Abstract
Momordica charantia Linn., commonly known as bitter gourd, has many protective roles due to its medicinal value as it contains bioactive components. However, this extract showed possible toxicity effect on zebrafish embryo. Thus this study was designed to differentiate the toxicity activities in two types of M. charantia sample which are Indian and Chinese M. charantia, as well as to compare between two different aqueous extraction methods, hot and cold aqueous method, using zebrafish embryo assay assessment. It was observed that the survival rate of zebrafish embryo decreased as the concentration of test extract increased for all samples of M. charantia. The LC50 values of hot aqueous Chinese M. charantia, hot aqueous Indian M. charantia, and cold aqueous Chinese M. charantia were 144.54 μg/ml, 199.53 μg/ml, and 251.19 μg/ml, respectively. However, cold aqueous Indian M. charantia has a higher LC50 which was not in the range of the tested concentration. Hatchability of Danio rerio embryo reduced as the concentration of M. charantia extract increased while no hatching was observed in the highest concentration (1000 μg/ml). Scoliosis of zebrafish larvae was only seen in higher concentrations (125-1000 μg/ml) of extract. The heartbeat of zebrafish larvae treated with M. charantia extract was within the normal range, 120-180 bpm, but at higher concentrations (125-1000 μg/ml) the heartbeat differed for all samples of test extract. Hence, although this plant extract was safe to be consumed due to its pharmaceutical effect, it still exhibited mild toxicity effect at higher concentration when it was evaluated on zebrafish embryo.
1. Introduction
Traditional medicine or folk medicine is a complementary and alternative medicine which is widely used in many countries. It gained popularity because people believe that traditional medicine has beneficial components which are made up of natural products. Based on their own mechanism of action, these natural sources can treat various diseases like allopathic drugs. Therefore, the main difference between conventional and folk medicine is that the former one is chemically treated and scientifically proven safe to be consumed. With conventional medicine the diagnosis is based on symptoms, whereas the folk medicine has a preventive approach as it is obtained from natural sources [1]. Despite minimal side effect due to a small amount of chemicals, heavy metals in the traditional medicine can eventually cause toxic effect over a high and prolonged consumption [2].
The toxic agent is mostly derived from sources like plants, animals, and microorganisms. As a toxic agent, it transmits the toxic substance through the various modes of transmission mainly via direct contact. A toxicology test is necessary, not only for allopathic medicine but also for complementary and alternative medicine to discover any adverse effects which are not known until the signs and symptoms develop upon high consumption. Some common adverse effects of traditional medicine include nausea, vomiting, weakness, dizziness, hypotension, paraesthesia of mouth and tongue, arrhythmia, and ventricular fibrillation [3]. Assessment of toxicity using toxicity testing assays can prove the safety of traditional medicine and promote its consumption.
Toxicity testing is accomplished to screen the chemical compositions of a sample to ensure that it contains no life-threatening components [4]. Furthermore, the importance of toxicity testing is to provide a dose-responsive curve against the toxicity effect, study the safety of components in the sample, and authenticate methods of investigating toxicity [5]. Zebrafish (Danio rerio) and its transparent embryos are among the apt models used to study the toxicity effect on the embryonic development. It is used to inspect the bioactive compounds of the sample through toxicity assay. Commonly used mammalian models have some drawbacks with higher cost and prolonged time for results, being yet ethically questionable. Moreover, genetically humans show great similarities of genomic sequences and brain patterning with the Danio rerio. Thus, this makes zebrafish embryo an advantageous assay in exploring many diversions of toxicology study yielding a prompt outcome [4].
Momordica charantia Linn., locally known as bitter gourd or bitter melon, is a type of vegetable-fruit used vastly as ethnomedicine. It is found in tropical regions, for instance, China, India, Tropical Africa, America, and Thailand. Bitter gourd has many medicinal values including antidiabetic, hypoglycemic, antitumor, antioxidant, antiulcer, analgesic, and antifertility properties [6]. The bioactive component in this unique fruit is answerable for the therapeutic properties.
There are two different types of bitter gourds, which are the Chinese and Indian bitter gourd with their own specialty. Bitter gourd is mostly eaten raw or with preservation in water to ensure that the medicinal components of the bitter gourd is preserved. It can be consumed by using hot or cold water depending on the individual's preference. For example, it is stated that, in China, the community consumed bitter gourd in combination with tea or hot water upon fermentation as it is believed that the bitter gourd has more natural weight losing properties than other allopathic drugs [7]. The cold bitter gourd is consumed as bitter gourd juice in combination with other fruits as well as vegetables.
Even with the increase in bitter gourd products which have been marketed, there are many cases of the adverse effect of bitter gourd consumption as it has the tendency of producing toxic effect. Thus, the aim of this study was to evaluate the effects of aqueous Momordica charantia Linn. exposure on zebrafish embryo through acute toxicity assay assessment.
1.1. Zebrafish Embryo Model
Zebrafish, scientifically known as Danio rerio, is a vertebrate animal model which is used widely in the field of Biomedical Science for toxicology testing [8]. It is used as an alternative animal model due to its advantages. Generally, the wide usage of zebrafish embryo is attributed to its short life cycle that produces rapid and prominent results [8]. The zebrafish embryo has fast embryogenesis process and produces a large number of eggs. Moreover, these eggs are highly permeable and highly tolerant to the penetration of chemical components. The embryo is transparent enough to observe the development of embryogenesis easily using the standard dissecting light microscope [9]. Some of the main parameters which are focused on during the toxicity assessment are survival rate, LC50 determination, hatching rate, scoliosis rate, and heart rate of the zebrafish embryo.
The survival rate refers to the ability of an organism to adapt in a particular environment according to its optimal condition requirement. Some of the ideal conditions which can affect the survival rate of zebrafish embryo include pH, temperature, chemistry, and hardness of water, as well as nitrogenous waste factor [10]. Higher survival shows that the zebrafish embryo is able to withstand the specific environment whereas low survival rate indicates that the embryo is unable to endure the surrounding atmosphere. The median lethal concentration was obtained from the mortality rate of the zebrafish embryo, in order to identify the safe concentration level for consumption of each M. charantia sample. In general, the higher LC50 values indicate that the toxicity level is low because higher concentration is needed to result in 50% of mortality rate of an organism, whereas a lower LC50 points out that the toxicity of the sample is more harmful because in lower concentration there is greater death of organism [10].
The zebrafish embryo has an external fertilization whereby the eggs will be deposited from the zebrafish yolk sack during the spawning process. The nonadherent embryo will then normally hatch within 48-72 hours post-fertilization [11]. The embryo is sensitive and has specific consideration for its development. The unfavorable condition can literally cause stress to the embryo affecting its growth and development stage resulting in morphological abnormalities [12]. One of the common morphological abnormalities is scoliosis. Scoliosis is the abnormal curvature of the spine causing the spine to have a “C” or “S” like shape. The scoliosis is basically a type of teratogenic effect. It is influenced by a few surrounding stress factors such as chemical compounds and heavy metals [13]. The presence of scoliosis literally indicates that the sample is toxic. The heartbeat of a developing wild-type Danio rerio begins at 36hpf [14]. The normal range of zebrafish heartbeat is between 120 and 180 beats per minute. Additionally, the heartbeat of zebrafish can be influenced by chemical factors, genetic-induced alteration, temperature, and many others [15]. These factors cause the heartbeat rate to alter resulting in bradycardia or tachycardia depending on the particular factor manipulating the zebrafish model.
2. Methods
2.1. Sample Collection
The Chinese M. charantia (C) and Indian M. charantia (I) samples were collected in Selangor Malaysia. Both plants were identified and authenticated by Institute of Bioscience, UPM (Plant Voucher Number: Chinese Bitter Melon: SK3160/17; Indian Bitter Melon: SK3157/17). The fresh fruit of M. charantia was sliced and oven-dried at 40 degrees Celsius (40°C) [16]. This was followed by powdering the dried sample.
2.2. Sample Preparation
Momordica charantia L. extraction was carried out using hot and cold aqueous extraction method for both types of M. charantia. The ground sample of M. charantia weighing 50 g was placed into a conical flask containing about 500 ml of distilled water. The dilution was based on the standard solvent to sample ratio of extraction preparation which is 1:10 [17]. Next, the diluted M. charantia sample in the conical flask was wrapped with an aluminum foil and placed in the water bath at 70°C for 24 hours [18]. Hot samples were abbreviated as hot aqueous (HA). As for the cold aqueous method, it was accomplished by keeping it at room temperature for 2 days [19]. Cold samples were abbreviated as cold aqueous (CA). The sample was then filtered using Whatman paper 1 and was placed at -20°C for freeze-drying in order to preserve and extend the shelf life of the sample collected.
2.3. Serial Dilution
Twofold serial dilution method was used to obtain a series of M. charantia extract samples with different concentrations. The stock solution was prepared by diluting 0.1 gram of M. charantia extract with 1000 μl of distilled water. From the stock solution, six successive series of concentration were produced, which were 15.625 μg/ml, 31.25 μg/ml, 62.5 μg/ml, 125 μg/ml, 250 μg/ml, and 500 μg/ml. On the other hand, as for the control treatment, 500 mg dose of paracetamol was used as a positive control, and distilled water was used as a negative control. The paracetamol sample was prepared through serial dilution using a similar method, producing the same series of concentration as the test extract [20].
2.4. Zebrafish Embryo Assay
One zebrafish embryo was transferred into each of the 96-well plates using a pasteur pipette at 24 hours post-fertilization (24hpf) [21]. Next, the treatment was given by pipetting the sample into each well in the 96-well plates based on different series of concentrations. The embryo development was observed after 24, 48, and 72 hours of treatment. The zebrafish embryo was viewed under the inverted microscope by focusing on a few parameters such as the survival rate, hatching rate, scoliosis, and heartbeat [22]. In addition, the number of heartbeats was counted for 15 seconds and then it was quantified by multiplying by four, to get the number of heartbeats per minute [23].
2.5. Data Analysis
The linear regression and one-way Analysis of Variance (ANOVA) were used to analyze the data obtained. The lethal concentration at 50% (LC50) of each M. charantia extract was calculated, through the linear regression graph of mortality rate in probit unit against the log concentration [24]. The one-way ANOVA was used to analyze the types of M. charantia extract affecting the survival rate, hatching rate, scoliosis, and heartbeat of zebrafish embryo influenced by different series of concentrations. Finally, all the data were interpreted as mean±SEM with a p value of <0.05 indicating the result to be statistically significant based on the statistical hypothesis testing used.
3. Results
3.1. Effect of M. charantia Extract on Survival Rate of Zebrafish Embryo
Based on the result in Figure 1, the survival rate of the zebrafish embryo was fluctuating as the concentration of hot aqueous Chinese M. charantia extract increased, yet it still exhibited a lower overall survival rate. The survival rate of embryo tested on hot aqueous Indian M. charantia extract showed that there was an inconsistent reduction as the concentration increased (Figure 2), followed by the survival rate of zebrafish embryo which gradually increased initially (31.25 μg/ml), and after that there was slight constant decline observed at higher concentration (250-1000 μg/ml) for cold aqueous Chinese M. charantia (Figure 3). It is also shown that the survival rate of embryo inconsistently decreased as the concentration of cold aqueous Indian M. charantia extract increased but a 100% survival rate was observed in the highest concentration (1000 μg/ml) (Figure 4). As for the paracetamol, the survival rate decreased as the concentration increased, but the overall survival rate was higher compared to all the test extracts (Figure 5).
Figure 1.
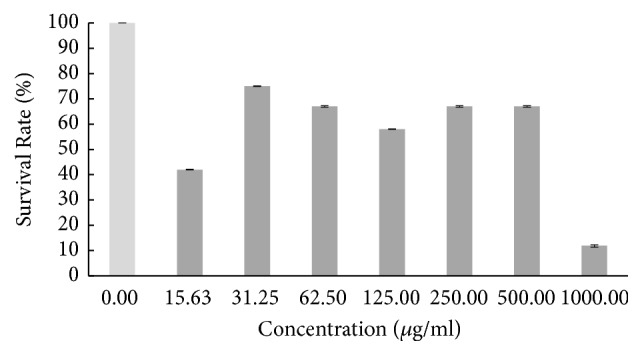
The survival rate of zebrafish embryo examined on hot aqueous Chinese M. charantia (CHA) at different series of concentration. Results were expressed as mean ± SEM of three independent experiments performed in triplicate.
Figure 2.
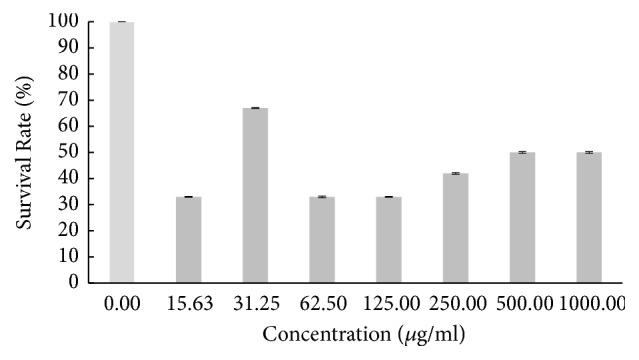
The survival rate of zebrafish embryo examined on hot aqueous Indian M. charantia (IHA) at different series of concentration. Results were expressed as mean ± SEM of three independent experiments performed in triplicate.
Figure 3.
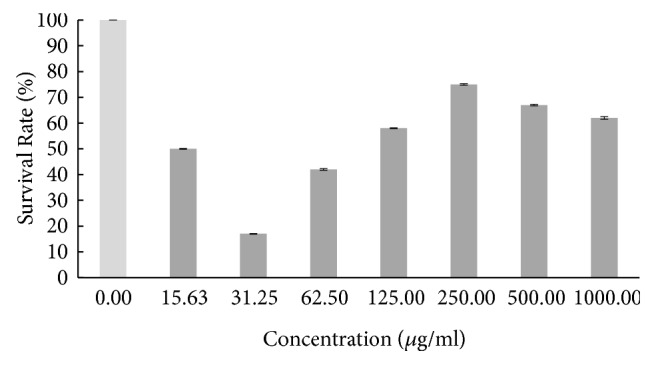
The survival rate of zebrafish embryo assessed on cold aqueous Chinese M. charantia (CCA) at various series of concentration. Results were expressed as mean ± SEM of three independent experiments performed in triplicate.
Figure 4.
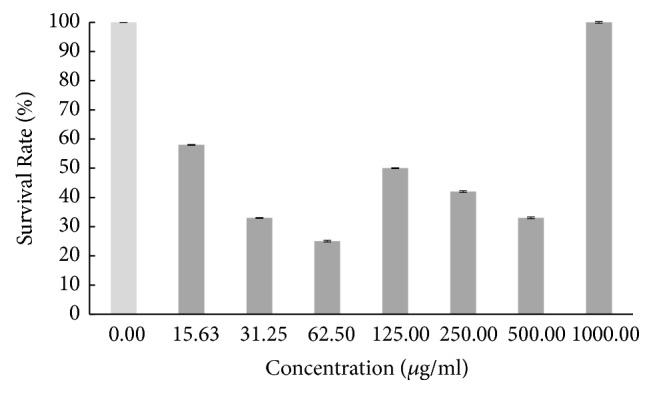
The survival rate of zebrafish embryo examined on cold aqueous Indian M. charantia (ICA) at different series of concentration.
Figure 5.
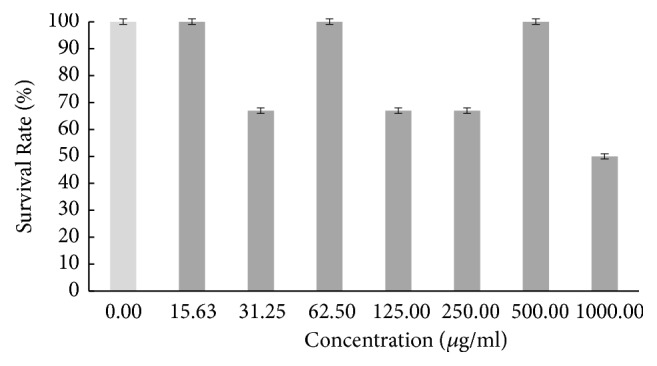
The survival rate of zebrafish embryo tested on paracetamol at different series of concentration. Results were expressed as mean ± SEM of three independent experiments performed in triplicate.
3.2. Effect of M. charantia Extract on Lethal Concentration at 50% of Survival (LC50) Values Assessed on Danio rerio Embryo Model
The LC50 value of hot aqueous Chinese M. charantia was 144.54 μg/ml and the extract with lower concentration than this value was considered safe to be consumed for the zebrafish embryo. For the hot aqueous Indian M. charantia, the concentration was below 199.53 μg/ml which was considered safe to be consumed by the zebrafish embryo. Furthermore, the LC50 of cold aqueous Chinese M. charantia was 251.19 μg/ml, more than that of the cold aqueous Indian M. charantia that was unavailable as the LC50 was out of the tested range. Paracetamol also showed unavailable data which is higher than the tested concentration, which indicates that it exhibits a lower toxicity effect (Figure 6).
Figure 6.
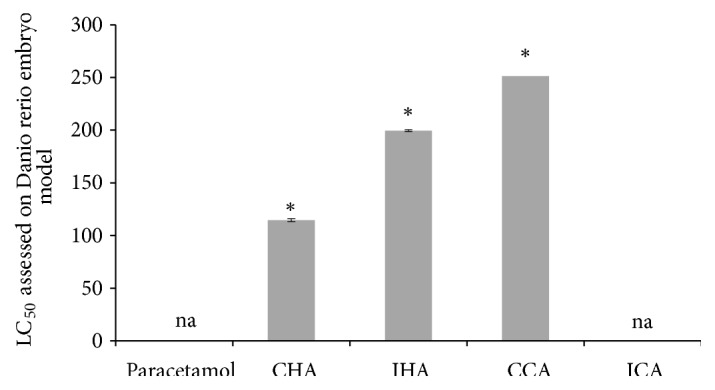
The lethal concentration at 50% of survival (LC50) values assessed on Danio rerio embryo model. Results were expressed as mean ± SEM of three independent experiments performed in triplicate. #p<0.05 compared with paracetamol.
3.3. Effect of M. charantia Extract on Hatching Rate of Danio rerio Embryo
According to the result obtained (Figure 7), the zebrafish embryo showed a delayed hatchability in all the samples of M. charantia test extract as the concentration of test extract increased. At lower concentration (0-62.5 μg/ml) of treatment solution, the hatching rate of the embryo was 100%, whereas, at the highest concentration (1000 μg/ml), there was no hatching observed. However, paracetamol showed a decline in hatching rate, yet delayed hatching was not seen.
Figure 7.
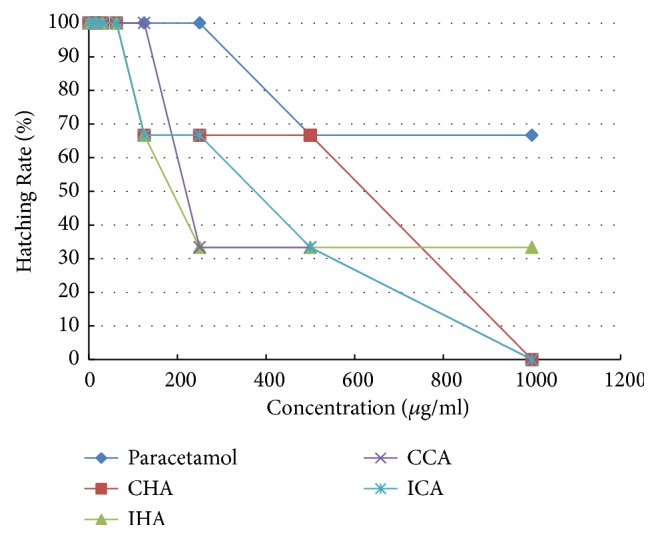
The hatching rate of zebrafish embryo examined on all test extracts and control solutions at various series of concentration. Results were expressed as mean ± SEM of three independent experiments performed in triplicate.
3.4. M. charantia Toxic Effect on the Scoliosis of Danio rerio Larvae
Based on the result presented (Figure 8), the scoliosis was only seen in the higher concentrations (125-1000 μg/ml) of all test extracts. There was no scoliosis seen in lower concentrations (0-62.5 μg/ml). The result showed that the cold aqueous Indian M. charantia exhibited a mild scoliosis effect on zebrafish larvae. However, the cold aqueous Chinese M. charantia expressed a higher scoliosis effect compared to all the other extracts. And as for both the hot the cold aqueous M. charantia extract, moderate scoliosis effect was observed on zebrafish larvae. No scoliosis was observed in positive control solution.
Figure 8.
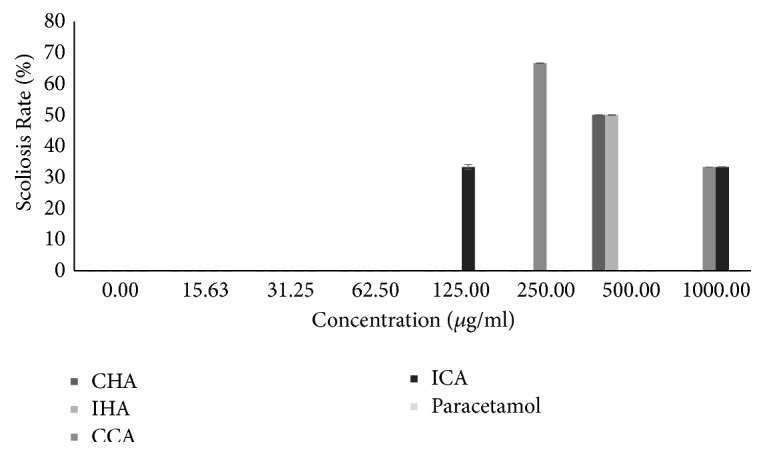
The scoliosis rate of zebrafish embryo tested on all test extracts and control solutions at different series of concentration. Results were expressed as mean ± SEM of three independent experiments performed in triplicate.
The scoliosis present in the hatched embryo compared to the normal hatched embryo was observed as in Figure 9.
Figure 9.
The normal appearance of hatched embryo (a) compared to the scoliosis present hatched embryo (b) at magnification of 500 μm using Microscope, OLYMPUS DX51; Camera, OLYMPUS DP72.
3.5. The Heartbeat of Zebrafish Larvae Treated with M. charantia Extract
All the M. charantia extracts presented a normal heartbeat, but at higher concentration of test extract, the heartbeat counting differed as shown in Figure 10. The hot aqueous Chinese M. charantia had an ideal heartbeat for all series of concentration, but at higher concentration (1000 μg/ml) the heartbeat counting eventually reduced. As for the hot aqueous Indian M. charantia, the overall heartbeat rate of zebrafish larvae decreased inconsistently as the concentration of test extract increased, which is specifically observed at 125 μg/ml until 1000 μg/ml. However, only the cold aqueous Chinese M. charantia showed that the heart rate increased within normal range, along with the higher concentration. Lastly, cold aqueous Indian M. charantia showed that the heartbeat decreased but remained within the normal range, as the concentration of test extract increased. Heartbeat was stable for the positive control.
Figure 10.
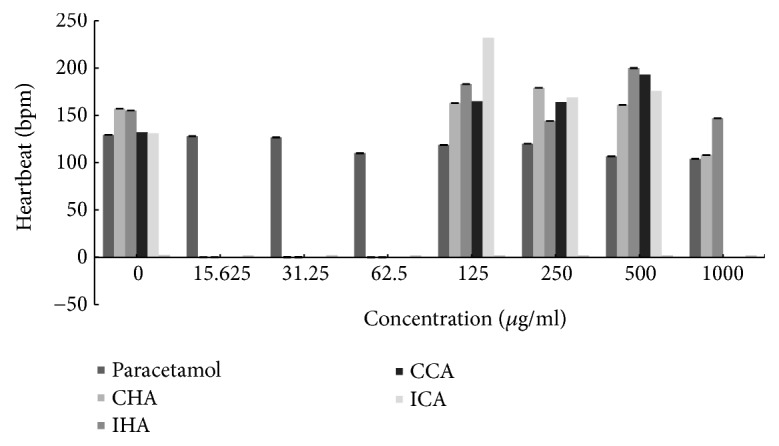
The heartbeat rate of zebrafish embryo examined on all test extracts and control solutions at various series of concentration.
4. Discussion
It can be summarised that the Indian M. charantia reflects a higher survival rate as compared to the Chinese M. charantia. This is because, although both the M. charantia extracts have the same components, they still have different amounts of total compounds present in the pulp of the fruit of M. charantia. Some of the main bioactive components are polysaccharides, peptides, protein, lipids, terpenoids, saponins, phenolics, and sterols, each with specific functional properties [25]. As stated, the protein content and total phenolic content of Indian M. charantia flesh are higher than those of the Chinese M. charantia flesh [26]. This may be one of the factors which contribute to the higher survival rate of Indian M. charantia which makes it a healthier choice as compared to the Chinese M. charantia.
As there is apparent difference between the two types of aqueous extraction method, the hot aqueous extraction method expressed a lower overall survival rate than the cold aqueous extraction samples. This is because the extraction method can influence the composition of extract due to different types of reaction during the aqueous extraction procedure. Specifically, flavonoid is one of the important elements in M. charantia as it possesses antioxidant property which removes the excess free radicals by acting as a neutralizing agent [25]. However, flavonoids extraction is known to be more efficient when the temperature is low because the higher temperature degrades the flavonoids component despite a rapid and high rate of reaction [27]. As reported, the absence of flavonoid compound in the phytochemical screening of M. charantia extract was proven using hot aqueous extraction method [28]. Thus, the absence of flavonoids can cause oxidative stress indirectly damaging the cellular molecules resulting in a lower survival rate of zebrafish embryo [29].
The study of the toxicity of M. charantia leaf using African catfish model reported that the survival rate of catfish decreased as the level of powdered leaf increased. The effect was also seen on blood components whereby the erythrocytes and Hb count decreased, whereas leukocytes count increased, as the powdered leaf of M. charantia increased [30]. Thus, this showed that the M. charantia generally provides a low survival rate as the amount of extracts increases. Meanwhile, the paracetamol used as the positive control had a higher survival rate compared to the other test extracts. The negative control showed 100% survival rate and this indicates that the negative control was an ideal condition for the survival of zebrafish embryo. This revealed that the negative control did not show any sort of toxicity effect, whereas the positive control exhibited a lower toxic effect as compared to all the test extracts seen on the survival rate of Danio rerio embryo.
The Indian M. charantia reflects a higher LC50 value which indicates that it is a better choice than the Chinese M. charantia as it ends in a lower toxicity level. In general, the Chinese M. charantia has lower protein and phenolic content as compared to Indian M. charantia [26]. This factor can eventually influence the mortality rate of the zebrafish embryo, causing the Chinese M. charantia to produce more toxic effects. Additionally, the cold aqueous showed a higher LC50 value than the hot aqueous method for both types of species. This suggested that the cold extraction method is a superior extraction method for M. charantia sample preparation. Apparently, the hot aqueous extract can degrade certain enzymes and molecules such as flavonoids which can induce toxicity effect despite higher extract yields [27]. Similarly, the hot aqueous extraction produces higher extract yields compared to cold aqueous extraction. Hence, the toxic compounds like cucurbitanes will be high in hot aqueous extract, causing it to exhibit more toxic effect affecting the mortality rate of zebrafish embryo [31].
In addition, coinciding with another study, the median lethal dose of M. charantia ethanolic extract is safe to be consumed below 2000 mg/kg of Sprague Dawley rats [32]. It is also stated that there might be potential toxic effect on blood, tissue, and organ specifically the liver if the M. charantia is consumed at a higher dose [32]. The LC50 value of paracetamol is higher than the test extract, because the mortality rate in treatment extract is higher than the mortality rate in control solution. This may be due to the components of M. charantia extract such as cucurbitane that is a type of protein causing a toxic effect and may disrupt the biological pathway of zebrafish embryo halting its development [25].
Moreover, the overall hatchability of zebrafish embryo declines as the concentration of all test extracts increases. Basically, the M. charantia is known to cause abortion, so this point can be correlated with the delayed hatching rate of zebrafish embryo observed. M. charantia contains α-momorcharin (α-MMC) and β-momorcharin (β-MMC) that contribute to antifertility activity [25]. The α-MMC mainly causes the termination of pregnancy during early stage which indirectly leads to miscarriage, while the β-MMC affects the adhesion and implantation of the embryo at the same time depressing the development of the embryo [25]. Hence, this may underlie the fact that the M. charantia generally disrupts the hatching rate of the embryo. Likewise a previous study on M. charantia leaf extract showed similar results on hatching rate [33]. The same observation was shown whereby the percentage of hatching rate reduced as the M. charantia extract concentration increased, which implied that the normal process of zebrafish embryo hatching rate was disturbed [33]. Another type of animal model embryo study using Fasciola hepatica, which is a type of trematode, reported that the embryogenesis of F. hepatica eggs was inhibited upon exposure to M. charantia extract [34]. This finding provided a promising source of anthelmintic agent and antifertility properties of M. charantia. Next, similar to test extract, the positive control decreased the hatching rate of zebrafish along with the increase in concentration. Oppositely, the negative control showed the highest hatching rate because it is the ideal condition for normal hatching process of zebrafish embryo [25].
Scoliosis of zebrafish larvae was only observed in the higher concentration of M. charantia extract. In general, the scoliosis rate of M. charantia might be due to the high amount of toxic compound present at a higher concentration as mentioned above. This composition of M. charantia may alter the development of vertebrae formation which leads to failure in spine development [35]. Other than toxic compound, the teratogenic effect of Danio rerio larvae can be affected due to the delayed hatching rate of embryo upon exposure to M. charantia [36]. A similar effect of scoliosis was observed in the previous study of M. charantia leaf extract tested on zebrafish embryo model where the teratogenic effect of M. charantia leaf extract was observed at higher concentration, specifically at 0.5% concentration [33]. Nevertheless, the compound of M. charantia which is responsible for this effect was not identified [33]. There was no scoliosis observed in negative control as well as all series of positive control. This manifested that the negative control and positive control do not reveal any toxic effect, in particular, scoliosis effect, as compared to all the test extracts. This finding contradicts that of the previous study where the acetaminophen on Danio rerio embryo instigated anomalies at a higher concentration causing impairment [37].
Furthermore, a study has proven that the M. charantia extract is a beneficial antihypertensive agent as it indirectly reduces blood pressure by decreasing heartbeat of zebrafish larvae. The M. charantia lowers the heart rate by inhibiting the angiotensin-converting enzyme (ACE) which is a type of enzymes that is responsible for converting angiotensin I to angiotensin II which constricts blood vessels. The inhibition of ACE leads to blood vessel dilation reducing the blood pressure as well as lowering the workload of heart. This influences the heart rate causing the heartbeat to slow down [38]. Another study reported that whole plant extract of M. charantia possessed antihypertensive properties, by normalizing the heart rate of hypertensive induced rats and decreasing the systemic arterial blood pressure. It was also reported that M. charantia acts as an antihypertensive agent, nevertheless this was not completely investigated [39]. Another study showed that bradycardia was observed in Zucker Obese (ZO) rats upon consumption of M. charantia [40]. Conversely, some studies reported slight increase of heart rate in the group of lean rats upon the consumption of this extract [40]. The previous result of increased heart rate was similar to that found in the cold aqueous Chinese M. charantia indicating that the low heart rate can also result in tachycardia. Lastly, both types of control solutions gave stable heartbeat within the normal range compared to the M. charantia extract.
5. Conclusion
In summary, the impact of M. charantia exposure on zebrafish embryo model through acute toxicity assay assessment was evaluated. A comparison of two different extraction methods showed that the cold aqueous extraction method manifested lower toxic effects compared to hot aqueous extraction. The Indian M. charantia was observed to possess a milder toxic effect compared to the Chinese M. charantia. Therefore, the results conclude that the cold aqueous Indian M. charantia is the best test extract as it exhibited lower toxicity effect in overall parameters such as survival rate, LC50 value determination, hatching rate, scoliosis, and heartbeat of the zebrafish embryo. Hence, although this plant extract is safe to be consumed due to its promising pharmaceutics, it still exhibits mild toxicity effect on a higher concentration as evaluated in the zebrafish embryo model. Thus, it is suggested that further experimental study on phytochemical screening should be conducted to identify the specific components of M. charantia which exhibit respective toxic effects.
Acknowledgments
This research was funded by Universiti Putra Malaysia for Project Grant Funding (GP-IPS/2016/9470400). The authors are thankful to Graduate Research Fellowship, UPM and Ministry of Higher Education Malaysia (MoHE).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there are no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Chidozie N. J., Okorie E. A., Reginald N. O., Chima O. E., Chukwuma N. I., Regina C. N. Effectiveness of traditional and conventional medicine in the treatment of diabetes: A comparative study of south-east, Nigeria. International Journal of Clinical Medicine Research. 2014;1(2):35–41. [Google Scholar]
- 2.National Center for Complementary and Alternative Medicine. Traditional Chinese Medicine: In Depth. 2015. [Google Scholar]
- 3.Hussin A. H. Adverse effects of herbs and drug-herbal interactions. Malaysian Journal of Pharmacy. 2001;1(2):39–44. [Google Scholar]
- 4.Bhattacharya S., Zhang Q., Carmichael P. L., Boekelheide K., Andersen M. E. Toxicity testing in the 21st century: Defining new risk assessment approaches based on perturbation of intracellular toxicity pathways. PLoS ONE. 2011;6(6):1–11. doi: 10.1371/journal.pone.0020887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arome D., Chinedu E. The importance of toxicity testing. Journal of Pharmaceutical and BioScience. 2014;4:146–148. [Google Scholar]
- 6.Raghavan Anilakumar K. Nutritional, pharmacological and medicinal properties of momordica charantia. International Journal of Nutrition and Food Sciences. 2015;4(1):75–83. doi: 10.11648/j.ijnfs.20150401.21. [DOI] [Google Scholar]
- 7.Deng Y., Tang Q., Zhang Y., Zhang R., Wei Z. Protective effect of Momordica charantiawater extract against liver injury in restraint-stressed mice and the underlying mechanism. Food & Nutrition Research. 2017;61(1):1–12. doi: 10.1080/16546628.2017.1348864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarvaiya V. N., Sadariya K. A., Rana M. P., Thaker A. M. Zebrafish as model organism for drug discovery and toxicity testing: a review. Veterinary Clinical Science. 2014;2(3):31–38. [Google Scholar]
- 9.Berry J. P., Gantar M., Gibbs P. D. L., Schmale M. C. The zebrafish (Danio rerio) embryo as a model system for identification and characterization of developmental toxins from marine and freshwater microalgae. Comparative Biochemistry and Physiology - C Toxicology and Pharmacology. 2007;145(1):61–72. doi: 10.1016/j.cbpc.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedayati A., Shaluei F., Jahanbakhshi A., Kolangi H., Alizadeh M. Retracted: Detection of LC50, NOEC and LOEC of some heavy metals (mercury, plumb and zinc) in freshwater fish Roach (Rutilus rutilus) Detection of LC50, NOEC and LOEC of some heavy metals (mercury, plumb and zinc) in freshwater fish Roach (Rutilus rutilus). Toxicology and Industrial Health. 2017;33(1):4–10. doi: 10.1177/0748233712457450. [DOI] [PubMed] [Google Scholar]
- 11.Sharmili S. V., Angelin A. J. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;3(6):6–11. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 12.Dubińska-Magiera M., Daczewska M., Lewicka A., Migocka-Patrzałek M., Niedbalska-Tarnowska J., Jagl K. Zebrafish: A model for the study of toxicants affecting muscle development and function. International Journal of Molecular Sciences. 2016;17(11):1–22. doi: 10.3390/ijms17111941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J., Freeman J. L. Zebrafish as a model for developmental neurotoxicity assessment: the application of the zebrafish in defining the effects of arsenic, methyl mercury, or lead on early neurodevelopment. Toxics. 2014;2(3):464–495. doi: 10.3390/toxics2030464. [DOI] [Google Scholar]
- 14.Pylatiuk C., Sanchez D., Mikut R., et al. Automatic zebrafish heartbeat detection and analysis for zebrafish embryos. Zebrafish. 2014;11(4):379–383. doi: 10.1089/zeb.2014.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Luca E., Zaccaria G. M., Hadhoud M., et al. ZebraBeat : A flexible platform for the analysis of the cardiac rate in zebrafish embryos. Scientific Reports. 2014;4(4898):1–13. [Google Scholar]
- 16.Tan E. S., Abdullah A., Maskat M. Y. Effect of drying methods on total antioxidant capacity of bitter gourd (Momordica charantia) fruit. Proceedings of the 2013 UKM Faculty of Science and Technology Post-Graduate Colloquium; July 2013; Malaysia. pp. 710–716. [Google Scholar]
- 17.Pandey A., Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. Journal of Pharmacognosy and Phytochemistry. 2014;2(5):115–119. [Google Scholar]
- 18.Tan S. P., Stathopoulos C., Parks S., Roach P. An optimised aqueous extract of phenolic compounds from bitter melon with high antioxidant capacity. Antioxidants. 2014;3(4):814–829. doi: 10.3390/antiox3040814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palit M., Hedge S. K., Bhat S. S. Effectiveness of mouthrinse formulated from aqueous extract of terminalia chebula on salivary streptococcus mutans count and pH among 8- to 12-year-old school children of karnataka: a randomized clinical trial. Journal of Clinical Pediatric Dentistry. 2016;9(4):349–354. doi: 10.5005/jp-journals-10005-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards H. Pharmaceutical Calculations for the Pharmacy Technician. 2018. Dilution and concentration; pp. 207–240. [Google Scholar]
- 21.Zhang X., Lu Z., Gelinas D., Ciruna B., Sun Y. Batch transfer of zebrafish embryos into multiwell plates. IEEE Transactions on Automation Science and Engineering. 2011;8(3):625–632. doi: 10.1109/TASE.2011.2121903. [DOI] [Google Scholar]
- 22.Ismail H. F., Hashim Z., Soon W. T., Rahman N. S. A., Zainudin A. N., Majid F. A. A. Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. Journal of Traditional and Complementary Medicine. 2017;7(4):452–465. doi: 10.1016/j.jtcme.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoage T., Ding Y., Xu X. Quantifying cardiac functions in embryonic and adult zebrafish. Methods in Molecular Biology. 2012;843:11–20. doi: 10.1007/978-1-61779-523-7_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent K. Probit analysis. Nature. 1948;161(4090):417–418. [Google Scholar]
- 25.Jia S., Shen M., Zhang F., Xie J. Recent advances in momordica charantia: Functional components and biological activities. International Journal of Molecular Sciences. 2017;18(12):1–25. doi: 10.3390/ijms18122555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Islam S., Jalaluddin M., Hettiarachchy N. S. Bio-active compounds of bitter melon genotypes (Momordica charantiaL.) in relation to their physiological functions. Function Foods in Heals and Disease. 2011;1(2):61–74. [Google Scholar]
- 27.Supraja P., Usha R. Antibacterial and phytochemical screening from leaf and fruit extracts of Momordica charantia. International Journal of Pharma and Bio Sciences. 2013;4(1):B787–B793. [Google Scholar]
- 28.Tan S. P., Parks S. E., Stathopoulos C. E., Roach P. D. Extraction of flavonoids from bitter melon. Food and Nutritional Sciences. 2014;5(2):458–465. [Google Scholar]
- 29.Mugoni V., Camporeale A., Santoro M. M. Analysis of oxidative stress in Zebrafish embryos. Journal of Visualized Experiments. 2014;7(89) doi: 10.3791/51328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morah N. I., Ekanem A., Ashipu L. B. Effect of Momordica charantia leaf on water parameters and survival rate of clarias gariepinus. International Journal of Current Research in Life Sciences. 2015;4(11):449–451. [Google Scholar]
- 31.Sultana B., Anwar F., Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14(6):2167–2180. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Husna R. N., Noriham A., Nooraain H., Azizah A. H., Amna O. F. Acute oral toxicity effects of Momordica charantia in sprague dawley rats. International Journal of Bioscience, Biochemistry and Bioinformatics. 2013;3(4):4–6. [Google Scholar]
- 33.Santos M. M. R., Agpaoa A. R., Sayson A. D., et al. Toxic and teratogenic effects of water leaf extract of momordica charantia in zebrafish (danio rerio) embryos. Der Pharma Chemica. 2017;9(6):119–122. [Google Scholar]
- 34.Pereira C. A. J., Oliveira L. L. S., Coaglio A. L., et al. Veterinary Parasitology Anti-helminthic activity of Momordica charantia L. against Fasciola hepatica eggs after twelve days of incubation in vitro. Veterinary Parasitology. 2016;228(8):160–166. doi: 10.1016/j.vetpar.2016.08.025. [DOI] [PubMed] [Google Scholar]
- 35.Arlet V., Odent T., Aebi M. Congenital scoliosis. European Spine Journal. 2003;12(5):456–463. doi: 10.1007/s00586-003-0555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dulay R. M. R., Vera J. S. D. Radical scavenging and teratogenic activities of stem-bark, leaves, and fruit rind of guyabano (annona muricata linn) Der Pharmacia Lettre. 2017;9(3):47–54. [Google Scholar]
- 37.David A., Pancharatna K. Effects of acetaminophen (paracetamol) in the embryonic development of zebrafish, Danio rerio. Journal of Applied Toxicology. 2009;29(7):597–602. doi: 10.1002/jat.1446. [DOI] [PubMed] [Google Scholar]
- 38.Alam M. A., Uddin R., Subhan N., Rahman M. M., Jain P., Reza H. M. Beneficial role of bitter melon supplementation in obesity and related complications in metabolic syndrome. Journal of Lipids. 2015;2015:18. doi: 10.1155/2015/496169.496169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ojewole J. A. O., Adewole S. O., Olayiwola G. Hypoglycaemic and hypotensive effects of Momordica charantia Linn (Cucurbitaceae) whole-plant aqueous extract in rats. Cardiovascular Journal of Africa. 2006;17(5):227–232. [PubMed] [Google Scholar]
- 40.Czompa A., Gyongyosi A., Szoke K., et al. Effects of momordica charantia (Bitter Melon) on ischemic diabetic myocardium. Molecules. 2017;22(488):1–15. doi: 10.3390/molecules22030488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.



