Abstract
(1) Background: The optimal timing of adjuvant chemotherapy (CT) in gastrointestinal malignancies is still a matter of debate. For colorectal cancer, it is recommended to start post-operative treatment within eight weeks. The objective of this study was to assess the clinical effects of starting adjuvant CT within or after 6–8 weeks post-surgery in colorectal, gastric, and pancreatic cancer. (2) Methods: MEDLINE, EMBASE, and the Cochrane Library were searched in December 2018. Publications comparing the outcomes of patients treated with adjuvant CT administered before (early) or after (delayed) 6–8 weeks post-surgery for colorectal, gastric, and pancreatic cancer were identified. The primary endpoint was overall survival (OS). (3) Results: Out of 8752 publications identified, 34 comparative studies assessing a total of 141,853 patients were included. Meta-analysis indicated a statistically significant increased risk of death with delayed CT (>6–8 weeks post-surgery) in colorectal cancer (hazard ratio (HR) = 1.27, 95% confidence interval (CI) 1.21–1.33; p <0.001). Similarly, for gastric cancer, delaying adjuvant CT was associated with inferior overall survival (HR = 1.2, 95% CI 1.04–1.38; p = 0.01). Conversely, the benefit of earlier CT was not evident in pancreatic cancer (HR = 1, 95% CI 1–1.01; p = 0.37). Conclusions: Starting adjuvant CT within 6–8 weeks post-surgery is associated with a significant survival benefit for colorectal and gastric cancer, but not for pancreatic cancer.
Keywords: colorectal cancer, gastric cancer, pancreatic cancer, adjuvant chemotherapy, timing, meta-analysis
1. Introduction
Surgical resection is the mainstay of treatment for most solid malignancies diagnosed at the localized stage. Unfortunately, disease recurrence is frequently encountered and mainly depends on the presence of clinically occult micrometastases at the time of surgery. Post-operative chemotherapy (CT) aims to eradicate these, thereby decreasing the possibility of recurrence.
The benefits of adjuvant CT have been clearly demonstrated in major gastrointestinal malignancies. Specifically, adding oxaliplatin to fluoropyrimidines has been associated with a significant survival gain in stage III radically resected colorectal cancer patients [1]. In gastric cancer, one of the largest meta-analyses concluded that adjuvant systemic therapy was associated with a 15% reduction in risk of death compared with surgery alone [2]. Finally, a very recent randomized phase III study compared an intensified triplet combination CT regimen (i.e., modified 5-fluorouracil, irinotecan, and oxaliplatin (FOLFIRINOX)) with the standard of care (i.e. Gemcitabine) in patients with resected pancreatic cancer. Although more toxic, FOLFIRINOX was shown to significantly improve both disease-free and overall survival [3].
The optimal time to initiate adjuvant CT is yet to be established and postoperative treatment is typically started once the patient has recovered from surgery. Major adjuvant randomized studies recommend initiation of CT within six to eight weeks after resection, and this has become an accepted approach. However, there is a lack of prospective trials that specifically evaluate whether starting the administration of adjuvant therapy after eight weeks compromises outcomes. To answer this important clinical question, we performed a systematic review of all available studies and a meta-analysis in gastrointestinal and pancreatic cancer settings.
2. Results
Our systematic literature search retrieved 8752 studies, 34 of which matched our inclusion criteria. These 34 studies corresponded to the post-hoc analyses of randomized controlled trials and cohorts and to retrospective studies. They included a total of 141,853 patients: 134,701 in the colon cancer cohort, as shown in Table 1 [4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]; 5121 in the gastric cancer cohort, as shown in Table 2 [26,27,28,29,30,31]; and 2031 in the pancreatic cancer cohort, as shown in Table 3 [32,33,34,35,36,37]. Among these studies, 22 were used for the colorectal cancer analysis and six were used for pancreatic and gastric cancer each. Table 1, Table 2 and Table 3 show the main characteristics of the studies and the cut-offs of the timing of adjuvant chemotherapy evaluated in each study. When the multivariate analysis was performed, the confounding factors typical of each study were considered (i.e. tumor extension, stage, nodal status, age, and sex).
Table 1.
Characteristics of included studies for colorectal cancer.
| Author/ Year |
N° pts | Type of Study | Median Follow up (months) | Country | Stage (%) | Comparison (weeks) | Type of Analysis | NOS Scale |
|---|---|---|---|---|---|---|---|---|
| Ahmed/ 2010 [4] |
663 | Retro | 54.6 | Canada | II–III (100) | < vs > 8 | Multi | 8 |
| Bayraktar/ 2011 [5] |
186 | Retro | 42.9 | US | II–III (100) | < vs > 8 | Multi | 8 |
| Becerra/ 2017 [6] |
1133 | Retro | NR | US | III (100) | < vs > 8 | Multi | 6 |
| Berglund/ 2008 [13] |
213 | Phase III | NR | Sweden | III (100) | < vs > 8 | Multi | NA |
| Bos/2015 [7] | 6620 | Retro | 60 | Netherlands | III (100) | < vs > 8 | Multi | 8 |
| Chau/2005 [14] | 801 | Phase III | 63.6 | UK | II–III (100) | < vs 8–12 | Multi | NA |
| Czaykowski/ 2011 [9] |
345 | Retro | 69.8 | Canada | III (100) | < vs > 8 | Multi | 8 |
| Cheung/ 2009 [8] |
6059 | Retro | NR | Canada | II–III (100) | < vs > 8 | Uni | 6 |
| Day/2014 [10] | 209 | Retro | 30 | UK | I–II (33), III (67) | < vs > 8 | Multi | 7 |
| Dos Santos/ 2013 [11] |
1318 | Retro | 41 | Brazil | II–III (100) | < vs > 8 | Multi | 8 |
| Gao/2018 [15] | 9722 | Retro | NR | US | III (100) | 5–8 vs > 8 | Multi | 6 |
| Hershman/ 2006 [16] |
4382 | Retro | NR | US | III (100) | < vs > 2–3 months | Multi | 6 |
| Kang/2013 [17] | 159 | Retro | 41.5 | Korea | III (100) | < vs 5–6 | Multi | 7 |
| Kim/2017 [18] | 5355 | Retro | 42.2 | Korea | II–III (100) | < vs > 8 | Multi | 7 |
| Klein/2015 [19] | 1827 | Retro | NR | Denmark | III (100) | 4–8 vs > 8 | Multi | 6 |
| Lima/2011 [20] | 1053 | Retro | NR | Canada | III (100) | < vs > 8 | Multi | 6 |
| Massarweh/ 2015 [12] |
51,331 | Retro | NR | US | III (100) | 8 vs 8–16 | Multi | 6 |
| Nachiappan/ 2015 [21] |
30,836 | Retro | 1–184* | UK | NR | < vs 8–16 | Multi | 7 |
| Peixoto/ 2015 [22] |
635 | Retro | 57.9 | Canada | III (100) | < vs > 8 | Multi | 8 |
| Sun/2016 [23] | 7794 | Retro | 61 | US | II–III (100) | < vs > 44 days | Multi | 8 |
| Tsai/2013 [24] | 1054 | Retro | 72.5 | Taiwan | III (100) | < vs ≥ 6 | Multi | 8 |
| Zeig-Owens/ 2009 [25] |
3006 | Retro | ≥48 | US | II–III (100) | < vs > 45 days | Multi | 8 |
* range of follow up; CSS, cancer-specific survival; Multi, multivariate; NOS, Newcastle–Ottawa scale; pts, patients; Retro, retrospective; RFS, relapse-free survival; Uni, univariate; vs, versus.
Table 2.
Characteristics of included studies for gastric cancer.
| Author/Year | N° pts | Type of Study | Median Follow up (months) | Country | Stage (%) | Comparison (weeks) | Type of Analysis | NOS Scale |
|---|---|---|---|---|---|---|---|---|
| Di Bartolomeo/ 2016 [26] |
1072 | Retro | 56.9 | Italy | Ib (8.2); II (31.8) III (41.6); IV (18.4) |
< vs > 8 | Multi | 8 |
| Fujitani/ 2017 [27] |
498 | Retro | NR | Japan | II (36.1); III (63.9) | < vs > 6 | Multi | 6 |
| Greenleaf/ 2016 [28] |
2332 | Retro | NR | US | I (11); II (30); III (50) | 8 vs > 8 | Multi | 6 |
| Park/ 2015 [29] |
840 | Retro | 34 | Korea | II (28.6); III (71.4) | 4–8 vs 8 | Multi | 7 |
| Qu/ 2015 [30] |
266 | Retro | 28 | China | IB (4.1); II (28.2) III (67.7) |
< vs > 6.4 | Multi | 6 |
| Yamamoto/ 2016 [31] |
113 | Retro | 47.6 | Japan | II (34.5); III (65.5) | < vs > 6 | Multi | 7 |
Multi, multivariate; NOS, Newcastle–Ottawa scale; pts, patients; Retro, retrospective; vs, versus.
Table 3.
Characteristics of included studies for pancreatic cancer.
| Author/Year | N° pts | Type of Study | Median Follow up (months) | Country | Stage (%) | Comparison (weeks) | Type of Analysis | NOS Scale |
|---|---|---|---|---|---|---|---|---|
| Kim/2017 [32] | 113 | Retro | 20.3 | Korea | - | < 6 vs > 6 | Multi | 6 |
| Lee/2017 [33] | 311 | Retro | 28 | Korea | I (4.1); II 94.2; III 1.6 | < 6 vs > 6 | Multi | 7 |
| Patel/2015 [34] | 34 | Retro | 22 | US | N0 38; N+ 62 | < 8 vs > 12 | Uni | 6 |
| Saeed/2016 [35] | 420 | Retro | 19.3 | US | I (8.5); II (87.1); III 4.2 | < 8 vs > 8 | Multi | 7 |
| Valle/2014 [36] | 985 | Phase III | 58.7 | Europe | I (10); II 29; III 58; IVa 4 | < 8 vs > 8 | Multi | 8 |
| Yabusaki/2016 [37] | 168 | Prosp | 24.5 | Japan | I–III (100) | < 8 vs > 8 | Multi | 7 |
Multi, multivariate; NOS, Newcastle–Ottawa scale; Prosp, prospective, pts, patients; Retro, retrospective; Uni, univariate; vs, versus.
All studies were retrospective, except for three randomized phase 3 trials and one prospective study. All articles were fully published between 2005 and 2017. In n = 25 studies, the comparison was made between more than versus (vs) less than eight weeks, in n = 5 studies between more vs less than six weeks, in n = 3 studies between 6.5 vs < 6.5 weeks, and in n = 1 study between 5–6 vs less than 5–6 weeks.
2.1. Effect of Delaying CT on Survival in Colorectal Cancer
Among colorectal cancer studies, the combined hazard ratio (HR) for delayed vs earlier adjuvant CT was 1.27 (95% confidence interval (CI) 1.21–1.33; p <0.001; Figure 1).
Figure 1.
Forest plot of association between delay of adjuvant chemotherapy beyond 6–8 weeks and survival in colorectal cancer.
As heterogeneity was found (I2 = 70%, p < 0.001), a random effect model was used. After removing the four studies with the largest weight sequentially, the HR ranged from 1.25 to 1.28 and remained significant in all cases. All studies except one reported the results as multivariate analyses.
2.2. Effect of Delaying CT on Survival in Gastric Cancer
By pooling the results of the six gastric cancer studies, the combined HR for delayed vs earlier adjuvant CT was 1.2 (95% CI 1.04–1.38; p = 0.01; Figure 2).
Figure 2.
Forest plot of association between delay of adjuvant chemotherapy beyond 6–8 weeks and survival in gastric cancer.
Again, there was evidence of heterogeneity (I2 = 90%, p < 0.001), therefore, a random effect model was used. After removing the study with the largest weight [28], the HR was 1.41 (95% CI 0.94–2.1, p = 0.09). All studies reported the results as multivariate analyses.
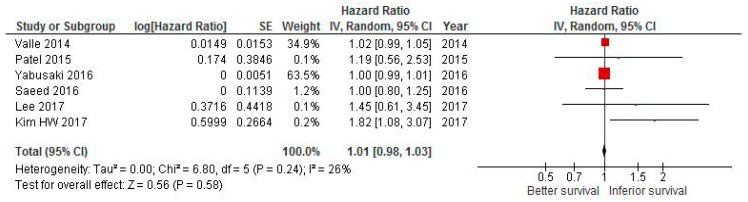
2.3. Effect of Delaying CT on Survival in Pancreatic Cancer
The pooled HR attained from six studies in pancreatic cancer was 1 (95% CI 1–1.01, p = 0.37; Figure 3).
Figure 3.
Forest plot of association between delay of adjuvant chemotherapy beyond 6–8 weeks and survival in pancreatic cancer.
There was no evidence of heterogeneity (I2 = 20%, p <0.001), so a fixed effect model was used. Removing the largest study (Reference [37]) did not change the final result.
2.4. Publication Bias
The funnel plot for the degree of asymmetry of the individual study results around the combined HR for overall survival (OS) in colorectal cancer studies is shown in Figure 4.
Figure 4.
Funnel plot of the relationship between the log hazard ratio (HR) and standard error of the log HR for overall survival (OS) in colorectal cancer studies.
The degree of asymmetry was not statistically significant according to the Egger method (p = 0.47). We used the trim and fill approach to adjust our estimate of effect size for potential asymmetry. The imputed estimate (HR =1.25; 95% CI, 1.18–1.32) was similar to that in the main analysis, indicating that the results are unlikely to be explained by publication bias.
3. Discussion
Most of the currently available evidence on the optimal timing of adjuvant CT is retrospective and derived from breast and colorectal cancer studies. For example, early initiation of adjuvant CT (within 20 days post-surgery) was shown to be associated with a significant improvement in disease-free survival in estrogen-receptor-negative premenopausal breast cancer patients [38]. Similarly, in stage III colorectal cancer, a recent study including 72,057 patients concluded that the maximum survival benefit of adjuvant CT was obtained when treatment was started within six to eight weeks [39]. Two previously published meta-analyses have also shown that delays beyond two months may compromise the effectiveness of CT [40,41].
In some cases, CT delays are caused by post-surgical complications and a retrospective cohort study of stage III colon cancer patients reported that 30–38% were surgeon-specific delays, while the vast majority were caused by medical oncologists and hospital-specific practices [6]. In a retrospective series of patients with stage I to III invasive breast cancer, it was mainly sociodemographic determinants that caused CT initiation delays of 91 or more days [42]. Interestingly, in cases of totally resected non-small-cell lung cancer, patients still benefited from delayed adjuvant CT when therapy was started up to four months after surgery [43]. It is not clear, however, whether there is any time point beyond which the benefits of adjuvant CT are lost for gastric and pancreatic cancers.
The results of this meta-analysis, which evaluated 34 studies and 141,853 patients, indicated that delaying the initiation of adjuvant CT beyond eight weeks post-surgery was associated with a 27% and 20% increased risk of death for colorectal and gastric cancer, respectively. For pancreatic cancer, no statistically significant difference was found for patients starting earlier compared with patients receiving post-operative treatment after two months. Noteworthy, the detrimental effect of delayed starting of adjuvant CT was independent by other main clinicopathological risk factors.
As the ultimate goal of any adjuvant therapy is to decrease the chance of recurrence by eradicating hidden malignant cells after surgery, a longer interval between surgery and adjuvant CT might facilitate the proliferation of micrometastases. There is also a strong biological rationale for the early activation of CT after curative surgery. Studies in animal models have shown that removal of the primary tumor can increase the number of circulating tumor cells and accelerate the growth of residual cells [44]. Additionally, surgery has been shown to enhance the production of oncogenic growth factors (i.e. transforming growth factor α) and to significantly reduce the immunotherapeutic effects of interleukin-2 and lymphokine-activated killer cells [45,46].
The kinetics of cellular proliferation also indicate that in vivo, the growth rate is at first rapid and then slows progressively [47]. Therefore, at least theoretically, early cytotoxic treatment is expected to be beneficial. Finally, according to the historic mathematic model by Goldie and Coldman [48], drug sensitivity is related to mutation rate and as tumor mutation rate increases over time, a longer time interval after surgery might increase the probability of the appearance of a resistant phenotype. For all these reasons, CT will be more effective if initiated promptly when tumor burden is low.
Unfortunately, in gastrointestinal malignancies, adjuvant therapy is often delayed due to post-surgical complications and poor general conditions. In particular, patients undergoing gastric and pancreatic surgery frequently present significant nutritional problems that compromise their adequate recovery and the subsequent initiation of adjuvant CT. In this regard, minimally invasive surgical techniques, such as laparoscopic gastrectomy, may help by accelerating recovery and facilitating a prompt return to normal bowel function and an early discharge from hospital [49]. Consequently, patients will have easier access to potentially curative adjuvant treatments.
As for colorectal and gastric cancer, the results of this meta-analysis are consistent with previous reports showing that eight weeks after surgery is a reasonable cut-off for recommending adjuvant CT activation. Specifically, a meta-analysis of 10 published studies involving 15,410 patients concluded that delaying adjuvant CT beyond 12 weeks after surgery was associated with decreased survival among patients with resected colorectal cancer [40]. Similarly, a recent study conducted in Asia involving 840 D2-resected stage 2 and 3 gastric cancer patients showed that delayed treatment of adjuvant CT after eight weeks was associated with worse survival outcomes than early and intermediate treatment initiation. Therefore, the start of adjuvant CT should be considered within eight weeks after radical resection [29].
A pancreatectomy is a surgical procedure associated with high rates of complications that negatively affect both time to adjuvant treatment initiation and long-term outcomes. A post-hoc analysis of the largest trial of adjuvant CT for pancreatic cancer (i.e., ESPAC-3) did not show any overall survival difference for patients who started CT earlier than eight weeks (compared to 12 weeks) following surgery [36]. The only prognostic factor was represented by the completion of all six cycles of planned adjuvant CT. Three subsequent large retrospective studies were concordant in demonstrating no detriment to survival for patients who had delayed initiation of adjuvant therapy greater than 12 weeks after surgery [50,51]. Consistent with these findings, our meta-analysis did not show any significant survival benefit associated with early initiation, confirming that in pancreatic cancer, factors related to the biology of the tumor may play a major role.
Our results need to be interpreted in the context of the study’s strengths and limitations. As a randomized controlled trial comparing the effect on survival of two different timings of initiation of adjuvant CT after surgery would not be feasible for ethical and clinical reasons, most of the included studies are derived from retrospective observations. Second, the reason for delaying CT for more than 6–8 weeks is unknown and could be potentially related to slow recovery after surgery or other morbidities. Eventually, the delay may potentially weaken the benefit of adjuvant therapy. Finally, the bad prognosis and the limited utility of older CT schedules can explain the negative effect of early initiation in pancreatic cancer. Otherwise, we can confirm that delaying adjuvant CT is potentially detrimental in stage III colorectal cancer and that this paper updates previous meta-analyses related to this topic with large case series. Furthermore, we can also provide evidence that in gastric cancer, if neoadjuvant CT is not scheduled, earlier initiation of postoperative CT could be useful.
4. Materials and Methods
4.1. Search Strategy and Inclusion Criteria
The current systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses statement [52]. We searched for relevant studies through database queries in PubMed, EMBASE, and the Cochrane Library (from inception to 16 December 2018) using the Medical Subject Headings: adjuvant chemotherapy AND (colorectal neoplasms or stomach neoplasm or pancreatic neoplasm) and survival and (timing OR initiation OR delay OR start OR time OR interval). Literature references were also scanned manually. To be eligible, studies had to include patients with resected gastric, colorectal, and pancreatic cancer and to assess the relationship between shorter (<6–8 weeks) and longer (>6–8 weeks) elapsed periods from surgery to the start of adjuvant CT and OS. Studies were excluded if they were abstracts or case reports, if they included patients treated with neoadjuvant therapy, and if they were written in a non-English language.
4.2. Data Extraction
Based on the title, keywords, and abstract, three reviewers (F.P., A.G., and M.G.) selected the studies by applying the inclusion criteria. When there was doubt regarding whether or not to select a study, a discussed was conducted to resolve this. The three reviewers assessed the full versions of the selected articles. When disagreements about inclusion were not resolved by consensus, a senior reviewer (A.Z.) was consulted. Figure 5 outlines the identification of studies for this systematic review and meta-analysis.
Figure 5.
Flow diagram of included studies.
The three reviewers extracted data from the included studies. For each article, the following data were extracted using Microsoft Word spreadsheets: Author and year of publication; the number of participants; country; stage of disease; description of the comparison; and outcome measures available. A quality assessment of all the studies included in the meta-analysis was performed according to the Newcastle–Ottawa scale (NOS). The total scores ranged from 0 (worst) to 9 (best) for cohort studies, with a score of at least 7 indicating high quality.
4.3. Statistical Analysis
The measure of effect in all studies was the HR for OS. For each study, the HR and 95% CI was estimated depending on the data provided in the publication. When different intervals between surgery and the start of CT were presented, any event occurring in general beyond 6–8 weeks was compared with all events occurring within the 6–8 weeks interval. Three different forest plots were created for each disease (colorectal, gastric, and pancreatic cancer). The homogeneity assumption in the meta-analysis was assessed by the Cochrane Chi2 statistic and I2 statistics were calculated for each result. The pooled HRs for death with early vs delayed CT were calculated using the fixed effect model/Mantel–Haenszel (M-H) method when there was minimal heterogeneity in the variables among the studies, and the Der Simonian–Laird method (random effect model) when there was significant heterogeneity (p for heterogeneity <0.1).
Each publication was weighted as a function of the inverse variance of each effect size and Chi2 and I2 test methods were utilized for the between-study heterogeneity of the HRs. The statistically significant differences were defined as <0.1 for the Chi2 p and greater than 50% for the I2 test. The publication bias was evaluated through Egger’s linear regression, Begg’s rank correlation, and funnel plots and a p-value <0.05 for the Egger’s or Begg’s tests was considered representative of significant statistical publication bias. In addition, the trim and fill approach was used to obtain an adjusted effect size that took into account the publication bias. All statistical analyses were performed with Review Manager (RevMan) (computer program) Version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
5. Conclusions
Findings from our study demonstrate that starting adjuvant CT within 6–8 weeks post-surgery is associated with a significant survival benefit in colorectal and gastric cancer. These results suggest that the timing of CT initiation is an important variable and that great efforts should be made to minimize post-surgical recovery time.
Author Contributions
Conceptualization, F.P. and A.Z.; methodology, F.P.; software, F.P.; validation, F.P., G.T. and A.Z.; formal analysis, F.P.; investigation, A.G.; resources, A.G.; data curation, M.L.; writing—original draft preparation, G.T., M.G., A.G.; writing—review and editing, L.T., C.P., M.R.; visualisation, M.G.; supervision, A.Z.; project administration, F.P.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Shah M.A., Renfro L.A., Allegra C.J., Andre T., de Gramont A., Schmoll H.J., Haller D.G., Alberts S.R., Yothers G., Sargent D.J. Impact of Patient Factors on Recurrence Risk and Time Dependency of Oxaliplatin Benefit in Patients With Colon Cancer: Analysis From Modern-Era Adjuvant Studies in the Adjuvant Colon Cancer End Points (ACCENT) Database. J. Clin. Oncol. 2016;34:843–853. doi: 10.1200/JCO.2015.63.0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diaz-Nieto R., Orti-Rodriguez R., Winslet M. Post-surgical chemotherapy versus surgery alone for resectable gastric cancer. Cochrane Database Syst. Rev. 2013 doi: 10.1002/14651858.CD008415.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy T., Hammel P., Hebbar M., Ben Abdelghani M., Wei A.C., Raoul J.L., Chone L., Francois E., Artru P., Biagi J.J., et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed S., Ahmad I., Zhu T., Arnold F.P., Faiz Anan G., Sami A., Yadav S.K., Alvi R., Haider K. Early discontinuation but not the timing of adjuvant therapy affects survival of patients with high-risk colorectal cancer: A population-based study. Dis. Colon Rectum. 2010;53:1432–1438. doi: 10.1007/DCR.0b013e3181e78815. [DOI] [PubMed] [Google Scholar]
- 5.Bayraktar U.D., Chen E., Bayraktar S., Sands L.R., Marchetti F., Montero A.J., Rocha-Lima C.M. Does delay of adjuvant chemotherapy impact survival in patients with resected stage II and III colon adenocarcinoma? Cancer. 2011;117:2364–2370. doi: 10.1002/cncr.25720. [DOI] [PubMed] [Google Scholar]
- 6.Becerra A.Z., Aquina C.T., Mohile S.G., Tejani M.A., Schymura M.J., Boscoe F.P., Xu Z., Justiniano C.F., Boodry C.I., Swanger A.A., et al. Variation in Delayed Time to Adjuvant Chemotherapy and Disease-Specific Survival in Stage III Colon Cancer Patients. Ann. Surg. Oncol. 2017;24:1610–1617. doi: 10.1245/s10434-016-5622-4. [DOI] [PubMed] [Google Scholar]
- 7.Bos A.C., van Erning F.N., van Gestel Y.R., Creemers G.J., Punt C.J., van Oijen M.G., Lemmens V.E. Timing of adjuvant chemotherapy and its relation to survival among patients with stage III colon cancer. Eur. J. Cancer. 2015;51:2553–2561. doi: 10.1016/j.ejca.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Cheung W.Y., Neville B.A., Earle C.C. Etiology of delays in the initiation of adjuvant chemotherapy and their impact on outcomes for Stage II and III rectal cancer. Dis. Colon Rectum. 2009;52:1054–1063. doi: 10.1007/DCR.0b013e3181a51173. discussion 1064. [DOI] [PubMed] [Google Scholar]
- 9.Czaykowski P.M., Gill S., Kennecke H.F., Gordon V.L., Turner D. Adjuvant chemotherapy for stage III colon cancer: Does timing matter? Dis. Colon Rectum. 2011;54:1082–1089. doi: 10.1097/DCR.0b013e318223c3d6. [DOI] [PubMed] [Google Scholar]
- 10.Day A.R., Middleton G., Smith R.V., Jourdan I.C., Rockall T.A. Time to adjuvant chemotherapy following colorectal cancer resection is associated with an improved survival. Colorectal Dis. 2014;16:368–372. doi: 10.1111/codi.12570. [DOI] [PubMed] [Google Scholar]
- 11.Dos Santos L.V., Faria T.M., Lima A.B., Abdalla K.C., de Moraes E.D., Cruz M.R., Lima J.P. Timing of adjuvant chemotherapy in colorectal cancer. Colorectal Dis. 2016;18:871–876. doi: 10.1111/codi.13306. [DOI] [PubMed] [Google Scholar]
- 12.Massarweh N.N., Haynes A.B., Chiang Y.J., Chang G.J., You Y.N., Feig B.W., Cormier J.N. Adequacy of the National Quality Forum’s Colon Cancer Adjuvant Chemotherapy Quality Metric: Is 4 Months Soon Enough? Ann. Surg. 2015;262:312–320. doi: 10.1097/SLA.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berglund A., Cedermark B., Glimelius B. Is it deleterious to delay the start of adjuvant chemotherapy in colon cancer stage III? Ann. Oncol. 2008;19:400–402. doi: 10.1093/annonc/mdm582. [DOI] [PubMed] [Google Scholar]
- 14.Chau I., Norman A.R., Cunningham D., Tait D., Ross P.J., Iveson T., Hill M., Hickish T., Lofts F., Jodrell D., et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann. Oncol. 2005;16:549–557. doi: 10.1093/annonc/mdi116. [DOI] [PubMed] [Google Scholar]
- 15.Gao P., Huang X.Z., Song Y.X., Sun J.X., Chen X.W., Sun Y., Jiang Y.M., Wang Z.N. Impact of timing of adjuvant chemotherapy on survival in stage III colon cancer: A population-based study. BMC Cancer. 2018;18:234. doi: 10.1186/s12885-018-4138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hershman D., Hall M.J., Wang X., Jacobson J.S., McBride R., Grann V.R., Neugut A.I. Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer. Cancer. 2006;107:2581–2588. doi: 10.1002/cncr.22316. [DOI] [PubMed] [Google Scholar]
- 17.Kang K.M., Hong K.S., Noh G.T., Oh B.Y., Chung S.S., Lee R.A., Kim K.H. Optimal time of initiating adjuvant chemotherapy after curative surgery in colorectal cancer patients. Ann. Coloproctol. 2013;29:150–154. doi: 10.3393/ac.2013.29.4.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y.W., Choi E.H., Kim B.R., Ko W.A., Do Y.M., Kim I.Y. The impact of delayed commencement of adjuvant chemotherapy (eight or more weeks) on survival in stage II and III colon cancer: A national population-based cohort study. Oncotarget. 2017;8:80061–80072. doi: 10.18632/oncotarget.17767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein M., Azaquoun N., Jensen B.V., Gogenur I. Improved survival with early adjuvant chemotherapy after colonic resection for stage III colonic cancer: A nationwide study. J. Surg. Oncol. 2015;112:538–543. doi: 10.1002/jso.24017. [DOI] [PubMed] [Google Scholar]
- 20.Lima I.S., Yasui Y., Scarfe A., Winget M. Association between receipt and timing of adjuvant chemotherapy and survival for patients with stage III colon cancer in Alberta, Canada. Cancer. 2011;117:3833–3840. doi: 10.1002/cncr.25954. [DOI] [PubMed] [Google Scholar]
- 21.Nachiappan S., Askari A., Mamidanna R., Munasinghe A., Currie A., Stebbing J., Faiz O. The impact of adjuvant chemotherapy timing on overall survival following colorectal cancer resection. Eur. J. Surg. Oncol. 2015;41:1636–1644. doi: 10.1016/j.ejso.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Peixoto R.D., Kumar A., Speers C., Renouf D., Kennecke H.F., Lim H.J., Cheung W.Y., Melosky B., Gill S. Effect of delay in adjuvant oxaliplatin-based chemotherapy for stage III colon cancer. Clin. Colorectal Cancer. 2015;14:25–30. doi: 10.1016/j.clcc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Sun Z., Adam M.A., Kim J., Nussbaum D.P., Benrashid E., Mantyh C.R., Migaly J. Determining the Optimal Timing for Initiation of Adjuvant Chemotherapy After Resection for Stage II and III Colon Cancer. Dis. Colon Rectum. 2016;59:87–93. doi: 10.1097/DCR.0000000000000518. [DOI] [PubMed] [Google Scholar]
- 24.Tsai W.S., Hsieh P.S., Yeh C.Y., Chiang J.M., Tang R., Chen J.S., Changchien C.R., Wang J.Y. Impact of chemotherapy-related prognostic factors on long-term survival in patients with stage III colorectal cancer after curative resection. Int. J. Clin. Oncol. 2013;18:242–253. doi: 10.1007/s10147-011-0370-8. [DOI] [PubMed] [Google Scholar]
- 25.Zeig-Owens R., Gershman S.T., Knowlton R., Jacobson J.S. Survival and time interval from surgery to start of chemotherapy among colon cancer patients. J. Registry Manag. 2009;36:30–41. quiz 61–32. [PubMed] [Google Scholar]
- 26.Di Bartolomeo M., Pietrantonio F., Rulli E., Poli D., Berenato R., Caporale M., Bajetta E., Floriani I. Impact on survival of timing and duration of adjuvant chemotherapy in radically resected gastric cancer. Tumori. 2016;102:e15–e19. doi: 10.5301/tj.5000480. [DOI] [PubMed] [Google Scholar]
- 27.Fujitani K., Kurokawa Y., Takeno A., Endoh S., Ohmori T., Fujita J., Yamasaki M., Takiguchi S., Mori M., Doki Y., et al. Time to initiation or duration of S-1 adjuvant chemotherapy; which really impacts on survival in stage II and III gastric cancer? Gastric Cancer. 2018;21:446–452. doi: 10.1007/s10120-017-0767-9. [DOI] [PubMed] [Google Scholar]
- 28.Greenleaf E.K., Kulaylat A.N., Hollenbeak C.S., Almhanna K., Wong J. Timing of Adjuvant Chemotherapy and Impact on Survival for Resected Gastric Cancer. Ann. Surg. Oncol. 2016;23:4203–4213. doi: 10.1245/s10434-016-5464-0. [DOI] [PubMed] [Google Scholar]
- 29.Park H.S., Jung M., Kim H.S., Kim H.I., An J.Y., Cheong J.H., Hyung W.J., Noh S.H., Kim Y.I., Chung H.C., et al. Proper timing of adjuvant chemotherapy affects survival in patients with stage 2 and 3 gastric cancer. Ann. Surg. Oncol. 2015;22:224–231. doi: 10.1245/s10434-014-3949-2. [DOI] [PubMed] [Google Scholar]
- 30.Qu J.L., Qu X.J., Li X., Zhang J.D., Teng Y.E., Jin B., Zhao M.F., Yu P., Liu J., Li D.Y., et al. Early initiation of fluorouracil-based adjuvant chemotherapy improves survival in patients with resectable gastric cancer. J. BUON. 2015;20:800–807. [PubMed] [Google Scholar]
- 31.Yamamoto M., Sakaguchi Y., Kinjo N., Yamaguchi S., Egashira A., Minami K., Ikeda Y., Morita M., Toh Y., Okamura T. S-1 Adjuvant Chemotherapy Earlier After Surgery Clinically Correlates with Prognostic Factors for Advanced Gastric Cancer. Ann. Surg. Oncol. 2016;23:546–551. doi: 10.1245/s10434-015-4868-6. [DOI] [PubMed] [Google Scholar]
- 32.Kim H.W., Lee J.C., Lee J., Kim J.W., Kim J., Hwang J.H. Early versus delayed initiation of adjuvant treatment for pancreatic cancer. PLoS ONE. 2017;12:e0173960. doi: 10.1371/journal.pone.0173960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee W., Yoon Y.S., Han H.S., Jang J.Y., Cho J.Y., Jung W., Kwon W., Choi Y., Kim S.W. Prognostic Relevance of the Timing of Initiating and the Completion of Adjuvant Therapy in Patients with Resected Pancreatic Ductal Adenocarcinoma. World J. Surg. 2017;41:562–573. doi: 10.1007/s00268-016-3798-1. [DOI] [PubMed] [Google Scholar]
- 34.Patel A.A., Nagarajan S., Scher E.D., Schonewolf C.A., Balasubramanian S., Poplin E., Moss R., August D., Carpizo D., Melstrom L., et al. Early vs. Late Chemoradiation Therapy and the Postoperative Interval to Adjuvant Therapy Do Not Correspond to Local Recurrence in Resected Pancreatic Cancer. Pancreat. Disord. Ther. 2015;5 doi: 10.4172/2165-7092.1000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saeed H., Hnoosh D., Huang B., Durbin E.B., McGrath P.C., Desimone P., Maynard E., Anthony L.B., Dineen S.P., Hosein P.J., et al. Defining the optimal timing of adjuvant therapy for resected pancreatic adenocarcinoma: A statewide cancer registry analysis. J. Surg. Oncol. 2016;114:451–455. doi: 10.1002/jso.24314. [DOI] [PubMed] [Google Scholar]
- 36.Valle J.W., Palmer D., Jackson R., Cox T., Neoptolemos J.P., Ghaneh P., Rawcliffe C.L., Bassi C., Stocken D.D., Cunningham D., et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: Ongoing lessons from the ESPAC-3 study. J. Clin. Oncol. 2014;32:504–512. doi: 10.1200/JCO.2013.50.7657. [DOI] [PubMed] [Google Scholar]
- 37.Yabusaki N., Fujii T., Yamada S., Murotani K., Sugimoto H., Kanda M., Nakayama G., Koike M., Fujiwara M., Kodera Y. The significance of relative dose intensity in adjuvant chemotherapy of pancreatic ductal adenocarcinoma-including the analysis of clinicopathological factors influencing relative dose intensity. Medicine (Baltimore) 2016;95:e4282. doi: 10.1097/MD.0000000000004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colleoni M., Bonetti M., Coates A.S., Castiglione-Gertsch M., Gelber R.D., Price K., Rudenstam C.M., Lindtner J., Collins J., Thurlimann B., et al. Early start of adjuvant chemotherapy may improve treatment outcome for premenopausal breast cancer patients with tumors not expressing estrogen receptors. The International Breast Cancer Study Group. J. Clin. Oncol. 2000;18:584–590. doi: 10.1200/JCO.2000.18.3.584. [DOI] [PubMed] [Google Scholar]
- 39.Turner M.C., Farrow N.E., Rhodin K.E., Sun Z., Adam M.A., Mantyh C.R., Migaly J. Delay in Adjuvant Chemotherapy and Survival Advantage in Stage III Colon Cancer. J. Am. Coll. Surg. 2018;226:670–678. doi: 10.1016/j.jamcollsurg.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 40.Biagi J.J., Raphael M.J., Mackillop W.J., Kong W., King W.D., Booth C.M. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: A systematic review and meta-analysis. JAMA. 2011;305:2335–2342. doi: 10.1001/jama.2011.749. [DOI] [PubMed] [Google Scholar]
- 41.Des Guetz G., Nicolas P., Perret G.Y., Morere J.F., Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur. J. Cancer. 2010;46:1049–1055. doi: 10.1016/j.ejca.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 42.Chavez-MacGregor M., Clarke C.A., Lichtensztajn D.Y., Giordano S.H. Delayed Initiation of Adjuvant Chemotherapy Among Patients With Breast Cancer. JAMA Oncol. 2016;2:322–329. doi: 10.1001/jamaoncol.2015.3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salazar M.C., Rosen J.E., Wang Z., Arnold B.N., Thomas D.C., Herbst R.S., Kim A.W., Detterbeck F.C., Blasberg J.D., Boffa D.J. Association of Delayed Adjuvant Chemotherapy With Survival After Lung Cancer Surgery. JAMA Oncol. 2017;3:610–619. doi: 10.1001/jamaoncol.2016.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gunduz N., Fisher B., Saffer E.A. Effect of surgical removal on the growth and kinetics of residual tumour. Cancer Res. 1979;39:3861–3865. [PubMed] [Google Scholar]
- 45.Eggermont A.M., Steller E.P., Sugarbaker P.H. Laparotomy enhances intraperitoneal tumour growth and abrogates the antitumor effects of interleukin-2 and lymphokine-activated killer cells. Surgery. 1987;102:71–78. [PubMed] [Google Scholar]
- 46.Ono I., Gunji H., Suda K., Iwatsuki K., Kaneko F. Evaluation of cytokines in donor site wound fluids. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1994;28:269–273. doi: 10.3109/02844319409022010. [DOI] [PubMed] [Google Scholar]
- 47.Frindel E., Malaise E.P., Alpen E., Tubiana M. Kinetics of cell proliferation of an experimental tumour. Cancer Res. 1967;27:1122–1131. [PubMed] [Google Scholar]
- 48.Goldie J.H., Coldman A.J. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat. Rep. 1979;63:1727–1733. [PubMed] [Google Scholar]
- 49.Vinuela E.F., Gonen M., Brennan M.F., Coit D.G., Strong V.E. Laparoscopic versus open distal gastrectomy for gastric cancer: A meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann. Surg. 2012;255:446–456. doi: 10.1097/SLA.0b013e31824682f4. [DOI] [PubMed] [Google Scholar]
- 50.Mirkin K.A., Greenleaf E.K., Hollenbeak C.S., Wong J. Time to the initiation of adjuvant chemotherapy does not impact survival in patients with resected pancreatic cancer. Cancer. 2016;122:2979–2987. doi: 10.1002/cncr.30163. [DOI] [PubMed] [Google Scholar]
- 51.Xia B.T., Ahmad S.A., Al Humaidi A.H., Hanseman D.J., Ethun C.G., Maithel S.K., Kooby D.A., Salem A., Cho C.S., Weber S.M., et al. Time to Initiation of Adjuvant Chemotherapy in Pancreas Cancer: A Multi-Institutional Experience. Ann. Surg. Oncol. 2017;24:2770–2776. doi: 10.1245/s10434-017-5918-z. [DOI] [PubMed] [Google Scholar]
- 52.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]