Abstract
Objectives:
We studied military health care provider (HCP) practices regarding reporting of adverse events following immunization (AEFI).
Methods:
A convenience sample of HCP was surveyed to assess familiarity with Vaccine Adverse Event Reporting System (VAERS), AEFI they were likely to report, methods used and preferred for reporting, and perceived barriers to reporting. We analyzed factors associated with HCP reporting AEFI to VAERS.
Results:
A total of 547 surveys were distributed with 487 completed and returned for an 89% response rate. The percentage of HCP aware of VAERS (54%) varied by occupation. 47% of respondents identified knowledge of at least one AEFI with only 34% of these indicating that they had ever reported to VAERS. More serious events were more likely to be reported. Factors associated with HCP reporting AEFIs in bivariate analysis included HCP familiarity with filing a paper VAERS report, HCP familiarity with filing an electronic VAERS report, HCP familiarity with VAERS, and time spent on immunization tasks. In a multivariable analysis, only HCP familiarity with filing a paper VAERS report was statistically significant (Odds ratio = 115.3; p < 0.001).
Conclusions:
Specific educational interventions targeted to military HCP likely to see AEFIs but not currently filing VAERS reports may improve vaccine safety reporting practices.
INTRODUCTION
The U.S. Department of Defense (DoD) has an immunization program providing service personnel with protection from a variety of pathogenic threats. Monitoring of adverse events following immunization (AEFI) is coordinated through the Military Vaccine (MILVAX) Agency, which includes the Vaccine Healthcare Centers Network (VHCN). The VHCN is an integrated part of the military health system with goals of enhancing vaccine safety, efficacy, and acceptability through programs and services that provide expert clinical consultation, care, safety surveillance, education, and research.1 Recent high-profile vaccine safety surveillance assessments have included the anthrax vaccine and the smallpox vaccine, where the DoD has been the primary user of these vaccines.2,3 Compared with adult civilians, service personnel are a highly vaccinated population and are routinely administered multiple concurrent vaccines on accession into basic training and predeployment. Another distinguishing factor about military immunization health care is frequent reliance upon special occupational groups (e.g., medics, immunization technicians) who are trained to administer vaccines and thus may see patients with AEFI which warrant reporting.
The DoD, through the VHCN, monitors potential AEFI primarily by using the Vaccine Adverse Event Reporting System (VAERS). Established in 1990, VAERS is a voluntary, post-licensure, national passive reporting system comanaged by the Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC), and serves as an early warning system to detect adverse events that may be related to vaccines.4,5 Importantly, as a passive system, VAERS is not designed to assess causal associations between vaccines and adverse events. Despite this limitation, VAERS is a valuable system for detecting potential vaccine safety concerns or “signals” that can then be investigated in further epidemiological studies.4,5 The main utility of VAERS is the identification of rare and severe AEFI, with one example being the rapid identification of increased intussusception reports following administration of the first-generation rotavirus vaccine.6
VAERS receives reports of possible AEFI from a wide variety of sources, including civilian and military health care providers (HCP), vaccine manufacturers, and the public. Traditionally, HCP and others have been encouraged to file paper reports for AEFI to VAERS, though web-based reports have also been facilitated since March 2002. HCP are required to report to VAERS any adverse event that is either listed by the vaccine manufacturer as a contraindication to further doses of the vaccine, or any adverse event that is listed in the Vaccine Injury Table and that occurs within the specified time period after vaccination with a vaccine listed from the table.7 Manufacturers are required to report all adverse events after U.S. licensed vaccines to VAERS.8 Military HCP are required to file a VAERS report for reactions that cause a service member either to lose more than 24 hours of duty time or require hospitalization.9 HCP (civilian and military) are also encouraged to report any other adverse event they consider to be clinically important. From 2000 to 2004, approximately 60% of all domestic reports came from either HCP or vaccine manufacturers, and approximately 8% came from vaccine recipients or their parent/guardian (CDC, unpublished data). During this same 5-year period, approximately 7.5% of all domestic reports were identified as military reports (service personnel or their dependents) but during 2006–2011, military reports only comprised approximately 5% of domestic reports (CDC, unpublished data).
Given the high level of vaccination among military personnel, identification and reporting of clinically significant AEFI is critical to identify any potential concerns. The need to better inform military HCP and service members about reporting AEFI to VAERS was a particular challenge for the DoD when the 1998 Anthrax Vaccine Immunization Program was implemented.9 However, military HCP awareness of and practices regarding reporting of AEFI is understudied.
The objective of this study was to evaluate the knowledge, attitudes, and practices of military HCP regarding AEFI identification and reporting to VAERS. The results of this survey provide a baseline against which a new survey could be compared.
METHODS
We performed a survey of a convenience sample of military HCP contacted through the VHCN. Enrollment of the majority of subjects occurred at Walter Reed Army Medical Center (Washington, DC), with additional enrollees at Wilford Hall Medical Center and Brooke Army Medical Center (both located in San Antonio, Texas). No specific training on VAERS was given as part of this study/protocol and the protocol was not designed to evaluate a specific VAERS training intervention. Survey questionnaires were distributed and completed, and data were collected in grouped settings, during military immunization training sessions, or at the respondents’ place of employment. Study participants included personnel in the military officer categories: Army Medical Corps (physicians), Army Medical Specialists (physician assistants), Army Medical Services Corps, Army Nurses Corps, and Army Veterinary Corps, as well as enlisted personnel in the Army Healthcare (91W) Aerospace Medical Services and Navy Medical Services specialties. We classified all participants into five categories based on their occupation: physicians, mid-level professionals, nurses, technicians, and others. An effort was made to target HCP who were regularly involved in administering vaccinations, had direct patient care responsibility (>10% time), or might identify persons with possible AEFI that would be reported to VAERS.
The survey was performed in 2005. No incentive was offered to participants. The self-administered questionnaire consisted of 23 closed-ended questions divided into the following three categories: (1) demographic, military, and professional characteristics; (2) knowledge, attitudes, and personal experiences of reporting AEFIs to VAERS; and (3) training received on reporting to VAERS and perceived barriers to and facilitators for reporting to VAERS.
STATISTICAL ANALYSIS
Descriptive statistics were computed for demographic variables. Questions with 5-choice Likert scales were grouped responses of comparable quality. For example, responses “very likely” and “extremely likely” were combined, responses of “somewhat likely” and “moderately were combined. We performed bivariate and multivariable analyses to evaluate the potential association between related to respondents’ demographic, military, and professional characteristics and whether they had reported an to VAERS. These factors included gender, age, current military status, current service group, occupational level, of years at current position, type of office location, time on immunization tasks, degree of familiarity with and specifically filing a paper report to VAERS and filing Internet VAERS report. Odds ratios (OR) and 95% confidence intervals (CI) are reported. For multivariable we used the stepwise method to select significant Missing data were excluded from the analysis. A significance level of 0.05 was adopted for all statistical significance tests. All analyses were performed using BASE 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
Study Population
From the sample of 547 potential participants invited to complete the survey, 512 surveys were returned, of which 487 were judged to contain usable data (representing a final response rate of 89.0%). Twenty-five surveys were judged to be unusable because they either did not contain responses to the majority of questions or the occupational characteristics of the respondent did not match those of the target population.
The demographics of our survey sample are given in Table I. The sample was evenly divided between males and females. The majority (82.3%) was aged between 18 and 45 years, with 17.7% aged 46 years or older. Approximately 66.9% of respondents were active duty, 10.6% in the National Guard or the Reserve, and 3.9% retired. Most respondents reported their military service branch as Army (61.6%), followed by Air Force (20.7%), others (14.1%), Navy (3.4%), and U.S. Public Health Service (0.2%). Occupational categories in the sample included physicians (12.6%), mid-level professionals (physician assistant, nurse practitioner) (3.5%), nurses (licensed vocational nurses, registered nurse, enlisted nurse) (26.5%), and technicians (medic/corpsman, immunization technician, or specialist) (50.0%), and others (7.4%). The majority (62.5%) of respondents had served in their same position for 5 years or less; however, 20.6% had served in their current position for more than 10 years. The majority (66.2%) of respondents reported their office was located in a hospital on a military base.
TABLE I.
Demographics of Military HCP Survey Respondents (N = 487)
| Characteristics | Number | Percent |
|---|---|---|
| Gender | ||
| Female | 244 | 50.1 |
| Male | 243 | 49.9 |
| Age (years)a | ||
| 18–25 | 114 | 23.5 |
| 26–35 | 192 | 39.5 |
| 36–45 | 94 | 19.3 |
| 46–55 | 68 | 14.0 |
| 56+ | 18 | 3.7 |
| Current Military Statusa | ||
| Active duty | 323 | 66.9 |
| Reserve Component | 51 | 10.6 |
| Retired | 19 | 3.9 |
| Other | 90 | 18.6 |
| Servicea | ||
| Army | 289 | 61.6 |
| Navy | 16 | 3.4 |
| Air Force | 97 | 20.7 |
| U.S. Public Health Service | 1 | 0.2 |
| Other | 66 | 14.1 |
| Occupationa | ||
| Physicians | 61 | 12.6 |
| Physician | 61 | 12.6 |
| Mid-Level Professionals | 17 | 3.5 |
| Physician Assistant | 3 | 0.6 |
| Nurse Practitioner | 14 | 2.9 |
| Nurses | 129 | 26.5 |
| Licensed Vocational Nurse | 47 | 9.7 |
| Registered Nurse | 75 | 15.4 |
| Enlisted Nurse | 7 | 1.4 |
| Technicians | 243 | 50 |
| Medic or Corpsman | 177 | 36.4 |
| Immunization Technician or Specialist | 66 | 13.6 |
| Others | 36 | 7.4 |
| Length of Time in Position (year)a | ||
| 0–2 | 168 | 35.0 |
| 3–5 | 132 | 27.5 |
| 6–10 | 81 | 16.9 |
| 11–15 | 35 | 7.3 |
| 16–20 | 25 | 5.2 |
| 20+ | 39 | 8.1 |
| Location of Office?a | ||
| Hospital on Base | 321 | 66.2 |
| Hospital off Base | 30 | 6.2 |
| Stand-Alone Clinic on Base | 70 | 14.4 |
| Stand-Alone Clinic off Base | 15 | 3.1 |
| Others | 49 | 10.1 |
There are missing data in the category.
Familiarity With VAERS
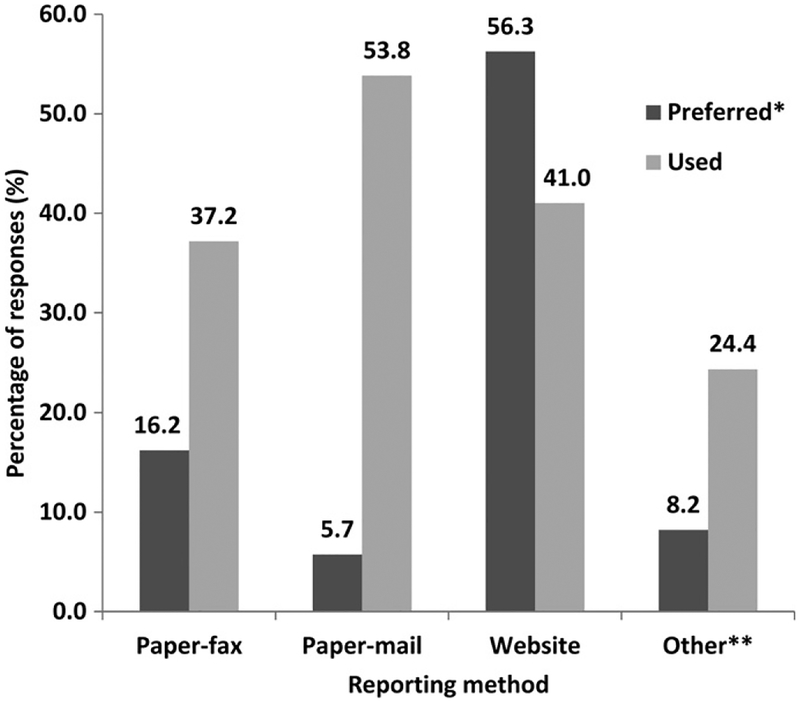
Overall, 53.9% of respondents were at least somewhat familiar with VAERS, although only 6.8% were very or extremely familiar with VAERS. With respect to the different reporting modalities, only 5.5% were very or extremely familiar with the procedure for filing a VAERS report using the paper form, and only 3.6% were very or extremely familiar with the procedure for filing a VAERS report using the Internet. A higher proportion of physicians (73.4%) than other occupational groups were at least somewhat familiar with VAERS. Of 78 HCP who identified and reported an AEFI to VAERS at least one time, a majority (91.0%) indicated the paper form (submitted by either fax or mail) as the method used, whereas 41.0% indicated they used electronic reporting via VAERS Web site (Fig. 1). When HCP were asked what would be the easiest method to report to VAERS, more than half of respondents (56.3%) indicated the electronic form through the VAERS Web site.
FIGURE 1.
Reporting methods for AEFI used and preferred by military HCP. *Each respondent was allowed to choose only one “preferred” method but multiple “used” methods. **Includes (but not limited to): referred to VHC/Allergy & Immunology, noted in patient’s medical chart, reported to a nurse, etc.
Likelihood of Reporting an AEFI to VAERS
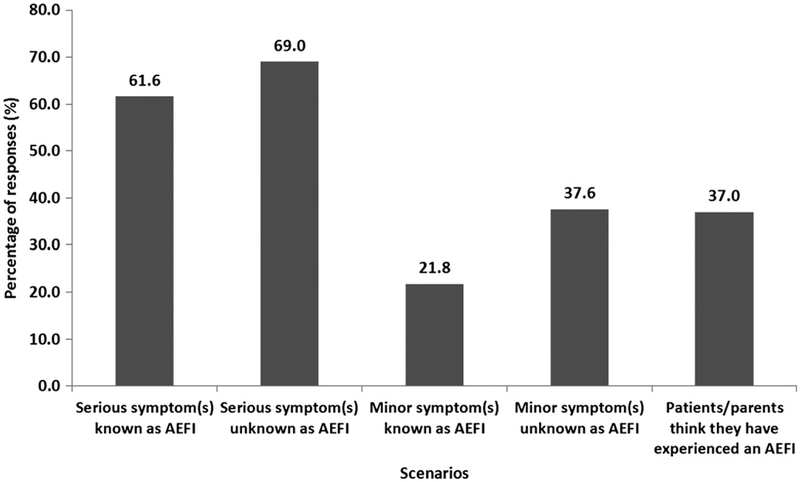
Most respondents indicated that they were very or extremely likely to report a serious AEFI to VAERS, whether or not the AEFI was known to be associated with immunizations (61.6% and 69.0%, respectively; Fig. 2). Substantially, fewer respondents indicated that they would be very or extremely likely to report a minor AEFI to VAERS, whether it was known or not known to be associated with immunizations. There was a trend toward higher proportions of respondents indicating that they would report AEFI when symptoms are unknown to occur after an immunization compared to symptoms that are known to occur, regardless of the severity of the symptoms.
FIGURE 2.
Scenarios when military HCP are very or extremely likely to report AEFI.
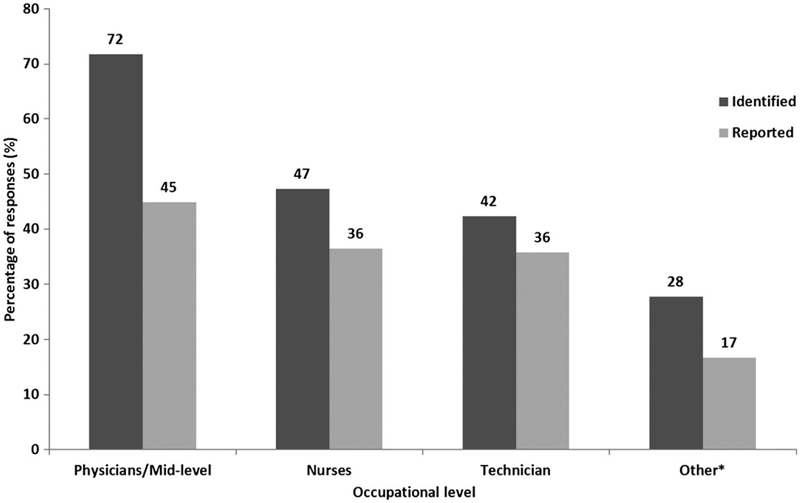
Across different occupational levels, we observed a decreasing trend (physicians/mid-level professionals > nurses > technicians > others) in the proportions of respondents who had ever identified an AEFI and who had ever reported an AEFI (Fig. 3).
FIGURE 3.
Identification and reporting of AEFI stratified by military HCP occupational level. *Includes LPN student, LVN student, medical student, nursing assistant, occupational therapy assistant, registered dietitian, psychologist, and instructor, etc.
Factors Related to HCP Reporting AEFI to VAERS
Among all respondents, 230 HCP indicated they had identified at least one AEFI, and 78 (33.9%) of those who identified an AEFI indicated they had reported to VAERS. Table II summarizes the results of our bivariate and multivariable analyses. Bivariate analysis identified 4 variables as significantly associated with the HCP reporting an AEFI to VAERS given that HCP had at least identified one AEFI. These factors included familiarity with the procedure for filing a paper report to VAERS, familiarity with the procedure for filing a report to VAERS using the Internet, familiarity with VAERS, and percentage of time at work spent on tasks related to immunization. In the multivariable analysis, HCP who reported that they were very/extremely familiar with filing a paper report to VAERS were more likely to report than those who were not at all familiar with that process (OR = 115.3; p < 0.0001). We did not find a significant association between persons who had filed a report to VAERS and the age of the respondents or the number of years they had worked at their current position. In addition, occupational category was not found to be associated with HCP reporting AEFI to VAERS.
TABLE II.
Factors Associated With HCP Reporting to VAERS After Identifying an AEFI
| Factors | Reporting Yes/Total n |
Bivariate Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | |||
| Familiarity With VAERS Paper Reporting | Not at All | 5/102 | 1 | |||||
| Moderate | 26/72 | 11.0 | (4.0, 30.4) | <0.0001 | 12.2 | (4.0, 37.4) | <0.0001 | |
| Very/Extremely | 46/54 | 111.6 | (34.6, 359.8) | 115.3 | (32.9, 404.1) | |||
| Familiarity With VAERS Internet Reporting | Not at All | 23/130 | 1 | |||||
| Moderate | 27/65 | 3.3 | (1.7, 6.4) | <0.0001 | ||||
| Very/Extremely | 27/33 | 20.9 | (7.8, 56.5) | |||||
| Familiarity With VAERS | Not at All | 3/78 | 1 | |||||
| Moderate | 24/85 | 9.836 | (2.8, 34.2) | <0.0001 | ||||
| Very/Extremely | 51/65 | 91.1 | (24.9,333.1) | |||||
| Time Spent on Immunization Tasks | 10% | 23/118 | 1 | |||||
| 25% | 9/28 | 2.2 | (0.9, 5.5) | |||||
| 50% | 6/14 | 3.1 | (1.0, 9.8) | <0.0001 | ||||
| 75% | 7/13 | 7.2 | (2.0, 26.8) | |||||
| >75% | 33/53 | 6.8 | (3.3, 14.0) | |||||
Potential Factors to Enhance the Likelihood of Reporting AEFI to VAERS
The questionnaire inquired about potential factors that would encourage reporting and make reporting easier. 59.1% of respondents indicated that more information on when it is appropriate to report to VAERS would make reporting AEFI much or extremely easier. Other measures reported to be helpful in making filing of VAERS reports much/extremely easier included providing more information on how to report to VAERS (58.1%), having someone with VAERS experience assist with the reporting process (55.6%), briefer forms to reduce the amount of time needed to fill the VAERS report (54.8%), clarification in the respondent’s office regarding whether his/her specific duties included reporting AEFI to VAERS (52.6%), more training in identifying AEFI (52.3%), and better access to materials needed to report to VAERS (52.2%). Of interest, only 37.0% of respondents indicated that a major consideration for them was confidentiality of medical records, and a similar minority (37.6%) thought that more encouragement from the leadership to report AEFI to VAERS would have an effect.
DISCUSSION
To our knowledge, this is the first report of a systematic evaluation of the knowledge, attitudes, and practices of military HCP regarding AEFI identification and reporting to VAERS. We found in this sample of military HCP that only 33% of respondents who had identified at least one AEFI had ever reported to VAERS. In addition, although the majority of military HCP were at least somewhat familiar with VAERS, 45% of sampled HCP did not have any knowledge about VAERS. Together, these findings suggest that military HCP knowledge and awareness of the process and practices regarding reporting of AEFI to VAERS is lacking, contributing to underreporting to VAERS, which may negatively impact the system’s public health function. Measures introduced in April 1999 following the implementation of 1998 Anthrax Vaccine Immunization Program to better inform military HCP and service members about reporting AEFI to VAERS included updating briefings to include information on reporting AEFI, and revising regulations to (1) make reporting requirements more inclusive, (2) clarify patient and HCP responsibilities, and (3) explain how to obtain and process VAERS forms.9 In addition, in July 1999, specific directions for reporting to VAERS together with specific treatment guidelines/algorithms for the management of local and systemic AEFI, pretreatment of vaccinees, and specialty referral processes within the DoD health care system were added as additional measures.9
Our study has some limitations. Our survey was conducted in 2005, and there may have been recent changes in military HCP awareness of and practices regarding reporting of AEFI to VAERS. We also used a convenience survey sample, which makes generalization of findings to the larger population of military personnel problematic. However, we did make an effort to target HCP who were regularly involved in administering vaccinations and might be more likely to identify and report potential AEFIs. Therefore, our results may represent a best-case scenario regarding military HCP knowledge of VAERS and likelihood of reporting AEFIs to VAERS.
We found a majority (91.0%) of respondents who had reported to VAERS indicated they used the VAERS paper form (submitted by either fax or mail) at least once as the method for reporting, and 41.0% indicated they had ever used electronic reporting via VAERS Web site. However, among all respondents, a majority (56.3%) indicated they would prefer Web site reporting over other reporting methods. This suggests that the latter group of military HCP may be receptive if a completely web-based online electronic VAERS reporting system was introduced and paper-based reporting was discontinued. However in this instance, a targeted educational initiative also for the minority 43.7% of respondents who state they do not prefer web reporting would be of critical importance. Of note, Internet-based reports have been shown to have better timeliness, potential to reduce transcription and data entry errors, and result in more accurate data.10 Lastly during the intervening period, the proportion of Internet-based VAERS reports has been consistently higher for military than nonmilitary reports, and there has also been a greater increase among military reports (43% vs. 18% in 2005 and 64% vs. 31% in 2013) (CDC, unpublished data), perhaps reflecting a higher level of awareness among DoD providers.
The majority of respondents indicated that they were very or extremely likely to report a serious AEFI to VAERS, whether or not the AEFI was known to be associated with immunizations (61.6% and 69.0%, respectively).
In the multivariable analysis, we found only HCP who reported that they were very/extremely or moderately familiar with filing a paper report to VAERS were more likely to report than those who were not at all familiar with that process. This finding is consistent with two published reports of civilian HCP knowledge and practices of reporting AEFI.11,12 In a survey of Canadian family physicians, Duclos et al11 found their reporting to the national AEFI monitoring system was significantly associated with knowledge of the reporting system and the reporting criteria. Whereas in a 2005 survey of U.S. office-based HCP, McNeil et al12 identified reporting to VAERS was significantly associated with the HCP being very familiar vs. not familiar with filing a paper VAERS report, the HCP primary care practice area of pediatrics vs. internal medicine and the HCP being very familiar vs. not familiar with when it was required to file a VAERS report.
To our knowledge, there is only one report of a direct comparison between military and civilian reporting AEFI to VAERS.13 This followed the U.S. National Smallpox Vaccination Program. In December 2002, the military began a mandatory vaccination of service members and, shortly thereafter in January 2003, vaccination was offered to civilian first responders who were mainly medical personnel. Although vaccination of military personnel has continued to date, the civilian vaccination program was suspended after the first year. McMahon et al13 found reporting rates for AEFI were significantly higher in civilian vs. military personnel ages <55 years (rate ratios ranged 4–27) and suggested that this difference may have resulted from differences in the level of stimulated reporting in these populations and their specific AEFI reporting practices, including the “threshold” for reporting.
More education of military HCP on the process of reporting to VAERS is needed. Since our survey was conducted, there have been improvements made to VAERS and more widespread use of electronic medical records, educational campaigns, and an influenza pandemic. VAERS served as the nation’s frontline monitoring system, providing the first available vaccine safety data after 2009-H1N1 vaccines were in use.14 Thus, our survey provides a baseline against which a new survey could be compared. Enhancements to VAERS are planned to improve the quality and timeliness of VAERS reporting, including expanded capacity for receiving electronic reports and planned evaluation of potentially enabling reporting directly from handheld data collection devices. A prototype computer application is also under development to operate with the electronic medical record to alert the HCP seeing a patient with a potential adverse event about recent vaccination and possibly filing a VAERS report (that can be automatically populated).15 Although the VAERS system may eventually transfer from a paper/telephone to a completely web-based reporting system, our survey suggests this should be done with caution, because we may lose those HCP who are extremely familiar with the paper form but not comfortable with the Internet.
The Military Health System has promoted efforts to standardize and enhance immunization health care education including VAERS education and positive encouragement for reporting in all medical settings. The Joint Immunization and Chemoprophylaxis Regulations updated in 2006 (http://www.uscg.mil/directives/cim/6000-6999/CIM_6230_4F.pdf) includes a summary of the earlier years’ focus on implementing the minimum standards for quality immunization health care in nontraditional sites.16 This also provides instruction on all service-specific vaccine delivery and personnel education requirements and includes information on VAERS reporting. Since its publication, the MILVAX/VHCN partnership (www.vaccines.mil and www.VHCinfo.org) has led efforts to enhance education about vaccines and reporting of AEFI to VAERS and when needed can assist in VAERS investigations and reporting. Immunization University and outreach educational programs (Immunization Leadership Course, Immunization Basic Course) are also provided regionally every 1 to 2 years. However, educational outreach to providers not involved in vaccine delivery is still a challenge as is evident from recent underreporting of myocarditis following smallpox vaccine, a well-recognized potentially serious adverse event. Although military HCP education on identifying AEFI and reporting to VAERS has been enhanced since 2005, ongoing efforts to improve understanding of the barriers to the reporting of AEFI are needed. Better understanding of the characteristics of military HCP may be useful to public health agencies as they plan improvements to VAERS.
ACKNOWLEDGMENTS
The authors thank the following individuals for their invaluable assistance with this study: Sandra L. Schneider, DrPH; Patricia A. Hutchinson, MD; John Grabenstein, PharmD, PhD; Mary C. Minor, FNP; Jeannette F. Williams, FNP; Sheila L. Hill, RN; Bryan L. Martin, DO; Christina Spooner, MS; Elaine Colen; and the supporting staff of the Vaccine Healthcare Centers Network (Walter Reed National Military Medical Center, Bethesda, MD; Womack Army Medical Center, Fort Bragg, NC; Wilford Hall Medical Center, San Antonio, TX; and Naval Medical Center Portsmouth, VA). The authors also thank RTI personnel for their assistance with the study and Frank DeStefano, MD MPH for critical review of the manuscript. The funding for this study was provided solely by the Centers for Disease Control and Prevention.
The views, findings, and conclusions in this report are those of the authors and do not reflect the official policy or position of the Centers for Disease Control and Prevention, the Departments of the Army/Navy/Air Force, the Department of Defense, or the U.S. Government. The use of trade names and commercial sources is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, or the U.S. Department of Health and Human Services. The protocol for this study was determined to be nonresearch and therefore did not require review by the Institutional Review Board of CDC or RTI International, and was approved by the DoD IRB.
REFERENCES
- 1.Vaccine Healthcare Centers Network. Available at http://www.vhcinfo.org; accessed June 6, 2013.
- 2.Centers for Disease Control and Prevention (CDC): Surveillance for adverse events associated with anthrax vaccination—U.S. Department of Defense, 1998–2000. MMWR Morb Mortal Wkly Rep 2000; 49: 341–5. [PubMed] [Google Scholar]
- 3.Grabenstein JD, Winkenwerder W Jr: US military smallpox vaccination program experience. JAMA 2003; 289: 3278–82. [DOI] [PubMed] [Google Scholar]
- 4.Varricchio F, Iskander J, DeStefano F, et al. : Understanding vaccine safety information from the Vaccine Adverse Event Reporting System (VAERS). Pediatr Infect Dis J 2004; 23: 287–94. [DOI] [PubMed] [Google Scholar]
- 5.Iskander JK, Miller ER, Chen RT: The role of the Vaccine Adverse Event Reporting System (VAERS) in monitoring vaccine safety. Pediatr Ann 2004; 33: 599–606. [DOI] [PubMed] [Google Scholar]
- 6.Murphy TV, Gargiullo PM, Massoudi MS, et al. : Intussusception among infants given an oral rotavirus vaccine. N Engl J Med 2001; 344: 1564–72. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. Health Resources and Services Administration. National Childhood Vaccine Injury Act, Vaccine Injury Table, 2011. Available at http://www.hrsa.gov/vaccinecompensation/vaccineinjurytable.pdf; accessed June 17, 2013.
- 8.Code of Federal Regulations Title 21, Vol 7. 21CFR600.80. Available at http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600; accessed June 17, 2013.
- 9.Medical Readiness: DOD Faces Challenges in Implementing its Anthrax Vaccine Immunization Program Letter Report GAO/NSAID-00–36. Washington, DC, U.S. General Accounting Office, October 1999. Available at http://www.globalsecurity.org/wmd/library/report/gao/nsiad-00-036.htm; accessed June 17, 2013. [Google Scholar]
- 10.Haber P, Iskander J, Walton K, Campbell SR, Kohl KS: Internet-based reporting to the vaccine adverse event reporting system: a more timely and complete way for providers to support vaccine safety. Pediatrics 2011; 127 (Suppl 1): S39–44. [DOI] [PubMed] [Google Scholar]
- 11.Duclos P, Hockin J, Pless R, Lawlor B: Reporting vaccine-associated adverse events. Are family physicians aware of criteria and procedures? Can Fam Physician 1997; 43: 1551–6. [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil MM, Li R, Pickering S, Real TM, Smith PJ, Pemberton MR: Who is unlikely to report adverse events after vaccinations to the Vaccine Adverse Event Reporting System (VAERS)? Vaccine 2013; 31: 2673–9. [DOI] [PubMed] [Google Scholar]
- 13.McMahon AW, Zinderman C, Ball R, Gupta G, Braun MM: Comparison of military and civilian reporting rates for smallpox vaccine adverse events. Pharmacoepidemiol Drug Safe 2007; 16: 597–604. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention: Safety of influenza A (H1N1) 2009 monovalent vaccines—United States, October 1–November 24, 2009. MMWR Morb Mortal Wkly Rep 2009; 58: 1351–6. [PubMed] [Google Scholar]
- 15.Hinrichsen VL, Kruskall B, O’Brien MQA, Lieu TA, Platt R: Vaccine Safety Datalink Team. Using electronic medical records to enhance detection and reporting of adverse events. J Am Med Inform Assoc 2007; 14: 731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention: Adult immunization programs in nontraditional settings: quality standards and guidance for program evaluation. A Report of the National Vaccine Advisory Committee. MMWR Morb Mortal Wkly Rep 2000; 49 (RR-01): 1–13. [PubMed] [Google Scholar]