Abstract
Introduction
Structural and functional changes that occur post myocardial infraction (MI) lead to the syndrome of heart failure (HF). However, their pathogenesis is poorly understood. Recently, alteration of the intestinal microbiota (dysbiosis) has emerged as a new candidate that may be correlated with risk of HF development. We hypothesized that selective gut modulation by probiotic administration may improve metabolic dysfunction and attenuate cardiac remodeling (CR) in MI subjects.
Methods
/Design: This article is presented in two sections: First, we provided a review of recent findings related to gut microbiota and CR and their association with probiotic supplementation. Secondly, we will conduct a randomized double-blinded controlled clinical trial in 46 Iranian patients with MI after successful percutaneous coronary intervention (PCI). The participants (age: ≥ 30 years; ejection fraction (EF) greater than 30) will be selected by a simple random sampling method and will be assigned to 3 months of 1.6* 109 CFU probiotic (Lactobacillus rhamnosus), or placebo groups (maltodextrin). The primary outcome is development of CR. The secondary outcomes measures include gut microbiota profile, biochemical variables and the safety of the probiotics supplementation. Also, echocardiography will be measured at baseline and following treatment. The data will be compared within and between groups using appropriate statistical methods.
Discussion
The results of this trial will provide evidence about the efficacy and safety of gut microbiota manipulation by probiotics in post-MI cardiac remodeling prevention.
Ethical issues
Present study protocol was approved by the regional committee of ethics in international branch of Tabriz University of Medical sciences (TBZMED) as a thesis proposal for PhD degree in Nutrition Sciences (IR.TBZMED.REC.1397.184).
Trial registration The Clinical trial was registered in the Iranian Registry of Clinical Trials (IRCT20121028011288N15).
Keywords: Cardiac remodeling, Heart failure, Microbiome, Myocardial infraction, Probiotics
1. Introduction
Heart failure (HF) is important public health problems in that its prevalence and incidence has been growing dramatically since past decades. In recent years [1,2], despite considerable pharmacotherapy, such as angiotensin-converting enzyme [3], the five-year survival rate is still unacceptably poor [4,5]. Although different diseases, such as hypertension and diabetes, might lead to heart failure [6], adverse cardiac remodeling (CR) [4] after myocardial infarction (MI), can predispose a person to HF development [7]. In addition, quality of life has not been appropriately improved by current therapeutic modalities [8,9]. So, preventing this process and alternative treatments may be useful. In the present study, we aimed to review the current literature on the modulatory effects of gut microbiota in HF. Subsequently, we will specifically focus on probiotic supplementation to investigate the CR and metabolic endotoxemia amelioration. In current study, we will debate recent findings that reveal mechanisms connecting the gut microbiota to low-grade inflammation in context of cardiovascular disease (CVD). Additionally, we will discuss the potential relationships between the gut microbiota with CR. Lastly, we talk over the potential gut modulation and their effects on CR process.
2. Cardiac remodeling
A process of regional and global structural and functional changes in the heart result from cardiac load or injury [10,11]. The process of cardiac remodeling is influenced by degree of cardiac damage, inflammatory process, neurohormonal activation and other factors which are still under investigation [12,13]. Moreover, there is general acceptance that CR might cause HF [7]. CR is assessed by echocardiography, and elevated circulating biomarkers, such as B-type natriuretic peptide (BNP) or matrix metalloproteinase (MMP) 9 and several new biomarkers which has been introduced in recent years [14,15]. As well as, progressive left ventricular (LV) dilatation post-MI assessed by diastolic and systolic dimensions (LVDD and LVDS, respectively) and ejection fraction through electrocardiography can be regarded as CR detection methods [16].
It has long been accepted that HF is associated with altered intestinal function. As well as, metabolic endotoxmia as a result of altered gut microbiota could influence HF [17]. More recently, preliminary animal CR model studies also demonstrated a direct mechanistic link between gut microbiota metabolites, and adverse ventricular dysfunction [17]. Indeed, it has been suggested that altered gut microbiota is prevalent in patients with HF [18]. However, the possible influence of gut modulation on HF pathogenesis is mostly unknown. At present, to the best of our knowledge, the association between gut metabolite and progress of HF has not been investigated. Given the proposed involvement of intestinal dysfunction and microbiota alterations in HF, we planned to investigate the association between gut microbiota, etiology and prognosis of CR process in MI patents.
3. Gut microbiota
The human gut microbiota has been identified as a possible novel risk factor [18]. Abnormal gut microbiota profiles (dysbiosis) have been associated with obesity, diabetes and CVD [19]. Exactly, in “metabolic endotoxemia” condition, lipopolysaccharides (LPSs) of gram-negative cell wall are able to induce systemic low-grade inflammation, insulin resistance, and increase cardiovascular risk [20]. The binding of LPS with Toll-like receptor (TLR) 4, exert innate immune system stimulation leading to proinflammatory response and subsequent metabolic disorders. Besides its role in inflammation [21,22], TLR4 induces production of MMP 9, which has been proposed to be a marker for CR process [23]. Therefore, it is thought that TLR4 plays an important role in post MI remodeling, however, so far, the effect of gut modulation on TLR4 and subsequent on CR processes has not been investigated. Probiotics administration may a novel therapeutic option to ameliorate LV remodeling in patients with MI. As pervious study proposed probiotics lead to decline in TLR4 stimulation [24,25].
In addition, bacterial components also cause inflammation and metabolic disease and certain bacterial metabolites such as Trimethylamine-N-oxide (TMAO) can accelerate cardiac remodeling process and cardiomyopathy [17]. Dysbiosis lead to increased TMAO levels which is a colorless amine oxide generated from choline, betaine, and carnitine by gut microbial metabolism [17].
Elevated TMAO levels predicted the risk of ventricular remodeling and heart failure development in humans and in several experimental studies [17,26,27]. There is not enough knowledge about the TMAO role in the CR process [26] and current evidence suggested that by affecting lipid and hormonal homeostasis TMAO may lead to the development of atherosclerosis [28]. Also, a recent study by Suzuki and colleague showed that elevated plasma TMAO levels not only caused inflammation and immune response but also directly contributed to progressive adverse ventricular remodeling [17,29].
Considering that the dysbiosis has significant effect on different aspects of CR processes that can lead to HF, it is important to understand approaches to modulate the gut microbiota (including the changes in diet or pre/probiotics supplementation). In this regard, several therapeutic methods have been developed to modulate and restore the gut microbiota [30]. Probiotics are live micro-organisms which can offer health benefits when administered in adequate amounts and used to rebalance the disturbed intestinal microbiota [31]. Several studies have been demonstrated that probiotics supplementation may be helpful for controlling the cardiovascular risk factors such as dyslipidemia, hypertension and inflammation [32,33]. However, there's a paucity of information regarding the potential benefit of probiotics on CR process. Recently, Lam et al. provided the primary evidence that probiotics is also cardio protective due to the observed reduction in size of infarct and improved left ventricular (LV) function after administration of probiotic Lactobacillus plantarum 24 h in ischemic rats. On the other hand, preliminary evidence proposed that gut modulation with probiotics, reduced the TMAO levels, secretion of inflammatory cytokines and the development of CR process [34].
4. Gut microbiota and cardiovascular disease
A number of preclinical studies have proposed that alterations in gut microbiota play an important role in the pathogenesis of cardiovascular disease (CVD). As well as, many studies showed that the gut microbiota also influences the whole metabolic activity of the body and its immune function [18]. Thus, modifying the gut microbiota with probiotics may be a new way for prevention and treatment of the cardiac remodeling in humans. It has long been recognized that probiotics supplementation leads to decrease in cardiovascular risk factor such as inflammation, hypercholesterolemia, hypertension, and metabolic disturbances [19]. On the other hand, probiotics administration may also influence on TMAO and metabolic consequence [35]. In addition, this is the first study to date in which we will assess the efficacy of probiotic in decreasing the level of endotoxin and intestinal metabolites (TMAO) and its effect on the cardiac remodeling process.
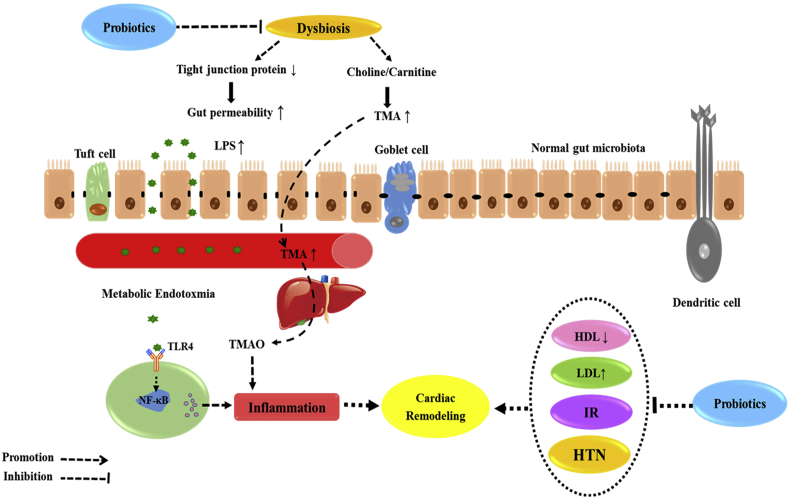
Briefly, dysbiosis can stimulate cardiac remodeling in three ways: first, through increased levels of lipopolysaccharides (LPS) and the development of metabolic endotoxmia. Secondly, by increasing cardiometabolic risk factors and inflammation in subjects with altered gut microbiota. Thirdly, through harmful intestinal metabolites, such as TMAO which directly promote the development and progression of cardiac remodeling and CHF. Preliminary evidence suggests that endotoxin concentrations are higher in adults with CVD compared with healthy individuals. As well as, modulation the gut microbiota by probiotics is an approach for reducing metabolic endotoxmia and attenuating the cardiac remodeling in rodents. On the other hand, probiotics may be able to improve the inflammation, lipid profile and ME which are the main risk factor for CVD, also, maybe offsetting cardiac remodeling process (Fig. 1).
Fig. 1.
Potential mechanism of dysbiosis and the onset of cardiac remodeling. These phenomena promote metabolic endotoxemia and initiate the development of low-grade inflammation and increased TMAO in the blood stream which might be attenuated by probiotics.
To the best of our knowledge, there is no clinical trial which explored whether probiotics administration can reduce cardiac remodeling process in patients with MI. Therefore, the primary objective of this study is to investigate the efficacy and safety of probiotics administration in preventing the development of post-MI remodeling in patent with successful percutaneous coronary intervention (PCI) via a parallel-group randomized double-blind placebo-controlled clinical trial. Left ventricular contractility and cardiac remodeling will be assessed by two-dimensional echocardiography and biomarkers will also be used to assess the remodeling.
5. The aims and hypotheses
5.1. General aim
The general aim of the current investigation is to determine the effects of three-month probiotic supplementation on preventing the development of post-MI remodeling and gut micro-biota composition through a double-blind, randomized, placebo-controlled clinical trial in adults with successful percutaneous coronary intervention (PCI).
6. The specific aims and hypotheses
6.1. Aim 1
To determine whether probiotic supplementation improves ventricular contractility in individuals with successful PCI. We hypothesize that Lactobacillus rhamnosus supplementation will attenuate cardiac remodeling in these individuals compared with placebo. Cardiac remodeling will be assessed by two-dimensional echocardiography (including left ventricular end diastolic diameter (LVEDD), right ventricular end diastolic diameter (RVEDD), left ventricular end-diastolic volume (LVEDV), right ventricular end-diastolic volume (RVEDV) and Ejection Fraction [4] and biomarkers (including TGF. Beta, NT pro BNP, MMP-9, Procolagen I).
6.2. Aim 2
To determine whether probiotic supplementation will improve inflammatory stress indices, oxidative stress indices and lipid profiles in individuals with MI. We hypothesize that probiotic supplementation will reduce the serum level of high sensitivity C-reactive protein (hs-CRP), Interleukin 1 beta (IL1B), total cholesterol [6], triglyceride (TG), low-density lipoprotein (LDL), (ox-LDL) and malondialdehyde (MDA) and improve high-density lipoprotein (HDL), total antioxidant capacity (TAC), and Interleukin 10 (IL-10) levels in these individuals.
6.3. Aim 3
To determine whether probiotic supplementation will improve metabolic endotoxemia and gut metabolite in individuals with MI. We hypothesize that probiotic supplementation will improve gut metabolites (TMAO) level and serum levels of endotoxemia (TLR4 and LPS) in these individuals.
6.4. Aim 4
To determine the changes in abundance and ratio of gut microbiota and their relation with cardiac remodeling during supplementation. We hypothesize that the Lactobacillus rhamnosus supplementation would attenuates the ventricular remodeling process after MI.
7. Methods/design
A two-arm parallel group randomized double-blind placebo controlled trial, was designed according to the CONSORT 2013 guidelines, and will be conducted over a period of one year from April 2018, in Tabriz, Iran. We will include 46 myocardial infarction patents who have been underwent successful percutaneous coronary intervention (PCI). The trial will be conducted at outpatient cardiology clinic of Shahid Madani Heart center affiliated to Tabriz University of Medical Sciences, Iran. All the patients will be screened by an expert cardiologist for eligibility which is presented in Table 1.
Table 1.
Criteria for participation in the study.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patients who provide written informed consent | Urgent need for revascularization procedure |
| Both gender | Significant renal dysfunction (serum creatinine above 1.5 mg/dl) |
| Subjects > 30 years of age | patients with severe liver and gastrointestinal diseases or diagnosis of diabetes mellitus |
| First episode of MI after successful PCI | allergy to studied agents; patients receiving immunosuppressive, anti-inflammatory and corticosteroid drugs; history of supplementation with pre/pro/symbiotic or antioxidants during or previous two months |
| An echocardiographic LV EF ≥ 30% | Heart failure (function class III and IV), Heart valve diseases |
| BMI > 25 kg/m2 | Refusal or inability to provide informal consent |
7.1. Allocation
Those who are willing to take part in the study will be carefully evaluated according to the inclusion criteria. Then, they will be requested to sign an informed consent and after that by using the Random Allocation Software, the subjects will be allocated into either probiotic or placebo group, stratified by sex, drugs intake and BMI. Participants and investigators will be blinded to the allocation of intervention (probiotic) or control (placebo) groups. Demographic information of the participants including age, height, weight, waist circumference, hip circumference will be collected. Primary and secondary outcomes measurement will be carried out at baseline and after intervention.
7.2. Interventions
The participants will be randomized into two equal groups. The groups are probiotic (containing the freeze-dried 1.6 × 109 CFU Lactobacillus rhamnosus) or placebo (maltodextrin). The appearance and labeling of drug product of the two capsule are identical. The subjects will be educated to use Lactobacillus rhamnosus capsules or placebo capsules together with their lunch daily for 12 weeks.
7.3. Randomization
Individuals will be suitably blinded in performing outcome measurements and type of supplement. Letter A or B will be assigned to supplements and are otherwise identical. The person responsible for preparing the supplement capsule (who is completely unrelated to the study) will be asked to assign a three-digit code to each of the two capsules (probiotic and placebo), and keep the codes for himself until the end of the study and data analyses. The subjects in both groups will receive a weight loss diet considering their dietary habit. They will be recommended not to modify their physical activity habits. To ensure that these habits have not been changed during the study, the participants will be trained to record physical activity diaries, which will be checked weekly during visits by a specialist's dietitian.
7.4. Follow-up
Study visits will be scheduled biweekly. During these visits, the researchers will check the compliance with dietary regime, supplements consumption and any side effects related to supplementation. Also, general questionnaire and a dietary recall questionnaire will be completed. A 24-h dietary recall questionnaire will be completed at the baseline, secondly middle of the intervention and also at the end of study.
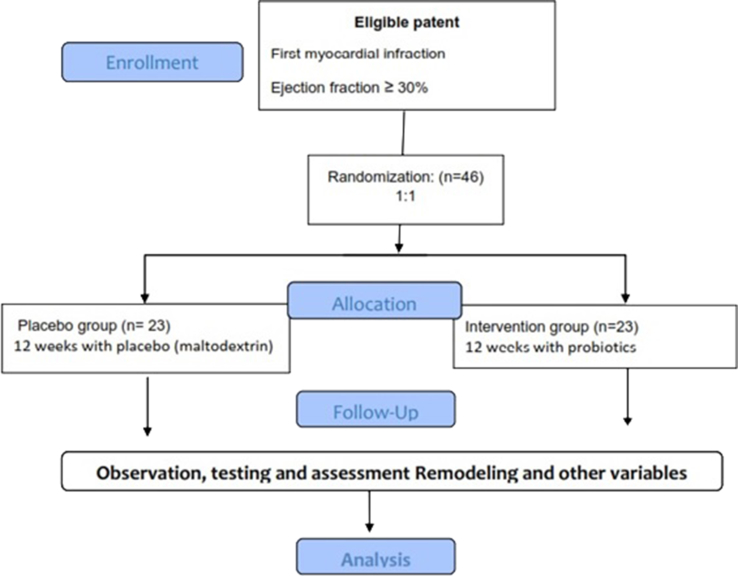
The supervisor will assess and report adverse events of trial interventions. Compliance will be assessed based on returned tablet counts. Participant who miss >10% of supplement dose at follow-up, will be excluded from the trial. Also, in the case of any changes in medication for example antibiotics consumption, or any other supplementation, the participants will be excluded from the study. An overview of study interventions and assessments is provided in Fig. 2.
Fig. 2.
Overall study algorithm of the Probiotic study.
7.5. Sample size
Calculation of sample size was based on a parallel two group randomized clinical trial with measurements of main outcomes at two time points. Considering the α = 0.05 for type one error rate, and to provide 80% power for demonstrating the superiority of the study agents compared with placebo, 46 subjects were estimated [36].
7.6. Statistical analysis
Statistical analysis of all data will be performed using the SPSS program, version 16.0 (SPSS Inc., Chicago, IL) and P value < 0.05 will be considered statistically significant. To assess the efficacy of this trial, both per protocol approach and intention-to treat [37] principle [38], will be analyzed.
7.7. Outcome measurements
The changes of the biomarkers and echocardiographic indices of cardiac remodeling, which will be measured by laboratory assessments and 2-Dimensional Echocardiography is the primary outcome of this study. Two-dimensional echocardiography will be conducted at Shahid Madani Heart center affiliated to Tabriz University of Medical Sciences. Some echocardiographic indices including LVEDV and RVEDV will be calculated according to the biplane Simpson's method.
7.8. Blood samples
After an overnight fasting, blood will be collected by the laboratory technician for measurement of biomarkers. The blood samples will be frozen at −80 °C until final processing. TGF. Beta, NT pro BNP, MMP-9, Procolagen I levels will be measured by specific ELISA kit. Measurement of total antioxidant capacity (TAC) and malondialdehyde (MDA) in serum will be done using Colorimetric Assay Kit. Lipid profile (TC, TG, LDL, HDL) and ox-LDL levels will be assessed by Pars Azmoon test kits (Pars Azmoon Inc, Tehran, Iran). Also serum levels of endotoxemia (TLR4 and LPS) and inflammatory stress indices (hs-CRP, IL1B, and IL-10) level will be measured using specific ELISA Kit.
7.9. Stool samples
The stool samples will be obtained at baseline and post intervention (after 3 months) in sterile plastic containers and will be delivered to the laboratory within 1 h of collection. Samples will be immediately frozen at −80 °C until assay. DNA will be extracted from fecal samples by DNA Extraction Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocols. Gut microbiota profile before and after probiotics supplementation will be determined by quantitative real-time polymerase chain reactions (RT-PCR) methodology via specific designed primers and Taqman probes.
7.10. Patient and public involvement
Patients and public were not involved in the design of this study., outcome measures, nor will they be involved with the conduct of the study. The recruitment plan was informed based on feedback from patients and public. A summary of the study results will be provided to each of the study participants. We will submit our findings for peer-review publication and presentation at national and international conferences.
7.11. Ethical issues & trial registration
Present study protocol was approved by the regional committee of ethics in international branch of Tabriz University of Medical sciences (TBZMED) as a thesis proposal for PhD degree in Nutrition Sciences (IR.TBZMED.REC.1397.184). Written informed consent will be taken from all the study subjects. The Clinical trial was registered in the Iranian Registry of Clinical Trials (IRCT20121028011288N15) and is available at http://en.irct.ir/user/trial/30121/view.
8. Discussion
The objective of this trial is to assess the effect of gut microbiota modulation on cardio metabolic risk factors and CR prevention after MI. The results of this study will provide evidence regarding the value of probiotic supplementation as an intervention for attenuating remodeling in patients with MI. As well as, we will evaluate the correlation between gut microbiota profile and CR processes in human subjects for the first time.
Despite the accepted roles of considerable pharmacotherapy such as ACE inhibitors in management of post-MI cardiac remodeling, the incidence rate is still noticeably high. Nevertheless, almost none of these therapeutic strategies cure the symptoms, and all of them cause great economic burdens to the patient and healthcare system (3, 4). Although the pattern of post-MI remodeling may exhibit a different picture, clinical guidelines recommend different methods to reduce the risk of cardiac remodeling in clinical trials. In CR process, during MI cell death and fibroblast recruitment take place resulting in collagen accumulation and cardiac fibrosis which lead to HF [39,40]. According to CR processes, fibrosis and inflammation are key pathological phenomenon of HF [41] and this study may show that a probiotic administration could attenuate this progression.
Disruption of gut microbiota profile has been implicated in many conditions and diseases such as diabetes and CVD. Previous evidence suggests that inhibition or treating these conditions might be achieved by manipulation of the gut microbiota [3]. Probiotic administration has been considered as an effective and safe approach for the treatment of various diseases such as cardiometabolic diseases [33]. Based on previous study, probiotics have shown effects on CVD through variety of mechanisms including production of organic acids, diminishing endotoxemia, inducing immune mediators, and by anti-inflammatory effect [33]. Evidence suggests that endotoxmia resulting from dysbiosis is a possible trigger of systemic inflammation which probably plays an important role in the pathogenesis and progression of HF [42]. In fact, LPS has been shown to exert proinflammatory effect which accelerates HF development [43]. Moreover, gut modulation with antibiotics or pre/probiotics improved gut permeability, reduced endotoxmia, lowered inflammation and increased quality of life in patients with HF [44]. In addition, it seems that gut modulation could attenuate CR process which might progress to HF [3,45]. With regard to HF, numerous studies have revealed that probiotics exert hypertrophic effects under ischemic conditions. Furthermore, clinical studies have shown that probiotic administration can potentially be helpful in prevention of cardiac failure and improvement of quality of life [3,46]. Our study maybe will be show a CR process, which was prevented by probiotics. On the other hand, the other important outcome of gut modulation with probiotics is that it can enable sensibly controlled metabolic endotoxmia and subsequent outcomes [47].
Recently, TMAO has been identified as a novel risk factor for the development of HF. It has been shown TMAO significantly elevated in CAD patients with HF compared to control subjects [28]. As well as, TMAO levels were strongly linked with gut microbiota profile and recently it has been shown that, gut modulation was associated with decrease in TMAO levels [17]. Based on recent findings, it is proposed that probiotics have beneficial effects on remodeling process. One notable mechanism of probiotics is its capability in attenuation of harmful gut metabolite such as TMAO which accelerate ventricular dysfunction. However, it has been proposed that TMAO levels were not associated with plasma LPS levels, suggesting that additional duplication effect of probiotics in HF prevention. These outcomes develop a hypothesis that guts modulation with probiotics supplementation might serve as an alternative method to treat CR process [17,26,27].
In addition, Lactobacillus rhamnosus as probiotics demonstrates significant benefits by anti-proliferative and anti-apoptotic effect in cardiomyocytes derived following myocardial infarction and attenuates myocardial hypertrophy and heart failure. More recently, Chao-Hung Lai et al. [48] showed that supplementation with multi-strain probiotics may attenuate cardiomyocyte fibrosis, cardiac hypertrophy and the autophagy-signaling pathway in high-fat diet-fed rats. In addition, previous studies showed that oral administration of probiotics promoted cardiac survival through activation of the PI3K/Akt pathway and decreased inflammatory markers and expression of fibrosis protein in hypertensive rats [49]. Indeed, probiotics supplementation may attenuate CR process through suppression of TLR-4-related inflammatory pathway and may have an anti-fibrosis effect [50].
However, the limitations of this study should also be considered. First, short duration of the intervention, as participants will be treated for only 12 weeks, whereas CR process development might progress in the longer period. Additionally, our sample size was relatively small. However, this study has a strong point which is the controlling main dietary substrates for TMAO formation. Secondly, we will measure the gut microbiome and gut metabolite together at the same time to firmly establish the gut microbiota as a risk factor for CR.
In conclusion, as far as we are aware, there is a lack of evidence regarding the management of post-MI cardiac remodeling. To the best of our knowledge, this is the first study which has been designed to assess the effects of probiotic supplementation in post-MI patients by measuring the major biomarkers and echocardiographic indices. The underlying mechanisms for these effects have not been understood yet; however, initial evidence suggests the amelioration of metabolic endotoxemia and decrease of low-grade inflammation and TMAO levels could attenuate these processes. Although our study has a relatively small sample size, this trial may play a vital role in providing basic clinical data in management of post-MI cardiac remodeling. In this trial using oral administration of probiotics, offers a new approach for post-MI cardiac remodeling. We also anticipate that probiotics supplementation will improve metabolic endotoxemia, lipid profile, and inflammatory status by altering the gut microbiota composition. If the results of the investigation show are effective, it will serve as a new strategy for CHF prevention. We hope this RCT will provide scientific evidence in supporting probiotics intervention for attenuating CR in MI patients.
Author contributions
Conceived and designed the experiments: JM MA. Performed the experiments: JM AG. Analyzed the data: JM VM MD. Wrote the paper: All of authors.
Conflicts of interest
The authors declare that they have no conflict of interest.
9. Trial status
The study was conceived and designed in 2018. The recruitment of the trial is ongoing.
Acknowledgements
We would like to thank all members of present study group for their ideas, suggestions, participation and support. As well as, the authors wish to thank Tabriz University of Medical Science for financial support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.conctc.2019.100364.
Abbreviations
- ACE
Angiotensin-converting enzyme
- MI
Myocardial infarction
- LV
Left ventricle
- EF
Ejection fraction
- ELISA
enzyme-linked immunosorbent assay
- hs-CRP
high-sensitive C-reactive protein
- HF
heart failure
Funding/support
The study was supported financially by Tabriz University of Medical Science as a thesis proposal for Ph.D. degree of the first author.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lloyd-Jones D., Adams R., Carnethon M., De Simone G., Ferguson T.B., Flegal K. Heart disease and stroke statistics—2009 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119(3) doi: 10.1161/CIRCULATIONAHA.108.191259. e21-e181. [DOI] [PubMed] [Google Scholar]
- 2.Roger V.L. Epidemiology of heart failure. Circ. Res. 2013;113(6):646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gan X.T., Ettinger G., Huang C.X., Burton J.P., Haist J.V., Rajapurohitam V. Probiotic administration attenuates myocardial hypertrophy and heart failure after myocardial infarction in the RatCLINICAL PERSPECTIVE. Circulation: Heart Fail. 2014;7(3):491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 4.Levy W.C., Mozaffarian D., Linker D.T., Sutradhar S.C., Anker S.D., Cropp A.B. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113(11):1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 5.Sadeghpour A., Pouraliakbar H., Azarfarin R., Ghavidel A.A., Zavareian S., Amirahmadi A. Mid-term patency in radial artery and saphenous vein after coronary artery bypass grafting in asymptomatic patients using 128-slice CT coronary angiography. Anesthesiology and pain medicine. 2015;5(1) doi: 10.5812/aapm.23799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grundy S.M., Benjamin I.J., Burke G.L., Chait A., Eckel R.H., Howard B.V. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 7.Cohn J.N., Ferrari R., Sharpe N. Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J. Am. Coll. Cardiol. 2000;35(3):569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 8.Dracup K., Walden J., Stevenson L., Brecht M. Quality of life in patients with advanced heart failure. J. Heart Lung Transplant.: the official publication of the International Society for Heart Transplantation. 1992;11(2 Pt 1):273–279. [PubMed] [Google Scholar]
- 9.Nieminen M.S., Dickstein K., Fonseca C., Serrano J.M., Parissis J., Fedele F. The patient perspective: quality of life in advanced heart failure with frequent hospitalisations. Int. J. Cardiol. 2015;191:256–264. doi: 10.1016/j.ijcard.2015.04.235. [DOI] [PubMed] [Google Scholar]
- 10.Sutton M.G.S.J., Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 11.Mckay R.G., Pfeffer M.A., Pasternak R.C., Markis J.E., Come P.C., Nakao S. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation. 1986;74(4):693–702. doi: 10.1161/01.cir.74.4.693. [DOI] [PubMed] [Google Scholar]
- 12.Nian M., Lee P., Khaper N., Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 2004;94(12):1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 13.Fraccarollo D., Galuppo P., Hildemann S., Christ M., Ertl G., Bauersachs J. Additive improvement of left ventricular remodeling and neurohormonal activation by aldosterone receptor blockade with eplerenone and ACE inhibition in rats with myocardial infarction. J. Am. Coll. Cardiol. 2003;42(9):1666–1673. doi: 10.1016/j.jacc.2003.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Halade G.V., Jin Y.-F., Lindsey M.L. Matrix metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol. Ther. 2013;139(1):32–40. doi: 10.1016/j.pharmthera.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masoumi G., Hidarpour E., Tabae A.S., Ziayeefard M., Azarasa A., Abneshahidi A., Anbardan S.J., Kashefi P. Evaluating hemodynamic outcomes of different dosages of intravenous nitroglycerin after coronary artery bypass graft surgery. Journal of research in medical sciences: the official journal of Isfahan University of Medical Sciences. 2011 Jul;16(7):910. [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng S., Xanthakis V., Sullivan L.M., Lieb W., Massaro J., Aragaam J. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122(6):570. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang W.W., Wang Z., Fan Y., Levison B., Hazen J.E., Donahue L.M. Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J. Am. Coll. Cardiol. 2014;64(18):1908–1914. doi: 10.1016/j.jacc.2014.02.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang W.W., Hazen S.L. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Investig. 2014;124(10):4204–4211. doi: 10.1172/JCI72331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntyre C.W., Harrison L.E., Eldehni M.T., Jefferies H.J., Szeto C.-C., John S.G. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011;6(1):133–141. doi: 10.2215/CJN.04610510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 21.Chow J.C., Young D.W., Golenbock D.T., Christ W.J., Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 1999;274(16):10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 22.Triantafilou M., Triantafilou K. Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol. 2002;23(6):301–304. doi: 10.1016/s1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- 23.Stapel H., Kim S.C., Osterkamp S., Knuefermann P., Hoeft A., Meyer R. Toll‐like receptor 4 modulates myocardial ischaemia–reperfusion injury: role of matrix metalloproteinases. Eur. J. Heart Fail. 2006;8(7):665–672. doi: 10.1016/j.ejheart.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Grabig A., Paclik D., Guzy C., Dankof A., Baumgart D., Erckenbrecht J. Escherichia coli strain Nissle 1917 ameliorates experimental colitis via toll-like receptor 2-and toll-like receptor 4-dependent pathways. Infect. Immun. 2006;74(7):4075–4082. doi: 10.1128/IAI.01449-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feleszko W., Jaworska J., Hamelmann E. Toll-like receptors—novel targets in allergic airway disease (probiotics, friends and relatives) Eur. J. Pharmacol. 2006;533(1–3):308–318. doi: 10.1016/j.ejphar.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 26.Tang W.W., Hazen S.L. Reply: trimethylamine-N-oxide and heart failure. J. Am. Coll. Cardiol. 2015;66(1):96–97. doi: 10.1016/j.jacc.2014.12.080. [DOI] [PubMed] [Google Scholar]
- 27.Trøseid M., Ueland T., Hov J., Svardal A., Gregersen I., Dahl C. Microbiota‐dependent metabolite trimethylamine‐N‐oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 2015;277(6):717–726. doi: 10.1111/joim.12328. [DOI] [PubMed] [Google Scholar]
- 28.Randrianarisoa E., Lehn-Stefan A., Wang X., Hoene M., Peter A., Heinzmann S.S. Relationship of serum trimethylamine N-oxide (TMAO) levels with early atherosclerosis in humans. Sci. Rep. 2016;6:26745. doi: 10.1038/srep26745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seldin M.M., Meng Y., Qi H., Zhu W., Wang Z., Hazen S.L. Trimethylamine N‐oxide promotes vascular inflammation through signaling of mitogen‐activated protein kinase and nuclear factor‐κB. Journal of the American Heart Association. 2016;5(2) doi: 10.1161/JAHA.115.002767. e002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins M.D., Gibson G.R. Probiotics, prebiotics, and synbiotics: approaches for modulating the microbial ecology of the gut. Am. J. Clin. Nutr. 1999;69(5) doi: 10.1093/ajcn/69.5.1052s. 1052s-7s. [DOI] [PubMed] [Google Scholar]
- 31.Tuohy K.M., Probert H.M., Smejkal C.W., Gibson G.R. Using probiotics and prebiotics to improve gut health. Drug Discov. Today. 2003;8(15):692–700. doi: 10.1016/s1359-6446(03)02746-6. [DOI] [PubMed] [Google Scholar]
- 32.Sun J., Buys N. Effects of probiotics consumption on lowering lipids and CVD risk factors: a systematic review and meta-analysis of randomized controlled trials. Ann. Med. 2015;47(6):430–440. doi: 10.3109/07853890.2015.1071872. [DOI] [PubMed] [Google Scholar]
- 33.Yoo J.Y., Kim S.S. Probiotics and prebiotics: present status and future perspectives on metabolic disorders. Nutrients. 2016;8(3):173. doi: 10.3390/nu8030173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boutagy N.E., Neilson A.P., Osterberg K.L., Smithson A.T., Englund T.R., Davy B.M. Probiotic supplementation and trimethylamine‐N‐oxide production following a high‐fat diet. Obesity. 2015;23(12):2357–2363. doi: 10.1002/oby.21212. [DOI] [PubMed] [Google Scholar]
- 35.Tang W., Hazen S.L. Probiotic therapy to attenuate weight gain and trimethylamine‐N‐Oxide generation: a cautionary tale. Obesity. 2015;23(12):2321–2322. doi: 10.1002/oby.21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajkumar H., Mahmood N., Kumar M., Varikuti S.R., Challa H.R., Myakala S.P. Effect of probiotic (VSL# 3) and omega-3 on lipid profile, insulin sensitivity, inflammatory markers, and gut colonization in overweight adults: a randomized, controlled trial. Mediat. Inflamm. 2014;2014 doi: 10.1155/2014/348959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miccoli R., Bianchi C., Odoguardi L., Penno G., Caricato F., Giovannitti M.G. Prevalence of the metabolic syndrome among Italian adults according to ATP III definition. Nutr. Metabol. Cardiovasc. Dis. 2005;15(4):250–254. doi: 10.1016/j.numecd.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Ten Have T.R., Normand S.L.T., Marcus S.M., Brown C.H., Lavori P., Duan N. Intent-to-treat vs. non-intent-to-treat analyses under treatment non-adherence in mental health randomized trials. Psychiatr. Ann. 2008;38(12) doi: 10.3928/00485713-20081201-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gajarsa J.J., Kloner R.A. Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities. Heart Fail. Rev. 2011;16(1):13–21. doi: 10.1007/s10741-010-9181-7. [DOI] [PubMed] [Google Scholar]
- 40.Fedak P.W., Verma S., Weisel R.D., Li R.-K. Cardiac remodeling and failure: from molecules to man (Part I) Cardiovasc. Pathol. 2005;14(1):1–11. doi: 10.1016/j.carpath.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Nicoletti A., Michel J.-B. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc. Res. 1999;41(3):532–543. doi: 10.1016/s0008-6363(98)00305-8. [DOI] [PubMed] [Google Scholar]
- 42.Escobedo G., López-Ortiz E., Torres-Castro I. Gut microbiota as a key player in triggering obesity, systemic inflammation and insulin resistance. Rev. Invest. Clin. 2014;66(5):450–459. [PubMed] [Google Scholar]
- 43.Danesh J., Wheeler J.G., Hirschfield G.M., Eda S., Eiriksdottir G., Rumley A. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 44.Dehghan P., Gargari B.P., Jafar-Abadi M.A., Aliasgharzadeh A. Inulin controls inflammation and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized-controlled clinical trial. Int. J. Food Sci. Nutr. 2014;65(1):117–123. doi: 10.3109/09637486.2013.836738. [DOI] [PubMed] [Google Scholar]
- 45.Pasini E., Aquilani R., Testa C., Baiardi P., Angioletti S., Boschi F. Pathogenic gut flora in patients with chronic heart failure. JACC (J. Am. Coll. Cardiol.): Heart Fail. 2016;4(3):220–227. doi: 10.1016/j.jchf.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Nagatomo Y., Tang W.W. Intersections between microbiome and heart failure: revisiting the gut hypothesis. J. Card. Fail. 2015;21(12):973–980. doi: 10.1016/j.cardfail.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Costanza A.C., Moscavitch S.D., Neto H.C.F., Mesquita E.T. Probiotic therapy with Saccharomyces boulardii for heart failure patients: a randomized, double-blind, placebo-controlled pilot trial. Int. J. Cardiol. 2015;179:348–350. doi: 10.1016/j.ijcard.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 48.Lai C.-H., Tsai C.-C., Kuo W.-W., Ho T.-J., Day C.-H., Pai P-y. Multi-strain probiotics inhibit cardiac myopathies and autophagy to prevent heart injury in high-fat diet-fed rats. Int. J. Med. Sci. 2016;13(4):277. doi: 10.7150/ijms.14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin P.-P., Hsieh Y.-M., Kuo W.-W., Lin Y.-M., Yeh Y.-L., Lin C.-C. Suppression of TLR-4-related inflammatory pathway and anti-fibrosis effects of probiotic-fermented purple sweet potato yogurt in hearts of spontaneously hypertensive rats. Chin. J. Physiol. 2013;56(3):174–183. doi: 10.4077/CJP.2013.BAB118. [DOI] [PubMed] [Google Scholar]
- 50.Lin P.-P., Hsieh Y.-M., Kuo W.-W., Lin Y.-M., Yeh Y.-L., Lin C.-C. Probiotic-fermented purple sweet potato yogurt activates compensatory IGF-IR/PI3K/Akt survival pathways and attenuates cardiac apoptosis in the hearts of spontaneously hypertensive rats. Int. J. Mol. Med. 2013;32(6):1319–1328. doi: 10.3892/ijmm.2013.1524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.