI] [PubMed] [Google Scholar]
- 37.Peterson G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 38.Bettendorff L., Peeters M., Jouan C., Wins P., Schoffeniels E. Determination of thiamin and its phosphate esters in cultured neurons and astrocytes using an ion-pair reversed-phase high-performance liquid chromatographic method. Anal. Biochem. 1991;198:52–59. doi: 10.1016/0003-2697(91)90505-n. [DOI] [PubMed] [Google Scholar]
- 39.Lanzetta P.A., Alvarez L.J., Reinach P.S., Candia O.A. An improved assay for nanomole amounts of inorganic phosphate. Anal. Biochem. 1979;100:95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]