Abstract
Background
Extracorporeal membrane oxygenation (ECMO) is often used in critical patients with severe myocardial failure. However, the mortality rate of patients on ECMO is often high. Recent studies have suggested that endothelial activation with subsequent vascular barrier breakdown is a critical pathogenic mechanism of organ damage and is related to the outcome of critical illness. This study aimed to determine whether endothelial biomarkers can be served as prognostic factors for the outcome of patients on ECMO.
Methods
This prospective study enrolled 23 critically ill patients on veno-arterial ECMO in the intensive care units of a tertiary care hospital between March 2014 and February 2015. Serum samples were tested for thrombomodulin, angiopoietin (Ang)-1, Ang-2, and vascular endothelial growth factor (VEGF). Demographic, clinical, and laboratory data were also collected.
Results
The overall mortality rate was 56.5%. The combination of Ang-2 at the time of ECMO support (day 0) and VEGF at day 2 had the ability to discriminate mortality (area under receiver operating characteristic curve [AUROC], 0.854; 95% confidence interval: 0.645–0.965).
Conclusions
In this study, we found that the combination of Ang-2 at day 0 and VEGF at day 2 was a modest model for mortality discrimination in this group of patients.
Keywords: Extracorporeal membrane oxygenation, Angiopoietin, Vascular endothelial growth factor, Endothelial biomarker
Introduction
Extracorporeal membrane oxygenation (ECMO) is often used in critical patients with severe myocardial failure (e.g., cardiogenic shock or myocarditis). It provides these patients with temporary circulatory support and has been utilized as a bridging therapy for further treatment. However, despite the rapid advances in the ECMO technique and post-operative care in recent decades, the mortality rate of patients on ECMO remains high [1–4].
Previous studies have shown that several intensive care unit (ICU) scoring systems have good ability in outcome prediction for patients on ECMO [1, 5, 6]. However, these scoring systems usually consist of many laboratory data and physiological measurements, and sometimes need complex calculation. Recently, several biomarkers have been applied to predict renal and neurologic outcomes in patients on ECMO [7, 8], but no particular biomarker is associated with mortality in this patient group.
Recent studies have shown that endothelial activation with subsequent vascular barrier breakdown is a critical pathogenic mechanism of organ damage and is related to the outcome of critical illness [9–12]. Thrombomodulin (TM) is a transmembranous glycoprotein found on the vascular endothelium [13]. It enhances thrombin-induced protein C activation and has roles in inflammation, coagulation, and fibrinolysis [14]. Soluble thrombomodulin levels are associated with mortality in patients with disseminated intravascular coagulation, sepsis, or acute respiratory distress syndrome [12, 15, 16]. Angiopoietin (Ang)-1, Ang-2, and vascular endothelial growth factor (VEGF) are proteins associated with angiogenesis. Ang-1 has an anti-inflammatory effect by limiting endothelium activation, while Ang-2 triggers an inflammatory response by activating the endothelium. Besides, Ang-1 downregulates VEGF expression and reduces thrombin-induced permeability [17–19]. In recent studies, low Ang-1 concentration and high Ang-2 concentration are associated with increased mortality in patients with sepsis [10, 20–22]. However, the relationship between VEGF level and mortality is discordant in different studies [12, 23, 24].
Although endothelial activation and injury are involved in organ damage and associated with the prognosis of critical illness, there has been no associated study on patients on ECMO. Therefore, this study aimed to determine whether the serum biomarkers of endothelial injury and activation could serve as prognostic factors for the outcome of patients on ECMO.
Materials and methods
Study population and data collection
The local Institutional Review Board (IRB) of Chang Gung Memorial Hospital approved the study protocol (IRB No. 103-1569C). The study was performed in the ICUs of a tertiary care hospital in Taiwan between March 2014 and February 2015. Patients who met the inclusion criteria were invited to participate in the study on the first day of ECMO support. Written informed consent was obtained from the next-of-kin of the patients before their participation. The following patients were excluded: pediatric patients younger than 18 years old, those with end stage renal disease undergoing regular renal replacement therapy, and those whose next-of-kin declined study enrollment. Besides, patients with veno-venous (V-V) ECMO support were also excluded due to different pathophysiologic changes between veno-arterial (V-A) and V-V ECMO. For patients with repeated ECMO support during hospitalization, we only collected the data on the first ECMO support. A total of 66 patients were screened during the study period, but the next-of-kin of 43 patients refused consent due to the critical condition of the patients. In total, 23 patients were enrolled.
The following data were prospectively collected: demographic data, indications for ECMO support, and outcomes. We utilized the worst physiological values on the day of ECMO support for physiological calculations. The primary study outcome was in-hospital mortality. Follow-up was performed at 6 months after hospital discharge via chart records or telephone interviews if necessary.
Sampling and quantifying serum biomarkers
Ten milliliters of blood were collected from each patient with routine blood tests performed at the time of ECMO support (day 0), the morning of the first post-ECMO day (day 1), and the morning of the second post-ECMO day (day 2). The blood samples were centrifuged at 1000 g for 5 min, and the supernatants were stored at − 80 °C. Serum biomarkers (Ang-1, Ang-2, VEGF, and TM) were quantified by an enzyme-linked immunosorbent assay (R&D system, Minneapolis, MN, USA) according to manufacturer instructions.
Clinical management
The ECMO device (Medtronic, Inc., Anaheim, CA) consisted of a centrifugal pump and a hollow-fiber microporous membrane oxygenator with an integrated heater. All ECMO circuits had a heparin-bound Carmeda bioactive surface. A silicone oxygenator (Medtronics, Minneapolis, MN, USA) was incorporated into the ECMO circuit. A 17–19 Fr percutaneous arterial (outflow) cannula and a 19–21 Fr percutaneous venous (inflow) cannula (DLP; Medtronic Inc., Minneapolis, MN) were chosen according to patients’ body size. Percutaneous access through the common femoral vein (inflow) and the common femoral artery (outflow) was preferred for V-A ECMO. If cyanosis was noted on the cannulated limb, an 8 Fr distal perfusion catheter would be implanted into the ipsilateral superficial femoral artery.
Statistical analysis
There was no sufficient power to test normality of continuous variables due to the small sample size of this study. Therefore, all statistical tests were done using nonparametric statistics. Descriptive statistics for continuous variables were expressed as median with interquartile range. Data between the survivors and non-survivors were compared using Mann-Whitney U test for continuous variables or Fisher’s exact test for categorical variables. The performance of discriminating mortality by those biomarkers at day 0, day 1, and day 2 of ECMO support was assessed using receiver operating characteristic (ROC) curve analysis. All statistical tests were two-tailed, and a value of P < 0.05 was considered statistically significant. No adjustment for multiple testing (multiplicity) was made in this study. Statistical analysis was conducted using SPSS 22 software (IBM SPSS, Armonk, NY: IBM Corp).
Results
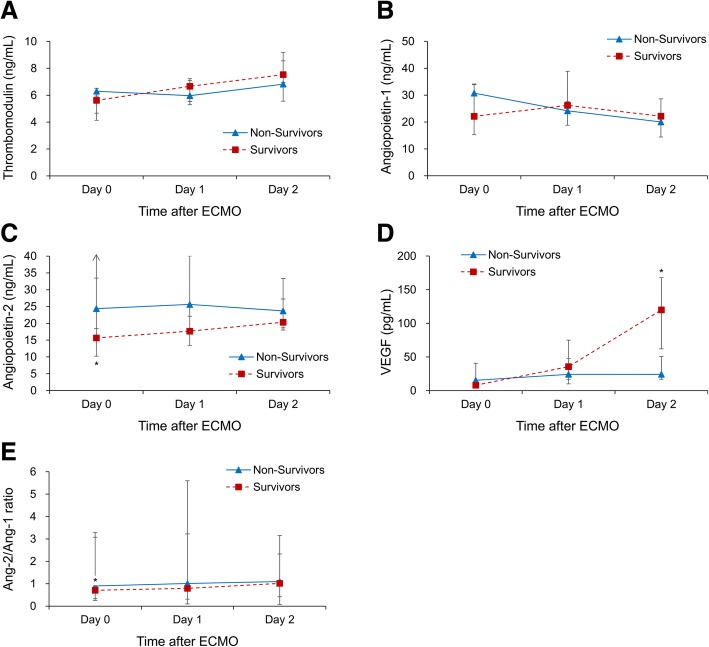
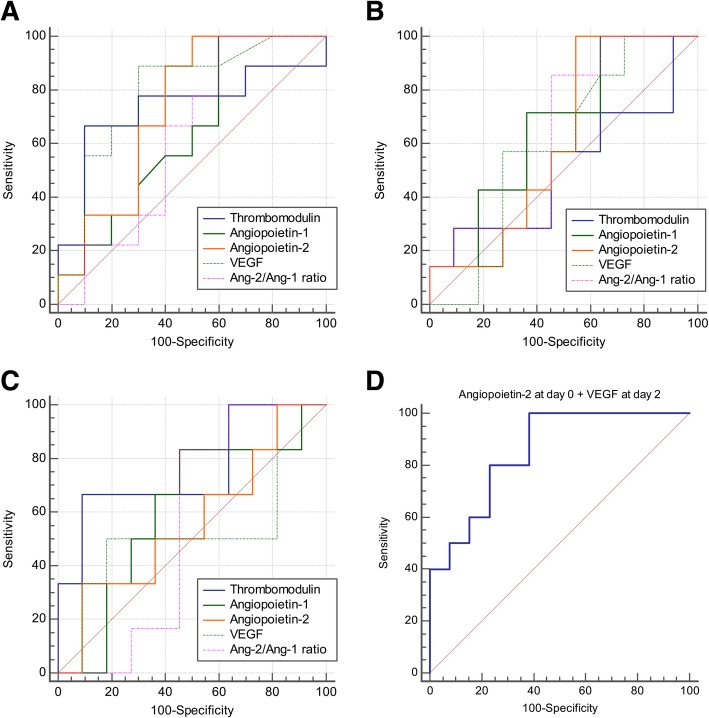
Between March 2014 and February 2015, 23 patients on ECMO support at the ICU were enrolled. The average age was 57 years and 19 (82.6%) were male. The in-hospital mortality rate was 56.5% (13/23). Table 1 presents the demographic data and clinical characteristics of the patients. Non-survivors had higher vasopressor/inotrope dose and higher Sequential Organ Failure Assessment (SOFA) score than survivors at the day of ECMO supplement. Table 2 shows the concentration changes of biomarkers at day 0, day 1, and day 2 of ECMO support. TM and Ang-1 concentrations showed no significant difference between survivors and non-survivors during the first 2 days. The Ang-2/Ang-1 ratio increased gradually in both groups and was higher in non-survivors. Notably, Ang-2 level decreased at day 0 (median: 15.7 vs. 24.4 ng/mL, P = 0.035) and VEGF level tremendously increased at day 2 (median: 119.9 vs. 24.2 pg/mL, P = 0.005) in the survivors as compared to non-survivors (Fig. 1). Figure 2 depicts the ROC curves of the four biomarkers in discriminating mortality at day 0, day 1, and day 2 of ECMO support. We found that the combined predictive probability of Ang-2 at day 0 and VEGF at day 2 had the ability of discriminating mortality (area under the ROC curve, 0.854; 95% confidence interval [CI], 0.645–0.965; as shown in Fig. 2d).
Table 1.
Patients’ demographic data and clinical characteristics
| Variable | All Patients (n = 23) | Non-Survivors (n = 10) | Survivors (n = 13) | P value |
|---|---|---|---|---|
| Age (years) | 57 (19) | 55 (7) | 58 (20) | 0.250 |
| Male sex, n (%) | 19 (82.6) | 7 (70) | 12 (92.3) | 0.281 |
| Diabetes mellitus, n (%) | 4 (17.4) | 1 (10) | 3 (23.1) | 0.604 |
| Coronary artery disease, n (%) | 15 (65.2) | 5 (50) | 10 (76.9) | 0.221 |
| Duration of ECMO support (days) | 5 (5) | 7 (11) | 4 (1) | 0.483 |
| Duration of ICU stay (days) | 11 (10) | 8 (11) | 17 (38) | 0.020 |
| Mechanical ventilation (days) | 8 (8) | 8 (11) | 8 (8) | 0.454 |
| IABP, n (%) | 18 (78.3) | 8 (80) | 10 (76.9) | 1.000 |
| Myocardial failure during operation | 10 (55.6) | 6 (75) | 4 (40) | 0.188 |
| Cardiogenic shock | 8 (44.4) | 2 (25) | 6 (60) | 0.188 |
| Indication for ECMO, n (%) | 0.119 | |||
| Postcardiotomy | 12 (52.2) | 5 (50) | 7 (53.8) | |
| Myocarditis | 1 (4.3) | 1 (10) | 0 (0) | |
| Acute myocardial infarction | 6 (26.1) | 1 (10) | 5 (38.5) | |
| Heart transplantation | 1 (4.3) | 1 (10) | 0 (0) | |
| Profound shock with desaturation | 2 (8.7) | 2 (20) | 0 (0) | |
| VT with cardiogenic shock | 1 (4.3) | 0 (0) | 1 (7.7) | |
| Complication of ECMO, n (%) | ||||
| Lower extremity ischemia | 2 (8.7) | 1 (10) | 1 (7.7) | 1.000 |
| Stroke | 1 (4.3) | 1 (10) | 0 (0) | 0.435 |
| Coma or brain hypoxia | 4 (17.4) | 4 (40) | 0 (0) | 0.024 |
| Significant bleeding | 8 (34.8) | 4 (40) | 4 (30.8) | 0.685 |
| Rethoractomy for bleeding | 5 (21.7) | 2 (20) | 3 (23.1) | 1.000 |
| Vasopressor/inotrope on ECMO 1st day | ||||
| Dopamine (μg/kg/min) | 0.0 (9.5) | 0.0 (4.7) | 0.0 (10.7) | 0.538 |
| Norepinephrine (μg/kg/min) | 0.1 (0.2) | 0.1 (0.3) | 0.0 (0.2) | 0.324 |
| Dobutamine (μg/kg/min) | 0.0 (6.3) | 5.0 (5.0) | 0.0 (0.0) | 0.032 |
| Epinephrine (μg/kg/min) | 0.1 (0.4) | 0.4 (0.4) | 0.0 (0.2) | 0.027 |
| Biochemistry data on ECMO 1st day | ||||
| MAP (mmHg) | 58 (19) | 55 (21) | 59 (14) | 0.306 |
| Diuresis (ml/kg/hr) | 0.9 (1.1) | 1.2 (1.0) | 0.9 (1.0) | 0.495 |
| SCr (mg/dL) | 1.4 (0.8) | 1.3 (0.5) | 1.5 (0.9) | 0.321 |
| WBC count (cu/mm) × 1000 | 16.0 (17.8) | 16.9 (12.3) | 15.7 (17.8) | 0.756 |
| Hemoglobin (g/dL) | 9.2 (1.5) | 9.1 (1.1) | 9.4 (2.0) | 0.710 |
| Platelets (× 109/L) | 9.7 (8.6) | 9.0 (7.9) | 10.2 (10.9) | 0.535 |
| Sodium (mEq/L) | 143 (18) | 147 (20) | 143 (11) | 0.456 |
| Potassium (mEq/L) | 3.2 (1.8) | 3.2 (2.0) | 3.6 (1.5) | 0.926 |
| Albumin (g/L) | 2.7 (0.7) | 2.8 (0.2) | 2.7 (1.1) | 1.000 |
| Lactate (mmol/L) | 79.4 (48.9) | 83.2 (75.2) | 75.3 (15.2) | 0.710 |
| PaO2/FiO2 | 384 (235) | 187 (411) | 395 (99) | 0.193 |
| AaDO2 | 237 (163) | 388 (382) | 235 (87) | 0.172 |
| APACHE II score | 23 (10) | 26 (10) | 23 (8) | 0.153 |
| SOFA score | 10 (5) | 11 (5) | 9 (2) | 0.026 |
| Acute kidney injury, n (%) | 18 (78.3) | 8 (80) | 10 (76.9) | 1.000 |
| KDIGO criteria (Stage 0/1/2/3) | 5/10/4/4 | 2/4/3/1 | 3/6/1/3 | 0.572 |
| Renal replacement therapy, n (%) | 10 (43.5) | 4 (40) | 6 (46.2) | 1.000 |
Continuous data were presented median (interquartile); ECMO extracorporeal membrane oxygenation, ICU intensive care unit, IABP intraaortic balloon pumping, VT ventricular tachycardia, MAP mean arterial pressure, SCr serum creatinine, WBC white blood cell, PaO2 partial pressure of oxygen, FiO2 fraction of inspired oxygen, AaDO2 alveolar-arterial oxygen tension difference, APACHE II acute physiology and chronic health evaluation II, SOFA sequential organ failure assessment, KDIGO kidney disease improving global outcomes
Table 2.
Patients’ endothelial biomarkers in the first 3 days
| Biomarker | All Patients (n = 23) | Non-Survivors (n = 10) | Survivors (n = 13) | P value |
|---|---|---|---|---|
| Thrombomodulin (ng/mL) | ||||
| Day 0 | 5.9 (2.4) | 6.3 (1.9) | 5.6 (1.8) | 0.420 |
| Day 1 | 6.3 (1.9) | 6.0 (1.9) | 6.7 (1.6) | 0.535 |
| Day 2 | 7.5 (2.7) | 6.8 (3.0) | 7.5 (2.2) | 0.215 |
| Angiopoietin-1 (ng/mL) | ||||
| Day 0 | 29.0 (16.1) | 30.8 (13.0) | 22.1 (18.7) | 0.203 |
| Day 1 | 24.9 (17.1) | 24.2 (8.8) | 26.2 (15.8) | 0.107 |
| Day 2 | 20.7 (13.8) | 20.1 (6.6) | 22.2 (9.5) | 0.172 |
| Angiopoietin-2 (ng/mL) | ||||
| Day 0 | 19.2 (24.8) | 24.4 (58.2) | 15.7 (23.2) | 0.035 |
| Day 1 | 24.7 (35.3) | 25.6 (29.0) | 17.7 (26.6) | 0.137 |
| Day 2 | 22.7 (15.4) | 23.7 (14.7) | 20.3 (9.2) | 0.577 |
| VEGF (pg/mL) | ||||
| Day 0 | 8.5 (13.7) | 15.3 (32.4) | 7.9 (2.1) | 0.071 |
| Day 1 | 33.0 (65.0) | 24.2 (37.6) | 35.6 (58.1) | 0.438 |
| Day 2 | 62.1 (119.2) | 24.2 (33.9) | 119.9 (105.8) | 0.005 |
| Ang-2/Ang-1 ratio | ||||
| Day 0 | 0.82 (1.82) | 0.90 (1.53) | 0.71 (2.20) | 0.470 |
| Day 1 | 1.01 (1.74) | 1.01 (3.67) | 0.79 (1.95) | 0.342 |
| Day 2 | 1.09 (0.64) | 1.10 (0.21) | 1.02 (1.54) | 0.763 |
Data were presented median (interquartile); VEGF vascular endothelial growth factor, Ang angiopoietin
Fig. 1.
Median values (lower limit of bar represents 25th percentile and upper limit of bar represents 75th percentile) of endothelial biomarkers in the non-survivors and survivors. * indicates P < 0.05 between non-survivors and survivors. ECMO, extracorporeal membrane oxygenation; VEGF, vascular endothelial growth factor; Ang, angiopoietin
Fig. 2.
Receiver operating characteristic curves (ROC) of discriminating mortality for (a) at day 0, (b) at day 1, (c) at day 2, and (d) combination of angiopoietin-2 at day 0 and VEGF at day 2. The area under ROC of angiopoietin-2 at day 0 + VEGF at day 2 was 0.854 (95% confidence interval, 0.645 to 0.965). VEGF, vascular endothelial growth factor; Ang, angiopoietin
Discussion
To our knowledge, this study is the first to investigate the relationship between endothelial biomarkers and mortality in patients on ECMO. In this study, we noticed a higher level of Ang-2 in non-survivors compared to that in survivors. Besides, we also observed that the combination of Ang-2 at day 0 and VEGF at day 2 showed a modest performance on mortality discrimination in patients on ECMO.
The initiation of ECMO brings an immediate and complex inflammatory reaction in patients, as seen in systemic inflammatory response syndrome. The inflammatory reaction then results in the widespread activation of the endothelium and induces pro-inflammatory cytokines secretion [25]. Moreover, active diseases that require ECMO support may be associated with endothelial inflammation, such as cardiotomy surgery and acute myocardial infarction. Non-pulsatile flow during aortic cross-cramping during cardiotomy is associated with diminished endothelial shear stress and reduced endothelial nitrogen oxide production, while intra-aortic balloon pump support provides steady pulsatile flow that induces a steady shear stress on the endothelial cells, thereby reducing endothelial activation and inflammatory response [26, 27]. Acute kidney injury following ECMO support is also related to endothelial injury [28]. Therefore, endothelial injury is an important issue in patients on ECMO.
Previous studies have shown that Ang-2 levels are associated with mortality in critically-ill patients [29–32]. Ang-2, a competitive antagonist of Ang-1, reacts with Tie2 receptor to maintain vascular stability. Upon inflammatory stimuli, Ang-2 is released from the Weibel-Palade bodies, causing capillary leakage and facilitating leukocyte migration [33]. In patients on ECMO, Ang-2 increases in response to early endothelial activation. Although it didn’t reveal a close relationship with acute kidney injury in our study, it still provided a potential marker for mortality prediction in patients on ECMO. Besides, Ang-2/Ang-1 ratio increases during capillary endothelial damage, and high Ang-2/Ang-1 ratio is related to poor outcome in patients with sepsis [20, 21]. In our study, the Ang-2/Ang-1 ratio increased gradually in both groups and was higher in non-survivors, which may implicate more severe endothelial damage in the non-survivor group.
VEGF is considered as an endothelial survival factor that prevents microvascular apoptotic cell loss in vitro [34]. Both low and high VEGF concentrations have been reported in critically-ill patients [24, 30, 35], and the significance of which is not fully understood. In our study, the VEGF concentration in the survivor group continued to increase over the first 72 h and was higher than the non-survivor group, which was similar to previous studies [24]. VEGF modulates the effect of Ang-2 in a context-dependent fashion: Ang-2 promotes basal lamina remodeling and endothelial cell proliferation at high VEGF concentration, but causes endothelial cell death and vessel regression if VEGF is inhibited [36]. In our study, we observed that survivors had significantly higher 72-h VEGF concentration compared to non-survivors. Higher VEGF concentration may modulate the Ang-2 effect and help endothelial cell proliferation and neovascularization, but the detailed relationship with mortality needs further studies to evaluate and confirm.
There are some limitations in our study. First, our study was performed at a tertiary care center with a small sample size. Although it was a prospective study, many next-of-kin of the patients declined to join the study at the time of ECMO support due to the critical condition of the patients. Large-scale studies at multiple centers should be performed to confirm these findings. Second, although we excluded patients on V-V ECMO support and only collected patients on V-A ECMO support, the diversity of the diseases indicated for ECMO support may still affect the results, and further subgroup investigations are needed to explore the relationship between specific diseases and endothelial biomarkers. Third, we did not compare the differences in endothelial biomarker levels with a control group because we could not find a group of patients with the same disease severity but without ECMO support.
In summary, we presented a relationship between endothelial biomarker changes and mortality in patients on V-A ECMO. The combination of Ang-2 at day 0 and VEGF at day 2 was a modest model for mortality discrimination in this group of patients. However, further larger studies are warranted due to the small sample size at a single tertiary-care medical center in this study.
Acknowledgements
The authors thank the staff of the Chang Gung Kidney Research Center and Chang Gung Memorial Hospital ICUs for their assistance.
Funding
No funding was received.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Ang
Angiopoietin
- AUROC
Area under receiver operating characteristic curve
- CI
Confidence interval
- ECMO
Extracorporeal membrane oxygenation
- ICU
Intensive care unit
- IRB
Institutional review board
- ROC
Receiver operating characteristic
- SOFA
Sequential organ failure assessment
- TM
Thrombomodulin
- V-A
Veno-arterial
- VEGF
Vascular endothelial growth factor
- V-V
Veno-venous
Authors’ contributions
TYT contributed to collecting data and manuscript drafting. KHT, CHC, and PCF revised the manuscript and conducted the statistical analysis. FCT and YYN helped with acquisition and interpretation of data. YCT, JTF, and CWY contributed to provide intellectual content of the work and involved in editing the manuscript. YCC contributed to the conception, design, and interpretation of data. All authors critically revised the manuscript. All authors have seen and approved the final draft of the manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from the next-of-kin of the patients before their participation. The study was approved by the local Institutional Review Board of Chang Gung Memorial Hospital approved the study protocol (Institutional Review Board No. 103-1569C).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lin CY, Tsai FC, Tian YC, et al. Evaluation of outcome scoring systems for patients on extracorporeal membrane oxygenation. Ann Thorac Surg. 2007;84:1256–1262. doi: 10.1016/j.athoracsur.2007.05.045. [DOI] [PubMed] [Google Scholar]
- 2.Chen YC, Tsai FC, Chang CH, et al. Prognosis of patients on extracorporeal membrane oxygenation: the impact of acute kidney injury on mortality. Ann Thorac Surg. 2011;91:137–142. doi: 10.1016/j.athoracsur.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 3.Aubron C, Cheng AC, Pilcher D, et al. Factors associated with outcomes of patients on extracorporeal membrane oxygenation support: a 5-year cohort study. Crit Care. 2013;17:R73. doi: 10.1186/cc12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zangrillo A, Landoni G, Biondi-Zoccai G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:172–178. [PubMed] [Google Scholar]
- 5.Tsai TY, Tsai FC, Chang CH, et al. Prognosis of patients on extracorporeal membrane oxygenation plus continuous arteriovenous hemofiltration. Chang Gung Med J. 2011;34:636–643. [PubMed] [Google Scholar]
- 6.D'Arrigo S, Cacciola S, Dennis M, et al. Predictors of favourable outcome after in-hospital cardiac arrest treated with extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Resuscitation. 2017;121:62–70. doi: 10.1016/j.resuscitation.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Zwiers AJ, Cransberg K, de Rijke YB, van Rosmalen J, Tibboel D, de Wildt SN. Urinary neutrophil gelatinase-associated lipocalin predicts renal injury following extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2015;16:663–670. doi: 10.1097/PCC.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 8.Bembea MM, Rizkalla N, Freedy J, et al. Plasma biomarkers of brain injury as diagnostic tools and outcome predictors after extracorporeal membrane oxygenation. Crit Care Med. 2015;43:2202–2211. doi: 10.1097/CCM.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 9.Aird WC. Vascular bed-specific hemostasis: role of endothelium in sepsis pathogenesis. Crit Care Med. 2001;29(7 Suppl):S28–S34. doi: 10.1097/00003246-200107001-00013. [DOI] [PubMed] [Google Scholar]
- 10.Mikacenic C, Hahn WO, Price BL, et al. Biomarkers of endothelial activation are associated with poor outcome in critical illness. PLoS One. 2015;10:e0141251. doi: 10.1371/journal.pone.0141251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson-Cohen C, Katz R, Price BL, et al. Association of markers of endothelial dysregulation Ang1 and Ang2 with acute kidney injury in critically ill patients. Crit Care. 2016;20:207. doi: 10.1186/s13054-016-1385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou PC, Filbin MR, Wang H, et al. Endothelial permeability and hemostasis in septic shock: results from the ProCESS trial. Chest. 2017;152:22–31. doi: 10.1016/j.chest.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maruyama I, Bell CE, Majerus PW. Thrombomodulin is found on endothelium of arteries, veins, capillaries, and lymphatics, and on syncytiotrophoblast of human placenta. J Cell Biol. 1985;101:363–371. doi: 10.1083/jcb.101.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conway EM. Thrombomodulin and its role in inflammation. Semin Immunopathol. 2012;34:107–125. doi: 10.1007/s00281-011-0282-8. [DOI] [PubMed] [Google Scholar]
- 15.Lin SM, Wang YM, Lin HC, et al. Serum thrombomodulin level relates to the clinical course of disseminated intravascular coagulation, multiorgan dysfunction syndrome, and mortality in patients with sepsis. Crit Care Med. 2008;36:683–689. doi: 10.1097/CCM.0B013E31816537D8. [DOI] [PubMed] [Google Scholar]
- 16.Sapru A, Calfee CS, Liu KD, et al. Plasma soluble thrombomodulin levels are associated with mortality in the acute respiratory distress syndrome. Intensive Care Med. 2015;41:470–478. doi: 10.1007/s00134-015-3648-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asahara T, Chen D, Takahashi T, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.RES.83.3.233. [DOI] [PubMed] [Google Scholar]
- 18.Lemieux C, Maliba R, Favier J, Theoret JF, Merhi Y, Sirois MG. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. 2005;105:1523–1530. doi: 10.1182/blood-2004-09-3531. [DOI] [PubMed] [Google Scholar]
- 19.Liu KL, Lee KT, Chang CH, Chen YC, Lin SM, Chu PH. Elevated plasma thrombomodulin and angiopoietin-2 predict the development of acute kidney injury in patients with acute myocardial infarction. Crit Care. 2014;18:R100. doi: 10.1186/cc13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ricciuto DR, dos Santos CC, Hawkes M, et al. Angiopoietin-1 and angiopoietin-2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit Care Med. 2011;39:702–710. doi: 10.1097/CCM.0b013e318206d285. [DOI] [PubMed] [Google Scholar]
- 21.Fang Y, Li C, Shao R, Yu H, Zhang Q, Zhao L. Prognostic significance of the angiopoietin-2/angiopoietin-1 and angiopoietin-1/Tie-2 ratios for early sepsis in an emergency department. Crit Care. 2015;19:367. doi: 10.1186/s13054-015-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher J, Douglas JJ, Linder A, Boyd JH, Walley KR, Russell JA. Elevated plasma angiopoietin-2 levels are associated with fluid overload, organ dysfunction, and mortality in human septic shock. Crit Care Med. 2016;44:2018–2027. doi: 10.1097/CCM.0000000000001853. [DOI] [PubMed] [Google Scholar]
- 23.van der Flier M, van Leeuwen HJ, van Kessel KP, Kimpen JL, Hoepelman AI, Geelen SP. Plasma vascular endothelial growth factor in severe sepsis. Shock. 2005;23:35–38. doi: 10.1097/01.shk.0000150728.91155.41. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson S, Pettilä V, Tenhunen J, et al. Vascular endothelial growth factor in severe sepsis and septic shock. Anesth Analg. 2008;106:1820–1826. doi: 10.1213/ane.0b013e31816a643f. [DOI] [PubMed] [Google Scholar]
- 25.Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20:387. doi: 10.1186/s13054-016-1570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macha M, Yamazaki K, Gordon LM, et al. The vasoregulatory role of endothelium derived nitric oxide during pulsatile cardiopulmonary bypass. ASAIO J. 1996;42:M800–M804. doi: 10.1097/00002480-199609000-00101. [DOI] [PubMed] [Google Scholar]
- 27.Onorati F, Santarpino G, Tangredi G, et al. Intra-aortic balloon pump induced pulsatile perfusion reduces endothelial activation and inflammatory response following cardiopulmonary bypass. Eur J Cardiothorac Surg. 2009;35:1012–1019. doi: 10.1016/j.ejcts.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 28.Basile DP. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 29.Gallagher DC, Parikh SM, Balonov K, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008;29:656–661. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kümpers P, Lukasz A, David S, et al. Excess circulating angiopoietin-2 is a strong predictor of mortality in critically ill medical patients. Crit Care. 2008;12:R147. doi: 10.1186/cc7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siner JM, Bhandari V, Engle KM, Elias JA, Siegel MD. Elevated serum angiopoietin 2 levels are associated with increased mortality in sepsis. Shock. 2009;31:348–353. doi: 10.1097/SHK.0b013e318188bd06. [DOI] [PubMed] [Google Scholar]
- 32.van der Heijden M, Pickkers P, van Nieuw Amerongen GP, et al. Circulating angiopoietin-2 levels in the course of septic shock: relation with fluid balance, pulmonary dysfunction and mortality. Intensive Care Med. 2009;35:1567–1574. doi: 10.1007/s00134-009-1560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Meurs M, Kümpers P, Ligtenberg JJ, Meertens JH, Molema G, Zijlstra JG. Bench-to-bedside review: angiopoietin signalling in critical illness - a future target? Crit Care. 2009;13:207. doi: 10.1186/cc7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 35.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, van Hinsbergh VW, Groeneveld AB. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008;63:903–909. doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 36.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99:11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.