Abstract
Immune cells, as a consequence of their plasticity, can acquire altered phenotype/functions within the tumor microenvironment (TME). Some of these aberrant functions include attenuation of targeting and killing of tumor cells, tolerogenic/immunosuppressive behavior and acquisition of pro-angiogenic activities. Natural killer (NK) cells are effector lymphocytes involved in tumor immunosurveillance. In solid malignancies, tumor-associated NK cells (TANK cells) in peripheral blood and tumor-infiltrating NK (TINK) cells show altered phenotypes and are characterized by either anergy or reduced cytotoxicity. Here, we aim at discussing how NK cells can support tumor progression and how induction of angiogenesis, due to TME stimuli, can be a relevant part on the NK cell-associated tumor supporting activities. We will review and discuss the contribution of the TME in shaping NK cell response favoring cancer progression. We will focus on TME-derived set of factors such as TGF-β, soluble HLA-G, prostaglandin E2, adenosine, extracellular vesicles, and miRNAs, which can exhibit a dual function. On one hand, these factors can suppress NK cell-mediated activities but, on the other hand, they can induce a pro-angiogenic polarization in NK cells. Also, we will analyze the impact on cancer progression of the interaction of NK cells with several TME-associated cells, including macrophages, neutrophils, mast cells, cancer-associated fibroblasts, and endothelial cells. Then, we will discuss the most relevant therapeutic approaches aimed at potentiating/restoring NK cell activities against tumors. Finally, supported by the literature revision and our new findings on NK cell pro-angiogenic activities, we uphold NK cells to a key host cellular paradigm in controlling tumor progression and angiogenesis; thus, we should bear in mind NK cells like a TME-associated target for anti-tumor therapeutic approaches.
Keywords: NK cells, tumor microenvironment, angiogenesis, tumor therapy, targeting immunotherapy, chemotherapy
1. Introduction
Strong evidences suggest that the presence of inflammatory cells within the TME plays a crucial role in the development and/or progression of tumors [1,2,3]. Among the host-dependent biological features of the tumor hallmarks defined by Hanahan and Weinberg [4], there are “evading immune destruction” and “tumor-promoting inflammation”, which together with the immune cell-mediated orchestration of angiogenesis, point out the key role of the immune system in neoplastic disease [3,4,5]. As a consequence of their functional plasticity, several immune cells, can modify upon stimuli delivered by the components of TME their phenotypic and functional features; this leads to a reduced killing of tumor cells, the expression of a tolerogenic/immunosuppressive behavior and the acquisition of pro-angiogenic activities, thus promoting tumor expansion [1,3,5,6,7].
NK cells are innate lymphocytes that can potentially control tumor growth by their cytotoxic activity [8,9]. Classical NK cells are distinct from innate lymphoid cells (ILCs) although they share with ILC1 several phenotypic features [10,11,12]; indeed, NK cells are key cytolytic effectors of innate immunity while ILC1 are generally non-cytotoxic or weakly cytotoxic [12] but they show a central role in response to certain infections and are also involved in tissue remodeling homeostasis, morphogenesis, metabolism, repair, and regeneration [10]. According to Vivier et al., ILC and NK cells originate from a common lymphoid progenitor (CLP) [11,12]. GATA3 or TOX/NFIL3/ID2/ETS1 drive the distinction between common innate lymphoid progenitor (CLIP) and the NK cell progenitor (NKP), respectively. Finally, T-bet/EOMES expression in NKPs govern NK cell differentiation [11,12]. Natural killer cell subsets can differ according to tissue distribution that is related to distinct homing properties and/or local maturation [13].
According to the surface expression of CD56 and CD16, two major peripheral blood NK subsets have been identified [8,9]. CD56dimCD16+ NK cells (90–95% of total circulating NK cells), endowed with cytotoxic activities by perforin and granzyme release and mediating antibody dependent cellular cytotoxicity (ADCC) and CD56brightCD16- NK cells (5–10% of total circulating NK cells), able in producing Th1 cytokines, such as IFN-γ and TNF-α [8,9]. Whether CD56dimCD16+ and CD56brightCD16- cell subsets can be definitely considered terminally differentiated NK cells, still represent a matter of debate. Strong evidence supports that CD56bright NK cells represent still an immature phenotype that is able to differentiate in CD56dim NK cells in vitro and in humanized murine models [13,14,15]. A distinct NK cell subset was found within the developing decidua known as decidual NK cells (dNK). dNK cells are able to acquire a tolerogenic and pro-angiogenic phenotype, identified as CD56superbrightCD16-VEGFhighPlGFhighCXCL8+ dNKs and are necessary to drive the spiral artery formation during the embryo development [16,17]. Alterations of the expression of relevant activating receptors such as the natural cytotoxicity receptors (NCRs: NKp30, NKp44, and NKp46) have been observed in blood from acute myeloid leukemia (AML) patients [18]; in addition, recent studies in breast [19], lung [20,21], colorectal cancer (CRC) [22,23], renal cell carcinoma [24], and gastrointestinal stromal tumors [25] have shown that intratumor NK cells display phenotypic and/or functional alterations compared with peripheral NK cells.
Neoplastic transformation significantly impacts on NK cell phenotype, localization, and functions. CD56brightCD16low/−Perflow NK cells appears to preferentially accumulate in solid cancers [2,5,20,21,22,26,27,28,29,30]. Recently, a new NK cell subset, termed CD56lowCD16low, has been described in the bone marrow (BM) and peripheral blood of pediatric healthy donors and leukemic transplanted patients. This CD56lowCD16low NK cell subset is supposed to represent an intermediate stage of differentiation between CD56highCD16+/− and CD56lowCD16high [31,32,33]. Elevated number of CD56lowCD16low NK cells have also been found in the BM on multiple myeloma patients, with decreased expression of activating receptors such as DNAM-1 and NKp30 and impaired cytolytic capabilities [15].
2. TME Factors Orchestrating NK Cell Activity
Tumor cells have developed several mechanisms to evade from NK cell immunosurveillance, through the modulation of cell surface molecules involved in their recognition and the release of immunosuppressive soluble factors such as TGF-β, HLA-G, prostaglandins and adenosine in the TME [34].
2.1. Selected Soluble Factors
2.1.1. TGF-β
Suppressive cytokines are crucial orchestrators in shaping NK cell anergy and exhaustion in tumors [35]. TGF-β is a major immunosuppressive cytokine present in the TME [36,37] and it is detected at high levels in different tumors [36]. The inhibitory effects of TGF-β on NK cells are well documented and act mainly by downregulating the expression of NKG2D [38] (Figure 1A). TGF-β has also been shown to inhibit CD16-mediated human NK cell IFN-γ production and ADCC through SMAD3 [39].
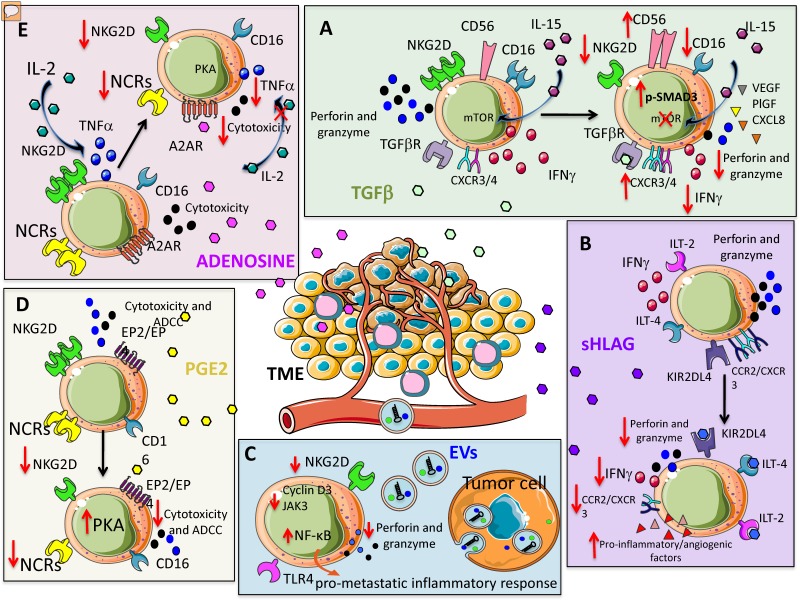
Figure 1.
Soluble factors within the tumor microenvironment (TME) orchestrating natural killer (NK) cell pro-tumor features. Several soluble or microvesicle/exosome-associated factors can impair NK cell-mediated anti-tumor activities. These factors can be produced by different components of the TME besides tumor cells. (A) TGF-β can inhibit interleukin-15 (IL-15) triggering of NK cells, impairing the mTOR signaling; this results in the reduction of NKG2D expression and consequent killing of NKG2DL+ tumor cells; also, CD16, perforins, granzymes and IFN-γ are downregulated. This revert NK cells to a pro-angiogenic phenotype characterized by the secretion of VEGF. (B) Soluble HLA-G interacting with the KIR2DL4, ILT-4 and ILT-2 inhibitory NK cell receptors shape the behavior of NK cells from cytotoxic to pro-angiogenic. (C) Tumor-derived extracellular vesicles (EVs) and exosomes expressing NKG2DL can induce NK cell anergy because they interact with NKG2D on NK cell surface; this impairs the binding of NK cells with NKG2DL present on tumor cells. (D) PGE2 recognizes EP2/EP specific receptors on cytotoxic NK cells leading to the decrement of expression of several activating receptors such as NKG2D and NCR. This leads to the inhibition of tumor target recognition and killing. (E) Likewise, PGE2, adenosine upon binding with A2AR receptors on NK cells induces the downregulation of NKG2D and NCR; this results in a reduced killing of tumor target cells expressing NKG2DL and/or NCRL.
NK cells from healthy donors, following exposure to TGF-β, exhibit alteration in their killing capability through inhibition of perforin and granzyme release [40,41,42]. Further, TGF-β-mediated alteration of degranulation capability (i.e., CD107a release) and reduction in Th1 cytokines have been described for NK cells in different tumors [36,37,38]. Mechanistically, Viel et al. demonstrated that the mammalian target of rapamycin (mTOR) is targeted in human and mouse NK cells (Figure 1A). The authors showed that treatment with TGF-β in vitro blocked interleukin-15 (IL-15)–induced activation of mTOR, resulting in downregulation of activatory receptors, reduced NK cell proliferation and cytotoxic activity [41]. Keskin et al. showed that TGF-β promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to dNK cells [43]. Again, TGF-β has been shown to upregulate CXCR3 and CXCR4 in tumor infiltrating NK cells [44], similar to what occurs for dNK cells [45,46,47].
Tumor-infiltrating and tumor-associated NK cells, in non-small cell lung cancer (NSCLC) patients, apart from functional anergy [21] can acquire the decidual-like CD56brightCD16- phenotype, endowed with pro-angiogenic functions [2,20]. TGF-β has been identified as a master angiogenic-switcher in NKs, being able to polarize CD56dimCD16+cytolytic NK cells toward the CD56brightCD16-VEGFhighPlGFhighCXCL8+IFNγlow NK cell subset [20] (Figure 1A).
Gao et al. demonstrated that TGF-β is able to convert NK cells (CD49a-CD49b+Eomes+) into ILC1 (CD49a+CD49b-Eomesint) [48]. Contrary to tumor immunization from NK cells, ILC1s were not able to control tumor growth. In this way, the tumor escapes the surveillance of the innate immune system by exploiting the sensitivity of NK cells to TGF-β to benefit from the plasticity of ILC1 in the TME [48]. Altogether, these data identify TGF-β as a relevant target to block the angiogenic switch in cancer patients and the inhibition of TGF-β signaling has been reported to preserve the function of highly activated, in vitro expanded NK cells in AML and CRC models [40] (Figure 1A).
Several cytokines, such as IL-2, IL-15, IL-21, IL-27, and IL-18, are generally referred as “immunostimulatory” for their ability of contrasting the effects of TGF-β1 [49]. Very unexpectedly, Casu et al. recently showed that IL-18, that is supposed to contrast immumosuppressive activities of TGF-β on NK cells, synergistically acts with TGF-β by contributing to the impairment of both NK cells recruitment and killing capability [49].
2.1.2. HLA-G
HLA-G is an immunoregulatory class I MHC molecule that have been found to be expressed by decidual trophoblasts [50,51] and in diverse tumor tissues [51,52,53]. Tumor and serum HLA-G expression levels have been reported to be an independent marker of poor prognosis in several tumors, including NSCLC, ovarian, breast, colorectal, esophageal, gastric, hepatocellular and endometrial cancers [52,54,55,56,57]. Several controversies emerged around the expression of HLA-G by tumors [58]. In particular, some commonly used monoclonal antibodies to HLA-G give false positive results [58].
Immunosuppressive activities of HLA-G on NK cells act by interacting with immunoglobulin-like transcripts ILT-2, ILT-4 and killer Ig-like immunoglobulin receptor (KIR) 2DL4, whose interactions result in dampened NK cytotoxicity [59,60,61] (Figure 1B). Interaction between HLA-G with KIR2DL4 [62] has been documented to induce resting NK cell stimulation to produce several pro-inflammatory and pro-angiogenic factors, via induction of a senescence-associated secretory phenotype [45].
Therefore, in a model of metastatic ovarian cancer, HLA-G has been reported to support tumor progression by reducing NK cell cytotoxicity [63]. HLA-G have been also found to downregulate CCR2 and CXCR3, but not CXCR4 expression on CD56bright NK cells, supporting the hypothesis that HLA-G is directly involved in NK cell recruitment by microenvironment [46] (Figure 1B).
2.1.3. Prostaglandin E2
Prostaglandin E2 (PGE2) is associated with enhancement of cancer cell survival, growth, migration, invasion, angiogenesis, and immunosuppression [47] and largely produced within the TME [64,65]. PGE2 has been reported to inhibit NK cell cytotoxicity by reducing the expression of NKG2D, NCRs (NKp30, NKp44, and NKp46), and ADCC [66,67]. PGE2-induced NK cell anergy was associated a cAMP-mediated PKA type I-dependent mechanism following the binding of PGE2 on EP2 and EP4 receptors [68] (Figure 1D). Co-culture experiments also demonstrated relevant contribution of melanoma cancer-associated fibroblasts in mediating NK cell inhibition through PGE2 release [69] (see below). Given the recent finding in solid cancers, showing that anergic and IFNγlow NK cells are subverted into pro-angiogenic NKs, it is conceivable that PGE2 can significantly contribute to the NK cell angiogenic switch. This will assume a dual role of PGE2 in supporting angiogenesis by direct action [70] and by polarizing anergic NK cells.
2.1.4. Adenosine
Adenosine is a soluble immunomodulatory molecule acting through adenosine receptors (A1, A2A, A2B, and A3) that have been found to be expressed on multiple immune subsets including NK cells [71,72,73,74]. Adenosine peaks during decidualization [75] and up to 20-fold increases in the extracellular fluid of solid carcinomas has been reported [76]. Extracellular adenosine accumulation is partially sustained by hypoxia associated with the modulation of enzymes implicated in adenosine metabolism, like adenosine kinase and endo-5′ nucleosidase. Once released in the extracellular environment, adenosine has been reported to impair NK cell normal function by decreasing IL-2-dependent TNF-α secretion, inhibiting cytotoxic granule exocytosis, repressing perforin and Fas ligand-mediated cytotoxic activity [34] (Figure 1E). Many of these effects are attributed to stimulation of the cyclic AMP/protein kinase A pathway, following the binding of adenosine to A2A receptors on NK cells [34].
Adenosine has been reported to affect the expression of activating NK cell receptors by suppressing the release of cytotoxic cytokines in NK cells stimulated with IL-2/NKp46, an effect mediated by the combination of adenylyl cyclase and PKA I, through adenosine A2A receptor signaling [77] (Figure 1E). Immune suppressive activities of adenosine have been also described in NK cells stimulated with either IL-2, IL-15, or a combination of IL-12 and IL-15, by downregulation of the activating receptors NKG2D and NKp30 [78].
Very recently, Young et al. showed that A2AR adenosine signaling suppresses NK cell maturation in the TME [79]. The authors observed that engagement of A2AR acts as a checkpoint, by limiting NK cell maturation. They found that global and NK cell-specific conditional deletion of A2AR resulted in increased number of terminally mature NK cells at homeostasis, after reconstitution, and in the TME [79]. These results demonstrate that A2AR-mediated adenosine signaling acts as an intrinsic negative regulator of NK cell maturation [79].
2.1.5. Extracellular Vesicles (EVs) and MicroRNAs (miRNAs)
Extracellular vesicles (EVs) are different submicron structures released by cells in a regulated fashion, that can be distinguished according to their size: apoptotic bodies (1000–5000 nm), microvesicles (200–1000 nm) and exosomes (30–150 nm) [80]. EVs are involved in intercellular communication between multiple cell types and substantially influence different physiological and pathological processes, including immune responses [81,82]. Diverse tumor cell-derived EVs (TEVs) can be differently up taken by NK cells [83]. EVs act as cargo containing miRNA that can bind to receptors of the Toll-like receptor (TLR) family on immune cells including NK cells, and are able to activate NF-κB and trigger a pro-metastatic inflammatory response [84]. TEVs can regulate NK cells, impairing their killing activity by down-regulating perforin/granzyme production and/or NKG2D ligand (NKG2DL) expression [44,85,86,87,88,89] (Figure 1C). The NKG2D/NKG2DL system plays an important role in tumor immune surveillance [87,88]. Berchem et al. showed that TEVs originating from hypoxic conditions exhibit strong immunosuppressive action on NK cells by delivering TGF-β, thus reducing NKG2D expression [90]. TEVs-associated TGF-β1 was linked with NK cell dysfunction in patients with AML [91]. Further studies reported that TEVs derived from diverse cancer cell lines, including mesothelioma, breast and prostate cancer cells, express NKG2DL and thereby down-regulate NKG2D expression on NK cells and CD8+ T cells, resulting in impaired cytotoxic effector functions [85,86]. It has also been shown that leukemia/lymphoma T and B cells secrete NKG2DL-expressing exosomes with the ability to impair the cytotoxic potency of NK and T cells from healthy donors [85,86]. In a similar manner, NKG2DL-bearing exosomes have been shown to be actively released by placental explants and play a role in the immune evasion of the fetus [92,93]. TEVs downregulate NKG2D expression on NKs also by shedding the NKG2DL on tumor cells, reducing NK cell activity [85,94,95]. TEVs may affect NK activity via other mechanisms including the down-modulation of IL-2-mediated pathways [96], suppressing perforin or cyclin D3 production [91] and Janus kinase (Jak)3 activation resulting in a failure of NK cell-mediated cytolysis [91].
Recent studies reported that EVs can activate immune cells. Viaud et al. demonstrated that dendritic cell (DC)-derived exosomes promote an IL-15Rα and NKG2D-dependent proliferation and activation of NK cells in a murine in vivo model, resulting in tumor regression. They also showed that a DC-derived exosome-based vaccine restored NKG2D-dependent functions of NK cells in half of the tested melanoma patients [63]. Oral cancer-derived EVs were able to promote the biological functions of NK cells, including proliferation, release of perforin and granzyme M, enhancing the cytotoxicity, thereby promoting their functions [97]. In another study, it was shown that EVs derived from genetically modified cells expressing IL-15, IL-18, and 4-1BBL, similar to their host cells, were able to increase NK cell cytotoxicity in tumor cells following a short time treatment (4 h). However, with an extended treatment time (48 h), these EVs inhibited the cytotoxicity of NK cells by acting on NKG2D, pointing out the dual effects of EVs on NK cells [98].
A better understanding of the mechanisms by which EVs influence the NK cell phenotype and function can open new possibilities for the use of EVs in controlling immune responses, either as a targeted therapy or as an adjuvant to modulate immune-based anti-cancer treatments.
MicroRNAs (miRNAs) are conserved non-coding single small in length (~22 nucleotides) stranded RNA molecules, now recognized as important regulators of several cellular processes, including immune function and cancer survival [99,100,101]. Beside their role in cancer, miRNAs have been shown to play a critical role in NK cell activation, effector response, and dysregulation in malignancies [102,103] (Table 1). However, the mechanism by which miRNAs regulates NK cell function is largely unknown. MiRNA profile impact on NK cell development and function was established in several studies through disrupting global miRNAs in mouse NK cells, resulting in decreased NK cell survival, maturation, and proliferation [103,104]. Pesce et al. found differentially expressed miRNAs in human NK cell subsets providing valuable clues of miRNA regulation in human NK cell maturation [105]. Specific miRNAs linked to the development and prognosis of several malignancies such as miR-15/16, miR-24, miR-29b miR-155, miR-150, miR-181, miR-483-1, miR-583 directly mediate NK cell development and differentiation by targeting specific genes [106,107] as described in Table 1. For instance, miR-181a/b regulates NK cell differentiation by targeting Notch signaling and upregulate IFN-γ production [108], while miR-15/16 family has been shown to directly target the murine IFN-γ 3′ UTR and the transcription factor c-Myb (Myb) affecting NK cell maturation program [106,109]. Additional miRNAs such as miR-146a negatively regulate IFN-γ production in NK cells by targeting IRAK1 and TRAF6, with subsequent inhibition of the NF-κB signaling cascade [110]. Similarly, it was shown that miRNA-146a reduce NK cell-mediated cytotoxicity and the production of interferon IFN-γ and TNF-α by targeting STAT1 [111]. In human NK cells, miRNA-155 overexpression was correlated with enhanced IFN-γ production and to directly downregulate SHIP1 (SH2-containing Inositol 5′-Phosphatase 1), a growth receptor expressed in CD56bright and CD56dim NK cell subsets [112,113,114]. In an AML mouse model, NK cells displayed high levels of miRNA-29b and reduced T-bet and EOMES associated with a block of CD56bright NK cell and cytokine-secreting potential [115]. A knockout mouse model of miRNA-29b and forced overexpression of T-bet and EOMES, primed NK cell development suggesting a key role of miRNA-29b in tumor evasion from NK cell surveillance [115].
Table 1.
Effects of microRNAs (miRNAs) on NK cell anti-tumor activities.
| miRNA and Role in NK Cells | ||||
|---|---|---|---|---|
| miRNA | Target | Role in NK Cell | System | References |
| miR-15/miR-16 | c-Myb IFN-γ |
NK cells maturation; IFN-γ production | mouse | [106,109] |
| miR-24 | Paxillin | Inhibition of IFN-γ, TNF-α and decreased cytotoxicity | human | [107] |
| miR-27a-5p | Prf1 GzmB Responsiveness to CCL4 (MIP1β) and CXCL8 (IL-8) |
NK cell cytoxicity | human | [117] |
| miR-29b | TBET EOMES |
Terminal differentiation; reduce CD56bright NK cell subset | human mouse |
[115,122] |
| miR-146a | IRAK1 TRAF6 STAT1 |
IFN-γ and TNF-α production | human | [110,111] |
| miR-146a-5p | KIR2DL1 KIR2DL2 |
NK cell activation, KIR and perforin expression | human | [105] |
| miR-150 | c-Myb. | Activation and maturation; increased expression of GZMB, KIR2DL2, CD16, CD56, NKG2D, NKp46 | human mouse |
[123,124] |
| miR-155 | SHIP1 | NK cell activation, IFN-γ production |
human mouse |
[113,114] |
| miR-181a/b | NOTCH1 | NK cell maturation and enhanced IFN-γ production | human | [108] |
| miR-182 | NKG2D NKG2A |
Upregulation of NKG2D and Perforin-1, downregulation of NKG2A | human | [121] |
| miR-483-3p | IGF-1 | NK cell development and cytotoxicity | human | [125] |
| miR-519a-3p | NKG2D ligands ULBP2 MICA | Impaired tumor cell recognition, NK activation, resistance toward granzyme B | human | [118] |
| miR-583 | IL2Rγ | NK cell differentiation | human | [126] |
| miR-615-5p | IGF-1 R | Decreased CD56dim, increased CD56bright NK cell subsets and reduced the cytotoxic markers NKG2D, TNF-α and perforins | human | [119] |
De-regulation of miRNAs that interfere with NK cell cytolytic activity (by targeting directly or indirectly granzyme B and perforin), such as miR-27a-5p, miR-146a-5p, miRNA-150, miRNA-519a-3p, and miRNA-615-5p, has been observed in different tumors [105,116,117,118,119]. Interestingly, Regis et al. showed that miR-27a-5p, apart from impacting on the effectors function of NKs, downregulates the expression of CX3CR1 in primary human NK cells via TGF-β. Affection of CX3CL1/CX3CR1, strongly impact on the recruitment of CD56dim NK cells, as a tumor strategy to escape immune recognition [120].
In NK cells of hepatocellular carcinoma (HCC) patients it was suggested that miRNA-182 may augment NK-cell cytotoxicity against liver cancer through perforin-1 up-regulation and by modulating NKG2D and NKG2A expressions [121].
The ability of miRNAs to hit simultaneously multiple tumor-promoting pathways make them attractive targets and miRNA regulation of NK cells may be a new therapeutic approach to treat cancer.
2.2. Cell-to-Cell Interactions
2.2.1. Macrophages
Among tumor-infiltrating innate immune cells, macrophages represent the best characterized and they are directly involved in many processes contributing to tumor initiation and progression [127]. Tumor-associated macrophages (TAM) with features of M2 macrophages can promote immunosuppression, fostering tumor invasion, angiogenesis, and lymphangiogenesis; thus, they support tumor progression and metastasis and are generally associated with poor prognosis [127,128,129]. Monocytes and macrophages are recruited in the tumor stroma to become TAMs by several inflammatory mediators, such as chemokines: CCL2, CCL5, and CXCL12, produced in TME by several cellular components including NK cells. Macrophages can inhibit NK cell-mediated activity by the release of soluble factors and cell-to-cell contact such as the expression of checkpoint blockade PD-L1 [130] (Figure 2A); on the other hand, NK cells could produce diverse inflammatory factors able to commit macrophage function. In this context, Bellora et al. studied in vitro cellular interactions between macrophages and NK cells [131]. These authors showed that following LPS stimulation both M0 and M2 macrophages became capable to fully activate NK cell by a cell-to-cell contact-dependent mechanism and through the DNAM-1 and 2B4 surface receptors [132] (Figure 2A). Further results by Mattiola et al. corroborated and deepened these findings [133].
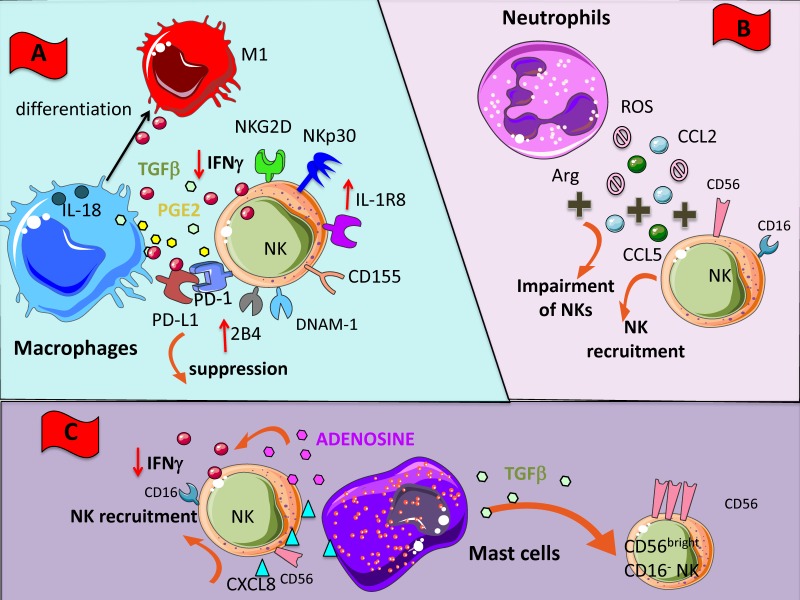
Figure 2.
NK cross-talk with innate immunity cellular components of TME. Within TME, NK cells can encounter different cells such as macrophages (A), neutrophils (B), and mast cells (C). All these cells have been shaped by the TME to exert an inhibitory effect on NK cells through the direct cell-to cell interaction or the soluble factors listed in Figure 1. (A) M2-like macrophages can deliver negative signals to NK cells by the release of TGF-β and PGE2 but also by the binding of PD-L1 to PD1; these interactions lead to downregulation of several NK cell activating receptors, such as NKG2D, NCR and DNAM1. (B) Neutrophils can impair NK cell functions via reactive oxigen species (ROS) and arginase (Arg) activity; they also secrete CCL2 and CCL5 chemokine favoring NK cell tissue localization; in turn, NK cells release CXCL8 which attract to the tumor site other neutrophils amplifying the inhibition. (C) Mast cells produce TGF-β and respond to CXCL8 produced by NK cells; this TGF-β inhibits the cytolytic activity of NK cells by the downregulation of activating receptors, similar to the effect of macrophages.
Macrophage-NK cell cross-talk in tumor setting has been investigated in ascites of ovarian cancer patients [134]. Results have revealed that whereas untreated TAMs induced inhibition of NK cell activity, the LPS stimulation of TAMs was able to restore NK cell effector functions against the NK-resistant OVACR-3 tumor cell line [134]. TAMs can dampen NK cell anti-tumor functions by realizing TGF-β and PGE2. Recently, it has been shown that in early lung adenocarcinoma lesions, a low level of activation of intralesional NK cells correlated with inactivated T cells, high grade of Treg cells and macrophages expressing the PPARγhigh suppressive phenotype [135]. Finally, new data have been added on the complex interplay between macrophages and NK cells, showing the relevance of the IL-1R8 on NK cell function during interaction with both macrophages and DCs. Indeed, the authors showed that IL-1R8-deficient NK cells in an IL-18-dependent manner were responsible of the anti-metastatic effect in two distinct tumor murine models [136] (Figure 2A). DCs can also play a key role in the regulation of NK cell activities in anti-tumor responses, mainly by a positive effect [137,138,139,140,141].
2.2.2. Neutrophils
Neutrophils are implicated in the acute phase of inflammation and they can acquire the capacity to extend their survival and promote cellular interaction with other leucocytes [142,143]. In the contest of the TME, neutrophils undergo a molecular reprogramming in response mainly to TGF-β that leads to the establishment of tumor-associated neutrophils (TANs or N2), endowed with pro-tumor features and pro-angiogenic capabilities [3,5,144,145,146,147]. The interplay between neutrophils and NK cells is becoming increasingly appreciated [148]. In different contexts the relationship between neutrophils and NK cells could result in both activation and suppression [149]. Several reports have documented that ROS and arginase I from neutrophils could induce functional impairment in NK cells [150,151] (Figure 2B). Interestingly, it has been reported that CD56brightCD16- NK cell subset is resistant to neutrophil-derived ROS, probably due to high anti-oxidative capacity of this subset than CD16+ NK cells, thereby TANs can induce selection and expansion of CD56brightCD16- NK cells [152]. Moreover, TANs can secrete CCL2 and CCL5 promoting NK cell recruitment in the TME [149,153,154] (Figure 2B). At the same time, CD56brightCD16- NK can release CXCL8 and favor neutrophil accumulation. It has been recently shown that NK cell can regulate tumor-promoting inflammation through functional modification of neutrophils. In a sarcoma transplantable murine model, NK cells have been shown to regulate neutrophil activities via IFN-γ, likely by an indirect mechanism and through the IL-17 axis. Therefore, tumor progression in a NK cell-depleted host is blocked when the IL-17A neutrophils axis associated with high levels of neutrophil-dependent VEGF are absent [155]. Finally, Spiegel et al. showed that TANs can support metastasis by interacting with NK by inhibiting NK cell-mediated cytotoxic activities and thus protecting intraluminal trapped tumor cells [156].
2.2.3. Mast Cells
Mast cells (MCs) are granulated tissue-resident cells, originating from hematopoietic innate immune system, which can exert protective immune responses against viral and microbial pathogens [157,158], but also adverse reactions in allergic diseases. In cancer patients, increased numbers of MCs have been detected inside the tumor and in peritumor tissues [159]; this increment is more evident in angiogenesis-associated with vascular tumors, such as hemangioma and hemangioblastoma, as well as with several hematological and solid tumors. The increased amount of MC in these tumors was associated with raised neovascularization, presence of MC-derived VEGF and FGF-2, and poor prognosis [160]. On the other hand, anti-tumor functions of MCs have been identified deriving from their ability to trigger target cell cytotoxicity by releasing TNF-α, by production of ROS, or by NK cells recruitment, as shown in a MC-derived CCL3-dependent orthotopic melanoma model [161]. MCs can induce NK cell recruitment also by the release of CXCL8 [162] (Figure 2C). The TGF-β production by MCs in the TME could be responsible of the switch from CD56dimCD16+ NK cell subset to the noncytotoxic CD56brightCD16- cell subset (Figure 2C). MCs can release adenosine in the extracellular milieu that has been reported to dampen NK cell activity by downregulating NKG2D and NKp30 expression and TNF-α/IFN-γ release [77,78] (Figure 2C).
Recently, the relevance of potential cross-talk between MCs and NK cells in the TME has been reported and in particular in the tumor angiogenic process [160,163]. Moreover, it has been shown that LPS-stimulated bone marrow MCs trigger cell contact-dependent IFN-γ secretion by NK cells, and this activation process is partly mediated by OX40L expression on MCs [164]. Several lines of evidence point out the role of NK cells and MCs in tumor growth and angiogenesis, given their ability to synergize in different pathological conditions [163].
2.2.4. Cancer-Associated Fibroblasts
Cancer-associated fibroblasts (CAFs) are the most abundant cell type within the active stroma of many cancer types [165,166,167]. Pro-tumorigenic activities of CAFs have been demonstrated and include induction of cell proliferation and progression by favoring metastasis [165,166,167]. CAFs are able to orchestrate tumor progression via secretion of various growth factors, cytokines, chemokines, and the degradation of extracellular matrix. Through gene expression and mass spectrometry analyses, several studies identified immunomodulatory activities of CAFs showing that their secretome is rich in several cytokines and chemokines endowed with immunosuppressive actions (IL-6, TGF-β, IL-1β, IL-10, IDO, and PGE2), inflammatory cell recruitment (CXCL1, 2, 5, 6, 9, 10, 12, CCL2, 3, 5,7, 20, and 26), and pro-angiogenic activities (VEGF, CXCL8, and FGFs) [168,169,170,171]. Indeed, CAFs exert relevant pro-tumorigenic activities supporting the cell metabolic reprogramming, as a consequence of the Warburg effects [172,173,174].
CAF/NK cell cross-talk results in the induction of NK cell immunosuppression by downregulation of NKG2D expression and functional anergy. CAF-derived TGF-β can acts on NK cells by inhibiting NK cell cytotoxicity, block IFN-γ release and can convert cytotoxic CD56dimCD16+ NKs towards the pro-angiogenic CD56brightCD16-VEGFhighPlGFhighCXCL8+IFN-γlow NKs [2,20] (Figure 3A). In melanoma patients MMPs released by CAFs support NK cell immunosuppression by selectively cleaving MICA and MICB on tumor cells [175]. CAFs are also an abundant source of PGE2, that has been reported to switch off NK cell activities in several cancers, by downregulating the expression of NKG2D, NKp30, NKp44 and decreasing perforin/granzyme B release [69,176,177] (Figure 3A).
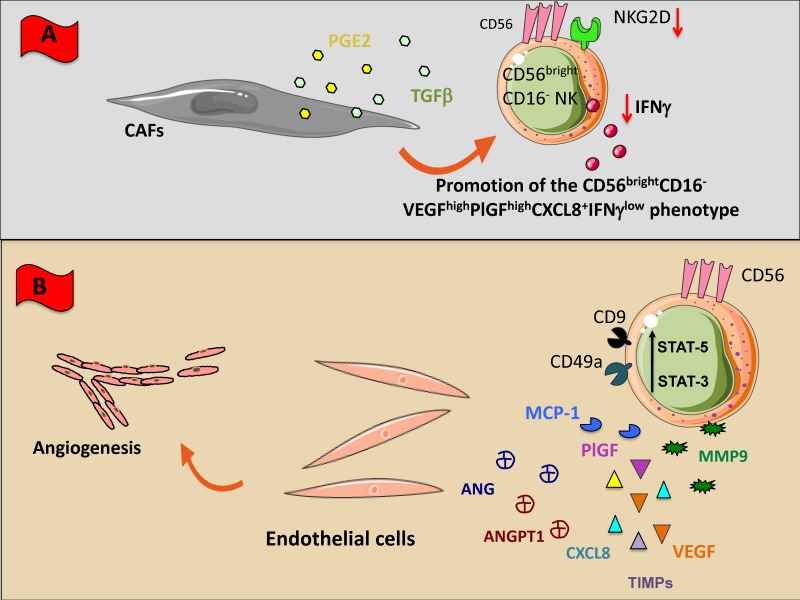
Figure 3.
NK cross-talk with stromal (non-immune) cellular components of TME. Within TME, NK cells can encounter different non-immunological stromal cells such as macrophages (A), cancer associated fibroblasts (CAFs), and endothelial cells (B). All these cells have been shaped by the TME to exert an inhibitory effect on NK cells through the direct cell-to cell interaction or the soluble factors listed in Figure 1. (A) CAFs downregulate NK cell functions releasing inhibiting factors including TGF-β, and PGE2; (B) Tumor infiltrating NK cells and NK cells present in peripheral blood express high levels of CD56 but negative or low expressing CD16 and cytolytic behavior; these NK cells typical of tumor patients produce several factors such as VEGF, Angiogenin (ANG), Angiopoietin-1 (ANGPT1), PIGF, CXCL8, and metalloproteinase which stimulate endothelial cell growth and angiogenesis.
2.2.5. Endothelial Cells
Interactions between NK and endothelial cells occur as early events during the immune surveillance of tissues, inflammatory responses and wound healing. In this scenario, most studies have been addressed to dNKs [16,17]. dNK cells have a CD56superbrightCD16-KIR+ phenotype, are poorly cytotoxic and produce large amounts of pro-angiogenic factors, such as VEGF, PlGF, CXCL8, and IL-10 [16,17]. Early on pregnancy, accumulating dNK represent 70% of the local lymphocytes and 30–40% of all decidual cells. We were the first in describing the pro-angiogenic activities on NK cells in different cancer types [2,5,20,22,29,178].
In patients with NSCLC we identify a polarized NK cell subset, defined as CD56brightCD16-VEGFhighPlGFhighCXCL8+IFN-γ− (Figure 3B) able to induce human umbilical vein endothelial cell (HUVEC) migration and the formation of capillary like structures [20]. These pro-angiogenic features were observed in NK cells from NSCLC tissues and patients’ peripheral blood, suggesting that angiogenic switch in NSCLC NKs occurs already at systemic level [2,20]. Interestingly, we found that NKs from patients with squamous cell carcinomas exhibited even higher pro-angiogenic activities as compared with those with adenocarcinomas [20].
NK cells from malignant pleural effusion have shown to be endowed with a decidual-like pro-angiogenic polarization, by acquiring a CD56brightCD16-CD49a+VEGF+ phenotype and are able to support the in vitro capillary-like structure formation in HUVECs [178]. Induction of a pro-angiogenic and decidual-like NK cell phenotype has been observed also in CRC patients. TINKs in CRC patients are polarized towards the CD56brightCD16-CD9+CD49a+ subset (Figure 3B) and can induce in vitro HUVEC proliferation, migration, and vessel formation [22]. Moreover, CRC TANKs, apart from exhibiting a decidual-like CD56+CD9+CD49a- phenotype, can release large amount of pro-angiogenic factors including VEGF, Angiogenin, Angiopoietin-1, CXCL8, MMP1, MMP9, TIMP-1, and support angiogenesis in vitro [22]. Interestingly, this CRC TANK polarization was associated with increased level of STAT3 and STAT5 and of note, inhibition of this axis by the anti-psychotic agent Pimozide, resulted in blocked release of VEGF and Angiogenin and functional inhibition of their pro-angiogenic activities [22].
3. NK Cells as a Therapeutic Tool: Current Strategies
The therapeutic potential of NK cells in cancer immunotherapy was firstly highlighted by Miller et al. in 2005, showing that the infusion of short-term IL-2-activated allogeneic haploidentical NK cells induce the remission in patients with refractory leukemia [179].
Despite this evidence, several concerns about the real feasibility of using NK cells in cancer immunotherapy have been pointed out. Indeed, the most of therapies are based on the antigen specificity that so far has been considered a unique property of T and B cells, even if the existence of a peculiar subset of Ly49+ NK cells characterized by a T cell-like immune memory has been recently demonstrated [180,181]. Moreover, the limited clonal abilities in vivo and tumor-homing capacity of NKs, along with their substantial phenotypic differences that cannot be recapitulated by animal models [182], still represented other relevant issues to study NK cell-based immunotherapeutic approaches.
Nevertheless, new advances in NK cells manipulation and novel findings of molecular mechanisms involved in NK cell anti-tumor activity have allowed the development of several approaches aimed at enhancing NK cytotoxicity against cancer cells.
3.1. Cytokines That Boost NK Cell Anti-Tumor Immunity
Due to its ability to enhance T cell as far as NK cell proliferation, homeostasis and cytotoxicity, IL-2 was the first cytokine employed in the clinic to boost immune responses in cancer patients (Figure 1A). Despite the high expectations, results from these studies demonstrated that the therapeutic anti-tumor potential of IL-2 administration was limited and especially when used at high doses, a relevant toxicity was observed [182]. Thus, other studies have focused on the use of low doses of IL-2 or antibody–cytokine fusion proteins also designated as immunocytokines characterized by a lower toxicity profile [183]. However, results obtained from these studies demonstrated that the enhancement of NK cell function was associated with Treg cell mobilization. In particular, Hirakawa et al. showed that low-dose of IL-2 induce STAT5 activation in Helios+ Treg cell and CD56brightCD16– NK cells in vitro and the selective expansion of these cell subsets was observed in GVHD patients upon IL-2 treatment [184]. Moreover, they also found the upregulation of Ki67 and CTLA-4 in NK cells and the increased expression of PD-1 in CD4+ and CD8+ T cells [184] (Figure 4A). Recently, a CEA-targeted IL-2 variant-based immunocytokine that abolishes CD25 binding, has been used to overcome the concomitant IL-2-based suppressive Treg cell activation with encouraging results [185]. Recently, a novel IL-2 variant, termed super-2, with increased binding affinity for IL-2Rβ has been proposed by Levin et al. who demonstrated in vivo the ability of their modified IL-2 to induce a higher expansion of cytotoxic T cells and a decreased activation of Treg cells [186]. To overcome Treg cell activation, IL-15, a cytokine that stimulates CD8+ T cells and non-terminally differentiated NK cells has been proposed as immunotherapeutic agent. The single-chain recombinant IL-15 (scIL-15) as far as soluble IL-15Rα have been used to stimulate NK proliferation and functions both in solid and hematologic malignancies [187,188] and are currently under evaluation by several ongoing clinical trials. An alternative cytokine-based approach used to boost NK cell cytolytic activity is IL-12 administration. IL-12 has been shown to promote IFN-γ release, migration and NK-mediated ADCC (Figure 4A), due to the induction of specific adhesion molecule including the selectin CD62L and the upregulation of KIRs and CD16 [189], however additional studies are needed to clarify the value of IL-12 use in the context of cancer therapy [190,191,192].
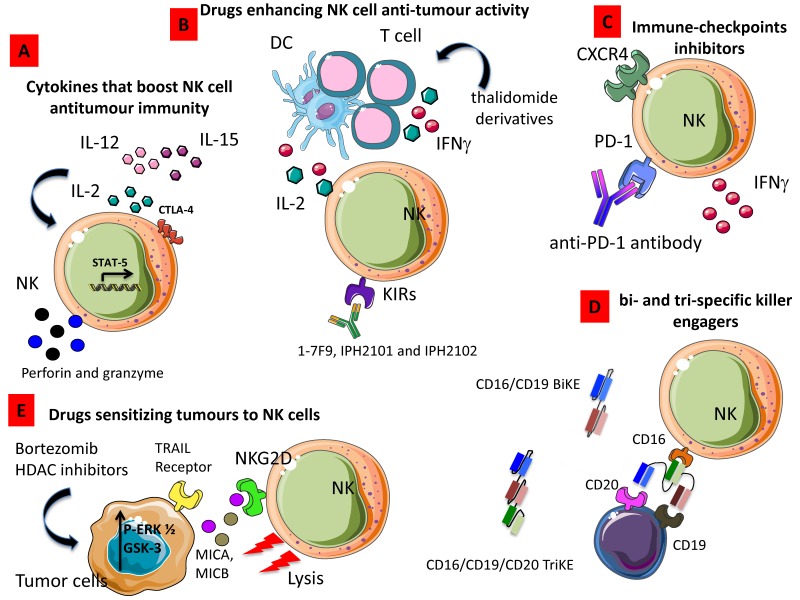
Figure 4.
Strategies enhancing NK cell anti-tumor activity. (A) Cytokines such as IL-2, IL-15, and IL-12 alone or in combination can increase production and release of cytolytic granule content boosting NK cell anti-tumor immunity. (B) Drugs, including thalidomide derivatives, enhance the production of IFN-γ, thus triggering NK cell-mediated cytolysis. (C) Antibody directed to immune-checkpoints inhibitors like PD-1 can relieve the brake to NK cell cytolysis. (D) Bi- and tri-specific killer engagers strongly activate NK cell-mediated killing of tumor target cells. (E) Drugs able to sensitize tumors to upregulate ligands of activating receptors can increment killing of tumor cells; indeed, histone deacetylase inhibitors (HDAC) trigger the expression of NKG2DL such as MICA and MICB on tumor cells, these cells are more easily recognized and killed by NKG2D+ NK cells.
3.2. Drugs Enhancing NK Cell Anti-Tumor Activity
Pre-clinical studies using thalidomide derivatives demonstrated that lenalidomide and pomalidomide can indirectly enhance NK cell cytotoxicity by activating the intracellular signaling of phosphoinositide-3 kinase (PI3K), followed by nuclear translocation of nuclear factor of activated T cells 2 (NFAT2) and activator protein 1 (AP-1) allowing the release of IL-2 and IFN-γ from T cells and DCs [193,194] (Figure 4B). Moreover, lenalidomide has been shown to induce the degradation of Ikaros and Aiolos, two transcription factors repressing IL-2 production in T cells [195] and concomitantly to up-regulate the expression of the NKG2D, DNAM-1 activating receptor, and ligands MICA and PVR/CD155 in human multiple myeloma (MM) cells [196].
On the basis of these pre-clinical and in vitro evidence, some clinical trials are focusing on the role of lenalidomide in enhancing NK cytotoxic abilities. However, a very recent study evaluating the effects of lenalidomide administration in MM patients showed that lenalidomide treatment neither activated NKs nor enhance degranulation or IFN-γ release by NK cells [197]. Another approach to increase the cytotoxicity of NKs is the disruption of inhibitory KIRs through mAb-mediated blockade. In this context, different mAbs have been developed, termed 1-7F9, IPH2101, and IPH2102 (Figure 4B). Two phase I clinical trial have demonstrated the low toxic profile of KIR-specific mAbs in cancer patients, while no significant changes were observed in the number of NK cells or have shown any reduction in KIR2D-positive NK cells upon treatment. Only a transient increases of serum TNF-α and MIP-1β was found in treated patients and a transient induction of CD69 expression on NK cells was observed [198,199].
3.3. Immune-Checkpoints Inhibitors
Benson et al. demonstrated that using the novel anti-PD-1 antibody, CT-011, is able to enhance NK cell functions against autologous and primary MM cells and increase NK trafficking by up-regulating CXCR4 [200] (Figure 4C). It has been demonstrated that disrupting PD-1 inhibitory pathway improved IFN-γ release by NK cells without enhancing their cytotoxic abilities [201] Figure 4C). Moreover, the ex vivo use of anti–PD-L1/PD-L2 mAbs was able to partially restore the degranulation of PD-1+ NK in presence of PD-L1/PD-L2+ OVCAR5 target cells [130]. Zhang et al. have also recently demonstrated that the blocking of TIGIT can prevent NK cell exhaustion and trigger NK cell-dependent tumor immunity in several mouse models [202].
3.4. Bi- and Tri-Specific Killer Engagers
These small molecules consist of a single heavy (VH) and light chain (VL) of the variable region of CD16 linked to one (BiKE) or two (TriKE) variable portions of several tumor antigens (Figure 4D), including CD20 and CD19 for non-Hodgkin’s lymphomas [203,204,205,206], CD19 and CD33 for different types of leukemia [207], CD30 for Hodgkin’s disease [208], EGF-R for EGF-R+ tumors [209], HER2/neu for metastatic breast cancer [210,211] and EpCAM for prostate, breast, colon, head, and neck carcinomas and MOV19 on ovarian cancer [212]. These agents improve cytotoxicity of NK cells by inducing the strong release of cytokines by NK cells and enhancing the NK-mediated ADCC [213].
3.5. Drugs Sensitizing Tumors to NK Cells
Drugs able to increase the susceptibility of tumor cells to NK cytotoxic effects have been recently proposed as an alternative approach. In particular, proteasome inhibitors, such as bortezomib, have been demonstrated to be able to induce the expression of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) receptors on tumor cells promoting their lysis by NK cell [214]. Moreover, in vitro treatment with bortezomib was also able to increase the expression of MICA/B, Nectin-2, and PVR expression on MM cells enhancing the sensitivity of MM cells to NK cell-mediated lysis [215] (Figure 4E).
Accordingly, in vitro studies using histone deacetylase (HDAC) inhibitors, including valproic acid, have demonstrated its ability to upregulates MICA/B and UL16-binding protein (ULBP) 2 on MM cells by inducing the phosphorylation of ERK ½ and in turn promoting their lysis by NKs [216] (Figure 4E). Skov and colleagues also demonstrated that the treatment of tumor cells with PXD101, suberoylanilide hydroxamic acid (SAHA), and trichostatin A, three HDAC inhibitors, induce MICA/B expression on Jurkat T cells by activating GSK-3 kinase signaling [217] and becoming targets for NKG2D-expressing cells like NK cells (Figure 4E).
3.6. Adoptively Infused NK Cells
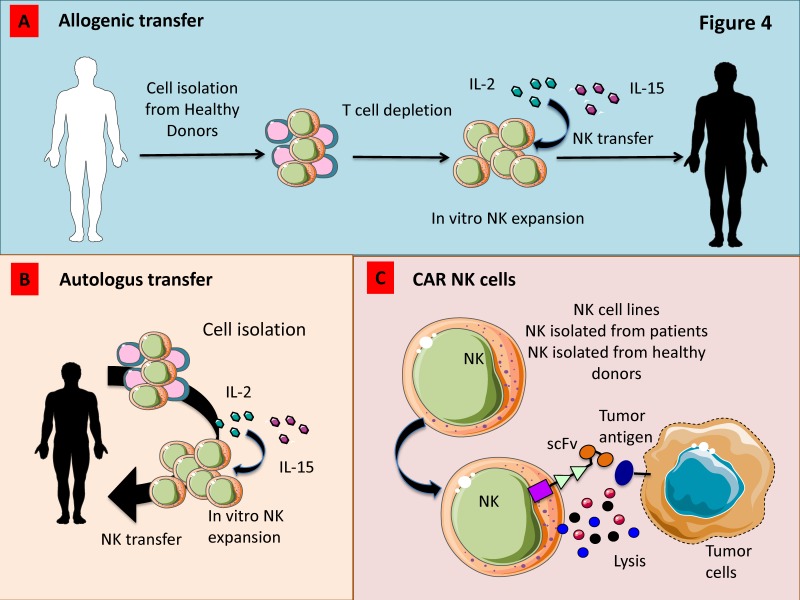
Allogeneic hematopoietic cell transplantation (HCT) is one of the most efficient therapeutic options in the context of myelodysplastic syndrome (MDS), AML, or chronic myelogenous leukemia (CML). This approach can result in durable remission of malignancies due to the development of graft versus leukemia (GVL) even if relapse is described in 40% of patients [218]. Immunotherapeutic strategies using adoptively transferred NK cells are particularly relevant for the possibility to pre-activate and manipulate NK cells prior to infusion (Figure 5A). Adoptive transfer of short-term allogeneic NK cells stimulated with 1000 IU/mL IL-2 for 8–16 h prior to infusion has been demonstrated to induce a clinical response in AML and MM patients [179,219]. The NKAML trial (Pilot Study of Haploidentical NK Transplantation for AML) demonstrated that the KIR-HLA-mismatched donor NK cell infusion reduces the risk of relapse in childhood AML with limited non-hematologic toxicity [220]. Shaffer et al. in a phase 2 study demonstrated the safety of haploidentical NK cell infusion after allogeneic HCT in 8 patients with relapsed or progressive AML or MDS. In this context, the authors showed transient responses in two patients and morphologic resolution of dysplasia in a third one [218]. Moreover, it has also been demonstrated that an improvement of the clinical response occurs whether adoptive cell transfer was followed by IL-2, which promote in vivo expansion of infused NK cells [179,221]. In contrast to short term activation, ex vivo expansion of peripheral NKs with media containing cytokines such as IL-2 and IL-15 allows to obtain high number of activated NK cells [222] (Figure 5A).
Figure 5.
Strategies to increase the anti-tumor activity of adoptively transferred NK cells for anti-cancer therapy. (A) Allogenic NK cells expanded ex vivo with IL-2 and/or IL-15 show a strong anti-tumor effect in patients which do not express the HLA-I allele recognized by inhibitory HLA-I receptors present on donor NK cells. (B) Also, autologous NK cells expanded ex vivo can be used to kill tumor cells. (C) Novel tools such as Chimeric Antigen Receptors (CAR) transduced into NK cells isolated from either healthy donors or patients can trigger transferred-NK cells to kill tumor cells. To avoid the variability of the NK cell activity from donor to donor, some NK cell lines have been transduced with CAR and used in clinical trials.
3.7. Genetic Modification of NK Cells
Some studies have been focused on cytokine gene transfer to promote NK cell survival (e.g., IL-2, IL-12, IL-15, and stem cell factor). In particular, engineered NK cell lines displayed an increased cytotoxic ability as well as proliferative rate, survival and in vivo anti-tumor activity [222]. In other investigations, NK cells were genetically manipulated to express a high-affinity variant of CD16a and it was demonstrated that a single-nucleotide polymorphism in CD16 that results in an amino acid substitution at position 158 (CD16-F158V) can bolster ADCC in vivo [223] (Figure 5B).
3.8. Chimeric Antigen Receptor (CAR)-Engineered NK Cells
Several pre-clinical and clinical trials have focused on the development of Chimeric Antigen Receptor (CAR)-modified T cells, while little is known on CAR-engineered NK cells. Studies focused on the therapeutic application of CAR-engineered NK cells use peripheral blood (PB-NK cells) or umbilical cord blood (UCB-NK cells) as source of NK cells (Figure 5C). This strategy showed a limited efficacy against solid tumors. Moreover, to collect sufficient number of NK cells, donors have to undergo repeated leukaphereses and have to be expanded in vitro by using feeder layers. In addition, NK cells collected are extremely variable, expressing different levels of markers such CD16 and KIRs [224]. As an alternative, the NK-92 cell line was also employed in clinical trials. The main concern on this strategy is represented by their mild stimulatory anti-cancer abilities, even if they still represent a valid therapeutic tool [224]. It has been demonstrated that also induced pluripotent stem cells (iPSC) can be used to generate a homogeneous NK cell population that can be genetically modified using both viral and non-viral methods to obtain CAR-NKs [225]. Moreover, Li et al. demonstrated that NK cells derived from human iPSCs expressing the transmembrane domain of NKG2D, the 2B4 co-stimulatory domain, and the CD3z signalling domain display a higher anti-tumor ability compared with PB-NK cells and T-CAR-iPSC-NK cells in an ovarian cancer xenograft model [225]. Most of the studies using CAR-engineered NKs have addressed their attention on CAR against CD19 and CD20 targeting B cell malignancies [226], demonstrating that CAR-expressing NK-92 cells effectively kill chronic lymphocytic leukaemia (CLL) cells and that such a cytotoxic response is significantly higher than that resulting from ADCC mediated by mAbs [226]. Esser et al. generated NK-92 cell expressing a disialogangliosideGD(2)-specific CAR for neuroblastoma (NB) treatment. These authors have demonstrated that CAR expression by gene-modified NK cells promote the recognition and elimination of NB cells. The efficacy of the treatment with GD(2)-specific NK was strictly dependent by antigen recognition and it can be reverted using anti-GD(2)or anti-idiotypic antibodies [227]. Recently, CXCR4-engineered NK cells with concomitant expression of EGFRvIII-specific CAR were generated by Muller et al. aiming to kill glioblastoma cells. By using in vitro and in vivo approaches, they have demonstrated that the expression of CXCR4 improve the migration to U87-MG glioblastoma cells resulting in a stronger anti-tumor effect compared with control groups [228]. Schönfeld et al. showed that NK-92/5.28.z ErbB2 (HER2)-specific NK cells efficiently lysed ErbB2-expressing tumor cells in vitro and were also able to reduce in vivo lung metastasis in a renal cell carcinoma model [229].
4. Conclusions
Altogether, it is clear that NK cells, besides the well-established anti-tumor effect, may support cancer both by immunosuppression and by supporting tumor angiogenesis. This unfavorable dark side of NK cells tightly depends on the interactions with the various cellular components of the host, placing TME as the major player in the tumor-immune escape and immune cell pro-tumor/pro-angiogenic-features. The cell-to-cell contact, cytokines, chemokines, immunomodulatory molecules, extracellular vesicles, play a key role in determining the final NK cell-mediated effect, leading to either proliferation or elimination of tumor cells. Thus, we propose NK cells as a relevant host-dependent hallmark of cancer and a key cellular paradigm in tumor progression and angiogenesis; thus, NK cells should be considered as a suitable target to modulate the immunosuppressive TME and try to trigger a more potent anti-tumor response.
Author Contributions
All authors listed made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the University of Insubria intramural grant FAR 2018 and the MIUR National Fund for Basic Research FFABR 2017 to L.M., and by the 5xmille 2014, 5xmille 2015 and 5xmille 2016 from Italian Ministry of Health to A.P.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Balkwill F.R., Capasso M., Hagemann T. The tumor microenvironment at a glance. J. Cell Sci. 2012;125:5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 2.Bruno A., Ferlazzo G., Albini A., Noonan D.M. A think tank of TINK/TANKs: Tumor-infiltrating/tumor-associated natural killer cells in tumor progression and angiogenesis. J. Natl. Cancer Inst. 2014;106:dju200. doi: 10.1093/jnci/dju200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruno A., Pagani A., Magnani E., Rossi T., Noonan D.M., Cantelmo A.R., Albini A. Inflammatory angiogenesis and the tumor microenvironment as targets for cancer therapy and prevention. Cancer Treat. Res. 2014;159:401–426. doi: 10.1007/978-3-642-38007-5_23. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Bruno A., Pagani A., Pulze L., Albini A., Dallaglio K., Noonan D.M., Mortara L. Orchestration of angiogenesis by immune cells. Front. Oncol. 2014;4:131. doi: 10.3389/fonc.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crusz S.M., Balkwill F.R. Inflammation and cancer: Advances and new agents. Nat. Rev. Clin. Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 7.Noonan D.M., De Lerma Barbaro A., Vannini N., Mortara L., Albini A. Inflammation, inflammatory cells and angiogenesis: Decisions and indecisions. Cancer Metastasis Rev. 2008;27:31–40. doi: 10.1007/s10555-007-9108-5. [DOI] [PubMed] [Google Scholar]
- 8.Cooper M.A., Fehniger T.A., Caligiuri M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/S1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 9.Vivier E., Tomasello E., Baratin M., Walzer T., Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 10.Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N., Mebius R.E., et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 11.Chiossone L., Dumas P.Y., Vienne M., Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018;18:671–688. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- 12.Vivier E., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N.J., Mebius R.E., et al. Innate Lymphoid Cells: 10 Years On. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Stabile H., Fionda C., Gismondi A., Santoni A. Role of Distinct Natural Killer Cell Subsets in Anticancer Response. Front. Immunol. 2017;8:293. doi: 10.3389/fimmu.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stabile H., Fionda C., Santoni A., Gismondi A. Impact of bone marrow-derived signals on NK cell development and functional maturation. Cytokine Growth Factor Rev. 2018;42:13–19. doi: 10.1016/j.cytogfr.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Vulpis E., Stabile H., Soriani A., Fionda C., Petrucci M.T., Mariggio E., Ricciardi M.R., Cippitelli M., Gismondi A., Santoni A., et al. Key Role of the CD56(low)CD16(low) Natural Killer Cell Subset in the Recognition and Killing of Multiple Myeloma Cells. Cancers. 2018;10:473. doi: 10.3390/cancers10120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blois S.M., Klapp B.F., Barrientos G. Decidualization and angiogenesis in early pregnancy: Unravelling the functions of DC and NK cells. J. Reprod. Immunol. 2011;88:86–92. doi: 10.1016/j.jri.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Hanna J., Goldman-Wohl D., Hamani Y., Avraham I., Greenfield C., Natanson-Yaron S., Prus D., Cohen-Daniel L., Arnon T.I., Manaster I., et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 18.Costello R.T., Sivori S., Marcenaro E., Lafage-Pochitaloff M., Mozziconacci M.J., Reviron D., Gastaut J.A., Pende D., Olive D., Moretta A. Defective expression and function of natural killer cell-triggering receptors in patients with acute myeloid leukemia. Blood. 2002;99:3661–3667. doi: 10.1182/blood.V99.10.3661. [DOI] [PubMed] [Google Scholar]
- 19.Mamessier E., Sylvain A., Thibult M.L., Houvenaeghel G., Jacquemier J., Castellano R., Goncalves A., Andre P., Romagne F., Thibault G., et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Investig. 2011;121:3609–3622. doi: 10.1172/JCI45816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno A., Focaccetti C., Pagani A., Imperatori A.S., Spagnoletti M., Rotolo N., Cantelmo A.R., Franzi F., Capella C., Ferlazzo G., et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia. 2013;15:133–142. doi: 10.1593/neo.121758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrega P., Morandi B., Costa R., Frumento G., Forte G., Altavilla G., Ratto G.B., Mingari M.C., Moretta L., Ferlazzo G. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer. 2008;112:863–875. doi: 10.1002/cncr.23239. [DOI] [PubMed] [Google Scholar]
- 22.Bruno A., Bassani B., D’Urso D.G., Pitaku I., Cassinotti E., Pelosi G., Boni L., Dominioni L., Noonan D.M., Mortara L., et al. Angiogenin and the MMP9-TIMP2 axis are up-regulated in proangiogenic, decidual NK-like cells from patients with colorectal cancer. FASEB J. 2018;32:5365–5377. doi: 10.1096/fj.201701103R. [DOI] [PubMed] [Google Scholar]
- 23.Rocca Y.S., Roberti M.P., Arriaga J.M., Amat M., Bruno L., Pampena M.B., Huertas E., Loria F.S., Pairola A., Bianchini M., et al. Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun. 2013;19:76–85. doi: 10.1177/1753425912453187. [DOI] [PubMed] [Google Scholar]
- 24.Schleypen J.S., Baur N., Kammerer R., Nelson P.J., Rohrmann K., Grone E.F., Hohenfellner M., Haferkamp A., Pohla H., Schendel D.J., et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin. Cancer Res. 2006;12:718–725. doi: 10.1158/1078-0432.CCR-05-0857. [DOI] [PubMed] [Google Scholar]
- 25.Delahaye N.F., Rusakiewicz S., Martins I., Menard C., Roux S., Lyonnet L., Paul P., Sarabi M., Chaput N., Semeraro M., et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat. Med. 2011;17:700–707. doi: 10.1038/nm.2366. [DOI] [PubMed] [Google Scholar]
- 26.Cantoni C., Huergo-Zapico L., Parodi M., Pedrazzi M., Mingari M.C., Moretta A., Sparatore B., Gonzalez S., Olive D., Bottino C., et al. NK Cells, Tumor Cell Transition, and Tumor Progression in Solid Malignancies: New Hints for NK-Based Immunotherapy? J. Immunol. Res. 2016;2016:4684268. doi: 10.1155/2016/4684268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levi I., Amsalem H., Nissan A., Darash-Yahana M., Peretz T., Mandelboim O., Rachmilewitz J. Characterization of tumor infiltrating natural killer cell subset. Oncotarget. 2015;6:13835–13843. doi: 10.18632/oncotarget.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messaoudene M., Fregni G., Fourmentraux-Neves E., Chanal J., Maubec E., Mazouz-Dorval S., Couturaud B., Girod A., Sastre-Garau X., Albert S., et al. Mature cytotoxic CD56(bright)/CD16(+) natural killer cells can infiltrate lymph nodes adjacent to metastatic melanoma. Cancer Res. 2014;74:81–92. doi: 10.1158/0008-5472.CAN-13-1303. [DOI] [PubMed] [Google Scholar]
- 29.Parisi L., Bassani B., Tremolati M., Gini E., Farronato G., Bruno A. Natural Killer Cells in the Orchestration of Chronic Inflammatory Diseases. J. Immunol. Res. 2017;2017:4218254. doi: 10.1155/2017/4218254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasero C., Gravis G., Granjeaud S., Guerin M., Thomassin-Piana J., Rocchi P., Salem N., Walz J., Moretta A., Olive D. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget. 2015;6:14360–14373. doi: 10.18632/oncotarget.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gismondi A., Stabile H., Nisti P., Santoni A. Effector Functions of Natural Killer Cell Subsets in the Control of Hematological Malignancies. Front. Immunol. 2015;6:567. doi: 10.3389/fimmu.2015.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helena S., Paolo N., Giovanna P., Cinzia F., Daria P., Letizia P.B., Pietro M., Franco L., Santoni A., Gismondi A. Reconstitution of multifunctional CD56(low)CD16(low) natural killer cell subset in children with acute leukemia given alpha/beta T cell-depleted HLA-haploidentical haematopoietic stem cell transplantation. Oncoimmunology. 2017;6:e1342024. doi: 10.1080/2162402X.2017.1342024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stabile H., Nisti P., Morrone S., Pagliara D., Bertaina A., Locatelli F., Santoni A., Gismondi A. Multifunctional human CD56 low CD16 low natural killer cells are the prominent subset in bone marrow of both healthy pediatric donors and leukemic patients. Haematologica. 2015;100:489–498. doi: 10.3324/haematol.2014.116053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baginska J., Viry E., Paggetti J., Medves S., Berchem G., Moussay E., Janji B. The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front. Immunol. 2013;4:490. doi: 10.3389/fimmu.2013.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peppa D., Micco L., Javaid A., Kennedy P.T., Schurich A., Dunn C., Pallant C., Ellis G., Khanna P., Dusheiko G., et al. Blockade of immunosuppressive cytokines restores NK cell antiviral function in chronic hepatitis B virus infection. PLoS Pathog. 2010;6:e1001227. doi: 10.1371/journal.ppat.1001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bierie B., Moses H.L. Tumor microenvironment: TGFbeta: The molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer. 2006;6:506–520. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 37.Pickup M., Novitskiy S., Moses H.L. The roles of TGFbeta in the tumor microenvironment. Nat. Rev. Cancer. 2013;13:788–799. doi: 10.1038/nrc3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castriconi R., Cantoni C., Della Chiesa M., Vitale M., Marcenaro E., Conte R., Biassoni R., Bottino C., Moretta L., Moretta A. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA. 2003;100:4120–4125. doi: 10.1073/pnas.0730640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trotta R., Dal Col J., Yu J., Ciarlariello D., Thomas B., Zhang X., Allard J., 2nd, Wei M., Mao H., Byrd J.C., et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J. Immunol. 2008;181:3784–3792. doi: 10.4049/jimmunol.181.6.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otegbeye F., Ojo E., Moreton S., Mackowski N., Lee D.A., de Lima M., Wald D.N. Inhibiting TGF-beta signaling preserves the function of highly activated, in vitro expanded natural killer cells in AML and colon cancer models. PLoS ONE. 2018;13:e0191358. doi: 10.1371/journal.pone.0191358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viel S., Marcais A., Guimaraes F.S., Loftus R., Rabilloud J., Grau M., Degouve S., Djebali S., Sanlaville A., Charrier E., et al. TGF-beta inhibits the activation and functions of NK cells by repressing the mTOR pathway. Sci. Signal. 2016;9:ra19. doi: 10.1126/scisignal.aad1884. [DOI] [PubMed] [Google Scholar]
- 42.Zaiatz-Bittencourt V., Finlay D.K., Gardiner C.M. Canonical TGF-beta Signaling Pathway Represses Human NK Cell Metabolism. J. Immunol. 2018;200:3934–3941. doi: 10.4049/jimmunol.1701461. [DOI] [PubMed] [Google Scholar]
- 43.Keskin D.B., Allan D.S., Rybalov B., Andzelm M.M., Stern J.N., Kopcow H.D., Koopman L.A., Strominger J.L. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc. Natl. Acad. Sci. USA. 2007;104:3378–3383. doi: 10.1073/pnas.0611098104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bobrie A., Colombo M., Raposo G., Thery C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 45.Rajagopalan S. HLA-G-mediated NK cell senescence promotes vascular remodeling: Implications for reproduction. Cell. Mol. Immunol. 2014;11:460–466. doi: 10.1038/cmi.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morandi F., Ferretti E., Castriconi R., Dondero A., Petretto A., Bottino C., Pistoia V. Soluble HLA-G dampens CD94/NKG2A expression and function and differentially modulates chemotaxis and cytokine and chemokine secretion in CD56bright and CD56dim NK cells. Blood. 2011;118:5840–5850. doi: 10.1182/blood-2011-05-352393. [DOI] [PubMed] [Google Scholar]
- 47.Wang D., Dubois R.N. Eicosanoids and cancer. Nat. Rev. Cancer. 2010;10:181–193. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao Y., Souza-Fonseca-Guimaraes F., Bald T., Ng S.S., Young A., Ngiow S.F., Rautela J., Straube J., Waddell N., Blake S.J., et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 2017;18:1004–1015. doi: 10.1038/ni.3800. [DOI] [PubMed] [Google Scholar]
- 49.Casu B., Dondero A., Regis S., Caliendo F., Petretto A., Bartolucci M., Bellora F., Bottino C., Castriconi R. Novel Immunoregulatory Functions of IL-18, an Accomplice of TGF-beta1. Cancers. 2019;11:75. doi: 10.3390/cancers11010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldman-Wohl D.S., Ariel I., Greenfield C., Hanoch J., Yagel S. HLA-G expression in extravillous trophoblasts is an intrinsic property of cell differentiation: A lesson learned from ectopic pregnancies. Mol. Hum. Reprod. 2000;6:535–540. doi: 10.1093/molehr/6.6.535. [DOI] [PubMed] [Google Scholar]
- 51.Gregori S., Amodio G., Quattrone F., Panina-Bordignon P. HLA-G Orchestrates the Early Interaction of Human Trophoblasts with the Maternal Niche. Front. Immunol. 2015;6:128. doi: 10.3389/fimmu.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin A., Yan W.H. Heterogeneity of HLA-G Expression in Cancers: Facing the Challenges. Front. Immunol. 2018;9:2164. doi: 10.3389/fimmu.2018.02164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rouas-Freiss N., Moreau P., Ferrone S., Carosella E.D. HLA-G proteins in cancer: Do they provide tumor cells with an escape mechanism? Cancer Res. 2005;65:10139–10144. doi: 10.1158/0008-5472.CAN-05-0097. [DOI] [PubMed] [Google Scholar]
- 54.Rutten M.J., Dijk F., Savci-Heijink C.D., Buist M.R., Kenter G.G., van de Vijver M.J., Jordanova E.S. HLA-G expression is an independent predictor for improved survival in high grade ovarian carcinomas. J. Immunol. Res. 2014;2014:274584. doi: 10.1155/2014/274584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheu J.J., Shih Ie M. Clinical and biological significance of HLA-G expression in ovarian cancer. Semin. Cancer Biol. 2007;17:436–443. doi: 10.1016/j.semcancer.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yie S.M., Hu Z. Human leukocyte antigen-G (HLA-G) as a marker for diagnosis, prognosis and tumor immune escape in human malignancies. Histol. Histopathol. 2011;26:409–420. doi: 10.14670/HH-26.409. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y., Zhao J., Qiu L., Zhang P., Li J., Yang D., Wei X., Han Y., Nie S., Sun Y. Co-expression of ILT4/HLA-G in human non-small cell lung cancer correlates with poor prognosis and ILT4-HLA-G interaction activates ERK signaling. Tumor Biol. 2016;37:11187–11198. doi: 10.1007/s13277-016-5002-5. [DOI] [PubMed] [Google Scholar]
- 58.Apps R., Gardner L., Moffett A. A critical look at HLA-G. Trends Immunol. 2008;29:313–321. doi: 10.1016/j.it.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 59.Chen B.G., Xu D.P., Lin A., Yan W.H. NK cytolysis is dependent on the proportion of HLA-G expression. Hum. Immunol. 2013;74:286–289. doi: 10.1016/j.humimm.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 60.Lin A., Xu H.H., Xu D.P., Zhang X., Wang Q., Yan W.H. Multiple steps of HLA-G in ovarian carcinoma metastasis: Alter NK cytotoxicity and induce matrix metalloproteinase-15 (MMP-15) expression. Hum. Immunol. 2013;74:439–446. doi: 10.1016/j.humimm.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 61.Wan R., Wang Z.W., Li H., Peng X.D., Liu G.Y., Ou J.M., Cheng A.Q. Human Leukocyte Antigen-G Inhibits the Anti-Tumor Effect of Natural Killer Cells via Immunoglobulin-Like Transcript 2 in Gastric Cancer. Cell. Physiol. Biochem. 2017;44:1828–1841. doi: 10.1159/000485819. [DOI] [PubMed] [Google Scholar]
- 62.Rajagopalan S., Long E.O. KIR2DL4 (CD158d): An activation receptor for HLA-G. Front. Immunol. 2012;3:258. doi: 10.3389/fimmu.2012.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Viaud S., Terme M., Flament C., Taieb J., Andre F., Novault S., Escudier B., Robert C., Caillat-Zucman S., Tursz T., et al. Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: A role for NKG2D ligands and IL-15Ralpha. PLoS ONE. 2009;4:e4942. doi: 10.1371/journal.pone.0004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harris S.G., Padilla J., Koumas L., Ray D., Phipps R.P. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/S1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 65.Kalinski P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holt D., Ma X., Kundu N., Fulton A. Prostaglandin E(2) (PGE (2)) suppresses natural killer cell function primarily through the PGE(2) receptor EP4. Cancer Immunol. Immunother. 2011;60:1577–1586. doi: 10.1007/s00262-011-1064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mao Y., Sarhan D., Steven A., Seliger B., Kiessling R., Lundqvist A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin. Cancer Res. 2014;20:4096–4106. doi: 10.1158/1078-0432.CCR-14-0635. [DOI] [PubMed] [Google Scholar]
- 68.Martinet L., Jean C., Dietrich G., Fournie J.J., Poupot R. PGE2 inhibits natural killer and gamma delta T cell cytotoxicity triggered by NKR and TCR through a cAMP-mediated PKA type I-dependent signaling. Biochem. Pharmacol. 2010;80:838–845. doi: 10.1016/j.bcp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 69.Balsamo M., Scordamaglia F., Pietra G., Manzini C., Cantoni C., Boitano M., Queirolo P., Vermi W., Facchetti F., Moretta A., et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc. Natl. Acad. Sci. USA. 2009;106:20847–20852. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y., Daaka Y. PGE2 promotes angiogenesis through EP4 and PKA Cgamma pathway. Blood. 2011;118:5355–5364. doi: 10.1182/blood-2011-04-350587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beavis P.A., Divisekera U., Paget C., Chow M.T., John L.B., Devaud C., Dwyer K., Stagg J., Smyth M.J., Darcy P.K. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc. Natl. Acad. Sci. USA. 2013;110:14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hatfield S.M., Kjaergaard J., Lukashev D., Belikoff B., Schreiber T.H., Sethumadhavan S., Abbott R., Philbrook P., Thayer M., Shujia D., et al. Systemic oxygenation weakens the hypoxia and hypoxia inducible factor 1alpha-dependent and extracellular adenosine-mediated tumor protection. J. Mol. Med. 2014;92:1283–1292. doi: 10.1007/s00109-014-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohta A., Gorelik E., Prasad S.J., Ronchese F., Lukashev D., Wong M.K., Huang X., Caldwell S., Liu K., Smith P., et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc. Natl. Acad. Sci. USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Young A., Ngiow S.F., Barkauskas D.S., Sult E., Hay C., Blake S.J., Huang Q., Liu J., Takeda K., Teng M.W.L., et al. Co-inhibition of CD73 and A2AR Adenosine Signaling Improves Anti-tumor Immune Responses. Cancer Cell. 2016;30:391–403. doi: 10.1016/j.ccell.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 75.Okada H., Tsuzuki T., Murata H. Decidualization of the human endometrium. Reprod. Med. Biol. 2018;17:220–227. doi: 10.1002/rmb2.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blay J., White T.D., Hoskin D.W. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–2605. [PubMed] [Google Scholar]
- 77.Raskovalova T., Lokshin A., Huang X., Jackson E.K., Gorelik E. Adenosine-mediated inhibition of cytotoxic activity and cytokine production by IL-2/NKp46-activated NK cells: Involvement of protein kinase A isozyme I (PKA I) Immunol. Res. 2006;36:91–99. doi: 10.1385/IR:36:1:91. [DOI] [PubMed] [Google Scholar]
- 78.Wang J., Matosevic S. Adenosinergic signaling as a target for natural killer cell immunotherapy. J. Mol. Med. 2018;96:903–913. doi: 10.1007/s00109-018-1679-9. [DOI] [PubMed] [Google Scholar]
- 79.Young A., Ngiow S.F., Gao Y., Patch A.M., Barkauskas D.S., Messaoudene M., Lin G., Coudert J.D., Stannard K.A., Zitvogel L., et al. A2AR Adenosine Signaling Suppresses Natural Killer Cell Maturation in the Tumor Microenvironment. Cancer Res. 2018;78:1003–1016. doi: 10.1158/0008-5472.CAN-17-2826. [DOI] [PubMed] [Google Scholar]
- 80.Brinton L.T., Sloane H.S., Kester M., Kelly K.A. Formation and role of exosomes in cancer. Cell. Mol. Life Sci. 2015;72:659–671. doi: 10.1007/s00018-014-1764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thery C., Ostrowski M., Segura E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 83.Huyan T., Du Y., Huang Q., Huang Q., Li Q. Uptake Characterization of Tumor Cell-derived Exosomes by Natural Killer Cells. Iran. J. Public Health. 2018;47:803–813. [PMC free article] [PubMed] [Google Scholar]
- 84.Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R., Lovat F., Fadda P., Mao C., Nuovo G.J., et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clayton A., Mitchell J.P., Court J., Linnane S., Mason M.D., Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J. Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 86.Clayton A., Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Mol. Dis. 2005;34:206–213. doi: 10.1016/j.bcmd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Gonzalez S., Lopez-Soto A., Suarez-Alvarez B., Lopez-Vazquez A., Lopez-Larrea C. NKG2D ligands: Key targets of the immune response. Trends Immunol. 2008;29:397–403. doi: 10.1016/j.it.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 88.Hayakawa Y., Smyth M.J. NKG2D and cytotoxic effector function in tumor immune surveillance. Semin. Immunol. 2006;18:176–185. doi: 10.1016/j.smim.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Lundholm M., Schroder M., Nagaeva O., Baranov V., Widmark A., Mincheva-Nilsson L., Wikstrom P. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: Mechanism of immune evasion. PLoS ONE. 2014;9:e108925. doi: 10.1371/journal.pone.0108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berchem G., Noman M.Z., Bosseler M., Paggetti J., Baconnais S., Le Cam E., Nanbakhsh A., Moussay E., Mami-Chouaib F., Janji B., et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-beta and miR23a transfer. Oncoimmunology. 2016;5:e1062968. doi: 10.1080/2162402X.2015.1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Whiteside T.L. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumor-derived exosomes) Biochem. Soc. Trans. 2013;41:245–251. doi: 10.1042/BST20120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hedlund M., Nagaeva O., Kargl D., Baranov V., Mincheva-Nilsson L. Thermal- and oxidative stress causes enhanced release of NKG2D ligand-bearing immunosuppressive exosomes in leukemia/lymphoma T and B cells. PLoS ONE. 2011;6:e16899. doi: 10.1371/journal.pone.0016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hedlund M., Stenqvist A.C., Nagaeva O., Kjellberg L., Wulff M., Baranov V., Mincheva-Nilsson L. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: Evidence for immunosuppressive function. J. Immunol. 2009;183:340–351. doi: 10.4049/jimmunol.0803477. [DOI] [PubMed] [Google Scholar]
- 94.Ashiru O., Boutet P., Fernandez-Messina L., Aguera-Gonzalez S., Skepper J.N., Vales-Gomez M., Reyburn H.T. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70:481–489. doi: 10.1158/0008-5472.CAN-09-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Y., Xiang X., Zhuang X., Zhang S., Liu C., Cheng Z., Michalek S., Grizzle W., Zhang H.G. Contribution of MyD88 to the tumor exosome-mediated induction of myeloid derived suppressor cells. Am. J. Pathol. 2010;176:2490–2499. doi: 10.2353/ajpath.2010.090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Filipazzi P., Burdek M., Villa A., Rivoltini L., Huber V. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Semin. Cancer Biol. 2012;22:342–349. doi: 10.1016/j.semcancer.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y., Qin X., Zhu X., Chen W., Zhang J., Chen W. Oral cancer-derived exosomal NAP1 enhances cytotoxicity of natural killer cells via the IRF-3 pathway. Oral Oncol. 2018;76:34–41. doi: 10.1016/j.oraloncology.2017.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Q., Huang Q., Huyan T., Wang Y., Huang Q., Shi J. Bifacial effects of engineering tumor cell-derived exosomes on human natural killer cells. Exp. Cell Res. 2018;363:141–150. doi: 10.1016/j.yexcr.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 99.Bhatia A., Kumar Y. Cellular and molecular mechanisms in cancer immune escape: A comprehensive review. Expert Rev. Clin. Immunol. 2014;10:41–62. doi: 10.1586/1744666X.2014.865519. [DOI] [PubMed] [Google Scholar]
- 100.Contreras J., Rao D.S. MicroRNAs in inflammation and immune responses. Leukemia. 2012;26:404–413. doi: 10.1038/leu.2011.356. [DOI] [PubMed] [Google Scholar]
- 101.Raisch J., Darfeuille-Michaud A., Nguyen H.T. Role of microRNAs in the immune system, inflammation and cancer. World J. Gastroenterol. 2013;19:2985–2996. doi: 10.3748/wjg.v19.i20.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu X., Wang Y., Sun Q., Yan J., Huang J., Zhu S., Yu J. Identification of microRNA transcriptome involved in human natural killer cell activation. Immunol. Lett. 2012;143:208–217. doi: 10.1016/j.imlet.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 103.Sullivan R.P., Leong J.W., Fehniger T.A. MicroRNA regulation of natural killer cells. Front. Immunol. 2013;4:44. doi: 10.3389/fimmu.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bezman N.A., Cedars E., Steiner D.F., Blelloch R., Hesslein D.G., Lanier L.L. Distinct requirements of microRNAs in NK cell activation, survival, and function. J. Immunol. 2010;185:3835–3846. doi: 10.4049/jimmunol.1000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pesce S., Squillario M., Greppi M., Loiacono F., Moretta L., Moretta A., Sivori S., Castagnola P., Barla A., Candiani S., et al. New miRNA Signature Heralds Human NK Cell Subsets at Different Maturation Steps: Involvement of miR-146a-5p in the Regulation of KIR Expression. Front. Immunol. 2018;9:2360. doi: 10.3389/fimmu.2018.02360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sullivan R.P., Leong J.W., Schneider S.E., Ireland A.R., Berrien-Elliott M.M., Singh A., Schappe T., Jewell B.A., Sexl V., Fehniger T.A. MicroRNA-15/16 Antagonizes Myb To Control NK Cell Maturation. J. Immunol. 2015;195:2806–2817. doi: 10.4049/jimmunol.1500949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang L.L., Zhang L.F., Shi Y.B. miR-24 inhibited the killing effect of natural killer cells to colorectal cancer cells by downregulating Paxillin. Biomed. Pharmacother. 2018;101:257–263. doi: 10.1016/j.biopha.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 108.Cichocki F., Felices M., McCullar V., Presnell S.R., Al-Attar A., Lutz C.T., Miller J.S. Cutting edge: microRNA-181 promotes human NK cell development by regulating Notch signaling. J. Immunol. 2011;187:6171–6175. doi: 10.4049/jimmunol.1100835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sullivan R.P., Leong J.W., Schneider S.E., Keppel C.R., Germino E., French A.R., Fehniger T.A. MicroRNA-deficient NK cells exhibit decreased survival but enhanced function. J. Immunol. 2012;188:3019–3030. doi: 10.4049/jimmunol.1102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang H., Zhang Y., Wu X., Wang Y., Cui H., Li X., Zhang J., Tun N., Peng Y., Yu J. Regulation of Human Natural Killer Cell IFN-gamma Production by MicroRNA-146a via Targeting the NF-kappaB Signaling Pathway. Front. Immunol. 2018;9:293. doi: 10.3389/fimmu.2018.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu D., Han Q., Hou Z., Zhang C., Zhang J. miR-146a negatively regulates NK cell functions via STAT1 signaling. Cell. Mol. Immunol. 2017;14:712–720. doi: 10.1038/cmi.2015.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Trotta R., Parihar R., Yu J., Becknell B., Allard J., 2nd, Wen J., Ding W., Mao H., Tridandapani S., Carson W.E., et al. Differential expression of SHIP1 in CD56bright and CD56dim NK cells provides a molecular basis for distinct functional responses to monokine costimulation. Blood. 2005;105:3011–3018. doi: 10.1182/blood-2004-10-4072. [DOI] [PubMed] [Google Scholar]
- 113.Trotta R., Chen L., Ciarlariello D., Josyula S., Mao C., Costinean S., Yu L., Butchar J.P., Tridandapani S., Croce C.M., et al. miR-155 regulates IFN-gamma production in natural killer cells. Blood. 2012;119:3478–3485. doi: 10.1182/blood-2011-12-398099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sullivan R.P., Fogel L.A., Leong J.W., Schneider S.E., Wong R., Romee R., Thai T.H., Sexl V., Matkovich S.J., Dorn G.W., 2nd, et al. MicroRNA-155 tunes both the threshold and extent of NK cell activation via targeting of multiple signaling pathways. J. Immunol. 2013;191:5904–5913. doi: 10.4049/jimmunol.1301950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mundy-Bosse B.L., Scoville S.D., Chen L., McConnell K., Mao H.C., Ahmed E.H., Zorko N., Harvey S., Cole J., Zhang X., et al. MicroRNA-29b mediates altered innate immune development in acute leukemia. J. Clin. Investig. 2016;126:4404–4416. doi: 10.1172/JCI85413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim N., Kim M., Yun S., Doh J., Greenberg P.D., Kim T.D., Choi I. MicroRNA-150 regulates the cytotoxicity of natural killers by targeting perforin-1. J. Allergy Clin. Immunol. 2014;134:195–203. doi: 10.1016/j.jaci.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim T.D., Lee S.U., Yun S., Sun H.N., Lee S.H., Kim J.W., Kim H.M., Park S.K., Lee C.W., Yoon S.R., et al. Human microRNA-27a* targets Prf1 and GzmB expression to regulate NK-cell cytotoxicity. Blood. 2011;118:5476–5486. doi: 10.1182/blood-2011-04-347526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Breunig C., Pahl J., Kublbeck M., Miller M., Antonelli D., Erdem N., Wirth C., Will R., Bott A., Cerwenka A., et al. MicroRNA-519a-3p mediates apoptosis resistance in breast cancer cells and their escape from recognition by natural killer cells. Cell Death Dis. 2017;8:e2973. doi: 10.1038/cddis.2017.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rahmoon M.A., Youness R.A., Gomaa A.I., Hamza M.T., Waked I., El Tayebi H.M., Abdelaziz A.I. MiR-615-5p depresses natural killer cells cytotoxicity through repressing IGF-1R in hepatocellular carcinoma patients. Growth Factors. 2017;35:76–87. doi: 10.1080/08977194.2017.1354859. [DOI] [PubMed] [Google Scholar]
- 120.Regis S., Caliendo F., Dondero A., Casu B., Romano F., Loiacono F., Moretta A., Bottino C., Castriconi R. TGF-beta1 Downregulates the Expression of CX3CR1 by Inducing miR-27a-5p in Primary Human NK Cells. Front. Immunol. 2017;8:868. doi: 10.3389/fimmu.2017.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abdelrahman M.M., Fawzy I.O., Bassiouni A.A., Gomaa A.I., Esmat G., Waked I., Abdelaziz A.I. Enhancing NK cell cytotoxicity by miR-182 in hepatocellular carcinoma. Hum. Immunol. 2016;77:667–673. doi: 10.1016/j.humimm.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 122.Blum W., Garzon R., Klisovic R.B., Schwind S., Walker A., Geyer S., Liu S., Havelange V., Becker H., Schaaf L., et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc. Natl. Acad. Sci. USA. 2010;107:7473–7478. doi: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bezman N.A., Chakraborty T., Bender T., Lanier L.L. miR-150 regulates the development of NK and iNKT cells. J. Exp. Med. 2011;208:2717–2731. doi: 10.1084/jem.20111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karlitepe A., Kabadayi H., Vatansever S., Gurdal M., Gunduz C., Ercan G. Anti-cancer efficiency of natural killer cells differentiated from human adipose tissue-derived mesenchymal stem cells and transfected with miRNA150. Exp. Oncol. 2017;39:212–218. doi: 10.31768/2312-8852.2017.39(3):212-218. [DOI] [PubMed] [Google Scholar]
- 125.Ni F., Sun R., Fu B., Wang F., Guo C., Tian Z., Wei H. IGF-1 promotes the development and cytotoxic activity of human NK cells. Nat. Commun. 2013;4:1479. doi: 10.1038/ncomms2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yun S., Lee S.U., Kim J.M., Lee H.J., Song H.Y., Kim Y.K., Jung H., Park Y.J., Yoon S.R., Oh S.R., et al. Integrated mRNA-microRNA profiling of human NK cell differentiation identifies MiR-583 as a negative regulator of IL2Rgamma expression. PLoS ONE. 2014;9:e108913. doi: 10.1371/journal.pone.0108913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sica A., Erreni M., Allavena P., Porta C. Macrophage polarization in pathology. Cell. Mol. Life Sci. 2015;72:4111–4126. doi: 10.1007/s00018-015-1995-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Galdiero M.R., Bonavita E., Barajon I., Garlanda C., Mantovani A., Jaillon S. Tumor associated macrophages and neutrophils in cancer. Immunobiology. 2013;218:1402–1410. doi: 10.1016/j.imbio.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 129.Qian B.Z., Pollard J.W. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pesce S., Greppi M., Tabellini G., Rampinelli F., Parolini S., Olive D., Moretta L., Moretta A., Marcenaro E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immunol. 2017;139:335–346. doi: 10.1016/j.jaci.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 131.Bellora F., Castriconi R., Dondero A., Reggiardo G., Moretta L., Mantovani A., Moretta A., Bottino C. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proc. Natl. Acad. Sci. USA. 2010;107:21659–21664. doi: 10.1073/pnas.1007654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bellora F., Castriconi R., Doni A., Cantoni C., Moretta L., Mantovani A., Moretta A., Bottino C. M-CSF induces the expression of a membrane-bound form of IL-18 in a subset of human monocytes differentiating in vitro toward macrophages. Eur. J. Immunol. 2012;42:1618–1626. doi: 10.1002/eji.201142173. [DOI] [PubMed] [Google Scholar]
- 133.Mattiola I., Pesant M., Tentorio P.F., Molgora M., Marcenaro E., Lugli E., Locati M., Mavilio D. Priming of Human Resting NK Cells by Autologous M1 Macrophages via the Engagement of IL-1beta, IFN-beta, and IL-15 Pathways. J. Immunol. 2015;195:2818–2828. doi: 10.4049/jimmunol.1500325. [DOI] [PubMed] [Google Scholar]
- 134.Bellora F., Castriconi R., Dondero A., Pessino A., Nencioni A., Liggieri G., Moretta L., Mantovani A., Moretta A., Bottino C. TLR activation of tumor-associated macrophages from ovarian cancer patients triggers cytolytic activity of NK cells. Eur. J. Immunol. 2014;44:1814–1822. doi: 10.1002/eji.201344130. [DOI] [PubMed] [Google Scholar]
- 135.Lavin Y., Kobayashi S., Leader A., Amir E.D., Elefant N., Bigenwald C., Remark R., Sweeney R., Becker C.D., Levine J.H., et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell. 2017;169:750–765. doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Molgora M., Bonavita E., Ponzetta A., Riva F., Barbagallo M., Jaillon S., Popovic B., Bernardini G., Magrini E., Gianni F., et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumor and anti-viral activity. Nature. 2017;551:110–114. doi: 10.1038/nature24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fernandez N.C., Lozier A., Flament C., Ricciardi-Castagnoli P., Bellet D., Suter M., Perricaudet M., Tursz T., Maraskovsky E., Zitvogel L. Dendritic cells directly trigger NK cell functions: Cross-talk relevant in innate anti-tumor immune responses in vivo. Nat. Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 138.Lucas M., Schachterle W., Oberle K., Aichele P., Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mingozzi F., Spreafico R., Gorletta T., Cigni C., Di Gioia M., Caccia M., Sironi L., Collini M., Soncini M., Rusconi M., et al. Prolonged contact with dendritic cells turns lymph node-resident NK cells into anti-tumor effectors. EMBO Mol. Med. 2016;8:1039–1051. doi: 10.15252/emmm.201506164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morandi B., Mortara L., Carrega P., Cantoni C., Costa G., Accolla R.S., Mingari M.C., Ferrini S., Moretta L., Ferlazzo G. NK cells provide helper signal for CD8+ T cells by inducing the expression of membrane-bound IL-15 on DCs. Int. Immunol. 2009;21:599–606. doi: 10.1093/intimm/dxp029. [DOI] [PubMed] [Google Scholar]
- 141.Morandi B., Mortara L., Chiossone L., Accolla R.S., Mingari M.C., Moretta L., Moretta A., Ferlazzo G. Dendritic cell editing by activated natural killer cells results in a more protective cancer-specific immune response. PLoS ONE. 2012;7:e39170. doi: 10.1371/journal.pone.0039170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Benoit T.G., Wilson G.R., Pryor N., Bull D.L. Isolation and pathogenicity of Serratia marcescens from adult house flies infected with Entomophthora muscae. J. Invertebr. Pathol. 1990;55:142–144. doi: 10.1016/0022-2011(90)90047-A. [DOI] [PubMed] [Google Scholar]
- 143.Jaillon S., Galdiero M.R., Del Prete D., Cassatella M.A., Garlanda C., Mantovani A. Neutrophils in innate and adaptive immunity. Semin. Immunopathol. 2013;35:377–394. doi: 10.1007/s00281-013-0374-8. [DOI] [PubMed] [Google Scholar]
- 144.Albini A., Bruno A., Noonan D.M., Mortara L. Contribution to Tumor Angiogenesis from Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front. Immunol. 2018;9:527. doi: 10.3389/fimmu.2018.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Andzinski L., Kasnitz N., Stahnke S., Wu C.F., Gereke M., von Kockritz-Blickwede M., Schilling B., Brandau S., Weiss S., Jablonska J. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int. J. Cancer. 2016;138:1982–1993. doi: 10.1002/ijc.29945. [DOI] [PubMed] [Google Scholar]
- 146.Shaul M.E., Fridlender Z.G. Neutrophils as active regulators of the immune system in the tumor microenvironment. J. Leukoc. Biol. 2017;102:343–349. doi: 10.1189/jlb.5MR1216-508R. [DOI] [PubMed] [Google Scholar]
- 147.Shaul M.E., Fridlender Z.G. Cancer related circulating and tumor-associated neutrophils—Subtypes, sources and function. FEBS J. 2018;285:4316–4342. doi: 10.1111/febs.14524. [DOI] [PubMed] [Google Scholar]
- 148.Molgora M., Supino D., Mavilio D., Santoni A., Moretta L., Mantovani A., Garlanda C. The yin-yang of the interaction between myelomonocytic cells and NK cells. Scand. J. Immunol. 2018;88:e12705. doi: 10.1111/sji.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Costantini C., Cassatella M.A. The defensive alliance between neutrophils and NK cells as a novel arm of innate immunity. J. Leukoc. Biol. 2011;89:221–233. doi: 10.1189/jlb.0510250. [DOI] [PubMed] [Google Scholar]
- 150.Shau H.Y., Golub S.H. Inhibition of lymphokine-activated killer- and natural killer-mediated cytotoxicities by neutrophils. J. Immunol. 1989;143:1066–1072. [PubMed] [Google Scholar]
- 151.Shau H.Y., Kim A. Suppression of lymphokine-activated killer induction by neutrophils. J. Immunol. 1988;141:4395–4402. [PubMed] [Google Scholar]
- 152.Harlin H., Hanson M., Johansson C.C., Sakurai D., Poschke I., Norell H., Malmberg K.J., Kiessling R. The CD16- CD56(bright) NK cell subset is resistant to reactive oxygen species produced by activated granulocytes and has higher antioxidative capacity than the CD16+ CD56(dim) subset. J. Immunol. 2007;179:4513–4519. doi: 10.4049/jimmunol.179.7.4513. [DOI] [PubMed] [Google Scholar]
- 153.Amano K., Hirayama M., Azuma E., Iwamoto S., Keida Y., Komada Y. Neutrophils induced licensing of natural killer cells. Mediat. Inflamm. 2015;2015:747680. doi: 10.1155/2015/747680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Araujo J.M., Gomez A.C., Aguilar A., Salgado R., Balko J.M., Bravo L., Doimi F., Bretel D., Morante Z., Flores C., et al. Effect of CCL5 expression in the recruitment of immune cells in triple negative breast cancer. Sci. Rep. 2018;8:4899. doi: 10.1038/s41598-018-23099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ogura K., Sato-Matsushita M., Yamamoto S., Hori T., Sasahara M., Iwakura Y., Saiki I., Tahara H., Hayakawa Y. NK Cells Control Tumor-Promoting Function of Neutrophils in Mice. Cancer Immunol. Res. 2018;6 doi: 10.1158/2326-6066.CIR-17-0204. [DOI] [PubMed] [Google Scholar]
- 156.Spiegel A., Brooks M.W., Houshyar S., Reinhardt F., Ardolino M., Fessler E., Chen M.B., Krall J.A., DeCock J., Zervantonakis I.K., et al. Neutrophils Suppress Intraluminal NK Cell-Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016;6:630–649. doi: 10.1158/2159-8290.CD-15-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chan C.Y., St John A.L., Abraham S.N. Plasticity in mast cell responses during bacterial infections. Curr. Opin. Microbiol. 2012;15:78–84. doi: 10.1016/j.mib.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang Z., Lai Y., Bernard J.J., Macleod D.T., Cogen A.L., Moss B., Di Nardo A. Skin mast cells protect mice against vaccinia virus by triggering mast cell receptor S1PR2 and releasing antimicrobial peptides. J. Immunol. 2012;188:345–357. doi: 10.4049/jimmunol.1101703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marone G., Varricchi G., Loffredo S., Granata F. Mast cells and basophils in inflammatory and tumor angiogenesis and lymphangiogenesis. Eur. J. Pharmacol. 2016;778:146–151. doi: 10.1016/j.ejphar.2015.03.088. [DOI] [PubMed] [Google Scholar]
- 160.Ribatti D., Crivellato E. Mast cells, angiogenesis, and tumor growth. Biochim. Biophys. Acta. 2012;1822:2–8. doi: 10.1016/j.bbadis.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 161.Oldford S.A., Haidl I.D., Howatt M.A., Leiva C.A., Johnston B., Marshall J.S. A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J. Immunol. 2010;185:7067–7076. doi: 10.4049/jimmunol.1001137. [DOI] [PubMed] [Google Scholar]
- 162.Tu J.F., Pan H.Y., Ying X.H., Lou J., Ji J.S., Zou H. Mast Cells Comprise the Major of Interleukin 17-Producing Cells and Predict a Poor Prognosis in Hepatocellular Carcinoma. Medicine. 2016;95:e3220. doi: 10.1097/MD.0000000000003220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ribatti D., Tamma R., Crivellato E. Cross talk between natural killer cells and mast cells in tumor angiogenesis. Inflamm. Res. 2019;68:19–23. doi: 10.1007/s00011-018-1181-4. [DOI] [PubMed] [Google Scholar]
- 164.Vosskuhl K., Greten T.F., Manns M.P., Korangy F., Wedemeyer J. Lipopolysaccharide-mediated mast cell activation induces IFN-gamma secretion by NK cells. J. Immunol. 2010;185:119–125. doi: 10.4049/jimmunol.0902406. [DOI] [PubMed] [Google Scholar]
- 165.Karagiannis G.S., Poutahidis T., Erdman S.E., Kirsch R., Riddell R.H., Diamandis E.P. Cancer-associated fibroblasts drive the progression of metastasis through both paracrine and mechanical pressure on cancer tissue. Mol. Cancer Res. 2012;10:1403–1418. doi: 10.1158/1541-7786.MCR-12-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Quail D.F., Joyce J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tao L., Huang G., Song H., Chen Y., Chen L. Cancer associated fibroblasts: An essential role in the tumor microenvironment. Oncol. Lett. 2017;14:2611–2620. doi: 10.3892/ol.2017.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bagordakis E., Sawazaki-Calone I., Macedo C.C., Carnielli C.M., de Oliveira C.E., Rodrigues P.C., Rangel A.L., Dos Santos J.N., Risteli J., Graner E., et al. Secretome profiling of oral squamous cell carcinoma-associated fibroblasts reveals organization and disassembly of extracellular matrix and collagen metabolic process signatures. Tumor Biol. 2016;37:9045–9057. doi: 10.1007/s13277-015-4629-y. [DOI] [PubMed] [Google Scholar]
- 169.De Boeck A., Hendrix A., Maynard D., Van Bockstal M., Daniels A., Pauwels P., Gespach C., Bracke M., De Wever O. Differential secretome analysis of cancer-associated fibroblasts and bone marrow-derived precursors to identify microenvironmental regulators of colon cancer progression. Proteomics. 2013;13:379–388. doi: 10.1002/pmic.201200179. [DOI] [PubMed] [Google Scholar]
- 170.Ljunggren H.G. Cancer immunosurveillance: NKG2D breaks cover. Immunity. 2008;28:492–494. doi: 10.1016/j.immuni.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 171.Torres S., Bartolome R.A., Mendes M., Barderas R., Fernandez-Acenero M.J., Pelaez-Garcia A., Pena C., Lopez-Lucendo M., Villar-Vazquez R., de Herreros A.G., et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin. Cancer Res. 2013;19:6006–6019. doi: 10.1158/1078-0432.CCR-13-1130. [DOI] [PubMed] [Google Scholar]
- 172.Avagliano A., Granato G., Ruocco M.R., Romano V., Belviso I., Carfora A., Montagnani S., Arcucci A. Metabolic Reprogramming of Cancer Associated Fibroblasts: The Slavery of Stromal Fibroblasts. Biomed. Res. Int. 2018;2018:6075403. doi: 10.1155/2018/6075403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Guido C., Whitaker-Menezes D., Capparelli C., Balliet R., Lin Z., Pestell R.G., Howell A., Aquila S., Ando S., Martinez-Outschoorn U., et al. Metabolic reprogramming of cancer-associated fibroblasts by TGF-beta drives tumor growth: Connecting TGF-beta signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle. 2012;11:3019–3035. doi: 10.4161/cc.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu D., Zhuo L., Wang X. Metabolic reprogramming of carcinoma-associated fibroblasts and its impact on metabolic heterogeneity of tumors. Semin. Cell Dev. Biol. 2017;64:125–131. doi: 10.1016/j.semcdb.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 175.Ziani L., Safta-Saadoun T.B., Gourbeix J., Cavalcanti A., Robert C., Favre G., Chouaib S., Thiery J. Melanoma-associated fibroblasts decrease tumor cell susceptibility to NK cell-mediated killing through matrix-metalloproteinases secretion. Oncotarget. 2017;8:19780–19794. doi: 10.18632/oncotarget.15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li T., Yi S., Liu W., Jia C., Wang G., Hua X., Tai Y., Zhang Q., Chen G. Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med. Oncol. 2013;30:663. doi: 10.1007/s12032-013-0663-z. [DOI] [PubMed] [Google Scholar]
- 177.Ziani L., Chouaib S., Thiery J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front. Immunol. 2018;9:414. doi: 10.3389/fimmu.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bosi A., Zanellato S., Bassani B., Albini A., Musco A., Cattoni M., Desio M., Nardecchia E., D’Urso D.G., Imperatori A., et al. Natural Killer Cells from Malignant Pleural Effusion Are Endowed with a Decidual-Like Proangiogenic Polarization. J. Immunol. Res. 2018;2018:2438598. doi: 10.1155/2018/2438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Miller J.S., Soignier Y., Panoskaltsis-Mortari A., McNearney S.A., Yun G.H., Fautsch S.K., McKenna D., Le C., Defor T.E., Burns L.J., et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 180.Lam V.C., Lanier L.L. NK cells in host responses to viral infections. Curr. Opin. Immunol. 2017;44:43–51. doi: 10.1016/j.coi.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wight A., Mahmoud A.B., Scur M., Tu M.M., Rahim M.M.A., Sad S., Makrigiannis A.P. Critical role for the Ly49 family of class I MHC receptors in adaptive natural killer cell responses. Proc. Natl. Acad. Sci. USA. 2018 doi: 10.1073/pnas.1722374115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Childs R.W., Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: The force awakens. Nat. Rev. Drug Discov. 2015;14:487–498. doi: 10.1038/nrd4506. [DOI] [PubMed] [Google Scholar]
- 183.Mortara L., Balza E., Bruno A., Poggi A., Orecchia P., Carnemolla B. Anti-cancer Therapies Employing IL-2 Cytokine Tumor Targeting: Contribution of Innate, Adaptive and Immunosuppressive Cells in the Anti-tumor Efficacy. Front. Immunol. 2018;9:2905. doi: 10.3389/fimmu.2018.02905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hirakawa M., Matos T.R., Liu H., Koreth J., Kim H.T., Paul N.E., Murase K., Whangbo J., Alho A.C., Nikiforow S., et al. Low-dose IL-2 selectively activates subsets of CD4(+) Tregs and NK cells. JCI Insight. 2016;1:e89278. doi: 10.1172/jci.insight.89278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Klein C., Waldhauer I., Nicolini V.G., Freimoser-Grundschober A., Nayak T., Vugts D.J., Dunn C., Bolijn M., Benz J., Stihle M., et al. Cergutuzumab amunaleukin (CEA-IL2v), a CEA-targeted IL-2 variant-based immunocytokine for combination cancer immunotherapy: Overcoming limitations of aldesleukin and conventional IL-2-based immunocytokines. Oncoimmunology. 2017;6:e1277306. doi: 10.1080/2162402X.2016.1277306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Levin A.M., Bates D.L., Ring A.M., Krieg C., Lin J.T., Su L., Moraga I., Raeber M.E., Bowman G.R., Novick P., et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine’. Nature. 2012;484:529–533. doi: 10.1038/nature10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dubois S., Patel H.J., Zhang M., Waldmann T.A., Muller J.R. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J. Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 188.Han K.P., Zhu X., Liu B., Jeng E., Kong L., Yovandich J.L., Vyas V.V., Marcus W.D., Chavaillaz P.A., Romero C.A., et al. IL-15:IL-15 receptor alpha superagonist complex: High-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine. 2011;56:804–810. doi: 10.1016/j.cyto.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lehmann D., Spanholtz J., Sturtzel C., Tordoir M., Schlechta B., Groenewegen D., Hofer E. IL-12 directs further maturation of ex vivo differentiated NK cells with improved therapeutic potential. PLoS ONE. 2014;9:e87131. doi: 10.1371/journal.pone.0087131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gokhale M.S., Vainstein V., Tom J., Thomas S., Lawrence C.E., Gluzman-Poltorak Z., Siebers N., Basile L.A. Single low-dose rHuIL-12 safely triggers multilineage hematopoietic and immune-mediated effects. Exp. Hematol. Oncol. 2014;3:11. doi: 10.1186/2162-3619-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gollob J.A., Veenstra K.G., Parker R.A., Mier J.W., McDermott D.F., Clancy D., Tutin L., Koon H., Atkins M.B. Phase I trial of concurrent twice-weekly recombinant human interleukin-12 plus low-dose IL-2 in patients with melanoma or renal cell carcinoma. J. Clin. Oncol. 2003;21:2564–2573. doi: 10.1200/JCO.2003.12.119. [DOI] [PubMed] [Google Scholar]
- 192.Trudeau C., Cotreau M.M., Stonis L., Dykstra K.H., Oestreicher J.L., Strahs A., Dorner A.J., Van Cleave V.H., Trepicchio W.L., Schwertschlag U.S. A single administration of recombinant human interleukin-12 is associated with increased expression levels of interferon-gamma and signal transducer and activator of transcription in healthy subjects. J. Clin. Pharmacol. 2005;45:649–658. doi: 10.1177/0091270005276116. [DOI] [PubMed] [Google Scholar]
- 193.Hayashi T., Hideshima T., Akiyama M., Podar K., Yasui H., Raje N., Kumar S., Chauhan D., Treon S.P., Richardson P., et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: Clinical application. Br. J. Haematol. 2005;128:192–203. doi: 10.1111/j.1365-2141.2004.05286.x. [DOI] [PubMed] [Google Scholar]
- 194.Lagrue K., Carisey A., Morgan D.J., Chopra R., Davis D.M. Lenalidomide augments actin remodeling and lowers NK-cell activation thresholds. Blood. 2015;126:50–60. doi: 10.1182/blood-2015-01-625004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gandhi A.K., Kang J., Havens C.G., Conklin T., Ning Y., Wu L., Ito T., Ando H., Waldman M.F., Thakurta A., et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.) Br. J. Haematol. 2014;164:811–821. doi: 10.1111/bjh.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fionda C., Abruzzese M.P., Zingoni A., Cecere F., Vulpis E., Peruzzi G., Soriani A., Molfetta R., Paolini R., Ricciardi M.R., et al. The IMiDs targets IKZF-1/3 and IRF4 as novel negative regulators of NK cell-activating ligands expression in multiple myeloma. Oncotarget. 2015;6:23609–23630. doi: 10.18632/oncotarget.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Besson L., Charrier E., Karlin L., Allatif O., Marcais A., Rouzaire P., Belmont L., Attal M., Lombard C., Salles G., et al. One-Year Follow-Up of Natural Killer Cell Activity in Multiple Myeloma Patients Treated with Adjuvant Lenalidomide Therapy. Front. Immunol. 2018;9:704. doi: 10.3389/fimmu.2018.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vey N., Bourhis J.H., Boissel N., Bordessoule D., Prebet T., Charbonnier A., Etienne A., Andre P., Romagne F., Benson D., et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120:4317–4323. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 199.Zelarney P.T., Belden E.L. Bovine interleukin 2: Production by an E-rosette-defined lymphocyte subpopulation. Vet. Immunol. Immunopathol. 1988;18:297–305. doi: 10.1016/0165-2427(88)90157-2. [DOI] [PubMed] [Google Scholar]
- 200.Benson D.M., Jr., Bakan C.E., Mishra A., Hofmeister C.C., Efebera Y., Becknell B., Baiocchi R.A., Zhang J., Yu J., Smith M.K., et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wiesmayr S., Webber S.A., Macedo C., Popescu I., Smith L., Luce J., Metes D. Decreased NKp46 and NKG2D and elevated PD-1 are associated with altered NK-cell function in pediatric transplant patients with PTLD. Eur. J. Immunol. 2012;42:541–550. doi: 10.1002/eji.201141832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang Q., Bi J., Zheng X., Chen Y., Wang H., Wu W., Wang Z., Wu Q., Peng H., Wei H., et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 2018;19:723–732. doi: 10.1038/s41590-018-0132-0. [DOI] [PubMed] [Google Scholar]
- 203.Glorius P., Baerenwaldt A., Kellner C., Staudinger M., Dechant M., Stauch M., Beurskens F.J., Parren P.W., Winkel J.G., Valerius T., et al. The novel tribody [(CD20)(2)xCD16] efficiently triggers effector cell-mediated lysis of malignant B cells. Leukemia. 2013;27:190–201. doi: 10.1038/leu.2012.150. [DOI] [PubMed] [Google Scholar]
- 204.Kellner C., Bruenke J., Horner H., Schubert J., Schwenkert M., Mentz K., Barbin K., Stein C., Peipp M., Stockmeyer B., et al. Heterodimeric bispecific antibody-derivatives against CD19 and CD16 induce effective antibody-dependent cellular cytotoxicity against B-lymphoid tumor cells. Cancer Lett. 2011;303:128–139. doi: 10.1016/j.canlet.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 205.Portner L.M., Schonberg K., Hejazi M., Brunnert D., Neumann F., Galonska L., Reusch U., Little M., Haas R., Uhrberg M. T and NK cells of B cell NHL patients exert cytotoxicity against lymphoma cells following binding of bispecific tetravalent antibody CD19 x CD3 or CD19 x CD16. Cancer Immunol. Immunother. 2012;61:1869–1875. doi: 10.1007/s00262-012-1339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Schubert I., Kellner C., Stein C., Kugler M., Schwenkert M., Saul D., Stockmeyer B., Berens C., Oduncu F.S., Mackensen A., et al. A recombinant triplebody with specificity for CD19 and HLA-DR mediates preferential binding to antigen double-positive cells by dual-targeting. MAbs. 2012;4:45–56. doi: 10.4161/mabs.4.1.18498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schubert I., Kellner C., Stein C., Kugler M., Schwenkert M., Saul D., Mentz K., Singer H., Stockmeyer B., Hillen W., et al. A single-chain triplebody with specificity for CD19 and CD33 mediates effective lysis of mixed lineage leukemia cells by dual targeting. MAbs. 2011;3:21–30. doi: 10.4161/mabs.3.1.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Felices M., Lenvik T.R., Davis Z.B., Miller J.S., Vallera D.A. Generation of BiKEs and TriKEs to Improve NK Cell-Mediated Targeting of Tumor Cells. Methods Mol. Biol. 2016;1441:333–346. doi: 10.1007/978-1-4939-3684-7_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ferrini S., Cambiaggi A., Sforzini S., Canevari S., Mezzanzanica D., Colnaghi M.I., Moretta L. Use of anti-CD3 and anti-CD16 bispecific monoclonal antibodies for the targeting of T and NK cells against tumor cells. Cancer Detect. Prev. 1993;17:295–300. [PubMed] [Google Scholar]
- 210.Gleason M.K., Verneris M.R., Todhunter D.A., Zhang B., McCullar V., Zhou S.X., Panoskaltsis-Mortari A., Weiner L.M., Vallera D.A., Miller J.S. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol. Cancer Ther. 2012;11:2674–2684. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shahied L.S., Tang Y., Alpaugh R.K., Somer R., Greenspon D., Weiner L.M. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. J. Biol. Chem. 2004;279:53907–53914. doi: 10.1074/jbc.M407888200. [DOI] [PubMed] [Google Scholar]
- 212.Vallera D.A., Zhang B., Gleason M.K., Oh S., Weiner L.M., Kaufman D.S., McCullar V., Miller J.S., Verneris M.R. Heterodimeric bispecific single-chain variable-fragment antibodies against EpCAM and CD16 induce effective antibody-dependent cellular cytotoxicity against human carcinoma cells. Cancer Biother. Radiopharm. 2013;28:274–282. doi: 10.1089/cbr.2012.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wiernik A., Foley B., Zhang B., Verneris M.R., Warlick E., Gleason M.K., Ross J.A., Luo X., Weisdorf D.J., Walcheck B., et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin. Cancer Res. 2013;19:3844–3855. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lundqvist A., Yokoyama H., Smith A., Berg M., Childs R. Bortezomib treatment and regulatory T-cell depletion enhance the antitumor effects of adoptively infused NK cells. Blood. 2009;113:6120–6127. doi: 10.1182/blood-2008-11-190421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Niu C., Jin H., Li M., Zhu S., Zhou L., Jin F., Zhou Y., Xu D., Xu J., Zhao L., et al. Low-dose bortezomib increases the expression of NKG2D and DNAM-1 ligands and enhances induced NK and gammadelta T cell-mediated lysis in multiple myeloma. Oncotarget. 2017;8:5954–5964. doi: 10.18632/oncotarget.13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wu X., Tao Y., Hou J., Meng X., Shi J. Valproic acid upregulates NKG2D ligand expression through an ERK-dependent mechanism and potentially enhances NK cell-mediated lysis of myeloma. Neoplasia. 2012;14:1178–1189. doi: 10.1593/neo.121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Skov S., Pedersen M.T., Andresen L., Straten P.T., Woetmann A., Odum N. Cancer cells become susceptible to natural killer cell killing after exposure to histone deacetylase inhibitors due to glycogen synthase kinase-3-dependent expression of MHC class I-related chain A and B. Cancer Res. 2005;65:11136–11145. doi: 10.1158/0008-5472.CAN-05-0599. [DOI] [PubMed] [Google Scholar]
- 218.Shaffer B.C., Le Luduec J.B., Forlenza C., Jakubowski A.A., Perales M.A., Young J.W., Hsu K.C. Phase II Study of Haploidentical Natural Killer Cell Infusion for Treatment of Relapsed or Persistent Myeloid Malignancies Following Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2016;22:705–709. doi: 10.1016/j.bbmt.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shi J., Tricot G., Szmania S., Rosen N., Garg T.K., Malaviarachchi P.A., Moreno A., Dupont B., Hsu K.C., Baxter-Lowe L.A., et al. Infusion of haplo-identical killer immunoglobulin-like receptor ligand mismatched NK cells for relapsed myeloma in the setting of autologous stem cell transplantation. Br. J. Haematol. 2008;143:641–653. doi: 10.1111/j.1365-2141.2008.07340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Rubnitz J.E., Inaba H., Ribeiro R.C., Pounds S., Rooney B., Bell T., Pui C.H., Leung W. NKAML: A pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J. Clin. Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Burns L.J., Weisdorf D.J., DeFor T.E., Vesole D.H., Repka T.L., Blazar B.R., Burger S.R., Panoskaltsis-Mortari A., Keever-Taylor C.A., Zhang M.J., et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: A phase I/II trial. Bone Marrow Transplant. 2003;32:177–186. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 222.Cheng M., Chen Y., Xiao W., Sun R., Tian Z. NK cell-based immunotherapy for malignant diseases. Cell. Mol. Immunol. 2013;10:230–252. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cartron G., Dacheux L., Salles G., Solal-Celigny P., Bardos P., Colombat P., Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- 224.Klingemann H., Boissel L., Toneguzzo F. Natural Killer Cells for Immunotherapy—Advantages of the NK-92 Cell Line over Blood NK Cells. Front. Immunol. 2016;7:91. doi: 10.3389/fimmu.2016.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lanuza P.M., Vigueras A., Olivan S., Prats A.C., Costas S., Llamazares G., Sanchez-Martinez D., Ayuso J.M., Fernandez L., Ochoa I., et al. Activated human primary NK cells efficiently kill colorectal cancer cells in 3D spheroid cultures irrespectively of the level of PD-L1 expression. Oncoimmunology. 2018;7:e1395123. doi: 10.1080/2162402X.2017.1395123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Boissel L., Betancur-Boissel M., Lu W., Krause D.S., Van Etten R.A., Wels W.S., Klingemann H. Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology. 2013;2:e26527. doi: 10.4161/onci.26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Esser R., Muller T., Stefes D., Kloess S., Seidel D., Gillies S.D., Aperlo-Iffland C., Huston J.S., Uherek C., Schonfeld K., et al. NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumor cells of neuroectodermal origin. J. Cell. Mol. Med. 2012;16:569–581. doi: 10.1111/j.1582-4934.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Muller N., Michen S., Tietze S., Topfer K., Schulte A., Lamszus K., Schmitz M., Schackert G., Pastan I., Temme A. Engineering NK Cells Modified with an EGFRvIII-specific Chimeric Antigen Receptor to Overexpress CXCR4 Improves Immunotherapy of CXCL12/SDF-1alpha-secreting Glioblastoma. J. Immunother. 2015;38:197–210. doi: 10.1097/CJI.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Schonfeld K., Sahm C., Zhang C., Naundorf S., Brendel C., Odendahl M., Nowakowska P., Bonig H., Kohl U., Kloess S., et al. Selective inhibition of tumor growth by clonal NK cells expressing an ErbB2/HER2-specific chimeric antigen receptor. Mol. Ther. 2015;23:330–338. doi: 10.1038/mt.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]