Abstract
Medical imaging modalities are used for different types of cancer detection and diagnosis. Recently, there have been a lot of studies on developing novel nanoparticles as new medical imaging contrast agents for the early detection of cancer. The aim of this review article is to categorize the medical imaging modalities accompanying with using nanoparticles to improve potential imaging for cancer detection and hence valuable therapy in the future. Nowadays, nanoparticles are becoming potentially transformative tools for cancer detection for a wide range of imaging modalities, including computed tomography (CT), magnetic resonance imaging, single photon emission CT, positron emission tomography, ultrasound, and optical imaging. The study results seen in the recent literature provided and discussed the diagnostic performance of imaging modalities for cancer detections and their future directions. With knowledge of the correlation between the application of nanoparticles and medical imaging modalities and with the development of targeted contrast agents or nanoprobes, they may provide better cancer diagnosis in the future.
Keywords: Cancer, diagnosis, medical imaging, nanoparticles
INTRODUCTION
At present, molecular imaging (MI) is an advanced imaging technique that provides detailed images of what is happening inside the body at the molecular and cellular levels. MI is sophisticated enough to characterize and measure the biological process at the cellular and subcellular of a living organism. Therefore, the mortality rate of cancer significantly increases for earlier detection of cancer, precise diagnosis of diseases, and enhancement of treatment results using appropriate imaging probes.[1]
MI probes can be divided into four groups: (a) phenotype probes, (b) targeted probes, (c) cell-tracking probes, and (d) reporter gene probes. The first category is utilized to determine the general features of malignant physiology, such as angiogenesis, cell proliferation, and apoptosis, and the expression of certain receptors in tumor cells. The second category is employed to image specific biomolecules, which are the characteristics of a tumor or even a class of tumors. In addition, the third category is used to localize and follow the movement of cells, which may be of importance for tumor survival. The cells can be labeled directly with tags or indirectly via the insertion of marker genes. Finally, it is the reporter gene probes which are used to monitor the action of genes in biological in vivo systems.
MI probes include targeting components such as antibodies,[2,3] dendrimers,[4] aptamers,[5] peptides,[6] or small molecules and signaling components, including radionuclides, fluorochromes for optical imaging, or paramagnetic chelates for magnetic resonance imaging (MRI).[7,8] Nanosized probes with unique properties have emerged as a potential class of MI.[9]
Clinicians use six imaging modalities to diagnose and treat cancer. However, only four of these imaging modalities, including computed tomography (CT), MRI, single-photon emission CT (SPECT), and positron emission tomography (PET), are widely used for three-dimensional (3D) in cancer staging.[10] Virtually, all four 3D imaging modalities suffer from deficiencies in sensitivity and resolution. With the possibility to solve several clinical problems in terms of scale and to enhance diagnosis ability, they were not designed to image small numbers of cancer cells. The aim of cancer imaging should be to detect the smallest possible number of cancer cells before the angiogenesis. Nowadays, nanoparticles have attracted significant attention as contrast agents for primary detection of cancer in the current imaging systems such as MI. Using nanoparticles, it is possible to (a) achieve great specificity toward a target (thus eliminating the hazard of side effects), (b) deliver a large payload quantity in a single dose, and (c) simultaneously deliver both imaging agents and therapeutics.
In this review article, recent advanced approaches in medical imaging modalities accompanying with using nanoparticles to improve potential imaging for cancer detection and hence valuable therapy in the future are considered.
POSITRON EMISSION TOMOGRAPHY
PET modality is designed to detect the paired 511 keV gamma rays generated from the annihilation event of a positron and an electron. The paired annihilation photons travel in opposite directions (180° apart) along a line. A PET detector surrounding the target can prepare signals and convert them into tomographic images. PET becomes a powerful tool for clinical diagnosis due to its high sensitivity, limitless depth of penetration, and quantitative capabilities.[10] In the clinical application, the PET scan is essential for cancer detection and staging as well as evaluation of the response of cancer to therapy. The main disadvantage of PET is the lack of anatomical information, and this has recently been compensated by merging these devices with either CT or MRI known as dual modalities imaging named PET/CT and PET/MRI.[11]
PET/CT has changed the diagnostic algorithm in oncology by substantially influencing the management of patients with cancer.[12] The most relevant biomarker for cancer among a variety of radiopharmaceuticals for molecular and metabolic imaging with PET is the fluorodeoxyglucose (FDG) (FDG-PET/CT). In contrast to conventional imaging modalities such as CT, ultrasound (US), and MRI, which detect tumors based on morphological alterations, PET detects and characterizes tumors based on molecular alterations and known as functional imaging modality. Following intravenous injection, FDG, similar to normal glucose, is taken up by cancer cells. The subsequent conversion of FDG to FDG-6-monophosphate by the intracellular enzyme hexokinase leads to trapping of the metabolite within the cancer cells.[13] This increased glucose metabolism in malignancies is mediated via increased expression and activity of glucose transporters in the cell membrane as well as via changes in the glycolytic enzyme expression and activity. This alteration in glucose metabolism represents one of the early events in carcinogenesis. PET/CT is also helpful in localizing tumors in cases where conventional diagnostic methods are unable to localize the cancer of unknown primary. For cancer staging, PET/CT offers many advantages over conventional imaging strategies. FDG-PET has high accuracy for staging cancers such as nonsmall cell lung, gastrointestinal tract, including colorectal and esophageal, thyroid, head and neck, and breast.[14,15,16]
New radioligand (68Ga-NOTA-AE105) in PET/CT was applied to detect the expression of urokinase-type plasminogen activator receptors on breast, urinary bladder, and prostate cancer, and no adverse events were reported with mentioned cancers after administration of this radioligand.[17]
Rowe et al.[18] conducted a study that finally introduced (2-(3-(1-carboxy-5-[(6-[18F] fluoro-pyridine- 3-carbonyl)-amino]-pentyl)-ureido) or [18F] DCFPyL, a novel low molecular weighted radiotracer. They reported that the novel radiotracer was targeted PSMA in patient with metastatic prostate cancer. Furthermore, Rowe et al.[19] have used biomarker including 18F-DCFBC using PET combination with MRI for the detection of prostate cancer.
In another study, Ellison et al.[20] introduced a novel image-guided radioarsenic-labeled thiolated mesoporous silica nanoparticle using PET scan for cancer patients. Moreover, for PET imaging of tumor vasculature, Chakravarty et al.[21] developed the hollow mesoporous silica nanoparticles conjugated Cu-1,4,7-triazacyclononane-1,4,7-triacetic acid-PEG-cRGDyK. Fabricated contrast agents such as 111In, 56Fe, and 14C conjugated with superparamagnetic iron oxide nanoparticles (SPION) for PET and SPECT imaging were done by Wang et al.[22] to evaluate their biodistribution in vivo. Using64Cu-doped PdCu@Au tripods as a photothermal therapy target for breast cancer was carried out by Pang et al.[23]
Interestingly, Pascual's group conjugated mesoporous silica nanoparticle with MUC1 aptamer as a radiolabelled tracer for PET and SPECT. They reported that 99mTc (S1-ap-MUC1-Tc) showed significant targeting in tumor-bearing mouse model.[24]
In addition, Zhao et al.[25] conjugated 199Au with D-Ala 1-peptide T-amide which could detect mouse triple-negative breast cancer and its malignancy using SPECT for in vivo studies. The 99mTc-labeled polyethylene glycol (PEG) iron oxide developed to display multicontrast agent for sentinel lymph node.
Recently, a dual probe,99mTc-radiolabeled nanosilica system conjugated with a trastuzumab half-chain for aggressive HER2-positive breast cancer was developed by Rainone et al.[26] as a theranostic probe. Moreover, SPECT and MRI provided presurgical information such as the location of the sentinel lymph node. It was reported that the multicontrast could be used for malignant melanoma as well as breast cancer imaging.[27]
PET can act as a guided device for different types of cancer treatment such as photothermal therapy. Furthermore, application of PET or SPECT in medicine allows researchers to investigate biodistribution and pharmacokinetics of tumor targeting biomarkers in the tumor site and also monitor its progression.
MAGNETIC RESONANCE IMAGING
The physical principle of MRI is the interaction of nuclei, which have a nonzero magnetic moment, within an external magnetic field. The spin of nuclei protons according to the strength of external magnetic field are affected by applying the radiofrequency (RF) pulse. When the RF pulse is turned off, the protons can return to the original state by transferring energy to the general structure of material and generating an RF signal. The entire process is known “relaxation.” After measuring the relaxation by receiver coils, the signals generated by relaxations will produce an image. The excellent anatomical resolution is the reason why MRI has attracted such great interest among medical professionals.
Over the past decades, the improvement in the instrument has brought MRI into a new era of MI. The excellent features of MRI include comparably high temporal and spatial resolution, high tissue contrast, nonionizing radiation, noninvasive, and simultaneous acquisition of anatomical and functional information.[28] Limitation of molecular MRI is low sensitivity which can improve using of imaging contrast agents.
SPIONs, ultrasmall SPIONs, Gd-encapsulated silicon microparticles, and Gd-ion-doped upconversion nanoparticles (UCNPs) are mainly domain of MRI contrast agents.[3]
A lot of work has been done on Gd-based contrast agents for MRI, which are among the world most recognized noninvasive techniques employed in clinical diagnosis. At ionic state, Gd is considered toxic but has less toxicity in its chelating form. Nanocarriers such as gadolinium oxide (Gd2O3) nanoparticles have been used by researchers to improve the T1 and T2 contrast of MRI.[3]
There is plenty of MR MI, which has proven the potential of this imaging modality. In recent years, using nanoparticle and nanotechnology is interest among researchers to increase MRI contrast in particular for cancer detection in early stages.
In earlier work, conjugation 9.2.27 monoclonal antibodies (Mabs) against human melanoma and WM53 Mabs against human leukemia cells with cyclic anhydride gadolinium-dietheylenetriaminepenta-acetic (DTPA) were performed by Shahbazi-Gahrouei et al.[29,30] Further, they introduced Gd-DTPA-C595 that promised contrast agents for the detection of MCF-7 breast cancer cells and Gd-hematoporphyrin for detection and diagnosis of melanoma, colorectal, as well as breast cancer in MRI.[31] In mentioned studies, lack of using nanoparticle technology was predicted by the researchers. Several years later, Mirzaei et al.[8] introduced dual probe, Anionic Linear Globular Dendrimer G2 with C595 Mab (Gd3+-ALGDG2-C595) as a therapeutic and imaging agent for breast cancer.
In this line, Shahbazi-Gahrouei et al.[32,33,34] conducted a study using SPIONs-C595 for the detection of MUC1-expressing ovarian cancer. Their findings revealed great tumor accumulation and detection of ovarian cancer by the nontoxic nanoprobe as a specific ovarian MRI contrast agent. Shahbazi-Gahrouei[35] has synthesized and applied a conjugated SPIONs-C595 against ovarian cell surface for early detection of ovarian cancer. In another study, he has introduced SPIONs conjugated J591 Mab and their experiments confirmed the high potential of the nanoprobe as a specific MRI contrast agent for the detection of PSMA-expressing prostate cancer.[36]
In an international work, authors[5,6] conducted a study on SPIONs conjugated with C595 Mab for early detection of overexpressed MUC1 breast cancer. Findings demonstrated signal intensity enhancement of MRI by T1 and T2 relaxation times in breast cancer cells (MCF-7). They concluded that SPIONs-C595 exhibited high-dual (T1 and T2) MRI contrast potential and might be applied as specific breast cancer (MCF-7) cell detection.
Due to extensive applications of magnetic iron oxide nanoparticles in biomedicine, many studies were performed under in vitro conditions and their cytotoxicity was investigated.[5,36] In this way, Keshtkar et al.[5] prepared a novel aptamer-conjugated nanoparticle using AS1411 aptamer which was conjugated to Fe3O4@Au nanoparticles on mouse mammary carcinoma (4T1) cells that overexpressed nucleolin. They found that the synthesized nanoprobe produced strongly darkened T2-weighted (90% reduction of signal intensity) in 4T1 cells at 45 μg/mL concentration.
Recently, Ghahremani et al. in 2018[36,37] introduced bovine serum albumin (BSA) capped gold nanoclusters targeted by AS1411 aptamer as a gold radiosensitizing (with size of 10–15 nm) candidate for megavoltage radiotherapy and biomedical imaging of breast cancer cells (4T1) targeting agent which its schematic preparation is shown in Figure 1.
Figure 1.
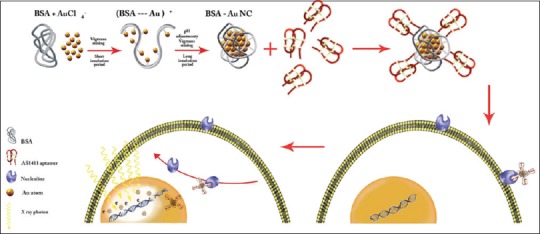
Schematic illustration of AS1411 aptamer conjugated bovine serum albumin (BSA)–gold nanoparticles (GNCs) preparation process and the mechanism of its internalization by cancer cells[37]
Liu et al. synthesized and applied hydrophilic CaF2:Yb, Er@CaF2:Gd nanoparticles modified using PEG-PAA di-block copolymer benefited from the presence of Gd only in the outer CaF2 layer of the nanoparticles Gd3+-doped CaF2-based core-shell nanoparticles for efficient magnetic resonance angiography and tumor diagnosis.[38]
Gd-based BSA hybrid-coated hollow gold nanoshells was applied for nanotheranostic agent due to its hollow and porous structures, hence possessing photodynamic/photothermal property and near-infrared (NIR) fluorescence/PA and excellent T1 contrast agent for MRI capability.[39]
A multimodal contrast agent for integrated preoperative MRI and intraoperative fluorescence image-guided surgery (FIGS) was introduced by Payne et al.,[40] in which self-assembled multimodal imaging nanoparticles were developed as a mixed micelle formulation using amphiphilic HA polymers functionalized with either Gd-DTPA for T1-weighted MRI. Payne et al. have employed simulated surgical phantoms that were routinely used to evaluate the depth at which NIR imaging agents could be detected by FIGS. Nanoprobe imaging agent efficacy was also evaluated in a human breast tumor xenograft model in nude mice. Future studies include different nontoxic nanoparticle agents as an MRI potential for both diagnosis and therapy.
COMPUTED TOMOGRAPHY
CT is an X-ray imaging modality which is very useful in medical imaging centers for cancer diagnosis. A CT scan image provides 2D cross-sectional image structures inside of the human body. Therefore, transparent tissues such as cancers can be imaged by CT. However, low signal-to-noise ratio reduced the ability of CT to distinguish between neighboring tissues. To overcome these disadvantages, using contrast media is established.
Several heavy atoms such as iodine, tungsten, and barium as contrast media are enhanced by the CT images due to great X-ray attenuation coefficient of mentioned metals. Moreover, somatostatin analog such as 1,4,7,10-tetraazocyclododecane-N, N′N″, N′″-tetraacetic acid PET/CT and 111In-octreotide improve the diagnostic of medullary thyroid and bone metastases.[41] Researchers have endeavored to develop and launch different types of contrast media as they are able to accumulate selectively at the target either chemically or physically interact with the desired site of the target. CT provides inexpensive and unmatched high spatial resolution images of anatomical structure as well as blood vessels. This imaging technique is also much faster and available than MRI.[42] CT always merges with other modalities for anatomical imaging such as MRI, SPECT, PET as well as MI to enhance functional imaging and to reflect essentially an anatomical conversion by employing novelties beacons that detect cellular events.[43]
For instance, SPECT/CT may help the oncologist for surgical decision by utilizing 99mTc-tri-peptide sequence of arginine-glycine-aspartic acid SPECT/CT for patients whom raised with lymph node metastatic.[44] Pandit-Taskar et al.[45] took advantage of the feature that 89Zr-DFO-huJ591 PET/CT-targeted PSMA for prostate cancer. They proved that developed imaging biomarker detected positive soft tissue sites and positive bone lesion for prostate cancer using PET/CT. The ability of the fabricated CT contrast agent, 2-deoxy-d-glucose (2-DG) labeled gold nanoparticle (Au-NP) was proved by Li et al.,[46] for the detection of human epithelial cancer cell. Aydogan[47] introduced 2-DG conjugated onto Au-NP as potential functional CT contrast for cancer detection.
Nanoparticles by surface modification have been functionalized to target affinity side of tumors by attaching to receptors such as the overexpression of folic acid or specific antigens on cancer cells. The mentioned operative approach for delivery of CT contrast media is called active targeting. Generally, there are three major applications of nanoparticles which utilize as X-ray contrast media in diagnosis; (a) blood pool, (b) passive targeting, and (c) active targeting. Shi et al.[48] used Au (III) ions to fabricate a novel dendrimer-stabilized Au-NP for imaging and targeting cancer cells. They found that Au-DSNPs targeted the cells that expressed folic acid and fluorescein isothiocyanate.
Iodinated gold nanoclusters (AuNCs-BSA-I) via BSA and chloramine-T was synthesized by Chen et al.[49] for fluorescence/CT imaging of human thyroid cancer. In a study, Nakagawa et al.[50] prepared PEG functionalized Au-NPs (Au-PEG), conjugated with the anti-HER2 antibody and showed its capable of functioning as CT imaging contrast media in breast cancer. Wei et al.[51] reported the use of dendrimer as modified small molecule of iodinated contrast media accompanying with inorganic nanoparticles improve blood circulation time of the contrast media which make it better for CT imaging. It was reported that methyl-orange-doped polystyrene Au-NP not only confirmed as CT contrast media but also suggested that it can be utilized for blood pool imaging.[52] Todays, Au-NPs for application in CT imaging due to its larger size, which resulted in longer circulation times and accumulation in tumor sites is more interested.
ULTRASOUND MOLECULAR IMAGING
It is a unique modality in the sense that it can be applied for both imaging and therapeutic purposes. In diagnostic purposes of US modality, one type of new contrast agent is microbubble (MB). MB can enhance the specificity and sensitivity of this modality for cancer detection. However, micrometer range size of MB is an obstacle for extravasation from the vasculature.
Common targets for molecular US imaging are surface receptor molecules expressed on the luminal side of activated endothelial either in response to inflammation or for angiogenic stimuli. Angiogenesis, a process of new blood vessels formation, plays the main role in tumor neovascularization. Neoangiogenesis is the initiation of tumors, which can be predicted by spreading cancer cells to other organs. Angiogenesis needs oxygen and other nutrients to remove useless cellular residues. Angiogenesis has a major role in tumor growth in many types of cancers. BR55, VEDFR2-specific US molecular contrast agent confirmed the simplifying of the prostate cancer detection in men employing clinical standard technology.[53]
Targeted MB in US imaging may enhance the permeability of cellular membrane. A novel MB-hydroxycamptothecin (10-HCPT) was developed by Li et al.[54] They reported that the injection of 10-HCPT-loaded MB and exposure to US might expand the drug concentration in tumor noteworthy, leading to a considerable boost in tumor inhibition rate (70.6%), contrast to entire 10-HCPT-loaded MB (47.8%) in addition to commercial HCPT administration (49.4%). Other studies also found that combining US and MB could significantly increase the transfection efficacy.[55,56]
Therefore, researchers are paying more attention to developing nanobubble (NB)-based UCAs for tumor US imaging. Nanosized bubbles with various shells composed of polymers or phospholipids and gas and liquid of solid cores have been applied in extravascular US imaging. One strategy for detection of the tumor is to apply specific antibody conjugated with MB targeting ligands. Yang et al.[57] observed that a novel NB antibody conjugates significantly detected the HER-expressing tumor and launched as specific MI as well as targeted therapy. Recently, Li et al.[58] conducted in-vivo and in vitro studies on fabricated percutaneous needle biopsy of the lung, neuropeptide Y modified nanosized bubbles. The results significantly presented that conjugated US contrast agent and targeted Y1 receptor overexpressed breast tumor with minimal toxicity in early stages [Figure 2]. It is important to know that among many new techniques, breast US imaging using contrast agents can assess the morphology, orientation, and internal structure for diagnosis of fatty breasts and also its difference from dense glandular structures.
Figure 2.
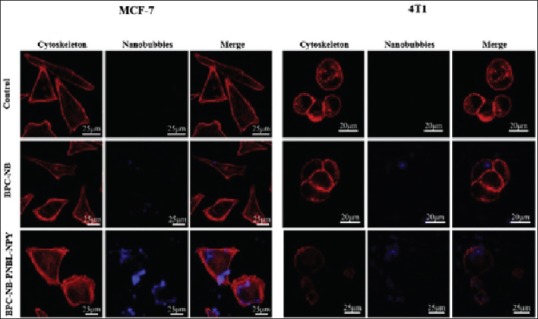
In vitro images of MCF-7 and 4T1 cells after 12 h incubation with 0.5 mg/mL biodegradable photoluminescent polymers nanobubbles (BPC-NB) and Pro30, Nle31, Bpe32, leu34 neuropeptide Y (BPC-NB-PNBL-NPY). NB are blue and cytoskeletons with rhodamine phalloidin are red[58]
OPTICAL IMAGING
Optical imaging is a noninvasive modality for looking inside the body, which significantly reduces the patient exposure to harmful radiation using nonionizing radiation such as visible, ultraviolet, and infrared light. These types of light generate images by exciting electrons without causing the damage that can occur with ionizing radiation used in some other imaging techniques. Optical imaging includes a variety of techniques such as endoscopy, optical coherence tomography (OCT), photoacoustic imaging, Raman spectroscopy, diffuse optical tomography, and superresolution microscopy.
Recently, many studies have performed on the application of nanoparticles in optical imaging. For instance, in a study, Xi et al.[59] designed a novel nuclear targeting nanoprobe, Au-NPs conjugated to SV-4. They showed that fabricated probe could assess the cell nucleus and hence provide the significant information in living cells which employed surface-enhanced Raman scattering for the research of drug delivery and cancer therapy. Further, complementary features of iron oxide and Au-NP attracted the attention of researcher to develop the multimodal probes for noninvasive imaging modalities. Reguera et al.[60] took advantage of this feature and discovered Janus magnetic-plasmonic nanoparticles as multipurpose versatile probe for surface-enhanced Raman scattering as well as MRI and CT. The Atabaev's group designed a nontoxic dual mode nanoprobe, Au, Gd-codoped yttria that successfully enhanced T1-weighted images. Simultaneously, the mentioned nanoprobe was used for optical imaging of L-929.[61] Nowadays, scientists put much effort to develop and synthesize new versatile nanoprobes to provide prominent information on the tumor by improving contrast for imaging. Xu et al.[62] demonstrated Au-Gds by biomineralization method and summarized the capability of Au-Gds as a trimodal agent for optical imaging, MRI, and CT. Kang et al.[63] combined both optical imaging and chemotherapeutic for cancer theranostic. For this purpose, they conducted a study on a novel theranostic NP, created mesoporous hollow organosilica (C-hMOS) nanoparticles, which could solve nonachieved key for cancer diagnostic and treatment using drug delivery [Figure 3].
Figure 3.
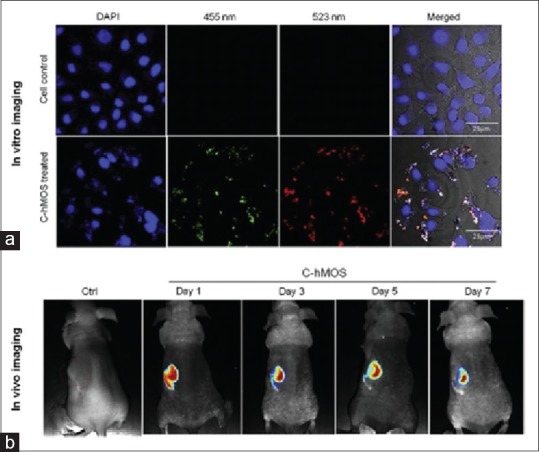
(a) Images of treated cells by 40 μg/ml dose of created mesoporous hollow organosilica (C-hMOS) nanoparticles, after a period of 4 h. To visualize nanoparticles inside cells, different wavelength used.(b) in vivo images of nude mouse which treated by C-hMOS and monitored for 7 days[63]
The principles of quantum dots in diagnostic and detection are reported in the literature by Pisanic et al.[64] They have presented a didactic overview of fundamental physical phenomena associated with quantum dots and paradigm examples of how these phenomena have been readily exploited for manifold uses in in vitro diagnostic assays and biodetection.
Synthesis of luminescent carbon dots (CDs) with ultrahigh quantum yield and inherent folate receptor-positive cancer cell targetability was performed by Liu et al.[65] They showed excellent photoluminescent activity, high photostability, and favorable biocompatibility of CDs and its great potential in biological and bioimaging studies.
The application of NIR-emitting quantum dots for HER2 localization in fixed and live cancer cells was assessed by Rizvi et al.[66] In this study, biocompatibility of SK-BR-3 and MCF-7 cell lines showed that NIR quantum dots anti-HER2-antibody bioconjugates successfully localized the studied cells at a concentration of 60 μg/mL after 1 and 24 h of exposure. They revealed that NIR quantum dot bioconjugates could be used for rapid localization of HER2 receptors and could potentially be used for targeted therapy as well as image-guided surgery.
In recent years, new fabrication and application of graphene quantum dots (GQDs) are interested among the researchers. In a study, Schroeder et al.[67] considered the ability of state-of-the-art use of GQDs in biological systems and health sciences. They reported that GQDs to be easily functionalized for use as a targeted multimodal treatment and imaging platform under in vitro and in vivo conditions with low toxicity. In another study, the conjugation of terephthalic acid on the surface of graphene quantum dots (TPA@GQDs) was performed by Hai et al.,[68] in which, TPA@GQDs was demonstrated by the quantitative fluorescent imaging of hydroxyl radical in living HeLa cells under different circumstances. The results showed that TPA@GQDs enabled the opportunities to study hydroxyl radical dynamics in living cells. They showed its low cytotoxicity and favorable biocompatibility and also recommended it as a potential image modality for cancer diagnosis.
CONCLUSION
In this review, a broad overview of nanoparticles applications in MI modalities is provided. These modalities are of great importance in the diagnosis of cancer in the early stages. Table 1 summarizes nanoprobes and targeting ligands in MI modalities for different cancer diagnosis. As can be seen from Table 1, MRI and PET are more applicable, in comparison to the other modalities for cancer diagnosis. Among all listed nanoprobes, Au-NPs have been applied and had unique role for use in drug delivery, targeting therapy, and MI.
Table1.
Nanoprobes (with their size) and targeting ligands used for cancer detection
| Nanoprobes and targeting ligand | Imaging modality | Cancer type |
|---|---|---|
| [18F] DCFPyL,[18] Cu-NOTA-PEG-cRGDyK (150-250 nm),[21] 64Cu-Doped PdCu@ Au,[23] 111In-SPION, 56Fe-SPION, and 14C-SPION,[22] 99mTc (S1-ap-MUC1-Tc)[24] | PET | Prostate, glioblastoma, colorectal,esophageal, thyroid, head and neck, breast, urinary bladder |
| 99mTcS1-ap-MUC1-Tc,[24] 56Fe-SPION, and 14C-SPION,[22] Silica,[26] 99mTc-3PRGD2[44] | SPECT | Mesothelioma, lymph node, breast, prostate, urinary bladder |
| SPIONs,[23] Gd-DTPA,[36] Gd3+-ALGDG2-C595 (61.6 nm),[5] 111In-SPION, 56Fe-SPION, and 14C-SPION Fe3O4@Au (74 nm),[36] AS1411 Ap-Au (10-15 nm),[37] CaF2:Yb, Er@CaF2:Gd (sub size 10 nm),[38] Gd-doped-Y2O3, SPION-C595 (87.4, 10-20 nm),[2,3,35] ICG-Au@BSA-Gd,[39] SPION-J59 (10-20 nm) 1[32] | MRI | Prostate, ovarian, breast, liver, glioblastoma, cervical, melanoma,colon, urinary bladder |
| 68Ga-DOTATATE,[44] 89Zr-DFO-huJ591,[48] Au PSNPs, Au-DENPs (17.8 nm),[51,52] 99mTc-3PRGD2,[44] 2-DG-Au[46,47] | CT | Lung, lymph nodes, bone, neuroglia |
| MB-10-HCPT,[54,55,56] NB-UCAs,[57] BPC-NB-PNBL-NPY[58] | Ultrasound | Colorectal, breast, liver |
| CDs, GQDs, TPA@GQDs,[68] Gold nanorods, Au-Gds (600 nm),[62] Gd-doped-Y2O3 (20 nm),[61] Au-Sv4[59] | Optical imaging | Lung, breast, cervical, colon |
PET=Positron emission tomography; SPECT=Single photon emission computed tomography; MRI=Magnetic resonance imaging; CT=Computerized tomography; CD=Carbon dots, Cu-NOTA=Cu-1,4,7-triazacyclononane-1,4,7-triacetic acid; GQD=Graphene quantum dot; SPION=Superparamagnetic iron oxide nanoparticle; CD=Carbon dot
Gold nanorods are another commonly used gold nanostructure in photothermal and NIR applications. Nowadays, Gd-doped-Y2O3 nanoprobes were used for simultaneous optical and T1-weighted MRI. Radiolabeling of SPIONs with the PET radioisotope (64Cu) and the SPECT radioisotope (67Ga) was used for a potential next generation of nontoxic multimodality PET/SPECT-MRI single probe imaging agents. These multimodality agents in the future would allow clinicians to predict the therapeutic effects, based on drug delivery in the tumor and also monitor the cancer progression in patients.
Currently, because of barriers in delivering, biological safety, and compatibility and the diversity between nanoparticles, there are only a few of MI contrast agents available in clinics. With the development of nanoparticle technology, it is predicted that most advancement will be achieved regarding to targeted MI agents. In the future, the application of nanoparticles has provided a platform for theranostic researches.
In addition, although a number of MI instruments are available, all have their limitations. For example, MRI is a useful clinical diagnostic tool, but it has poor sensitivity. Therefore, more focus should be performed on using multimodality imaging such as PET/MRI techniques to overcome these limitations. Multimodality imaging has many advantages, but some problems such as the difficulty of designing these combined systems still exist.
From the point of educational view for all medical students and nanotechnology researchers who are interested in cancer imaging and therapy, this review article could be useful. The limitation of this article is that it has not covered every aspect of all imaging modalities for cancer detection in details.
Financial support and sponsorship
This work is a part of projects (No: 194116 and 196053) which was financially supported by the Isfahan University of Medical Sciences, Isfahan, Iran.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ahmadi A, Salehi F. Evaluation of observed and the expected incidence of common cancers: An experience from Southwestern of Iran, 2010-2014. J Res Med Sci. 2018;23:4. doi: 10.4103/jrms.JRMS_788_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moradi Khaniabadi P, Shahbazi-Gahrouei D, Jaafar MS, Majid AM, Moradi Khaniabadi B, Shahbazi-Gahrouei S, et al. Magnetic iron oxide nanoparticles as T2 MR imaging contrast agent for detection of breast cancer (MCF-7) cell. Avicenna J Med Biotechnol. 2017;9:181–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Khaniabadi P, Majid A, Asif M, Khaniabadi B, Shahbazi-Gahrouei D, Jaafar M. Breast cancer cell targeted MR molecular imaging probe: Anti-MUC1 antibody-based magnetic nanoparticles. J Physics Conf Series. 2017;851:012014. [Google Scholar]
- 4.Mirzaei M, Mohagheghi M, Shahbazi-Gahrouei D, Khatami A. Gd3+-anionic linear globular dendrimer-G2-C595 a dual novel nanoprobe for MR imaging and therapeutic agent: An in vitro study. J Biomol Res Therap. 2012;1:1000103. [Google Scholar]
- 5.Keshtkar M, Shahbazi-Gahrouei D, Mehrgardi MA, Aghaei M, Khoshfetrat SM. Synthesis and cytotoxicity assessment of gold-coated magnetic iron oxide nanoparticles. J Biomed Phys Eng. 2018;8:357–64. [PMC free article] [PubMed] [Google Scholar]
- 6.Sun X, Li Y, Liu T, Li Z, Zhang X, Chen X, et al. Peptide-based imaging agents for cancer detection. Adv Drug Deliv Rev. 2017;110(111):38–51. doi: 10.1016/j.addr.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mirzaei M, Mohagheghi M, Shahbazi-Gahrouei D. Synthesis and development of Gd3+‑ALGDG 2-C595 as MR imaging contrast agent. J Biomater Nanobiotech. 2013;4:22–9. [Google Scholar]
- 8.Muthu MS, Leong DT, Mei L, Feng SS. Nanotheranostics-application and further development of nanomedicine strategies for advanced theranostics. Theranostics. 2014;4:660–77. doi: 10.7150/thno.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Zhen Z, Todd T, Chu PK, Xie J. Nanoparticles for improving cancer diagnosis. Mater Sci Eng R Rep. 2013;74:35–69. doi: 10.1016/j.mser.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khemthongcharoen N, Jolivot R, Rattanavarin S, Piyawattanametha W. Advances in imaging probes and optical microendoscopic imaging techniques for early in vivo cancer assessment. Adv Drug Deliv Rev. 2014;74:53–74. doi: 10.1016/j.addr.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Lewis GD, Chiang SB, Butler EB, Teh BS. The utility of positron emission tomography/computed tomography in target delineation for stereotactic body radiotherapy for liver metastasis from primary gastric cancer: An illustrative case report and literature review. J Gastrointest Oncol. 2017;8:E39–42. doi: 10.21037/jgo.2017.01.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lonsdale MN, Beyer T. Dual-modality PET/CT instrumentation-today and tomorrow. Eur J Radiol. 2010;73:452–60. doi: 10.1016/j.ejrad.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Buck AK, Herrmann K, Stargardt T, Dechow T, Krause BJ, Schreyögg J, et al. Economic evaluation of PET and PET/CT in oncology: Evidence and methodologic approaches. J Nucl Med Technol. 2010;38:6–17. doi: 10.2967/jnmt.108.059584. [DOI] [PubMed] [Google Scholar]
- 14.Cheen Hoe AK, Hamzah F, Abdul Khader MA. Incidental follicular thyroid carcinoma detected on F-18 FDG PET CT imaging for breast cancer staging: A case report. Malays J Med Sci. 2014;21:75–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Miyazaki T, Sohda M, Higuchi T, Tanaka N, Suzuki S, Sakai M, et al. Effectiveness of FDG-PET in screening of synchronous cancer of other organs in patients with esophageal cancer. Anticancer Res. 2014;34:283–7. [PubMed] [Google Scholar]
- 16.Khan N, Oriuchi N, Higuchi T, Zhang H, Endo K. PET in the follow-up of differentiated thyroid cancer. Br J Radiol. 2003;76:690–5. doi: 10.1259/bjr/31538331. [DOI] [PubMed] [Google Scholar]
- 17.Skovgaard D, Persson M, Brandt-Larsen M, Christensen C, Madsen J, Klausen TL, et al. Safety, dosimetry, and tumor detection ability of 68Ga-NOTA-AE105: First-in-human study of a novel radioligand for uPAR PET imaging. J Nucl Med. 2017;58:379–86. doi: 10.2967/jnumed.116.178970. [DOI] [PubMed] [Google Scholar]
- 18.Rowe SP, Macura KJ, Mena E, Blackford AL, Nadal R, Antonarakis ES, et al. PSMA-based [(18) F] DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol. 2016;18:411–9. doi: 10.1007/s11307-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowe SP, Gage KL, Faraj SF, Macura KJ, Cornish TC, Gonzalez-Roibon N, et al. 18F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J Nucl Med. 2015;56:1003–10. doi: 10.2967/jnumed.115.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellison PA, Chen F, Goel S, Barnhart TE, Nickles RJ, DeJesus OT, et al. Intrinsic and stable conjugation of thiolated mesoporous silica nanoparticles with radioarsenic. ACS Appl Mater Interfaces. 2017;9:6772–81. doi: 10.1021/acsami.6b14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakravarty R, Goel S, Hong H, Chen F, Valdovinos HF, Hernandez R, et al. Hollow mesoporous silica nanoparticles for tumor vasculature targeting and PET image-guided drug delivery. Nanomedicine (Lond) 2015;10:1233–46. doi: 10.2217/nnm.14.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Kumar R, Nagesha D, Duclos RI, Jr, Sridhar S, Gatley SJ, et al. Integrity of (111) In-radiolabeled superparamagnetic iron oxide nanoparticles in the mouse. Nucl Med Biol. 2015;42:65–70. doi: 10.1016/j.nucmedbio.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Pang B, Zhao Y, Luehmann H, Yang X, Detering L, You M, et al. 64Cu-doped pdCu@Au tripods: A Multifunctional nanomaterial for positron emission tomography and image-guided photothermal cancer treatment. ACS Nano. 2016;10:3121–31. doi: 10.1021/acsnano.5b07968. [DOI] [PubMed] [Google Scholar]
- 24.Pascual L, Cerqueira-Coutinho C, García-Fernández A, de Luis B, Bernardes ES, Albernaz MS, et al. MUC1 aptamer-capped mesoporous silica nanoparticles for controlled drug delivery and radio-imaging applications. Nanomedicine. 2017;13:2495–505. doi: 10.1016/j.nano.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Pang B, Luehmann H, Detering L, Yang X, Sultan D, et al. Gold nanoparticles doped with (199) au atoms and their use for targeted cancer imaging by SPECT. Adv Healthc Mater. 2016;5:928–35. doi: 10.1002/adhm.201500992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rainone P, Riva B, Belloli S, Sudati F, Ripamonti M, Verderio P, et al. Development of 99mTc-radiolabeled nanosilica for targeted detection of HER2-positive breast cancer. Int J Nanomedicine. 2017;12:3447–61. doi: 10.2147/IJN.S129720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Tian Y, Shan D, Gong A, Zeng L, Ren W, et al. Neuropeptide Y Y1 receptor-mediated biodegradable photoluminescent nanobubbles as ultrasound contrast agents for targeted breast cancer imaging. Biomaterials. 2017;116:106–17. doi: 10.1016/j.biomaterials.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Usman MS, Hussein MZ, Fakurazi S, Ahmad Saad FF. Gadolinium-based layered double hydroxide and graphene oxide nano-carriers for magnetic resonance imaging and drug delivery. Chem Cent J. 2017;11:47. doi: 10.1186/s13065-017-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahbazi-Gahrouei D, Williams M, Rizvi S, Allen BJ. In vivo studies of Gd-DTPA-monoclonal antibody and gd-porphyrins: Potential magnetic resonance imaging contrast agents for melanoma. J Magn Reson Imaging. 2001;14:169–74. doi: 10.1002/jmri.1168. [DOI] [PubMed] [Google Scholar]
- 30.Shahbazi-Gahrouei D. Gadolinium-porphyrins: New potential magnetic resonance imaging contrast agents for melanoma detection. J Res Med Sci. 2006;11:217–23. [Google Scholar]
- 31.Shahbazi-Gahrouei D, Khodamoradi E. Porphyrin-based agents: Potential MR imaging contrast agents for colorectal (HT29/219) detection in mice. J Res Med Sci. 2007;7:1015–20. [Google Scholar]
- 32.Abdolahi M, Shahbazi-Gahrouei D, Laurent S, Sermeus C, Firozian F, Allen BJ, et al. Synthesis and in vitro evaluation of MR molecular imaging probes using J591 mAb-conjugated SPIONs for specific detection of prostate cancer. Contrast Media Mol Imaging. 2013;8:175–84. doi: 10.1002/cmmi.1514. [DOI] [PubMed] [Google Scholar]
- 33.Shahbazi-Gahrouei D, Abdolahi M. Detection of MUC1-expressing ovarian cancer by C595 monoclonal antibody-conjugated SPIONs using MR imaging. ScientificWorldJournal. 2013;2013:609151. doi: 10.1155/2013/609151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shahbazi-Gahrouei D, Abdolahi M. Superparamagnetic iron oxide-C595: Potential MR imaging contrast agents for ovarian cancer detection. J Med Phys. 2013;38:198–204. doi: 10.4103/0971-6203.121198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shahbazi-Gahrouei D. A novel method for quantitative analysis of anti – MUCI expressing ovarian cancer cell surface based on magnetic cell separation. J Med Sci. 2012;12:256–66. [Google Scholar]
- 36.Ghahremani F, Kefayat A, Shahbazi-Gahrouei D, Motaghi H, Mehrgardi MA, Haghjooy-Javanmard S, et al. AS1411 aptamer-targeted gold nanoclusters effect on the enhancement of radiation therapy efficacy in breast tumor-bearing mice. Nanomedicine (Lond) 2018;13:2563–78. doi: 10.2217/nnm-2018-0180. [DOI] [PubMed] [Google Scholar]
- 37.Ghahremani F, Shahbazi-Gahrouei D, Kefayat A, Motaghi H, Mehrgardi MA, Javanmard SH. AS1411 aptamer conjugated gold nanoclusters as a targeted radiosensitizer for megavoltage radiation therapy of 4T1 breast cancer cells. RSC Adv. 2018;8:4249–58. [Google Scholar]
- 38.Liu K, Yan X, Xu YJ, Dong L, Hao LN, Song YH, et al. Sequential growth of caF2Yb, Er@CaF2Gd nanoparticles for efficient magnetic resonance angiography and tumor diagnosis. Biomater Sci. 2017;5:2403–15. doi: 10.1039/c7bm00797c. [DOI] [PubMed] [Google Scholar]
- 39.You Q, Sun Q, Yu M, Wang J, Wang S, Liu L, et al. BSA-bioinspired gadolinium hybrid-functionalized hollow gold nanoshells for NIRF/PA/CT/MR quadmodal diagnostic imaging-guided photothermal/Photodynamic cancer therapy. ACS Appl Mater Interfaces. 2017;9:40017–30. doi: 10.1021/acsami.7b11926. [DOI] [PubMed] [Google Scholar]
- 40.Payne WM, Hill TK, Svechkarev D, Holmes MB, Sajja BR, Mohs AM, et al. Multimodal imaging nanoparticles derived from hyaluronic acid for integrated preoperative and intraoperative cancer imaging. Contrast Media Mol Imaging. 2017;2017:9616791. doi: 10.1155/2017/9616791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaga LY, Cunha ML, Campos Neto GC, Garcia MR, Yang JH, Camacho CP, et al. 68Ga-DOTATATE PET/CT in recurrent medullary thyroid carcinoma: A lesion-by-lesion comparison with 111In-octreotide SPECT/CT and conventional imaging. Eur J Nucl Med Mol Imaging. 2017;44:1695–701. doi: 10.1007/s00259-017-3701-9. [DOI] [PubMed] [Google Scholar]
- 42.You S, Jung HY, Lee C, Choe YH, Heo JY, Gang GT, et al. High-performance dendritic contrast agents for X-ray computed tomography imaging using potent tetraiodobenzene derivatives. J Control Release. 2016;226:258–67. doi: 10.1016/j.jconrel.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 43.Jambor I, Kuisma A, Ramadan S, Huovinen R, Sandell M, Kajander S, et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-naF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2016;55:59–67. doi: 10.3109/0284186X.2015.1027411. [DOI] [PubMed] [Google Scholar]
- 44.Jin X, Liang N, Wang M, Meng Y, Jia B, Shi X, et al. Integrin imaging with 99mTc-3PRGD2 SPECT/CT shows high specificity in the diagnosis of lymph node metastasis from non-small cell lung cancer. Radiology. 2016;281:958–66. doi: 10.1148/radiol.2016150813. [DOI] [PubMed] [Google Scholar]
- 45.Pandit-Taskar N, O’Donoghue JA, Durack JC, Lyashchenko SK, Cheal SM, Beylergil V, et al. A phase I/II study for analytic validation of 89Zr-J591 immunoPET as a molecular imaging agent for metastatic prostate cancer. Clin Cancer Res. 2015;21:5277–85. doi: 10.1158/1078-0432.CCR-15-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Chaudhary A, Chmura SJ, Pelizzari C, Rajh T, Wietholt C, et al. A novel functional CT contrast agent for molecular imaging of cancer. Phys Med Biol. 2010;55:4389–97. doi: 10.1088/0031-9155/55/15/013. [DOI] [PubMed] [Google Scholar]
- 47.Aydogan B, Li J, Rajh T, Chaudhary A, Chmura SJ, Pelizzari C, Wietholt C, et al. AuNP-DG: Deoxyglucose-labeled gold nanoparticles as X-ray computed tomography contrast agents for cancer imaging. Mol Imaging Biol. 2010;12:463–7. doi: 10.1007/s11307-010-0299-8. [DOI] [PubMed] [Google Scholar]
- 48.Shi X, Wang SH, Van Antwerp ME, Chen X, Baker JR., Jr Targeting and detecting cancer cells using spontaneously formed multifunctional dendrimer-stabilized gold nanoparticles. Analyst. 2009;134:1373–9. doi: 10.1039/b902199j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Zhu H, Huang X, Wang P, Zhang F, Li W, et al. Novel iodinated gold nanoclusters for precise diagnosis of thyroid cancer. Nanoscale. 2017;9:2219–31. doi: 10.1039/c6nr07656d. [DOI] [PubMed] [Google Scholar]
- 50.Nakagawa T, Gonda K, Kamei T, Cong L, Hamada Y, Kitamura N, et al. X-ray computed tomography imaging of a tumor with high sensitivity using gold nanoparticles conjugated to a cancer-specific antibody via polyethylene glycol chains on their surface. Sci Technol Adv Mater. 2016;17:387–97. doi: 10.1080/14686996.2016.1194167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei P, Chen J, Hu Y, Li X, Wang H, Shen M, et al. Dendrimer-stabilized gold nanostars as a multifunctional theranostic nanoplatform for CT imaging, photothermal therapy, and gene silencing of tumors. Adv Healthc Mater. 2016;5:3203–13. doi: 10.1002/adhm.201600923. [DOI] [PubMed] [Google Scholar]
- 52.Zhou B, Yang J, Peng C, Zhu J, Tang Y, Zhu X, et al. PEGylated polyethylenimine-entrapped gold nanoparticles modified with folic acid for targeted tumor CT imaging. Colloids Surf B Biointerfaces. 2016;140:489–96. doi: 10.1016/j.colsurfb.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 53.Smeenge M, Tranquart F, Mannaerts CK, de Reijke TM, van de Vijver MJ, Laguna MP, et al. First-in-human ultrasound molecular imaging with a VEGFR2-specific ultrasound molecular contrast agent (BR55) in prostate cancer: A safety and feasibility pilot study. Invest Radiol. 2017;52:419–27. doi: 10.1097/RLI.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 54.Li P, Zheng Y, Ran H, Tan J, Lin Y, Zhang Q, et al. Ultrasound triggered drug release from 10-hydroxycamptothecin-loaded phospholipid microbubbles for targeted tumor therapy in mice. J Control Release. 2012;162:349–54. doi: 10.1016/j.jconrel.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 55.Dijkmans PA, Juffermans LJ, Musters RJ, van Wamel A, ten Cate FJ, van Gilst W, et al. Microbubbles and ultrasound: From diagnosis to therapy. Eur J Echocardiogr. 2004;5:245–56. doi: 10.1016/j.euje.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Taniyama Y, Tachibana K, Hiraoka K, Aoki M, Yamamoto S, Matsumoto K, et al. Development of safe and efficient novel nonviral gene transfer using ultrasound: Enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther. 2002;9:372–80. doi: 10.1038/sj.gt.3301678. [DOI] [PubMed] [Google Scholar]
- 57.Yang H, Cai W, Xu L, Lv X, Qiao Y, Li P, et al. Nanobubble-affibody: Novel ultrasound contrast agents for targeted molecular ultrasound imaging of tumor. Biomaterials. 2015;37:279–88. doi: 10.1016/j.biomaterials.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Tian Y, Shan D, Gong A, Zeng L, Ren W, et al. Neuropeptide Y Y1 receptor-mediated biodegradable photoluminescent nanobubbles as ultrasound contrast agents for targeted breast cancer imaging. Biomaterials. 2017;116:106–17. doi: 10.1016/j.biomaterials.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 59.Xie W, Wang L, Zhang Y, Su L, Shen A, Tan J, et al. Nuclear targeted nanoprobe for single living cell detection by surface-enhanced Raman scattering. Bioconjug Chem. 2009;20:768–73. doi: 10.1021/bc800469g. [DOI] [PubMed] [Google Scholar]
- 60.Reguera J, Jiménez de Aberasturi D, Henriksen-Lacey M, Langer J, Espinosa A, Szczupak B, et al. Janus plasmonic-magnetic gold-iron oxide nanoparticles as contrast agents for multimodal imaging. Nanoscale. 2017;9:9467–80. doi: 10.1039/c7nr01406f. [DOI] [PubMed] [Google Scholar]
- 61.Atabaev TS, Lee JH, Shin YC, Han DW, Choo KS, Jeon UB, et al. Eu, gd-codoped yttria nanoprobes for optical and T1-weighted magnetic resonance imaging. Nanomaterials (Basel) 2017;7 doi: 10.3390/nano7020035. pii: E35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu C, Wang Y, Zhang C, Jia Y, Luo Y, Gao X, et al. AuGd integrated nanoprobes for optical/MRI/CT triple-modal in vivo tumor imaging. Nanoscale. 2017;9:4620–8. doi: 10.1039/c7nr01064h. [DOI] [PubMed] [Google Scholar]
- 63.Kang MS, Singh RK, Kim TH, Kim JH, Patel KD, Kim HW, et al. Optical imaging and anticancer chemotherapy through carbon dot created hollow mesoporous silica nanoparticles. Acta Biomater. 2017;55:466–80. doi: 10.1016/j.actbio.2017.03.054. [DOI] [PubMed] [Google Scholar]
- 64.Pisanic TR, 2nd, Zhang Y, Wang TH. Quantum dots in diagnostics and detection: Principles and paradigms. Analyst. 2014;139:2968–81. doi: 10.1039/c4an00294f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu H, Li Z, Sun Y, Geng X, Hu Y, Meng H, et al. Synthesis of luminescent carbon dots with ultrahigh quantum yield and inherent folate receptor-positive cancer cell targetability. Sci Rep. 2018;8:1086. doi: 10.1038/s41598-018-19373-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rizvi SB, Rouhi S, Taniguchi S, Yang SY, Green M, Keshtgar M, et al. Near-infrared quantum dots for HER2 localization and imaging of cancer cells. Int J Nanomedicine. 2014;9:1323–37. doi: 10.2147/IJN.S51535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schroeder KL, Goreham RV, Nann T. Graphene quantum dots for theranostics and bioimaging. Pharm Res. 2016;33:2337–57. doi: 10.1007/s11095-016-1937-x. [DOI] [PubMed] [Google Scholar]
- 68.Hai X, Guo Z, Lin X, Chen X, Wang J. Fluorescent TPA@ GQDs probe for sensitive assay and quantitative imaging of hydroxyl radical in living cells. ACS Applied Materials and Interfaces. 2018 doi: 10.1021/acsami.7b16094. [DOI] [PubMed] [Google Scholar]


