Abstract
Background:
The present study was conducted to determine antimicotic susceptibility of Candida species (sp.) from patients with symptomatic candiduria.
Materials and Methods:
Identification of Candida sp. and determination of efficacy of most routine antifungals were done using polymerase chain reaction-restriction fragment length polymorphism method and E-test, respectively.
Results:
The results from susceptibility test reveal that caspofungin and amphotericin B have high antifungal activity against both albicans (100% and 96%, respectively) and nonalbicans (95.11% and 72.72%, respectively) isolates.
Conclusion:
The present study suggests that caspofungin and amphotericin B have the excellent ability to eradicate both Candida groups that showed decreased susceptibility to other compounds.
Keywords: Antifungal drugs, Candida species, polymerase chain reaction-restriction fragment length polymorphism, symptomatic candiduria
INTRODUCTION
Candiduria has been considered as a challenging condition for clinicians because of the complex relationship between its colonization and infection. The complication occurs during long-term hospitalization, especially in individuals who are admitted in the intensive care unit setting that may lead to changes in etiologic agent to nonalbicans Candida (NAC).[1] This pictorial variation has created a new and serious complication because a broad spectrum of NAC isolates are typically less susceptible to routine antimicotic.[2] Clinically, amphotericin B and fluconazole were prescribed as the superior choice for the treatment of candiduria.[3] On the other side, a serious concern remains due to the intrinsic resistance of Candida glabrata and Candida krusei isolates to fluconazole.[4]
In the present study, we aimed to determine the in vitro antifungal susceptibility profile of Candida sp. from patients with symptomatic candiduria against amphotericin B, fluconazole, itraconazole, voriconazole, and caspofungin.
MATERIALS AND METHODS
Subjects
The experimental study was conducted in 2017. The patients who were suspected of sepsis, candiduria, and the presence of pyuria were enrolled for this study. Therefore, 500 midstream of first-void urine and indwelling urinary catheter were collected.
Identification of Candida sp.
Identification of Candida sp. was performed based on direct examination, colony color, and yeast counts superior to 105 UFC/mL on CHROM Agar Candida medium (CHROM agar, France) at 35°C for 24 h.
The polymerase chain reaction-restriction fragment length polymorphism (RFLP) was performed based on the amplification of ITS1-5.8SrDNA-ITS2 region and MspI (Fermentas, USA) restriction enzyme. Restriction fragments were separated by 2% agarose gel electrophoresis.
Antimicotic susceptibility assay
The susceptibility of amphotericin B, fluconazole, itraconazole, voriconazole, and caspofungin was performed using RPMI 1640 agar-based E-test method (BioMeriéux, Sweden).[5] Candida albicans ATCC 24433 was used as the reference control.
RESULTS
Totally, 89 (17.8%) urine samples were positive for Candida species which collected from 43 (48.3%) male and 46 (51.6%) female individuals were positive for symptomatic candiduria. The mean age of participants in this study was 57.66 ± 22.30. Fever experience was recorded in 70.8% of patients while other clinical manifestations such as abdominal pain, renal pain, and dysuria were observed in 12.4%, 5.6%, and 6.7% of cases, respectively.
RFLP fingerprint analysis revealed that C. albicans (n = 56;63%) is the predominant causative agent isolated from urine followed by Candida tropicalis (n = 24; 27%), Candida parapsilosis (5; 5.6%), C. glabrata (n = 2; 2.2%), and C. krusei (n = 2; 2.2%) [Figure 1].
Figure 1.
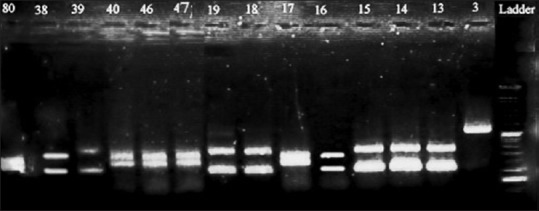
Polymerase chain reaction-restriction fragment length polymorphism fingerprint with MspI restriction enzyme: Candida albicans: 80, 40, 46, 47, 17; Candida tropicalis: 38, 39, 19, 18, 13, 14, 15; Candida parapsilosis: 3; Candida krusei: 16
The results from antimycotic susceptibility tests are presented in Table 1.
Table 1.
A head-to-head comparison of five antifungal susceptibilities profile in Candida sp.
| Species (n) | Antifungals | E-test | ||
|---|---|---|---|---|
| S | I | R | ||
| Candida albicans (56) | AMB | 96 | 0 | 4 |
| VCZ | 92 | 8 | 0 | |
| ICZ | 77.82 | 16 | 6.8 | |
| FCZ | 90 | 10 | 0 | |
| CAS | 100 | 0 | 0 | |
| Candida tropicalis (24) | AMB | 83.33 | 16.66 | 8.33 |
| VCZ | 70.80 | 20 | 9.2 | |
| ICZ | 66.6 | 68.33 | 16.66 | |
| FCZ | 75 | 25 | 8.33 | |
| CAS | 95.11 | 0 | 5.89 | |
| Candida parapsilosis (5) | AMB | 90 | 8 | 2 |
| VCZ | 60 | 40 | 0 | |
| ICZ | 40 | 0 | 0 | |
| FCZ | 40 | 60 | 0 | |
| CAS | 100 | 0 | 0 | |
| Candida krusei (2) | AMB | 100 | 0 | 0 |
| VCZ | 100 | 0 | 0 | |
| ICZ | 0 | 100 | 0 | |
| FCZ | 0 | 60 | 40 | |
| CAS | 100 | 0 | 0 | |
| Candida glabrata (2) | AMB | 100 | 0 | 0 |
| VCZ | 100 | 0 | 0 | |
| ICZ | 0 | 50 | 50 | |
| FCZ | 0 | 0 | 100 | |
| CAS | 100 | 0 | 0 | |
AMB=Amphotericin B; FCZ=Fluconazole; ICZ=Itraconazole; VCZ=Voriconazole; CAS=Caspofungin; S=Susceptible; I=Intermediate; R=Resistance
DISCUSSION
In line with previous studies regardless of asymptomatic and symptomatic candiduria,[6,7,8] the current results based on RFLP pattern revealed that C. albicans (63%) is still the predominant causative agent isolated from urine followed by C. tropicalis (27%), C. parapsilosis (5.6%), C. glabrata, and C. krusei (2.2%).
An overall look through the results from susceptibility test reveals that caspofungin and amphotericin B have high antimicotic activity against both albicans (100%–96%, respectively) and non albicans (95.11% and 72.72%, respectively) isolates. In line with other investigations,[8,9,10,11] the present finding suggests that caspofungin and amphotericin B are suitable alternatives in all cases of Candida sp. that showed resistance to azolic compounds.
It has been well established that C. albicans is intrinsically sensitive to a broad range of antimicotic classes and resistance must be acquired.[8,9,10] In concordance with this finding, results in this study show that C. albicans is largely susceptible to all antimicotic agents that were used here except itraconazole. In practice, itraconazole is normally more effective than fluconazole, and it should be prescribed for cases with fluconazole-resistant isolates. Here, emerging resistant isolates were probably associated with previous exposure to fluconazole.
Regarding susceptibility results, decreased susceptibility to voriconazole was exclusively limited to C. tropicalis. In several studies, voriconazole-resistant C. tropicalis were clinically isolated.[12] In contrast with the present findings, recent investigations in candiuria indicated that no resistance was found among C. tropicalis.[8,10]
The rate of resistance to itraconazole varies between NAC isolates; here, the highest rate of resistance to itraconazole was found in C. tropicalis and C. parapsilosis species. The resistance to itraconazole has been reported in C. tropicalis and C. glabrata isolated from urine specimen.[1]
Similar to previous findings in the current investigation, C. glabrata and C. krusei showed the decreased sensitivity against fluconazole.[1,8] Interestingly, the high fluconazole resistance was determined for C. tropicalis and C. parapsilosis. Decreased fluconazole sensitivity to C. tropicalis was reported.[1,8,10,13] Since, multiple treatment guidelines recommend fluconazole as a first-line antifungal for candiduria emerging resistance to fluconazole in NAC has complicated treatment and is a warning for clinicians and public health authorities.
Finally, the present study suggests that caspofungin and amphotericin B have the excellent ability to eradicate both Candida groups that showed decreased susceptibility to other compounds. Taken together, widespread use of antimicotic, emerging new pathogens in addition to misidentification of fungal agents, may lead to poorer clinical outcomes and difficulty of treatment and is an alarm for clinician and public health authorities.
Financial support and sponsorship
This research has been financially supported by Iran University of Medical Sciences grant No: 25619.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This research has been financially supported by Iran University of Medical Sciences grant No: 25619.
REFERENCES
- 1.Singla N, Gulati N, Kaistha N, Chander J. Candida colonization in urine samples of ICU patients: Determination of etiology, antifungal susceptibility testing and evaluation of associated risk factors. Mycopathologia. 2012;174:149–55. doi: 10.1007/s11046-011-9514-7. [DOI] [PubMed] [Google Scholar]
- 2.Bicmen C, Doluca M, Gulat S, Gunduz AT, Tuksavul F. Species level identification and antifungal susceptibility of yeasts isolated from various clinical specimens and evaluation of integral system yeasts plus. New Microbiol. 2012;35:327–34. [PubMed] [Google Scholar]
- 3.Voltan AR, Fusco-Almeida AM, Mendes-Giannini MJ. Candiduria: epidemiology, resistance, classical and alternative antifungals drugs. SOJ Microbiol Infect Dis. 2014;2:1–7. [Google Scholar]
- 4.Richter SS, Galask RP, Messer SA, Hollis RJ, Diekema DJ, Pfaller MA, et al. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol. 2005;43:2155–62. doi: 10.1128/JCM.43.5.2155-2162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zareifar S, Badiee P, Haddadi P, Abdolkarimi B. Susceptibility pattern of anti-Candida drugs in the pediatric patients with acute leukemia. Iran J Ped Hematol Oncol. 2017;7:1–8. [Google Scholar]
- 6.Esmailzadeh A, Zarrinfar H, Fata A, Sen T. High prevalence of candiduria due to non-albicans Candida species among diabetic patients: A matter of concern? J Clin Lab Anal. 2018;32:e22343. doi: 10.1002/jcla.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zarei-Mahmoudabadi A, Zarrin M, Ghanatir F, Vazirianzadeh B. Candiduria in hospitalized patients in teaching hospitals of Ahvaz. Iran J Microbiol. 2012;4:198–203. [PMC free article] [PubMed] [Google Scholar]
- 8.Toner L, Papa N, Aliyu SH, Dev H, Lawrentschuk N, Al-Hayek S. Candida growth in urine cultures: a contemporary analysis of species and antifungal susceptibility profiles. QJM: Int J Med. 2015;109:325–9. doi: 10.1093/qjmed/hcv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarei Mahmoudabadi A, Rezaei-Matehkolaei A, Ghanavati F. The susceptibility patterns of Candida species isolated from urine samples to posaconazole and caspofungin. Jundishapur J Microbiol. 2015;8:e24298. doi: 10.5812/jjm.24298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Freitas AR, Baeza LC, Faria MG, Dota KF, Godoy Martínez P, Svidzinski TI, et al. Yeasts isolated from nosocomial urinary infections: Antifungal susceptibility and biofilm production. Rev Iberoam Micol. 2014;31:104–8. doi: 10.1016/j.riam.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Zarei-Mahmoudabadi A, Zarrin M, Beheshti Fard M. Antifungal susceptibility of Candida species isolated from candidura. Jundishapur J Microbiol. 2013;6:24–8. [Google Scholar]
- 12.Fothergill AW, Sutton DA, McCarthy DI, Wiederhold NP. Impact of new antifungal breakpoints on antifungal resistance in Candida species. J Clin Microbiol. 2014;52:994–7. doi: 10.1128/JCM.03044-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seifi Z, Azish M, Salehi Z, Zarei Mahmoudabadi A, Shamsizadeh A. Candiduria in children and susceptibility patterns of recovered Candida species to antifungal drugs in Ahvaz. J Nephropathol. 2013;2:122–8. doi: 10.12860/JNP.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]


