Abstract
Microbial communities are responsible for the unique functional properties of chocolate. During microbial growth, several antimicrobial and antioxidant metabolites are produced and can influence human wellbeing. In the last decades, the use of starter cultures in cocoa fermentation has been pushed to improve nutritional value, quality, and the overall product safety. However, it must be noted that unpredictable changes in cocoa flavor have been reported between the different strains from the same species used as a starter, causing a loss of desirable notes and flavors. Thus, the importance of an accurate selection of the starter cultures based on the biogenic effect to complement and optimize chocolate quality has become a major interest for the chocolate industry. This paper aimed to review the microbial communities identified from spontaneous cocoa fermentations and focused on the yeast starter strains used in cocoa beans and their sensorial and flavor profile. The potential compounds that could have health-promoting benefits like limonene, benzaldehyde, 2-phenylethanol, 2-methylbutanal, phenylacetaldehyde, and 2-phenylethyl acetate were also evaluated as their presence remained constant after roasting. Further research is needed to highlight the future perspectives of microbial volatile compounds as biomarkers to warrant food quality and safety.
Keywords: fermentation, functional volatile compounds, starter culture, yeast, roasting, chocolate, cocoa beans
1. Introduction
Certainly, people have been changing their food consumption patterns and lifestyle over the last decade [1]. To counteract unhealthy food choices, functional food has emerged as a strategy to increase the consciousness of the relationship between diet and disease/health to consumers. The objective of a successful functional food is to target a specific group of consumers and to meet their health demands without compromising flavor, taste, and color. In this context, the most used bioactive compounds in the food industry include alkaloids, anthocyanins, carotenoids, flavonoids, glucosinolates, isoflavones, phenolic acids, hydrolysate proteins, tannins, and phytochemical terpenes [2].
Volatile organic compounds (VOCs) are organic molecules that include esters, alcohols, aldehydes, ketones, phenols, terpenes, etc. These VOCs are synthesized naturally by a broad number of plants or microorganisms (as secondary metabolites) to enable interactions with their environment. In addition, it has been demonstrated that VOCs provide health benefits to consumers [3]. Health benefits provided by the microbial communities can be either direct or indirect. The difference between these two concepts is the ingestion of a live microorganism (direct) or the ingestion of microbial metabolites (indirect or biogenic effect) [4]. Undeniably, a biogenic effect is commonly observed in fermented foods such as chocolate.
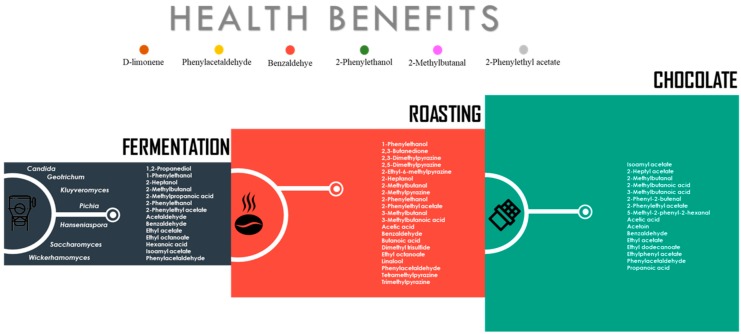
The production of microbial metabolites in cocoa beans begins during fermentation. In this process, microorganisms, encompassing bacteria and yeasts, serve to confer taste, texture, and desirable aromas to the final product. An effective cocoa fermentation develops when a correct microbial succession of yeasts, lactic acid bacteria (LAB), and acetic acid bacteria (AAB) takes place [5,6]. The success of these dynamics is due to the nutrient content of the cocoa pulp that is used as an optimal substrate for the microbial growth, and yeasts are considered the first microorganisms growing at the beginning of the fermentation process, producing ethanol, organic acids and VOCs, that contribute as precursors of chocolate flavor [7]. For those reasons, yeasts have been widely used as starter cultures in cocoa beans with the aim to enrich the sensorial quality of chocolate. However, the modulation of the remarkable complexity of microbial communities in cocoa beans to obtain an optimal flavor fingerprinting as well as understanding the metabolic and regulatory networks concerning the production of secondary metabolites are still not clear. In this context, the present review aims to describe the development of the microbes in fermented cocoa beans, and to evaluate the individual capacities of yeast species to form aroma compounds to enhance flavor perception and nutritional or healthy values. More importantly, it assesses the most frequently identified VOCs during the three different steps of chocolate elaboration, including fermentation, roasting, and the final product, chocolate (Figure 1). It is important to clarify that the VOCs identified in the fermentation and final product were the most frequently identified VOCs in inoculated cocoa beans with yeast, while the most frequently identified VOCs during roasting were assessed from non-inoculated cocoa beans.
Figure 1.
Tracking volatile compounds from chocolate.
2. Microbial Composition of Fermented Cocoa Beans
The fermentation step is considered a key stage that influences the flavor potential of cocoa beans. Existing scientific data shows the complexity of the composition of the microbial population on fermented cocoa beans, that varies depending on: plant variety; environmental conditions; post-harvesting processing; type of fermentation; and agricultural practices [6,8,9,10]. The bacteria population often present during cocoa fermentation are mainly composed by LAB mostly belonging to the Lactobacillus and Leuconostoc genera, as well as AAB such as members of the genus Acetobacter [11,12,13,14,15,16,17,18,19,20,21,22,23,24]. In addition, some species belonging to Bacillus have also been rarely isolated from fermented cocoa beans. Despite the lower complexity of the bacteria population in the cocoa-fermented system, several yeasts have been identified including species belonging to Candida, Debaromyces, Geotrichum, Hanseniaspora, Kluyveromyces, Pichia, Saccharomyces, Rhodotorula, Saccharomycopsis, and Wickerhamomyces [6,12,14,15,17,18,20,21,22,23,24,25,26,27,28,29,30,31]. Besides yeast species, filamentous fungi belonging to Aspergillus, Mucor, Neurospora, Penicillium, and Rhizopus are also often reported [6,23]. Interestingly, there are some discrepancies between the relative abundance of microbial communities reported in fermented cocoa beans from different origins and fermented from different types of fermentations (box, heap). Nonetheless, a recent study suggested that not only the most abundant microbial species could affect the production of organic molecules, but also rare species were involved [6].
2.1. Yeasts Species Used as Starter during Cocoa Fermentation
Some yeasts and fungi are considered a safe source of ingredients and additives for food processing because they have a positive image with consumers [32]. The interest in yeasts as starter cultures has arisen in recent years especially in relation to the addition of Saccharomyces, Pichia, Kluyveromyces, Candida, and Torulaspora to fermented cocoa beans [6,25,28,30,31,33,34,35,36,37]. Yeasts that are being used to ferment cocoa beans are shown in Table 1. It should be noted that starter cultures used to drive cocoa fermentation processes have been applied only in a few cocoa-producing countries such as Brazil, Malaysia, Indonesia, and Cameroon [6,25,28,30,31,33,34,35,36,38]. The importance of standardized cocoa fermentation process has become controversial, considering that the environmental conditions are difficult to control in most of the cocoa-producing countries. Therefore, the choice of selecting cocoa fermenting starters, discriminated based on origin and microbial communities, should be a logical choice from the available options.
Table 1.
Functional yeasts used as starters in cocoa fermentation.
| Genera/Species | Year | Country | Type of Cocoa Bean | Type of Fermentation | Amount | VOCs | Sensorial Analysis | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| F | C | ||||||||
| Kluyveromyces marxianus MMIII-41 | 2008 | Brazil | NM | Plastic basket | 45 kg | - | - | + | Leal et al., 2008 [28] |
| Saccharomyces cerevisiae UFLA CA11 | 2014 | Brazil | PH16 | Wooden box | 60 kg | + | - | - | Ramos et al., 2014 [33] |
| Saccharomyces cerevisiae UFLA CA11 | 2014 | Brazil | PS1030 | Wooden box | 60 kg | + | - | - | Ramos et al., 2014 [33] |
| Saccharomyces cerevisiae UFLA CA11 | 2014 | Brazil | FA13 | Wooden box | 60 kg | + | - | - | Ramos et al., 2014 [33] |
| Saccharomyces cerevisiae UFLA CA11 | 2014 | Brazil | PS1319 | Wooden box | 60 kg | + | - | - | Ramos et al., 2014 [33] |
| Saccharomyces cerevisiae, Pichia kluyveri and Hanseniaspora uvarum | 2015 | Brazil | PS1319 | Wooden box | 100 kg | - | - | + | Batista et al., 2015 [30] |
| Candida sp. | 2015 | Malaysia | NM | Basket | 5 kg | - | - | - | Mahazar et al., 2015 [36] |
| Saccharomyces cerevisiae H19 | 2015 | Malaysia | NM | Basket | 50 kg | - | + | + | Meersman et al., 2016 [37] |
| Saccharomyces cerevisiae H28 | 2015 | Malaysia | NM | Basket | 50 kg | - | + | + | Mersman et al., 2016 [37] |
| Saccharomyces cerevisiae H37 | 2015 | Malaysia | NM | Basket | 50 kg | - | + | + | Mersman et al., 2016 [37] |
| Saccharomyces cerevisae var. chevalieri | 2015 | Indonesia | Forastero | Plastic bags | NM | - | - | - | Cempaka et al., 2014 [35] |
| Saccharomyces cerevisiae | 2016 | Brazil | CCN51 | Wooden box | 100 kg | - | + | + | Menezes et al., 2016 [34] |
| Saccharomyces cerevisiae | 2016 | Brazil | CEPEC2004 | Wooden box | 100 kg | - | + | + | Menezes et al., 2016 [34] |
| Saccharomyces cerevisiae | 2016 | Brazil | FA13 | Wooden box | 100 kg | - | + | + | Menezes et al., 2016 [34] |
| Saccharomyces cerevisiae | 2016 | Brazil | PS1030 | Wooden box | 100 kg | - | + | + | Menezes et al., 2016 [34] |
| Torulaspora delbrueckii | 2017 | Brazil | PS1319 | Wooden box | 300 kg | - | + | + | Visintin et al., 2017 [31] |
| T. delbrueckii | 2017 | Brazil | SJ02 | Wooden box | 300 kg | - | + | + | Visintin et al., 2017 [31] |
| S. cerevisiae and T. delbrueckii | 2017 | Brazil | PS1319 | Wooden box | 300 kg | - | + | + | Visintin et al., 2017 [31] |
| Pichia kudriavzevii LPB06 | 2017 | Brazil | NM | Lab scale | 400 g | + | - | - | Pereira et al., 2017 [25] |
| Pichia kudriavzevii LPB07 | 2017 | Brazil | NM | Lab scale | 400 g | + | - | - | Pereira et al., 2017 [25] |
| Saccharomyces cerevisiae | 2018 | Cameroon | Forastero | Wooden box | 200 kg | + | - | - | Mota-Gutierrez et al., 2018 [6] |
| Saccharomyces cerevisiae | 2018 | Cameroon | Forastero | Heap | 100 kg | + | - | - | Mota-Gutierrez et al., 2018 [6] |
| Saccharomyces cerevisiae and T. delbrueckii | 2018 | Cameroon | Forastero | Wooden box | 200 kg | + | - | - | Mota-Gutierrez et al., 2018 [6] |
| Saccharomyces cerevisiae and T. delbrueckii | 2018 | Cameroon | Forastero | Heap | 100 kg | + | - | - | Mota-Gutierrez et al., 2018 [6] |
Abbreviations: NM, not mentioned; F, Fermented cocoa volatile compounds profile; C, Chocolate volatile compounds profile from inoculated cocoa beans; PH16 (Porto hibrido/Sao Jose da Vitoria, Brazil), PS1030 (Porto Seguro/Urucuca, Brazil), FA13 (Angola/Itahuípe Brazil), PS1319 (Bahia, Brazil), CCN51 (Ecuador), CEPEC2004 (Ilhéus/Bahia, Brazil), SJ02 (Bahia, Brazil), Witches broom- resistant varieties; +, Presence; -, Absence; VOCs, volatile organic compounds.
The most frequently used yeast culture in fermented cocoa beans is Saccharomyces cerevisiae. This yeast has the capability to assimilate and ferment reducing sugars and citric acid, produce aroma substances and killer-like toxins, and it has a high pectinolytic activity and can prevent microbial pathogen growth [5,7,10,39,40,41,42]. Despite the well-known Saccharomyces, non-Saccharomyces yeast (Kluyveromyces, Hanseniaspora, Pichia, and Torulaspora) have also shown a relevant pectinolytic activity and increased the aroma complexity in wine [43,44]. However, these species exhibit a lower ethanol yield, and sugar consumption compared to S. cerevisiae [45]. Regardless of this characteristic, several studies have used mixed yeast cultures to inoculate fermented cocoa beans (Table 1) [6,31,46]. However, the combination of different yeast species often results in unpredictable compounds produced and/or different microbial communities, which can affect both the chemical and ecological population of fermented cocoa beans. The unpredictable changes, specifically from the ecological point of view, might be explained by the antagonistic ability of some yeast, such as S. cerevisiae, to inhibit the growth of non-Saccharomyces species (Hanseniaspora guilliermondii, Torulaspora delbrueckii, Kluveromyces marxianus, and Lachancea thermotolerants) by the production of antimicrobial peptides with a 4.0, 4.5, and 6.0 kDa [47]. Therefore, the selection of starter cultures to produce chocolate plays an important role not only in the modulation of the microbial communities, but rather to achieve optimal sensorial properties, such as cocoa, malty, and fruity flavors [6,31,48]. To this regard, future research is needed to elucidate the variability at the strain level that contributes an added value to the cocoa fermentation [25].
2.2. Quality Evaluation of the Chocolate Produce from Inoculated Cocoa Beans
Contrasting findings on the sensory analysis of chocolate produced from cocoa beans inoculated with yeasts has been recently assessed (Table 1) [28,30,31,34,38]. In detail, the consumer panel from Brazil and Malaysia described chocolates inoculated with K. marxianus (Brazil), S. cerevisiae (Malayzia and Brazil), T. delbrueckii, and a mixed culture of S. cerevisiae and T. delbrueckii (Brazil) with better desirable notes, flavor attributes, and global acceptability compared with chocolate produced from spontaneous cocoa bean fermentation [28,31,38]. In contrast, coffee and sour attributes with a worse acceptance were described from the chocolate produced in Brazil inoculated with a mixture of three yeast starters (S. cerevisiae, P. kluyveri, and H. uvarum) during cocoa fermentation [30]. Interestingly, the chocolate produced from different cocoa varieties originated from Brazil inoculated with S. cerevisiae during fermentation were clearly discriminated based on the perceptible attributes of each variety [31,34]. However, the lack of the small number of published studies regarding the sensory analysis of chocolate produced from inoculated cocoa beans with yeast species during fermentation from different countries to improve sensorial attributes are not conclusive.
3. Changes in the Nutrient Composition from Fermented to Roasted Cocoa Beans
The transformation of the nutrient content of cocoa beans during fermentation plays an important role in the development of selected attributes in the final product (chocolate). Fats, proteins, and carbohydrates are the main macronutrients found in cocoa seeds (Table 2) [49,50,51,52,53]. Beans also contain amines that are already present in the unfermented dried cocoa and as expected their amount increased after fermentation and decreased after thermal cocoa processing [54,55]. The first step in processing cocoa beans is to ferment amino acids and oligopeptides, and reduce sugars (Table 2). This step is crucial for the development of the quality cocoa flavor that depends on the balance of organic compounds. In general, the biochemical processes involved over the fermentation and roasting of cocoa beans comprise the hydrolysis of sucrose and proteins, oxidation and hydrolysis of phenolic compounds, biosynthesis of alkaloids, amino acids, release of alcohols (that are also oxidized into acetic and lactic acid), and the breakdown of fatty acids [6,49,50,56,57].
Table 2.
Nutritional composition of cocoa beans expressed as g/kg.
| Source | Origin | Variety | Genetic Material | Carbohydrates | Lipids | Proteins | |||
|---|---|---|---|---|---|---|---|---|---|
| Sucrose | Fructose | Glucose | Total Carbohydrates | ||||||
| Afoakwa et al., 2013 [56] | Ghana | NM | Unfermented | 155.00 | 552.00 | 216.00 | |||
| Efraim et al., 2010 [50] | Brazil | Forastero | Unfermented | 548.20 | 238.80 | ||||
| Afoakwa et al., 2013 [56] | Ghana | NM | Fermented | 210.00 | 534.00 | 188.00 | |||
| Efraim et al., 2010 [50] | Brazil | Forastero | Fermented | 556.00 | 169.90 | ||||
| Redgwell et al., 2003 [52] | Ghana | NM | Dry cocoa beans | 1.58 | 4.18 | 0.62 | |||
| Redgwell et al., 2003 [52] | Ivory Coast | NM | Dry cocoa beans | 1.55 | 2.80 | 0.80 | |||
| Redgwell et al., 2003 [52] | Ecuador | NM | Dry cocoa beans | 4.83 | 1.72 | 0.84 | |||
| Gu et al., 2013 [53] | Papua New Guinea | Trinitario | Roasted | 458.60 | |||||
| Gu et al., 2013 [53] | Indonesia | Trinitario | Roasted | 498.50 | |||||
| Gu et al., 2013 [53] | China | Trinitario | Roasted | 392.40 | |||||
| Gu et al., 2013 [53] | China | Trinitario | Roasted | 434.40 | |||||
| Redgwell et al., 2003 [52] | Ghana | NM | Roasted | 1.41 | 0.60 | 0.05 | |||
| Redgwell et al., 2003 [52] | Ivory Coast | NM | Roasted | 2.03 | 0.44 | 0.05 | 134.40 | ||
| Redgwell et al., 2003 [52] | Ecuador | NM | Roasted | 6.24 | 0.61 | 0.11 | 181.70 | ||
Abbreviations: NM, not mentioned.
3.1. Composition of Volatile Compounds from Cocoa Beans
More than 600 different VOCs have been identified in chocolate flavor. Substances such as aliphatic esters, polyphenols, unsaturated aromatic carbonyls, diketopiperazines, pyrazines, and theobromine are developed, and these compounds provide the characteristic chocolate flavor [49].
3.1.1. VOCs Associated with Inoculated Cocoa Beans
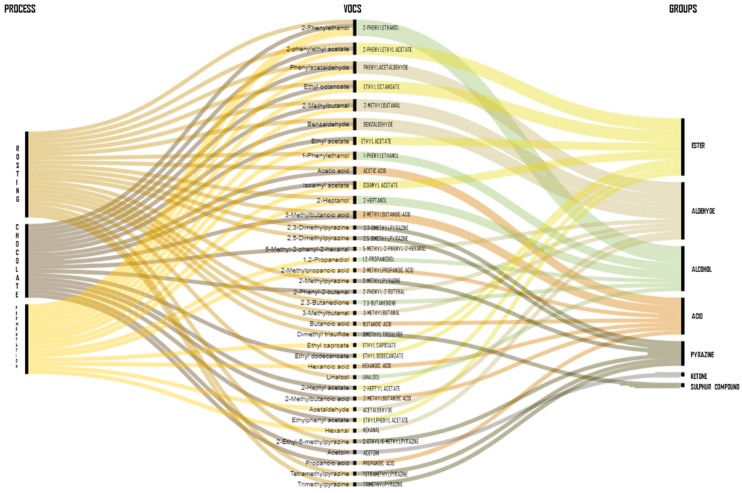
A total of twenty VOCs profiles from inoculated cocoa beans with yeast starters (n = 10) and from chocolate produced from inoculated cocoa beans also with yeasts (n = 10) has been recently reported from ten different cocoa varieties using eleven different yeast strains over the world (Table 1). The identified VOCs from five different cocoa varieties inoculated with different yeasts during fermentation originated from Cameroon and Brazil were used to create a list of all the identified compounds. The data were treated as dummy variables indicating whether the VOCs were identified and only the most frequently reported were used to increase our knowledge of the probable VOCs formed when cocoa beans are inoculated with yeasts [6,25,33]. As expected, esters, alcohols, and aldehydes were the three major VOCs groups characterized in fermented cocoa beans inoculated with yeast (Figure 2).
Figure 2.
Most identified and abundant volatile compounds in fermented and roasted cocoa beans and chocolate.
In detail, the most predominant VOCs among the three studies were ethyl acetate, benzaldehyde, hexanoic acid, and the key aromatic markers for chocolate (2-heptanol, 2-phenylethanol, 2-phenylethyl acetate, and phenylacetaldehyde) [48], while the most abundant compounds at the end of the fermentation were ethyl octanoate, 1-butanol, 1-pentanol, phenylacetaldehyde, ethyl acetate, isoamyl acetate, limonene, and acetic acid (Table 3) [6,25,33]. It is important to highlight that recently, it has been demonstrated that the volatilome profile of cocoa beans fermented in boxes increased the production of alcohols and esters compared to heap fermentations [6]. However, not only the type of fermentation could influence the volatilome profile. It has been shown that the effect of the yeast starter on different cocoa varieties also influences the relative percentage of VOCs, such as 2-phenylethanol and ethyl acetate [33]. Concerning the dynamics of VOCs, it has been reported that the concentrations of limonene-epoxide and 1-butanol decreased over the fermentation time, while ethyl acetate, limonene, benzaldehyde, benzyl alcohol, acetoin, 3-methyl-1-butanol, acetic acid, and the key-aroma markers (phenylacetaldehyde, 2-heptanol and, 2-phenylethanol) increased (Table 3). The development of VOCs during cocoa fermentation and the appropriate selection of starter culture play a crucial role especially for consumers that follow a raw-food diet. Fermented cocoa beans are a suitable food product for this new trend towards raw foods and desirable attributes should also be met after fermentation [58].
Table 3.
Concentration ranges (µg/kg) of volatile compounds of raw, fermented, and roasted cocoa beans and chocolate.
| Volatile Aroma Compounds | Raw Beans [6,33] | End of Fermentation [6,33] | Roasting [59,60,61,62,63,64] | Chocolate [31,34,37] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldehydes | ||||||||||||
| 2-Methylbutanal | 0.70 | - | 1.24 | 0.49 | - | 1.46 | 111.00 | - | 4500.00 | 0.21 | - | 38.30 |
| Acetaldehyde | 0.02 | - | 0.85 | 0.00 | - | 0.18 | 285.00 | - | 285.00 | 0.60 | - | 41.70 |
| Benzaldehyde | 0.21 | - | 0.55 | 0.59 | - | 0.75 | 28.00 | - | 895.00 | 2.77 | - | 53.50 |
| Decanal | 0.03 | - | 0.06 | 0.02 | - | 0.04 | 1.00 | - | 1.00 | |||
| Dodecanal | 0.00 | - | 0.02 | 0.00 | - | 0.01 | 0.10 | - | 0.50 | |||
| Furfural | 0.00 | - | 0.24 | 0.00 | - | 0.25 | 26.00 | - | 87.00 | |||
| Hexanal | 0.02 | - | 3.65 | 0.01 | - | 6.55 | ||||||
| Nonanal | 0.14 | - | 0.19 | 0.09 | - | 0.14 | 46.00 | - | 46.00 | 0.05 | - | 1.52 |
| Phenylacetaldehyde | 4.06 | - | 6.09 | 3.49 | - | 12.37 | 60.00 | - | 5500.00 | 0.06 | - | 0.15 |
| (E)-2-Undecenal | 0.00 | - | 0.01 | 0.00 | - | 0.05 | ||||||
| 2-Phenyl-2-butenal | 0.00 | - | 0.00 | 0.00 | - | 0.05 | ||||||
| Alcohols | ||||||||||||
| (Z)-3-Hexen-1-ol | 0.00 | - | 37.65 | 0.01 | - | 0.02 | ||||||
| 1,2-Propanediol | 0.00 | - | 0.00 | 0.07 | - | 0.35 | 1.10 | - | 1.70 | |||
| 1-Butanol | 3.20 | - | 33.26 | 0.91 | - | 10.50 | ||||||
| 1-Decanol | 0.01 | - | 0.01 | 0.01 | - | 0.01 | ||||||
| 1-Dodecanol | 0.02 | - | 0.17 | 0.05 | - | 0.38 | ||||||
| 1-Heptadecanol | 0.03 | - | 0.10 | 0.06 | - | 0.21 | ||||||
| 1-Heptanol | 0.04 | - | 0.05 | 0.00 | - | 0.00 | 0.03 | - | 0.05 | |||
| 1-Hexanol | 0.21 | - | 0.43 | 0.15 | - | 0.22 | ||||||
| 1-Octanol | 0.06 | - | 0.09 | 0.09 | - | 0.17 | ||||||
| 1-Octen-3-ol | 0.03 | - | 0.05 | 0.00 | - | 0.18 | ||||||
| 1-Pentanol | 0.13 | - | 0.83 | 0.07 | - | 0.14 | ||||||
| 1-Phenylethanol | 0.29 | - | 0.55 | 0.22 | - | 0.34 | ||||||
| 1-Propanol | 0.00 | - | 1.01 | 0.02 | - | 1.02 | ||||||
| 2,3-Butanediol | 0.00 | - | 9.60 | 0.00 | - | 2.07 | 62.00 | - | 356.00 | 35.40 | - | 65.35 |
| 2-Ethyl-1-hexanol | 0.31 | - | 0.49 | 0.14 | - | 0.34 | 0.37 | - | 0.71 | |||
| Furfuryl alcohol | 0.00 | - | 0.00 | 0.00 | - | 10.71 | 0.49 | - | 0.90 | |||
| 2-Heptanol | 0.35 | - | 0.54 | 0.00 | - | 8.97 | 32.00 | - | 1070.00 | 0.00 | - | 0.00 |
| 2-Hexanol | 0.42 | - | 1.13 | 0.07 | - | 0.18 | ||||||
| 2-Methyl-1-butanol | 0.00 | - | 3.36 | 0.00 | - | 2.75 | 0.10 | - | 3.70 | |||
| 2-Methyl-1-propanol | 0.00 | - | 0.22 | 0.00 | - | 10.33 | ||||||
| 2-Nonanol | 0.04 | - | 0.06 | 0.16 | - | 0.78 | 1.00 | - | 1.00 | |||
| 2-Pentanol | 25.70 | - | 47.70 | 1.52 | - | 4.32 | 0.47 | - | 0.47 | |||
| 2-Phenylethanol | 0.31 | - | 0.55 | 0.00 | - | 6.87 | 63.00 | - | 7500.00 | 3.60 | - | 142.00 |
| 3-Methyl-1-butanol | 1.09 | - | 1.30 | 0.88 | - | 1.86 | 27.00 | - | 238.00 | 0.10 | - | 27.10 |
| 3-Methyl-1-pentanol | 0.63 | - | 7.64 | 0.00 | - | 3.08 | ||||||
| Benzyl alcohol | 0.04 | - | 0.05 | 0.03 | - | 0.07 | 104.00 | - | 104.00 | 0.20 | - | 0.23 |
| Ethanol | 2.17 | - | 3.89 | 1.25 | - | 3.81 | 124.00 | - | 124.00 | 4.06 | - | 6.71 |
| Isobutanol | 0.10 | - | 1.54 | 0.06 | - | 0.14 | ||||||
| Methanol | 0.00 | - | 15.74 | 0.00 | - | 24.41 | 9068.00 | - | 9068.00 | |||
| (E)-3-Hexen-1-ol | 0.00 | - | 43.75 | |||||||||
| Acids | ||||||||||||
| 2-Methylpropanoic acid | 0.00 | - | 0.00 | 0.00 | - | 0.60 | 79.00 | - | 79.00 | 7.70 | - | 48.80 |
| 3-Methylbutanoic acid | 0.05 | - | 0.10 | 3.51 | - | 9.20 | 86.00 | - | 9700.00 | 0.10 | - | 48.10 |
| Acetic acid | 0.68 | - | 1.30 | 4.33 | - | 28.40 | 5.60 | - | 330000.00 | 734.00 | - | 2555.70 |
| Butanoic acid | 0.00 | - | 7.36 | 0.00 | - | 13.10 | 21.00 | - | 570.00 | 1.30 | - | 2555.70 |
| Decanoic acid | 0.00 | - | 1.32 | 0.00 | - | 0.00 | ||||||
| Heptanoic acid | 0.00 | - | 9.79 | 0.00 | - | 0.09 | 31.00 | - | 31.00 | |||
| Hexanoic acid | 0.16 | - | 2.71 | 0.00 | - | 0.50 | 116.00 | - | 116.00 | 0.40 | - | 1.47 |
| Nonanoic acid | 0.00 | - | 10.28 | 0.00 | - | 0.00 | 0.10 | - | 0.10 | |||
| Octanoic acid | 0.03 | - | 0.06 | 0.11 | - | 0.27 | ||||||
| Ketones | ||||||||||||
| 2-Heptanone | 0.66 | - | 1.28 | 0.88 | - | 3.61 | 85.00 | - | 140.00 | 1.10 | - | 5.20 |
| 2-Pentanone | 1.55 | - | 9.73 | 1.01 | - | 2.23 | ||||||
| 2-Undecanone | 0.04 | - | 0.05 | 0.00 | - | 0.03 | 1.00 | - | 1.00 | |||
| Acetoin | 0.38 | - | 0.47 | 1.23 | - | 5.98 | 14.00 | - | 1143.00 | 1.99 | - | 505.20 |
| Acetophenone | 1.17 | - | 3.06 | 0.81 | - | 2.31 | 14.00 | - | 225.00 | |||
| Esters | ||||||||||||
| 1,2-Propanediol diacetate | 6.50 | - | 8.11 | 1.21 | - | 2.53 | ||||||
| Isoamyl acetate | 0.00 | - | 56.50 | 0.00 | - | 17.65 | ||||||
| 2,3-Butanediol diacetate | 0.15 | - | 0.30 | 0.03 | - | 1.20 | ||||||
| 2-Pentanol acetate | 1.42 | - | 2.55 | 1.78 | - | 3.93 | ||||||
| Diethyl malate | 0.00 | - | 0.00 | 0.18 | - | 0.44 | ||||||
| Diethyl succinate | 0.06 | - | 11.65 | 0.00 | - | 0.93 | ||||||
| Ethyl acetate | 0.00 | - | 18.45 | 0.00 | - | 22.82 | 66.00 | - | 66.00 | 1.40 | - | 28.90 |
| Ethyl benzoate | 0.00 | - | 0.02 | 0.13 | - | 0.24 | 2.10 | - | 2.10 | |||
| Ethyl butanoate | 0.26 | - | 3.99 | 0.06 | - | 4.18 | ||||||
| Ethyl caproate | 0.17 | - | 0.22 | 0.43 | - | 0.94 | ||||||
| Ethyl dodecanoate | 0.00 | - | 1.64 | 0.38 | - | 1.77 | 24.00 | - | 24.00 | |||
| Ethyl octanoate | 0.00 | - | 0.03 | 0.00 | - | 74.29 | 3.30 | - | 143.00 | 0.09 | - | 19.10 |
| Ethyl pyruvate | 0.00 | - | 0.88 | 1.78 | - | 20.88 | ||||||
| Ethyl-o-toluate | 0.00 | - | 0.00 | 0.33 | - | 0.62 | ||||||
| Furfuryl acetate | 1.59 | - | 27.04 | 0.13 | - | 3.57 | ||||||
| Hexyl acetate | 0.00 | - | 0.01 | 0.00 | - | 0.04 | ||||||
| Isoamyl benzoate | 0.10 | - | 0.19 | 0.02 | - | 0.56 | ||||||
| Isobutyl acetate | 0.14 | - | 1.97 | 0.06 | - | 1.98 | ||||||
| Methyl octanoate | 0.10 | - | 0.15 | 0.00 | - | 0.00 | ||||||
| Mono-ethyl succinate | 0.00 | - | 1.98 | 0.00 | - | 0.52 | ||||||
| Hexyl butanoate | 0.00 | - | 0.05 | 0.00 | - | 0.00 | ||||||
| Phenyl acetate | 0.00 | - | 0.54 | 0.00 | - | 0.14 | ||||||
| α-Phenylethyl acetate | 0.00 | - | 0.45 | 0.17 | - | 0.89 | 34.00 | - | 930.00 | 2.60 | - | 37.10 |
| Propyl acetate | 0.00 | - | 0.09 | 0.00 | - | 1.34 | ||||||
| β-Phenylethyl acetate | 0.03 | - | 0.12 | 0.73 | - | 1.69 | ||||||
| Terpenes | ||||||||||||
| Carveol | 0.01 | - | 0.05 | 0.00 | - | 0.00 | ||||||
| (Z)-Linalool oxide pyranoid | 0.10 | - | 0.15 | 0.06 | - | 0.17 | ||||||
| (Z)-Linalool oxide furanoid | 0.03 | - | 0.10 | 0.00 | - | 0.10 | 21.00 | - | 21.00 | |||
| Nerylacetone | 0.02 | - | 0.04 | 0.00 | - | 0.00 | ||||||
| Limonene | 6.65 | - | 12.37 | 6.43 | - | 30.60 | ||||||
| Geraniol | 0.00 | - | 0.31 | 0.00 | - | 0.00 | ||||||
| Limonene epoxide | 0.29 | - | 0.90 | 0.00 | - | 0.02 | ||||||
| Sabinene | 0.05 | - | 0.17 | 0.05 | - | 0.30 | ||||||
| α-Caryophyllene | 0.08 | - | 0.09 | 0.07 | - | 0.18 | ||||||
| α-Citral | 0.03 | - | 0.10 | 0.02 | - | 0.15 | ||||||
| α-Limonene diepoxide | 0.00 | - | 0.02 | 0.00 | - | 0.02 | ||||||
| β-Caryophyllene | 0.01 | - | 0.02 | 0.01 | - | 0.03 | ||||||
| β-Citronellol | 0.00 | - | 0.00 | 0.00 | - | 0.41 | ||||||
| β-Myrcene | 1.98 | - | 2.32 | 0.96 | - | 3.14 | 66.00 | - | 66.00 | |||
| (E)-β-ocimene | 0.08 | - | 0.34 | 0.06 | - | 0.51 | ||||||
| Lactones | ||||||||||||
| Δ-Decalactone | 0.00 | - | 0.20 | |||||||||
| Other compounds | ||||||||||||
| 1,1-Diethoxyethane | 0.06 | - | 21.65 | 0.12 | - | 5.83 | ||||||
| o-Guaiacol | 0.00 | - | 0.01 | 0.02 | - | 0.62 | 230.00 | - | 230.00 | |||
| Phenol | 0.02 | - | 0.03 | 0.02 | - | 0.37 | 7.00 | - | 7.00 | |||
| trans-Methyl dihydrojasmonate | 0.02 | - | 0.04 | 0.02 | - | 0.04 | ||||||
Values are expressed as concentration ranges (µg/kg). Not statistical analysis was applied due to unbalanced sample size. Different color showed decrease (light blue) or increase (light green) of selected VOC concentrations.
Despite the development of VOCs in inoculated cocoa fermentations, the volatilome profile of chocolate produced from cocoa beans originated from Brazil and Malaysia, inoculated with S. cerevisiae and T. delbrueckii, and a mixed culture of these two yeasts at the beginning of the fermentation has been assessed (Table 1) [31,34,38]. Interesting observations can be made regarding the most frequently identified VOCs in the fermented cocoa beans inoculated with yeasts and the chocolate produced also from inoculated cocoa beans with yeasts, which support the idea that some VOCs produced during fermentation can remain after processing (Figure 2). Remarkably, acetic acid was the most abundant VOC during fermentation and remained the most abundant VOC in chocolate followed by acetoin and 2-phenylethanol (Table 3) [31,34,37].
Several limitations were noted during the collection of the reported VOCs in both the inoculated fermented cocoa beans and the chocolate produced from different inoculated cocoa beans. The first limitation was related to the incongruency of the total number of VOCs and terpenoids reported, whereas some studies have not reported any terpenoids and the total number of VOCs identified vary from 34 to 72 compounds [6,25,31,33,34,37]. Second, studies that identified VOCs in inoculated fermented cocoa beans and chocolate are limited. Although there are no studies that have been tracking the presence of VOCs over the whole chocolate process, this review provides us with an idea of which VOCs are only formed during the fermentation of cocoa beans inoculated with yeast species and could probably remain in the end product. It is worth noting that future research in the identification of VOCs may further increase our knowledge on the role of yeasts, particularly if they increase the production of esters, aldehydes, and terpenoids. This could heighten the positive impacts of yeasts during cocoa fermentation.
3.1.2. Dynamics of VOCs during Roasting
Roasting of cocoa beans is used to diminish moisture and acidity by reducing concentrations of volatile acids such as acetic acid and water [49]. However, the degree of this reduction depends on the time/temperature conditions used [59]. Several chemical reactions such as Maillard and Strecker reactions play an important role during roasting to develop the characteristic aroma and flavor of chocolate [55]. These reactions reduce sugars and amino acids to produce mainly heterocyclic groups such as aldehydes and pyrazines. Indeed, roasting has been shown to be a more effective amine generator than fermentation and it has been observed that the fermentation process supplied precursors for Strecker aldehyde formation. Overall, these reactions also depend on temperature and pH, in which higher temperatures increase amine generation [49,55,59].
Enormous progress is currently being made in the identification of VOCs during roasting [48,59,60,61,62,63,64,65]. In detail, a total of 243 VOCs has recently been reported from three different cocoa varieties originating from ten different countries (Table 4). The most frequently identified and abundant VOCs in roasted cocoa beans are acetic acid, 3-methylbutanoic acid, benzaldehyde, and the key aromatic compounds (2-heptanol, 2-phenylethanol, phenylacetaldehyde, and 2-methylbutanal, Table 3) [59,60,61,62,63,64]. Interestingly, we observed that the key aromatic compounds (2-phenylethyl acetate, phenylacetaldehyde, and 2-heptanol), benzaldehyde, acetic acid, as well as trimethylpirazine and 3-methylbutanal, formed during inoculated fermentations, were still present after the roasting process [48,59,60,61,62,63,64,65].
Table 4.
Overview of the volatile organic compounds of roasted cocoa beans from different origins under different roasting conditions.
| Source | Country | Variety | Equipment | Roasting conditions | |
|---|---|---|---|---|---|
| Temperature (°C) | Time (min) | ||||
| Bonhevi et al., 2005 [60] | Ghana, Cameroon, Ivory Coast, Brazil and Ecuador | NM | GC-MS | 130 | 48 |
| Ramli et al., 2006 [59] | Malaysia | NM | GC-MSD | 150 | 30 |
| Frauendorfer and Shieberle, 2008 [63] | Grenada | Criollo | HRGC-MS | 95 | 14 |
| Huang and Barringer, 2011 [61] | Ecuador | NM | SIFT-MS | 150 | 30 |
| Van Durme et al., 2016 [62] | Ghana and Tanzania | NM | HS-SPME-GC-MS | 150 | 30 |
| Magagna et al., 2018 [65] | Mexico | NM | HS-SPME-GCxGC-MS | 100–130 | 20–40 |
| Tan and Kerr, 2018 [64] | United States of America | Forastero | GC-MS and ANN-based-e-nose | 135 | 0–40 |
| Magagna et al., 2017 [48] | Ecuador and Mexico | Trinitario hybrids | GCxGC-MS, GCx2GC-MS/FID | nm | nm |
Abbreviations: nm: Not mentioned, SIFT-MS: Selected ion flow tube-mass spectrometry, GC-MS: Gas chromatograph-mass spectrometer, ANN: Artificial neural network, GC-MSD: Gas chromatography-Mass selective detector, HRGC-MS: High-resolution gas chromatography-mass spectrometry, HS-SPME: Head-space solid-phase micro-extraction, FID: Flame ionization detector.
In terms of the concentration changes in VOCs during roasting, it has been shown that the key odorants formed during fermentation 2-heptanol, 2-phenylethyl acetate, 2-phenylethanol, butanoic acid, and ethyl 2-methylbutanoate remained nearly constant during the roasting process, while the formation of pyrazines, a by-product of Maillard reaction, mainly occurs during roasting [48,63,64]. It should also be noted that the loss and development of limonene, ethyl acetate, benzaldehyde, and 2-methylbutanal after thermal processing remains unclear.
4. Synthesis of VOCs by Fungal Communities and their Potential Health Benefits
Research on microbial flavor generation has tremendously increased over the last two decades and special attention was given to understand the microbial processes or microbial strategies to produce flavor compounds [25,27,66,67,68,69]. Interestingly, VOCs have been traditionally used and added to food products more for pleasure and consumers’ acceptability than for nutritional reasons. However, microorganisms and their metabolites produced have been also exploited for their tremendous potential to provide health benefits in humans. In fact, it has been recently pointed out the potential health benefits contributed mainly by VOCs in plant foods [3].
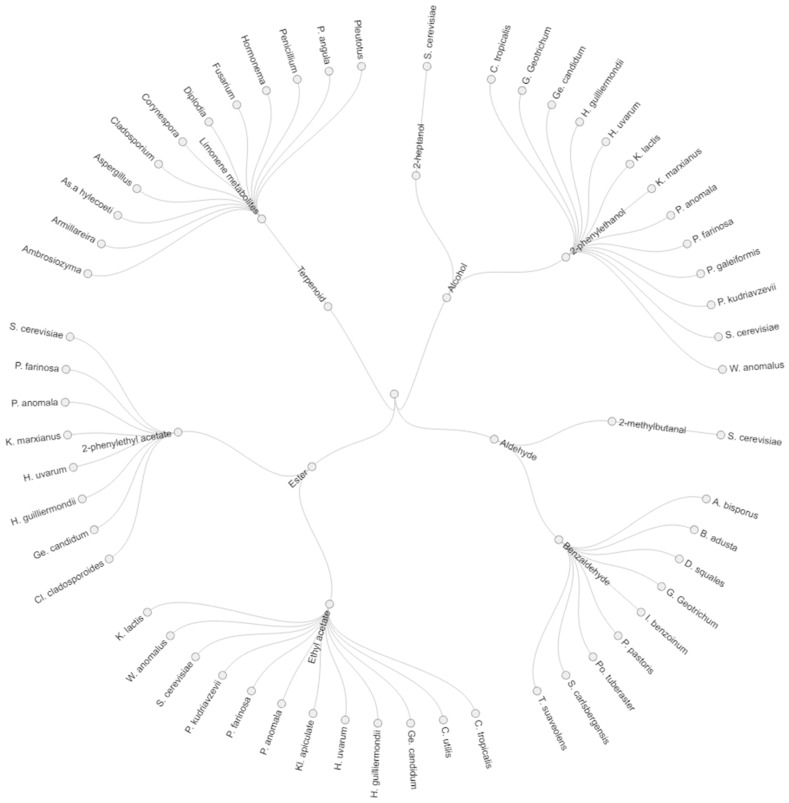
Volatile organic compounds can be synthesized by biological process (microorganisms during fermentation), chemical reactions (synthetic and semi-synthetic) or plant extracts, depending on the type of compound that needs to be synthesized. Concerning biological processes, the microbial metabolism includes the transformation of natural precursor (sugars, organic acids, amino acids, and fatty acids) to a wide range of flavor molecules such as aliphatics, aromatics, terpenes, lactones, O-heterocycles, and S- and N-containing compounds [68]. This review focuses on the formation of six VOCs (2-phenylethanol, phenylacetaldehyde, 2-methylbutanal, benzaldehyde, limonene, and 2-phenylethyl acetate) that are formed during inoculated cocoa fermentations with yeasts, and remain present after roasting, and the potential health benefits of these VOCs. Overall, a total of 36 fungi have been described as producers of the selected VOCs, as shown in Figure 3. In detail, S. cerevisiae, P. anomala, H. uvarum, H. guilliermondii, and Galactomyces geotrichum have been demonstrated to produce the majority of the selected VOCs, while a high variation between species of Candida and Pichia has been reported (Figure 3).
Figure 3.
Yeast producer of selected key aromatic compounds in cocoa beans. Abbreviations: S: Saccharomyces, P: Pichia, C: Candida, G: Galactomyces, Ge: Geotrichum, H: Hanseniaspora, K: Kluyveromyces, W: Wickerhamomyces, A: Agaricus, B: Bjerkandera, D: Dichomitus, I: Ischnoderma, Po: Polyporus, T: Trametes. Kl: Kloeckera, Cl: Cladosporium, As: Ascoide.
Fusel alcohols are generally synthetized by the yeast’s Ehrlich pathway by the conversion of reducing sugars; this pathway contains a three-enzyme cascade that converts valine, leucine, and isoleucine into their corresponding alcohols [68]. The microbial production of L-phenylalanine to 2-phenylethanol (a rose like odor) involves the transamination of the amino acid to phenylpyruvate, decarboxylation to phenylacetaldehyde, and reduction to alcohol by yeast species [27,66,70,71,72,73,74], while the synthesis of secondary alcohols, such as 2-heptanol can be obtained from 2-heptanone (Table 5) [75]. Regarding the potential health benefits, 2-phenylethanol has been demonstrated to inhibit the growth of Gram-negative bacteria and filamentous fungi [76,77].
Table 5.
Summary table of the yeast producer of selected key aromatic compounds in cocoa beans.
| Group | VOCs | Microorganism | Reference |
|---|---|---|---|
| Alcohol | 2-heptanol | Saccharomyces cerevisiae | Cappaert and Laroche, 2004 [75] |
| 2-phenylethanol | Candida tropicalis | Koné et al., 2016 [27] | |
| Galactomyces geotrichum | Koné et al., 2016 [27] | ||
| Geotrichum candidum | Janssens et al., 1992 [66] | ||
| Hanseniaspora guilliermondii | Moreira et al., 2005 [73] | ||
| Hanseniaspora uvarum | Moreira et al., 2005 [73] | ||
| Kluyveromyces lactis | Janssens et al., 1992 [66], Fabre et al., 1997 [74] | ||
| Kluyveromyces marxianus | Janssens et al., 1992 [66], Whittmann et al., 2002 [72], Etschman et al., 2005 [71], Fabre et al., 1997 [74] | ||
| Pichia anomala | Janssens et al., 1992 [66] | ||
| Pichia farinosa | Janssens et al., 1992 [66] | ||
| Pichia galeiformis | Koné et al., 2016 [27] | ||
| Pichia kudriavzevii | Koné et al., 2016 [27] | ||
| Saccharomyces cerevisiae | Kim et al., 2014 [70], Koné et al., 2016 [27], Schwan and Wheals, 2004 [7], Moreira et al., 2005 [73], Fabre et al., 1997 [74] | ||
| Wickerhamomyces anomalus | Koné et al., 2016 [27] | ||
| Aldehydes | 2-methylbutanal | Saccharomyces cerevisiae | Janssens et al., 1992 [66], Larroy et al., 2002 [78] |
| Benzaldehyde | Agaricus bisporus | Janssens et al., 1992 [66] | |
| Bjerkandera adusta | Lapadatescu et al., 1997 [79] | ||
| Dichomitus squales | Lapadatescu et al., 1997 [79] | ||
| Galactomyces geotrichum | Koné et al., 2016 [27] | ||
| Ischnoderma benzoinum | Lapadatescu et al., 1997 [79] | ||
| Pichia pastoris | Berger, 2007 [68] | ||
| Polyporus tuberaster | Kawabe and Morita, 1994 [80] | ||
| Saccharomyces carlsbergensis | Pal et al., 2009 [81] | ||
| Phenylacetaldehyde | Kluyveromyces marxianus | Etschman et al., 2005 [71] | |
| Acetobacter | Berger, 2007 [68] | ||
| Ester | Ethyl acetate | Candida tropicalis | Koné et al., 2016 [27] |
| Candida utilis | Janssens et al., 1992 [66] | ||
| Geotrichum candidum | Janssens et al., 1992 [66] | ||
| Hanseniaspora guilliermondii | Rojas et al., 2001 [82] | ||
| Hanseniaspora uvarum | Rojas et al., 2001 [82] | ||
| Kloeckera apiculate | Schwan and Wheals, 2004 [7] | ||
| Pichia anomala | Janssens et al., 1992 [66], Rojas et al., 2001 [82] | ||
| Pichia farinosa | Janssens et al., 1992 [66] | ||
| Pichia kudriavzevii | Koné et al., 2016 [27], Pereira et al., 2017 [25] | ||
| Saccharomyces cerevisiae | Janssens et al., 1992 [66], Koné et al., 2016 [27], Rojas et al., 2001 [82], Schwan and Wheals, 2004 [7] | ||
| Wickerhamomyces anomalus | Koné et al., 2016 [27] | ||
| Kluyveromyces lactis | Van Laere et al., 2008 [83] | ||
| 2-Phenylethyl acetate | Cladosporium cladosporoides | Janssens et al., 1992 [66] | |
| Geotrichum candidum | Janssens et al., 1992 [66] | ||
| Hanseniaspora guilliermondii | Rojas et al., 2001 [82], Moreira et al., 2005 [73] | ||
| Hanseniaspora uvarum | Rojas et al., 2001 [82] | ||
| Kluyveromyces marxianus | Janssens et al., 1992 [66], Whittmann et al., 2002 [72], Etschman et al., 2005 [71] | ||
| Pichia anomala | Janssens et al., 1992 [66], Rojas et al., 2001 [82] | ||
| Pichia farinosa | Janssens et al., 1992 [66] | ||
| Saccharomyces cerevisiae | Kone et al., 2016 [27], Rojas et al., 2001 [82] | ||
| Terpenoid | Limonene | Ascoidea hylecoeti | Janssens et al., 1992 [66] |
| Limonene metabolites (terpineol, verbenol) |
Armillareira,
Aspergillus Cladosporium |
Duetz et al., 2003 [84], Janssens et al., 1992 [66] | |
| Limonene metabolites (limonene-1,2-epoxide) |
Corynespora
Diplodia |
Duetz et al., 2003 [84] | |
| Limonene metabolites (verbenone) | Hormonema | Berger, 2007 [68] | |
| Limonene metabolites (carvone, carveol) |
Penicillium
Pleutotus |
Janssens et al., 1992 [66], Duetz et al., 2003 [84] | |
| Limonene metabolites |
Pichia angula
Ambrosiozyma Fusarium |
Janssens et al., 1992 [66] Berger, 2007 [68] |
Besides 2-phenylethanol, other VOCs such as benzaldehyde and its derivates have been used as preservatives [85,86]. However, this compound is also able to induce antitumor activity in human cells [85,86,87,88] and antioxidant activity [53,89]. Concerning the conversion of benzyl alcohol or L-phenylalanine into benzaldehyde, this conversion has been attributed not only to yeasts but also to the basidiomycetes’ activity (Table 5) [27,66,79,80,81]. In general, aldehydes can be produced by the oxidation of alcohols such as 2-methylbutanol, 3-methylbutanol, and 2-methyl-1-propanol derived from short-chain aliphatic aldehydes such as acetaldehyde, 2-methyl-1-propanal, 2-methylbutanal, and 3-methylbutanal efficiently produced by the metabolism of yeast [66,78].
Regarding the biosynthesis and conversion of monoterpenes, it has been associated with the basidiomycetes’ metabolism. Limonene is produced by plants as a defense for pathogens, and this transforms into other monoterpenoids such as carvone, terpineol, perillyl alcohol, limonene epoxide, and verbenone, which can be associated with the activity of several fungal species (Table 5) [66,68,84]. Interestingly, recent in vivo and in vitro studies have reported anticarcinogenic and antinociceptive activity of limonene [90,91,92,93,94,95,96], and this compound has also been used as a preservative [97].
Last but not least, the well-known ester 2-phenylethyl acetate is recognized for its antimicrobial activity [98]. In general, the biotransformation of esters includes a more complex catabolic reaction, and it comprises the esterification of amino acids or short-chain aliphatic fatty acid and terpenyl alcohol into the desired flavor ester. The transformation of 2-phenylethyl acetate is usually metabolized from amino acids, such as phenylalanine and/or phenylpyruvic acid also from yeast species (Table 5) [66,73,82]. Besides 2-phenylethyl acetate, ethyl acetate is formed from the esterification of leucine, isoleucine or valine, and a natural aliphatic alcohol has been attributed to the activity of yeast (Table 5) [7,25,27,73,82,83].
Overall, the potential health effect of the selected VOCs synthesized by chemical reactions or biological processes have been linked to prevent or delay diseases or the growth of undesirable microorganisms. In summary, it has been reported from in vivo and in vitro studies the anticarcinogenic and the antinociceptive activity of limonene, [90,91,92,93,94,95,96], the antitumor activity of benzaldehyde [85,86,87,88], and antioxidant activity of benzaldehyde and its derivates [99,100]. In addition, 2-phenylethanol [76,77,101], 2-phenylethyl acetate [98], limonene [97], benzaldehydes, and derivates [89,99,100,102] have been widely used as preservatives.
While most studies have focused on describing the capacity of VOCs to prevent, slow or inhibit the growth of microorganisms, tumors, or cells to provide health benefits, recent literature has demonstrated the capacity of these compounds to stimulate communication with the limbic system of the brain via neurons through oral routes and olfactory receptors in the nose, which changed mood and emotions by creating a sedative effect for the reduction of stress and anxiety, and finally by reducing the pain perception [103]. On the other hand, dysfunction of the chemosensory activities were highly related to differences in dietary behaviors, including loss of appetite, unintended weight loss, malnutrition, and well-known psychiatric and neurological disorders [104,105,106,107,108,109]. More important is the fact that this loss has been reported to affect the general population and it remains undiagnosed in some patients [104,110]. In this regard, 2-phenylethanol has been used to counteract the olfactory dysfunction due to multiple etiologies [111,112,113,114,115,116,117]. However, the mechanism of action of the improvements of the smell progresses and the association of chemosensory function with dietary and health outcomes remains unclear. There is no doubt that individuals with this dysfunction, highly observed in neurological diseases such as Parkinson’s, are more likely to experience a hazardous event and are the major concern for public health. Considering the positive effect of the single compounds, also produced by microbial communities during cocoa fermentation, this review hypothesizes that the consumption of chocolate produced from inoculated cocoa beans with yeasts could provide a positive health effect to consumers. However, more comprehensive studies are required to confirm the potential effect of VOCs from chocolate in human health.
In terms of international legal regulations, according to the Join FAO/WHO expert committee on food additives, all the VOCs proposed in this review are categorized as flavoring agents and do not represent a safety concern since they are predictably metabolized efficiently into innocuous products and their estimated daily intake are below the threshold for daily human intake [118].
5. Conclusions
Microbial communities in, on, and around our food are essential for exploring the interaction between the food system and its inter-connectedness with human health. Tracking the production of functional compounds produced by microbes will serve to improve the formation of desirable compounds. Future perspectives on the selection of the best candidate starter cultures possessing genes coding for oral usage to acquire desirable compounds and the mechanism underlying flavor perception linked to nutritional or health values need to be assessed. The findings of the present review and future analyses of VOCs may help to inform researchers, policy makers, the chocolate industry, and the general public to explore yeasts as proper producers of important VOCs to improve quality and health.
Author Contributions
Conceptualization, J.M.-G., L.C.; Validation, L.C., I.F., L.B.-P.; Investigation, J.M.-G., L.B.-P.; Writing-original Draft Preparation, J.M.-G.; Writing; Review and Editing, L.C., I.F., L.B.-P.; Supervision, L.C.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Schmieder R., Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27:863–864. doi: 10.1093/bioinformatics/btr026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun-Waterhouse D., Wadhwa S.S. Industry-relevant approaches for minimising the bitterness of bioactive compounds in functional foods: A Review. Food Bioprocess Technol. 2013;6:607–627. doi: 10.1007/s11947-012-0829-2. [DOI] [Google Scholar]
- 3.Ayseli M.T., Ipek Ayseli Y. Flavors of the future: Health benefits of flavor precursors and volatile compounds in plant foods. Trends Food Sci. Technol. 2016;48:69–77. doi: 10.1016/j.tifs.2015.11.005. [DOI] [Google Scholar]
- 4.Gobbetti M., Di Cagno R., de Angelis M. Functional microorganisms for functional food quality. Crit. Rev. Food Sci. Nutr. 2010;50:716–727. doi: 10.1080/10408398.2010.499770. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen D.S., Teniola O.D., Ban-Koffi L., Owusu M., Andersson T.S., Holzapfel W.H. The microbiology of Ghanaian cocoa fermentations analysed using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 2007;114:168–186. doi: 10.1016/j.ijfoodmicro.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Mota-Gutierrez J., Botta C., Ferrocino I., Giordano M., Bertolino M., Dolci P., Cannoni M., Cocolin L. Dynamics and biodiversity of bacterial and yeast communities during the fermentation of cocoa beans. Appl. Environ. Microbiol. 2018:AEM.01164-18. doi: 10.1128/AEM.01164-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwan R.F., Wheals A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 2004;44:205–221. doi: 10.1080/10408690490464104. [DOI] [PubMed] [Google Scholar]
- 8.Camu N., De Winter T., Verbrugghe K., Cleenwerck I., Vandamme P., Takrama J.S., Vancanneyt M., De Vuyst L. Dynamics and biodiversity of populations of lactic acid bacteria and acetic acid bacteria involved in spontaneous heap fermentation of cocoa beans in Ghana. Appl. Environ. Microbiol. 2007;73:1809–1824. doi: 10.1128/AEM.02189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camu N., De Winter T., Van Schoor A., De Bruyne K., Vandamme P., Takrama J.S., Addo S.K., De Vuyst L. Influence of turning and environmental contamination on the dynamics of populations of lactic acid and acetic acid bacteria involved in spontaneous cocoa bean heap fermentation in Ghana. Appl. Environ. Microbiol. 2008;74:86–98. doi: 10.1128/AEM.01512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jespersen L., Nielsen D.S., Hønholt S., Jakobsen M. Occurrence and diversity of yeasts involved in fermentation of West African cocoa beans. FEMS Yeast Res. 2005;5:441–453. doi: 10.1016/j.femsyr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Papalexandratou Z., Vrancken G., De Bruyne K., Vandamme P., De Vuyst L. Spontaneous organic cocoa bean box fermentations in Brazil are characterized by a restricted species diversity of lactic acid bacteria and acetic acid bacteria. Food Microbiol. 2011;28:1326–1338. doi: 10.1016/j.fm.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Papalexandratou Z., De Vuyst L. Assessment of the yeast species composition of cocoa bean fermentations in different cocoa-producing regions using denaturing gradient gel electrophoresis. FEMS Yeast Res. 2011;11:564–574. doi: 10.1111/j.1567-1364.2011.00747.x. [DOI] [PubMed] [Google Scholar]
- 13.Kostinek M., Ban-Koffi L., Ottah-Atikpo M., Teniola D., Schillinger U., Holzapfel W.H., Franz C.M.A.P. Diversity of predominant lactic acid bacteria associated with cocoa fermentation in Nigeria. Curr. Microbiol. 2008;56:306–314. doi: 10.1007/s00284-008-9097-9. [DOI] [PubMed] [Google Scholar]
- 14.Miescher Schwenninger S., Freimüller Leischtfeld S., Gantenbein-Demarchi C. High-throughput identification of the microbial biodiversity of cocoa bean fermentation by MALDI-TOF MS. Lett. Appl. Microbiol. 2016;63:347–355. doi: 10.1111/lam.12621. [DOI] [PubMed] [Google Scholar]
- 15.Ho V.T.T., Zhao J., Fleet G. The effect of lactic acid bacteria on cocoa bean fermentation. Int. J. Food Microbiol. 2015;205:54–67. doi: 10.1016/j.ijfoodmicro.2015.03.031. [DOI] [PubMed] [Google Scholar]
- 16.García-Alamilla P., Lagunes-Gálvez L.M., Barajas-Fernández J., García-Alamilla R., Garcia-Armisen T., Papalexandratou Z., Hendryckx H., Camu N., Vrancken G., De Vuyst L., et al. Diversity of the total bacterial community associated with Ghanaian and Brazilian cocoa bean fermentation samples as revealed by a 16 S rRNA gene clone library. Appl. Microbiol. Biotechnol. 2010;87:2281–2292. doi: 10.1007/s00253-010-2698-9. [DOI] [PubMed] [Google Scholar]
- 17.Papalexandratou Z., Falony G., Romanens E., Jimenez J.C., Amores F., Daniel H., De Vuyst L. Species diversity, community dynamics, and metabolite kinetics of the microbiota associated with traditional Ecuadorian spontaneous cocoa bean fermentations. Appl. Environ. Microbiol. 2011;77:7698–7714. doi: 10.1128/AEM.05523-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papalexandratou Z., Lefeber T., Bahrim B., Seng O., Daniel H., De Vuyst L. Acetobacter pasteurianus predominate during well-performed Malaysian cocoa bean box fermentations, underlining the importance of these microbial species for a successful cocoa bean fermentation process. Food Microbiol. 2013;35:73–85. doi: 10.1016/j.fm.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Bortolini C., Patrone V., Puglisi E., Morelli L. Detailed analyses of the bacterial populations in processed cocoa beans of different geographic origin, subject to varied fermentation conditions. Int. J. Food Microbiol. 2016;236:98–106. doi: 10.1016/j.ijfoodmicro.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Miguel M.G.D.C.P., de Castro Reis L.V., Efraim P., Santos C., Lima N., Schwan R.F. Cocoa fermentation: Microbial identification by MALDI-TOF MS, and sensory evaluation of produced chocolate. LWT—Food Sci. Technol. 2017;77 doi: 10.1016/j.lwt.2016.11.076. [DOI] [Google Scholar]
- 21.de Melo Pereira G.V., Magalhães K.T., de Almeida E.G., da Silva Coelho I., Schwan R.F. Spontaneous cocoa bean fermentation carried out in a novel-design stainless steel tank: Influence on the dynamics of microbial populations and physical-chemical properties. Int. J. Food Microbiol. 2013;161:121–133. doi: 10.1016/j.ijfoodmicro.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Illeghems K., De Vuyst L., Papalexandratou Z., Weckx S. Phylogenetic analysis of a spontaneous cocoa bean fermentation metagenome reveals new insights into its bacterial and fungal community diversity. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0038040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Veiga Moreira I.M., Gabriela M., Miguel P., Ferreira W., Ribeiro D., Freitas R. Microbial succession and the dynamics of metabolites and sugars during the fermentation of three different cocoa (Theobroma cacao L.) hybrids. Food Res. Int. 2013;54:9–17. doi: 10.1016/j.foodres.2013.06.001. [DOI] [Google Scholar]
- 24.Hamdouche Y., Guehi T., Durand N., Kedjebo K.B.D., Montet D., Meile J.C. Dynamics of microbial ecology during cocoa fermentation and drying: Towards the identification of molecular markers. Food Control. 2015;48:117–122. doi: 10.1016/j.foodcont.2014.05.031. [DOI] [Google Scholar]
- 25.Pereira G.V.M., Alvarez J.P., de Neto D.P.C., Soccol V.T., Tanobe V.O.A., Rogez H., Góes-Neto A., Soccol C.R. Great intraspecies diversity of Pichia kudriavzevii in cocoa fermentation highlights the importance of yeast strain selection for flavor modulation of cocoa beans. LWT—Food Sci. Technol. 2017;84:290–297. doi: 10.1016/j.lwt.2017.05.073. [DOI] [Google Scholar]
- 26.Arana-Sánchez A., Segura-García L.E., Kirchmayr M., Orozco-Ávila I., Lugo-Cervantes E., Gschaedler-Mathis A. Identification of predominant yeasts associated with artisan Mexican cocoa fermentations using culture-dependent and culture-independent approaches. World J. Microbiol. Biotechnol. 2015;31:359–369. doi: 10.1007/s11274-014-1788-8. [DOI] [PubMed] [Google Scholar]
- 27.Koné M.K., Guéhi S.T., Durand N., Ban-kof L., Berthiot L., Tachon A.F., Brou K., Boulanger R., Montet D. Contribution of predominant yeasts to the occurrence of aroma compounds during cocoa bean fermentation. Food Res. Int. 2016;89:910–917. doi: 10.1016/j.foodres.2016.04.010. [DOI] [Google Scholar]
- 28.Leal G.A., Gomes L.H., Efraim P., De Almeida Tavares F.C., Figueira A. Fermentation of cacao (Theobroma cacao L.) seeds with a hybrid Kluyveromyces marxianus strain improved product quality attributes. FEMS Yeast Res. 2008;8:788–798. doi: 10.1111/j.1567-1364.2008.00405.x. [DOI] [PubMed] [Google Scholar]
- 29.Crafack M., Mikkelsen M.B., Saerens S., Knudsen M., Blennow A., Lowor S., Takrama J., Swiegers J.H., Petersen G.B., Heimdal H., et al. Influencing cocoa flavour using Pichia kluyveri and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int. J. Food Microbiol. 2013;167:103–116. doi: 10.1016/j.ijfoodmicro.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Batista N.N., Ramos C.L., Ribeiro D.D., Pinheiro A.C.M., Schwan R.F. Dynamic behavior of Saccharomyces cerevisiae, Pichia kluyveri and Hanseniaspora uvarum during spontaneous and inoculated cocoa fermentations and their effect on sensory characteristics of chocolate. LWT—Food Sci. Technol. 2015;63:221–227. doi: 10.1016/j.lwt.2015.03.051. [DOI] [Google Scholar]
- 31.Visintin S., Ramos L., Batista N., Dolci P., Schwan F., Cocolin L. Impact of Saccharomyces cerevisiae and Torulaspora delbrueckii starter cultures on cocoa beans fermentation. Int. J. Food Microbiol. 2017;257:31–40. doi: 10.1016/j.ijfoodmicro.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Fleet G.H. Yeasts in foods and beverages: Impact on product quality and safety. Curr. Opin. Biotechnol. 2007;18:170–175. doi: 10.1016/j.copbio.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Ramos C.L., Dias D.R., Miguel M.G.D.C.P., Schwan R.F. Impact of different cocoa hybrids (Theobroma cacao L.) and S. cerevisiae UFLA CA11 inoculation on microbial communities and volatile compounds of cocoa fermentation. Food Res. Int. 2014;64:908–918. doi: 10.1016/j.foodres.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 34.Menezes A.G.T., Batista N.N., Ramos C.L., e Silva A.R.D.A., Efraim P., Pinheiro A.C.M., Schwan R.F. Investigation of chocolate produced from four different Brazilian varieties of cocoa (Theobroma cacao L.) inoculated with Saccharomyces cerevisiae. Food Res. Int. 2016;81:83–90. doi: 10.1016/j.foodres.2015.12.036. [DOI] [Google Scholar]
- 35.Cempaka L., Aliwarga L., Purwo S., Kresnowati M.T.A.P. Dynamics of cocoa bean pulp degradation during cocoa bean fermentation: Effects of yeast starter culture addition. J. Math. Fundam. Sci. 2014;46:14–25. doi: 10.5614/j.math.fund.sci.2014.46.1.2. [DOI] [Google Scholar]
- 36.Mahazar N.H., Sufian N.F., Meor Hussin A.S., Norhayati H., Mathawan M., Rukayadi Y. Candida sp. as a starter culture for cocoa (Theobroma cacao L.) beans fermentation. Int. Food Res. J. 2015;22:1783–1787. [Google Scholar]
- 37.Meersman E., Steensels J., Struyf N., Paulus T., Saels V., Mathawan M., Allegaert L., Vrancken G., Verstrepen K.J. Tuning chocolate flavor through development of thermotolerant Saccharomyces cerevisiae starter cultures with increased acetate ester. Appl. Environ. Microbiol. 2016;82:732–746. doi: 10.1128/AEM.02556-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meersman E., Steensels J., Paulus T., Struyf N., Saels V., Mathawan M., Koffi J., Vrancken G., Verstrepen K.J. Breeding strategy to generate robust yeast starter cultures for cocoa pulp fermentations. Appl. Environ. Microbiol. 2015;81:6166–6176. doi: 10.1128/AEM.00133-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniel H.M., Vrancken G., Takrama J.F., Camu N., De Vos P., De Vuyst L. Yeast diversity of Ghanaian cocoa bean heap fermentations. FEMS Yeast Res. 2009;9:774–783. doi: 10.1111/j.1567-1364.2009.00520.x. [DOI] [PubMed] [Google Scholar]
- 40.de Melo Pereira G.V., Miguel M.G.D.C.P., Ramos C.L., Schwan R.F. Microbiological and physicochemical characterization of small-scale cocoa fermentations and screening of yeast and bacterial strains to develop a defined starter culture. Appl. Environ. Microbiol. 2014;78:5395–5405. doi: 10.1128/AEM.01144-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samagaci L., Ouattara H., Niamké S., Lemaire M. Pichia kudrazevii and Candida nitrativorans are the most well-adapted and relevant yeast species fermenting cocoa in Agneby-Tiassa, a local Ivorian cocoa producing region. Food Res. Int. 2016;89:773–780. doi: 10.1016/j.foodres.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 42.Branco P., Francisco D., Chambon C., Hébraud M., Arneborg N., Almeida M.G., Caldeira J., Albergaria H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl. Microbiol. Biotechnol. 2014;98:843–853. doi: 10.1007/s00253-013-5411-y. [DOI] [PubMed] [Google Scholar]
- 43.Jayani R.S., Saxena S., Gupta R. Microbial pectinolytic enzymes: A review. Process Biochem. 2005;40:2931–2944. doi: 10.1016/j.procbio.2005.03.026. [DOI] [Google Scholar]
- 44.Whitener M.E.B., Stanstrup J., Carlin S., Divol B., Du Toit M., Vrhovsek U. Effect of non-Saccharomyces yeasts on the volatile chemical profile of Shiraz wine. Aust. J. Grape Wine Res. 2017;23:179–192. doi: 10.1111/ajgw.12269. [DOI] [Google Scholar]
- 45.Contreras A., Hidalgo C., Henschke P.A., Chambers P.J., Curtin C., Varela C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014;80:1670–1678. doi: 10.1128/AEM.03780-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Batista N.N., Ramos C.L., Dias D.R. The impact of yeast starter cultures on the microbial communities and volatile compounds in cocoa fermentation and the resulting sensory attributes of chocolate. J. Food Sci. Technol. 2016;53:1101–1110. doi: 10.1007/s13197-015-2132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albergaria H., Francisco D., Gori K., Arneborg N., Gírio F. Saccharomyces cerevisiae CCMI 885 secretes peptides that inhibit the growth of some non-Saccharomyces wine-related strains. Appl. Microbiol. Biotechnol. 2010;86:965–972. doi: 10.1007/s00253-009-2409-6. [DOI] [PubMed] [Google Scholar]
- 48.Magagna F., Guglielmetti A., Liberto E., Reichenbach S.E., Allegrucci E., Gobino G., Bicchi C., Cordero C. Comprehensive chemical fingerprinting of high-quality cocoa at early stages of processing: Effectiveness of combined untargeted and targeted approaches for classification and discrimination. J. Agric. Food Chem. 2017;65:6329–6341. doi: 10.1021/acs.jafc.7b02167. [DOI] [PubMed] [Google Scholar]
- 49.Afoakwa E.O., Paterson A., Fowler M., Ryan A., Ohene E., Paterson A., Fowler M., Ryan A., Fowler M., Ryan A. Flavor formation and character in cocoa and chocolate: A critical review. Crit. Rev. Food Sci. Nutr. 2008;48:840–857. doi: 10.1080/10408390701719272. [DOI] [PubMed] [Google Scholar]
- 50.Efraim P., Pezoa-García N.H., Calil D., Jardim P., Nishikawa A., Haddad R., Eberlin M.N. Influência da fermentação e secagem de amêndoas de cacau no teor de compostos fenólicos e na aceitação sensorial. Ciência Tecnol. Aliment. 2010;30:142–150. doi: 10.1590/S0101-20612010000500022. [DOI] [Google Scholar]
- 51.Caligiani A., Cirlini M., Palla G., Ravaglia R., Arlorio M. GC-MS detection of chiral markers in cocoa beans of different quality and geographic origin. Chirality Pharmacol. Biol. Chem. Consequences Mol. Asymmetry. 2007;334:329–334. doi: 10.1002/chir.20380. [DOI] [PubMed] [Google Scholar]
- 52.Redgwell R.J., Trovato V., Curti D. Cocoa bean carbohydrates: Roasting-induced changes and polymer interactions. Food Chem. 2003;80:511–516. doi: 10.1016/S0308-8146(02)00320-5. [DOI] [Google Scholar]
- 53.Gu F., Tan L., Wu H., Fang Y., Xu F., Chu Z., Wang Q. Comparison of cocoa beans from China, Indonesia and Papua New Guinea. Foods. 2013;2:183–197. doi: 10.3390/foods2020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pätzold R., Brückner H., Oracz J., Nebesny E., Sukha D.A., Butler D.R., Umaharan P., Boult E., Miescher Schwenninger S., Freimüller Leischtfeld S., et al. Assessment methodology to predict quality of cocoa beans for export. J. Agric. Food Chem. 2008;56:773–780. doi: 10.1002/chir. [DOI] [Google Scholar]
- 55.Granvogl M., Bugan S., Schieberle P. Formation of amines and aldehydes from parent amino acids during thermal processing of cocoa and model systems: New insights into pathways of the strecker reaction. J. Agric. Food Chem. 2006;54:1730–1739. doi: 10.1021/jf0525939. [DOI] [PubMed] [Google Scholar]
- 56.Afoakwa E.O., Quao J., Takrama J., Budu A.S. Chemical composition and physical quality characteristics of Ghanaian cocoa beans as affected by pulp pre-conditioning and fermentation. J. Food Sci. Technol. 2013;50:1097–1105. doi: 10.1007/s13197-011-0446-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Afoakwa E.O., Kongor J.E., Takrama J.F., Budu A.S. Changes in acidification, sugars and mineral composition of cocoa pulp during fermentation of pulp pre-conditioned cocoa (Theobroma cacao) beans. Int. Food Res. J. 2013;20:1215–1222. [Google Scholar]
- 58.Scott-Thomas C. Raw Food on the Rise. [(accessed on 15 January 2019)]; Available online: https://www.foodnavigator.com/Article/2015/06/09/Raw-food-on-the-rise.
- 59.Ramli N., Hassan O., Said M., Samsudin W., Idris N.A. Influence of roasting conditions on volatile flavor of roasted Malaysian cocoa beans. J. Food Process. Preserv. 2006;30:280–298. doi: 10.1111/j.1745-4549.2006.00065.x. [DOI] [Google Scholar]
- 60.Bonhevi J.S. Investigation of aromatic compounds in roasted cocoa powder. Eur. Food Res. Tecnhonol. 2005;221:19–29. doi: 10.1007/s00217-005-1147-y. [DOI] [Google Scholar]
- 61.Huang Y., Barringer S.A. Monitoring of cocoa volatiles produced during roasting by selected ion flow tube-mass spectrometry (SIFT-MS) J. Food Sci. 2011;76:279–286. doi: 10.1111/j.1750-3841.2010.01984.x. [DOI] [PubMed] [Google Scholar]
- 62.Van Durme J., Ingels I., De Winne A. Online roasting hyphenated with gas chromatography-mass spectrometry as an innovative approach for assessment of cocoa fermentation quality and aroma formation potential. Food Chem. 2016;205:66–72. doi: 10.1016/j.foodchem.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 63.Frauendorfer F., Schieberle P. Changes in key aroma compounds of criollo cocoa. J. Agric. Food Chem. 2008;56:10244–10251. doi: 10.1021/jf802098f. [DOI] [PubMed] [Google Scholar]
- 64.Tan J., Kerr W.L. Determining degree of roasting in cocoa beans by artificial neural network (ANN)-based electronic nose system and gas chromatography/mass spectrometry (GC/MS) J. Sci. Food Agric. 2018;98:3851–3859. doi: 10.1002/jsfa.8901. [DOI] [PubMed] [Google Scholar]
- 65.Magagna F., Liberto E., Reichenbach S.E., Tao Q., Carretta A., Cobelli L., Giardina M., Bicchi C., Cordero C. Advanced fingerprinting of high-quality cocoa: Challenges in transferring methods from thermal to differential-flow modulated comprehensive two dimensional gas chromatography. J. Chromatogr. A. 2018;1536:122–136. doi: 10.1016/j.chroma.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 66.Janssens L., De Pooter H.L., Schamp N.M., Vandamme E.J. Production of flavours by microorganisms. Process Biochem. 1992;27:195–215. doi: 10.1016/0032-9592(92)80020-4. [DOI] [Google Scholar]
- 67.Hagedorn S., Kaphammer B. Microbial biocatalysis in the generation of flavor and fragrance chemicals. Annu. Rev. Microbiol. 1994;46:1561–1563. doi: 10.1146/annurev.mi.48.100194.004013. [DOI] [PubMed] [Google Scholar]
- 68.Berger R.G. Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability. Springer; Berlin, Germany: 2007. [Google Scholar]
- 69.Schrader J., Etschmann M.M.W., Sell D., Hilmer J.-M., Rabenhorst J. Applied biocatalysis for the synthesis of natural flavour compounds: Curent industrial processes and future prospects. Biotechnol. Lett. 2004:463–472. doi: 10.1023/B:BILE.0000019576.80594.0e. [DOI] [PubMed] [Google Scholar]
- 70.Kim B., Cho B.R., Hahn J.S. Metabolic engineering of Saccharomyces cerevisiae for the production of 2-phenylethanol via Ehrlich pathway. Biotechnol. Bioeng. 2014;111:115–124. doi: 10.1002/bit.24993. [DOI] [PubMed] [Google Scholar]
- 71.Etschmann M.M.W., Sell D., Schrader J. Production of 2-phenylethanol and 2-phenylethylacetate from L-phenylalanine by coupling whole-cell biocatalysis with organophilic pervaporation. Biotechnol. Bioeng. 2005;92:624–634. doi: 10.1002/bit.20655. [DOI] [PubMed] [Google Scholar]
- 72.Wittmann C., Hans M., Bluemke W. Metabolic physiology of aroma-producing Kluyveromyces marxianus. Yeast. 2002;19:1351–1363. doi: 10.1002/yea.920. [DOI] [PubMed] [Google Scholar]
- 73.Moreira N., Mendes F., Hogg T., Vasconcelos I. Alcohols, esters and heavy sulphur compounds production by pure and mixed cultures of apiculate wine yeasts. Int. J. Food Microbiol. 2005;103:285–294. doi: 10.1016/j.ijfoodmicro.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 74.Fabre C.E., Blanc P.J., Goma G. Screening of yeasts producing 2-phenylethylalcohol. Biotechnol. Tech. 1997;11:523–525. doi: 10.1023/A:1018422302176. [DOI] [Google Scholar]
- 75.Cappaert L., Larroche C. Oxidation of a mixture of 2-(R) and 2-(S)-heptanol to 2-heptanone by Saccharomyces cerevisiae in a biphasic system. Biocatal. Biotransform. 2004;22:291–296. doi: 10.1080/10242420400011992. [DOI] [Google Scholar]
- 76.Chen H., Fink G.R. Feedback control of morphogenesis in fungi by aromatic alcohols. Genes Dev. 2006;20:1150–1161. doi: 10.1101/gad.1411806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fraud S., Rees E.L., Mahenthiralingam E., Russell A.D., Maillard J.Y. Aromatic alcohols and their effect on Gram-negative bacteria, cocci and mycobacteria (1) J. Antimicrob. Chemother. 2003;51:1435–1436. doi: 10.1093/jac/dkg246. [DOI] [PubMed] [Google Scholar]
- 78.Larroy C., Parés X., Biosca J.A. Characterization of a Saccharomyces cerevisiae NADP(H)-dependent alcohol dehydrogenase (ADHVII), a member of the cinnamyl alcohol dehydrogenase family. Eur. J. Biochem. 2002;269:5738–5745. doi: 10.1046/j.1432-1033.2002.03296.x. [DOI] [PubMed] [Google Scholar]
- 79.Lapadatescu C., Feron G., Vergoignan C., Djian A., Durand A., Bonnarme P. Influence of cell immobilization on the production of benzaldehyde and benzyl alcohol by the white-rot fungi Bjerkandera adusta, Ischnoderma benzoinum and Dichomitus squalens. Appl. Microbiol. Biotechnol. 1997;47:708–714. doi: 10.1007/s002530050999. [DOI] [Google Scholar]
- 80.Kawabe T., Morita H. Production of benzaldehyde and benzyl alcohol by the mushroom Polyporus tuberaster K2606. J. Agric. Food Chem. 1994;42:2556–2560. doi: 10.1021/jf00047a034. [DOI] [Google Scholar]
- 81.Pal S., Park D.H., Plapp B.V. Activity of yeast alcohol dehydrogenases on benzyl alcohols and benzaldehydes. Characterization of ADH1 from Saccharomyces carlsbergensis and transition state analysis. Chem. Biol. Interact. 2009;178:16–23. doi: 10.1016/j.cbi.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rojas V., Gil J.V., Piñaga F., Manzanares P. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int. J. Food Microbiol. 2001;70:283–289. doi: 10.1016/S0168-1605(01)00552-9. [DOI] [PubMed] [Google Scholar]
- 83.Van Laere S.D.M., Saerens S.M.G., Verstrepen K.J., Van Dijck P., Thevelein J.M., Delvaux F.R. Flavour formation in fungi: Characterisation of KlAtf, the Kluyveromyces lactis orthologue of the Saccharomyces cerevisiae alcohol acetyltransferases Atf1 and Atf2. Appl. Microbiol. Biotechnol. 2008;78:783–792. doi: 10.1007/s00253-008-1366-9. [DOI] [PubMed] [Google Scholar]
- 84.Duetz W.A., Bouwmeester H., Beilen J.B., Witholt B. Biotransformation of limonene by bacteria, fungi, yeasts, and plants. Appl. Microbiol. Biotechnol. 2003;61:269–277. doi: 10.1007/s00253-003-1221-y. [DOI] [PubMed] [Google Scholar]
- 85.Ariyoshi-Kishino K., Hashimoto K., Amano O., Saitoh J., Kochi M., Sakagami H. Tumor-specific cytotoxicity and type of cell death induced by benzaldehyde. Anticancer Res. 2010;30:5069–5076. [PubMed] [Google Scholar]
- 86.Stringer T., Therrien B., Hendricks D.T., Guzgay H., Smith G.S. Mono- and dinuclear (η6-arene) ruthenium (II) benzaldehyde thiosemicarbazone complexes: Synthesis, characterization and cytotoxicity. Inorg. Chem. Commun. 2011;14:956–960. doi: 10.1016/j.inoche.2011.03.041. [DOI] [Google Scholar]
- 87.Su W., Zhou Q., Huang Y., Huang Q., Huo L., Xiao Q., Huang S., Huang C., Chen R., Qian Q., et al. Synthesis, crystal and electronic structure, anticancer activity of ruthenium (II) arene complexes with thiosemicarbazones. Appl. Organomet. Chem. 2013;27:307–312. doi: 10.1002/aoc.2977. [DOI] [Google Scholar]
- 88.Liu Y., Sakagami H., Hashimoto K., Kikuchi H., Amano O., Ishibara M., Yu G. Tumor-specific cytotoxicity and type of cell death induced by beta-cyclodextrin benzaldehyde inclusion compound. Anticancer Res. 2008;28:229–236. [PubMed] [Google Scholar]
- 89.Alamri A., El-Newehy M.H., Al-Deyab S.S. Biocidal polymers: Synthesis and antimicrobial properties of benzaldehyde derivatives immobilized onto amine-terminated polyacrylonitrile. Chem. Cent. J. 2012;6:1–13. doi: 10.1186/1752-153X-6-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rabi T., Bishayee A. d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. J. Carcinog. 2009;8:9. doi: 10.4103/1477-3163.51368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vigushin D.M., Poon G.K., Boddy A., English J., Halbert G.W., Pagonis C., Jarman M., Coombes R.C. Phase I and pharmacokinetic study of d-limonene in patients with advanced cancer. Cancer Research Campaign Phase I/II Clinical Trials Committee. Cancer Chemother. Pharmacol. 1998;42:111–117. doi: 10.1007/s002800050793. [DOI] [PubMed] [Google Scholar]
- 92.Igimi H., Watanabe D., Yamamoto F., Asakawa S., Toraishi K., Shimura H. A useful cholesterol solvent for medical dissolution of gallstones. Gastroenterol. Jpn. 1992;27:536–545. doi: 10.1007/BF02777791. [DOI] [PubMed] [Google Scholar]
- 93.De Almeida A.A.C., Costa J.P., De Carvalho R.B.F., De Sousa D.P., De Freitas R.M. Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Res. 2012;1448:56–62. doi: 10.1016/j.brainres.2012.01.070. [DOI] [PubMed] [Google Scholar]
- 94.do Amaral J.F., Silva M.I.G., de Aquino Neto M.R.A., Neto P.F.T., Moura B.A., de Melo C.T.V., de Sousa F.C.F. Antinociceptive effect of the monoterpene R-(+)-limonene in mice. Biol. Pharm. Bull. 2007;30:1217–1220. doi: 10.1248/bpb.30.1217. [DOI] [PubMed] [Google Scholar]
- 95.Giri R.K., Parija T., Das B.R. D-limonene chemoprevention of hepatocarcinogenesis in AKR mice: Inhibition of c-jun and c-myc. Oncol. Rep. 1999;6:1123–1127. doi: 10.3892/or.6.5.1123. [DOI] [PubMed] [Google Scholar]
- 96.Kaji I., Tatsuta M., Lishi H., Baba M., Inoue A., Kasugai H. Inhibition by d-limonene of experimental hepatocarcinogenesis in sprague-dawley rats does not involve P21ras plasma membrane association. Int. J. Cancer. 2001;93:441–444. doi: 10.1002/ijc.1353. [DOI] [PubMed] [Google Scholar]
- 97.Vuuren S.V., Viljoen A.M. Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combinatio. Flavours Fragr. J. 2007;22:540–544. doi: 10.1002/ffj.1843. [DOI] [Google Scholar]
- 98.Jirovetz L., Buchbauer G., Schmidt E., Denkova Z., Slavchev A., Stoyanova A., Geissler M. Purity, antimicrobial activities and olfactory evaluations of 2-phenylethanol and some derivatives. J. Essent. Oil Res. 2008;20:82–85. doi: 10.1080/10412905.2008.9699429. [DOI] [Google Scholar]
- 99.Ullah I., Khan A.L., Ali L., Khan A.R., Waqas M., Hussain J., Lee I.J., Shin J.H. Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 2015;53:127–133. doi: 10.1007/s12275-015-4632-4. [DOI] [PubMed] [Google Scholar]
- 100.Wang J., Liu H., Zhao J., Gao H., Zhou L., Liu Z., Chen Y., Sui P. Antimicrobial and antioxidant activities of the root bark essential oil of Periploca sepium and its main component 2-hydroxy-4-methoxybenzaldehyde. Molecules. 2010;15:5807–5817. doi: 10.3390/molecules15085807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lingappa B.T., Prasad M., Lingappa Y., Hunt D.F., Biemann K. Phenethyl alcohol and tryptophol: Autoantibiotics produced by the fungus Candida albicans. Science. 1969;163:192–194. doi: 10.1126/science.163.3863.192. [DOI] [PubMed] [Google Scholar]
- 102.Wang Q., Ke L., Xue C., Luo W., Chen Q. Inhibitory kinetics of p-substituted benzaldehydes on polyphenol oxidase from the fifth instar of Pieris rapae L. Tsinghua Sci. Technol. 2007;12:400–404. doi: 10.1016/S1007-0214(07)70060-3. [DOI] [Google Scholar]
- 103.Wilkinson S.M., Love S.B., Westcombe A.M., Gambles M.A., Burgess C.C., Cargill A., Young T., Maher E.J., Ramirez A.J. Effectiveness of aromatherapy massage in the management of anxiety and depression in patients with cancer: A multicenter randomized controlled trial. J. Clin. Oncol. 2007;25:532–539. doi: 10.1200/JCO.2006.08.9987. [DOI] [PubMed] [Google Scholar]
- 104.Hoffman H.J., Rawal S., Li C., Duffy V.B. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): First-year results for measured olfactory dysfunction. Rev. Endocr. Metab. Disord. 2017;17:221–240. doi: 10.1007/s11154-016-9364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Naka A., Riedl M., Luger A., Hummel T., Mueller C.A. Clinical significance of smell and taste disorders in patients with diabetes mellitus. Eur. Arch. Oto-Rhino-Laryngol. 2010;267:547–550. doi: 10.1007/s00405-009-1123-4. [DOI] [PubMed] [Google Scholar]
- 106.Aschenbrenner K., Scholze N., Joraschky P., Hummel T. Gustatory and olfactory sensitivity in patients with anorexia and bulimia in the course of treatment. J. Psychiatr. Res. 2008;43:129–137. doi: 10.1016/j.jpsychires.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 107.Skrandies W., Zschieschang R. Olfactory and gustatory functions and its relation to body weight. Physiol. Behav. 2015;142:1–4. doi: 10.1016/j.physbeh.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 108.Clepce M., Reich K., Gossler A., Kornhuber J., Thuerauf N. Olfactory abnormalities in anxiety disorders. Neurosci. Lett. 2012;511:43–46. doi: 10.1016/j.neulet.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 109.Kershaw J.C., Mattes R.D. Nutrition and taste and smell dysfunction. World J. Otorhinolaryngol.-Head Neck Surg. 2018;4:3–10. doi: 10.1016/j.wjorl.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pekala K., Chandra R.K., Turner J.H. Efficacy of olfactory training in patients with olfactory loss: A systematic review and meta-analysis. Int. Forum Allergy Rhinol. 2016;6:299–307. doi: 10.1002/alr.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang R.S., Twu C.W., Liang K.L. The effect of olfactory training on the odor threshold in patients with traumatic anosmia. Am. J. Rhinol. Allergy. 2017;31:317–322. doi: 10.2500/ajra.2017.31.4466. [DOI] [PubMed] [Google Scholar]
- 112.Hummel T., Reden K.R.J., Hähner A., Weidenbecher M., Hüttenbrink K.B. Effects of olfactory training in patients with olfactory loss. Laryngoscope. 2009;119:496–499. doi: 10.1002/lary.20101. [DOI] [PubMed] [Google Scholar]
- 113.Haehner A., Tosch C., Wolz M., Klingelhoefer L., Fauser M., Storch A., Reichmann H., Hummel T. Olfactory training in patients with Parkinson’s disease. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0061680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Geißler K., Reimann H., Gudziol H., Bitter T., Guntinas-Lichius O. Olfactory training for patients with olfactory loss after upper respiratory tract infections. Eur. Arch. Oto-Rhino-Laryngol. 2014;271:1557–1562. doi: 10.1007/s00405-013-2747-y. [DOI] [PubMed] [Google Scholar]
- 115.Damm M., Pikart L.K., Reimann H., Burkert S., Göktas Ö., Haxel B., Frey S., Charalampakis I., Beule A., Renner B., Hummel T., et al. Olfactory training is helpful in postinfectious olfactory loss: A randomized, controlled, multicenter study. Laryngoscope. 2014;124:826–831. doi: 10.1002/lary.24340. [DOI] [PubMed] [Google Scholar]
- 116.Kollndorfer K., Kowalczyk K., Hoche E., Mueller C.A., Pollak M., Trattnig S., Schöpf V. Recovery of olfactory function induces neuroplasticity effects in patients with smell loss. Neural Plast. 2014;2014 doi: 10.1155/2014/140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Konstantinidis I., Tsakiropoulou E., Bekiaridou P., Kazantzidou C., Constantinidis J. Use of olfactory training in post-traumatic and postinfectious olfactory dysfunction. Laryngoscope. 2013;123 doi: 10.1002/lary.24390. [DOI] [PubMed] [Google Scholar]
- 118.Evaluation of Certain Food Additives: Fifty-Ninth Report of the Joint FAO/WHO Expert Committee on Food Additives. [(accessed on 10 January 2019)]; Available online: https://apps.who.int/iris/bitstream/handle/10665/42601/WHO_TRS_913.pdf?sequence=1&isAllowed=y.