Abstract
Computed tomography has an established role in the evaluation of a variety of cardiac disorders, including congenital heart diseases. The current generation of high-speed scanners produces volumetric data at low doses of radiation. The interpretation of cardiac anatomy, however, is generally limited to multiplanar assessment of two-dimensional images. The volume rendering technique provides an excellent three-dimensional demonstration of external morphology but offers little information about the intracardiac anatomy. The alternative approach of virtual cardiac dissection, which is a modification of volume rendering, on the other hand, provides crucial insights regarding the intracardiac anatomy. At present, virtual cardiac dissection requires expensive software packages. These software packages are not available in all countries, thus limiting its use in routine clinical care. We present here the details of a newly developed technique that permits virtual cardiac dissection using a personal computer and open-source software. Our technique involves no additional cost and can be achieved in the comfort of the office or operating room of the cardiologist, radiologist, or cardiac surgeon. This enhanced three-dimensional visualization of intracardiac anatomy will surely improve the understanding of the morphological details of both normal and malformed hearts. In addition, by permitting assessment in projections with which modern-day cardiologists and cardiac surgeons are conversant, it is likely to improve clinical decision-making. We illustrate here its potential utility in the morphologic assessment of the atrial septum and its deficiencies, along with malformations of the ventricular outflow tracts, including common arterial trunk.
Keywords: Computed tomography, congenital heart disease, virtual cardiac dissection
INTRODUCTION
There has been unprecedented growth in the field of cardiac imaging. While cross-sectional echocardiography remains the imaging modality of choice for most patients with cardiac disease, multimodal imaging is increasingly used for the assessment of complex congenital malformations.[1,2] Computed tomography, for example, rapidly provides high-quality information needed for optimal management. Despite the acquisition of volumetric data by computed tomography, the assessment of cardiac anatomy is largely based on multiplanar interpretation of two-dimensional images. There has been limited focus on the potential to provide three-dimensional visualization of intracardiac anatomy from these datasets. It has been shown, nonetheless, that the preparation of patient-specific three-dimensional printed models of the heart can facilitate the understanding of intracardiac anatomy.[3,4,5] Three-dimensional printing, however, requires dedicated software, special material for printing, and the availability of personnel who are expert in both the medical and engineering aspects of the process.[4,5]
Virtual cardiac dissection is another means of demonstrating the pertinent intracardiac anatomy.[6,7] To date, although the information obtained is revolutionary in illustrating the details of cardiac structure, the technique requires specialized software packages. The cost of the necessary software packages, such as Ziostation 2 (Ziosoft Inc, Belmont, USA) and TeraRecon (Foster City, California, USA), along with the required hardware, currently remains a challenge. We describe here our technique for achieving virtual cardiac dissection using open-source software at no additional cost. The advantages of such visualization of intracardiac anatomy at low cost are self-evident. In this introductory description, we illustrate here its value in demonstrating the extent of the normal atrial septum and its deficiencies and in lesions involving the ventricular outflow tracts. We also demonstrate its value in defining the arrangement of pulmonary arteries in patients with common arterial trunk.
VIRTUAL CARDIAC DISSECTION USING A PERSONAL COMPUTER
Origin of the idea
Volume rendering is often used in clinical practice for three-dimensional representation of cardiac anatomy. Most clinicians and investigators, however, have ignored its potential in demonstrating intracardiac anatomy. In recent years, the availability of specialized software packages has made it possible to illustrate details of intracardiac anatomy.[6,7,8,9,10] These software packages, however, are not available in all countries. When available, they are prohibitively costly. This has prompted us to develop our own low-cost method of virtual cardiac dissection.
The hardware and the software
Horos is an open-source version of OsiriX (Pixmeo, Switzerland), a Mac-based software, which allows processing of three-dimensional datasets.[11,12] It is freely available under GNU lesser general public license, version 3.[13] The advantage of the software package is that it permits multiplanar and three-dimensional reconstruction of cardiac anatomy. The technique of low-cost virtual cardiac dissection was developed and tested on a laptop notebook computer, specifically a MacBook Pro (Retina, 13-inch, early 2015). The computer has a 2.7 GHz Intel Core i5 processor and 8GB of random access memory. It uses an Intel Iris Graphics 6100 card (Apple Inc., Cupertino, California, USA), and macOS High Sierra version 10.13.1 to run the Horos software.
Volume rendering technique
Having imported the datasets into the Horos database, they are assessed for quality. Those with optimal chamber opacification and minimal artifacts are then chosen for virtual cardiac dissection. The datasets are first subjected to volume rendering. In this technique of three-dimensional representation, the cross-sectional images are stacked to produce a virtual cube of volume within the computer screen.[14] Unlike other techniques of three-dimensional visualization, which use only a portion of available information, volume rendering incorporates information from the entire dataset.[15,16,17] The resultant virtual volume thus produces high fidelity images with preserved spatial orientation.[18,19]
The display of the virtual volume can be adjusted by altering the window settings, specifically the level and width of the window. Only tissues with attenuation within the selected range of Hounsfield units are displayed. The display of the virtual volume can be further improved by altering the opacity and color of various components using computer algorithms called transfer functions.[18,19] Most of the software packages do not permit easy access to these transfer function. Instead, a variety of predefined combinations of window settings and transfer functions, commonly known as “presets,” are usually made available. Application of these presets quickly transforms the image to demonstrate external cardiac morphology. The intracardiac anatomy, however, remains obscure.
Modifications to achieve virtual cardiac dissection
Once the benefits of varying the window settings and transfer functions became obvious to us, we used the 16-bit color lookup table (CLUT), editor for modifying the display of the virtual volume. The 16-bit CLUT editor, available within the program, permits quantitative manipulation of the window settings and the transfer functions in a fashion almost akin to digital photo editors. In its current form, however, the system is not as automated. The editor contains a gray mountain, representing the spread of attenuation values in Hounsfield units throughout the dataset, and a color map. The X-axis of this gray mountain maps out the image in a linear fashion along a density curve, with the density varying according to the characteristics of the tissues and the opacification of the contrast. The Y-axis, on the other hand, is the transparency function, with the top being completely opaque and the bottom being completely transparent.[20] The sideways boundaries of the color map, which determine the range of Hounsfield units visible on the screen, thus define the tissues that are visible on the screen. The height of the color map defines the opacity of different structures in the virtual volume. The visualization can then be further customized by right-left or up-down shifting of the color maps or of the individual points on the curve. New color maps can also be added and edited to improve quality of visualization. We have summarized the steps used for creating virtual cardiac dissections in Online Video 1.
The thickness of various cardiac structures is obviously different. Differential manipulation of the curves, therefore, is necessary to illustrate different cardiac tissues. Visualization of the thin leaflets of the cardiac valves, for example, mandates rightward shift of the color map compared to visualization of thicker structures, such as the subvalvular apparatus and the myocardium. Simultaneous change on the screen guides further adjustment of the color map settings. Once an optimal visualization of the intracardiac anatomy is achieved, these settings of the color maps and transfer functions can be stored as additional presets for future use. These presets, when saved, are useful in achieving rapid reconstructions in subsequent datasets. In our early experience, the virtual dissection from one dataset took 30–40 min. At present, with the help of our predefined presets, we are now able to achieve desired reconstructions in <15 min.
Interactive editing of the virtual volume for clinical evaluation
After optimizing the visualization, the displayed virtual volume can be further edited for displaying intracardiac anatomy in clinically relevant projections. This is achieved by cutting through the volume by shifting the three orthogonal planes. This process of clip editing permits real-time multiplanar editing of the volume. The orthogonal planes can also be angulated to replicate oblique views with cranial or caudal tilt. The clip editing, and the movement of the clipping planes, is guided by simultaneously changing of the coordinates visible on the screen. In addition, the orientation of the direction cube on the screen also helps in recreating clinically relevant projections.
CLINICAL APPLICATIONS
In our experience, we have found the technique of virtual cardiac dissection useful in the setting of both congenital and valvular heart diseases. To illustrate its potential value, we have selected a few examples involving congenital cardiac malformations.
The atrial septum and interatrial communications
Interatrial communications are one of the most common groups of congenital cardiac malformations. Not all of the interatrial communications, however, are within the confines of the atrial septum. Some, therefore, are not strictly described as “atrial septal defects.” It is an understanding of the extent of the atrial septum that underlies the appreciation of the difference between true atrial septal defects and those that permit interatrial shunting outside the atrial septum. Over and above these semantic considerations, the surrounds of the atrial septum also provide important landmarks to guide procedures performed by interventional cardiologists, electrophysiologists, and cardiac surgeons. An understanding of the extent of the atrial septum, its borders, therefore, is a key to achieving successful interventional or surgical repair. Virtual cardiac dissection also provides evidence that supports the recent observations made by developmental biologists. The so-called “septum secundum,” as shown previously in many textbooks, is no more than the superior interatrial fold. It is located between the attachments of the caval veins to the right atrium and the insertion of the right pulmonary veins to the left atrium.[21] This interatrial fold is normally overlapped by the cranial margin of the primary atrial septum, which forms the floor of oval fossa. The fold forms its posterosuperior rim [Figure 1]. When traced anteriorly, the fold is directly related to the aortic root [Figure 2]. When traced posteriorly, the rim becomes directly continuous with the wall of the inferior caval vein. All of these important landmarks are well shown by virtual cardiac dissection [Figure 1]. In addition, such dissection reveals the true secondary septal component, which is the anteroinferior buttress of the oval fossa.[21] The anteroinferior buttress separates the floor of the fossa from the orifice of the coronary sinus inferiorly and from the hinge of the septal leaflet of the tricuspid valve anteriorly. The mouth of the coronary sinus is separated from the mouth of the inferior caval vein by an additional infolding of the venous walls, which is known as the sinus septum and which is again well demonstrated by virtual cardiac dissection [Figure 2]. Virtual cardiac dissection then shows that it is the deficiency of the floor of the oval fossa or the primary atrial septum, which is responsible for producing the most common type of interatrial communication. Of necessity, such defects are positioned within the rims of the fossa and are aptly called oval fossa defects. Virtual cardiac dissection also helps in demonstrating the relationship of such defects with adjacent structures, notably the aortic root [Figure 2], a feature of importance when planning interventional closure.
Figure 1.
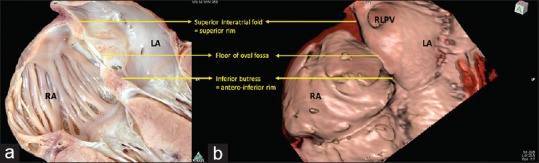
An image obtained using virtual cardiac dissection (a) with one of the heart specimens sectioned in a comparable view (b). Both panels clearly demonstrate that the superior rim of the oval fossa is no more than the superior interatrial fold between the connections of the superior caval vein to the right atrium, and the right upper pulmonary vein to the left atrium. The oval fossa lies between this superior rim and the anteroinferior buttress, which anchors the fossa to the atrioventricular junction. ICV: Inferior caval vein, LA: Left atrium, RA: Right atrium, RAA: Right atrial appendage, RLPV: Right lower pulmonary vein, RPA: Right pulmonary artery
Figure 2.
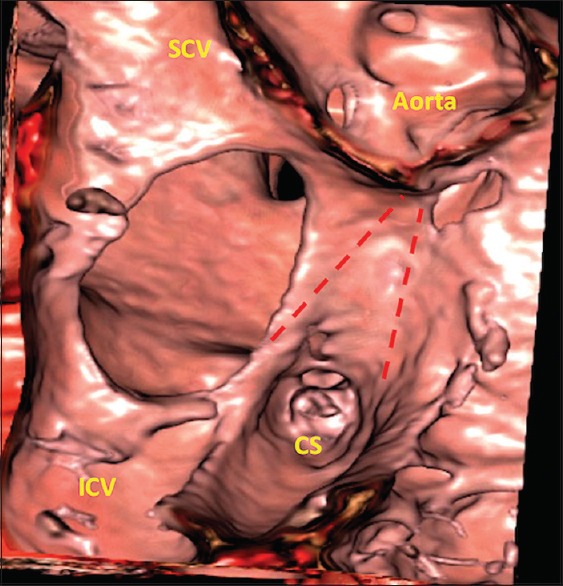
Virtual cardiac dissection in a patient with an atrial septal defect within the oval fossa, as seen in right anterior oblique projection, demonstrates the relationship of the defect with the rims of the fossa, providing important information for prospective interventional closure. The details of triangle of Koch and tendon of Todaro are also well demonstrated (dotted red lines). CS: Coronary sinus, ICV: Inferior caval vein, SCV: Superior caval vein
Similarly, virtual dissection provides enhanced visualization of the defects that provide shunting between the atrial chambers outside the confines of oval fossa. The superior sinus venosus defect, for example, can be shown in this fashion to be anomalous connection of one or more right-sided pulmonary veins to the posterior wall of the superior caval vein. The interatrial communication thus produced is no more than the orifice of the anomalous pulmonary veins, which continue to retain their left atrial connection [Figure 3 and Online Video 2].
Figure 3.
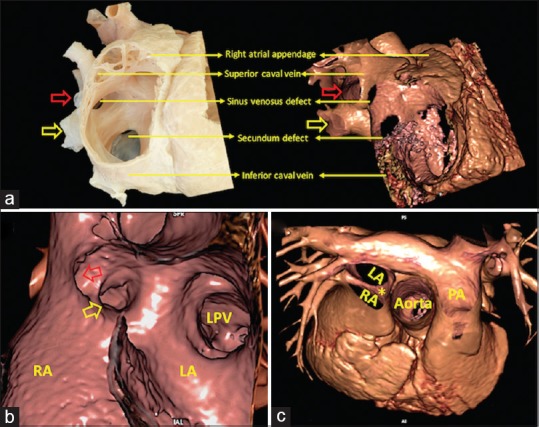
Virtual cardiac dissection from a patient with a superior sinus venosus interatrial communication comparable to a heart specimen sectioned in an equivalent view (a) shows that the defect lies beyond the confines of atrial septum. Virtual cardiac dissection in left anterior oblique projection (b) illustrates anomalous connection of the right upper pulmonary vein (red arrow), while the right lower pulmonary vein (yellow arrow) connects normally. (c) The orifice of the superior caval vein overriding the superior rim of the atrial septum (yellow asterisk). LA: Left atrium, LPV: Left pulmonary vein, PA: Pulmonary artery, RA: Right atrium
The interventricular communication versus the ventricular septal defect
Unlike the atrial septum, lesions involving the ventricular septum are much more complex, both in terms of their morphology, and the approaches needed for their treatment. For the purpose of current review, we have confined our discussion to interventricular communications located in the subaortic region. The essence of many such defects, as seen for example in tetralogy of Fallot, is overriding of the crest of the muscular ventricular septum by the aortic root [Figure 4]. In such situations, the surgeon places a patch within the cavity of the right ventricle to restore continuity between the left ventricle and the overriding aortic root. In effect, the surgeon closes the right-hand margin of the cone of space that exists beneath the overriding aortic root [Figure 4b]. The left-hand margin of the cone is never closed during surgical repair since it is the outflow tract for the left ventricle. All these details are well exhibited on virtual cardiac dissection [Figure 4a and b].
Figure 4.
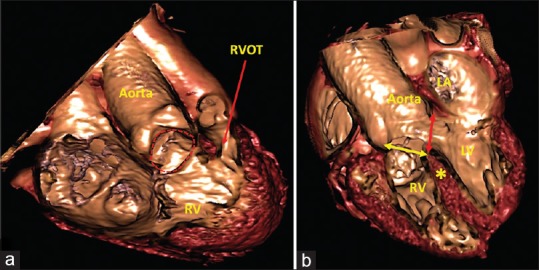
Virtual cardiac dissection from an adolescent boy with Tetralogy of Fallot. (a) An image in right anterior oblique projection with cranial angulation shows the ventricular septal defect (red dotted line) and narrowing of the right ventricular outflow tract. The relationship of the ventricular septal defect with the tricuspid valve apparatus is also well seen. (b) Equivalent to fluoroscopic left anterior oblique projection with cranial angulation shows a large subaortic ventricular septal defect with overriding of the aortic root over the crest of the muscular septum (yellow asterisk). Surgeons will have to close the defect at its right ventricular margin (yellow double-headed arrow) to connect aorta to the left ventricle. The left ventricular margin (red double-headed arrow) is the outflow tract for the left ventricle, and can never be closed. LV: Left ventricle, RV: Right ventricle
The situation is more complex in the setting of double outlet right ventricle when both arterial trunks arise exclusively from the morphologically right ventricle. In this setting, the key to surgical correction is again the location of the communication between the ventricles, which can occupy subaortic, subpulmonary, doubly committed, or noncommitted locations.[22] In the setting of double outlet right ventricle, however, it is this communication between the ventricles that is usually described as the “ventricular septal defect” [Figure 5a]. The virtual cardiac dissection clearly illustrate that the communication thus described as the “ventricular septal defect,” in reality, is the outlet for the left ventricle [Figure 5b]. This so-called “ventricular septal defect,” described more accurately as the interventricular communication,[22] is never closed by the surgeon during operative intervention, or at least, it is not closed unless an alternative route is created to provide the outlet for the left ventricle. The area that is analogous to the “ventricular septal defect” as seen in the setting of tetralogy of Fallot [Figure 5 and Online Video 1] extends to the rightward margin of the aortic root. It is this area that will be closed by the surgeon when creating an extensive tunnel to place the aortic root in continuity with the cavity of the left ventricle. Virtual cardiac dissection also shows the likely relationship of the newly created tunnel to the leaflets of the tricuspid valve [Figure 5].
Figure 5.
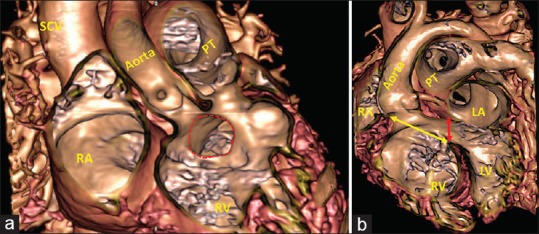
Virtual cardiac dissection from a 20-month-old girl with double outlet right ventricle shows the morphology of the subaortic interventricular communication (red dotted line) in anteroposterior (a) and left anterior oblique projection (b). (a) In the anteroposterior plane, it shows the defect opens to the right ventricle directly beneath the aortic root. (b) In the left anterior oblique projections, it shows that the area (yellow double-headed arrow) around which the surgeon will create a tunnel to reconnect the aortic root with the left ventricle is analogous to the ventricular septal defect as seen in the setting of overriding of the aortic root [Figure 4]. In reality, the area currently described as the “ventricular septal defect” is the outlet for the morphologically left ventricle (red double-headed arrow). Virtual dissection shows why it is more appropriate to use the term interventricular communication in the setting of double outlet right ventricle. LA: Left atrium, LV: Left ventricle, RA: Right atrium, RV: Right ventricle, PT: Pulmonary trunk, SCV: Superior caval vein
Common arterial trunk
The arrangement of pulmonary arteries in patients with common arterial trunk is highly variable. It forms the basis of two well-known systems used for classification systems.[23,24] In clinical practice, however, it is frequent to find a discrepancy between the descriptions provided during preoperative imaging and the surgical findings. This is particularly the case in differentiating the presence of a common pulmonary orifice, with a short confluent pulmonary segment, often called “Type I ½,” from the arrangement with closely separated orifices for the right and left pulmonary arteries, the so-called “Type II” variant.[25] Even detailed assessment of computed tomography, including the evaluation of external morphology by volume rendering, can prove insufficient in distinguishing these features. Virtual cardiac dissection, on the other hand, by permitting accurate visualization of the internal anatomy, provides necessary information to show precise fashion of origin of the right and left pulmonary arteries from the common arterial trunk [Figure 6].
Figure 6.
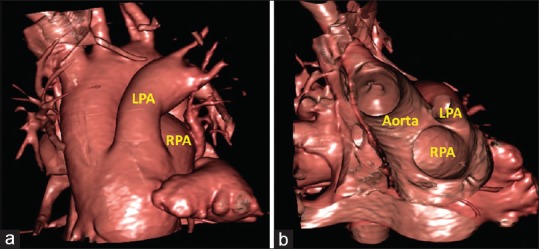
Virtual cardiac dissection obtained from a patient with common arterial trunk. It is not possible from the external view (a) to demonstrate the precise arrangement of the orifices of the left and right pulmonary arteries. The internal view (b), in anteroposterior projection with cranial tilt, on the other hand, provides an unequivocal demonstration of the closely separated locations of the orifices of the pulmonary arteries. LPA: Left pulmonary artery, RPA: Right pulmonary artery
DISCUSSION
We submit that the images we have produced by virtual dissection provide details of cardiac morphology comparable to those obtained using much costlier equipment. As with the expensive alternatives, furthermore, they provide the detail in attitudinally appropriate orientation.[26,27] As we have shown, the ability to display the images in any desired projection makes the technique useful in the day-to-day understanding not only of normal anatomy but also the anatomy of malformed hearts. Cardiologists, for example, can view the images in the fluoroscopic views to which they are accustomed, while surgeons can view the morphology in orientations as seen in the operating room [Online Video 2]. We predict that the technique has great potential to improve preprocedural clinical planning for interventional cardiologists, electrophysiologists, and cardiac surgeons. We recognize, nonetheless, that new technique is not without its limitations. As with any application depending on the use of computers, the ability of others to use our technique will depend on our ability to describe the steps required to manipulate the software. We hope that our attempts, including our video (online Video 1), will have provided the necessary guidance. The ability to produce high-quality virtual cardiac dissection is largely dependent on the quality of the computed tomographic datasets. Even with recent advancements, the spatial resolution of computed tomography is not always sufficient to display thinner cardiac structures, especially in children and patients with higher heart rates. The ability is further limited by the variability in the level of attenuation achieved during the computed tomographic study. At present, there is no way of controlling the level of attenuation. In our initial experience, when assessing 25 computed tomography datasets selected in a random fashion, accurate virtual cardiac dissection was achievable in two-thirds. We also recognize the limitation related to the time it takes for creating images of relevant cardiac structures since manual manipulation of “color maps” or “curves” is mandatory. As we have emphasized, therefore, an initial learning curve will be required to obtain an understanding of the functioning of various controls and editing tools. Once those seeking to use the technique become conversant with the required editing, we predict that the use of saved presets will provide relatively rapid virtual dissection. This, in turn, should bring the knowledge of the details of the anatomy of the normal and abnormal heart to the fingertips of all those who have access to computed tomographic datasets.
Our technique has been developed using Horos software on a Macintosh personal computer. We believe that, with the appropriate use of CLUT editing, it will be possible to prepare comparable reconstructions on a Windows platform. We also believe that a similar technique can be employed for analysis of the three-dimensional datasets obtained from magnetic resonance angiography. If these goals can be achieved, the use of virtual dissection can revolutionize the way the cardiologists, cardiac radiologists and cardiac surgeons assess the datasets now provided by modern-day computed tomographic and magnetic resonance imaging scanners. Also, integration of the virtual cardiac dissection technique with the recently developed image fusion technology[28] can surely be expected to provide intracardiac anatomic landmarks for easy and reliable performance of interventional procedures.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos Available on: www.annalspc.com
Acknowledgments
We are indebted to Dr. Shumpei Mori, from University of Kobe, Japan, for introducing us to virtual cardiac dissection. We also thank Professors Anita Saxena, SS Kothari, the late Rajnish Juneja, and S Ramakrishnan from the Department of Cardiology, AIIMS, New Delhi, for providing relevant inputs at various stages of developing the technique. We acknowledge the help received from Professors Sanjiv Sharma, Gurpreet Singh Gulati, Priya Jagia, and Dr. Sanjeev Kumar from the Department of Cardiovascular Radiology and Endovascular Interventions, AIIMS, New Delhi, in providing computed tomographic datasets.
REFERENCES
- 1.Simpson J, Lopez L, Acar P, Friedberg MK, Khoo NS, Ko HH, et al. Three-dimensional echocardiography in congenital heart disease: An expert consensus document from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:1–27. doi: 10.1016/j.echo.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Burchill LJ, Huang J, Tretter JT, Khan AM, Crean AM, Veldtman GR, et al. Noninvasive imaging in adult congenital heart disease. Circ Res. 2017;120:995–1014. doi: 10.1161/CIRCRESAHA.116.308983. [DOI] [PubMed] [Google Scholar]
- 3.Vukicevic M, Mosadegh B, Min JK, Little SH. Cardiac 3D printing and its future directions. JACC Cardiovasc Imaging. 2017;10:171–84. doi: 10.1016/j.jcmg.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kappanayil M, Koneti NR, Kannan RR, Kottayil BP, Kumar K. Three-dimensional-printed cardiac prototypes aid surgical decision-making and preoperative planning in selected cases of complex congenital heart diseases: Early experience and proof of concept in a resource-limited environment. Ann Pediatr Cardiol. 2017;10:117–25. doi: 10.4103/apc.APC_149_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valverde I, Gomez-Ciriza G, Hussain T, Suarez-Mejias C, Velasco-Forte MN, Byrne N, et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: An international multicentre study. Eur J Cardiothorac Surg. 2017;52:1139–48. doi: 10.1093/ejcts/ezx208. [DOI] [PubMed] [Google Scholar]
- 6.Mori S, Spicer DE, Anderson RH. Revisiting the anatomy of the living heart. Circ J. 2016;80:24–33. doi: 10.1253/circj.CJ-15-1147. [DOI] [PubMed] [Google Scholar]
- 7.Mori S, Fukuzawa K, Takaya T, Takamine S, Ito T, Fujiwara S, et al. Clinical cardiac structural anatomy reconstructed within the cardiac contour using multidetector-row computed tomography: Atrial septum and ventricular septum. Clin Anat. 2016;29:342–52. doi: 10.1002/ca.22546. [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Anderson RH, Nishii T, Matsumoto K, Loomba RS. Isomerism in the setting of the so-called “heterotaxy”: The usefulness of computed tomographic analysis. Ann Pediatr Cardiol. 2017;10:175–86. doi: 10.4103/apc.APC_171_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suranyi P, Varga-Szemes A, Hlavacek AM. An overview of cardiac computed tomography in adults with congenital heart disease. J Thorac Imaging. 2017;32:258–73. doi: 10.1097/RTI.0000000000000281. [DOI] [PubMed] [Google Scholar]
- 10.Rames JD, Kavarana MN, Schoepf UJ, Hlavacek AM. The utility of computed tomographic angiography in a neonate on extracorporeal membrane oxygenation with extreme cyanosis after Blalock-Taussig shunt. Ann Pediatr Cardiol. 2017;10:209–11. doi: 10.4103/0974-2069.205137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ratib O, Rosset A. Open-source software in medical imaging: development of OsiriX. Int J Comput Assist Radiol Surg. 2006;1:187–96. [Google Scholar]
- 12.Spiriev T, Nakov V, Laleva L, Tzekov C. OsiriX software as a preoperative planning tool in cranial neurosurgery: A step-by-step guide for neurosurgical residents. Surg Neurol Int. 2017;8:241. doi: 10.4103/sni.sni_419_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. [Last accessed on 2018 Apr 05]. Available from: https://www.horosproject.org/download-horos/
- 14.Calhoun PS, Kuszyk BS, Heath DG, Carley JC, Fishman EK. Three-dimensional volume rendering of spiral CT data: Theory and method. Radiographics. 1999;19:745–64. doi: 10.1148/radiographics.19.3.g99ma14745. [DOI] [PubMed] [Google Scholar]
- 15.Heath DG, Soyer PA, Kuszyk BS, Bliss DF, Calhoun PS, Bluemke DA, et al. Three-dimensional spiral CT during arterial portography: Comparison of three rendering techniques. Radiographics. 1995;15:1001–11. doi: 10.1148/radiographics.15.4.7569120. [DOI] [PubMed] [Google Scholar]
- 16.Ney DR, Drebin RA, Fishman EK, Magid D. Volumetric rendering of computed tomographic data: principles and techniques. IEEE Comput Graph Appl. 1990;10:24–32. [Google Scholar]
- 17.Fishman EK, Ney DR, Heath DG, Corl FM, Horton KM, Johnson PT, et al. Volume rendering versus maximum intensity projection in CT angiography: What works best, when, and why. Radiographics. 2006;26:905–22. doi: 10.1148/rg.263055186. [DOI] [PubMed] [Google Scholar]
- 18.Uhl JF. Three-dimensional modelling of the venous system by direct multislice helical computed tomography venography: Technique, indications and results. Phlebology. 2012;27:270–88. doi: 10.1258/phleb.2012.012J07. [DOI] [PubMed] [Google Scholar]
- 19.Luccichenti G, Cademartiri F, Pezzella FR, Runza G, Belgrano M, Midiri M, et al. 3D reconstruction techniques made easy: Know-how and pictures. Eur Radiol. 2005;15:2146–56. doi: 10.1007/s00330-005-2738-5. [DOI] [PubMed] [Google Scholar]
- 20.OsiriX User Manual. [Last accessed on 2018 Apr 05]. Available from: http://www.osirix-viewer.com/UserManualIntroduction.pdf .
- 21.Jensen B, Spicer DE, Sheppard MN, Anderson RH. Development of the atrial septum in relation to postnatal anatomy and interatrial communications. Heart. 2017;103:456–62. doi: 10.1136/heartjnl-2016-310660. [DOI] [PubMed] [Google Scholar]
- 22.Ebadi A, Spicer DE, Backer CL, Fricker FJ, Anderson RH. Double-outlet right ventricle revisited. J Thorac Cardiovasc Surg. 2017;154:598–604. doi: 10.1016/j.jtcvs.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 23.Collett RW, Edwards JE. Persistent truncus arteriosus; a classification according to anatomic types. Surg Clin North Am. 1949;29:1245–70. doi: 10.1016/s0039-6109(16)32803-1. [DOI] [PubMed] [Google Scholar]
- 24.Van Praagh R, Van Praagh S. The anatomy of common aorticopulmonary trunk (truncus arteriosus communis) and its embryologic implications. A study of 57 necropsy cases. Am J Cardiol. 1965;16:406–25. doi: 10.1016/0002-9149(65)90732-0. [DOI] [PubMed] [Google Scholar]
- 25.Russell HM, Jacobs ML, Anderson RH, Mavroudis C, Spicer D, Corcrain E, et al. A simplified categorization for common arterial trunk. J Thorac Cardiovasc Surg. 2011;141:645–53. doi: 10.1016/j.jtcvs.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Cook AC, Anderson RH. Attitudinally correct nomenclature. Heart. 2002;87:503–6. doi: 10.1136/heart.87.6.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson RH. The time has come to describe cardiac structures as seen during life, and not as perceived in the autopsy room. Heart Rhythm. 2015;12:515–6. doi: 10.1016/j.hrthm.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 28.Balzer J, Zeus T, Hellhammer K, Veulemans V, Eschenhagen S, Kehmeier E, et al. Initial clinical experience using the EchoNavigator(®)-system during structural heart disease interventions. World J Cardiol. 2015;7:562–70. doi: 10.4330/wjc.v7.i9.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


