Abstract
KRAS-driven non-small cell lung cancer (NSCLC) patients have no effective targeted treatment. In this study, we aimed to investigate targeting epidermal growth factor receptor (EGFR) as a therapeutic approach in KRAS-driven lung cancer cells. We show that ablation of EGFR significantly suppressed tumor growth in KRAS-dependent cells and induced significantly higher expression of CX chemokine receptor 7 (CXCR7) and activation of MAPK (ERK1/2). Conversely, rescue of EGFR led to CXCR7 downregulation in EGFR−/− cells. Dual EGFR and CXCR7 inhibition led to substantial reduction of MAPK (pERK) and synergistic inhibition of cell growth. Analysis of two additional EGFR knockout NSCLC cell lines using CRISPR/Cas9 revealed genotype dependency of CXCR7 expression. In addition, treatment of different cells with gefitinib increased CXCR7 expression in EGFRwt but decreased it in EGFRmut cells. CXCR7 protein expression was detected in all NSCLC patient samples, with higher levels in adenocarcinoma as compared to squamous cell lung carcinoma and healthy control cases. In conclusion, EGFR and CXCR7 have a crucial interaction in NSCLC, and dual inhibition may be a potential therapeutic option for NSCLC patients.
Keywords: epidermal growth factor receptor (EGFR), CXCR7, KRAS, lung cancer, targeted therapy, gene editing
1. Introduction
Lung cancer is the leading cause of cancer related death worldwide [1]. Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancers [2]. Different options for the treatment of patients with NSCLC are available, including surgery, radiotherapy, chemotherapy, immunotherapy, and tyrosine kinase inhibitors (TKIs). Gefitinib, erlotinib, and afatinib are the most commonly used TKIs directed against mutant epidermal growth factor receptor (EGFR) [3]. Despite high response rates in EGFR-mutant NSCLC patients, the majority of the patients cannot benefit from EGFR-TKIs, as the frequency of the activating mutations is about 10% in the non-Asian population [4]. The objective response rate in patients with wild-type EGFR treated with TKIs (7.2%) is significantly lower as compared to the chemotherapy (16.8%) group [5].
KRAS is mutated in 20–30% of NSCLC cases and is involved in the regulation of cell proliferation. Mutations in KRAS mostly occur in codon 12 and 13 and are associated with a worse prognosis. Patients with mutant KRAS have worse response to EGFR-TKIs, radiotherapy, and adjuvant chemotherapy [6]. Therapeutic approaches targeting KRAS are still limited with low clinical efficacy. Therefore, KRAS-mutant tumors are considered as “undruggable.”
There is ample evidence that resistance to the EGFR-TKIs can be related to reactivation of the MAPK/ERK signaling pathway [7]. The reactivation of MAPK/ERK signaling limits the response to EGFR-TKIs and leads to resistance [8,9]. Single targeted therapy for MAPK/ERK associated pathways, such as anti-BRAF, -MEK and -KRAS, is inefficient because tumor cells can acquire resistance within a short time by activating alternative pathways [10,11]. Pre-clinical and clinical studies using combination therapy have shown more promising results in TKI-resistant tumors with alterations in MAPK/ERK pathways, for instance, the dual inhibition of KRAS/FAS, KEAP1/NRF2, BRAF/MEK, and BRAF/RTK [7,10,11,12,13].
Crosstalk between G protein-coupled receptor (GPCRs) and EGFR contributes to tumor cell progression [14]. CX chemokine receptor 7 (CXCR7) is a new member of GPCRs [15], which for a long time had been considered as a receptor for vasoactive intestinal peptide and as a decoy receptor [16,17]. Recently, CXCR7 has been classified as a novel receptor for CX chemokine ligand (CXCL) 12 [18], CXCL11, human macrophage migration inhibitory factor (MIF) [19], and Dickkopf-3 [20]. CXCR7 facilitates tumor development and progression [21,22]. CXCR7 is also involved in the formation of metastasis in lung cancer patients/mouse models [21,23,24]. CXCR7 may interact with EGFR and promote MAPK signaling and tumor cell progression [25,26,27,28]. Interestingly, one study shows that secreted MIF binds to EGFR [29] and inhibits its activation. Another study showed that MIF binds to CXCR7 and promotes ERK signaling [19]. In addition, co-localization of CXCR7 with EGFR leads to EGFR phosphorylation [28]. Despite these studies, the mechanisms behind CXCR7-EGFR crosstalk and how CXCR7 behaves during EGFR targeted treatment are not clear.
In this study, we aimed to explore EGFR knockout as a therapeutic option in EGFR wild-type and KRAS mutated lung cancer cells. To the best of our knowledge, this is the first study showing that wild-type EGFR plays a significant role in growth of KRAS-dependent cancer cells. We identified overexpression of CXCR7 as a bypass mechanism in EGFR−/− cells by promoting MAPK signaling. Both EGFR and CXCR7 inhibition showed synergetic suppression of cancer cell growth. Furthermore, we revealed that CXCR7 was increased in EGFRwt but decreased in EGFRmut cell lines after treatment with gefitinib. We also show that CXCR7 expression is higher in adenocarcinoma (ADC) than squamous cell lung carcinoma (SQCC) in patients with NSCLC and healthy lung tissue. Hence, dual inhibition of EGFR and CXCR7 might be a potential treatment strategy for NSCLC.
2. Materials and Methods
2.1. Cell Culture
A549 (wild-type EGFR, mutant KRAS) was purchased from ATCC. H1650 (mutant EGFR, wild-type KRAS) and H1299 (wild-type EGFR, wild-type KRAS) cell lines were kindly provided by Dr. Klaas Kok (Department of Genetics, University Medical Center Groningen, Groningen, The Netherlands). The HCC827 (mutant EGFR, wild-type KRAS) cell line was a gift from Dr. Martin Pool (Department of Medical Oncology, University Medical Center Groningen, Groningen, The Netherlands). The cells cultured either in DMEM (A549) or RPMI-1640 (H1650, H1299, and HCC827) containing 1% penicillin/streptomycin supplemented with 10% fetal bovine serum (FBS) (Costar Europe, Badhoevedorp, The Netherlands) at 37 °C with 5% CO2. Cells were stimulated with 10 ng/mL epidermal growth factor (EGF) (R & D Systems, Minneapolis, MN, USA) for 15 min or with recombinant human SDF-1α (CXCL12) (300-28A) (PeproTech, Rocky Hill, CT, USA) for 3 min, where indicated.
2.2. Anti-Tumor Reagents
Cetuximab was purchased from Merck (Dietikon, Switzerland). Gefitinib was purchased from Sigma-Aldrich (Zwijndrecht, The Netherlands); Y27632 was purchased from Tocris Bioscience (Bristol, UK). Sunitinib, selumetinib, and vemurafenib were purchased from LC Laboratories (Woburn, MA, USA). C646 was purchased from Axon Medchem (Groningen, the Netherlands). SAHA and entinostat were purchased from Selleckchem (Munich, Germany). Cis-Diamineplatinum (II) dichloride and staurosporine were purchased from Sigma (Aldrich, Nederland). Doxrubicin was purchased from Teva Pharmaceuticals. VI83, which inhibits PDGFRß, was a kind gift from Vichem Laboratories (Budapest, Hungary). CXCR7 inhibitor was a kind gift from Professor Rob Leurs and Professor Martine J. Smit (Vrije Universiteit Amsterdam, Amsterdam, The Netherlands). All drugs were diluted and aliquoted in dimethyl sulfoxide (DMSO), stored at −20 °C.
2.3. Transfection and Establishment of EGFR−/− Cell Lines
A pool of two CRISPR/Cas9 EGFR knockout (KO) plasmids (Santa Cruz Biotechnology, Dallas, TX, USA), each encoding the Cas9 nuclease and a 20-nucleotide guide RNA (gRNA) targeting exons 2 and 3 of the EGFR, were used to establish EGFR−/− lung cancer cell lines (Table S1). For transfection, 3 × 105 cells were seeded in a single well of a 6-well plate. The next day, cells were transfected with CRISPR/Cas9 plasmids pool using Lipofectamine 3000 (Invitrogen, Carlsbad, USA) according to the manufacturer’s instructions with 3 μg of CRISPR/Cas9 plasmids. These plasmids contain GFP (Santa Cruz Biotechnology, Dallas, TX, USA) and puromycin resistance genes. Puromycin was used to select cells with successful uptake of the plasmid. Culture medium was changed one day after transfection, followed by puromycin selection with 2 μg/mL for the next three days. Single cells were seeded into 96-well plates and after three weeks of culture single cell wells were tested for EGFR knockout by Sanger sequencing, Western blot, and fluorescence-activated cell sorting (FACS) analysis. We could not establish HCC827 EGFR−/− cells, which is probably due to the strong dependence of these cells to EGFR signaling [30,31].
2.4. Western Blot
Cells were lysed using ELB-softer (150 mM NaCl, 50 mM Hepes pH = 7.5, 5 mM EDTA, 0.1% NP-40) with Protease Inhibitor Cocktail (Thermo Fisher Scientific, Waltham, MA, USA), and PhosSTOP Phosphatase Inhibitor Cocktail (Roche, Mannheim, Germany). Protein concentrations were measured using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. Twenty micrograms of each sample were separated by pre-cast 5–15% SDS-PAGE (Bio-Rad, Hercules, CA, USA) and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% skimmed milk in PBST for 1 h at room temperature (RT) and incubated overnight at 4 °C with the following primary antibodies—MAPK (Erk) Antibody (#9102, 1:1000), Akt Antibody (#9272, 1:1000), Phospho-EGF Receptor (Tyr1068) (#2234, 1:1000), p(Thr308)-Akt (#9275, 1:1000), Phospho-Akt (Ser473) (#9271, 1:1000), Phospho-MAPK (pERK) (#9101, 1:1000), and β-Actin (#4967, 1:10000) from Cell Signaling Leiden, The Netherlands, anti-EGFR (1005, sc-03-G, 1:1000) from Santa Cruz Biotechnology Inc., and anti-CXCR7 (GTX100027, 1:1000) from GeneTex, Irvine, CA, USA—followed by treatment with the secondary antibodies goat anti-rabbit HRP (#P0448, 1:2000) or rabbit anti-mouse HRP (#P0260, 1:2000), depending on the primary antibody, from DakoCytomation, Glostrup, Denmark, for 1 h at RT. The bands were visualized using the Western Lightning Plus-ECL kit (PerkinElmer, Waltham, MA, USA) and quantified by GeneSnap image analysis software (SynGene, Frederick, MD, USA).
2.5. DNA Isolation, Polymerase Chain Reaction (PCR), and Sequencing
Cells were harvested by trypsin and washed with PBS. DNA was isolated using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Exons 2 and 3 of the EGFR were amplified by PCR using standard procedures. PCR was performed using 150 ng of genomic DNA in a final volume of 25 µL containing 1× PCR buffer, 0.25 µL of Pfu DNA polymerase (Thermo Fisher Scientific, Waltham, MA, USA) and 100 nM primers (Table S2). The PCR program comprised one cycle of 98 °C for 2 min, 30 cycles of 98 °C for 10 s, of 59 °C for 15 s, and of 72 °C for 30 s, and one cycle of 72 °C for 10 min. PCR products were analyzed on 1.5% agarose gel. PCR products were purified using the Wizard SV Gel and PCR Clean-Up Kit (Promega, Madison, WI, USA) according to the company’s protocol and sequenced at Macrogen Europe (Amsterdam, The Netherlands).
2.6. Colony Formation Assay
Total numbers of 500 or 1000 cells were seeded in a single well of a 6-well plate and treated with 100 nM cetuximab or 5 μM gefitinib for 12 days at 37 °C in a humidified CO2 incubator. The medium was then removed and cells were washed with PBS, followed by cell fixation using 4% formaldehyde. Cells were stained with 1% crystal violet and colonies were counted. Experiments were performed in triplicate and repeated at least three times.
To test the effects of EGF and CXCR7 inhibitor on cell proliferation, 10,000 cells were seeded in a 12-well plate and grown for 6 days at 37 °C in a humidified CO2 incubator. The medium was removed, and cells were washed with PBS, followed by cell fixation using 4% formaldehyde. Cells were stained with 1% crystal violet. For quantification of the staining, 1 mL of 10% acetic acid was used for each well to extract the dye, and the absorbance was measured at wavelength of 590 nm.
2.7. Wound Healing Assay
The A549 EGFRwt/wt and EGFR−/− cells were seeded in 6-well plates at a density of 7 × 105 cells per well and starved overnight in DMEM containing 1% FBS. A wound was gently made by scraping the cells with a sterile 200 μL pipette tip. The detached cells were removed using PBS. Pictures were taken at 0 h, 12 h, and 24 h using a CK2 inverted microscope (Olympus, Tokyo, Japan). Experiments were performed in triplicate and repeated at least three times.
2.8. Cell Migration Assay
A total number of 1 × 104 cells in DMEM containing 1% FBS was added to the top Transwell insert. The 24-well plate wells were filled with a 750 μL culture medium. The Transwell insert was gently added to the 24-well plate and incubated at 37 °C with 5% CO2 for 20–24 h. Medium and remaining cells were carefully removed from the top of the membrane. The insert membrane was stained with 0.5% crystal violet. Invaded cells were photographed randomly and counted. Experiments were performed in triplicate and repeated at least three times.
2.9. MTS Assay
A total of 3 × 103 cells were seeded per well in 96-well plates and cultured for 24 h. Cells were then treated with appropriate drugs for 72 h. Next, cells were incubated at 37 °C with a medium containing MTS for 90 min following the protocol of CellTiter 96 AQueous One Solution Reagent (Promega, Madison, WI, USA). The absorbance was determined at a wavelength of 490 nm using a Synergy H1 plate reader (BioTek, Winooski, VT, USA). Experiments were performed in triplicate and repeated at least three times.
2.10. Flow Cytometric Analysis
Cells were harvested, washed twice with standard FACS buffer (PBS/1%FBS), and incubated with a primary antibody (cetuximab) or a human IgG isotype as a negative control (Invitrogen, Carlsbad, CA, USA) for 1 h on ice. Cells were then washed with FACS buffer, followed by 1 h incubation with a goat anti-human IgG (H + L) Cross-Adsorbed secondary antibody, Alexa Fluor 488. EGFR membrane expression was determined using a FACSCalibur flow cytometer (BD, Franklin Lakes, NJ, USA).
2.11. RNA Isolation and Quantitative Reverse Transcriptase PCR (qRT-PCR)
Cells were harvested by trypsin and washed with PBS. RNA was isolated using the Maxwell LEV simply RNA Cells/Tissue Kit (Promega, Madison, WI, USA). RNA concentrations were measured using NanoDrop (Thermo Fisher Scientific, Waltham, USA). cDNA was synthesized from 200 ng of total RNA using the Reverse Transcription kit (Promega, Madison, WI, USA) according to the manufacturer’s instruction.
qRT-PCR was performed using 20 ng of cDNA as input and SensiMix SYBRkit (Bioline, Taunton, MA, USA) in an ABI Prism 7900HT Sequence Detection System (Thermo Fisher Scientific, Waltham, MA, USA). Primer sets are listed in Table S2. Data analysis was performed using SDS v.2.3 software (Applied Biosystems, Foster City, CA, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels were measured and used as reference for data normalization.
2.12. CXCR7 Knockdown Using siRNAs
Approximately 3 × 105 cells per well were cultured for 24 h in a 6-well plate. Cells were then transfected with a mixture of CXCR7-specific validated siRNAs (Thermo Fisher Scientific, Waltham, USA) and negative control siRNAs (Thermo Fisher Scientific, Waltham, WI, USA) using Lipofectamine 3000 (Invitrogen, Carlsbad, USA) according to the manufacturer’s protocol. Total RNA was isolated 72 h after transfection. Experiments were repeated three times.
2.13. EGFR Rescue in A549 EGFR−/− Cells
A total of 3 × 105 A549 EGFR−/− cells per well were cultured for 24 h in a 6-well plate. Cells were transfected with pCDNA6A-EGFR wild-type plasmids using Lipofectamine 3000 (Invitrogen, Carlsbad, USA). Culture medium was changed one day after transfection, followed by blasticidin selection with an appropriate concentration for the next three days. EGFR expression level was determined by Western blot. Experiments were repeated three times. pCDNA6A-EGFR wild-type plasmid was a gift from Mien-Chie Hung (Addgene plasmid #42665).
2.14. Patient Tumor Samples and Immunohistochemistry (IHC)
A total of 47 patients with lung cancer were included in the study. A tissue microarray (TMA) containing 43 formalin fixed paraffin embedded (FFPE) primary lung tumor samples from the Department of Pathology, University Medical Center Groningen (UMCG), and 4 additional FFPE primary lung tumor samples from The First Hospital of Lanzhou University were used to detect CXCR7 protein expression using IHC. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Briefly, 4 μm FFPE sections were deparaffinized using xylene for 10 min. Next, slides were incubated with Tris/HCl (pH = 9) in a microwave. After blocking endogenous peroxidase activity with hydrogen peroxide, slides were incubated with the anti-CXCR7 primary rabbit polyclonal antibody (GTX100027, GeneTex, Irvine, USA) for 1 h at RT. Slides were then incubated with peroxidase-labeled goat anti-rabbit secondary and rabbit anti-goat tertiary antibodies (Dako, Denmark) for 30 min at RT. Visualization was performed using ImmPACT NovaRED Peroxidase (HRP) Substrate (Vector Laboratories, Burlingame, CA, USA) followed by hematoxylin staining. Scoring of the slides was performed by an experienced pulmonary pathologist (WT).
2.15. Statistical Analysis
The data are presented as mean ± SD, except where otherwise indicated. Data are derived from three or more independent experiments (unless otherwise indicated), and statistical analysis was performed using GraphPad software v.5.0 (GraphPad Software, La Jolla, CA, USA). Results were analyzed by a two-tailed unpaired student’s t-test unless otherwise noted. A chi-square test was used to determine significance in IHC results. p-values less than 0.05 were considered significant. The synergic analysis was performed using CompuSyn Program (ComboSyn, Paramus, NJ, USA).
3. Results
3.1. EGFR Knockout in A549 Cell Line
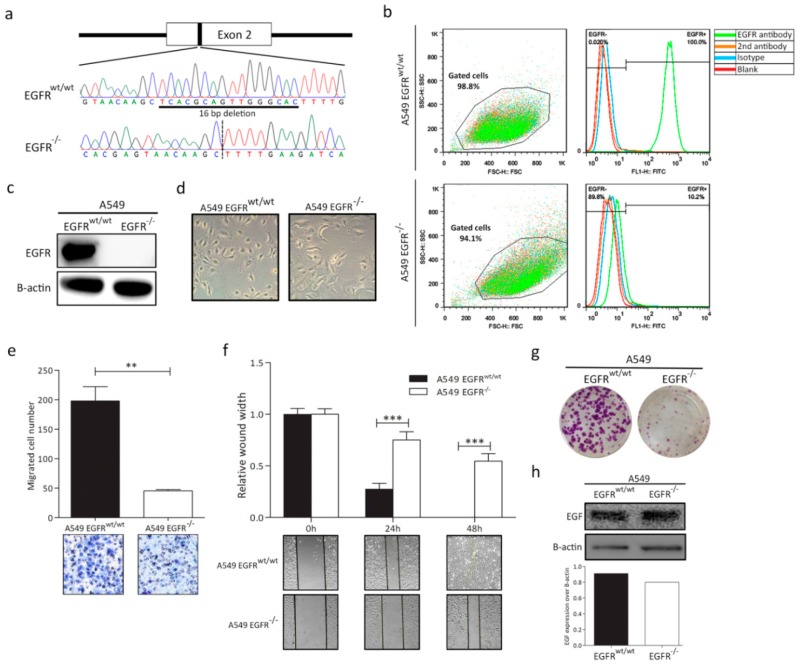
A549 is an adenocarcinoma cell line that contains a wild-type EGFR and mutant KRAS. To generate an A549 EGFR knockout cell lines, a CRISPR/Cas9 approach was applied using three gRNAs targeting exons 2 and 3 of EGFR (Table S1). Sanger sequencing revealed a 16 bp deletion in exon 2 of EGFR, which resulted in a premature stop codon, L79X (Figure 1a). Another independent clone with 1 bp insertion in exon 3 was shown in Figure S1. FACS and Western blot showed expression of EGFR in the wild-type A549 cells, while it was totally absent in the EGFR knockout cell line (Figure 1b,c and Figure S2).
Figure 1.
CRISPR/Cas9-mediated epidermal growth factor receptor (EGFR) knockout in non-small cell lung cancer (NSCLC) A549 cells and characterization of A549 EGFRwt/wt and EGFR−/− cells. (a) Schematic diagram of guide RNAs (gRNAs) targeting exon 2 of the EGFR gene in A549 cells and validation by Sanger sequencing. (b) FACS analysis shows EGFR expression in EGFRwt/wt and EGFR−/− cells. (c) Western blot demonstrating EGFR expression in EGFRwt/wt and absence of EGFR protein in EGFR−/− cells. (d) Morphologic changes of EGFRwt/wt and EGFR−/− cells. Magnification: 10x(e) Cell migration analysis using a Transwell assay. (f) Wound healing assay to evaluate wound closure and cell migration ability at different time points. (g) Colony formation of EGFRwt/wt and EGFR−/− cells. (h) Western blot showing EGF expression in A549 EGFRwt/wt and EGFR−/− cells. Data in bar graphs are represented as mean ± SD (n ≥ 3); two-tailed unpaired student’s t-test: * p-values < 0.05; ** p-values < 0.01; *** p-values < 0.001; ns: not significant.
3.2. EGFR Plays a Significant Role in Cell Progression in KRAS-Dependent Lung Cancer Cells
To characterize the phenotypic effect of EGFR loss in KRAS-dependent NSCLC cells, we examined the proliferation and migration ability of the A549 EGFRwt/wt and EGFR−/− cells. We observed the morphological changes in EGFR−/− cells. Compared to the normal spindle shaped A549 cells, A549 EGFR−/− cells showed a more flat and irregular polygonal shape (Figure 1d). A549 EGFR−/− cells showed significant attenuated proliferation and migration properties (Figure 1e–g and Figure S3). To exclude the interference of endogenous EGF, we examined the expression of EGF by Western blot. We did not observe any obvious change in EGF levels in A549 EGFRwt/wt and EGFR−/− cells (Figure 1h).
3.3. EGFR Knockout Does Not Significantly Affect Drug Sensitivity
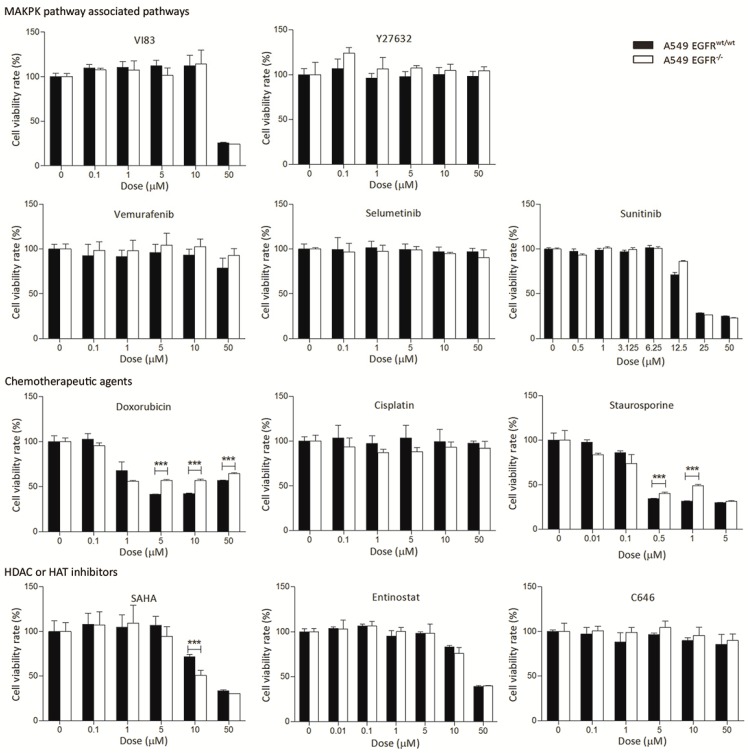
To determine drug response changes in A549 EGFRwt/wt and EGFR−/− cells, we tested a series of targeted and chemotherapy drugs, including anti VEGFR, BRAF, PDGFR, MEK, and HDAC/HAT, cisplatin, doxorubicin, and staurosporine. At low to intermediate doses of the drugs, we did not observe substantial differences between EGFRwt/wt and EGFR−/− cells (Figure 2). At higher levels, A549 EGFR−/− cells showed slightly more resistance to doxorubicin and cisplatin than EGFRwt/wt cells did. We also observed more resistance to staurosporine, a well-known apoptosis inducer, in A549 EGFR−/− cells (Figure 2). However, the EGFR−/− cells were more sensitive to SAHA as compared to the EGFRwt/wt cells at a dose of 10 µM.
Figure 2.
Treatment of the A549 EGFRwt/wt and EGFR−/− cells with different drugs. Cells were treated with drugs at indicated concentrations for 72 h and cell viability was determined with an MTS assay. For staurosporine, cell viability was determined after 24 h due to its toxicity. Data in bar graphs are represented as mean ± SD (n ≥ 3); two-tailed unpaired student’s t-test: * p-values < 0.05; ** p-values < 0.01; *** p-values < 0.001; ns: not significant.
3.4. Effect of EGFR Loss on Downstream Pathways
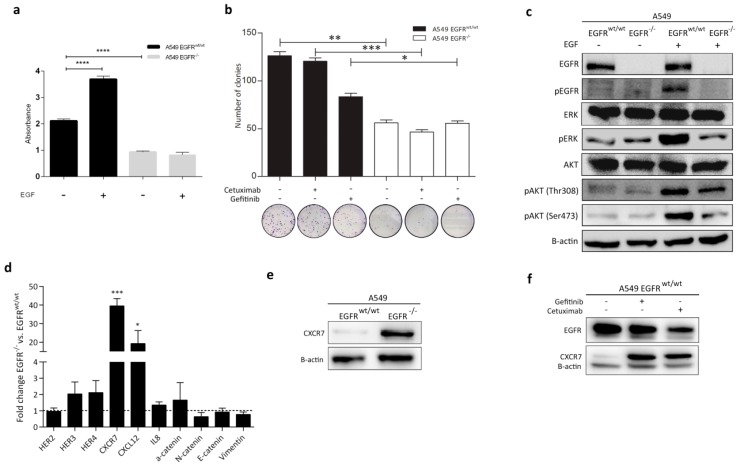
To test the effect of EGF on cell growth, we performed a clonogenic proliferation assay in the presence and absence of EGF in A549 EGFRwt/wt and EGFR−/− cells. As expected, EGF can stimulate EGFRwt/wt cell proliferation but did not affect it in EGFR−/− cells (Figure 3a). Thus, EGFR loss resulted in decreased cell proliferation with or without EGF (Figure 3a). EGFR loss also led to a stronger inhibition of cell proliferation as compared to gefitinib and cetuximab treatment (Figure 3b). As EGFR plays crucial roles in the PI3K-Akt and MAPK/ERK pathways, we determined changes in these two key pathways after EGFR loss. We investigated expression of EGFR, pEGFR, Akt, pAkt (Thr308 and Ser473), and MAPK (pERK) by Western blot. After stimulation with EGF, the induction of pEGFR, pERK, and pAkt (Thr308 and Ser473) was substantially lower in A549 EGFR−/− cells in comparison with EGFRwt/wt cells (Figure 3c). In contrast, pERK levels were slightly higher in A549 EGFR−/− cells than the wild-type cells in the absence of EGF (Figure 3c).
Figure 3.
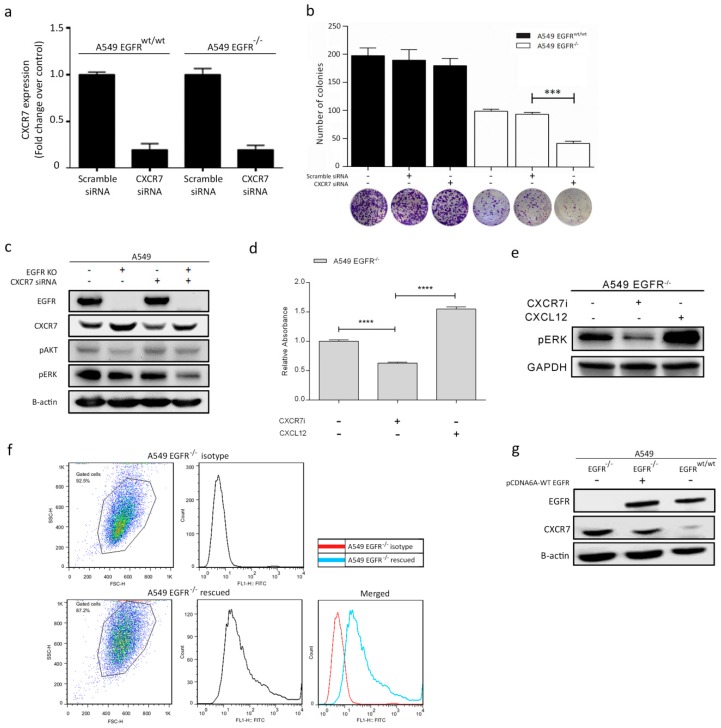
Effect of different treatments on cell proliferation and expression of EGFR downstream signaling proteins in A549 EGFRwt/wt and EGFR−/− cells. (a) Proliferation analysis of EGFRwt/wt and EGFR−/− cells in the presence and absence of EGF. Cells were cultured in serum-starved medium (2% serum). (b) Colony formation ability of EGFRwt/wt and EGFR−/− cells before and after treatment. (c) Western blot analysis of EGFR downstream proteins before and after stimulation with EGF for 15 min. (d) Relative mRNA expression of the selected genes. (e) Western blot analysis of CXCR7 in EGFRwt/wt and EGFR−/− cells. (f) CXCR7 protein expression in EGFRwt/wt cells treated with 0.5 µM gefitinib and 100 nM cetuximab for 72 h. Data in bar graphs are represented as mean ± SD (n ≥ 3); two-tailed unpaired student’s t-test: * p-values < 0.05; ** p-values < 0.01; *** p-values < 0.001.
3.5. CXCR7 Is Significantly Upregulated in A549 EGFR−/− Cells and EGFRwt/wt Cells Treated with EGFR Inhibitors
We performed qRT-PCR on a panel of genes associated with the EGFR/MAPK pathway and epithelial-mesenchymal transition (EMT) (Table S2). Surprisingly, CXCR7 showed a marked upregulation at the RNA and protein levels in A549 EGFR−/− cells (Figure 3d,e and Figure S4). In addition, we observed overexpression of CXCL12 (19-fold), a main ligand for CXCR7, in EGFR−/− cells (Figure 3d). Treatment of the EGFRwt/wt cells with gefitinib or cetuximab for 72 h resulted in an increased CXCR7 expression level (Figure 3f). This shows that inhibition or loss of EGFR induces CXCR7 expression in A549 cells and may subsequently contribute to tumor survival.
In contrast to CXCR7, moderate changes were observed in the expression of HER3 (twofold higher) and HER4 (twofold higher) in A549 EGFR−/− cells as compared to EGFRwt/wt cells (Figure 3d). Differences in HER2 mRNA levels were negligible. We did not observe any significant changes in the expression of EMT-related markers. Only the levels of α-catenin mRNA were slightly increased by 1.6-fold in A549 EGFR−/− cells (Figure 3d).
3.6. Dual Inhibition of CXCR7 and EGFR Downregulates MAPK (ERK1/2) and Suppresses Proliferation
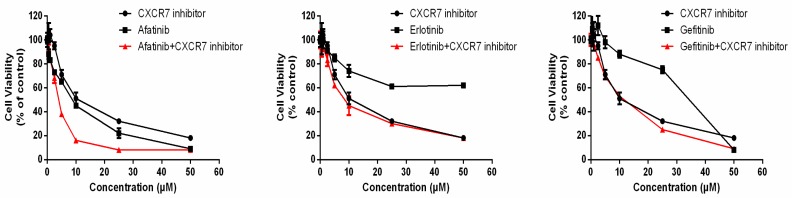
We investigated the synergistic inhibitory effect of gefitinib, erlotinib, and afatinib with a CXCR7 inhibitor. We observed a significant synergistic inhibitory effect of CXCR7 inhibitor combined with afatinib (Figure 4 and Table 1). However, a combination of CXCR7 inhibitor with erlotinib or gefitinib did not show an obvious inhibitory effect (Figure 4).
Figure 4.
Dual inhibition of CXCR7 and EGFR. Combination of afatinib and CXCR7 inhibitor induces a synergistic inhibitory effect in A549 cell line. Cells were treated with drugs at indicated concentrations for 72 h, and cell viability was determined with an MTS assay.
Table 1.
Effect of concomitant combination of EGFR-TKI (TKI: tyrosine kinase inhibitor) and CXCR7 inhibitor on A549 cells based on the Chou and Talalay method.
| Combination Treatment | TKIs (µM) | CXCR7i (µM) | FA | CI | Effect |
|---|---|---|---|---|---|
| Afatinib + CXCR7i | 10 | 10 | 0.84 | 0.47 | Synergistic |
| Erlotinib + CXCR7i | 10 | 10 | 0.55 | 0.74 | Moderately Synergistic |
| Gefitinib + CXCR7i | 10 | 10 | 0.47 | >1.1 | Antagonistic |
FA represents the fraction of growth effect of drug-treated cells compared with control cells and CI represents the combination index. CI = 1: additivity; CI > 1: antagonism; CI < 1: synergism.
Next, we knocked down CXCR7 by siRNAs in A549 EGFRwt/wt and EGFR−/− cells to explore whether cell proliferation can be further suppressed (Figure 5a,b). We showed that CXCR7 knockdown alone did not change A549 EGFRwt/wt cell growth. However, CXCR7 knockdown significantly decreased proliferation of A549 EGFR−/− cells (Figure 5b).
Figure 5.
The interaction of EGFR and CXCR7 and their effect on downstream pathways and cell proliferation in A549 EGFRwt/wt and EGFR−/− cells. (a) CXCR7 mRNA levels after transient transfection with either scramble siRNA or CXCR7 siRNA. (b) Colony formation assay to determine the effect of CXCR7 knockdown on cell proliferation. (c) Western blot analysis of CXCR7, pAKT, and pERK protein expression levels after CXCR7 knockdown. (d) Colony formation assay to determine the effects of the CXCR7 inhibitor and CXCL12 on cell proliferation. (e) Western blot analysis of pERK after treatment of EGFR−/− cells with CXCR7 inhibitor (2 μM) for 24 h and CXCL12 (100 ng/mL) for 3 min. (f) FACS analysis shows EGFR expression in A549 EGFR−/− following transfection with EGFR pCDNA. (g) Western blot analysis of EGFR and CXCR7 in A549 EGFR−/− cells transfected with EGFR pCDNA, EGFR−/−, and EGFRwt/wt cells. Data in bar graphs are represented as mean ± SD (n ≥ 3); two-tailed unpaired student’s t-test: * p-values < 0.05; ** p-values < 0.01; *** p-values < 0.001; ns: not significant.
To gain insight into the CXCR7-dependent mechanisms that lead to tumor cell proliferation, we checked MAPK (ERK1/2) expression. CXCR7 knockdown decreased pERK levels in A549 EGFR−/− cells as compared to EGFRwt/wt cells (Figure 5c). Moreover, CXCR7 inhibition can further restrain the proliferation of EGFR−/− cells (p < 0.001) (Figure 5d) and decrease pERK levels (Figure 5e). Conversely, stimulation of CXCR7 with its ligand CXCL12 promoted proliferation of A549 EGFR−/− cells (Figure 5d) and increase pERK levels (Figure 5e). Taken together, our results suggest that CXCR7 induces proliferation by enhancing pERK levels in A549 EGFR−/− cells.
3.7. Rescue of EGFR Leads to CXCR7 Downregulation in A549 EGFR−/− Cells
To further confirm the interaction between EGFR and CXCR7, we rescued EGFR in A549 EGFR−/− cells. Overexpression of wild-type EGFR in A549 EGFR−/− cells restored EGFR membrane expression (Figure 5f). In the control groups, EGFR signals were not detected. Interestingly, CXCR7 expression dramatically decreased after the reintroduction of EGFR to levels slightly above those in EGFRwt/wt cells (Figure 5g). These data suggest a tight functional regulatory link between EGFR and CXCR7.
3.8. CXCR7 Expression, Altered by the Ablation of EGFR, Is Associated with the Genotype of NSCLC
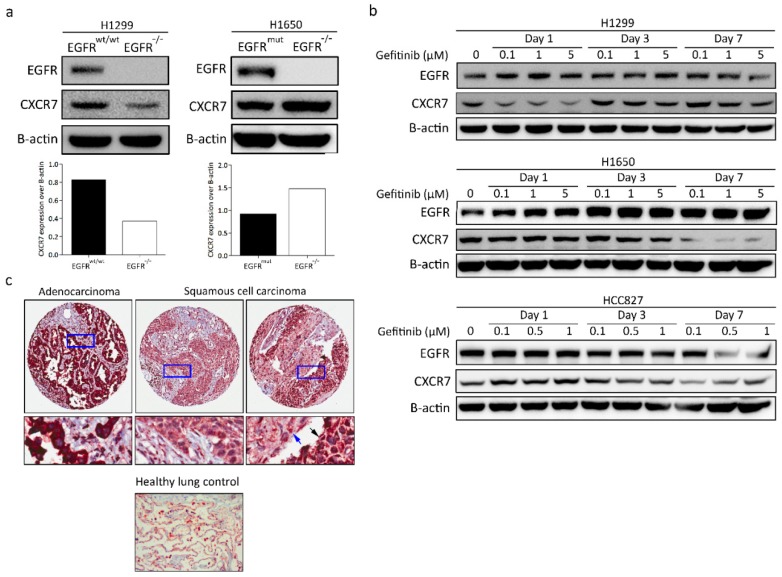
We next determined whether CXCR7 shows similar effects in lung cancer cells with different genetic backgrounds. Knockout of EGFR in H1299 cells, with wild-type EGFR, led to a dramatic decrease in CXCR7 levels (Figure 6a). Knockout of EGFR in H1650, with exon 19 deletions in EGFR, revealed higher expression of CXCR7.
Figure 6.
CXCR7 expression in different NSCLC cell lines with or without gefitinib treatment and CXCR7 expression in NSCLC patients. (a) Western blot analysis of H1299, H1650 EGFRwt/wt, and EGFR−/− cells. (b) Western blot analysis of H1299, H1650, and HCC827 cells treated with different doses of gefitinib over time. (c) Immunohistochemical staining of CXCR7 in NSCLC primary tumor samples (n = 47). The blue and black arrows show the expression of CXCR7 in mature and less differentiated squamous cell lung carcinoma (SQCC), respectively.
Next, we treated H1299, H1650, and HCC827 cells with gefitinib to investigate changes in the expression levels of CXCR7. H1299 is resistant to gefitinib (IC50, 40 µmol/L) [32]. In contrast to the H1299 EGFR−/− cells, H1299 parental cells showed a decreased CXCR7 expression level after one-day treatment with gefitinib and an increased expression with a low dose of gefitinib three and seven days after the start of treatment (Figure 6b).
Interestingly, both CXCR7 and EGFR showed a highly dose- and time-dependent expression pattern in H1650 cells after treatment with gefitinib (Figure 6b). Moreover, EGFR and CXCR7 expression showed a clear negative correlation upon gefitinib treatment. Particularly, CXCR7 expression was decreased, whereas EGFR expression increased after treatment with different doses of gefitinib over time in H1650 (Figure 6b). Both EGFR and CXCR7 expression increased after one day of treatment with gefitinib (Figure 6b). However, in EGFR-mutant NSCLC cell lines (HCC827 and H1650), EGFR and CXCR7 expression substantially decreased after longer treatment (three and seven days) with gefitinib in a dose-dependent manner (Figure 6b). Thus, in EGFR-mutant NSCLC cell lines (HCC827 and H1650), CXCR7 expression levels decreased with gefitinib treatment. Conversely, in EGFR wild-type NSCLC cell lines (H1299 and A549), CXCR7 expression levels increased after gefitinib treatment (Figure 3f and Figure 6b). Together, these data indicate that CXCR7 and EGFR have a close interaction that is dependent on the EGFR genotype.
3.9. CXCR7 Is Highly Expressed in Primary Lung Adenocarcinoma
To investigate the clinical relevance of CXCR7 expression in NSCLC patients, we evaluated CXCR7 expression in 47 primary NSCLC patients by IHC. Immunostaining revealed strong cytoplasmic expression of CXCR7 in 74.5% (35/47), weak expression in 17% (8/47), and heterogeneous expression (weak and strong) in 8.5% (4/47) of the tumor samples (Figure 6c and Table 2). CXCR7 expression was weak in the healthy lung tissue (Figure 6c).
Table 2.
CXCR7 expression in lung cancer patients.
| Subtype | Strong (%) | Weak (%) | Strong-Weak (%) | Total | p-Value |
|---|---|---|---|---|---|
| Histology | 0.005 * | ||||
| ADC | 23 (92) | 2 (8) | 0 | 25 | |
| SQCC | 8 (44.5) | 6 (33.3) | 4 (22.2) | 18 | |
| Other | 4 (100) | 0 | 0 | 4 |
* Chi-square test.
CXCR7 expression was strong in the vast majority of the ADCs (92%). In contrast to ADC cases, only 40.5% of SQCC tumors showed strong CXCR7 expression. Approximately 33.3% had a weak expression, and 22.2% of the SQCC tumors showed an intra-heterogeneous CXCR7 expression pattern. In this heterogeneous group, the expression of CXCR7 was weaker in more differentiated tumor cells as compared to the less differentiated ones (Figure 6c and Table 2). Other subtypes (NSCLC NOS and pleomorphic carcinoma; n = 4) showed strong expression. These results suggest that CXCR7 may play a role in the development of different histological subtypes of NSCLC tumors.
4. Discussion
KRAS and EGFR are commonly mutated in NSCLC. However, whether targeting EGFR is an option for the treatment of KRAS-dependent NSCLC is not clear. In this study, we showed that EGFR plays a critical role in the proliferation of KRAS-dependent cells. Furthermore, we report a novel genotype-dependent crosstalk between EGFR and CXCR7 in NSCLC cells. We showed that EGFR disruption or blockage can lead to upregulation of CXCR7 and activation of the MAPK signaling pathway, resulting in outgrowth or survival advantage of the KRAS-dependent cells. We also demonstrated that dual inhibition of EGFR and CXCR7 suppressed tumor cell growth and reduced MAPK activity by downregulation of pERK levels. Dual inhibition of EGFR and CXCR7 by inhibitors leads to a synergistic effect, which may provide a novel therapeutic approach for NSCLC patients. We also showed that CXCR7 is overexpressed in ADC patients as compared to the SQCC cases.
KRAS-mutant tumor cells appear to rely on KRAS for cell proliferation [33]. In this study, we showed that EGFR also plays an essential role in the survival of NSCLC cells harboring KRAS mutations. We also observed morphological changes in EGFR−/− lung tumor cells as well as substantial reduction in migration ability of these cells. It is not surprising that previous studies have shown that gefitinib is not efficient for the treatment of NSCLC patients with wild-type EGFR, because gefitinib has a higher affinity to the mutated version of EGFR, such as L858R point mutation, as compared to wild-type EGFR [34]. Anti-EGFR therapy with EGFR blocking antibody cetuximab shows resistance due to dysregulation of EGFR and activation of other HER family receptors [35]. Thus, these studies do not show that wild-type EGFR is not necessary for tumor proliferation. Based on our results, wild-type EGFR is important for proliferation and migration of KRAS-mutant tumor cells.
We used 11 inhibitors, including MAPK associated inhibitors, chemotherapeutic agents, HDAC and HAT inhibitors, and an apoptosis inducer agent to evaluate the drug response in A549 EGFRwt/wt and EGFR−/− cells. However, we did not observe significant differences between the two genotypes. These results indicate the ineffectiveness of clinically available drugs for the treatment of KRAS-mutant NSCLC cells. In contrast, simultaneous inhibition (inhibitors or knockdown) of EGFR and CXCR7 significantly attenuated cancer cell growth. Based on our results, we propose a potential novel strategy for the treatment of KRAS-dependent NSCLC by dual inhibition of EGFR and CXCR7. Further in vivo studies are needed to identify appropriate delivery methods that can efficiently and specifically target EGFR and CXCR7 in tumor cells [36,37].
CXCR7 contributes to angiogenesis and cell growth in lung tumors [21,23,38,39]. In addition, overexpression of CXCR7 was associated with poorer prognosis in lung cancer patients [33]. The latter effect was probably induced by the activation of the MAPK pathway [28,40]. We showed that EGFR blockage or knockout leads to reduced growth and a significant increase in CXCR7 levels in A549 cells. CXCR7 knockdown decreased pERK levels and further suppressed proliferation of A549 EGFR−/− cells. Dual inhibition of EGFR and CXCR7 by inhibitors can achieve a synergistic inhibitory effect. Thus, our observations suggest that CXCR7 overexpression is one of the survival mechanisms in KRAS-dependent tumor cells with EGFR loss or cells treated with EGFR-TKIs. Interestingly, we only observed the inhibitory effect of concomitant combination of CXCR7 inhibitor and afatinib, but not gefitinib or erlotinib. One possible explanation is that afatinib is an irreversible HER family inhibitor. Gefitinib and elortinib only have a strong inhibitory effect on mutant EGFR, but afatinib can effectively inhibit both wild-type and mutant EGFR [41,42]. It has previously been shown that afatinib can suppress tumor growth in KRAS-mutant NSCLCs, but not erlotinib or gefitinib. Interestingly, our in vitro results are in concordance with a previously published in vivo study [43]. These observations demonstrate that dual inhibition of CXCR7 and EGFR have a synergistic therapeutic effect in KRAS-mutant NSCLC cells.
CXCL12 is involved in cell proliferation and brain metastasis in lung cancer patients [39]. CXCL12 is overexpressed in NSCLC cell lines and primary lung cancer [44] and may promote tumor cell migration and growth through the ERK pathway [45]. We observed an increased expression of CXCL12, a shared ligand of CXCR7 and CXCR4 with a higher affinity towards CXCR7, in A549 EGFR−/− cells. CXCR7 knockdown is highly efficient in the suppression of cell proliferation of A549 EGFR−/− cells, as shown in our study. However, other ligands of CXCR7 such as CXCL11, MIF, and Dickkopf-3 may also influence CXCR7-mediated cell proliferation, which requires further investigation. We did not observe significant changes in the expression levels of other HER family receptors including HER2, HER3, and HER4 in A549 EGFR−/− cells. Thus, our data suggest that HER family member receptors are not significantly affected by EGFR loss.
Our results show that MAPK/pERK is continuously activated in both EGFR−/− and EGFRwt/wt cells. This is indicative of an EGFR-independent survival mechanism, which may be partly explained by the presence of KRAS activating mutations in these cells. KRAS mutations can lead to constant activation of RAS/RAF/MEK/ERK [46]. Furthermore, as EGFR is upstream of the RAS pathway, loss of EGFR may not affect activation of RAS/RAF/MEK/ERK introduced by mutant KRAS. Notably, one study showed that KRAS pathways coordinate the transduction of CXCL12/CXCR4/CXCR7 and influence growth of pancreatic cancer [47]. It will be of great interest to explore whether CXCR7 expression, altered by the ablation of EGFR, is associated with KRAS in NSCLC. We showed that EGF can still stimulate pAkt in EGFR−/− cells. This observation indicates that EGF-induced phosphorylation of Akt may be independent of EGFR, which to our knowledge has not been reported previously. One hypothesis is that EGF stimulates pAkt via CXCR7, because previous studies have shown that CXCR7 associated pathways can induce Akt phosphorylation [48,49], and we observed CXCR7 overexpression in A549 EGFR−/− cells. In addition, one recent study reported that Akt activation is related to EGFR drug resistance in lung cancer [50]. Given the importance of Akt for tumor survival and growth [51], future studies are needed to explore the crosstalk between EGF-EGFR and CXCL12-CXCR7 in the MAPK pathway.
We found a close inverse relationship between EGFR and CXCR7 expression (Figure 7). When EGFR is expressed in the A549 EGFR−/− cells, a decreased expression of CXCR7 is observed. Mechanisms leading to CXCR7 upregulation in EGFR−/− cells are still not clear. Previous studies showed that the CXCR7 promoter contains five NF-kB binding sites and activated NF-kB can upregulate CXCR7 [52]. Thus, one potential mechanism can be that CXCR7 is transcriptionally activated by NF-kB. Several studies have shown that NF-kB activation drives survival of tumor cell treated with EGFR-TKIs [53,54,55,56]. Another possibility is that CXCR7 overexpression may be related to the mutational status of KRAS [47].
Figure 7.
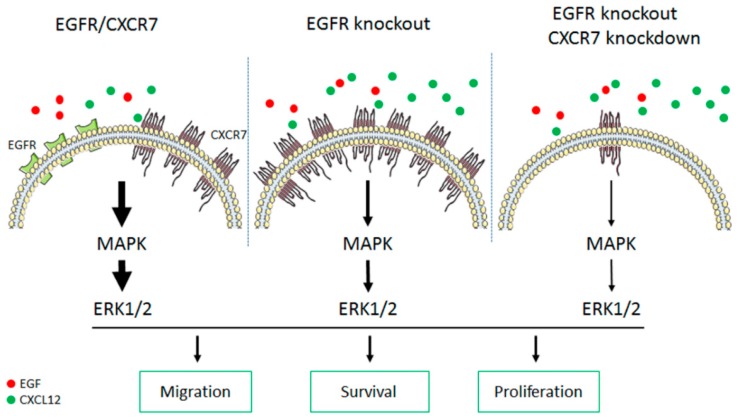
The proposed model of CXCR7-EGFR crosstalk and its effect on the MAPK signaling pathway. EGFR-ERK and CXCR7-ERK signaling pathways promote tumor cell development (left). Ablation of EGFR leads to overexpression of CXCR7/CXCL12 and subsequently stimulates the ERK pathway, resulting in tumor cell survival (middle). Dual inhibition of EGFR and CXCR7 further suppresses ERK signaling and synergistically inhibits tumor growth (right). Thickness of the arrow shows strength of the signaling. The number of solid dots (in red or green) shows the amount of the endogenous epidermal growth factor (EGF) or CXCL12.
We showed that upon treatment with gefitinib, CXCR7 expression dramatically decreased in EGFR-mutant NSCLC cell lines, but, conversely, it is increased in EGFR wild-type NSCLC cell lines. Blocking mutant EGFR with TKIs can substantially inhibit MAPK/ERK and suppress tumor cell proliferation [57,58]. This indicates that EGFR-TKIs may reduce pERK and inhibit tumor cell proliferation partially through downregulation of CXCR7. Moreover, insensitivity or acquired resistance of EGFR wild-type NSCLC cells for TKIs might be due to increased CXCR7 levels. Our results show that CXCR7 expression is highly related to the genetic background of the lung cancer cells, at least in the three cell lines we tested, suggesting the importance of personalized therapy in lung cancer treatment. Importantly, we observed higher expression of CXCR7 in lung ADCs (92% strongly expressed CXCR7) as compared to lung SQCC cases (40.5% strongly expressed CXCR7). One study, which performed a meta-analysis in nearly 3000 patients, showed that EGFR was less expressed in ADC (39%) than in SQCC (58%) [59]. Given this study and our results, there may be a correlation between EGFR and CXCR7 in patients with NSCLC. Moreover, higher expression of CXCR7 in the less differentiated SQCC tumors may be due to its critical role during tumor initiation and the differentiation process of this tumor subtype. Higher expression of CXCR7 in lung cancer tissues as compared to the healthy lung tissue suggests CXCR7 may be a target for lung cancer treatment.
5. Conclusions
Our data show that wild-type EGFR plays a significant role in KRAS-mutant NSCLC cancer cells and revealed CXCR7 upregulation as a potential survival mechanism in KRAS-mutant cells upon EGFR loss (Figure 7). Our findings suggest that dual inhibition of EGFR and CXCR7 may be a promising therapeutic strategy for at least a subset of patients with NSCLC.
Acknowledgments
The authors gratefully acknowledge Rob Leurs and Martine J. Smit for providing the CXCR7 inhibitors. We acknowledge Petra E. van der Wouden for technical assistance during experiments. Bin Liu and Siwei Chen have received a PhD research fellowship from the China Scholarship Council.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/11/4/455/s1, Figure S1: Schematic diagram of gRNAs targeting exon3 of the EGFR gene in A549 cells and validation by Sanger sequencing for clone 1, Figure S2: Western blot demonstrating EGFR expression in EGFRwt/wt and absence of EGFR protein in EGFR−/− cells (Clone 1), Figure S3: Wound healing assay to evaluate wound closure and cell migration ability at different time points (Clone 1), Figure S4: qPCR and Western blot analysis of CXCR7 in A549 EGFRwt/wt and EGFR−/− cells (Clone 1), Table S1: List of gRNA sequences for CRISPR/Cas9, Table S2: List of the primer sets used for qRT-PCR.
Author Contributions
H.J.H., B.L., and A.S. designed the study. B.L., A.S., S.S., R.S., D.C., and S.C. performed the experimental work and analyzed data. B.N.M. evaluated PCR analyses. A.v.d.B., W.T., A.J.v.d.W., and W.H. provided the patient materials. W.T. scored IHC staining of the patient tumor samples. B.L. wrote the manuscript. All the authors analyzed the data. H.J.H supervised the project.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Wee P., Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers. 2017;9:52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchetti A., Martella C., Felicioni L., Barassi F., Salvatore S., Chella A., Camplese P.P., Iarussi T., Mucilli F., Mezzetti A., et al. EGFR Mutations in Non–Small-Cell Lung Cancer: Analysis of a Large Series of Cases and Development of a Rapid and Sensitive Method for Diagnostic Screening with Potential Implications on Pharmacologic Treatment. J. Clin. Oncol. 2005;23:857–865. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 5.Lee J.K., Hahn S., Kim D.W., Suh K.J., Keam B., Kim T.M., Lee S.H., Heo D.S. Epidermal growth factor receptor tyrosine kinase inhibitors vs conventional chemotherapy in non–small cell lung cancer harboring wild-type epidermal growth factor receptor: A meta-analysis. JAMA. 2014;311:1430–1437. doi: 10.1001/jama.2014.3314. [DOI] [PubMed] [Google Scholar]
- 6.Riely G.J., Marks J., Pao W. KRAS mutations in non-small cell lung cancer. Proc. Am. Thorac. Soc. 2009;6:201–205. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- 7.Krall E.B., Wang B., Munoz D.M., Ilic N., Raghavan S., Niederst M.J., Yu K., Ruddy D.A., Aguirre A.J., Kim J.W., et al. KEAP1 loss modulates sensitivity to kinase targeted therapy in lung cancer. Elife. 2017;6:e18970. doi: 10.7554/eLife.18970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ercan D., Xu C., Yanagita M., Monast C.S., Pratilas C.A., Montero J., Butaney M., Shimamura T., Sholl L., Ivanova E.V., et al. Reactivation of ERK Signaling causes resistance to EGFR kinase inhibitors. Cancer Discov. 2012;2:934–947. doi: 10.1158/2159-8290.CD-12-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saber A., Liu B., Ebrahimi P., Haisma H.J. CRISPR/Cas9 for overcoming drug resistance in solid tumors. DARU J. Pharm. Sci. 2019 doi: 10.1007/s40199-019-00240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B., Saber A., Haisma H.J. CRISPR/Cas9: A powerful tool for identification of new targets for cancer treatment. Drug Discov. Today. 2019 doi: 10.1016/j.drudis.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Nazarian R., Shi H., Wang Q., Kong X., Koya R.C., Lee H., Chen Z., Lee M.-K., Attar N., Sazegar H., et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–977. doi: 10.1038/nature09626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mou H., Moore J., Malonia S.K., Li Y., Ozata D.M., Hough S., Song C.-Q., Smith J.L., Fischer A., Weng Z., et al. Genetic disruption of oncogenic Kras sensitizes lung cancer cells to Fas receptor-mediated apoptosis. Proc. Natl. Acad. Sci. USA. 2017;114:3648–3653. doi: 10.1073/pnas.1620861114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Planchard D., Smit E.F., Groen H.J.M., Mazieres J., Besse B., Helland Å., Giannone V., D’Amelio A.M., Zhang P., Mookerjee B., et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. doi: 10.1016/S1470-2045(17)30679-4. [DOI] [PubMed] [Google Scholar]
- 14.Bhola N.E., Grandis J.R. Crosstalk between G-protein-coupled receptors and epidermal growth factor receptor in cancer. Front. Biosci. 2008;13:1857–1865. doi: 10.2741/2805. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Martín L., Sánchez-Mateos P., Cabañas C. CXCR7 impact on CXCL12 biology and disease. Trends Mol. Med. 2013;19:12–22. doi: 10.1016/j.molmed.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Libert F., Parmentier M., Lefort A., Dinsart C., Van Sande J., Maenhaut C., Simons M.J., Dumont J.E., Vassart G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989;244:569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- 17.Sreedharan S.P., Robichon A., Peterson K.E., Goetzl E.J. Cloning and expression of the human vasoactive intestinal peptide receptor. Proc. Natl. Acad. Sci. USA. 1991;88:4986–4990. doi: 10.1073/pnas.88.11.4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns J.M., Summers B.C., Wang Y., Melikian A., Berahovich R., Miao Z., Penfold M.E.T., Sunshine M.J., Littman D.R., Kuo C.J., et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J. Exp. Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alampour-Rajabi S., El Bounkari O., Rot A., Muller-Newen G., Bachelerie F., Gawaz M., Weber C., Schober A., Bernhagen J. MIF interacts with CXCR7 to promote receptor internalization, ERK1/2 and ZAP-70 signaling, and lymphocyte chemotaxis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015;29:4497–4511. doi: 10.1096/fj.15-273904. [DOI] [PubMed] [Google Scholar]
- 20.Issa Bhaloo S., Wu Y., LE Bras A., Yu B., Gu W., Xie Y., Deng J., Wang Z., Zhang Z., Kong D., et al. Binding of Dickkopf-3 to CXCR7 Enhances Vascular Progenitor Cell Migration and Degradable Graft Regeneration. Circ. Res. 2018;123:451–466. doi: 10.1161/CIRCRESAHA.118.312945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao Z., Luker K.E., Summers B.C., Berahovich R., Bhojani M.S., Rehemtulla A., Kleer C.G., Essner J.J., Nasevicius A., Luker G.D., et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc. Natl. Acad. Sci. USA. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J., Shiozawa Y., Wang J., Wang Y., Jung Y., Pienta K.J., Mehra R., Loberg R., Taichman R.S. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J. Biol. Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 23.Iwakiri S., Mino N., Takahashi T., Sonobe M., Nagai S., Okubo K., Wada H., Date H., Miyahara R. Higher expression of chemokine receptor CXCR7 is linked to early and metastatic recurrence in pathological stage I nonsmall cell lung cancer. Cancer. 2009;115:2580–2593. doi: 10.1002/cncr.24281. [DOI] [PubMed] [Google Scholar]
- 24.Würth R., Bajetto A., Harrison J.K., Barbieri F., Florio T. CXCL12 modulation of CXCR4 and CXCR7 activity in human glioblastoma stem-like cells and regulation of the tumor microenvironment. Front. Cell. Neurosci. 2014;8:144. doi: 10.3389/fncel.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wani N.A., Nasser M.W., Ahirwar D.K., Zhao H., Miao Z., Shilo K., Ganju R.K. C-X-C motif chemokine 12/C-X-C chemokine receptor type 7 signaling regulates breast cancer growth and metastasis by modulating the tumor microenvironment. Breast Cancer Res. 2014;16:R54. doi: 10.1186/bcr3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin L., Han M.-M., Wang F., Xu L.-L., Yu H.-X., Yang P.-Y. CXCR7 stimulates MAPK signaling to regulate hepatocellular carcinoma progression. Cell Death Dis. 2014;5:e1488. doi: 10.1038/cddis.2014.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salazar N., Muñoz D., Kallifatidis G., Singh R.K., Jordà M., Lokeshwar B.L. The chemokine receptor CXCR7 interacts with EGFR to promote breast cancer cell proliferation. Mol. Cancer. 2014;13:198. doi: 10.1186/1476-4598-13-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh R.K., Lokeshwar B.L. The IL-8 regulated Chemokine Receptor CXCR7 Stimulates EGFR Signaling to Promote Prostate Cancer Growth. Cancer Res. 2011;71:3268–3277. doi: 10.1158/0008-5472.CAN-10-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng Y., Li X., Qian X., Wang Y., Lee J.-H., Xia Y., Hawke D.H., Zhang G., Lyu J., Lu Z. Secreted and O-GlcNAcylated MIF binds to the human EGF receptor and inhibits its activation. Nat. Cell Biol. 2015;17:1348–1355. doi: 10.1038/ncb3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiessling M.K., Schuierer S., Stertz S., Beibel M., Bergling S., Knehr J., Carbone W., de Vallière C., Tchinda J., Bouwmeester T., et al. Identification of oncogenic driver mutations by genome-wide CRISPR-Cas9 dropout screening. BMC Genom. 2016;17:723. doi: 10.1186/s12864-016-3042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makinoshima H., Takita M., Matsumoto S., Yagishita A., Owada S., Esumi H., Tsuchihara K. Epidermal Growth Factor Receptor (EGFR) Signaling Regulates Global Metabolic Pathways in EGFR-mutated Lung Adenocarcinoma. J. Biol. Chem. 2014;289:20813–20823. doi: 10.1074/jbc.M114.575464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rho J.K., Choi Y.J., Ryoo B.-Y., Na I.I.I., Yang S.H., Kim C.H., Lee J.C. p53 enhances gefitinib-induced growth inhibition and apoptosis by regulation of Fas in non-small cell lung cancer. Cancer Res. 2007;67:1163–1169. doi: 10.1158/0008-5472.CAN-06-2037. [DOI] [PubMed] [Google Scholar]
- 33.Bryant K.L., Mancias J.D., Kimmelman A.C., Der C.J. KRAS: Feeding pancreatic cancer proliferation. Trends Biochem. Sci. 2014;39:91–100. doi: 10.1016/j.tibs.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yun C.-H., Boggon T.J., Li Y., Woo M.S., Greulich H., Meyerson M., Eck M.J. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: Mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wheeler D.L., Huang S., Kruser T.J., Nechrebecki M.M., Armstrong E.A., Benavente S., Gondi V., Hsu K.-T., Harari P.M. Mechanisms of acquired resistance to cetuximab: Role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bremer E., van Dam G.M., de Bruyn M., van Riezen M., Dijkstra M., Kamps G., Helfrich W., Haisma H. Potent Systemic Anticancer Activity of Adenovirally Expressed EGFR-Selective TRAIL Fusion Protein. Mol. Ther. 2018;16:1919–1926. doi: 10.1038/mt.2008.203. [DOI] [PubMed] [Google Scholar]
- 37.Maussang D., Mujić-Delić A., Descamps F.J., Stortelers C., Vanlandschoot P., Stigter-van Walsum M., Vischer H.F., van Roy M., Vosjan M., Gonzalez-Pajuelo M., et al. Llama-derived single variable domains (nanobodies) directed against chemokine receptor CXCR7 reduce head and neck cancer cell growth in vivo. J. Biol. Chem. 2013;288:29562–29572. doi: 10.1074/jbc.M113.498436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner P.L., Hyjek E., Vazquez M.F., Meherally D., Liu Y.F., Chadwick P.A., Rengifo T., Sica G.L., Port J.L., Lee P.C., et al. CXCL12 and CXCR4 in adenocarcinoma of the lung: Association with metastasis and survival. J. Thorac. Cardiovasc. Surg. 2009;137:615–621. doi: 10.1016/j.jtcvs.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 39.Cavallaro S. CXCR4/CXCL12 in Non-Small-Cell Lung Cancer Metastasis to the Brain. Int. J. Mol. Sci. 2013;14:1713–1727. doi: 10.3390/ijms14011713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajagopal S., Kim J., Ahn S., Craig S., Lam C.M., Gerard N.P., Gerard C., Lefkowitz R.J. Beta-arrestin- but not G protein-mediated signaling by the “decoy” receptor CXCR7. Proc. Natl. Acad. Sci. USA. 2010;107:628–632. doi: 10.1073/pnas.0912852107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgillo F., Della Corte C.M., Fasano M., Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open. 2016;1:e000060. doi: 10.1136/esmoopen-2016-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Arcangelo M., Hirsch F.R. Clinical and comparative utility of afatinib in non-small cell lung cancer. Biologics. 2014;8:183–192. doi: 10.2147/BTT.S40567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moll H.P., Pranz K., Musteanu M., Grabner B., Hruschka N., Mohrherr J., Aigner P., Stiedl P., Brcic L., Laszlo V., et al. Afatinib restrains K-RAS–driven lung tumorigenesis. Sci. Transl. Med. 2018;10:eaao2301. doi: 10.1126/scitranslmed.aao2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wald O., Shapira O.M., Izhar U. CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic roles and therapeutic potential. Theranostics. 2013;3:26–33. doi: 10.7150/thno.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hisao I., Sunaga N., Shimizu Y., Yanagitani N., Kaira K., Tomizawa Y., Mori M. SDF-1/CXCL12 overexpression and its role in the development of lung cancer. Cancer Res. 2008;68:5104. [Google Scholar]
- 46.Roberts P.J., Der C.J. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 47.Heinrich E.L., Lee W., Lu J., Lowy A.M., Kim J. Chemokine CXCL12 activates dual CXCR4 and CXCR7-mediated signaling pathways in pancreatic cancer cells. J. Transl. Med. 2012;10:68. doi: 10.1186/1479-5876-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chatterjee M., Borst O., Walker B., Fotinos A., Vogel S., Seizer P., Mack A., Alampour-Rajabi S., Rath D., Geisler T., et al. Macrophage migration inhibitory factor limits activation-induced apoptosis of platelets via CXCR7-dependent Akt signaling. Circ. Res. 2014;115:939–949. doi: 10.1161/CIRCRESAHA.115.305171. [DOI] [PubMed] [Google Scholar]
- 49.Kumar R., Tripathi V., Ahmad M., Nath N., Mir R.A., Chauhan S.S., Luthra K. CXCR7 mediated Giα independent activation of ERK and Akt promotes cell survival and chemotaxis in T cells. Cell. Immunol. 2012;272:230–241. doi: 10.1016/j.cellimm.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 50.Jacobsen K., Bertran-Alamillo J., Molina M.A., Teixido C., Karachaliou N., Pedersen M.H., Castellvi J., Garzon M., Codony-Servat C., Codony-Servat J., et al. Convergent Akt activation drives acquired EGFR inhibitor resistance in lung cancer. Nat. Commun. 2017;8:410. doi: 10.1038/s41467-017-00450-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luo J., Manning B.D., Cantley L.C. Targeting the PI3K-Akt pathway in human cancer: Rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/S1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 52.Tarnowski M., Kucia M., Ratajczak M.Z. Isolation and Functional Analysis of CXCR7 Promoter—A Novel Receptor for Stromal Derived Factor-1 (SDF-1): Different Regulation of Expression in Human Hematopoietic Cells Versus Pediatric Sarcomas. Blood. 2009;114:4583. [Google Scholar]
- 53.Shostak K., Chariot A. EGFR and NF-kappaB: Partners in cancer. Trends Mol. Med. 2015;21:385–393. doi: 10.1016/j.molmed.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Bivona T.G., Hieronymus H., Parker J., Chang K., Taron M., Rosell R., Moonsamy P., Dahlman K., Miller V.A., Costa C., et al. FAS and NF-kappaB signalling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471:523–526. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galvani E., Sun J., Leon L.G., Sciarrillo R., Narayan R.S., Sjin R.T.T., Lee K., Ohashi K., Heideman D.A.M., Alfieri R.R., et al. NF-kappaB drives acquired resistance to a novel mutant-selective EGFR inhibitor. Oncotarget. 2015;6:42717–42732. doi: 10.18632/oncotarget.3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blakely C.M., Pazarentzos E., Olivas V., Asthana S., Yan J.J., Tan I., Hrustanovic G., Chan E., Lin L., Neel D.S., et al. NF-κB activating complex engaged in response to EGFR oncogene inhibition drives tumor cell survival and residual disease in lung cancer. Cell Rep. 2015;11:98–110. doi: 10.1016/j.celrep.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maemondo M., Inoue A., Kobayashi K., Sugawara S., Oizumi S., Isobe H., Gemma A., Harada M., Yoshizawa H., Kinoshita I., et al. Gefitinib or Chemotherapy for Non–Small-Cell Lung Cancer with Mutated EGFR. N. Engl. J. Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 58.Okamoto K., Okamoto I., Okamoto W., Tanaka K., Takezawa K., Kuwata K., Yamaguchi H., Nishio K., Nakagawa K. Role of survivin in EGFR inhibitor-induced apoptosis in non-small cell lung cancers positive for EGFR mutations. Cancer Res. 2010;70:10402–10410. doi: 10.1158/0008-5472.CAN-10-2438. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura H., Kawasaki N., Taguchi M., Kabasawa K. Survival impact of epidermal growth factor receptor overexpression in patients with non-small cell lung cancer: A meta-analysis. Thorax. 2006;61:140–145. doi: 10.1136/thx.2005.042275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.