Abstract
Acute hepatopancreatic necrosis disease (AHPND) is a newly emergent penaeid shrimp disease which can cause 70–100% mortality in Penaeus vannamei and Penaeus monodon, and has resulted in enormous economic losses since its appearance. AHPND is caused by the specific strains of Vibrio parahaemolyticus that harbor the pVA1 plasmid and express PirAvp and PirBvp toxins. These two toxins have been reported to form a binary complex. When both are present, they lead to the death of shrimp epithelial cells in the hepatopancreas and cause the typical histological symptoms of AHPND. However, the binding mode of PirAvp and PirBvp has not yet been determined. Here, we used isothermal titration calorimetry (ITC) to measure the binding affinity of PirAvp and PirBvp. Since the dissociation constant (Kd = 7.33 ± 1.20 μM) was considered too low to form a sufficiently stable complex for X-ray crystallographic analysis, we used alternative methods to investigate PirAvp-PirBvp interaction, first by using gel filtration to evaluate the molecular weight of the PirAvp/PirBvp complex, and then by using cross-linking and hydrogen-deuterium exchange (HDX) mass spectrometry to further understand the interaction interface between PirAvp and PirBvp. Based on these results, we propose a heterotetrameric interaction model of this binary toxin complex. This model provides insight of how conformational changes might activate the PirBvp N-terminal pore-forming domain and should be helpful for devising effective anti-AHPND strategies in the future.
Keywords: Vibrio parahaemolyticus, AHPND, PirAvp, PirBvp, protein-protein interaction
1. Introduction
Vibrio parahaemolyticus is a common halophilic Gram-negative bacterium that can be found in estuarine, marine and coastal environments. Recently, however, some new virulent strains of this opportunistic marine pathogen were identified as the causative agent of acute hepatopancreatic necrosis disease (AHPND) in shrimp. AHPND has a high mortality rate in shrimp (70–100% in Penaeus monodon and Penaeus vannamei), leading to catastrophic drops in shrimp production and enormous economic losses that impact the whole industry. Shrimp infected with AHPND show some readily observable symptoms, such as lethargy, an empty stomach and midgut, and a pale to white atrophied hepatopancreas [1], while the characteristic histological symptom of AHPND is the sloughing of HP tubule epithelial cells into the HP tubule lumens [1,2]. However, due to the absence of obvious bacterial colonies in the hepatopancreas tube lumens in the initial stage of acute infection of V. parahaemolyticus [1,3,4], Tran et al. proposed that AHPND symptoms were not induced by the bacteria themselves, but the toxins secreted by the bacteria [1]. Subsequent reverse gavage experiments further confirmed this hypothesis [1,5].
In addition to the specific strains of V. parahaemolyticus, several other Vibrio species such as V. harveyi, V. campbelli, V. owensii, and V. punensis were also found to cause AHPND [6,7,8,9]. These and other reports further showed that all these AHPND-causing pathogens harbor plasmids that contain the pirA and pirB toxin genes which are homologs of the Photorhabdus insect-related (Pir) binary toxins [6,7,8,9,10]. Durán-Avelar et al. even reported that a non-Vibrio bacterium, Microccocus luteus, also harbors the pirA and pirB toxin genes [11]. These two toxin proteins were confirmed to be the key factors that cause AHPND symptoms by Lee et al., who demonstrated that PirAvp/PirBvp are sufficient to induce the typical symptoms of AHPND by feeding shrimp with either the recombinant PirAvp/PirBvp proteins or with E. coli that expressed both PirAvp and PirBvp [10]. In addition to showing that PirAvp and PirBvp form a complex, we also used structural analysis (i.e., X-ray crystallography) to show that the assembled PirAvp and PirBvp structure was similar to that of the Bacillus thuringiensis Cry insecticidal toxins: the N-terminal and C-terminal of PirBvp correspond to the pore-forming domain I and the receptor-binding domain II of Cry protein, respectively, while PirAvp corresponds to Cry toxin domain III, which is the sugar-binding domain [10,12]. However, we failed to obtain the crystal of the PirAvp/PirBvp complex for subsequent structural analysis, and the interaction model between PirAvp and PirBvp therefore remained unclear. Here, we use alternative methods, such as isothermal titration calorimetry (ITC), gel filtration, cross-linking mass spectrometry, and hydrogen–deuterium exchange (HDX), to investigate the interface between PirAvp and PirBvp. We expect that with a better understanding of this interface, it will be possible to develop more effective strategies against AHPND in the future.
2. Results and Discussion
2.1. PirAvp and PirBvp Have a Low Binding Affinity
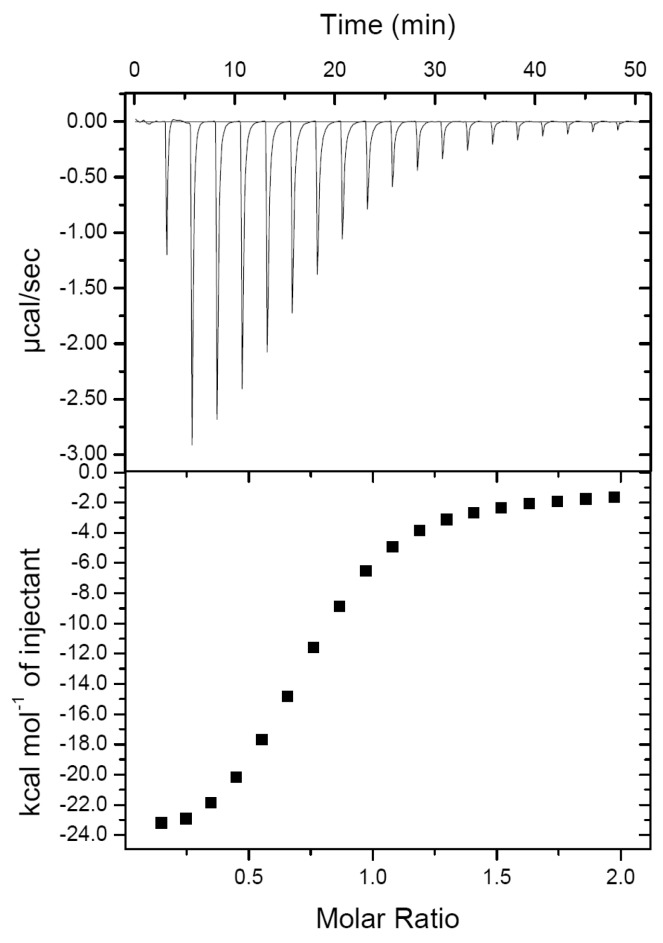
Since PirAvp and PirBvp need to bind to each other to achieve their cytotoxic activity [10], an understanding of their binding mode should be useful for designing an anti-AHPND strategy, for example by using a small compound, peptide or antibody to block the interfaces of PirAvp and PirBvp to prevent formation of the toxic complex. In our previous report, we confirmed that PirAvp could interact with PirBvp [10]. Unfortunately, we were unable to obtain any crystal for the PirAvp/PirBvp complex, and in fact we found crystals only for PirBvp; neither PirAvp nor the complex was detected [10]. This result suggested that the complex is unstable. In the present study, to confirm this result, we used isothermal titration calorimetry (ITC) to determine the binding affinity of PirAvp and PirBvp and obtained a value of 7.33 ± 1.20 μM (Figure 1). Other thermodynamic parameters from the ITC assay are shown in Table 1. In general, this binding affinity is not good enough to maintain a stable complex for a long time, which might explain why we were unable to obtain a useful crystal of this complex for X-ray crystallography. In addition, the calculated binding stoichiometry ratio (N) was 0.74 ± 0.01, suggesting that some protein molecules may be inactive. For these reasons, we decided to use alternative methods to investigate the binding model for PirAvp and PirBvp.
Figure 1.
Determination of the binding affinity between PirAvp and PirBvp by an isothermal titration calorimetry (ITC) assay. The dissociation constant (Kd) between PirAvp and PirBvp was determined as 7.33 ± 1.20 μM. Other thermodynamic parameters for the PirAvp/PirBvp interaction are shown in Table 1. The data were collected from triplicate experiments. All three experiments produced very similar results; only a single experiment is shown in the Figure.
Table 1.
Thermodynamic parameters for the interaction between PirAvp and PirBvp.
| ΔH (kcal/mol) | ΔG (kcal/mol) | Kd (μM) | N |
|---|---|---|---|
| −25.69 ± 1.42 | −7.01 ± 0.10 | 7.33 ± 1.20 | 0.74 ± 0.01 |
2.2. Calculation of the Native Molecular Weights of PirAvp, PirBvp and the PirAvp/PirBvp Complex by Gel Filtration
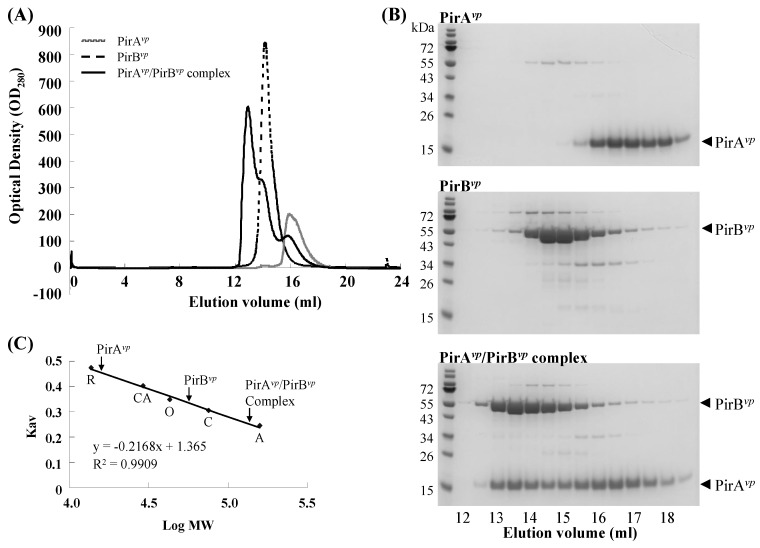
To determine the PirAvp/PirBvp binding ratio, we used gel filtration chromatography to evaluate the native molecular weights of PirAvp, PirBvp and the PirAvp/PirBvp complex. The observed molecular weights of PirAvp and PirBvp were 15.75 kDa and 56.12 kDa, respectively (Figure 2 and Table 2). These values are close to their theoretical molecular weights and suggest that PirAvp and PirBvp both appear as monomers in the solution. The matching theoretical and evaluated molecular weights of PirAvp and PirBvp also indicate that the results of this gel filtration analysis are reliable. We also found that the observed molecular weight of the PirAvp/PirBvp complex (136.08 kDa) was similar to the theoretical molecular weight of the heterodimer/heterodimer interaction (132.59 kDa) (Table 2), suggesting that PirAvp and PirBvp may interact with each other to form a heterotetramer. We note that, while we used a molar ratio of the input PirAvp and PirBvp of more than 1:1 (about 1.7:1), there were still unbound, free-form PirAvp and PirBvp monomers left over, as evidenced by the asymmetry of the PirAvp/PirBvp complex peak (Figure 2A). This result was consistent with our ITC results, which also implied that some of the purified recombinant PirAvp and/or PirBvp failed to interact to form the PirAvp/PirBvp complex. Both experiments therefore suggest that some of the PirAvp and PirBvp might somehow have been rendered inactive. The presence of free-form PirAvp and PirBvp monomers might also have been due to the dissociation of the complex, because as noted above, the PirAvp/PirBvp complex is unstable and its components have a low binding affinity.
Figure 2.
The native molecular weights of the PirAvp, PirBvp and PirAvp/PirBvp complex were estimated by gel filtration analysis. (A,B) Compared to PirAvp and PirBvp, the PirAvp/PirBvp complex has a larger molecular weight and thus appeared in a smaller elution volume. (C) The proteins provided in the gel filtration calibration kits were used to create a plot of Kav against log MW. Using this standard curve, the molecular weights of PirAvp, PirBvp and the PirAvp/PirBvp complex were calculated. A, C, O, CA, and R represented aldolase (158 kDa), conalbumin (75 kDa), ovalbumin (43 kDa), carbonic anhydrate (29 kDa), and ribonuclease A (13.7 kDa), respectively.
Table 2.
A summary of the gel filtration results.
| Protein | Theoretical MW (kDa) | Estimated MW (kDa) |
|---|---|---|
| PirAvp (N-His10) | 15.19 | 15.75 |
| PirBvp (C-His6) | 51.13 | 56.12 |
| PirAvp/PirBvp complex | 132.59 | 136.08 |
We further investigated the possible stoichiometry between PirAvp and PirBvp by using an alternative method. As shown in Figure S1A, two dilution series of known amounts of PirAvp and PirBvp were separated by SDS-PAGEs, and by using Image J software (https://imagej.nih.gov/ij/download.html), the intensities of the protein bands were quantified and used to produce standard curves. Serial dilutions of the complex that was collected from the gel filtration assay described above were separated by another SDS-PAGE (Figure S1B), and the intensities of the protein bands were quantified using the same procedures. In Figure S1B, only Lane 5 contained amounts of PirAvp and PirBvp proteins that were located within the confidence intervals given by the standard curves. The protein quantities in Lane 5 were then used with the observed molecular weights of PirAvp and PirBvp (Table 2) to calculate the number of PirAvp and PirBvp molecules, and the molar ratio of PirAvp and PirBvp was determined as 1.1 (Figure S1C and Table S1). This binding stoichiometry was close to the 2:2 ratio proposed above.
2.3. Determination of the Interface between PirAvp and PirBvp Using Cross-Linking Coupled Mass Spectrometry Analysis
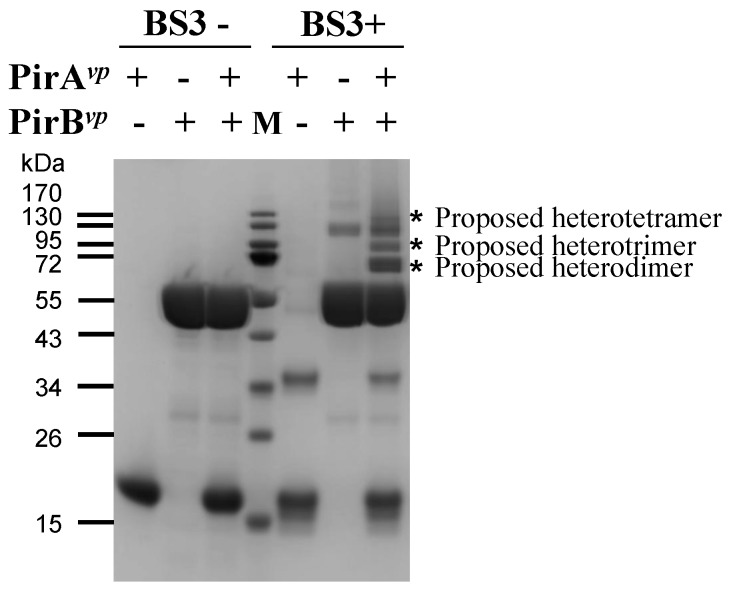
Cross-linking coupled mass spectrometry analysis is widely used as an alternative way to investigate the interaction between macromolecules [13,14,15,16,17,18], and we used this technique to study the interface between PirAvp and PirBvp. The crosslinker used in this assay is bis(sulfosuccinimidyl)suberate BS3, which is an amine-to-amine crosslinker with an arm length of 11.4 Å. BS3 crosslinks the amine groups on two lysines when the distance between them is shorter than BS3′s arm length. Since 11.4 Å is such a short distance, the BS3 crosslinked lysines must be very close to each other, and thus probably part of the interface of the two interacting proteins [16,17].
As shown in Figure 3, shifted protein bands of around 70 kDa, 85 kDa and 140 kDa (asterisks) were found in the lane of the crosslinked PirAvp and PirBvp. These protein bands were close to the theoretical molecular weights of the heterodimer (1 PirAvp/1 PirBvp, 66.31 kDa), heterotrimer (2 PirAvp/1 PirBvp, 81.48 kDa) and heterotetramer (2 PirAvp/2 PirBvp, 132.59 kDa), respectively. This result also suggests that the PirAvp/PirBvp oligomers might be unstable, perhaps because of the low binding affinity. The major protein band (proposed heterodimer) was excised and subjected to in-gel digestion procedures using trypsin plus chymotrypsin. The digested peptides were further analyzed by a NanoLC-nanoESI-MS/MS (LTQ-Orbitrap Elite) coupled with MassMatrix software analysis. The result revealed that the residues Lys 67 and Lys 70 of PirAvp were crosslinked with Lys 394 of PirBvp (Table 3) and implies that these lysine residues should be localized in the dimeric interface of PirAvp and PirBvp.
Figure 3.
PirAvp and PirBvp can form a complex and can be crosslinked by cross-linker BS3. PirAvp, PirBvp and PirAvp + PirBvp were treated with or without BS3. The crosslinked PirAvp and PirBvp shifted to a higher location as indicated by the asterisk. M: protein marker.
Table 3.
Identified crosslinked peptides of the PirAvp and PirBvp proteins.
| Crosslinked Lysine Residues | PP/PP2/PPtag Score | MW (obs) (Da) | MW (Da) | Assigned Peptide Sequence | |
|---|---|---|---|---|---|
| PirAvp Peptide (Chain A) |
PirBvp Peptide (Chain B) |
||||
| PirAvpK67-PirBvpK394 | 29.8/15.4/4.5 | 2749.3991 | 2749.4212 | GAPFMAGGWK(67) | TFVVGENSGK(394)PSVRL |
| PirAvpK70-PirBvpK394 | 61.7/20.4/11.1 | 2637.4449 | 2637.4471 | VAK(70)SHVVQR | TFVVGENSGK(394)PSVR |
| 33.0/18.4/9.6 | 2637.4466 | 2637.4471 | VAK(70)SHVVQR | TFVVGENSGK(394)PSVR | |
| 34.5/14.9/5.2 | 2638.4289 | 2638.4505 | VAK(70)SHVVQR | TFVVGENSGK(394)PSVR | |
| 34.4/14.6/4.4 | 2638.4332 | 2638.4505 | VAK(70)SHVVQR | TFVVGENSGK(394)PSVR | |
2.4. Hydrogen-Deuterium Exchange (HDX) Coupled Mass Spectrometry Analysis of the PirAvp/PirBvp Interface
In addition to the cross-linking mass spectrometry analysis, we also used HDX mass spectrometry to investigate the interface between PirAvp and PirBvp. HDX has been used in structural biology research for nearly 30 years [19], and its principles and other information have been reviewed in detail [20]. The basic principle is that the closer the location of the hydrogen/deuterium to the surface of the molecule, the higher the exchange rate. Conversely, the exchange rate of interacting regions in a protein complex is lower because the participating residues are buried within the complex [20]. Thus, a comparison of the hydrogen–deuterium exchange rates of the PirAvp/PirBvp complex with the exchange rates of PirAvp and PirBvp alone should reveal which areas of PirAvp and PirBvp interact with each other or undergo structural changes during formation of the PirAvp/PirBvp complex. Since the exchange between hydrogen and deuterium was close to equilibrium in the later stages of the reaction (Figure S2), we focused on analyzing the data at 10, 40, and 80 s after the addition of deuterium. Our criteria were as follows: (1) if two-thirds of these three time points showed more than a 1.4-fold difference in the hydrogen–deuterium exchange rates between the single protein and complex, we considered this region to be heavily involved in the PirAvp/PirBvp complex formation; (2) peptides showing a difference of between 1.2–1.4-fold at two or three time points were considered to be interacting regions closer to the surface; (3) peptides with less than 1.2-fold difference were considered not to be involved in the interaction. Results are shown in Table 4. In Figure 4, the interacting regions are colored blue, and the regions not showing significant differences in hydrogen–deuterium exchange rates are colored red.
Table 4.
(A) The PirAvp peptides that were identified in HDX coupled mass spectrometry analysis. (B) The PirBvp peptides that were identified in HDX coupled mass spectrometry analysis.
| (A) | ||||
| Identified Peptide Sequences Derived from PirAvp | Deuterium Incorporation Fold | Classification | ||
| 10 s | 40 s | 80 s | ||
| 2-SNNIKHETDYSHD-14 | 1.2 | 1.0 | 1.0 | Not involved in binding |
| 15-WTVEPNGGVTEVDSKHTPIIPEVGRS-40 | Involved in binding; in the center of the interface or deep within the complex | |||
| 15-WTVEPNGGVTEVDSKHTPIIPEVG-38 | 1.6 | 1.6 | 1.4 | |
| 15-WTVEPNGGVTEVDSKHTPIIPEVGRSVD-42 | 1.5 | 1.5 | 1.4 | |
| 26-VDSKHTPIIPEVGRSVD-42 | 1.7 | 1.8 | 1.5 | |
| 41-VDIENTGRGEL-51 | 1.0 | 1.0 | 1.0 | Not involved in binding |
| 52-TIQYQWGAPFMAGGWKVAKSHVVQRDET-79 | Involved in binding; edge of the interface or near the surface of the complex | |||
| 52-TIQYQWGAPFMAGGWKVAKSHVVQRDET-79 | 1.2 | 1.2 | 1.2 | |
| 66-WKVAKSHVVQRDET-79 | 1.2 | 1.2 | 1.2 | |
| 80-YHLQRPDNAF-89 | 1.1 | 1.1 | 1.2 | Not involved in binding |
| 89-FYHQRIVVINNGASRGF-105 | Not involved in binding | |||
| 89-FYHQRIVVINNGASRG-104 | 1.1 | 1.2 | 1.1 | |
| 90-YHQRIVVINNGASRGF-105 | 1.1 | 1.2 | 1.1 | |
| (B) | ||||
| Identified Peptide Sequences Derived from PirBvp | Deuterium Incorporation Fold | Classification | ||
| 10 s | 40 s | 80 s | ||
| 11-SLTEFNPNNARKSYL-25 | 0.9 | 1.0 | 1.0 | Not involved in binding |
| 36-AFKAMVSFGLSNIPYAGGF-54 | Not involved in binding | |||
| 36-AFKAMVSF-43 | 1.1 | 1.0 | 1.0 | |
| 41-VSFGLSNIPYAGGF-54 | 1.0 | 1.1 | 1.2 | |
| 59-WNIFWPNTPNEPDIE-73 | 1.2 | 0.8 | 0.8 | Not involved in binding |
| 87-VDESIIDAINGILDSKIKETRDKIQDINE-115 | Not involved in binding | |||
| 87-VDESIIDAINGIL-99 | 1.5 | - | 1.7 | |
| 100-DSKIKETRDKIQDINE-115 | 1.1 | 1.1 | 1.2 | |
| 116-TIENFGYAAAKDDYIGL-132 | 1.0 | 0.8 | 0.7 | Exposed after complex formation |
| 178-DYKDEFGFTDSDVHKLTRNIDKL-200 | Not involved in binding | |||
| 178-DYKDEFGFTDSDVHKLTRNIDKL-200 | 1.0 | 1.1 | 1.2 | |
| 185-FTDSDVHKLTRNIDKL-200 | 1.0 | 1.1 | 1.4 | |
| 214-WADNDSYNNANQD-226 | 1.1 | 1.4 | 1.4 | Involved in binding; in the center of the interface or deep within the complex |
| 234-GARSWCTVHGFEHMLIWQKIKELKKVDVFVHSNLISYSPAVGFPSGNF-281 | Not involved in binding | |||
| 234-GARSWCTVHGFEHM-247 | 1.0 | 0.9 | 0.9 | |
| 248-LIWQKIKELKKVDVFVHSNL-267 | 0.9 | 1.0 | 1.1 | |
| 268-ISYSPAVGFPSGNF-281 | 1.1 | 1.1 | 1.2 | |
| 290-DEIPQPLKPNM-300 | 1.4 | 1.4 | 1.3 | Involved in binding; in the center of the interface or deep within the complex |
| 301-FGERRNRIVKIESW-314 | 1.1 | 1.1 | 1.1 | Not involved in binding |
| 322-YNRVGRLKL-330 | 1.3 | 1.6 | 1.8 | Involved in binding; in the center of the interface or deep within the complex |
| 337-VVELGKAHKYDEHYQS-352 | 0.8 | 1.0 | 1.1 | Not involved in binding |
| 375-RIVFHFSDDRT-385 | 0.9 | 0.9 | 0.9 | Not involved in binding |
| 386-FVVGENSGKPSVRLQL-401 | 1.1 | 1.3 | 1.3 | Involved in binding; edge of the interface or near the surface of the complex |
| 409-MLADQEGSDKVAA-421 | Involved in binding; in the center of the interface or deep within the complex | |||
| 409-MLADQEGSDKVAA-421 | 1.3 | 1.9 | 1.8 | |
| 410-LADQEGSDKVAA-421 | 1.5 | 1.8 | 1.9 | |
| 426-YELFHPDEF-434 | 1.5 | 1.2 | 1.0 | Involved in binding; in the center of the interface or deep within the complex |
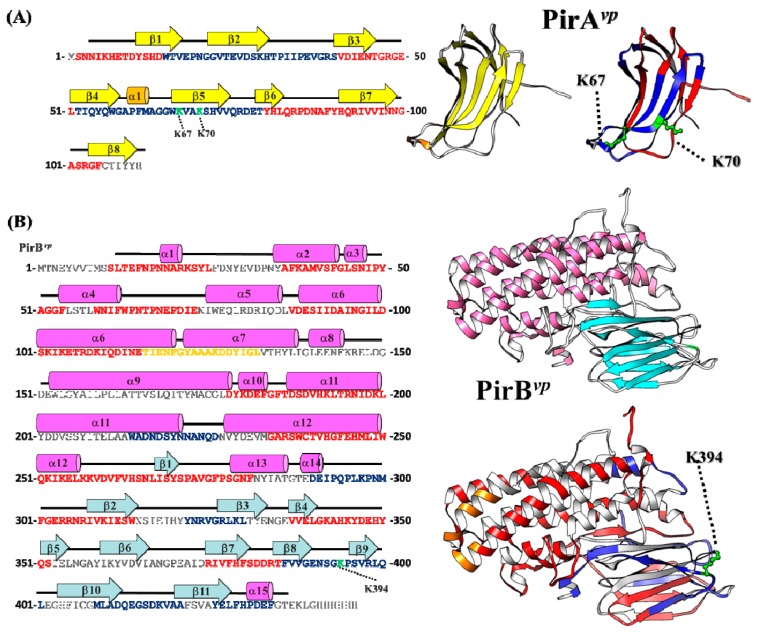
Figure 4.
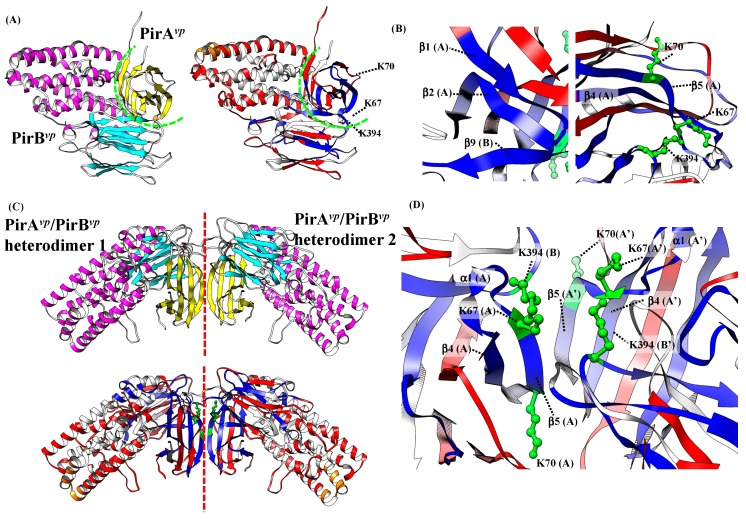
The interacting regions of (A) PirAvp and (B) PirBvp. Based on the BS3-crosslinking and HDX results, the proposed interacting surfaces of PirAvp and PirBvp are shown. (Left) The secondary structural elements of PirAvp and PirBvp are shown above the amino acid sequences. In PirAvp, orange cylinders and yellow arrows represent α-helices and β-sheets, respectively. In PirBvp, magenta cylinders and cyan arrows represent the α-helices and β-sheets. The BS3-crosslinked lysines are colored green. The regions thought to be involved or not involved in the interaction are colored blue and red, respectively. (Right) The crystal structures of PirAvp and PirBvp. The putatively pore-forming domain in the N-terminal region that is thought to become exposed due to conformational changes after formation of the heterodimer is colored orange.
Figure 4A shows that there are two major regions of PirAvp that interact with PirBvp. This model is based on the fact that the peptide 52-TIQYQWGAPFMAGGWK(67)VAK(70)SHVVQRDET-79 from PirAvp showed decreased deuterium incorporation in PirAvp/PirBvp complex compared to stand alone PirAvp (Table 4A), suggesting that this region may be involved in the PirAvp/PirBvp interaction. As mentioned above, this region also includes the Lys 67 and Lys 70 of PirAvp that can be cross-linked with Lys 394 on PirBvp. In addition, the relatively small difference in exchange rate (~1.2X) implies that this region may be located on the edge of the interface between PirAvp and PirBvp, and may not be embedded deeply in the complex. Another PirAvp peptide, 15-WTVEPNGGVTEVDSKHTPIIPEVGRS-40, showed distinctly lower hydrogen–deuterium exchange rates (differences of more than 1.4X), suggesting that this region may also be involved in the interaction between PirAvp and PirBvp and may be embedded deep in the interior of the complex.
Meanwhile, the PirBvp-derived peptide 386-FVVGENSGK(394)PSVRLQL-401 also showed a decreased deuterium incorporation (~1.2X) after the complex formation. This region also contains the Lys 394 that can be crosslinked with PirAvp, which further supports its probable involvement in the interface between PirAvp and PirBvp. The significantly decreased hydrogen-deuterium exchange rates of other PirBvp-derived peptides (214-WADNDSYNNANQD-226, 290-DEIPQPLKPNM-300, 322-YNRVGRLKL-330, 409-MLADQEGSDKVAA-421 and 426-YELFHPDEF-434) in the PirAvp/PirBvp complex imply that these regions too may be involved in the complex formation and/or embedded in the depths of the complex. Most of these peptides are localized on the antiparallel β-sheets (β-8, 9, 10 and 11) found in the C-terminal domain of PirBvp, suggesting that this PirBvp domain mediates the major interaction to PirAvp. It is also worth noting that the hydrogen-deuterium exchange rate of peptide 116-TIENFGYAAAKDDYIGL-132 derived from the N-terminus of PirBvp was progressively increased after complex formation, suggesting that this region may be exposed after PirAvp/PirBvp interaction (Figure 4). Since the N-terminus of PirBvp was similar to the pore-forming domain I of Cry toxin [10,12], this exposed region (colored orange in Figure 4B) perhaps acts as a fusion peptide that participates in membrane insertion or pore formation on the host cell membrane.
2.5. Proposed PirAvp/PirBvp Binding Model Using Cross-Linking Coupled Mass Spectrometry and HDX Analysis
We next used the information from cross-linking coupled mass spectrometry and HDX analysis to generate a PirAvp/PirBvp binding model. After using the Z-DOCK server (http://zdock.umassmed.edu/), to generate possible models based on the three cross-linked lysines, these candidate models were evaluated in terms of the HDX analysis. Figure 5A shows a proposed PirAvp/PirBvp heterodimer that was the best fit to these requirements. In this heterodimeric model, the β-sheet 2 of PirAvp (20-GGVTEDSKH-30) was predicted to interact to β-sheet 9 region of PirBvp (395- PSVRLQ-400) (Figure 5B, left). Additionally, the β-sheet 5 of PirAvp region (66-WKVAKSHV-73) is close to the loop region between β-sheet 8 and 9 of PirBvp (390-ENSGKP-395), which suggests an interaction between them (Figure 5B; right). Notably, in this proposed PirAvp/PirBvp heterodimer, there are still some available binding regions on PirAvp (i.e., the β-sheet 4-α-helix 1-β-sheet 5 regions of PirAvp; 52-TIQYQWGAPFMAGGWKVAKSHVVQRDET-79, as determined by HDX analysis). These regions may be involved in higher orders of PirAvp/PirBvp oligomeric formation.
Figure 5.
Proposed PirAvp/PirBvp binding mode. (A) Proposed PirAvp/PirBvp heterodimer. The green dotted line indicates the heterodimeric interface between PirAvp and PirBvp. (B) Two regions that may be involved in the formation of the PirAvp/PirBvp heterodimer. (C) Proposed PirAvp/PirBvp heterotetramer. The red dotted line indicates the interface between the two PirAvp/PirBvp heterodimers. (D) Details of the possible interface of the proposed PirAvp/PirBvp heterotetramer. In this model, the binding region between the two heterodimers depends on the interaction of the â-sheet 4-α-helix 1-â-sheet 5 regions of the two PirAvp proteins.
In the gel filtration analysis (Figure 2), we found that PirAvp and PirBvp form a tetramer in solution. The proposed PirAvp/PirBvp heterodimer was subsequently used to predict their tetrameric conformation. A predicted PirAvp/PirBvp heterotetramer that uses a similar docking strategy is shown in Figure 5C. In the resulting PirAvp/PirBvp heterotetramer, two PirAvp/PirBvp heterodimers bind to each other by using the β-sheet 4-α-helix 1-β-sheet 5 regions of PirAvp (52-TIQYQWGAPFMAGGWKVAKSHVVQRDET-79) (Figure 5D).
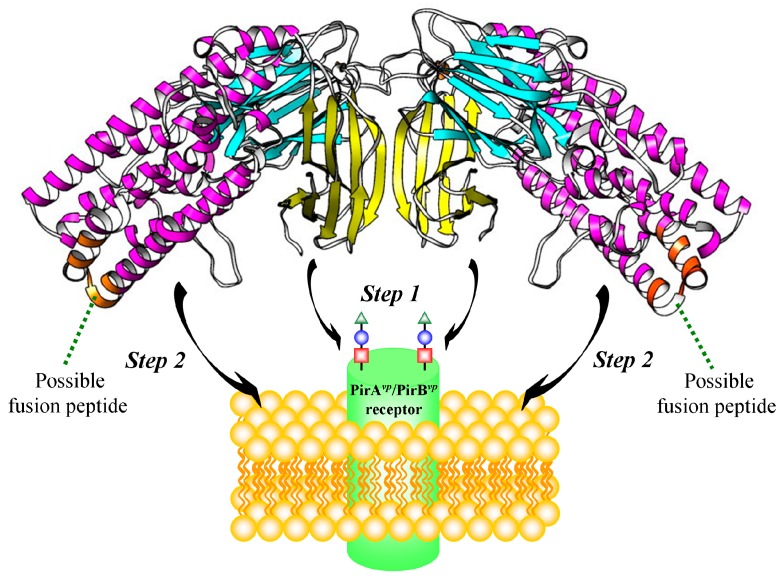
Since the structural topology of PirAvp/PirBvp complex is very similar to Cry toxins [10], we speculate that PirAvp/PirBvp toxin might use a similar mechanism to damage host cells. For example, during the pore-formation by Cry 1A toxin, the GalNAc sugar on the aminopeptidaseN (APN) receptor is firstly recognized by Cry domain III, and the receptor is bound by Cry domain II [21,22,23]. Subsequently, this receptor-bound Cry toxin undergoes a proteolytic cleavage which induces the oligomerization of Cry toxin, and finally, the toxin inserts to cell membrane and forms a pore on the membrane [21,22,23]. According to the proposed heterotetramer structure, the PirAvp/PirBvp heterodimer may form a heterotetramer through PirAvp-PirAvp interaction (Figure 5C). The other side of PirAvp may participate in receptor recognition and binding. After the receptor binding, and possibly after the PirAvp/PirBvp complex forms a higher-order oligomer, PirBvp may be pulled toward the cell membrane, where it inserts into the membrane using its α-helix to efficiently form a transmembrane pore (Figure 6). We noted above that the low binding affinity between PirAvp and PirBvp may directly affect the stability of the heterotetramer. However, the stability may be improved after the PirAvp/PirBvp complex recognizes and interacts with its receptor. In addition, all of our experiments were performed in the absence of any membranes, and it is possible that, as suggested above, after the PirAvp/PirBvp complexes undergo receptor recognition/binding with an actual cell membrane, the heterotetramer might form a larger oligomer and its subunit stoichiometry may even be different from that of the free complex.
Figure 6.
Schematic representation of the proposed binding mechanism of the heterotetrameric PirAvp/PirBvp toxin with its receptor. The PirAvp/PirBvp heterotetramer first uses PirAvp to recognize and bind with a receptor on the host cell membrane (Step 1), after which the newly-exposed N-terminus region of PirBvp (orange) is pulled toward the cell membrane (Step 2) where it inserts into the membrane using its α-helix and initiates the process of pore formation.
Since both PirAvp and PirBvp have been reported as secreted proteins [10,24], they are therefore good targets for anti-AHPND drug design. For pore-forming toxins, structural insights into the protein components can provide useful information on conformation rearrangement, the binding interface between receptor and ligand, and the oligomerization of the toxin [25]. Depending on the structural information, different strategies can be used to prevent pore formation. For example, in developing a drug to treat infection with multidrug-resistant strains of Staphylococcus aureus, the interaction of natural compounds (e.g., oroxylin A, oroxin A and oroxin B) with S. aureus Hla may prevent the loop transition during the pore formation, and therefore inhibit the haemolytic activity of Hla [26,27]; an antibody against the receptor binding domains of S. aureus Hla also prevents the toxin from recognizing the cell membrane and blocks its binding with the receptor [28]. Meanwhile, as part of a strategy against necrotic enteritis caused by Clostridium perfringens, recombinant toxoids can be used as vaccines that trigger immune responses, and structural information on the toxin or pore architecture will be helpful in the design of site-directed mutations [29,30]. In the case of PirAvp/PirBvp, based on the interface and heterotetrameric binding model proposed here, another possible option is to use in silico screening to identify small compounds that may be able to block the interaction of PirAvp and PirBvp. Further, the structural biology approach used here should be useful not only for designing anti-AHPND strategies in the future, but also as a platform to study other Pir toxins and provide structural insights that can be applied to the control of other pests or vector mosquitoes.
3. Materials and Methods
3.1. Construction and Recombinant Protein Purification
The coding regions of pirAvp and pirBvp (accession no. KP324996, regions 35028 to 35363 and 33699 to 35015, respectively) were constructed into pET16b and pET21b vectors, and the resulting recombinant plasmids were named pirAvp-pET16b and pirBvp-pET21b, respectively. These plasmids were then transformed into E. coli BL21(DE3) strain to express the N-terminal tagged His10-PirAvp and the C-terminal tagged His6-PirBvp. The cultures of pirAvp-pET16b or pirBvp-pET21b transformed BL21(DE3) were incubated overnight and then subcultured into fresh LB medium with dilution ratios of 1:250 and 1:50, respectively. The cultures were then grown at 37 °C until the OD600 reached to 0.4. To induce protein expression, IPTG was added to a final concentration of 1 mM and the cultures were incubated at 16 °C for 20 h. The cells were then collected and subjected to protein purification procedures. For the N-terminal tagged His10-PirAvp, cells were resuspended with lysis buffer (20 mM Tris, pH 7.4, 100 mM NaCl, 20 mM imidazole, 100 μg/mL lysozyme, 10 μg/mL DNase I, 1 mM PMSF) and homogenized with sonication. After removing the cell debris, the supernatant was filtered through a 0.45 μm filter and loaded onto a 5 mL HisTrap HP column (GE Healthcare, Chicago, IL, USA). The column was washed with wash buffer (20 mM Tris, pH 7.4, 100 mM NaCl, 20 mM imidazole), and the His-tagged protein was eluted with a imidazole gradient (from 20 mM to 500 mM). The eluted His10-PirAvp was further purified with a Superdex 75 column using the gel filtration buffer (30 mM Tris, pH 7.4, 100 mM NaCl, 1 mM DTT, 5% glycerol). The C-terminal tagged His6-PirBvp protein was purified using the same procedures, except that the buffers were as follows: lysis buffer (20 mM Tris, pH 8.0, 300 mM NaCl, 20 mM imidazole, 100 μg/mL lysozyme, 10 μg/mL DNase I, 1 mM PMSF), wash buffer (20 mM Tris, pH 8.0, 300 mM NaCl, 20 mM imidazole), elution buffer (20 mM Tris, pH 8.0, 300 mM NaCl, 20–500 mM imidazole), and gel filtration buffer (20 mM Tris, pH 8.0, 300 mM NaCl). Protein concentrations were determined by the Bradford method.
3.2. Determination of the Binding Affinity between PirAvp and PirBvp by Isothermal Titration Calorimetry (ITC)
After the N-terminal tagged His10-PirAvp and the C-terminal tagged His6-PirBvp were both dialyzed against the same reaction buffer (30 mM Tris, pH8.0, 100 mM NaCl, 5% glycerol, 1mM DTT), they were respectively loaded into the sample cell and syringe of an iTC200 (GE Healthcare) instrument. For titration, 2 μL of 1100 μM PirBvp was injected into the cell containing 110 μM PirAvp every 150 s for a total of 19 injections at 25 °C. All data except the first injection point were included to calculate the thermodynamic parameters. The enthalpy (ΔH), binding entropy (ΔS), equilibrium constant (1/Kd), and stoichiometric ratio (N) were obtained by using the computer software ORIGIN 7 (GE Healthcare, Chicago, IL, USA, 2002). Gibbs free energy (ΔG) was calculated using the equation: ΔG =ΔH − TΔS.
3.3. Determination of the Native Molecular Weights of PirAvp, PirBvp and PirAvp/PirBvp Complex by Using Gel Filtration
For the single proteins, N-terminal tagged His10-PirAvp or C-terminal tagged His6-PirBvp were separately diluted with the reaction buffer (30 mM Tris, pH8.0, 100 mM NaCl, 5% glycerol, 1mM DTT) to a final concentrations of 65.83 μM (1 mg/mL) and 39.12 μM (2 mg/mL), respectively. The proteins were then loaded onto an Enrich SEC 650 10 × 300 column (BioRad, Hercules, CA, USA), eluted with reaction buffer, and a continuous series of 0.5 mL eluted samples were collected. For the PirAvp/PirBvp complex, N-terminal tagged His10-PirAvp and C-terminal tagged His6-PirBvp were mixed in reaction buffer to final concentrations of 65.83 μM (1 mg/mL) and 39.12 μM (2 mg/mL), respectively, incubated at 25 °C for 15 min and analyzed with the same column under the same conditions. The fractions corresponding to the peaks in the diagrams were analyzed with SDS-PAGE. To create a standard curve, native proteins from two gel filtration calibration kits (GE Healthcare), including aldolase (A; 158 kDa), conalbumin (C; 75 kDa), ovalbumin (O; 43 kDa), carbonic anhydrate (CA; 29 kDa), and ribonuclease A (R; 13.7 kDa) were also analyzed with the same column, and the logarithm of molecular weight (log MW) of each protein was plotted against its Kav using the calculation:
| Kav = (Ve − Vo)/(Vt − Vo) | (1) |
where Ve is the elution volume, Vo is the column void volume (determined using blue dextran 2000), and Vt is the total column bed volume (24 mL for the Enrich SEC 650 10 × 300 column).
3.4. Determination of the Binding Stoichiometry of PirAvp and PirBvp by Densitometric Analysis
To achieve this analysis, we first collected the PirAvp/PirBvp complex from the gel filtration fractions that eluted at 12.5 to 13 mL to avoid any possible contamination of the PirBvp monomer. The complex sample was then serially diluted and separated in SDS-PAGE. Different quantities of PirAvp (1–6 μg) and PirBvp (2–8 μg) were also separated by SDS-PAGE. Computer software Image J (https://imagej.nih.gov/ij/download.html) was used to quantify the signal intensities of all of the protein bands. Standard curves were generated for PirAvp and PirBvp and used to calculate the amounts of PirAvp and PirBvp that dissociated from the PirAvp/PirBvp complex. Only serially diluted complex samples for which the amount of protein of both PirAvp and PirBvp were located within the confidence intervals were used for these calculations. Finally, the amounts of PirAvp and PirBvp were divided by their observed molecular weights (as obtained from the gel filtration analysis) to give the molar ratio of PirAvp and PirBvp in the complex.
3.5. Cross-Linking Coupled Mass Spectrometry Analysis of PirAvp and PirBvp
Before being subjected to a cross-linking reaction, the recombinant PirAvp and PirBvp were dialyzed against 1X PBS to remove Tris, the presence of which can inhibit the activity of bissulfosuccinimidyl suberate (BS3). PirAvp was then mixed with PirBvp in PBS at a concentration of 13.2 μM each. To serve as a control, PirAvp and PirBvp were also diluted with PBS separately. After incubating for 15 min at 25 °C, BS3 was added to the mixtures to a final concentration of 1 mM. The mixtures were then incubated at 25 °C for an additional 60 min and separated in SDS-PAGE. As loading controls, the PirAvp, PirBvp and PirAvp + PirBvp were incubated without BS3 and separated with the same SDS-PAGE.
For mass spectrometry analysis, the shifted bands of the crosslinked PirAvp and PirBvp were excised from the SDS-PAGE and digested with trypsin coupled with chymotrypsin in accordance with standard in-gel digestion procedures. The digested peptides were then analyzed with a NanoLC-nanoESI-MS/MS (LTQ-Orbitrap Elite, Thermo Fisher Scientific, Waltham, MA, USA) using the standard protocol of the Common Mass Spectrometry Facilities of the Institute of Biological Chemistry at Academia Sinica [31,32] and subjected to data analysis using the Massmatrix software [33].
3.6. Hydrogen-Deuterium Exchange (HDX) Mass Spectrometry Analysis of PirAvp and PirBvp
To initiate the hydrogen–deuterium exchange, the recombinant PirAvp, PirBvp (15 pmol each) and PirAvp/PirBvp protein complex (15 pmol: 15pmol) were separately diluted in the exchange buffer (99.9% D2O in PBS, pH 7.4) at a ratio of 1:10 at room temperature. To quench the HD exchange, 3.5 μL sample (1.5 pmol of target protein) was mixed with 6.5 μL pre-chilled quenching buffer [to a final concentration of 1.5 M guanidine hydrochloride, 150 mM tris(2-carboxyethyl)phosphine, and 0.8% formic acid] at 10, 40, 80, 180, 600, 1800, and 3600 s after deuterium was added. The mixture was immediately loaded onto a pepsin column for online digestion, and the digested peptides were then desalted using a reverse-phase column (Zorbax 300SB-C18, 0.3 × 5 mm; Agilent Technologies, Wilmington, DE, USdA). The desalted peptides were further separated on a customized HydroRP column using a linear gradient of 8–95% HPLC buffer (99.9% acetonitrile, 0.1% formic acid, 0.025% trifluoroacetic acid) for 14.5 min with a flow rate of 0.5 μL/min. The LC apparatus was coupled with a 2D linear ion trap mass spectrometer (Orbitrap Classic; Thermo Fisher Scientific, Waltham, MA, USA) and the full-scan MS was performed in the Orbitrap over a m/z range of 350 to 1600 Da and a resolution of 60,000 at m/z 400. The ion signal of [Si(CH3)2O]6H+ at m/z 536.165365 was served as the lock mass for internal calibration. The peptides were ionized at an electrospray voltage of 1.8 kV, and the temperature of the capillary was set to 200 °C. To control the accumulated time or ions, the automatic gain control of MS and MS/MS were 1,000 ms (full scan) and 150 ms (MS/MS), or 1 × 106 ions (full scan) and 2 × 103 ions (MS/MS), respectively.
3.7. Peptide Identification and HDX Data Analysis
The computer software Proteome Discoverer (Version 1.4, Thermo Fisher Scientific, Waltham, MA, USA, 2012) was used for peptide identification, and the SEQUEST search engine was used for the MS/MS spectra searching against the single protein database (i.e., PirAvp or PirBvp). For peptide identification, the mass tolerance was 10 ppm for intact peptide masses and 0.5 Da for CID fragment ions. Peptide-spectrum matches (PSM) were then filtered based on high confidence and a peptide identification search engine rank of 1 to ensure an overall false discovery rate below 0.01. For HDX profile analysis, the peptide identification template was first generated based on the LC-MS/MS result of target protein identification. The template was then preloaded in ExMS module installed in the MATLAB environment. To calculate the deuterium atom number in each peptide, the HDX MS spectra were loaded and analyzed. The result was then presented as an average value of deuterium incorporation based on two independent experiments.
3.8. Molecular Docking Analysis of PirAvp/PirBvp Complex
To identify the binding mode of PirAvp and PirBvp, we performed a protein-protein docking analysis. First, potential binding poses were generated by uploading the crystal structures of the two proteins to the ZDOCK server (http://zdock.umassmed.edu/) [34]. A PirAvp/PirBvp complex that satisfied the cross-linking distance criteria from the predicted poses was selected as the binding mode. Next, the complex structure was uploaded to the ZDOCK server to generate potential binding poses for the PirAvp/PirBvp heterodimer complexes. Finally, the complex structure was evaluated by the HDX results: the binding regions where the hydroge-deuterium exchange rate became lower after PirAvp/PirBvp complex formation should be found inside the complex, while regions where the exchange rate was unchanged should not be involved in the interaction. The resulting pdb files of PirAvp/PirBvp heterodimer and heterotetramer were included in the supporting files for a reference.
Acknowledgments
We thank Shu-Chuan Jao of the Biophysics Core Facility, Department of Academic Affairs and Instrument Service at Academia Sinica for her suggestions in ITC analysis. Cross-linking coupled mass spectrometry analysis was assisted by Shu-Yu Lin of the Academia Sinica Common Mass Spectrometry Facilities located at the Institute of Biological Chemistry. We are indebted to Paul Barlow for his helpful comments on the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6651/11/4/233/s1, Figure S1: Calculation of the stoichiometry of PirAvp and PirBvp by using SDS-PAGE coupled with densitometric analysis. Figure S2: HDX profiles.doc, PDB files: PirAB dimer model.pdb and PirAB tetramer model.pdb. Table S1: Calculation of the molar ratio of PirAvp / PirBvp in Lane 5 of Figure S2B.
Author Contributions
Conceptualization, Y.-F.C., C.-F.L. and H.-C.W. (Hao-Ching Wang); Data curation, S.-J.L. and H.-C.W. (Hao-Ching Wang); Formal analysis, S.-J.L., K.-C.H. and H.-C.W. (Hao-Ching Wang); Funding acquisition, C.-F.L. and H.-C.W. (Hao-Ching Wang); Investigation, S.-J.L. and Y.-L.C.; Methodology, H.-C.W. (Hao-Ching Wang); Project administration, C.-F.L. and H.-C.W. (Hao-Ching Wang); Resources, C.-F.L. and H.-C.W. (Hao-Ching Wang); Software, K.-C.H. and H.-C.W. (Hao-Ching Wang); Supervision, C.-F.L. and H.-C.W. (Hao-Ching Wang); Writing–original draft, S.-J.L. and H.-C.W. (Hao-Ching Wang); Writing–review & editing, S.-J.L., Y.-F.C., T.-P.K., H.-C.W. (Han-Ching Wang) and H.-C.W. (Hao-Ching Wang).
Funding
This research was funded by Ministry of Science and Technology (grant numbers: MOST 107-2313-B-038-001, MOST 105-2313-B-006-003-MY3, MOST 106-2633-B-006-004, MOST 107-3017-F-006-001, MOST 108-3017-F-006-001). This work was also financially supported by the “International Center for the Scientific Development of Shrimp Aquaculture” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Conflicts of Interest
The authors declare no conflicts of interest.
Key Contribution
The model of this marine bacterial binary toxin PirAvp/PirBvp not only elucidates the interface between PirAvp and PirBvp, but also suggests that two PirAvp/PirBvp heterodimers may form a higher order heterotetramer through PirAvp-PirAvp interaction, and the toxin may recognize and bind to the receptor via the other side of PirAvp. After receptor binding, PirBvp may be pulled toward the cell membrane, where it is inserted into the membrane via the newly exposed α-helix upon PirAvp interaction, initiating the pore formation process. The structure-function relationship should be useful for developing anti-AHPND strategies in the future.
References
- 1.Tran L., Nunan L., Redman R., Mohney L., Pantoja C., Fitzsimmons K., Lightner D. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013;105:45–55. doi: 10.3354/dao02621. [DOI] [PubMed] [Google Scholar]
- 2.Lightner D.V., Redman R.M., Pantoja C.R., Noble B.L., Tran L. Early mortality syndrome affects shrimp in Asia. Glob. Aquac. Advocate. 2012;15:40. [Google Scholar]
- 3.Gomez-Gil B., Soto-Rodríguez S., Lozano R., Betancourt-Lozano M. Draft genome sequence of Vibrio parahaemolyticus Strain M0605, which causes severe mortalities of shrimps in Mexico. Genome Announc. 2014;2:e00055-14. doi: 10.1128/genomeA.00055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soto-Rodriguez S.A., Gomez-Gil B., Lozano-Olvera R., Betancourt-Lozano M., Morales-Covarrubias M.S. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp (Litopenaeus vannamei) in Northwestern Mexico. Appl. Environ. Microbiol. 2015;81:1689–1699. doi: 10.1128/AEM.03610-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sirikharin R., Taengchaiyaphum S., Sanguanrut P., Chi T.D., Mavichak R., Proespraiwong P., Nuangsaeng B., Thitamadee S., Flegel T.W., Sritunyalucksana K. Characterization and PCR detection of binary, Pir-Like toxins from Vibrio parahaemolyticus isolates that cause acute hepatopancreatic necrosis disease (AHPND) in shrimp. PLoS ONE. 2015;10:e0126987. doi: 10.1371/journal.pone.0126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kondo H., Van P.T., Dang L.T., Hirono I. Draft genome sequence of non-Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome Announc. 2015;3:e00978-15. doi: 10.1128/genomeA.00978-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dong X., Wang H., Xie G., Zou P., Guo C., Liang Y., Huang J. An isolate of Vibrio campbellii carrying the pirVP gene causes acute hepatopancreatic necrosis disease. Emerg. Microbes Infect. 2017;6:e2. doi: 10.1038/emi.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L., Xiao J., Xia X., Pan Y., Yan S., Wang Y. Draft genome sequence of Vibrio owensii strain SH-14, which causes shrimp acute hepatopancreatic necrosis disease. Genome Announc. 2015;3:e01395-e15. doi: 10.1128/genomeA.01395-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Restrepo L., Bayot B., Arciniegas S., Bajaña L., Betancourt I., Panchana F., Muñoz A.R. PirVP genes causing AHPND identified in a new Vibrio species (Vibrio punensis) within the commensal Orientalis clade. Sci. Rep. 2018;8:13080. doi: 10.1038/s41598-018-30903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee C.T., Chen I.T., Yang Y.T., Ko T.P., Huang Y.T., Huang J.Y., Huang M.F., Lin S.J., Chen C.Y., Lin S.S., et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA. 2015;112:10798–10803. doi: 10.1073/pnas.1503129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durán-Avelar M.J., Vázquez-Reyes A., González-Mercado A.L., Zambrano-Zaragoza J.F., Ayón-Pérez M.F., Agraz-Cibrián J.M., Gutiérrez-Franco J., Vibanco-Pérez N. pirA- and pirB-like gene identification in Micrococcus luteus strains in Mexico. J. Fish Dis. 2018;41:1667–1673. doi: 10.1111/jfd.12874. [DOI] [PubMed] [Google Scholar]
- 12.Lin S.J., Hsu K.C., Wang H.C. Structural insights into the cytotoxic mechanism of Vibrio parahaemolyticus PirAvp and PirBvp toxins. Mar. Drugs. 2017;15:373. doi: 10.3390/md15120373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rappsilber J., Siniossoglou S., Hurt E.C., Mann M. A generic strategy to analyze the spatial organization of multi-protein complexes by cross-linking and mass spectrometry. Anal. Chem. 2000;72:267–275. doi: 10.1021/ac991081o. [DOI] [PubMed] [Google Scholar]
- 14.Denison C., Kodadek T. Toward a general chemical method for rapidly mapping multi-protein complexes. J. Proteome Res. 2004;3:417–425. doi: 10.1021/pr034071j. [DOI] [PubMed] [Google Scholar]
- 15.Sharon M., Taverner T., Ambroggio X.I., Deshaies R.J., Robinson C.V. Structural organization of the 19S proteasome lid: Insights from MS of intact complexes. PLoS. Biol. 2006;4:e267. doi: 10.1371/journal.pbio.0040267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guilliam T.A., Bailey L.J., Brissett N.C., Doherty A.J. PolDIP2 interacts with human PrimPol and enhances its DNA polymerase activities. Nucleic Acids Res. 2016;44:3317–3329. doi: 10.1093/nar/gkw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komolov K.E., Du Y., Duc N.M., Betz R.M., Rodrigues J.P.G.L.M., Leib R.D., Patra D., Skiniotis G., Adams C.M., Dror R.O., et al. Structural and Functional Analysis of a β2-Adrenergic Receptor Complex with GRK5. Cell. 2017;169:407–421. doi: 10.1016/j.cell.2017.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisz D.A., Liu H., Zhang H., Thangapandian S., Tajkhorshid E., Gross M.L., Pakrasi H.B. Mass spectrometry-based crosslinking study shows that the Psb28 protein binds to cytochrome b559 in Photosystem II. Proc. Natl. Acad. Sci. USA. 2017;114:2224–2229. doi: 10.1073/pnas.1620360114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paterson Y., Englander S.W., Roder H. An antibody binding site on cytochrome c defined by hydrogen exchange and two-dimensional NMR. Science. 1990;249:755–759. doi: 10.1126/science.1697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oganesyan I., Lento C., Wilson D.J. Contemporary hydrogen deuterium exchange mass spectrometry. Methods. 2018;144:27–42. doi: 10.1016/j.ymeth.2018.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Soberón M., Pardo L., Muñóz-Garay C., Sánchez J., Gómez I., Porta H., Bravo A. Pore formation by Cry toxins. Adv. Exp. Med. Biol. 2010;677:127–142. doi: 10.1007/978-1-4419-6327-7_11. [DOI] [PubMed] [Google Scholar]
- 22.Xu C., Wang B.C., Yu Z., Sun M. Structural insights into Bacillus thuringiensis Cry, Cyt and parasporin toxins. Toxins. 2014;6:2732–2770. doi: 10.3390/toxins6092732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins J.L., Lee M.K., Valaitis A.P., Curtiss A., Dean D.H. Bivalent sequential binding model of a Bacillus thuringiensis toxin to gypsy moth aminopeptidase N receptor. J. Biol. Chem. 2000;275:14423–14431. doi: 10.1074/jbc.275.19.14423. [DOI] [PubMed] [Google Scholar]
- 24.Tinwongger S., Nochiri Y., Thawonsuwan J., Nozaki R., Kondo H., Awasthi S.P., Hinenoya A., Yamasaki S., Hirono I. Virulence of acute hepatopancreatic necrosis disease PirAB-like relies on secreted proteins not on gene copy number. J. Appl. Microbiol. 2016;121:1755–1765. doi: 10.1111/jam.13256. [DOI] [PubMed] [Google Scholar]
- 25.Dal Peraro M., van der Goot F.G. Pore-forming toxins: Ancient, but never really out of fashion. Nat. Rev. Microbiol. 2016;14:77–92. doi: 10.1038/nrmicro.2015.3. [DOI] [PubMed] [Google Scholar]
- 26.Dong J., Qiu J., Zhang Y., Lu C., Dai X., Wang J., Li H., Wang X., Tan W., Luo M., et al. Oroxylin A inhibits hemolysis via hindering the self-assembly of α-hemolysin heptameric transmembrane pore. PLoS Comput. Biol. 2013;9:e1002869. doi: 10.1371/journal.pcbi.1002869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu J., Wang D., Zhang Y., Dong J., Wang J., Niu X. Molecular modeling reveals the novel inhibition mechanism and binding mode of three natural compounds to staphylococcal α-hemolysin. PLoS ONE. 2013;8:e80197. doi: 10.1371/journal.pone.0080197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foletti D., Strop P., Shaughnessy L., Hasa-Moreno A., Casas M.G., Russell M., Bee C., Wu S., Pham A., Zeng Z., et al. Mechanism of action and in vivo efficacy of a human-derived antibody against Staphylococcus aureus á-hemolysin. J. Mol. Biol. 2013;425:1641–1654. doi: 10.1016/j.jmb.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Fernandes da Costa S.P., Savva C.G., Bokori-Brown M., Naylor C.E., Moss D.S., Basak A.K., Titball R.W. Identification of a key residue for oligomerisation and pore-formation of Clostridium perfringens NetB. Toxins. 2014;6:1049–1061. doi: 10.3390/toxins6031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bokori-Brown M., Hall C.A., Vance C., Fernandes da Costa S.P., Savva C.G., Naylor C.E., Cole A.R., Basak A.K., Moss D.S., Titball R.W. Clostridium perfringens epsilon toxin mutant Y30A-Y196A as a recombinant vaccine candidate against enterotoxemia. Vaccine. 2014;32:2682–2687. doi: 10.1016/j.vaccine.2014.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H.L., Chiang I.C., Liang S.Y., Lee D.Y., Chang G.D., Wang K.Y., Lin S.Y., Shih Y.L. Quantitative proteomics analysis reveals the min system of Escherichia coli modulates reversible protein association with the inner membrane. Mol. Cell Proteomics. 2016;15:1572–1583. doi: 10.1074/mcp.M115.053603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naveen V., Hsiao C.D. NrdR Transcription regulation: Global proteome analysis and its role in Escherichia coli viability and virulence. PLoS ONE. 2016;11:e0157165. doi: 10.1371/journal.pone.0157165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H., Freitas M.A. MassMatrix: A database search program for rapid characterization of proteins and peptides from tandem mass spectrometry data. Proteomics. 2009;9:1548–1555. doi: 10.1002/pmic.200700322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pierce B.G., Wiehe K., Hwang H., Kim B.H., Vreven T., Weng Z. ZDOCK server: Interactive docking prediction of protein-protein complexes and symmetric multimers. Bioinformatics. 2014;30:1771–1773. doi: 10.1093/bioinformatics/btu097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.