Abstract
A protein extract was generated from the macroalga Ulva lactuca, which was subsequently hydrolysed using the food-grade enzyme papain and angiotensin-converting Enzyme I and renin inhibitory peptides identified using a combination of enrichment strategies employing molecular weight cutoff filtration and mass spectrometry analysis. The generated hydrolysates with the most promising in vitro activity were further purified using preparative RP-HPLC and characterised. The 1 kDa hydrolysate (1 kDa-UFH), purified and collected by preparative RP-HPLC at minutes 41‒44 (Fr41‒44), displayed statistically higher ACE-I inhibitory activities ranging from 96.91% to 98.06%. A total of 48 novel peptides were identified from these four fractions by LC-MS/MS. A simulated gastrointestinal digestion of the identified peptide sequences was carried out using in silico enzyme cleavage simulation tools, resulting in 86 peptide sequences that were further assessed for their potential activity, toxicity and allergenicity using multiple predictive approaches. All the peptides obtained in this study were predicted to be non-toxic. However, 28 out of the 86 novel peptides released after the in silico gastrointestinal digestion were identified as potential allergens. The potential allergenicity of these peptides should be further explored to comply with the current labelling regulations in formulated food products containing U. lactuca protein hydrolysates.
Keywords: seaweed protein, protein hydrolysate, ACE-I, renin, allergenicity, in silico analysis, functional food, bioactive peptide, bioinformatics
1. Introduction
Cardiovascular disease (CVD) is the main cause of death in Europe [1], and among CVD, hypertension has become a problem of global, epidemic proportions [2]. Long-term hypertension results in increased risk of stroke, heart attack, arterial aneurysm and kidney failure [3,4]; even moderate hypertension can shorten the life span [3]. Hypertension is a global problem that influences the health of the population and also exerts a substantial influence on the economy. The direct medical costs associated with CVD in the United States in 2010 were valued at approximately 273 billion USD and this figure is estimated to reach 818 billion USD by 2030 [5].
The inhibition of enzymes such as renin (EC 3.4.23.15) and angiotensin-I-converting enzyme (ACE-I, EC 3.4.15.1) is known to be effective at lowering blood pressure [6]. These enzymes operate within a number of metabolic pathways including the renin‒angiotensin‒aldosterone system (RAAS), the renin‒chymase system (RCS), the kinin‒nitric oxide system (KNOS) and the neutral endopeptidase system (NEPS) [7]. Synthetic drugs that target renin and ACE-I are available for the clinical treatment of hypertension. These drugs include captopril, enalapril and lisinopril. Despite the efficiency of these drugs, adverse side effects including dry cough, taste disturbances, skin rashes, renal failure, congenital malformations and angioneurotic oedema have been reported [8,9]. Thus, it is necessary to find safer enzyme inhibitors for the prevention and remedy of hypertension from natural products [10].
Bioactive peptides or cryptides are sequences of between 2 and 30 amino acids. These peptides show no activity within the parent protein, but after their release by different methods, i.e., enzymatic hydrolysis or fermentation, they may show beneficial biological activities. Thus, bioactive peptides obtained from different food protein sources are considered a promising alternative to synthetic drugs [2] and these food-based strategies to control high blood pressure can be considered an excellent intervention to improve health and wellness and decrease health care costs [3]. Numerous renin and ACE-I-inhibitory peptides have been obtained previously by hydrolysis of animal proteins from milk [11], eggs [12] and blood [6,13]. Several peptides were also identified from the macroalga Palmaria palmata [14] and indeed terrestrial crops [15,16].
Macroalgae are a rich source of multiple biologically active compounds including polysaccharides and proteins [17,18]. Chlorophyta (green) and Rhodophyta (red) macroalgae are the most promising algae to be exploited as sources of protein for functional food and feed development [17,19]. Amongst the green macroalgae, Ulva sp. is a promising biomass as it has a worldwide distribution [20] and levels of protein documented in the literature of between 7% and 33% of the dry weight of the algal plant [21,22]. Apart from its total protein content, marine organisms have adapted to extreme environmental conditions including high salt concentration and pressure conditions and the composition and primary sequences of amino acids of these marine proteins are different from those of land proteins [10]. Thus, marine macroalgae are interesting protein resources for the generation of novel antihypertensive (renin and ACE-I inhibitory) peptides by enzymatic hydrolysis [10].
The aim of this study was to generate and characterise novel bioactive protein hydrolysates from U. lactuca as this macroalgae is considered a relatively untapped source of biologically active molecules. The protein extracted from Ulva sp. was hydrolysed using the food-grade enzyme papain; enriched using 1, 3 and 10 kDa molecular weight cutoff (MWCO) filtration membranes; and further purified using preparative RP-HPLC. The antihypertensive activities of the fractions was measured in vitro for renin and ACE-I inhibition and active fractions subsequently characterised using mass spectrometry (LC/MS/MS). The amino acid sequence of peptides contained in these fractions was determined and they were subsequently assessed for their bitterness, resistance to gastrointestinal digestion, potential toxicity and estimated allergenicity using multiple in silico predictive tools.
2. Results and Discussion
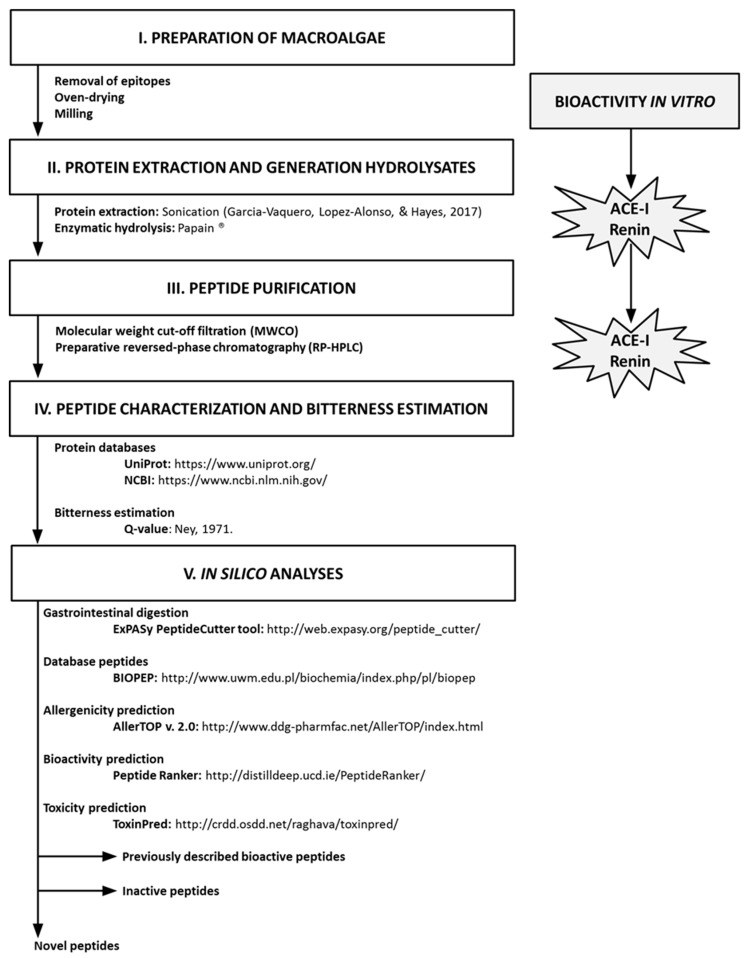
The methodological approaches used in this study to generate and identify bioactive peptides from Ulva sp. are schematically represented in Figure 1 and further described in Section 3.
Figure 1.
Schematic representation of the procedures used to generate and identify bioactive peptides from Ulva lactuca using in vitro and in silico tools.
2.1. Protein Extraction
The extracts generated from Ulva sp. had a protein content of 69.19 ± 1.44%, as assessed by the BCA method. Previous studies extracting protein from macroalgae Himanthalia elongata using a sonication water bath also reported similar protein contents (63.38 ± 0.49%) in the algal extracts [23]. Moreover, the yields of total protein extracted from Ulva sp. were 4649.98 ± 96.68 mg of protein per 100 g of dried biomass.
2.2. Generation of Hydrolysates and Antihypertensive Activities In Vitro
A papain hydrolysate of the crude Ulva protein was generated in order to release biologically active peptides or cryptides from the parent proteins. Previous reports emphasised the need to generate and use protein hydrolysates as the presence of intact or partially hydrolysed proteins can induce immune-mediated allergic reactions in sensitive individuals [24]. Although the need for amino acids could also be supplied by mixtures of synthetic amino acids, the generation of protein hydrolysates remains the most promising source of peptides [24]. The protein hydrolysates can be produced at a large scale industrially, and the peptides generated have good absorption and stability when compared to free amino acids, particularly glutamine, tyrosine and cysteine [24].
To concentrate and purify the different peptides generated during the enzymatic hydrolysis, the full hydrolysate was filtered through MWCO filtration units of 1, 3, and 10 kDa separately, generating three additional ultra-filtered fractions, namely 1 kDa-UFH, 3 kDa-UFH and 10 kDa-UFH. The control of the molecular size of the peptides generated after a protein hydrolysis is an essential step in the development of dietary products [24]. MWCO filtration has been proven to be the most efficient post-hydrolysis procedure to separate non-hydrolysed proteins, high MW peptides or residues of the proteolytic enzymes added to perform the hydrolysis [24].
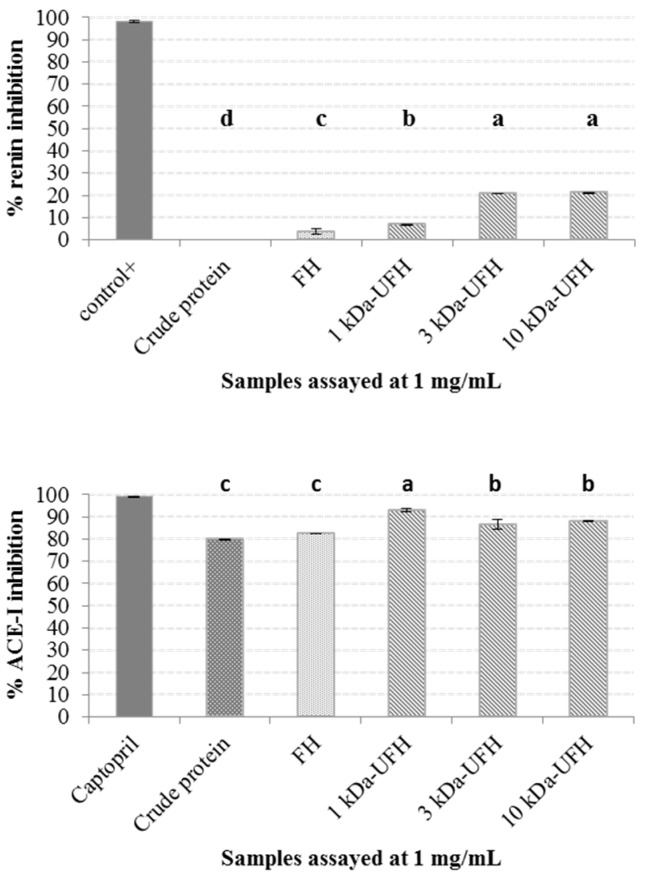
The in vitro renin and ACE-I inhibitory activities of crude protein, full hydrolysate (FH) and the three ultra-filtered fractions (1 kDa-UFH, 3 kDa-UFH and 10 kDa-UFH) are shown in Figure 2. When assayed for renin inhibition, the crude protein did not inhibit renin and the full hydrolysate inhibited renin by 3.70 ± 1.39% compared to the specific renin inhibitor, Z-Arg-Arg-Pro-Phe-His-Sta-Ile-His-Lys-(Boc)-OMe, which was used as the positive control. Ultrafiltration enhanced renin inhibitory activity and the different fractions inhibited renin as follows: 3 kDa by 20.79 ± 0.15%; 10 kDa-UFH by 21.19 ± 0.27% and 1 kDa-UFH by 6.89 ± 0.18%. The renin inhibitory activities were all less than 20% and compared negatively to previously identified renin inhibitors such as hydrolysates from the macroalga Palmaria palmata [14] or other terrestrial crops such as Avena sativa [25].
Figure 2.
In vitro renin and angiotensin-I-converting enzyme (ACE-I) inhibitory activities of crude protein, full hydrolysate (FH) and ultra-filtered hydrolysates (1, 3 and 10 kDa-UFH) generated from Ulva sp. The samples and positive controls (the renin inhibitor Z-Arg-Arg-Pro-Phe-His-Sta-Ile-His-Lys-(Boc)-OMe and the ACE-I inhibitor captopril) were assayed at 1 mg/mL. The results are expressed as mean ± standard deviation of the mean (SEM). The different letters in the figure indicate statistical significant differences (P < 0.05) between the different samples tested.
In the case of ACE-I, crude Ulva sp. protein inhibited ACE-I by 79.87 ± 0.18% at a concentration of 1 mg/mL and after hydrolysis with papain the ACE-I inhibition activity of the FH was increased to 82.37 ± 0.05% when assayed at 1 mg/mL concentrations compared to the initial protein. Following ultrafiltration, ACE-I inhibition also increased, with the maximum inhibitory activity observed for the 1 kDa-UFH (93.03 ± 0.87%) followed by both the 3 kDa-UFH (86.64 ± 2.17%) and 10 kDa-UFH (88.12 ± 0.02%) when all fractions were assayed at a concentration of 1 mg/mL. These results are in agreement with previous reports that describe the structure of peptides with ACE-I inhibitory activities as short amino acid sequences, including di and tri-peptides [26] or even up to 10 amino acid residues [27]. The low concentration of small peptides in the FH compared to the ultra-filtered fractions and the blocking effect of large peptides on the antihypertensive activity of small peptides, could explain these results. Moreover, the ACE-I inhibitory activities of all the fractions, particularly the 1 kDa-UFH, were higher than other promising peptide fractions obtained from terrestrial plants and animal proteins. Gangopadhyay, Wynne, O’Connor, Gallagher, Brunton, Rai and Hayes [15] generated protein hydrolysates from barley (Hordeum vulgare) and purified the hydrolysate using membranes, being the most promising fractions the 3 kDa fraction that inhibited ACE-I by 70.37 ± 0.67%, followed by the 10 kDa UFH (57.42 ± 4.68%) and the full hydrolysate (47.23 ± 2.62%). Similarly to our results, Lafarga, Rai, O’connor and Hayes [6] generated hydrolysates from bovine haemoglobin proteins and the highest ACE-I inhibition was appreciated in the peptides contained in the 1 kDa fraction that showed approximately 40% of ACE-I inhibition, compared to the 3 and 10 kDa samples.
2.3. Purification by Preparative Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC) and Antihypertensive Activity In Vitro
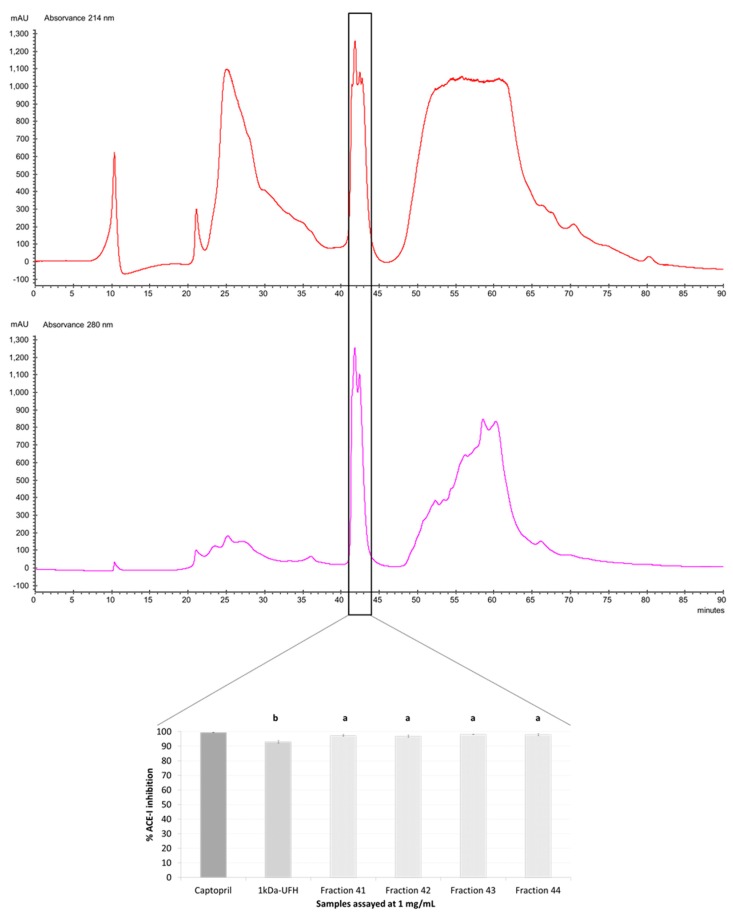
To further purify the most promising peptides contained in the 1 kDa-UFH fraction, preparative RP-HPLC was performed, the peptides were collected every minute and the separation was monitored at 214 nm, wavelength of absorbance of peptide bonds, and 280 nm, which is the wavelength of choice to detect residues of aromatic amino acids [28]. The RP-HPLC chromatogram obtained for the 1 kDa-UFH fraction of Ulva sp. is presented in Figure 3. The chromatogram at 214 nm shows main absorbance peaks for peptide bonds at minutes 9‒11, 21‒30, 41‒44 and a final plateau at 52‒62 min. However, when monitoring the absorbance at 280 nm, the main peaks related to the presence of aromatic amino acids were mainly appreciated at minutes 41‒44 and the previously observed plateau at minutes 52‒62 decreased significantly. The presence of branched amino acids at the N-terminal position and aromatic amino acid residues at the C-terminal position in peptides has been described as one key structural feature governing the ACE-I inhibitory activity of peptides [29]. Thus, peptides containing amino acids such as leucine, valine, alanine and the aromatic amino acids tyrosine, phenylalanine or tryptophan are likely to inhibit ACE by competing for binding to the catalytic sites of the enzyme [30,31]. From the chromatogram shown in Figure 3, using RP-HPLC monitored at 280 nm, the fractions collected at minutes 41‒44 were likely to content high amounts of aromatic amino acids and, thus, have a strong potential to inhibit ACE-I. When the peptide fractions 41‒44 were assayed at 1 mg/mL for ACE-I inhibition, the antihypertensive activity of all these fractions was statistically higher compared to the previously assayed 1 kDa-UFH. The average ACE-I of all the fractions was in all cases higher than 95%, close to the levels of inhibition of ACE-I of the captopril used as a positive control. The peptides contained in these four peptide fractions (Fr41‒44) were identified by HPLC-MS/MS.
Figure 3.
Preparative reversed-phase HPLC (RP-HPLC) chromatogram obtained from the 1 kDa-UFH monitored at wavelengths 214 and 280 nm. The ACE-I inhibitory activities of captopril, 1 kDa-UFH and the samples from RP-HPLC collected at minutes 41‒44 (Fr41‒Fr44) are also presented at the end of the figure. The results are expressed as mean ± standard deviation of the mean (SEM). The different letters in the figure indicate statistical significant differences (P < 0.05) between the samples tested.
2.4. Identification of Peptides Using HPLC-MS/MS and Bitterness Estimation
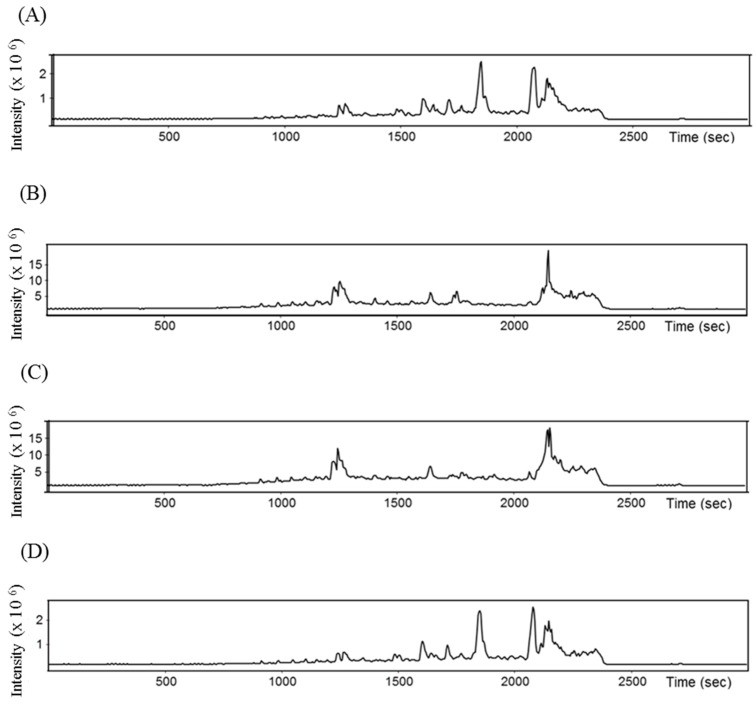
A total of 48 peptides were identified from the four selected fractions (Fr41‒44) of 1 kDa-UFH showing potent ACE-I inhibitory activities. The amino sequences of these novel peptides and their modifications were further characterized by the observed and calculated molecular masses, theoretical and observed mass/charge (m/z), and charge (z) as it is shown in Table 1. The total ion chromatogram after the HPLC separation in mass spectrometry is also presented in Figure 4. The amino acid sequences had green algae origin and were compared to previously reported peptides in the BIOPEP database [32]. All the peptides identified were novel and not previously reported in this database. As these peptides are intended for oral intake, one factor that could influence consumption when incorporated into a food product could be the taste [24]. The process of protein hydrolysis generates short peptides that are normally bitter and not accepted by Western consumers, regardless of the potential health benefits of the products [33]. The Q-values were calculated for the identified peptides based on the method of Ney [34], who estimates the bitterness of the peptides on the basis of the solubility data of each individual amino acid. When a peptide has a molecular weight (MW) lower than 6 kDa and the Q-value of the peptide exceeds 1400 cal/mol, the sequence is predicted to be bitter. The peptides identified in Fr41‒44 have generally Q-values below the threshold of bitterness (see Table 1). Only the sequences SAGVLPWK, GAAPTPPSPPPATKPSTPPKPPT, IECCLLFALV, PVGCLPK, DAVEIWRVK, DEVIPGAL, PKPPALCN and PPNPPNPPN with Q-values ranging from 1440 to 1743.33 cal/mol could be predicted to be bitter. Although this estimation method can be applied to the majority of the known peptides, a few authors have also suggested that the bitterness of the peptides could be related to the MW of the amino acid sequences rather than the Q-value [35].
Table 1.
Peptides identified by HPLC-MS/MS from Fr41‒44 collected by RP-HPLC of Ulva sp. protein hydrolysates with papain. The Q-values are an estimation of the bitterness of the peptides and are calculated and summarised in the last column.
| Samples | Amino Acid Sequences | Modifications | Observed MW |
Calculated MW |
Observed m/z |
Theoretical m/z |
Charge (+) |
Database | Accessions | Q-Values |
|---|---|---|---|---|---|---|---|---|---|---|
| Fr41 | TGGSLHAA | 712.41 | 712.35 | 357.21 | 357.18 | 2 | NCBInr | XP_005851095.1; EFN58993.1 | 607.5 | |
| VVPKAAPPPN | 988.76 | 988.57 | 495.39 | 495.29 | 2 | NCBInr | XP_005843189.1; EFN51087.1 | 1343 | ||
| ATKPAN | 600.41 | 600.32 | 301.21 | 301.17 | 2 | NCBInr | XP_001696486.1; XP_001696477.1 | 1001.67 | ||
| SGAASASGAA | 748.40 | 748.34 | 375.21 | 375.17 | 2 | NCBInr | RRRRRKXZ51346.1 | 377 | ||
| HSAVFAAS | 788.51 | 788.38 | 395.26 | 395.20 | 2 | NCBInr | GAX77766.1 | 883.75 | ||
| NGNAASPGQPPLEVH | 1486.84 | 1486.72 | 744.43 | 744.37 | 2 | NCBInr | RRRRRJAC80986.1 | 960 | ||
| HQWGGAPQH | 1016.69 | 1016.46 | 509.35 | 509.24 | 2 | NCBInr | XP_002500034.1; ACO61292.1 | 794.44 | ||
| KFKGMNHINDIEKFK | 1847.96 | 1847.97 | 616.99 | 617.00 | 3 | UniProt | sp|B9DRW5 | 1304 | ||
| GAVHAMVDTAKIHKGKK | 1789.95 | 1790.00 | 597.66 | 597.67 | 3 | UniProt | RRRRRsp|Q3TC72 | 1048.23 | ||
| Fr42 | AGGPNQPPN | 850.48 | 850.39 | 426.25 | 426.20 | 2 | NCBInr | XP_001694488.1; EDP02483.1 | 941.11 | |
| AANITVPAAN | 940.58 | 940.50 | 471.30 | 471.26 | 2 | NCBInr | XP_002957564.1; EFJ41334.1 | 1062 | ||
| DSLSAIGGAPDG | 1058.45 | 1058.49 | 530.23 | 530.25 | 2 | NCBInr | JAC65094.1 | 885.83 | ||
| SAGVLPWK | 856.50 | 856.48 | 429.26 | 429.25 | 2 | NCBInr | KXZ46138.1 | 1500 | ||
| DGLIDGL | 694.35 | 701.36 | 695.36 | 702.37 | 1 | UniProt | tr|A0A1C9C803|A0A1C9C803_9FLOR | 1270 | ||
| GDLIDVA | 694.35 | 701.36 | 695.36 | 702.37 | 1 | UniProt | tr|M1VAH3|M1VAH3_CYAM1 | 1270 | ||
| NQVTNLIVA | 970.46 | 970.54 | 486.24 | 486.28 | 2 | UniProt | tr|A0A1G4NVL3|A0A1G4NVL3_9FLOR | 1091.11 | ||
| GSASGAFVY | 857.46 | 857.39 | 429.74 | 429.70 | 2 | UniProt | tr|M1V5F7|M1V5F7_CYAM1 | 972.22 | ||
| Fr43 | HGPPPPSPYRSAAGRAAL | 1800.90 | 1800.94 | 601.31 | 601.32 | 3 | NCBInr | XP_005846003.1; EFN53901.1 | 1297.22 | |
| EAEPAEAA | 786.37 | 786.34 | 394.19 | 394.18 | 2 | NCBInr | XP_005852249.1; EFN60147.1 | 898.75 | ||
| SIAGVAAFIG | 904.48 | 904.50 | 453.25 | 453.26 | 2 | NCBInr | JAC64130.1 | 1251 | ||
| YAAKMRK | 866.54 | 866.48 | 434.28 | 434.25 | 2 | NCBInr | XP_007512502.1; CCO17102.1 | 1337.14 | ||
| DDLKGTF | 794.38 | 794.38 | 398.20 | 398.20 | 2 | NCBInr | XP_003058074.1; EEH58025.1 | 1155.71 | ||
| GGVYGTSAR | 866.50 | 866.42 | 434.26 | 434.22 | 2 | NCBInr | XP_001700029.1; EDP07725.1 | 722.22 | ||
| GSECMWFAS | 1016.33 | 1016.37 | 509.17 | 509.19 | 2 | NCBInr | XP_001693035.1; EDP03604.1 | 923.33 | ||
| AGFSYFGESS | 1050.45 | 1050.43 | 526.23 | 526.22 | 2 | NCBInr | XP_002951597.1; EFJ47408.1 | 957 | ||
| DLLALRELDVACN | 1443.77 | 1443.74 | 722.89 | 722.88 | 2 | NCBInr | JAT73014.1 | 1167.69 | ||
| NGGDLPGAL | 812.42 | 812.40 | 407.22 | 407.21 | 2 | NCBInr | XP_003055625.1; EEH60877.1 | 968.89 | ||
| ALLQQQAQMAAALPLPP | 1759.90 | 1759.97 | 587.64 | 587.66 | 3 | NCBInr | XP_005847720.1; EFN55618.1 | 1299.41 | ||
| LTPCAVPE | 828.43 | 828.41 | 415.22 | 415.21 | 2 | NCBInr | KXZ44026.1 | 1383.75 | ||
| ERTGRVAM | 918.48 | 918.47 | 460.25 | 460.24 | 2 | NCBInr | KXZ43890.1 | 771.25 | ||
| LNCALK | 660.43 | 660.36 | 331.22 | 331.19 | 2 | NCBInr | XP_001416982.1; ABO95275.1 | 1176.67 | ||
| GAAPTPPSPPPATKPSTPPKPPT | 2190.15 | 2190.17 | 731.06 | 731.06 | 3 | NCBInr | XP_002958348.1; EFJ40570.1 | 1558.69 | ||
| IECCLLFALV | 1122.55 | 1122.58 | 562.28 | 562.30 | 2 | NCBInr | GAX82307.1 | 1585 | ||
| PVGCLPK | 712.40 | 712.39 | 357.21 | 357.20 | 2 | NCBInr | JAC75907.1 | 1550 | ||
| DEDESSFGK | 1012.47 | 1012.40 | 507.24 | 507.21 | 2 | UniProt | tr|R7QMS5|R7QMS5_CHOCR | 712.22 | ||
| GASPVTFVFT | 1024.49 | 1024.52 | 513.25 | 513.27 | 2 | UniProt | tr|M1VHB3|M1VHB3_CYAM1 | 1295 | ||
| AGDLGAYG | 722.39 | 722.32 | 362.20 | 362.17 | 2 | UniProt | tr|A0A1X6NU59|A0A1X6NU59_PORUM | 911.25 | ||
| RSARVRVGSTAT | 1259.69 | 1259.71 | 630.85 | 630.86 | 2 | UniProt | tr|A0A1X6NKL7|A0A1X6NKL7_PORUM | 665.83 | ||
| INNNKIITNL | Deamidated (N)@2 | 1156.65 | 1156.65 | 579.33 | 579.33 | 2 | UniProt | tr|A0A1Z1MP80|A0A1Z1MP80_9FLOR | 1323 | |
| DAVEIWRVK | 1114.58 | 1114.61 | 558.30 | 558.31 | 2 | UniProt | tr|R7QMS0|R7QMS0_CHOCR | 1488.89 | ||
| Fr44 | DEVIPGAL | 812.43 | 812.43 | 407.22 | 407.22 | 2 | NCBInr | GAX84002.1 | 1440 | |
| DATFCGDLDDA | 1141.54 | 1141.42 | 571.78 | 571.72 | 2 | NCBInr | XP_001697003.1; EDP00695.1 | 830 | ||
| PKPPALCN | 838.47 | 838.44 | 420.24 | 420.23 | 2 | NCBInr | KXZ44308.1 | 1562.5 | ||
| PPNPPNPPN | 942.56 | 942.46 | 472.29 | 472.24 | 2 | NCBInr | XP_002955492.1; EFJ43345.1 | 1743.33 | ||
| TPALVSQLH | 964.50 | 964.53 | 483.26 | 483.27 | 2 | NCBInr | XP_011396301.1; KFM23431.1 | 1195.56 | ||
| TMSDRFL | 868.51 | 868.41 | 435.26 | 435.21 | 2 | NCBInr | XP_002499460.1; ACO60718.1 | 1160 | ||
| AAGAAPLL | 682.44 | 682.40 | 342.23 | 342.21 | 2 | NCBInr | AAW51128.1 | 1297.5 | ||
| LIKELDSN | 930.62 | 930.50 | 466.32 | 466.26 | 2 | UniProt | tr|A0A1Z1MG56|A0A1Z1MG56_9FLOR | 1303.75 |
Figure 4.
Total ion chromatogram (TIC) separation of the selected HPLC fractions for mass spectrometry analysis. (A) corresponds to Fr41, (B) to Fr42, (C) to Fr43 and (D) to Fr44.
2.5. In Silico Analyses
2.5.1. In Silico GI Enzymatic Digestion
Several issues that will influence the future applicability and usage of bioactive peptides include the water solubility of the molecules, the stability of the products and the bioavailability of the peptides when passing through biological barriers such as the GI tract [6]. The resistance of peptides to the enzymatic degradation in the GI tract will determine the bioavailability of the initial peptides. Moreover, novel amino acid sequences could be released from the parent peptide during the process of digestion and may exert toxic, allergenic or other potent biological activities when absorbed. After the in silico enzymatic simulations performed, only a few peptides remained intact, namely ATKPAN, SGAASASGAA (from Fr41), AGGPNQPPN, AANITVPAAN (identified in Fr42), EAEPAEAA, GAAPTPPSPPPATKPSTPPKPPT (obtained in Fr43) and PPNPPNPPN (Fr44). All the peptides obtained from the in silico enzymatic simulation were further compared with the BIOPEP database [32] to identify previously reported bioactive peptides. There were 16 di- and tri-peptides released after the GI enzymatic simulation previously reported as bioactives (see Table 2).
Table 2.
Bioactive peptides generated after the in silico gastrointestinal digestion of the identified peptide from UIva sp. already available in the scientific literature. The data were attained from the BIOPEP database (http://www.uwm.edu.pl/biochemia/index.php/pl/biopep) on 14 February 2019.
| Amino Acid Sequences | Bioactivity In Vitro * | Reference |
|---|---|---|
| AA | ACE-I inhibitor | Cushman et al. [37] |
| AG | ACE-I inhibitor | Cheung, Wang, Ondetti, Sabo and Cushman [29] |
| AS | DPP IV inhibitor | Lan et al. [38] |
| DG | ACE-I inhibitor | Meisel, Walsh, Murray and Fitzgerald [7] |
| GA | ACE-I inhibitor | Cheung, Wang, Ondetti, Sabo and Cushman [29] |
| GD | ACE-I inhibitor | Cheung, Wang, Ondetti, Sabo and Cushman [29] |
| GGV | HMG-CoA reductase inhibitor | Soares, Mendonça, de Castro, Menezes and Arêas [36] |
| GK | ACE-I inhibitor | Cheung, Wang, Ondetti, Sabo and Cushman [29] |
| GM | ACE-I inhibitor | Cheung, Wang, Ondetti, Sabo and Cushman [29] |
| IG | ACE-I inhibitor | Cheung, Wang, Ondetti, Sabo and Cushman [29] |
| IH | DPP IV inhibitor | Lan, Ito, Ohno, Motoyama, Ito and Kawarasaki [38] |
| NH | DPP IV inhibitor | Lan, Ito, Ohno, Motoyama, Ito and Kawarasaki [38] |
| PK | DPP IV inhibitor | Lan, Ito, Ohno, Motoyama, Ito and Kawarasaki [38] |
| TM | DPP IV inhibitor | Lan, Ito, Ohno, Motoyama, Ito and Kawarasaki [38] |
| VK | DPP IV inhibitor | Lan, Ito, Ohno, Motoyama, Ito and Kawarasaki [38] |
| ACE-I inhibitor | Wang and De Mejia [39] | |
| VR | DPP IV inhibitor | Nongonierma and FitzGerald [40] |
| ACE-I inhibitor | Gómez-Ruiz et al. [41] |
* Abbreviations in the table correspond to: angiotensin-I-converting enzyme (ACE-I), dipeptidyl peptidase IV inhibitor (DPP IV inhibitor), 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor (HMG-CoA reductase inhibitor).
The main biological activities described for these amino acid sequences are antihypertensive (ACE-I inhibitors) and anti-diabetic (dipeptidyl peptidase IV inhibitors) peptides. Furthermore, the sequence GGV was described as an inhibitor of the enzyme 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase) and could have a hypocholesterolemic effect [36].
2.5.2. In Silico Prediction of Allergenicity and Bioactivity
Food allergy is a huge concern in the development of nutraceuticals and the formulation of novel and safe food products [42]. Allergic reactions start when immunoglobulin E binds and activates mast and basophil cells upon the contact with food allergens. These activated cells release granules containing inflammatory mediators and other molecules, generating an immediate allergic reaction that could be life-threatening [43]. Immunoglobulin E-mediated food allergies affect between 3% and 8% of children and 1–3% of adults in developed countries and their prevalence and severity are increasing. The most commonly described sources of food allergens are milk, eggs and wheat, which produce reactions that are normally overcome with age as the patients acquire tolerance. However, peanuts, tree nuts and fish allergies often persist over a lifetime [43]. The potential allergenicity of proteins from macroalgae has not yet been fully explored. Recently, Polikovsky, Fernand, Sack, Frey, Müller and Golberg [42] reported the first study on the existence of potential allergens in proteins extracted from Ulva sp. The authors suggested that the potential allergenicity of Ulva sp. proteins will depend on the extraction conditions as the protocols of extraction will influence the mass transfer of molecules from the macroalgal biomass to the extract. Moreover, the European Food Safety Authority (EFSA) favours the use of in silico tools to predict the potential allergenicity of food proteins [44].
In the current study, the 70 novel peptides released after the in silico GI enzymatic digestion were further analysed for their potential allergenicity in silico using AllerTop version 2.0 (http://www.ddg-pharmfac.net/AllerTOP). This in silico prediction tool is based on the transformation of amino acid descriptors of the protein strings into uniform vectors, followed by using the k nearest neighbours algorithm (kNN, k = 1) to classify the peptides based on a set containing 2427 known allergens and 2427 non-allergens [45]. The amino acid sequences that could be described as probably allergenic are summarised in Table 3, together with their nearest allergenic protein, while the probably non-allergenic 42 peptides are summarised in Table 4.
Table 3.
Peptides identified as having the potential to cause allergy obtained after in silico gastrointestinal digestion and assessment using online tools ToxinPred and Peptideranker. The toxicological, bioactivity scores and the nearest protein identified in different databases are also summarised for each peptide.
| Amino Acid Sequence | MW (Da) |
SVM Scores a | PeptideRanker Scores b | Nearest Protein |
|---|---|---|---|---|
| AANITVPAAN | 940.4978 | −1.3 | 0.144457 | NCBI GI number 2154734 |
| AAPPPN | 565.627 | −0.46 | 0.792864 | NCBI GI number 33323477 |
| AGD | 261.235 | −0.8 | 0.283992 | UniProtKB accession number O01949 |
| AGGPNQPPN | 850.3933 | −0.72 | 0.621643 | NCBI GI number 33323477 |
| CGD | 293.295 | −0.79 | 0.646691 | NCBI GI number 102834 |
| DD | 248.192 | −0.79 | 0.098852 | UniProtKB accession number Q17282 |
| DDA | 319.271 | −0.8 | 0.127455 | UniProtKB accession number O01949 |
| DEVIPGA | 699.759 | −0.45 | 0.185675 | NCBI GI number 543491 |
| EVH | 383.404 | −0.82 | 0.0380427 | NCBI GI number 21465915 |
| GASPVT | 530.579 | −0.97 | 0.247962 | NCBI GI number 2500822 |
| GESS | 378.339 | −0.76 | 0.0716116 | NCBI GI number 32363197 |
| GGAPQH | 565.586 | −0.86 | 0.388533 | UniProtKB accession number P86254 |
| GSASGA | 448.433 | −0.93 | 0.250858 | NCBI GI number 543482 |
| GSECM | 525.592 | −0.42 | 0.554283 | NCBI GI number 162927 |
| IDVA | 416.475 | −0.84 | 0.0871288 | NCBI GI number 539716 |
| IECC | 466.568 | −0.25 | 0.448357 | NCBI GI number 14285595 |
| IITN | 459.543 | −0.84 | 0.106012 | UniProtKB accession number Q93YG7 |
| IK | 259.349 | −0.8 | 0.0974139 | NCBI GI number 157829757 |
| INNNK | 601.66 | −0.81 | 0.0938778 | NCBI GI number 1083651 |
| NGNAASPGQPPL | 1122.203 | −0.78 | 0.505873 | NCBI GI number 33323477 |
| NQVTN | 574.591 | −1.01 | 0.0559664 | UniProtKB accession number P00709 |
| SAIGGAPDG | 743.771 | −0.67 | 0.397354 | NCBI GI number 285005077 |
| SAR | 332.36 | −0.81 | 0.278711 | NCBI GI number 81890324 |
| TGR | 332.36 | −0.8 | 0.276659 | UniProtKB accession number Q7M1M4 |
| TPAL | 400.475 | −0.8 | 0.342157 | NCBI GI number 18203509 |
| VAM | 319.419 | −0.8 | 0.264259 | NCBI GI number 9072 |
| VSQ | 332.357 | −0.83 | 0.0492778 | NCBI GI number 2147092 |
| VVPK | 441.571 | −0.88 | 0.0810705 | NCBI GI number 2133755 |
a SVM scores obtained from in silico analyses using ToxinPred (http://crdd.osdd.net/raghava/toxinpred/). Data retrieved on 15 February 2019. b Biological activity scores obtained from in silico analyses using Peptideranker (http://distilldeep.ucd.ie/PeptideRanker/). Data retrieved on 14 February 2019.
Table 4.
Probably non-allergenic peptides obtained after in silico gastrointestinal digestion. The toxicological, bioactivity scores and the nearest protein identified in different databases are also summarised for each peptide.
| Amino Acid Sequence | MW (Da) |
SVM Scores a |
PeptideRanker Scores b |
Nearest Protein |
|---|---|---|---|---|
| AAAL | 344.411 | −0.84 | 0.28869 | UniProtKB accession number A9UGV6 |
| AAGAAP | 456.499 | −1.01 | 0.352249 | UniProtKB accession number Q7M1V0 |
| AAK | 288.347 | −0.77 | 0.113614 | UniProtKB accession number P27807 |
| AAL | 273.332 | −0.84 | 0.310569 | UniProtKB accession number A9UGV6 |
| AAS | 247.251 | −0.82 | 0.123531 | UniProtKB accession number A2WQG7 |
| AM | 220.287 | −0.8 | 0.74549 | UniProtKB accession number Q8IWT0 |
| ATKPAN | 600.32312 | −0.88 | 0.129647 | UniProtKB accession number Q8WYQ3 |
| CN | 235.258 | −0.8 | 0.63423 | UniProtKB accession number P01052 |
| DAT | 305.288 | −0.81 | 0.0954274 | UniProtKB accession number Q9NNX6 |
| DAVEI | 545.59 | −0.93 | 0.0603561 | UniProtKB accession number Q9T2R4 |
| DEDESS | 680.58 | −0.78 | 0.0365686 | UniProtKB accession number Q9S8K0 |
| DS | 220.182 | −0.8 | 0.0878061 | UniProtKB accession number O75366 |
| DSN | 334.286 | −0.78 | 0.104772 | UniProtKB accession number P31358 |
| DVACN | 520.558 | −0.68 | 0.203726 | UniProtKB accession number Q7M2H1 |
| EAEPAEAA | 394.193 | −0.99 | 0.0830328 | UniProtKB accession number Q9S8F6 |
| ER | 303.318 | −0.8 | 0.0704548 | UniProtKB accession number Q9T2R8 |
| GAAPTPPSPPPATKPSTPPKPPT | 731.0588 | −0.69 | 0.378489 | UniProtKB accession number Q9S8M0 |
| GAVH | 382.42 | −0.77 | 0.173142 | UniProtKB accession number Q9S8I6 |
| GPPPPSP | 647.729 | −0.11 | 0.870562 | UniProtKB accession number Q7M1V1 |
| GTF | 323.349 | −0.8 | 0.84905 | UniProtKB accession number P04234 |
| GTSAR | 490.517 | −0.89 | 0.151592 | UniProtKB accession number Q7M282 |
| IDG | 303.315 | −0.78 | 0.288785 | UniProtKB accession number P86001 |
| INDIEK | 730.816 | −0.97 | 0.0849714 | UniProtKB accession number P86006 |
| IVA | 301.386 | −0.79 | 0.0757333 | UniProtKB accession number Q9S8N5 |
| NCA | 306.337 | −0.77 | 0.425512 | UniProtKB accession number A6N1B4 |
| NGGDL | 474.471 | −0.76 | 0.567577 | UniProtKB accession number P55857 |
| PGA | 243.263 | −0.81 | 0.674335 | UniProtKB accession number Q9S906 |
| PKPPAL | 621.778 | −0.7 | 0.715386 | UniProtKB accession number Q7M1U2 |
| PLPP | 422.525 | −1.04 | 0.876691 | UniProtKB accession number Q7M1V1 |
| PPNPPNPPN | 942.455933 | −0.4 | 0.855282 | UniProtKB accession number Q9S8M0 |
| PVGCL | 487.615 | −0.63 | 0.734232 | UniProtKB accession number P83184 |
| QQQAQM | 732.809 | −0.92 | 0.216075 | UniProtKB accession number Q9UL45 |
| SAAGR | 460.49 | −0.85 | 0.341095 | UniProtKB accession number P80825 |
| SAGVL | 445.516 | −0.99 | 0.38464 | UniProtKB accession number A9UGV7 |
| SAV | 275.305 | −0.82 | 0.0814653 | UniProtKB accession number Q09Y74 |
| SDR | 376.37 | −0.83 | 0.242439 | UniProtKB accession number Q9BXJ1 |
| SGAASASGAA | 748.335144 | −1.17 | 0.2792 | UniProtKB accession number Q7M1V0 |
| SIAGVAA | 587.674 | −1.28 | 0.134975 | UniProtKB accession number A9UGV6 |
| TGGS | 320.302 | −0.77 | 0.17105 | UniProtKB accession number Q15517 |
| TPCAVPE | 715.819 | −0.88 | 0.224438 | UniProtKB accession number Q9T2Q0 |
| VDTAK | 532.594 | −0.34 | 0.0513549 | UniProtKB accession number Q9T2R4 |
| VGSTAT | 534.567 | −1.03 | 0.0691571 | UniProtKB accession number Q10ST8 |
a SVM scores obtained from in silico analyses using ToxinPred (http://crdd.osdd.net/raghava/toxinpred/). Data retrieved on 15 February 2019. b Biological activity scores from in silico analyses using Peptideranker (http://distilldeep.ucd.ie/PeptideRanker/). Data retrieved on 14 February 2019.
This information will be useful to prevent allergic systemic reactions produced when the allergens cross the intestinal mucosa and enter the circulation, creating a reaction that may compromise the circulatory and nervous systems [43]. The European Food Safety Authority (EFSA) favours the use of in silico tools to predict the initial potential allergenicity of food proteins [44]. The results on the allergenicity of these peptides suggest that papain hydrolysates from Ulva sp. could result in potentially allergenic peptides after GI digestion and, thus, this parameter should be further assessed in vitro and in vivo to comply with the current food allergen labelling European regulations such as the Regulation (EU) No.1169/2011.
The potential of the allergenic and non-allergenic peptides to be bioactive is also summarised in Table 3 and Table 4, as analysed using PeptideRanker. Several peptides scored high in peptide ranker (>0.6); in particular, peptides GPPPPSP and GTF, with scores over 0.8, show potential for further in vivo evaluation as some of the peptides assayed in vitro did not perform as well as expected using in vivo models [46].
2.5.3. In Silico Prediction of Toxicity
The protein hydrolysates of this study were generated from edible parts of Ulva sp. destined for human consumption and the enzyme papain is a food-grade molecule obtained from plant (Carica papaya). Moreover, protein hydrolysates generated using similar enzymatic procedures from animal [6,13] and other edible protein sources such as plants [15] and algae [14] have not been reported to pose a serious health risk to consumers. None of the identified peptides in this study were predicted to be toxic using ToxinPred (http://crdd.osdd.net/raghava/toxinpred/) as seen in the negative SVM scored summarised in Table 3 and Table 4. The toxicity assessments should be further explored using cell lines and animal studies before the product is made available for human consumption. However, protein hydrolysates and low MW peptides are generally considered non-toxic [6] and are less allergenic than full or partially hydrolysed proteins [24]. Protein hydrolysates have been used to develop hypoallergenic infant formulas [47], but also in specific diets to treat patients with metabolic disorders affecting amino acid digestion, absorption and metabolism [24]. Other uses of protein hydrolysates include the treatment of malnutrition associated with age [48] or other diseases such as cancer, trauma, burns and hepatic encephalopathies, mainly due to the easy absorption of short peptides [24].
3. Material and Methods
3.1. Biological Materials
The macroalgae Ulva lactuca was kindly supplied by Portomuiños (Galicia, Spain). Macroalgae were collected at Muxía (A Coruña, Galicia, Spain) on 10 June 2013. Following harvest, the seaweeds were cleaned and epitopes removed, oven-dried, milled and vacuum preserved until further analysis.
3.2. Chemicals
Ammonium sulphate, formic acid (FA), trifluoroacetic acid (TFA), acetonitrile (ACN), HPLC-grade water CHROMASOLV®, dimethyl sulfoxide (DMSO), papain (EC 3.4.22.2) from Carica papaya ≥3 U/mg, QuantiPro BCA assay kit and the inhibitors of ACE-I (Captopril©) and renin (Z-Arg-Arg-Pro-Phe-His-Sta-Ile-His-Lys-(Boc)-OMe) were all supplied by Sigma-Aldrich (Dublin, Ireland). The ACE-I and renin inhibitory screening assay kits were supplied by Dojindo Laboratories (Kumamoto, Japan) and Cayman Chemical Company (Ann Arbor, MI, USA), respectively. All the other chemicals used were of analytical grade.
3.3. Protein Extraction and Quantification
Crude protein was extracted following the method previously described by Garcia-Vaquero, Lopez-Alonso and Hayes [23]. Briefly, 10 g of dried seaweed were suspended in 1 L of ultrapure water and ultra-sonicated for 1 h (Branson 3510EMT, Branson Ultrasonics Corporation, Danbury, CT, USA) and left overnight on a magnetic stirrer plate (IKA RCT basic safety control) at 4 °C. The solution was then centrifuged at 10,000× g for 1 h and the supernatant decanted. The pellet fraction was re-suspended in 0.5 L of ultrapure water and subjected to a second extraction procedure as described above. Supernatants from both days were pooled together; the solution was then saturated to 80% with ammonium sulphate for 1 h at 4 °C and centrifuged at 20,000× g for 1 h to precipitate the protein. The protein precipitates were subsequently dialyzed using Thermo Scientific™ SnakeSkin™ dialysis tubing, 3.5 kDa MWCO (Thermo Fisher Scientific, Hudson, NH, USA) against ultrapure water at 4 °C. Dialyzed protein extracts were freeze-dried and stored at ‒20 °C until further analysis.
The protein contents in the crude extracts of Ulva lactuca were determined using the QuantiPro BCA Assay Kit (Sigma-Aldrich, Saint Louis, MO, USA). Samples were prepared according to the manufacturer’s instructions using Greiner 96-well flat-bottom plates (Sigma) and absorbance values were read at 562 nm in a microplate reader (FLUOstar Omega, BMG Labtech, Offenburg, Germany).
3.4. Protein Hydrolysis and Molecular Weight Cutoff Filtration
Ulva sp. hydrolysates of the crude protein extracts were prepared using a 1 L bioreactor with temperature and pH control (Bioflo 110, New Brunswick Scientific Co Inc., Edison, NJ, USA). The crude protein was dispersed in ultrapure water at a concentration of 0.01 g/mL at a total volume of 0.5 L. The hydrolysis was carried out by the addition of 1% papain®. Conditions used included stirring at 300 rpm, pH 6 and temperature maintained at 60 °C for a 24 h period. The hydrolysis was stopped by heating the mixture to 95 °C for 10 min in a water bath. The full hydrolysate (FH) was further concentrated using 10 kDa, 3 kDa and 1 kDa MWCO membranes obtaining three fractions, namely 1 kDa-UFH, 3 kDa-UFH and 10 kDa-UFH, respectively. All the hydrolysates were freeze dried, vacuum-packed and stored at ‒20 °C until further use.
3.5. Preparative Reversed-Phase High-Performance Liquid Chromatography (RP-HPLC)
The 1 KDa-UFH was further purified by preparative RP-HPLC (Varian Pro-Star, Agilent Technologies, USA) using a Luna® C18 (Phenomenex®, 5 µm 100 μm × 21.2 mm). Prior to analysis, samples were re-dissolved (5% w/v) in HPLC-grade water with 0.1% TFA and filtered through 0.2 μm filters (Agilent Captiva, Agilent Technologies, Santa Clara, CA, USA). The column was equilibrated with HPLC-grade water with 0.1% TFA at a flow rate of 1 mL/min for 10 min. Following the injection (1 mL) the sample was separated using a linear gradient of ACN (2–98% v/v, 90 min) containing 0.1% TFA at a flow rate of 1 mL/min. The absorbance was monitored at wavelengths 214 and 280 nm and the eluted fractions were collected every minute. The solvents contained in the fractions (ACN and TFA) were vapored under nitrogen and the peptide fractions were freeze-dried.
3.6. In Vitro Antihypertensive Activities
3.6.1. ACE-I Inhibition Assay
The ACE-I inhibitory activity of the samples was measured according to a method based on the detection of the amount of 3-hydroxybutyrate (3-HB) generated from 3-hydroxybutyryl-Gly-Gly-Gly (3HB-GGG). The measurement was done using the materials enclosed in an ACE-I inhibition kit-WST (Dojindo Laboratories) and prepared according to the manufacturer’s instructions. All fractions were assayed at a concentration of 1 mg/mL in HPLC-grade water in triplicate and standard deviations of the mean (SEM) were calculated. Briefly, the enzyme working solution was prepared by dissolving enzyme B in 2 mL of HPLC-grade water and adding 1.5 mL of this solution to enzyme A. The indicator working solution was prepared by dissolving enzyme C and coenzyme with 3 mL of HPLC-grade water each and adding 2.8 mL of each of them to the indicator solution to prepare the indicator working solution. Negative control or blank 1 was prepared by adding 20 μL of HPLC-grade water and 20 μL of substrate buffer. Reagent blank 2 wells were prepared by adding 40 μL of HPLC-grade water and 20 μL of substrate buffer. Inhibitor wells were prepared by adding 20 μL of the unknown sample and 20 μL of substrate buffer. The ACE-I inhibitor captopril© was used as a positive control at the same concentration and following the same procedure as the samples in the inhibitor wells. The enzymatic reaction was started by adding 20 μL of enzyme working solution to each inhibitor and negative control wells. The plate was covered and incubated at 37 °C. After 1 h, 200 μL of indicator working solution was added to each well and the microwell plate was further incubated at room temperature for 10 min. The absorbance of the reaction was measured with a microplate reader (FLUOstar Omega, BMG Labtech, Offenburg, Germany) at 450 nm. The ACE-I inhibition percentage for each sample was calculated using Equation (1):
| % InhibitionACE-I = [(Ablank1 − Ainhibitor)/(Ablank1 − Ablank2)] × 100. | (1) |
3.6.2. Renin Inhibition Assay
The renin inhibition assay was performed using an enzymatic kit (Cayman Chemical Company) and following the manufacturer’s recommendations. Ulva sp. proteins, hydrolysates and the positive control (Z-Arg-Arg-Pro-Phe-His-Sta-Ile-His-Lys-(Boc)-OMe) were dissolved in DMSO at a concentration of 1 mg/mL. The assay started by transferring 10 μL of samples and control to a microplate, followed by the addition of 20 μL renin substrate, 150 μL assay buffer and 10 μL renin. The plates were incubated at 37 °C for 15 min and the fluorescence of the reaction was read at excitation wavelengths of 340 nm and emission wavelengths of 500 nm in a microplate reader (FLUOstar Omega, BMG Labtech, Offenburg, Germany). The percentage of renin inhibition was calculated using Equation (2):
| % Inhibitionrenin = [(100% Initial activity − Inhibitor)/100% Initial activity] × 100. | (2) |
3.7. Identification of Peptides by Mass Spectrometry
ACE-I inhibitory and renin inhibitory fractions were characterised using liquid chromatography and tandem mass spectrometry (LC-MS/MS). The samples were re-suspended in 50 μL in 2% ACN containing 0.1% TFA and 5 µL were injected into a Nano-LC Ultra 1D Plus system (Eksigent AB Sciex, Dublin, CA, USA) and pre-concentrated using a trap column (C18-CL Eksigent AB Sciex, 3 µm, 350 μm × 0.5 mm) for 5 min using 0.1% TFA at a flow rate of 3 µL/min. The samples were loaded onto an analytical column (C18-CL Nikkyo, 3 µm, 75 μm × 12 cm) equilibrated in 0.1% FA in water. Elution was carried out using a linear gradient from 5% to 35% of solvent B in A for 45 min (solvent A: 0.1% FA in water; solvent B: ACN containing 0.1% FA) at a flow rate of 0.3 μL/min. The peptides were analysed using nanoESI qQTOF (nanoelectrospray ionization in a quadrupole/time-of-flight TripleTOF 5600+ system from AB Sciex Instruments (Framingham, MA, USA)). The sample was ionised by applying 2.8 kV to the spray emitter and the analyses were carried out in a data-dependent mode. MS1 scans were acquired from 350 to 1250 m/z for 250 ms, the quadrupole resolution was set to ‘UNIT’ for MS2 experiments and the scans were acquired from 100 to 1500 m/z for 50 ms in ‘high sensitivity’ mode. The switch criteria used were charge (1+ to 5+), minimum intensity and 70 counts per second (cps). Up to 25 ions were selected for fragmentation after each survey scan and the dynamic exclusion was set to 15 s.
The data from LC-MS/MS were further processed using ProteinPilot version 5.0 search engine (AB Sciex, Framingham, MA, USA) using the default parameters to generate the peak list directly from the 5600 TripleTOF 5600+ files. The Paragon algorithm (Shilov et al. [49]) of ProteinPilot version 5.0 was used to search in Uniprot and NCBI_GreenAlgae databases, selecting no enzyme specificity, no taxonomy restriction and with the search effort of the software set to “Thorough”.
3.8. In Silico Analyses
The bitterness of the characterised peptides was estimated by the “Q rule” proposed by Ney [34]. This estimation method is based on the calculation of a Q-value for each peptide on the basis of its amino acid composition and the solubility data of each of the individual amino acids of the peptide sequences.
Furthermore, characterised peptides from Ulva sp. were assessed for potential cleavage by GI tract enzymes including pepsin pH 1.3 and pH > 2.0 (EC 3.4.23.1), trypsin (EC 3.4.21.4) and chymotrypsin (EC 3.4.21.1) using the ExPASy PeptideCutter tool (http://web.expasy.org/peptide_cutter/). The novel peptide sequences generated after the GI in silico digestion were also compared against the BIOPEP database (http://www.uwm.edu.pl/biochemia/index.php/pl/biopep) to identify the previously reported active sequences [32]. The allergenicity of the novel sequences after the GI in silico digestion was predicted using AllerTOP version 2.0 (http://www.ddg-pharmfac.net/AllerTOP/index.html). The toxicity of all the peptides was scored in ToxinPred (http://crdd.osdd.net/raghava/toxinpred/) using a virtual scanning method (VSM) Swiss-Prot based and a SVM threshold of 0.0 [50]. The potential biological activity of all the peptides was predicted using the scores calculated in PeptideRanker (http://distilldeep.ucd.ie/PeptideRanker/) [51].
3.9. Statistical Analyses
All the statistical analyses were performed using SPSS version 24.0. The biological activities in vitro of the different protein, hydrolysate and fractions were analysed using a univariate general linear model and LSD post hoc tests. In all the cases the criterion for statistical significance was P < 0.05.
4. Conclusions
This study shows the great potential of Ulva sp. protein hydrolysates generated using the food-grade enzyme papain as a source of bioactive peptides. The hydrolysates and fractions generated had low renin and high ACE-I inhibitory activities in vitro. The peptides of the most active hydrolysates were fully identified and in silico tools including ExPASy PeptideCutter, ToxinPred and AllerTOP were used to predict the resistance of the peptides to gastrointestinal enzymatic digestion, toxicity and allergenicity of the peptides, respectively. Using this mixed in vitro‒in silico approach, 48 novel peptides were identified and 86 novel amino acid sequences were generated after the in silico gastrointestinal simulation. Although the peptides were predicted to be non-toxic, several sequences were predicted to be probably allergenic. Further in vivo studies will be needed to confirm the antihypertensive activity of the hydrolysate and the safety of the identified bioactive peptides.
Acknowledgments
This project was supported by the project BioAlgae, funded by Teagasc (grant number: NFNY6889-142). The authors thank Portomuiños for supplying seaweed for use in this work. Marco García-Vaquero was in receipt of a postdoctoral fellowship granted by The Barrié Foundation in Galicia (Spain) for the period 2014‒2016 and currently works within the “Macroalgal Fibre Initiative Project” funded by Science Foundation Ireland (SFI) [grant number: 14/IA/2548].
Author Contributions
M.G.-V. achieved funds, designed and performed the experiments and wrote the manuscript. M.H. collaborated by providing funding and also designing and reviewing the manuscript. L.M. performed the LC-MS/MS.
Funding
This research was funded by the Barrié Foundation (Galicia, Spain) during the period 2014-2016 and by the project BioAlgae funded by Teagasc (grant number: NFNY6889-142).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Callow A.D. Cardiovascular disease 2005—The global picture. Vasc. Pharmacol. 2006;45:302–307. doi: 10.1016/j.vph.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Iwaniak A., Minkiewicz P., Darewicz M. Food-originating ACE inhibitors, including antihypertensive peptides, as preventive food components in blood pressure reduction. Compr. Rev. Food Sci. Food Saf. 2014;13:114–134. doi: 10.1111/1541-4337.12051. [DOI] [PubMed] [Google Scholar]
- 3.Decker E.A., Park Y. Healthier meat products as functional foods. Meat Sci. 2010;86:49–55. doi: 10.1016/j.meatsci.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Johnston C.I. Franz Volhard Lecture. Renin-angiotensin system: A dual tissue and hormonal system for cardiovascular control. J. Hypert. 1992;10:S13–S26. doi: 10.1097/00004872-199212007-00002. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich P.A., Trogdon J.G., Khavjou O.A., Butler J., Dracup K., Ezekowitz M.D., Finkelstein E.A., Hong Y., Johnston S.C., Khera A. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 6.Lafarga T., Rai D.K., O’connor P., Hayes M. Generation of bioactive hydrolysates and peptides from bovine hemoglobin with in vitro renin, angiotensin-I-converting enzyme and dipeptidyl peptidase-IV inhibitory activities. J. Food Biochem. 2016;40:673–685. doi: 10.1111/jfbc.12259. [DOI] [Google Scholar]
- 7.Meisel H., Walsh D., Murray B., Fitzgerald R. ACE inhibitory peptides, nutraceutical proteins and peptides in health and disease. In: Mine Y., Shahidi F., editors. Nutraceutical Science and Technology. CRC Press; New York, NY, USA: 2006. [Google Scholar]
- 8.Cooper W.O., Hernandez-Diaz S., Arbogast P.G., Dudley J.A., Dyer S., Gideon P.S., Hall K., Ray W.A. Major congenital malformations after first-trimester exposure to ACE inhibitors. N. Engl. J. Med. 2006;354:2443–2451. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 9.Pryde P.G., Sedman A.B., Nugent C.E., Barr M. Angiotensin-converting enzyme inhibitor fetopathy. J. Am. Soc. Nephrol. 1993;3:1575–1582. doi: 10.1681/ASN.V391575. [DOI] [PubMed] [Google Scholar]
- 10.He H.-L., Liu D., Ma C.-B. Review on the angiotensin-I-converting enzyme (ACE) inhibitor peptides from marine proteins. Appl. Biochem. Biotechnol. 2013;169:738–749. doi: 10.1007/s12010-012-0024-y. [DOI] [PubMed] [Google Scholar]
- 11.Nagpal R., Behare P., Rana R., Kumar A., Kumar M., Arora S., Morotta F., Jain S., Yadav H. Bioactive peptides derived from milk proteins and their health beneficial potentials: An update. Food Funct. 2011;2:18–27. doi: 10.1039/C0FO00016G. [DOI] [PubMed] [Google Scholar]
- 12.Abeyrathne E., Lee H., Jo C., Nam K., Ahn D. Enzymatic hydrolysis of ovalbumin and the functional properties of the hydrolysates. Poult. Sci. 2014;93:2678–2686. doi: 10.3382/ps.2014-04155. [DOI] [PubMed] [Google Scholar]
- 13.Lafarga T., O’Connor P., Hayes M. Identification of novel dipeptidyl peptidase-IV and angiotensin-I-converting enzyme inhibitory peptides from meat proteins using in silico analysis. Peptides. 2014;59:53–62. doi: 10.1016/j.peptides.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald C.N., Mora-Soler L., Gallagher E., O’Connor P., Prieto J., Soler-Vila A., Hayes M. Isolation and characterization of bioactive pro-peptides with in vitro renin inhibitory activities from the macroalga Palmaria palmata. J. Agric. Food Chem. 2012;60:7421–7427. doi: 10.1021/jf301361c. [DOI] [PubMed] [Google Scholar]
- 15.Gangopadhyay N., Wynne K., O’Connor P., Gallagher E., Brunton N.P., Rai D.K., Hayes M. In silico and in vitro analyses of the angiotensin-I converting enzyme inhibitory activity of hydrolysates generated from crude barley (Hordeum vulgare) protein concentrates. Food Chem. 2016;203:367–374. doi: 10.1016/j.foodchem.2016.02.097. [DOI] [PubMed] [Google Scholar]
- 16.Li G.-H., Qu M.-R., Wan J.-Z., You J.-M. Antihypertensive effect of rice protein hydrolysate with in vitro angiotensin I-converting enzyme inhibitory activity in spontaneously hypertensive rats. Asia Pac. J. Clin. Nutr. 2007;16:275–280. [PubMed] [Google Scholar]
- 17.Garcia-Vaquero M., Hayes M. Red and green macroalgae for fish and animal feed and human functional food development. Food Rev. Int. 2016;32:15–45. doi: 10.1080/87559129.2015.1041184. [DOI] [Google Scholar]
- 18.Garcia-Vaquero M., Rajauria G., Tiwari B., Sweeney T., O’Doherty J. Extraction and yield optimisation of fucose, glucans and associated antioxidant activities from Laminaria digitata by applying response surface methodology to high intensity ultrasound-assisted extraction. Mar. Drugs. 2018;16:257. doi: 10.3390/md16080257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Vaquero M. Novel Proteins for Food, Pharmaceuticals, and Agriculture: Sources, Applications, and Advances. Volume 139 Wiley; New York, NY, USA: 2018. Seaweed Proteins and Applications in Animal Feed. [Google Scholar]
- 20.Edwards M., Hanniffy D., Heesch S., Hernandez-Kantun J., Queguineur B., Ratcliff J., Soler-Vila A., Wan A. Macroalgae Fact-Sheets. NUI; Galway, Ireland: 2014. [Google Scholar]
- 21.Fleurence J., Morançais M., Dumay J. Proteins in Food Processing. Elsevier; New York, NY, USA: 2018. Seaweed proteins; pp. 245–262. [Google Scholar]
- 22.Frikha F., Kammoun M., Hammami N., Mchirgui R., Belbahri L., Gargouri Y., Miled N., Ben-Rebah F. Chemical composition and some biological activities of marine algae collected in Tunisia. Cienc. Mar. 2011;37:113–124. doi: 10.7773/cm.v37i2.1712. [DOI] [Google Scholar]
- 23.Garcia-Vaquero M., Lopez-Alonso M., Hayes M. Assessment of the functional properties of protein extracted from the brown seaweed Himanthalia elongata (Linnaeus) SF Gray. Food Res. Int. 2017;99:971–978. doi: 10.1016/j.foodres.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 24.Clemente A. Enzymatic protein hydrolysates in human nutrition. Trends Food Sci. Technol. 2000;11:254–262. doi: 10.1016/S0924-2244(01)00007-3. [DOI] [Google Scholar]
- 25.Bleakley S., Hayes M., O’Shea N., Gallagher E., Lafarga T. Predicted release and analysis of novel ACE-I, renin, and DPP-IV inhibitory peptides from common oat (Avena sativa) protein hydrolysates using in silico analysis. Foods. 2017;6:108. doi: 10.3390/foods6120108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu J., Aluko R.E., Nakai S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure−activity relationship study of di-and tripeptides. J. Agric. Food Chem. 2006;54:732–738. doi: 10.1021/jf051263l. [DOI] [PubMed] [Google Scholar]
- 27.Wu J., Aluko R.E., Nakai S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure-activity relationship modeling of peptides containing 4-10 amino acid residues. QSAR Comb. Sci. 2006;25:873–880. doi: 10.1002/qsar.200630005. [DOI] [PubMed] [Google Scholar]
- 28.Dong M.W., Tran A.D. Factors influencing the performance of peptide mapping by reversed-phase high-performance liquid chromatography. J. Chromatogr. A. 1990;499:125–139. doi: 10.1016/S0021-9673(00)96968-1. [DOI] [PubMed] [Google Scholar]
- 29.Cheung H.-S., Wang F.-l., Ondetti M.A., Sabo E.F., Cushman D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J. Biol. Chem. 1980;255:401–407. [PubMed] [Google Scholar]
- 30.He H.-L., Chen X.-L., Wu H., Sun C.-Y., Zhang Y.-Z., Zhou B.-C. High throughput and rapid screening of marine protein hydrolysates enriched in peptides with angiotensin-I-converting enzyme inhibitory activity by capillary electrophoresis. Bioresour. Technol. 2007;98:3499–3505. doi: 10.1016/j.biortech.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 31.Moayedi A., Mora L., Aristoy M.-C., Hashemi M., Safari M., Toldrá F. ACE-inhibitory and antioxidant activities of peptide fragments obtained from tomato processing by-products fermented using Bacillus subtilis: Effect of amino acid composition and peptides molecular mass distribution. Appl. Biochem. Biotechnol. 2017;181:48–64. doi: 10.1007/s12010-016-2198-1. [DOI] [PubMed] [Google Scholar]
- 32.Minkiewicz P., Dziuba J., Iwaniak A., Dziuba M., Darewicz M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008;91:965–980. [PubMed] [Google Scholar]
- 33.Grasso S., Brunton N., Lyng J., Lalor F., Monahan F. Healthy processed meat products–Regulatory, reformulation and consumer challenges. Trends Food Sci. Technol. 2014;39:4–17. doi: 10.1016/j.tifs.2014.06.006. [DOI] [Google Scholar]
- 34.Ney K. Voraussage der Bitterkeit von Peptiden aus deren Aminosäurezu-sammensetzung. Prediction of bitterness of peptides from their amino acid composition. Z. Lebensm. Unters. Forsch. 1971;147:64–68. doi: 10.1007/BF01879606. [DOI] [Google Scholar]
- 35.Cho M.J., Unklesbay N., Hsieh F.-H., Clarke A.D. Hydrophobicity of bitter peptides from soy protein hydrolysates. J. Agric. Food Chem. 2004;52:5895–5901. doi: 10.1021/jf0495035. [DOI] [PubMed] [Google Scholar]
- 36.Soares R.A.M., Mendonça S., de Castro L.Í.A., Menezes A.C., Arêas J.A.G. Major peptides from amaranth (Amaranthus cruentus) protein inhibit HMG-CoA reductase activity. Int. J. Mol. Sci. 2015;16:4150–4160. doi: 10.3390/ijms16024150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cushman D., Cheung H., Sabo E., Ondetti M. Angiotensin Converting Enzyme Inhibitors. Mechanisms of Action and Clinical Implications. Urban & Schwarzenberg; Munich, Germany: 1981. Angiotensin converting enzyme inhibitors: Evolution of a new class of antihypertensive drugs; pp. 3–25. [Google Scholar]
- 38.Lan V.T.T., Ito K., Ohno M., Motoyama T., Ito S., Kawarasaki Y. Analyzing a dipeptide library to identify human dipeptidyl peptidase IV inhibitor. Food Chem. 2015;175:66–73. doi: 10.1016/j.foodchem.2014.11.131. [DOI] [PubMed] [Google Scholar]
- 39.Wang W., De Mejia E.G. A new frontier in soy bioactive peptides that may prevent age-related chronic diseases. Compr. Rev. Food Sci. Food Saf. 2005;4:63–78. doi: 10.1111/j.1541-4337.2005.tb00075.x. [DOI] [PubMed] [Google Scholar]
- 40.Nongonierma A.B., FitzGerald R.J. Inhibition of dipeptidyl peptidase IV (DPP-IV) by proline containing casein-derived peptides. J. Funct. Foods. 2013;5:1909–1917. doi: 10.1016/j.jff.2013.09.012. [DOI] [Google Scholar]
- 41.Gómez-Ruiz J.Á., Ramos M., Recio I. Identification of novel angiotensin-converting enzyme-inhibitory peptides from ovine milk proteins by CE-MS and chromatographic techniques. Electrophoresis. 2007;28:4202–4211. doi: 10.1002/elps.200700324. [DOI] [PubMed] [Google Scholar]
- 42.Polikovsky M., Fernand F., Sack M., Frey W., Müller G., Golberg A. In silico food allergenic risk evaluation of proteins extracted from macroalgae Ulva sp. with pulsed electric fields. Food Chem. 2019;276:735–744. doi: 10.1016/j.foodchem.2018.09.134. [DOI] [PubMed] [Google Scholar]
- 43.Valenta R., Hochwallner H., Linhart B., Pahr S. Food allergies: The basics. Gastroenterology. 2015;148:1120–1131.e4. doi: 10.1053/j.gastro.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.EFSA Panel on Genetically Modified Organisms (GMO Panel) Scientific opinion on the assessment of allergenicity of GM plants and microorganisms and derived food and feed. EFSA J. 2010;8:1700. doi: 10.2903/j.efsa.2010.1700. [DOI] [Google Scholar]
- 45.Dimitrov I., Bangov I., Flower D.R., Doytchinova I. AllerTOP v. 2—A server for in silico prediction of allergens. J. Mol. Model. 2014;20:2278. doi: 10.1007/s00894-014-2278-5. [DOI] [PubMed] [Google Scholar]
- 46.Wu J., Liao W., Udenigwe C.C. Revisiting the mechanisms of ACE inhibitory peptides from food proteins. Trends Food Sci. Technol. 2017;69:214–219. doi: 10.1016/j.tifs.2017.07.011. [DOI] [Google Scholar]
- 47.Høst A., Halken S. Hypoallergenic formulas—When, to whom and how long: After more than 15° years we know the right indication! Allergy. 2004;59:45–52. doi: 10.1111/j.1398-9995.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- 48.Nygård L.A.K., Mundal I., Dahl L., Benth J.Š., Rokstad A.M.M. Nutrition and physical performance in older people—Effects of marine protein hydrolysates to prevent decline in physical performance: A randomised controlled trial protocol. BMJ Open. 2018;8:e023845. doi: 10.1136/bmjopen-2018-023845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shilov I.V., Seymour S.L., Patel A.A., Loboda A., Tang W.H., Keating S.P., Hunter C.L., Nuwaysir L.M., Schaeffer D.A. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol. Cell. Proteom. 2007;6:1638–1655. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]
- 50.Gupta S., Kapoor P., Chaudhary K., Gautam A., Kumar R., Raghava G.P. Open Source Drug Discovery Consortium. In silico approach for predicting toxicity of peptides and proteins. PLoS ONE. 2013;8:e73957. doi: 10.1371/journal.pone.0073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mooney C., Haslam N.J., Pollastri G., Shields D.C. Towards the improved discovery and design of functional peptides: Common features of diverse classes permit generalized prediction of bioactivity. PLoS ONE. 2012;7:e45012. doi: 10.1371/journal.pone.0045012. [DOI] [PMC free article] [PubMed] [Google Scholar]