Abstract
An unguarded atrioventricular orifice is an extremely rare congenital anomaly characterized by the absence of the atrioventricular valve in varying proportions. While atresia of the mitral or aortic valves are usually described as causes for hypoplastic left heart, our case highlights the role of free atrioventricular valve regurgitation and consequent volume loss of the left heart, giving rise to a small left ventricle. There was an associated double-outlet right ventricle and Type B aortic interruption. While we have attempted to discuss the complex management options in this scenario, the parents decided to withdraw further care.
Keywords: Congenital mitral valve absence, double-outlet right ventricle, hypoplastic left ventricle
INTRODUCTION
An unguarded atrioventricular orifice is a rare condition with attendant free regurgitation across the atrioventricular orifice. The effects of congenital atrioventricular valve regurgitation may have a significant role in the maldevelopment of the involved atrial and ventricular chambers and also the great arteries.
CASE REPORT
A male baby, born by cesarean section at term to a primigravida mother, with a birth weight of 1.8 kg, was referred to our center for suspected congenital heart disease in view of cyanosis and respiratory distress on the third postnatal day. Routine antenatal scans were reportedly normal; although, no dedicated fetal cardiac ultrasound was performed. There was no history of birth asphyxia. The baby was tachypneic, had mild chest retractions; pre- and postductal saturations were 87%. All peripheral pulses were well felt, and there was a soft-systolic murmur at the apex.
Two-dimensional echocardiogram showed abdominal situs solitus with levocardia, atrioventricular concordance, normal systemic venous drainage to the right atrium, and pulmonary veins draining to the left atrium. A large fossa ovalis defect showed left to right shunt [Figure 1a]. The mitral valve was absent; with only a ridge of tissue on the lateral aspect of the left atrioventricular groove [Figures 1b, 2 and Video 1]. There was free mitral regurgitation (peak regurgitant velocity of 1.8 m/s) and no mitral inflow obstruction [Figure 1c, d and Video 2]. The mitral annulus measured 5 mm in diameter (Z score = −3.48, Parameter[z]). No great artery arose from the morphologic left ventricle, which was hypoplastic and nonapex forming. The ventricular septum was intact, and the significant septal push to the left was better appreciated in diastole. The great arteries arose from the morphologic right ventricle [Videos 3 and 4]. This anatomic rarity is also noteworthy for the absence of a ventricular septal defect in the setting of a double-outlet right ventricle. As a corollary, an obligatory left-to-right shunt at the level of the ventricular septal defect in double-outlet right ventricle seems not necessary with mitral valve agenesis. There was moderate tricuspid regurgitation, with a dilated right atrium, and dilated hypertrophied right ventricle [Figure 3a]. Although the moderator band was well seen, the septal attachment of the leaflet could not be well-delineated, and the chordae and papillary muscles of the right ventricle were elongated. The Aorta was anterior and slightly to the right of the pulmonary artery at the level of the semilunar valves [Figure 3b]. The right ventricle gave rise to a dilated pulmonary trunk [Figure 3c]. Peak pulmonary regurgitation velocity of 3 m/s estimated the mean pulmonary artery pressure at 36 mm Hg. Sweeping further anteriorly, the aorta was also seen to arise from the right ventricle [Figure 3d]. There was no obstruction to flow across the tri-leaflet aortic valve or aortic regurgitation. Aortic arch was left-sided; however, the arch could not be traced beyond the left common carotid artery [Figure 4a and b]. The pulmonary artery and ductal view showed the dilated branch pulmonary arteries and a large ductus shunting bidirectionally and continuing as the descending aorta (Type B Interruption) [Figure 4c and d]. Biventricular systolic function was normal.
Figure 1.
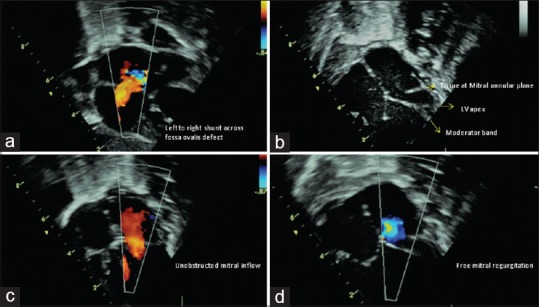
(a) Subcostal view showing the left-to-right shunt across fossa ovalis defect. (b) Apical four-chamber view in diastole. Arrow points to the lateral ridge of tissue at the plane of the mitral annulus. No valve is seen. Dashed-arrow points to the intact ventricular septum and shows the left ventricle reaching half way to the apex formed by the right ventricle. Bold arrow shows the moderator band in the right ventricle with its septal and parietal attachments. (c) Unobstructed laminar mitral inflow. (d) Free mitral regurgitation
Figure 2.
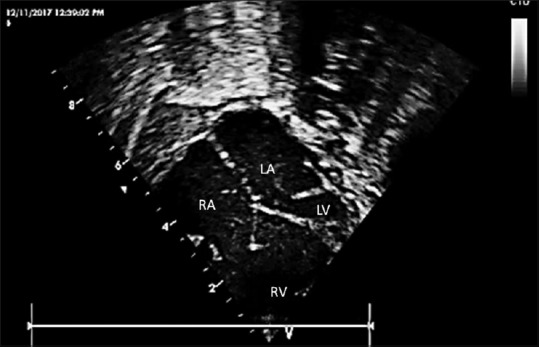
An enlarged view showing the crux of the heart with intact ventricular septum, a small left ventricle, and ridge of tissue on the lateral aspect of the mitral annular plane. Right atrium, left atrium, dilated right ventricle, and hypoplastic left ventricle are labeled within the figure
Figure 3.
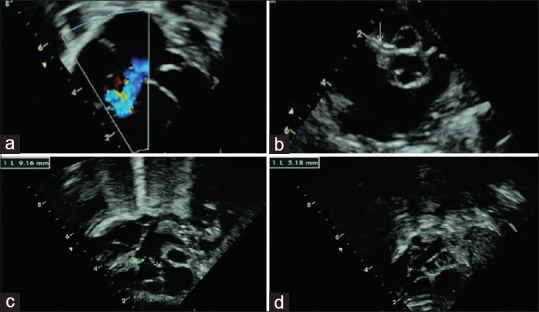
(a) Moderate-to-severe tricuspid regurgitation jet. (b) Parasternal short-axis view showing antero-posterior relationship of the great arteries. Arrow points to the origin of the right coronary artery from the anterior aorta. (c) Inflow-to-outflow sweep of the right ventricle showing a dilated pulmonary trunk with good-sized branch pulmonary arteries, arising from the dilated right ventricle. (d) Further anterior sweep opens the aorta arising from the right ventricle. The aortic annulus measures slightly more than half of the pulmonary annulus
Figure 4.
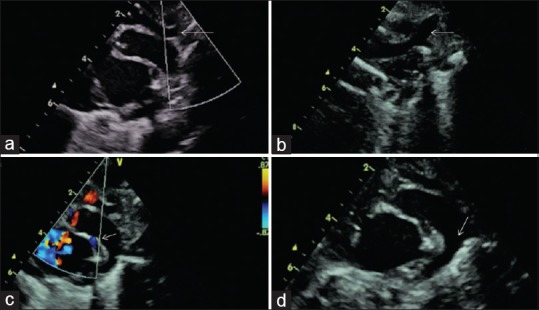
(a) Suprasternal view shows the transverse arch, not traceable below the point shown. (b) Left aortic arch with the dilated left common carotid artery. (c) Color Doppler showing antegrade flow across both the aorta and the pulmonary artery. Note the dilated pulmonary artery in comparison to the aorta. The ductus continues as the descending aorta. Color flow into the left pulmonary artery is shown by the arrow. (d) Arrow points to the large ductus continuing as the descending aorta
The complexity of the heart disease and possible surgical options were explained to the parents. However, in view of uncertain outcomes, long-term morbidity and the need for multiple-staged surgeries, they chose to offer comfort care at home.
DISCUSSION
Congenital absence of the mitral valve (unguarded mitral orifice) is an extremely rare congenital malformation. Initial reports of this condition were in the setting of atrioventricular discordance with an L-posed aorta.[1] There has been only one other report of this condition with concordant atrioventricular connection.[2] Almost all previously described cases have been associated with the double-outlet right ventricle, as in our case.[3] The atrioventricular valves are thought to develop from endocardial cushions between day 37 and 42 of life. Molecular mechanisms have identified the absence of certain genes such as connexin-45, leading to valve agenesis in mice.[4] The exact reason for agenesis of only one atrioventricular valve is still unknown. The presence of only a ridge of tissue on the lateral aspect of the left atrioventricular junction, a smaller effective annulus and the absence of an outflow tract from the left ventricle could have all contributed to hypoplasia of the left ventricle. In the absence of an outflow tract from the left ventricle, any blood which enters the left atrioventricular orifice has nowhere else to traverse (the ventricular septum being intact) and would egress back into the left atrium during systole, contributing to a significant volume loss of the left ventricle. The left ventricle was thus thin walled and reached only halfway to the apex, formed by the right ventricle. The finding is in stark contrast to ventricular dilatation seen with regurgitant atrioventricular valves of ventricles that support either the systemic or pulmonary circulation. We offered initial palliation in the form of arch reconstruction, pulmonary artery banding with the division of the ductus, and atrial septectomy. However, a freely regurgitant left atrioventricular orifice would still be significantly disadvantageous for a future Fontan pathway. Hence, exclusion of the left ventricle (similar to a Starne's procedure) was also considered. Although previous reports have suggested the survival of a few beyond the neonatal period, successful treatment of the condition has not been reported.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos available on: www.annalspc.com
REFERENCES
- 1.Brenner JI, Bharati S, Winn WC, Jr, Lev M. Absent tricuspid valve with aortic atresia in mixed levocardia (atria situs solitus, L-loop). A hitherto undescribed entity. Circulation. 1978;57:836–40. doi: 10.1161/01.cir.57.4.836. [DOI] [PubMed] [Google Scholar]
- 2.Su JA, Ho J, Wong PC. Unguarded mitral orifice associated with hypoplastic left heart syndrome. Cardiol Young. 2015;25:1002–5. doi: 10.1017/S1047951114001334. [DOI] [PubMed] [Google Scholar]
- 3.Kishi K, Katayama H, Ozaki N, Odanaka Y, Masuda M, Nemoto S, et al. Fatal cardiac anomaly of unguarded mitral orifice with asplenia syndrome. J Cardiol Cases. 2017;15:6–9. doi: 10.1016/j.jccase.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong EJ, Bischoff J. Heart valve development: Endothelial cell signaling and differentiation. Circ Res. 2004;95:459–70. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


