Abstract
Autophagy as a primary homeostatic and catabolic process is responsible for the degradation and recycling of proteins and cellular components. The mechanism of autophagy has a crucial role in several cellular functions and its dysregulation is associated with tumorigenesis, tumor–stroma interactions, and resistance to cancer therapy. A growing body of evidence suggests that autophagy is also a key regulator of the tumor microenvironment and cellular immune response in different types of cancer, including colorectal cancer (CRC). Furthermore, autophagy is responsible for initiating the immune response especially when it precedes cell death. However, the role of autophagy in CRC and the tumor microenvironment remains controversial. In this review, we identify the role of autophagy in tumor microenvironment regulation and the specific mechanism by which autophagy is implicated in immune responses during CRC tumorigenesis and the context of anticancer therapy.
Keywords: Autophagy, colorectal cancer, immunotherapy, tumor stroma, tumor microenvironment
1. Introduction
Colorectal cancer (CRC) is the third most frequently diagnosed malignancy and the second leading cause of cancer-related deaths in the U.S.A. and worldwide [1]. By 2030, the estimated global burden of CRC is expected to reach more than 2.2 million new cases and 1.1 million deaths [2]. Despite significant advances in standard of care therapies, the 5-year survival rate for patients diagnosed with metastatic CRC remains very poor, at approximately 12% [1]. Among others, autophagy is a major mechanism which is strongly associated with tumorigenesis in different types of cancer, including CRC.
The mechanism of autophagy has been identified as a catabolic process with an essential role to digest proteins and dysfunctional cellular organelles [3]. Numerous steps related to autophagy include membrane trafficking vesicles, essential autophagy proteins, a double membrane organelle, which is called an autophagosome, and fusion with lysosomes to create the autophagolysosome. Autophagolysosome is a fundamental structure responsible for degrading the luminal content [4]. The role of autophagy is extended from cellular homeostasis to tumor development [5,6].
Many genes and proteins are crucial for the initiation and progression of autophagy. Genes, like Beclin-1, LC3, ATG5, and ATG6, have a crucial role for autophagy from normal function to CRC, where these genes have been reported with high expression. Furthermore, these autophagy gene-markers are associated with a more aggressive CRC phenotype [7].
Various morphological changes characterize the autophagy process. In the first step of autophagy, which is called initiation or nucleation, the phagophore, a double membrane structure, is formed through the activation of the class-III PI3K-Beclin-1 complex. Elongation is the next step in the autophagy process. This step is characterized by the arising of the phagophore from different double membrane organelles, such as the Endoplasmic Reticulum (ER), Golgi, and mitochondria. The phagophore starts to enclose the cytosolic cargos, leading to the formation of the autophagosome. The formation of the phagophore is highlighted by different Atgs, p62/ SQSTM1 (an adaptor protein responsible for the docking of specific cargoes), and the lipid-modification of LC3I to LC3II. The maturation step and the following fuse step include the autophagosome formation, which eventually fuses with lysosomes to form autolysosomes. Finally, during the degradation step, lysosomal/vacuolar hydrolases digest autolysosomal products and release them in the cytosol [4].
Over the last years, many studies have been conducted that support the dual role of autophagy in CRC. Autophagy appears to be responsible for maintaining the energy homeostasis in cells, which is required for several cellular functions, such as proliferation [8], angiogenesis, migration [9], and EMT (epithelial-mesenchymal transition) phenotype [10]. Autophagy is identified to be upregulated in a hypoxic region of already established tumors, where the energy demands are increased [11]. Moreover, cancer cells of high graded tumors appear to be addicted to autophagy to maintain their energy balance [12,13]. Numerous studies report the impact of autophagy in cancer patients’ response to chemotherapy. Increasing levels of autophagy are linked with inadequate response to chemotherapeutic drugs and dismal survival rates [14].
In different cancer types, such as CRC, a single-nucleotide polymorphism, in autophagy-related genes, like ATG16L1, is associated with a reduction of autophagy and a significant negative predictive value for patients’ survival with metastatic disease [15,16]. Besides, monoallelic deletion of other crucial autophagy genes, such as Beclin-1, which leads to autophagy reduction, has been identified in several diseases, such as cancer and Alzheimer’s [17,18,19]. Other studies highlight the positive impact of monoallelic deletion or total loss of other genes, such as ATG5, ATG7, and ATG4C, in cancer development [20]. In addition, KRAS, an essential oncogene in CRC development, is strongly associated with autophagy [21]. Cancer cells of KRAS-dependent tumors use autophagy in order to support the growth of cancer cells under stressful conditions in hypoxic regions of tumors [8]. All these studies highlight the dual role of autophagy as a tumor promoter or tumor suppressor mechanism. The accumulation of dysfunctional proteins and cellular organelles through the reduction of autophagy increases the risk for malignant transformation. Furthermore, low basic levels of autophagy are required for cell survival as was identified through experiments with a knockout of different autophagy genes, such as ATG genes, Beclin-1, or AMBRA1 [22,23]. Autophagy is responsible for recycling cellular components and producing energy and pro-oncogenic factors [24]. Different stage of tumors, anti-cancer treatment, mutations in ATGs, and oncogenes are closely associated with autophagy and its controversial role in tumorigenesis. Further study is required in order to address the link between autophagy and hallmarks of cancer.
Furthermore, the increasing levels of autophagy, in these regions, are strongly associated with the regulation of the immune response in the tumor microenvironment [11,25]. The microenvironment of different malignant tumors, including CRC, is characterized by numerous cell types (including immune, tumor, and other types of cells). All these stroma cell types utilize a different extent of autophagy. Therefore, focusing on autophagy and its role in the tumor microenvironment for the discovery of novel anti-cancer therapeutic targets should be further elucidated [11,26]. The role of autophagy in developing an immune response against tumor cells is far more complex. Therefore, autophagy may be a promising therapeutic target in combination with other anti-neoplastic drugs and immunotherapy in the context of this unique cellular composition of the tumor microenvironment.
2. The Major Players in the Tumor Microenvironment
For years, solid cancers were considered as a mass of homogenous cancer cells [27]. Cancer evolution and resistance to treatment is caused by tumor heterogeneity. Over the past decade, it has become increasingly clear that there is a wild diversity of cells with tangled and branching pedigrees in the same tumor. One section of a tumor might be dense with cells containing a particular oncogene mutation, whereas another section might have vastly different mutation backgrounds driving their growth [28]. Tumors should be perceived as separate tissues with a different and more complex cellular network with specialized or dedifferentiated malignant cell types, fibroblasts, tumor stem cells, immune, and endothelial cells. This complex network is characterized as a tumor stroma with unique potential for anticancer therapy [29].
2.1. The Heterogeneity of the Tumor Microenvironment
The vast majority of solid tumors are composed of not only malignant cells, but also of fibroblasts. It is widely accepted that tumorigenesis is a multistep process, the progression of which depends on a sequential accumulation of mutations within tissue cells. Moreover, tumor initiation is associated with the activation of different stromal, endothelial and mesenchymal cells, fibroblasts, and immunogenic cells [30,31]. It is well known that tumor heterogeneity is associated with the more aggressive phenotype and a lack of response against anti-cancer therapy in different types of cancer, including CRC [32].
2.2. The Role of T-Lymphocytes
The major effectors of the immune response against tumor cells are the cytotoxic CD8+ T-lymphocytes or T-cells (CTL). The abundance of T-cells is a decisive prognostic factor for the response of chemotherapy and immunotherapy in cancer patients especially at early stages of the disease, where patients have a strong effector T cell response and more frequently present a high Immunoscore [33,34]. CTLs are responsible for killing hostile cells, such as tumor cells [35]. Type 1 of T-helper cells (Th-1) regulates the activation of CTL and Th-2 initiates humoral immunity [36]. In many studies, the activation of the immune system and tumor-infiltrating lymphocytes (TILs) are used for the grading of the tumor and as a putative prognostic marker for CRC patients. The characterization is based on TILs, tumor invasion, spread to the lymph nodes, and the tumor staging system [33,35].
Many studies have identified that the activation of CTL is inhibited by the PD-L1/PD1 axis interaction in CRC tumors with the Mismatch repair deficiency/Microsatellite instability -high MMRd/MSI-H phenotype [37,38,39]. The clinical effectiveness of anti-PD1 monoclonal antibodies is beneficial for this subgroup of patients [40]. In contrast, with MSI-H CRC tumors, in almost all MSS CRC tumors, inhibition of the PD-L1/ PD1 axis has no significant clinical effect, thus underlining the complexity of this immunosuppressive mechanism [41].
A particular group of lymphocytes that are strongly associated with tumors is the regulatory T-cells [42]. The role of Tregs (regulatory T cells) is controversial because of the genetic and phenotypic differentiation of T-cells. The Treg-specific DNA hypo-methylated regions contribute to the stable expression of Treg function-associated key genes, including Foxp3. Accordingly, FoxP3 robustly represses different genes, including Il2, contributing to Treg suppressive activity. In tumors, it is critical to deplete FOXP3 high CD45RA_CD25 high effector Treg cells, which are firmly installed with the Treg-type hypo-methylation and are most suppressive [43]. The origin of Tregs can be either directly from the thymus (tTreg) or by peripheral differentiation (pTreg) of conventional T lymphocytes [44]. The majority of Tregs are characterized by a high expression of specific biomarkers such as FOXP3, IL-2 receptor alpha chain, CD25 IL-10, TGF-β, and IL-35. Also, proteins, like CTLA-4 (cytotoxic T-lymphocyte–associated antigen 4), PD-1 (programmed death 1), and GITR (the receptor of glucocorticoid-induced tumor necrosis factor), have been identified in the surface of Tregs [45,46,47]. It is well known that molecules, like IL-27 and IL-33, are stimulators of Tregs in CRC through TGF-β-mediated differentiation of Tregs [44].
The primary role of Tregs is to control inflammation and maintain peripheral tolerance in immune homeostasis. Furthermore, FOXP3+ Tregs are crucial in the inhibition of the cytotoxic effect of T-cells in many cancer types, including CRC [42]. The lack of FOXP3+ Tregs and the ratio of CD3+/FOXP3+ T cell may be a prognostic marker for clinical outcomes in patients with CRC [48].
2.3. The Role of Tumor-Associated Myeloid Cells
Different cell types, such as cancer-associated fibroblasts (CAFs) and tumor-associated macrophages (TAMs), in the tumor microenvironment, regulate tumor growth, invasion, and the metastatic phenotype of cancer cells [49,50]. Many studies support the hypothesis that bone marrow-derived cells (TANs, TAMs, and myeloid-derived suppressor cells or MDSCs) are closely associated with the progression of the tumor [50,51].
Two different sub-populations of TAMs, the anti-tumorigenic and pro-tumorigenic or M1 and M2 phenotype, respectively, with high plasticity, have already been identified [52,53]. The most common myeloid infiltrate in solid tumors is composed of myeloid-derived suppressor cells (MDSCs) and tumor-associated macrophages (TAMs). These cells promote tumor growth through their inherent immunosuppressive activity, neoangiogenesis, and mediation of epithelial-mesenchymal transition. Several small molecules are already used in order to inhibit the tumorigenic action of these cells [52]. It is well known that neutrophils regulate the tumor microenvironment through the production of several immunogenic, angiogenic, and inflammatory factors, such as matrix metalloproteinases (MMPs), Vascular endothelial growth factor (VEGF), neutrophil elastase, and hepatocyte growth factor [54,55,56]. The number of neutrophils in peripheral blood is already evaluated as a negative clinical progression marker in various malignant tumors, including CRC [57]. The two different types of neutrophils, N1 and N2 neutrophils, have been associated with tumor progression. N1 neutrophils reduce tumor immunosuppression through the production of several molecules, such as TNF-α, ROS (Reactive oxygen species), ICAM-1 (Intercellular Adhesion Molecule 1), and Fas. In contrast, N2 neutrophils, increase tumorigenicity through the production of MMP-9, VEGF, and several chemokines [58].
Myeloid-derived suppressor cells or MDSCs have an immunosuppressive ability that is triggered by inflammation. MDSCs are abundant in different tumors types with a critical role in tumor progression [56]. Tumors produce several chemokines, such as CCL2 and CCL5, which regulate the migration of MDSCs in tumors [59]. Several studies support the idea that tumors attract MDSCs in the tumor microenvironment. MDSCs suppress the anti-tumor activity of the immune system through the activation of different genes associated with arg1 (Arginase 1), fatty acid oxidation (FAO), and ROS [60]. Furthermore, MDSCs seem to inhibit both antigen-specific and nonspecific (CD3/CD28) proliferative responses in the tumor microenvironment in both ROS-dependent and independent ways. Also, MDSCs inhibit the stimulation of CD3/CD28 T-cells through the production of NO (Nitric Oxide) and Arg1 [61]. In the tumor microenvironment, MDSCs are converted into nonspecific suppressor cells through the up-regulation of iNOS (inducible nitric oxide synthase) and arginase I. These enzymes are known to be actively involved in T cell suppression in a way that does not require antigen-specific contact between MDSC and T cells to inhibit their function [62].
Several studies over the last years highlight the impact of autophagy in MDSCs’ survival in the tumor microenvironment. Glycolytic metabolism is strongly associated with the metabolism of MDSCs [63]. Glycolysis prevents the AMPK-ULK1, a key player in autophagy regulation, which increases the GM-CSF (granulocyte macrophage colony-stimulating factor) expression and supports the development of MDSCs in the tumor microenvironment [64]. Furthermore, MDSCs activate autophagy through phosphorylation of AMPK. The initiation of autophagy increases several anti-apoptotic factors, such as BCL-2 (B-cell lymphoma 2) and MCL-1 (Myeloid cell leukemia 1), which promotes multiple myeloma (MM) progression [65].
2.4. Cancer-Associated Fibroblasts (CAFs)
Cancer-associated fibroblasts or CAFs represent a heterogeneous group of cells. They are responsible for the remodeling of the extracellular matrix (ECM) and support the invasion and metastasis of cancer cells [66]. Different molecules, such as FAP (fibroblast activation protein) and alpha-smooth muscle actin (a-SMA), have been already used as markers of activated CAFs and other fibroblasts [67]. CRC transcriptome studies associate the presence of CAFs with poor outcomes of patients, thus underlining the clinical significance of CAFs as a prognostic marker. Furthermore, the differentiation of CAFs and induction of the fibrogenic phenotype is regulated by the signaling pathway of TGF-β, mechanical stress, and fibronectin [68,69,70].
2.5. Angiogenesis and Neo-Vascularization Process in Tumor Stroma
It is well known that the stroma of CRC is also the scaffold for the development of tumor-associated blood vessels. Mesenchymal cell type, such as fibroblasts and immune cells, are responsible for supplying the VEGF with tumors cells [71]. Other molecules, like MMPs and associated proteases, that are expressed by immunosuppressive myeloid cells (IMCs) and CAFs appear to be increased in the tumor microenvironment. These enzymes help neo-angiogenesis by altering the ECM and proteolytic activation of embedded angiogenic factors (FGF and VEGF) [72].
2.6. Other Immune Cell Types in the Tumor Microenvironment
Several studies identified many other immune cell types in the tumor microenvironment of CRC. Immune cell types that appear in CRC microenvironment, like neutrophils, mast cells, natural killer (NK) cells, or eosinophils, did not appear to have a significant role in the impact of the clinical progression of CRC patients [73,74].
3. The Role of Autophagy in Stroma Development, Inflammation, and the Immunity Response
It has been proven that autophagy affects the microenvironment of the tumor and vice versa. These microenvironmental factors include cytokines, hypoxia, and inflammation in the tumor environment [75]. In response to stress conditions in the tumor microenvironment, autophagy is activated to maintain and supply energy. Additionally, digestion of intracellular components prevents the accumulation of toxic cellular remnants.
Cancer cells coexist with their microenvironment and the role of autophagy in modulating their interactions with other cell types may be a target for the modulation of autophagy, as a potential anti-cancerous treatment [76]. Autophagy is also a key factor in the function of APCs and T-cells. Autophagy is implicated in the presentation of antigens in both MHC-I and MHC-II in Dendritic cells (DCs). Finally, autophagy contributes to the functional activity of immune cells by creating T-cell memory, depending on autophagy [77].
3.1. The Role of Inflammation in Colorectal Cancer Development
Chronic inflammation is a high-risk factor for cancer. Patients with inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), have a three-fold increased risk of developing CRC. This type of cancer is known as “colitis-associated colorectal cancer (CAC)” [78]. Activation of Toll-like receptor 4 (TLR4) promotes the development of colitis-associated cancer through activation of the Cox-2 and EGFR signaling pathway [79]. Cancer development is due to the non-neoplastic inflammatory epithelium. Mutations in essential genes (c-src, p53, K-RAS, β-catenin, and APC) are caused by inflammation as well as DNA damage, which then leads to CAC onset in patients with IBD. Moreover, inflammation triggers signaling pathways, such as STAT3 (Signal transducer and activator of transcription 3) and β-catenin, which causes proliferation and remodeling of epithelial cells and then promotes tumor growth [80]. The CAC microenvironment is a complex system of various types of cells, cytokines, and signaling molecules that play a significant role in tumorigenesis. Immune cells develop many individual functions in the CAC microenvironment. Macrophages promote CAC tumorigenesis and the development of reactive oxygen species (ROS), IL-5, and nitric oxide synthase (NOS) [80]. Tregs and Th17 cells have tumor-promoting activity during CAC [81,82] formation while CD8+ T cells serve a protective role against CAC oncogenesis [83]. TAMs and CADs regulate the production of cytokines, such as IL-6, IL-8, IL-10, and IFN-γ, in the tumor microenvironment. Cytokines are key molecules to the development of inflammation during tumor progression [84]. Several studies support that autophagy is triggered via inflammation. In addition, NLRP3 (NLR Family Pyrin Domain Containing 3) inflammasome (a mitochondrion that is damaged depending on the structure) is negatively regulated by autophagy with IL-1b and IL-18 production and subsequent inflammation response under control [25].
3.2. Hypoxia-Induced Autophagy in the Tumor Microenvironment
Many studies have shown that many types of tumors are found under hypoxic conditions [4,26]. Autophagy in a hypoxic environment in tumors depends on the duration and percentage of hypoxia. Under moderate and chronic hypoxia, hypoxia-induced factor-1 (HIF-1a) and PKC-JNK regulate autophagy [85]. Since hypoxia results in BNIP3 or REDD1 being dependent on autophagy, the question arises as to whether there is an association between BNIP3, HIF-1, and/or REDD1. Many published data support the notion that HIF-1α can up-regulate BNIP3 transcription. Enhanced BNIP3 then interferes with the Beclin1 and BCL2-forming complex and further suppresses Rheb-mTOR [86,87]. Hypoxia raises the levels of REDD1, which then separates the 14-3-3 proteins from the TSC2 complex and finally reduces mTOR [87]. Also, a stress sensor, Ataxia Telangiectasia Mutated (ATM), was verified as being involved in the REDD1-modulated mTOR signaling. Under the hypoxic environment, ATM (Ataxia Telangiectasia Mutated) (-/-) MEFs perform decreased expressions of HIF-1α and REDD1. Overall, it is suggested that hypoxia-induced ATM activation results in increased HIF-1α-BNIP3 and REDD1 to increase autophagy through the inhibition of mTOR [87,88].
3.3. The Cross-Talk between Autophagy and Antigen Presenting Cells
Activation of the anticancer T-cell is induced by identifying the antigenic tumor peptides present on the cell surface of professional APCs, like DCs. However, autophagy through DCs and macrophages affects the surface expression of the MHC-I and peptide complex. For example, the expression of MHC-I in embryo mice DCs and macrophages was upregulated during inhibition of autophagy using chemical inhibitors or downregulation of the main autophagy genes [89,90]. This adjustment was attributed to the slower internalization of classical MHC class I molecules, leading to increased CD8+ T cell stimulation [90]. Hence, in the absence of autophagy, MHC-I molecules appear more consistently expressed and less degenerated [91]. Equally, DCs from mice lacking VPS34 (vacuolar protein sorting-associated protein 34) expressed more MHC-I on the cell surface as well as MHC-II [92]. In contrast, surface expression of MHC-II in macrophages was downregulated when inhibiting autophagy using 3-Methyladenine (3-MA) [91]. Autophagy is associated with the cross-presentation of antigens in DCs. Cross-presentation is a process that permits the loading of MHC-I into DCs with extracellular antigens, which is essential for activating, for example, CTL responses in melanoma [91,93,94,95]. The cross-presentation capability of bone marrow-derived dendritic cells (DCs) is characterized by increased levels of autophagy [90,96].
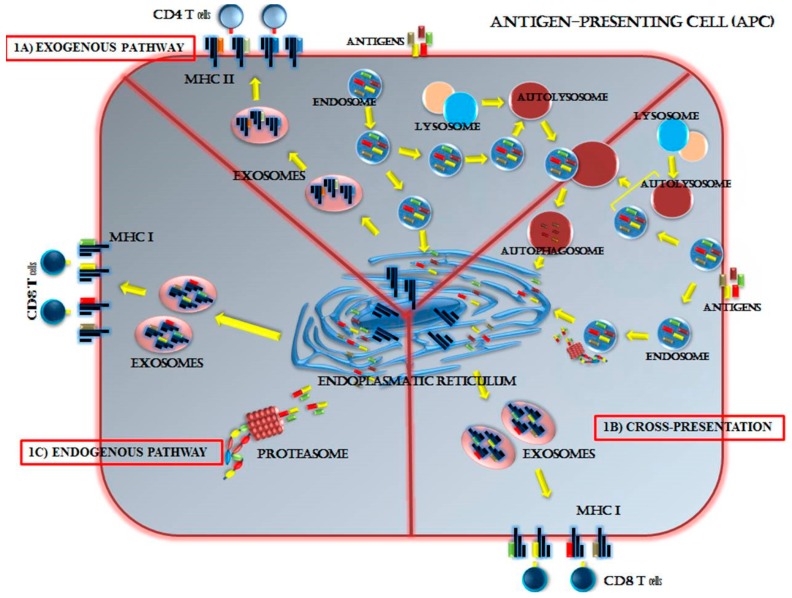
Antigen presentation in MHC-II was similarly altered in the inhibition of autophagy with reduced DC treatment mediated by an immunodominant mycobacterial peptide with the reduced presentation of vaccinia virus Ankara antigens and herpes simplex virus (HSV) antigens [97,98]. Accordingly, antigen-specific T-cell responses were down-regulated. Thus, inhibition of autophagy modified the peptide pool presented in MHC and reducing the presentation of immunodominant epitopes [99]. Although, inhibition of autophagy up-regulates surface expression of MHC-I, it also changes the group of immunogenic peptides presented on MHC. Thus, the effect on surface expression of MHC-I and II is less well-confirmed, which has been best determined in the context of the so-called cross-presentation in DCs [93,100,101]. As it was mentioned before, increased levels of autophagy characterize the cross-presentation capability of DCs compared with DCs that do not cross-present antigens, and the autoimmune inhibition that reduces the cross-presentation of MHC-I mediated MHC-I [102,103]. Inhibition of autophagy modified the presentation of the different peptides in MHC and appeared to change the pool of immunodominant epitopes of these peptides. Further mechanistic studies are needed to define how autophagy serves as a target for MHC class I cross-presentation. The central role of autophagy in antigen-presenting cells (APCs) is presented in Figure 1.
Figure 1.
The role of autophagy in the presentation of immunogenic peptides in antigen-presenting cells (APCs). Autophagy has a vital role in the degradation of proteins in order for APCs to use them as antigenic peptides on Major Histocompatibility Complex (MHC)-I and II. Three distinct pathways of antigen processing by the APC have been identified: Exogenous (1A), cross-presentation (1B), and endogenous (1C) pathway. In the exogenous pathway, different antigens and peptides are produced outside the APC and placed on the MHC class II for recognition by CD4+ T cells. The exogenous pathway occurs in macrophages, dendritic cells, and B cells. The endogenous pathway loads cell-produced antigenic peptides onto MHC class I for recognition by CD8+ T cells. The endogenous pathway is responsible for immune recognition of peptides from the virus or self-digested peptides. The endogenous pathway characterizes many cell types, not just APCs, allowing for sensing of viral infection in all cell types. In the cross-presentation pathway, different peptides, from endocytosis and the autophagy degradation pathway, are loaded on MHC class I for recognition by CD8+ T cells. The peptides originate from the surrounding cell environment of tumor apoptotic bodies. This pathway targets virus-infected cells other than APCs and the tumor. The cross-presentation pathway is identified as the most efficient in dendritic cells.
In general, peptides are cleaved and digest from proteins through proteasome in the endogenous pathway. In the exogenous and cross-presentation pathway, the endocytotic peptides are closely associated with autophagy. Endosomes fuse with the autolysosomes in order to digest the peptides and the neo-antigens are loaded onto MHC I and II in the endoplasmic reticulum (ER).
In the already developed tumor microenvironment, M2-phenotype tumor-associated macrophages (TAMs) promote angiogenesis, growth, and metastasis of tumor and cancer cells [104]. However, different studies support that M1 macrophages inhibit tumor growth [58]. The latest reports have shown that autophagy plays a crucial role in the production and polarization of macrophages. Deficiency of TLR2 strongly inhibits autophagy and leads to the biosynthesis of the M2 macrophage, which in turn promotes oncogenesis [58,105]. Moreover, the initiation of autophagy in TAM can increase the radiosensitivity of CRC, inhibit proliferation, and trigger apoptotic cell death [106].
Thus, autophagy in TAM can play a crucial role in cancer suppression. Also, the role of other native immune cells, such as NK cells and neutrophils, plays a vital role in the tumorigenesis of CRC. For example, tumor-associated neutrophils (TANs) facilitate the onset and development of CAC and increase autophagy in neutrophils, which are associated with increased migration of cancer cells [91]. Several in vivo studies suggest that inhibition of autophagy in tumor cells reduces the development of tumors by facilitating the removal of cancer cells via NK cells [107]. Analogous results have also been observed in other types of cancers, such as renal cell carcinoma and melanoma [81].
3.4. Autophagy—A Key Regulator of T-Cell Activation
The adaptive immune system includes the identification of pathogen or tumor proteins and their presence in MHC molecules by antigen-presenting cells (APCs). For this aim, MHC class I molecules are recognized by T cell receptors (TCRs) in CD8+ T cells. Subsequently, MHC class II molecules are recognized by TCRs in CD4+ T cells [90,91,92]. T cells are activated and differentiated into various types of effector T cells, including Tregs, Th cells, and cytotoxic T cells. Tregs produce anti-inflammatory cytokines, like IL-10 and TGF-β. Also, Th-cells can produce pro-inflammatory cytokines, such as IL-2, IL-5, IL-13, and IL-17A, and interferon gamma (INF-γ). Cytotoxic T cells cause the apoptosis of infected or malignant cells with the release of perforin and granzymes [81,108].
It has been reported that autophagy enhances the adaptive immune response by facilitating APC recognition and preserving the function, survival, and homeostasis of T cells among others [77]. T cell homeostasis involves the clearance of T cells deficient in autophagy [109]. For example, the loss of VPS34 accumulates ROS, which causes an increase in pre-apoptotic protein expression and robust apoptosis of these T cells [110]. Also, depletion of VPS34 also prevents the normal operation of Tregs. Moreover, the deletion of ATG5 and Beclin 1 results in inefficient proliferation and disordered function of CD8+ and CD4+ T cells, respectively, following TCR stimulation [111,112]. On the contrary, autophagy contributes to the maintenance of the survival and function of T cell lymphocytes CD8+ [113].
4. The Current State of Immunotherapy in CRC Patients
The treatment for CRC patients with early-stage disease is surgical removal of tumors. Chemotherapy usually follows the surgery for more advanced disease [114]. Recently, it has been shown that immunotherapy amplifies the immune responses against tumors and it has already been used for patients with solid tumors [115].
In the last few years, many immunomodulating agents have been developed that show significant efficacy. The FDA (Food and Drug Administration) has already approved immune checkpoint inhibitors, such as ipilimumab (an anti-CTLA-4 MoAbs), nivolumab, and pembrolizumab (anti-PD-1 MoAbs) or atezolizumab, avelumab, and durvalumab (anti-PD-L1 MoAbs) for different types of cancer, like melanoma, lung cancer, and renal cell carcinoma. They have recently shown promising activity as a treatment for CRC, although efficacy is reserved for a specific subset of patients [116,117].
It is well known that PD-L1, on tumor and stromal cells, suppresses the antitumor activity of the immune system through stabilization of TNF-α [118]. Furthermore, the PD-1/PD-L1 axis regulates inhibition of the immune response and leads T-cells to exhaustion and apoptotic cell death [119,120]. Wang et al. have shown that metastatic colorectal cancer (mCRC) has higher levels of PD-L1 [121]. Furthermore, dysregulation of signaling pathways, like PI3K-AKT, or chromosomal amplification of the 9q24.1 locus regulates the expression of PD-L1 and PD-L2 in different types of gastrointestinal cancers [120,122].
It is well known that the MSI phenotype in CRC varies according to the stage of the disease. CRC patients with mismatch repair (MMR) deficiency (15% to 20% of stage II/III CRCs) have a better prognosis. Metastatic CRC with deficient MMR represent around 5% and is associated with a poor prognosis [123]. Predictive biomarkers, like MMR and microsatellite status, a mutation in proto-oncogenes, and the expression of PD-L1 have already been used to classify patients in whom immunotherapy might be more beneficial [116,124]. Unfortunately, the percentage of patients with gastrointestinal cancer who will acquire durable clinical responses remains limited. The response rate for CRC patients with mismatch repair deficiency is less than 50% [125] and less than 30% for gastroesophageal cancer [125,126].
In many types of cancer, immunotherapy has been proven as a prominent therapeutic approach. Moreover, in the last few years, significant advances have also been made in CRC. An anti-CTLA-4 monoclonal antibody (tremelimumab) has proven useful for CRC patients, obtaining one 6-month strong response [127]. In a phase II trial, three groups of patients were formed according to their microsatellite status—MSI-H, non-MSI-H, and MSS CRC—in order to test the clinical activity of anti-PD1 MoAb, Pembrolizumab. The immune-related objective response rate (ORR) and immune-related 6-month PFS progression-free survival (PFS) rate were 40% and 78%, respectively, for mismatch repair–deficient (dMMR) colorectal cancers and 0% and 11% for mismatch repair-proficient colorectal cancers patients. The KEYNOTE-177 phase III trial evaluated the above results in patients with dMMR mCRC after treatment with Pembrolizumab versus standard therapy. In Checkmate 142, treatment with Nivolumab alone or in combination with Ipilimumab was tested in metastatic CRC patients according to the microsatellite status. In the update published on Lancet, 31% of CRC patients who were treated with Nivolumab had an objective response, with a disease control rate of 69% for 12 weeks or longer [123]. The combinatorial treatment of Nivolumab and Ipilimumab showed a 55% ORR, while the disease control rate for 12 weeks or longer was 80% [128,129].
The first anti-PD-L1 monoclonal antibody with FDA approval is atezolizumab. This is a fully humanized antibody which targets explicitly PD-L1. It is currently approved for patients with metastatic NSCLC and metastatic urothelial carcinoma with disease progression after treatment of platinum-based chemotherapy [130,131]. Atezolizumab shows response rates higher for patients with PD-L1 positive tumors [132,133]. A similar antibody is durvalumab. The safety and tolerability of durvalumab alone or in combination with tremelimumab have already been tested in a phase I trial for patients with CRC. Promising results have been presented in patients with PD-L1-expressing tumors with microsatellite instability [120,133,134]. These kinds of tumors are characterized by a higher number of infiltrated immune cells.
Furthermore, anti-PD-L1 therapy is more efficient in combination because of the differential expression of PD-1 and PD-L1 in the tumor microenvironment. On the other hand, several types of cancers, such as melanoma and breast cancer, are characterized by PD-L1 expression in both tumors and infiltrating immune cells [120]. The other, a less studied ligand of PD-1 is PD-L2. PD-L2 has been identified to be expressed in macrophages, B-cells, and dendritic cells [124,135]. In CRC, the expression of PD-L2 is approximately 40% and it is regulated by glycosylation and IFNγ [136]. Further, ongoing studies are evaluating the combinations of PD-1, PD-L1, and/or CTLA-4 monoclonal antibodies with other chemotherapeutic molecules, which will re-activate the immune system against CRC tumors (Table 1).
Table 1.
Clinical studies with immunotherapy for patients with Please define this term if appropriate.
| Number of Study | Immune Target | Agent/Compound | Phase of Study |
|---|---|---|---|
| NCT01876511 | PD-1 | Pembrolizumab | II |
| NCT02981524 | PD-1 | Cyclophosphamide followed by Pembrolizumab | II |
| NCT03657641 | PD-1 | Pembrolizumab + Vicriviroc | I/II |
| NCT03631407 | PD-1 | Pembrolizumab + Regorafenib | II |
| NCT03475004 | PD-1 | Pembrolizumab, Bevacizumab, and Binimetinib | II |
| NCT03658772 | PD-1 | Pembrolizumab + grapiprant | I |
| NCT03519412 | PD-1 | Pembrolizumab + temozolomide | II |
| NCT02713373 | PD-1 | Pembrolizumab + cetuximab | I/II |
| NCT02375672 | PD-1 | Pembrolizumab + FOLFOX | II |
| NCT03332498 | PD-1 | Pembrolizumab + Ibrutinib | I/II |
| NCT02851004 | PD-1 | Pembrolizumab + SBRT | I/II |
| NCT02837263 | PD-1 | Pembrolizumab + BBI609 | I |
| NCT02992912 | PD-1 | Atezolizumab + stereotactic ablative radiotherapy | II |
| NCT03712943 | PD-1 | Nivolumab + Regorafenib | I |
| NCT03711058 | PD-1 | Nivolumab + Copanlisib | I/II |
| NCT03414983 | PD-1 | Nivolumab, Oxaliplatin, Leucovorin, Fluorouracil, Bevacizumab | II/III |
| NCT02860546 | PD-1 | Nivolumab + TAS-102 | II |
| NCT03026140 | PD-1 and CTLA-4 | Nivolumab + Ipilimumab +/− celecoxib | I/III |
| NCT03693846 | PD-1 and CTLA-4 | Nivolumab + Ipilimumab | II |
| NCT03104439 | PD-1 and CTLA-4 | Nivolumab + Ipilimumab + radiotherapy | II |
| NCT03377361 | PD-1 and CTLA-4 | Nivolumab + Ipilimumab + Trametinib | I/II |
| NCT03832621 | PD-1 and CTLA-4 | Nivolumab, Ipilimumab, Temozolomide | II |
| NCT02327078 | PD-1 and IDO | Nivolumab + Epacadostat | VII |
| NCT02983578 | PD-L1 | AZD9150 + MEDI4736 | II |
| NCT02982694 | PD-L1 | Atezolizumab + Bevacizumab | II |
| NCT02777710 | PD-L1 | Durvalumab + Pexidartinib | I |
| NCT03827044 | PD-L1 | Avelumab | III |
| NCT02669914 | PD-L1 | Durvalumab | II |
| NCT02754856 | PD-L1 and CTLA-4 | MEDI4736 + Tremelimumab | I |
| NCT03202758 | PD-L1 and CTLA-4 | Durvalumab, Tremelimumab, and FOLFOX | I/II |
NCT, national clinical trial; PD-1, programmed cell death-1; PD-1, programmed cell death-1 ligand; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; IDO, indoleamine-pyrrole 2,3-dioxygenase.
Several studies associate the expression of PD-L1 with PD-L2 and with the geographical association of different types of immune cells. The protein levels of PD-L1 and PD-L2 are associated with the response of anti-PD1 MoAbs. Thus, PD-L2 may be a promising target in immunotherapeutic schemes for CRC [137,138]. It is well known that increasing levels of CD73 block the activation of lymphocytes via increasing adenosine levels. Thus, inhibition of CD73 enhances the therapeutic effect of anti-PD1 and anti-CTL4 monoclonal antibodies [139]. Furthermore, several studies, have explored the relationship between the inhibition of PD-1/CTLA-4 and the increasing levels of CD8+ and CD4+ T cells and cytokines, Tregs inhibition, and other molecules essential for T-cell function [120,140].
5. Targeting Autophagy—A Promising Anti-Cancer Strategy
5.1. The Main Autophagy Inhibitors in Cancer Therapy
Different studies in the last years support the concept of the protective role of autophagy against a different type of cancer therapy, like radiotherapy, chemotherapy, and immunotherapy [141]. The crucial role of autophagy is to regulate the energy and metabolic balance of cancer cells [17] and through the impairment of cell death [142]. Years of efforts have led to the development of molecules that inhibit autophagy. Because of the crucial role of autophagy in cancer cell initiation and progression, the inhibition of autophagy has been shown to be beneficial in anticancer treatment.
Chloroquine (CQ) and its derivative, hydroxychloroquine (HCQ), is one of the most well-known inhibitors that target the fusion of the autophagosome with a lysosome. Over the last years, different clinical trials have attempted to evaluate the clinical significance of autophagy inhibition with CQ or HCQ in several types of cancers [76]. Unfortunately, these clinical trials failed to provide clinically significant benefits because of a lack of consistent inhibition of autophagy with CQ and its derivative, HCQ [143]. However, the combination of autophagy inhibition with other agents provides some encouraging results [76,144]. The combination of HCQ with chemotherapy, like gemcitabine, in pancreatic adenocarcinoma reduced the level of tumor marker 19-9 around 60% [145]. Furthermore, inhibition of autophagy may also have benefits in immunotherapy. The combination of CQ with IL-2 has proven effective with limited toxicity in a preclinical murine hepatic metastasis model. Moreover, this combinatorial scheme increases long term survival and the proliferation and infiltration of immune cells in the liver and spleen [141].
The clinical response of CQ and HCQ appears to vary widely. CQ and its derivative, CHQ, are not specific inhibitors of autophagy [141] and this appears to affect the bioavailability of other drugs by altering the tumor pH [143,146]. Also, the lack of a specific biomarker, which evaluates the inhibition of autophagy, add to the difficulties of these autophagy inhibitors to provide clinically significant results. New, more specific autophagy inhibitors may provide benefits for patients [76,141].
A more potent autophagy inhibitor is Lys05, a dimeric for of Chloroquine. Lys05 alters the acidification of the lysosomes and causes impairment of lysosomal enzymes. It can be used in lower doses. Thus, it is more tolerated and associated with stronger anti-tumor activity [147]. Another autophagy inhibitor is SAR405. SAR405 is a specific kinase inhibitor of Vps18 and Vps34. Vps34 and Beclin-1 regulate the initiation of the autophagy process. Inhibition of Vps34 leads to dysfunctional lysosome and vesicle trafficking activity [148]. Several studies support that inhibition of Beclin-1 reduces tumor growth and enhances anti-tumor NK cell activity. Decreasing levels of Beclin-1 leads tumor cells to overexpress CCL5 cytokine, which regulates the trafficking of NK cells to the tumors [141]. SBI-0206965 is a highly selective, small molecule inhibitor for ULK1 (Unc-51 like kinase-1). This molecule inhibits autophagy through the reduction of ULK1-mediated phosphorylation events in cells. In vivo experiments support the antitumor activity of SBI-0206965 via inhibition of autophagy in different types of cancer [149]. Several other drugs, such as verteporfin, clomipramine, and desmethylclomipramine (DCMI), have been FDA-approved for use in therapy. All these agents alter the acidification of lysosomes or block autophagosome-lysosome fusion [150]. Specifically, autophagy inhibition through DCMI enhances the efficacy of doxorybicin in in vitro studies [151]. Another potent autophagy inhibitor is spautin-1. The mechanism by which spautin-1 inhibits autophagy has already been identified. It inhibits two ubiquitin-specific peptidases, USP10 and USP13, which regulate the deubiquitination of Beclin-1 in Vps34 complexes. Thus, autophagy initiation is inhibited [152]. Due to the strong association of autophagy with the tumor microenvironment and the immune response against tumors, autophagy inhibition may have a negative effect on the adaptive antitumor immunity against tumors. Starobinets et al. (2016) identified that adaptive antitumor immunity is not adversely associated with autophagy inhibition in breast and melanoma cancer models. Thus, autophagy inhibitors can be safely combined with other chemotherapeutic drugs, such as anthracyclines, and still trigger a productive antitumor T cell response against tumors [153].
5.2. Activators of Autophagy for Cancer Therapy
The current review attempts to extensively analyze the role of autophagy in the development of the tumor microenvironment and anti-cancer immunotherapy. In many cases, it is well understood that autophagy has a crucial role in the anti-tumor immune response in CRC. Autophagy not only regulates the antigen presentation in MHC I and II, but it has also been associated with apoptotic cell death in some cases. Due to the multifaceted role of autophagy in cancer, several molecules that induce autophagy have been developed in order to have benefits in anti-cancer therapy.
The most well-known autophagy activators are rapamycin and rapalogs (everolimus, temsirolimus, and deforolimus—analogs of rapamycin). They are inhibitors of mTOR and mTORC1, respectively, and consequently activate autophagy [154]. In endometrial cancer cells, everolimus has been identified as a suppressor of proliferation, especially when it is combined with paclitaxel [155]. Rapamycin was reported to enhance radiation therapy in A549 lung cancer cells through the induction of autophagy and delaying of DNA damage repair [156]. Rapamycin and rapalogs are putative therapeutic molecules that act through autophagy induction, especially when combined with other anti-neoplastic drugs. The clinical application of autophagy activators requires further investigation [155].
Another compound which reduces cell proliferation through the induction of autophagy is metformin. Inhibition of autophagy with specific autophagy inhibitors or knockdown of Beclin-1 reversed the cytotoxic effects of metformin. Furthermore, metformin was identified to increase TNF-related apoptosis-inducing ligand (TRAIL)-dependent apoptosis in lung adenocarcinoma cells through the induction of autophagy machinery [152]. In a BRCA1-deficient mammary tumor model, the combination of metformin with spautin-1 sensitizes BRCA1-deficient breast tumors to mitochondrial disruptors. It is well known that these two agents target different aspects of mitochondrial function and thus it may partially explain the contradictory observation of an autophagy inhibitor (spautin-1) with an autophagy inducer (metformin) in the reduction of cell viability [157].
Obatoclax, a molecule that specifically targets the Bcl-2 family, has been identified as an anti-cancer agent against hematologic malignancies [158]. The main anticancer mechanism of Obatoclax is strongly associated with autophagy induction. Furthermore, Obatoclax stimulates the assembly of necrosomes in the membranes of autophagosomes and consequently induces necroptosis [154]. Several studies have established natural alkaloids, such as isoliensinine, cepharanthine, and liensinine, as inducers of autophagy in cancer [159]. Alkaloids regulate autophagy through phosphorylation of AMPK and inhibition of mTOR. These kinds of alkaloids have been reported to induce apoptotic cell death in apoptosis-resistant MEFs [154].
Herein, we provide two summarized tables about small molecules that inhibit or activate autophagy. Regulation of autophagy is already used in research to develop new chemotherapeutic strategies with immunotherapy for different types of cancer, including CRC (Table 2 and Table 3).
Table 2.
Commonly used molecules inhibiting autophagy. Small molecules that have been identified as inhibitors of autophagy and the main mechanism of action.
| Compound | Autophagy Inhibitors |
|---|---|
| Mechanism of Action | |
| Bafilomycin A1 | Inhibitor of v-ATPase, inhibition of lysosomal acidification |
| Concanamycin A | Inhibitor of v-ATPase, inhibition of lysosomal acidification |
| Azithromycin | Inhibitor of v-ATPase, inhibition of lysosomal acidification |
| 3-Methyladenine (3-MA) | Inhibitor of class III PI3K |
| Chloroquine (CQ) | Neutralizes the acidic pH of intracellular vesicles |
| Hydroxy-chloroquine (HCQ) | CQ derivative-Neutralizes the acidic pH of intracellular vesicles |
| Lys05 | CQ derivative-alter the acidification of the lysosomes |
| SAR405 | Kinase inhibitor of Vps18 and Vps34 |
| SBI-0206965 | Inhibitor of ULK1 |
| Verteporfin | Inhibit acidification of lysosomes |
| Clomipramine | Inhibit acidification of lysosomes |
| desmethylclomipramine (DCMI) | Inhibit Autophagosome-Lysosome fusion |
| Paclitaxel | Microtubule stabilizer- inhibits phosphorylation of VPS34 at T159 |
| SAHA | Interact in autophagosome-lysosome fusion |
| Monensin | Inhibit autophagosome-lysosome fusion |
| Sputin-1 | Inhibits the activity of ubiquitin-specific peptidases, USP10 and USP13 |
| SP600125 | Inhibition of JNK—reduction of Beclin-1 |
| U0126 | Inhibitor of MEK1 and MEK2 |
| Wortmannin | PI3K inhibitor |
| LY294002 | PI3K inhibitor |
| SB202190 | Cross-inhibition of the PI3K/mTOR and MAPKs pathway |
| SB203580 | Inhibit autophagy by interfering with the trafficking of Atg9 |
| MHY1485 | mTOR activator |
Table 3.
Commonly used molecules to induce autophagy. Small molecules that have been identified as autophagy inducers and the primary mechanism of action.
| Compound/Molecule | Autophagy Inducers |
|---|---|
| Mechanism of Action | |
| Rapamycin | mTORC1 inhibitor |
| Temsirolimus | mTORC1 inhibitor |
| Deforolimus | mTORC1 inhibitor |
| Everolimus | mTORC1 inhibitor |
| Metformin | AMPK activator |
| Obatoclax | Inhibitor of Bcl-2 family proteins |
| isoliensinine | Natural alkaloid |
| cepharanthine | Natural alkaloid |
| liensinine | Natural alkaloid |
| Perifosine | AKT inhibitor |
| Tat–Beclin-1 peptide | Releases beclin-1 into cytoplasm-regulate autophagosome formation |
| Lithium | Increase the levels of Beclin-1/VPS34 complexes |
| GDC-0980 | Dual inhibitor of PI3K and mTORC1 |
| GDC-0941 | Inhibitor of class I PI3K |
| fluspirilene | Antagonists of L-type Ca2+ channels |
| verapamil | Antagonists of L-type Ca2+ channels |
| loperamide | Antagonists of L-type Ca2+ channels |
| nimodipine | Antagonists of L-type Ca2+ channels |
| amiodarone | Antagonists of L-type Ca2+ channels |
6. Conclusions
In the last decade, autophagy has been strongly associated with tumorigenesis in colorectal cancer. The dual role of autophagy as survival and a pro-death mechanism has become a field of research in order to develop more effective therapeutic schemes against cancer. In established tumors, autophagy has a vital role as a survival mechanism, especially in the hypoxic regions of tumors. It is well known that tumors are characterized by a highly heterogeneous population of cancer, mesenchymal, immune, and stromal cells in a complex structure, which is identified as the tumor microenvironment. A growing body of evidence supports the hypothesis that autophagy regulates not only the metabolic function of cancer cells, but also other types of cells in the tumor microenvironment. Autophagy has a crucial role as a regulator of immune responses by sustaining the activation, homeostasis, and biological functions of different immune cells, such as T-cells, macrophages, and antigen presenting cells. Moreover, the impact of autophagy on tumor cells has also been observed in the active participation in intracellular and extracellular antigen processing for MHC-I and/or MHC-II presentation. Besides, autophagy has also been reported to associate with the cross-presentation of neo-antigens for MHC-I presentation and the internalization process. Several studies support autophagy as a potential target to strengthen or attenuate the effects of immunotherapy against different types of cancer, including CRC. In the future, efforts should be focused on how to regulate autophagy in the tumor microenvironment in order to strengthen the response of the immune system and overcome anti-tumor immune resistance in immunotherapy for colorectal cancer.
Abbreviations
| ATGs | Autophagy-related genes |
| CAFs | Cancer-associated fibroblasts |
| CRC | Colorectal cancer |
| CTLs | Cytotoxic T lymphocytes |
| CTLA-4 | cytotoxic T-lymphocyte-associated antigen-4 |
| CQ | Chloroquine |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| HCQ | Hydroxyl-chloroquine |
| mCRC | metastatic Colorectal cancer |
| MDSCs | Myeloid-derived suppressor cell |
| MHC I and II | Major histocompatibility complex I and II |
| MoAbs | Monoclonal antibodies |
| NSCLC | Non-small cell lung cancer |
| MMRd | Mismatch repair deficiency |
| MSI-H | Microsatellite instability-High |
| MSS | Microsatellite stable |
| TANs | Tumor-associated neutrophils |
| TAMs | Tumor-associated macrophages |
| TCR | T-cell receptor |
| TILs | Tumor-infiltrating lymphocytes |
| Tregs | Regulatory T cells |
| 3-MA | 3-Methyladenine |
Author Contributions
E.K., P.S. and G.K. made substantial contributions to acquisition, analysis and interpretation of data. A.G.P. and M.V.K. made substantial contributions in the conception, design and interpretation of the data as well as in drafting the manuscript and revising it critically for important intellectual content.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N., Ohsumi Y., Yoshimori T. Autophagosome Formation in Mammalian Cells. Cell Struct. Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 4.Koustas E., Karamouzis M.V., Mihailidou C., Schizas D., Papavassiliou A.G. Co-targeting of EGFR and autophagy signaling is an emerging treatment strategy in metastatic colorectal cancer. Cancer Lett. 2017;396:94–102. doi: 10.1016/j.canlet.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Pandurangan A.K., Divya T., Kumar K., Dineshbabu V., Velavan B., Sudhandiran G., AshokKumar P. Colorectal carcinogenesis: Insights into the cell death and signal transduction pathways: A review. J. Gastrointest. Oncol. 2018;10:244–259. doi: 10.4251/wjgo.v10.i9.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroemer G., Mariño G., Levine B. Autophagy and the integrated stress response. Mol. Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burada F., Nicoli E.R., Ciurea M.E., Uscatu D.C., Ioana M., Gheonea D.I. Autophagy in colorectal cancer: An important switch from physiology to pathology. World J. Gastrointest. Oncol. 2015;7:271–284. doi: 10.4251/wjgo.v7.i11.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koustas E., Sarantis P., Papavassiliou A.G., Karamouzis M. V Upgraded role of autophagy in colorectal carcinomas. World J. Gastrointest. Oncol. 2018;10:367–369. doi: 10.4251/wjgo.v10.i11.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaaf M.B., Houbaert D., Meçe O., Agostinis P. Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ. 2019;26:665–679. doi: 10.1038/s41418-019-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colella B., Faienza F., Di Bartolomeo S. EMT Regulation by Autophagy: A New Perspective in Glioblastoma Biology. Cancers. 2019;11:312. doi: 10.3390/cancers11030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X., Yu D.-D., Yan F., Jing Y.-Y., Han Z.-P., Sun K., Liang L., Hou J., Wei L.-X. The role of autophagy induced by tumor microenvironment in different cells and stages of cancer. Cell Biosci. 2015;5:14. doi: 10.1186/s13578-015-0005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer. 2012;12:401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo J.Y., White E. Autophagy, metabolism, and cancer. Cold Spring Harb. Symp. Quant. Biol. 2016;81:73–78. doi: 10.1101/sqb.2016.81.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mellor H.R., Harris A.L. The role of the hypoxia-inducible BH3-only proteins BNIP3 and BNIP3L in cancer. Cancer Metastasis Rev. 2007;26:553–566. doi: 10.1007/s10555-007-9080-0. [DOI] [PubMed] [Google Scholar]
- 15.Huijbers A., Plantinga T.S., Joosten L.A.B., Aben K.K.H., Gudmundsson J., Heijer M.D., Kiemeney L.A.L.M., Netea M.G., Hermus A.R.M.M., Netea-Maier R.T. The effect of the ATG16L1 Thr300Ala polymorphism on susceptibility and outcome of patients with epithelial cell-derived thyroid carcinoma. Endocr. Relat. Cancer. 2012;19:L15–L18. doi: 10.1530/ERC-11-0302. [DOI] [PubMed] [Google Scholar]
- 16.Huang C.-Y., Huang S.-P., Lin V.C., Yu C.-C., Chang T.-Y., Lu T.-L., Chiang H.-C., Bao B.-Y. Genetic variants of the autophagy pathway as prognostic indicators for prostate cancer. Sci. Rep. 2015;5:14045. doi: 10.1038/srep14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun C.W., Lee S.H. The Roles of Autophagy in Cancer. Int. J. Mol. Sci. 2018;19:3466. doi: 10.3390/ijms19113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miracco C., Cosci E., Oliveri G., Luzi P., Pacenti L., Monciatti I., Mannucci S., De Nisi M.C., Toscano M., Malagnino V., et al. Protein and mRNA expression of autophagy gene Beclin 1 in human brain tumours. Int. J. Oncol. 2007;30:429–436. [PubMed] [Google Scholar]
- 19.Pickford F., Masliah E., Britschgi M., Lucin K., Narasimhan R., Jaeger P.A., Small S., Spencer B., Rockenstein E., Levine B., et al. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J. Clin. Investig. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariño G., Salvador-Montoliu N., Fueyo A., Knecht E., Mizushima N., López-Otín C. Tissue-specific Autophagy Alterations and Increased Tumorigenesis in Mice Deficient in Atg4C/Autophagin-3. J. Biol. Chem. 2007;282:18573–18583. doi: 10.1074/jbc.M701194200. [DOI] [PubMed] [Google Scholar]
- 21.Oikonomou E., Koustas E., Goulielmaki M., Pintzas A. BRAF vs. RAS oncogenes: Are mutations of the same pathway equal? Differential signalling and therapeutic implications. Oncotarget. 2015;5:11752–11777. doi: 10.18632/oncotarget.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cianfanelli V., D’Orazio M., Cecconi F. AMBRA1 and BECLIN 1 interplay in the crosstalk between autophagy and cell proliferation. Cell Cycle. 2015;14:959–963. doi: 10.1080/15384101.2015.1021526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue Z., Jin S., Yang C., Levine A.J., Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo J.Y., Chen H.-Y., Mathew R., Fan J., Strohecker A.M., Karsli-Uzunbas G., Kamphorst J.J., Chen G., Lemons J.M., Karantza V., et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev. 2011;25:460–470. doi: 10.1101/gad.2016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhong Z., Sanchez-Lopez E., Karin M. Autophagy, Inflammation and Immunity: A Troika Governing Cancer and Its Treatment. Cell. 2016;166:288–298. doi: 10.1016/j.cell.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degenhardt K., Mathew R., Beaudoin B., Bray K., Anderson D., Chen G., Mukherjee C., Shi Y., Gélinas C., Fan Y., et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell. 2006;10:51–64. doi: 10.1016/j.ccr.2006.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerlinger M., Rowan A.J., Horswell S., Math M., Larkin J., Endesfelder D., Gronroos E., Martinez P., Matthews N., Stewart A., et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molinari C., Marisi G., Passardi A., Matteucci L., De Maio G., Ulivi P. Heterogeneity in Colorectal Cancer: A Challenge for Personalized Medicine? Int. J. Mol. Sci. 2018;19:3733. doi: 10.3390/ijms19123733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietras K., Östman A. Hallmarks of cancer: Interactions with the tumor stroma. Exp. Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 30.Tape C.J. The Heterocellular Emergence of Colorectal Cancer. Trends Cancer. 2017;3:79–88. doi: 10.1016/j.trecan.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fridman W.H., Zitvogel L., Sautès-Fridman C., Kroemer G. The immune contexture in cancer prognosis and treatment. Nat. Rev. Clin. Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 32.Kather J.N., Halama N., Jaeger D. Genomics and emerging biomarkers for immunotherapy of colorectal cancer. Semin. Cancer Biol. 2018;52:189–197. doi: 10.1016/j.semcancer.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Bupathi M., Wu C. Biomarkers for immune therapy in colorectal cancer: Mismatch-repair deficiency and others. J. Gastrointest. Oncol. 2016;7:713–720. doi: 10.21037/jgo.2016.07.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mlecnik B., Bindea G., Kirilovsky A., Angell H.K., Obenauf A.C., Tosolini M., Church S.E., Maby P., Vasaturo A., Angelova M., et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci. Transl. Med. 2016;8:327. doi: 10.1126/scitranslmed.aad6352. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa K., Tanaka K., Homma Y., Nojiri K., Kumamoto T., Takeda K., Endo I. Low Infiltration of Peritumoral Regulatory T Cells Predicts Worse Outcome Following Resection of Colorectal Liver Metastases. Ann. Surg. Oncol. 2015;22:180–186. doi: 10.1245/s10434-014-3974-1. [DOI] [PubMed] [Google Scholar]
- 36.Yu P., Fu Y.X. Tumor-infiltrating T lymphocytes: Friends or foes? Lab. Investig. 2006;86:231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 37.Hu Z., Ma Y., Shang Z., Hu S., Liang K., Liang W., Xing X., Wang Y., Du X. Improving immunotherapy for colorectal cancer using dendritic cells combined with anti-programmed death-ligand in vitro. Oncol. Lett. 2018;15:5345–5351. doi: 10.3892/ol.2018.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pauken K.E., Wherry E.J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh P.P., Sharma P.K., Krishnan G., Lockhart A.C. Immune checkpoints and immunotherapy for colorectal cancer. Gastroenterol. Rep. 2015;3:289–297. doi: 10.1093/gastro/gov053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Derhovanessian E., Maier A.B., Beck R., Jahn G., Hähnel K., Slagboom P., De Craen A.J.M., Westendorp R.G.J., Pawelec G. Hallmark Features of Immunosenescence Are Absent in Familial Longevity. J. Immunol. 2010;185:4618–4624. doi: 10.4049/jimmunol.1001629. [DOI] [PubMed] [Google Scholar]
- 41.Koustas E., Papavassiliou A.G., Karamouzis M.V. The role of autophagy in the treatment of BRAF mutant colorectal carcinomas differs based on microsatellite instability status. PLoS ONE. 2018;13:e0207227. doi: 10.1371/journal.pone.0207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y., Colello J., Jarjour W., Zheng S.G. Cellular Metabolic Regulation in the Differentiation and Function of Regulatory T Cells. Cells. 2019;8:188. doi: 10.3390/cells8020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morikawa H., Sakaguchi S. Genetic and epigenetic basis of Treg cell development and function: From a FoxP3-centered view to an epigenome-defined view of natural Treg cells. Immunol. Rev. 2014;259:192–205. doi: 10.1111/imr.12174. [DOI] [PubMed] [Google Scholar]
- 44.Herk E.H., Velde A.A. Treg subsets in inflammatory bowel disease and colorectal carcinoma. Characteristics, role and therapeutic targets. J. Gastroenterol. Hepatol. 2016;31:1393–1404. doi: 10.1111/jgh.13342. [DOI] [PubMed] [Google Scholar]
- 45.Hori S., Nomura T., Sakaguchi S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 46.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 47.Salama P., Phillips M., Grieu F., Morris M., Zeps N., Joseph D., Platell C., Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 48.Sinicrope F.A., Rego R.L., Ansell S.M., Knutson K.L., Foster N.R., Sargent D.J. Intraepithelial Effector (CD3+)/Regulatory (FoxP3+) T-Cell Ratio Predicts a Clinical Outcome of Human Colon Carcinoma. Gastroenterology. 2009;137:1270–1279. doi: 10.1053/j.gastro.2009.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao D., Mittal V. The role of bone-marrow-derived cells in tumor growth, metastasis initiation and progression. Trends Mol. Med. 2009;15:333–343. doi: 10.1016/j.molmed.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mantovani A., Cassatella M.A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 52.Szebeni G.J., Vizler C., Nagy L.I., Kitajka K., Puskas L.G., Tanaka T., Shimizu M. Pro-Tumoral Inflammatory Myeloid Cells as Emerging Therapeutic Targets. Int. J. Mol. Sci. 2016;17:1958. doi: 10.3390/ijms17111958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaul M.E., Fridlender Z.G. Neutrophils as active regulators of the immune system in the tumor microenvironment. J. Leukoc. Biol. 2017;102:343–349. doi: 10.1189/jlb.5MR1216-508R. [DOI] [PubMed] [Google Scholar]
- 54.Houghton A.M., Rzymkiewicz D.M., Ji H., Gregory A.D., Egea E.E., Metz H.E., Stolz D.B., Land S.R., Marconcini L.A., Kliment C.R., et al. Neutrophil Elastase-Mediated Degradation of IRS-1 Accelerates Lung Tumor Growth. Nat. Med. 2010;16:219–223. doi: 10.1038/nm.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galdiero M.R., Varricchi G., Loffredo S., Mantovani A., Marone G. Roles ofneutrophils in cancer growth and progression. J. Leukoc. Biol. 2018;103:457–464. doi: 10.1002/JLB.3MR0717-292R. [DOI] [PubMed] [Google Scholar]
- 56.Wislez M., Rabbe N., Marchal J., Milleron B., Crestani B., Mayaud C., Antoine M., Soler P., Cadranel J. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: Role in tumor progression and death. Cancer Res. 2003;63:1405–1412. [PubMed] [Google Scholar]
- 57.Li Z., Zhao R., Cui Y., Zhou Y., Wu X. The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I–III colon cancer. Sci. Rep. 2018;8:9453. doi: 10.1038/s41598-018-27896-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mizuno R., Kawada K., Itatani Y., Ogawa R., Kiyasu Y., Sakai Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int. J. Mol. Sci. 2019;20:529. doi: 10.3390/ijms20030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R., Kaiser E.A., Snyder L.A., Pollard J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar V., Patel S., Tcyganov E., Gabrilovich D.I. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37:208–220. doi: 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haverkamp J.M., Crist S.A., Elzey B.D., Cimen C., Ratliff T.L. In vivo suppressive function of myeloid-derived suppressor cells is limited to the inflammatory site. Eur. J. Immunol. 2011;41:749–759. doi: 10.1002/eji.201041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corzo C.A., Condamine T., Lu L., Cotter M.J., Youn J.-I., Cheng P., Cho H.-I., Celis E., Quiceno D.G., Padhya T., et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J. Exp. Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang G.M., Tan Y., Wang H., Peng L., Chen H.T., Meng X.J., Li L.L., Liu Y., Li W.F., Shan H. The relationship between autophagy and the immune system and its applications for tumor immunotherapy. Mol. Cancer. 2019;18:17. doi: 10.1186/s12943-019-0944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li W., Tanikawa T., Kryczek I., Xia H., Li G., Wu K., Wei S., Zhao L., Vatan L., Wen B., et al. Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell Metab. 2018;28:87–103.e6. doi: 10.1016/j.cmet.2018.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Veirman K., Menu E., Maes K., De Beule N., De Smedt E., Maes A., Vlummens P., Fostier K., Kassambara A., Moreaux J., et al. Myeloid-derived suppressor cells induce multiple myeloma cell survival by activating the AMPK pathway. Cancer Lett. 2019;442:233–241. doi: 10.1016/j.canlet.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 66.Xing F. Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front. Biosci. 2010;15:166. doi: 10.2741/3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koliaraki V., Pallangyo C.K., Greten F.R., Kollias G. Mesenchymal Cells in Colon Cancer. Gastroenterology. 2017;152:964–979. doi: 10.1053/j.gastro.2016.11.049. [DOI] [PubMed] [Google Scholar]
- 68.Grillo A.R., Scarpa M., D’Inca R., Brun P., Scarpa M., Porzionato A., De Caro R., Martines D., Buda A., Angriman I., et al. TAK1 is a key modulator of the profibrogenic phenotype of human ileal myofibroblasts in Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;309:443–454. doi: 10.1152/ajpgi.00400.2014. [DOI] [PubMed] [Google Scholar]
- 69.Hawinkels L.J.A.C., Paauwe M., Verspaget H.W., Wiercinska E., Van Der Zon J.M., Van Der Ploeg K., Koelink P.J., Lindeman J.H.N., Mesker W., Ten Dijke P., et al. Interaction with colon cancer cells hyperactivates TGF-β signaling in cancer-associated fibroblasts. Oncogene. 2014;33:97–107. doi: 10.1038/onc.2012.536. [DOI] [PubMed] [Google Scholar]
- 70.Calon A., Espinet E., Palomo-Ponce S., Tauriello D.V.F., Iglesias M., Céspedes M.V., Sevillano M., Nadal C., Jung P., Zhang X.H.F., et al. Dependency of Colorectal Cancer on a TGF-β-Driven Program in Stromal Cells for Metastasis Initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Connell J.T., Sugimoto H., Cooke V.G., MacDonald B.A., Mehta A.I., LeBleu V.S., Dewar R., Rocha R.M., Brentani R.R., Resnick M.B., et al. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. USA. 2011;108:16002–16007. doi: 10.1073/pnas.1109493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bonnans C., Chou J., Werb Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Charoentong P., Finotello F., Angelova M., Mayer C., Efremova M., Rieder D., Hackl H., Trajanoski Z. Pan-cancer Immunogenomic Analyses Reveal Genotype-Immunophenotype Relationships and Predictors of Response to Checkpoint Blockade. Cell Rep. 2017;18:248–262. doi: 10.1016/j.celrep.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 74.Halama N., Braun M., Kahlert C., Spille A., Quack C., Rahbari N., Koch M., Weitz J., Kloor M., Zoernig I., et al. Natural Killer Cells are Scarce in Colorectal Carcinoma Tissue Despite High Levels of Chemokines and Cytokines. Clin. Cancer Res. 2011;17:678–689. doi: 10.1158/1078-0432.CCR-10-2173. [DOI] [PubMed] [Google Scholar]
- 75.Vaupel P., Mayer A. Hypoxia and anemia: Effects on tumor biology and treatment resistance. Transfus. Clin. Biol. 2005;12:5–10. doi: 10.1016/j.tracli.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 76.Levy J.M.M., Towers C.G., Thorburn A. Targeting Autophagy in Cancer Therapy. Nat. Rev. Cancer. 2016;17:528–542. doi: 10.1038/nrc.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Durães F.V., Niven J., Dubrot J., Hugues S., Gannagé M. Macroautophagy in Endogenous Processing of Self- and Pathogen-Derived Antigens for MHC Class II Presentation. Front. Immunol. 2015;6:79. doi: 10.3389/fimmu.2015.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bernstein C.N., Blanchard J.F., Kliewer E., Wajda A. Cancer risk in patients with inflammatory bowel disease: A population-based study. Cancer. 2001;91:854–862. doi: 10.1002/1097-0142(20010215)91:4<854::AID-CNCR1073>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 79.Fukata M., Chen A., Vamadevan A.S., Cohen J., Breglio K., Krishnareddy S., Hsu D., Xu R., Harpaz N., Dannenberg A.J., et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chaturvedi M.M., Sung B., Yadav V.R., Kannappan R., Aggarwal B.B. NF-κB addiction and its role in cancer: One size does not fit all. Oncogene. 2011;30:1615–1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu Y., Yao J., Xie J., Liu Z., Zhou Y., Pan H., Han W. The role of autophagy in colitis-associated colorectal cancer. Signal Transduct. Target. Ther. 2018;3:31. doi: 10.1038/s41392-018-0031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ning C., Li Y.-Y., Wang Y., Han G.-C., Wang R.-X., Xiao H., Li X.-Y., Hou C.-M., Ma Y.-F., Sheng D.-S., et al. Complement activation promotes colitis-associated carcinogenesis through activating intestinal IL-1β/IL-17A axis. Mucosal Immunol. 2015;8:1275–1284. doi: 10.1038/mi.2015.18. [DOI] [PubMed] [Google Scholar]
- 83.Olguín J.E., Medina-Andrade I., Molina E., Vázquez A., Pacheco-Fernández T., Saavedra R., Pérez-Plasencia C., Chirino Y.I., Vaca-Paniagua F., Arias-Romero L.E., et al. Early and partial reduction in CD4+Foxp3+ regulatory T cells during colitis-associated colon cancer induces CD4+ and CD8+ T cell activation inhibiting tumorigenesis. J. Cancer. 2018;9:239–249. doi: 10.7150/jca.21336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ngabire D., Kim G.-D. Autophagy and Inflammatory Response in the Tumor Microenvironment. Int. J. Mol. Sci. 2017;18:2016. doi: 10.3390/ijms18092016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bellot G., Garcia-Medina R., Gounon P., Chiche J., Roux D., Pouysségur J., Mazure N.M. Hypoxia-Induced Autophagy Is Mediated through Hypoxia-Inducible Factor Induction of BNIP3 and BNIP3L via Their BH3 Domains. Mol. Cell. Biol. 2009;29:2570–2581. doi: 10.1128/MCB.00166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin A., Yao J., Zhuang L., Wang D., Han J., Lam E.W., Network T.R., Gan B. The Foxo-BNIP3 axis exerts a unique regulation of mTORC1 and cell survival under energy stress. Oncogene. 2014;33:3183–3194. doi: 10.1038/onc.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y.-Y., Feun L.G., Thongkum A., Tu C.-H., Chen S.-M., Wangpaichitr M., Wu C., Kuo M.T., Savaraj N. Autophagic Mechanism in Anti-Cancer Immunity: Its Pros and Cons for Cancer Therapy. Int. J. Mol. Sci. 2017;18:1297. doi: 10.3390/ijms18061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Çam H., Easton J.B., High A., Houghton P.J. mTORC1 signaling under hypoxic conditions is controlled by ATM-dependent phosphorylation of HIF-1α. Mol. Cell. 2010;40:509–520. doi: 10.1016/j.molcel.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Valečka J., Almeida C.R., Su B., Pierre P., Gatti E. Autophagy and MHC-restricted antigen presentation. Mol. Immunol. 2018;99:163–170. doi: 10.1016/j.molimm.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Keller C.W., Loi M., Ligeon L.-A., Gannage M., Lunemann J.D., Münz C. Endocytosis regulation by autophagy proteins in MHC restricted antigen presentation. Curr. Opin. Immunol. 2018;52:68–73. doi: 10.1016/j.coi.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 91.Folkerts H., Hilgendorf S., Vellenga E., Bremer E., Wiersma V.R. The multifaceted role of autophagy in cancer and the microenvironment. Med. Res. Rev. 2019;39:517–560. doi: 10.1002/med.21531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parekh V.V., Wu L., Boyd K.L., Williams J.A., Gaddy J.A., Olivares-Villagómez D., Cover T.L., Zong W.-X., Zhang J., Van Kaer L. Impaired autophagy, defective T cell homeostasis and a wasting syndrome in mice with a T cell-specific deletion of Vps34. J. Immunol. 2013;190:5086–5101. doi: 10.4049/jimmunol.1202071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loi M., Müller A., Steinbach K., Niven J., Barreira da Silva R., Paul P., Ligeon L.A., Caruso A., Albrecht R.A., Becker A.C., et al. Macroautophagy Proteins Control MHC Class I Levels on Dendritic Cells and Shape Anti-viral CD8+ T Cell Responses. Cell Rep. 2016;15:1076–1087. doi: 10.1016/j.celrep.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 94.Germic N., Frangez Z., Yousefi S., Simon H.-U. Regulation of the innate immune system by autophagy: Monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019;26:715–727. doi: 10.1038/s41418-019-0297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Münz C. Autophagy proteins in antigen processing for presentation on MHC molecules. Immunol. Rev. 2016;272:17–27. doi: 10.1111/imr.12422. [DOI] [PubMed] [Google Scholar]
- 96.Mintern J.D., Macri C., Chin W.J., Panozza S.E., Segura E., Patterson N.L., Zeller P., Bourges D., Bedoui S., McMillan P.J., et al. Differential use of autophagy by primary dendritic cells specialized in cross-presentation. Autophagy. 2015;11:906–917. doi: 10.1080/15548627.2015.1045178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loi M., Ligeon L.-A., Münz C. MHC Class I Internalization via Autophagy Proteins. Methods Mol. Biol. 2019;1880:455–477. doi: 10.1007/978-1-4939-8873-0_29. [DOI] [PubMed] [Google Scholar]
- 98.Thiele F., Tao S., Zhang Y., Muschaweckh A., Zollmann T., Protzer U., Abele R., Drexler I. Modified vaccinia virus Ankara-infected dendritic cells present CD4+ T-cell epitopes by endogenous major histocompatibility complex class II presentation pathways. J. Virol. 2015;89:2698–2709. doi: 10.1128/JVI.03244-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bronietzki A.W., Schuster M., Schmitz I. Autophagy in T-cell development, activation and differentiation. Immunol. Cell Biol. 2015;93:25–34. doi: 10.1038/icb.2014.81. [DOI] [PubMed] [Google Scholar]
- 100.Nedjic J., Aichinger M., Mizushima N., Klein L. Macroautophagy, endogenous MHC II loading and T cell selection: The benefits of breaking the rules. Curr. Opin. Immunol. 2009;21:92–97. doi: 10.1016/j.coi.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 101.Khan N., Vidyarthi A., Pahari S., Negi S., Aqdas M., Nadeem S., Agnihotri T., Agrewala J.N. Signaling through NOD-2 and TLR-4 Bolsters the T cell Priming Capability of Dendritic cells by Inducing Autophagy. Sci. Rep. 2016;6:19084. doi: 10.1038/srep19084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tey S.-K., Khanna R. Autophagy mediates transporter associated with antigen processing-independent presentation of viral epitopes through MHC class I pathway. Blood. 2012;120:994–1004. doi: 10.1182/blood-2012-01-402404. [DOI] [PubMed] [Google Scholar]
- 103.Lee H.K., Mattei L.M., Steinberg B.E., Alberts P., Lee Y.H., Chervonsky A., Mizushima N., Grinstein S., Iwasaki A. In Vivo Requirement for Atg5 in Antigen Presentation by Dendritic Cells. Immunity. 2010;32:227–239. doi: 10.1016/j.immuni.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Galdiero M.R., Bonavita E., Barajon I., Garlanda C., Mantovani A., Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 105.Yang M., Liu J., Shao J., Qin Y., Ji Q., Zhang X., Du J. Cathepsin S-mediated autophagic flux in tumor-associated macrophages accelerate tumor development by promoting M2 polarization. Mol. Cancer. 2014;13:43. doi: 10.1186/1476-4598-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shao L.-N., Xing C.-G., Yang X.-D., Young W., Zhu B.-S., Cao J.-P. Effects of autophagy regulation of tumor-associated macrophages on radiosensitivity of colorectal cancer cells. Mol. Med. Rep. 2016;13:2661–2670. doi: 10.3892/mmr.2016.4820. [DOI] [PubMed] [Google Scholar]
- 107.Viry E., Baginska J., Berchem G., Noman M.Z., Medves S., Chouaib S., Janji B. Autophagic degradation of GZMB/granzyme B: A new mechanism of hypoxic tumor cell escape from natural killer cell-mediated lysis. Autophagy. 2014;10:173–175. doi: 10.4161/auto.26924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shibutani S.T., Saitoh T., Nowag H., Münz C., Yoshimori T. Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 2015;16:1014–1024. doi: 10.1038/ni.3273. [DOI] [PubMed] [Google Scholar]
- 109.Oral O., Yedier O., Kilic S., Gozuacik D. Involvement of autophagy in T cellbiology. Histol. Histopathol. 2017;32:11–20. doi: 10.14670/HH-11-785. [DOI] [PubMed] [Google Scholar]
- 110.Willinger T., Flavell R.A. Canonical autophagy dependent on the class III phosphoinositide-3 kinase Vps34 is required for naive T-cell homeostasis. Proc. Natl. Acad. Sci. USA. 2012;109:8670–8675. doi: 10.1073/pnas.1205305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu X., Araki K., Li S., Han J.H., Ye L., Tan W.G., Konieczny B.T., Bruinsma M.W., Martinez J., Pearce E.L., et al. Autophagy is essential for effector CD8(+) T cell survival and memory formation. Nat. Immunol. 2014;15:1152–1161. doi: 10.1038/ni.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reed M., Morris S.H., Jang S., Mukherjee S., Yue Z., Lukacs N.W. Autophagy-inducing protein beclin-1 in dendritic cells regulates CD4 T cell responses and disease severity during respiratory syncytial virus infection. J. Immunol. 2013;191:2526–2537. doi: 10.4049/jimmunol.1300477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Henson S.M., Lanna A., Riddel N.E., Franzese O., Macaulay R., Griffiths S.J., Puleston D.J., Watson A.S., Simon A.K., Tooze S.A., et al. P38 signaling inhibits mTORC1-independent autophagy in senescent human CD8+ T cells. J. Clin. Investig. 2014;124:4004–4016. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Venook A. Critical Evaluation of Current Treatments in Metastatic Colorectal Cancer. Oncologist. 2005;10:250–261. doi: 10.1634/theoncologist.10-4-250. [DOI] [PubMed] [Google Scholar]
- 115.Le D.T., Durham J.N., Smith K.N., Wang H., Bartlett B.R., Aulakh L.K., Lu S., Kemberling H., Wilt C., Luber B.S., et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou C., Zhang J. Immunotherapy-based combination strategies for treatment of gastrointestinal cancers: Current status and future prospects. Front. Med. 2019;13:12–23. doi: 10.1007/s11684-019-0685-9. [DOI] [PubMed] [Google Scholar]
- 117.Arora S.P., Mahalingam D. Immunotherapy in colorectal cancer: For the select few or all? J. Gastrointest. Oncol. 2018;9:170–179. doi: 10.21037/jgo.2017.06.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lim S.-O., Li C.-W., Xia W., Cha J.-H., Chan L.-C., Wu Y., Chang S.-S., Lin W.-C., Hsu J.-M., Hsu Y.-H., et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell. 2016;30:925–939. doi: 10.1016/j.ccell.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Juneja V.R., McGuire K.A., Manguso R.T., LaFleur M.W., Collins N., Haining W.N., Freeman G.J., Sharpe A.H. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 2017;214:895–904. doi: 10.1084/jem.20160801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yaghoubi N., Soltani A., Ghazvini K., Hassanian S.M., Hashemy S.I. PD-1/ PD-L1 blockade as a novel treatment for colorectal cancer. Biomed. Pharmacother. 2019;110:312–318. doi: 10.1016/j.biopha.2018.11.105. [DOI] [PubMed] [Google Scholar]
- 121.Bin Wang H., Yao H., Li C.S., Liang L.X., Zhang Y., Chen Y.X., Fang J.-Y., Xu J., Fang J. Rise of PD-L1 expression during metastasis of colorectal cancer: Implications for immunotherapy. J. Dig. Dis. 2017;18:574–581. doi: 10.1111/1751-2980.12538. [DOI] [PubMed] [Google Scholar]
- 122.O’Donnell J.S., Massi D., Teng M.W., Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin. Biol. 2018;48:91–103. doi: 10.1016/j.semcancer.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 123.Battaglin F., Naseem M., Lenz H.J., Salem M.E. Microsatellite instability in colorectal cancer: Overview of its clinical significance and novel perspectives. Clin. Adv. Hematol. Oncol. 2018;16:735–745. [PMC free article] [PubMed] [Google Scholar]
- 124.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kang Y.K., Boku N., Satoh T., Ryu M.H., Chao Y., Kato K., Chung H.C., Chen J.S., Muro K., Kang W.K., et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 126.Fuchs C.S., Doi T., Jang R.W., Muro K., Satoh T., Machado M., Sun W., I Jalal S., A Shah M., Metges J.-P., et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer: Phase 2 Clinical KEYNOTE-059 Trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chung K.Y., Fong L., Venook A., Beck S.B., Dorazio P., Criscitiello P.J., Healey D.I., Huang B., Gómez-Navarro J., Saltz L.B., et al. Phase II Study of the Anti-Cytotoxic T-Lymphocyte–Associated Antigen 4 Monoclonal Antibody, Tremelimumab, in Patients with Refractory Metastatic Colorectal Cancer. J. Clin. Oncol. 2010;28:3485–3490. doi: 10.1200/JCO.2010.28.3994. [DOI] [PubMed] [Google Scholar]
- 128.Le D.T., Uram J.N., Wang H., Bartlett B., Kemberling H., Eyring A., Skora A., Azad N.S., Laheru D.A., Donehower R.C., et al. PD-1 blockade in tumors with mismatch repair deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Overman M.J., Lonardi S., Wong K.Y.M., Lenz H.-J., Gelsomino F., Aglietta M., Morse M.A., Van Cutsem E., McDermott R., Hill A., et al. Durable Clinical Benefit with Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 130.Rico G.T., Price T.J. Atezolizumab for the treatment of colorectal cancer: The latest evidence and clinical potential. Opin. Biol. Ther. 2018;18:449–457. doi: 10.1080/14712598.2018.1444024. [DOI] [PubMed] [Google Scholar]
- 131.Calles A., Aguado G., Sandoval C., Álvarez R. The role of immunotherapy in small cell lung cancer. Clin. Transl. Oncol. 2019:1–16. doi: 10.1007/s12094-018-02011-9. [DOI] [PubMed] [Google Scholar]
- 132.Herbst R.S., Soria J.-C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S., Sosman J.A., McDermott D.F., Powderly J.D., Gettinger S.N., et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Link J.T., Overman M.J. Immunotherapy Progress in Mismatch Repair–Deficient Colorectal Cancer and Future Therapeutic Challenges. Cancer J. 2016;22:190–195. doi: 10.1097/PPO.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 134.Emambux S., Tachon G., Junca A., Tougeron D. Results and challenges of immune checkpoint inhibitors in colorectal cancer. Expert Opin. Biol. Ther. 2018;18:561–573. doi: 10.1080/14712598.2018.1445222. [DOI] [PubMed] [Google Scholar]
- 135.Zhong X., Tumang J.R., Gao W., Bai C., Rothstein T.L. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for VH11/VH12 and phosphatidylcholine binding. Eur. J. Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 136.Wang H., Yao H., Li C., Liang L., Zhang Y., Shi H., Zhou C., Chen Y., Fang J.-Y., Xu J. PD-L2 expression in colorectal cancer: Independent prognostic effect and targetability by deglycosylation. Oncoimmunology. 2017;6:e1327494. doi: 10.1080/2162402X.2017.1327494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guo P.-D., Sun Z.-W., Lai H.-J., Yang J., Wu P.-P., Guo Y.-D., Sun J. Clinicopathological analysis of PD-L2 expression in colorectal cancer. OncoTargets Ther. 2018;11:7635–7642. doi: 10.2147/OTT.S177329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Taube J.M., Klein A., Brahmer J.R., Xu H., Pan X., Kim J.H., Chen L., Pardoll D.M., Topalian S.L., Anders R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014;20:5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Seto T., Sam D., Pan M. Mechanisms of Primary and Secondary Resistanceto Immune Checkpoint Inhibitors in Cancer. Med. Sci. 2019;7:14. doi: 10.3390/medsci7020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Curran M.A., Montalvo W., Yagita H., Allison J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Janji B., Berchem G., Chouaib S. Targeting Autophagy in the Tumor Microenvironment: New Challenges and Opportunities for Regulating Tumor Immunity. Front. Immunol. 2018;9:887. doi: 10.3389/fimmu.2018.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Qian H.-R., Shi Z.-Q., Zhu H.-P., Gu L.-H., Wang X.-F., Yang Y. Interplay between apoptosis and autophagy in colorectal cancer. Oncotarget. 2017;8:62759–62768. doi: 10.18632/oncotarget.18663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rosenfeld M.R., Ye X., Supko J.G., Desideri S., A Grossman S., Brem S., Mikkelson T., Wang D., Chang Y.C., Hu J., et al. A phase I/II trial of hydroxychloroquine in conjunction with radiation therapy and concurrent and adjuvant temozolomide in patients with newly diagnosed glioblastoma multiforme. Autophagy. 2014;10:1359–1368. doi: 10.4161/auto.28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goulielmaki M., Koustas E., Moysidou E., Vlassi M., Sasazuki T., Shirasawa S., Zografos G., Oikonomou E. BRAF associated autophagy exploitation: BRAF and autophagy inhibitors synergise to efficiently overcome resistance of BRAF mutant colorectal cancer cells. Oncotarget. 2015;7:9188–9221. doi: 10.18632/oncotarget.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boone B.A., Bahary N., Zureikat A.H., Moser A.J., Normolle D.P., Wu W.C., Singhi A.D., Bao P., Bartlett D.L., Liotta L.A., et al. Safety and Biologic Response of Pre-operative Autophagy Inhibition in Combination with Gemcitabine in Patients with Pancreatic Adenocarcinoma. Ann. Surg. Oncol. 2015;22:4402–4410. doi: 10.1245/s10434-015-4566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pellegrini P., Strambi A., Zipoli C., Hägg-Olofsson M., Buoncervello M., Linder S., De Milito A. Acidic extracellular pH neutralizes the autophagy-inhibiting activity of chloroquine: Implications for cancer therapies. Autophagy. 2014;10:562–571. doi: 10.4161/auto.27901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Amaravadi R.K., Winkler J.D. Lys05: A new lysosomal autophagy inhibitor. Autophagy. 2012;8:1383–1384. doi: 10.4161/auto.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ronan B., Flamand O., Vescovi L., Dureuil C., Durand L., Fassy F., Bachelot M.-F., Lamberton A., Mathieu M., Bertrand T., et al. A highly potent and selective Vps34 inhibitor alters vesicle trafficking and autophagy. Nat. Chem. Biol. 2014;10:1013–1019. doi: 10.1038/nchembio.1681. [DOI] [PubMed] [Google Scholar]
- 149.Egan D.F., Chun M.G., Vamos M., Zou H., Rong J., Miller C.J., Lou H.J., Raveendra-Panickar D., Yang C.-C., Sheffler D.J., et al. Small Molecule Inhibition of the Autophagy Kinase ULK1 and Identification of ULK1 Substrates. Mol. Cell. 2015;59:285–297. doi: 10.1016/j.molcel.2015.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vakifahmetoglu-Norberg H., Xia H.-G., Yuan J. Pharmacologic agents targeting autophagy. J. Clin. Investig. 2015;125:5–13. doi: 10.1172/JCI73937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rossi M., Munarriz E.R., Bartesaghi S., Milanese M., Dinsdale D., Guerra-Martin M.A., Bampton E.T.W., Glynn P., Bonanno G., Knight R.A., et al. Desmethylclomipramine induces the accumulation of autophagy markers by blocking autophagic flux. J. Cell Sci. 2009;122:3330–3339. doi: 10.1242/jcs.048181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu J., Xia H., Kim M., Xu L., Li Y., Zhang L., Cai Y., Norberg H.V., Zhang T., Furuya T., et al. Beclin1 Controls the Levels of p53 by Regulating the Deubiquitination Activity of USP10 and USP13. Cell. 2011;147:223–234. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Starobinets H., Ye J., Broz M., Barry K., Goldsmith J., Marsh T., Rostker F., Krummel M., Debnath J. Antitumor adaptive immunity remains intact following inhibition of autophagy and antimalarial treatment. J. Clin. Investig. 2016;126:4417–4429. doi: 10.1172/JCI85705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang Z.J., Chee C.E., Huang S., Sinicrope F.A. The Role of Autophagy in Cancer: Therapeutic Implications. Mol. Cancer Ther. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Byun S., Lee E., Lee K.W. Therapeutic Implications of Autophagy Inducers in Immunological Disorders, Infection, and Cancer. Int. J. Mol. Sci. 2017;18:1959. doi: 10.3390/ijms18091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang H., Li D., Li X., Ou X., Liu S., Zhang Y., Ding J., Xie B. Mammalian target of rapamycin inhibitor RAD001 sensitizes endometrial cancer cells to paclitaxel-induced apoptosis via the induction of autophagy. Oncol. Lett. 2016;12:5029–5035. doi: 10.3892/ol.2016.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yeo S.K., Paul R., Haas M., Wang C., Guan J.-L. Improved efficacy of mitochondrial disrupting agents upon inhibition of autophagy in a mouse model of BRCA1-deficient breast cancer. Autophagy. 2018;14:1214–1225. doi: 10.1080/15548627.2018.1460010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Opydo-Chanek M., Gonzalo O., Marzo I. Multifaceted anticancer activity of BH3 mimetics: Current evidence and future prospects. Biochem. Pharmacol. 2017;136:12–23. doi: 10.1016/j.bcp.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 159.Law B.Y.K., Chan W.K., Xu S.W., Wang J.R., Bai L.P., Liu L., Wong V.K.W. Natural small-molecule enhancers of autophagy induce autophagic cell death in apoptosis-defective cells. Sci. Rep. 2014;4:5510. doi: 10.1038/srep05510. [DOI] [PMC free article] [PubMed] [Google Scholar]