Key Points
Question
What is the national incidence of cutaneous and metastatic cutaneous squamous cell carcinoma in England?
Findings
In this national population-based study, the age-standardized rates for the first registered cutaneous squamous cell carcinoma from 2013 through 2015 were 77.3 per 100 000 person-years in men and 34.1 per 100 000 person-years in women. After a maximum follow-up of 36 months, 1.1% of women and 2.4% of men with a cutaneous squamous cell carcinoma developed metastatic cutaneous squamous cell carcinoma.
Meaning
These data are essential for informing future health care planning and evaluating skin cancer prevention policies.
This population-based study assesses the national incidence of cutaneous and metastatic cutaneous squamous cell carcinoma in England from 2013 through 2015.
Abstract
Importance
Cutaneous squamous cell carcinoma (cSCC) is the most common skin cancer with metastatic potential, but epidemiologic data are poor. Changes to the National Cancer Registration and Analysis Service (NCRAS) in England have allowed more accurate data analysis of primary and metastatic cSCC since 2013.
Objective
To assess the national incidence of cSCC and metastatic cSCC (mcSCC) in England from 2013 through 2015.
Design, Setting, and Participants
This national population-based study identified a cohort of patients with cSCC and mcSCC in England from January 1, 2013, through December 31, 2015. Patients were identified using diagnostic codes derived from pathology reports in the NCRAS. Data were analyzed from March 1, 2017, through March 1, 2018.
Main Outcomes and Measures
Incidence rates across sex and risk factors for cSCC were derived from the NCRAS data. Risk of occurrence of mcSCC among the population with cSCC was assessed with Cox proportional hazards regression analysis to determine indicators of mcSCC.
Results
Among the 76 977 patients with first primary cSCC in 2013 through 2015 (62.7% male; median age, 80 years [interquartile range, 72-86 years]), the age-standardized rates for the first registered cSCC in England from 2013 through 2015 were 77.3 per 100 000 person-years (PY) (95% CI, 76.6-78.0) in male patients and 34.1 per 100 000 PY (95% CI, 33.7-34.5) in female patients. Increased primary cSCC tumor count was observed in older, white male patients in lower deprivation quintiles. After a maximum follow-up of 36 months, cumulative incidence of mcSCC developed in 1.1% of women and 2.4% of men with a primary cSCC. Significant increases in the risk of metastasis with adjusted hazard rates of approximately 2.00 were observed in patients who were aged 80 to 89 years (hazard ratio [HR], 1.23; 95% CI, 1.07-1.43), 90 years or older (HR, 1.35; 95% CI, 1.09-1.66), male (HR, 1.79; 95% CI, 1.52-2.10), immunosuppressed (HR, 1.99; 95% CI, 1.64-2.42), and in higher deprivation quintiles (HR for highest quintile, 1.64; 95% CI, 1.35-2.00). Primary cSCC located on the ear (HR, 1.70; 95% CI, 1.42-2.03) and lip (HR, 1.85; 95% CI, 1.29-2.63) were at highest risk of metastasis.
Conclusions and Relevance
This study presents the first national study of the incidence of mcSCC. With limited health care resources and an aging population, accurate epidemiologic data are essential for informing future health care planning, identifying high-risk patients, and evaluating skin cancer prevention policies.
Introduction
Keratinocyte cancers include basal cell carcinoma and cutaneous squamous cell carcinoma (cSCC). They are the most common cancer in people of European ancestry; cSCCs represent only 20% of all keratinocyte cancers,1 but owing to the risk of metastatic cSCC (mcSCC), cSCC represents the most common cause of mortality due to keratinocyte cancers.2
Epidemiologic data on keratinocyte cancers have historically been of poor quality. Most cancer registries do not register keratinocyte cancers owing to their high volume and the complexity of accurately registering multiple tumors per patient.3,4,5 Hence, estimates of cSCC incidence often rely on population surveys or medical claims data.6,7,8,9 Despite this lack of data, evidence suggests that incidence rates of cSCC are increasing rapidly worldwide in light-skinned populations.5
Regarding mcSCC, epidemiologic data are rare and generally restricted to studies including at best several hundred patients with cSCC or small series of high-risk patients such as organ transplant recipients.10,11 The absence of nationwide good-quality registration of cSCC and mcSCC hampers the planning and evaluation of prevention, staging, and assessment of treatment cost-efficiency.6 Moreover, therapeutic options for mcSCC are limited, with minimal progress being made.
Changes in cancer registration processes in England in 2013, including the introduction of nationalized and automated cSCC registration, has enabled the creation of a population-based nationwide dataset specific for cSCC and mcSCC that is unique in the world.2,5,7,12,13 The objective of this study was to report incidence for cSCC and mcSCC in England from the National Cancer Registration and Analysis Service (NCRAS) data.
Methods
Study Design, Setting, and Participants
Cutaneous SCC
Data for this cohort of patients with cSCC and mcSCC in England were provided by the NCRAS (January 1, 2013, through December 31, 2015). National Health Service pathology laboratories are required and all private pathology laboratories in England are recommended to submit all pathology reports of cancer to the NCRAS. These pathology reports are enhanced with information from the Patient Administration System and Cancer Outcomes and Services Dataset to create a cancer record. No ethical approval or informed consent for this study was required; data are collected and reported by cancer registries using the statutory power provided by section 251 of the National Health Service Act 2006.14
Cutaneous SCCs were identified using topographical code C44 from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10); and morphology codes 8050 to 8052, 8070 to 8078, and 8082 to 8084; and behavior code 3 (malignant) from the International Classification of Diseases for Oncology, Second Edition (ICD-O-2). In situ cSCC, Bowen disease, mucosal cSCC, and genital cSCC were excluded. The date of the pathologic sample was interpreted as the date of diagnosis. Before 2013, cSCC registry data collection is less complete and these data were therefore not used.
Patients with nodal or distant mcSCC from 2013 to 2015 were identified from the group of 93 890 patients diagnosed with a cSCC during the study period based on the following selection criteria.
Search for keywords: review of all patients for whom the pathology reports included words such as metastatic, metastasis, FNA (fine-needle aspiration), fine needle, dissection, parotid, core biopsy, node biopsy, Tru-cut, needle aspirate, or lymph node.
Cancer Outcomes and Services Data set staging data: review of all patients for whom staging from the seventh edition of the American Joint Committee on Cancer was reported as a nodal or a metastatic stage above 0.11
Hospital Episode Statistics operation code data: review of all patients who have hospital operation codes for undergoing lymph node biopsies and dissection from 2013 to 2015 (Office of Population Censuses and Surveys Classification of Interventions and Procedures Edition 4 codes T8 or Y20).
Mortality data from the Office for National Statistics: review of all patients reported to have died of nonmelanoma skin cancers from 2013 to 2015.
In addition, we reviewed pathology reports of 1859 patients in which ICD-10 codes C07 (malignant parotid neoplasm), C08 (malignant salivary glands neoplasm), or C76 to C80 (malignant neoplasms of ill-defined, secondary, and unspecified sites) or ICD-O-2 behavior code 6 (metastatic) were used to identify possible mcSCC. This endeavor resulted in 1566 confirmed cases of mcSCC following review of 6168 patients’ registry data.
All mcSCC were histologically confirmed based on the information provided in the pathology report (clinical and pathologic information), topography, lymphatic drainage of the primary tumor, and lack of presence of other potential primary cancers, with the exception of 1 patient who had radiologically confirmed mcSCC documented on the pathology report. All mcSCC cases were reviewed by a dermatologist with or without a consultant pathologist. For multiple potential primary cSCCs, the primary site was chosen based on clinical judgement using the above information as well as the time from primary tumor diagnosis to metastasis. Only nodal and distant metastases were included in the study owing to the complexity in confirming in transit metastases vs recurrence of the primary cancer. Metastatic cSCCs of unknown origin or potentially from other primary sources were excluded, as were patients who developed a recurrence of mcSCC from 2013 through 2015 where metastasis was originally diagnosed before 2013. When the primary tumor pathology report was missing (64 of 1566), the date and site was assumed based on clinical information provided in the pathology report from the metastasis, historically registered tumors at NCRAS (with pathology reports no longer available), and operation codes from Hospital Episode Statistics with diagnosis ICD-10 code C44. After this process, the presumed primary pathology date was not available for only 19 (1.2%) of the 1566 patients.
Variables
Patient demographics including age, sex, and ethnicity were analyzed from the NCRAS. Deprivation quintiles were calculated using the patients’ lower-layer super output area at diagnosis linked to the Index of Multiple Deprivation 2015. The Index of Multiple Deprivation 2015 is based on socially similar (assessed through housing type) geographical units of, on average, 1500 residents in England; there are 32 844 units in England. For each unit, an index is calculated based on income; employment; educational level, skills, and training; health deprivation and disability; crime; barriers to housing and services; and living environment.15
Ethnicity was self-reported and coded using the Patient Administration System. To assess for immunosuppression, registry data and Hospital Episode Statistics were analyzed for diagnosis or operation codes associated with hematologic malignant disease, HIV, or solid organ transplant before the date of primary tumor diagnosis.
Sensitivity Analysis
To assess the sensitivity of identifying mcSCC, 1260 patients with primary cSCC from 2013 to 2015 were randomly selected from an Excel spreadsheet (version 2010; Microsoft Corp) using the random number generator. Review of the patient’s pathology reports by a dermatologist (Z.C.V.) found no further mcSCC.
Statistical Analysis
Data were analyzed from March 1, 2017, through March 1, 2018. The NCRAS data were extracted using an SQL Developer environment (version 4.1.5.21; Oracle). We used Stata software (version 14; StataCorp) for statistical analyses.
Age-standardized incidence rates were computed using the 2013 European Standard Population and reported per 100 000 person-years (PY). The time to metastasis was defined as the interval from the date of the first cSCC diagnosis from the 2013-2015 study period to the date of diagnosis of metastasis (with a minimum of 1 day). The survival of patients with mcSCC began from the date of metastasis diagnosis.
The cumulative risk of mcSCC occurrence and mortality during the 2013-2015 study period was determined using the Kaplan-Meier method. Metastatic cSCC in which the tumor identified as the primary source occurred before 2013 was excluded from risk of occurrence and Cox proportional hazards regression analysis models. In these models, patient follow-up started from the date of the first cSCC during the 2013-2015 study period and ended when they died or were lost to or unavailable for follow-up. Patients were followed up until December 31, 2016, for vital status and December 31, 2015, for metastasis risk. Total number of patients with cSCC lost to follow-up were 5665 (6.0%), which represents patients who have left the country or for whom vital status could not be confirmed on National Health Service digital files at the end of follow-up.
Risk factors included in multivariate analysis included age, sex, site of the primary tumor, deprivation quintile, and immunosuppression. For the risk of death, the presence of mcSCC was considered in the following 2 complementary ways: one model with a binomial variable for absence or presence of mcSCC, and another with the absence of mcSCC and, when an mcSCC was present, the likely primary cSCC from which the mcSCC arose. Results were expressed as hazard ratios (HRs) with 95% CIs for time to outcomes. Bidirectional P < .05 and 95% CIs of HRs not including 1.00 were deemed statistically significant.
Results
Cutaneous SCC
In 2013 through 2015, 93 890 patients living in England were diagnosed with a cSCC. Of these, 76 977 patients (82.0%) had a first registered primary cSCC (62.7% male and 37.3% female; median age, 80 years [interquartile range {IQR}, 72-86 years]), representing age-standardized rates of 77.3 per 100 000 PY (95% CI, 76.6-7.80) in male patients and 34.1 per 100 000 PY (95% CI, 33.7-34.5) in female patients (eFigure in the Supplement). The remaining 16 913 patients with cSCCs (18.0%) had a diagnosis of first primary cSCC before 2013 and at least 1 subsequent cSCC from 2013 through 2015.
The median age at onset for the first primary cSCC was 78 years (IQR, 71-84 years) in male patients and 80 years (IQR, 71-87 years) in female patients (Table 1). Primary cSCCs occurred predominantly in patients 70 years and older (78.8%) and those from white ethnic groups (89.0%). The incidence of first primary cSCC significantly increased from the most (10.6%) to the least (26.7%) deprived quintiles of the population. Compared with women, men had cSCC considerably more frequently on the ears (15.8% vs 1.3%) and scalp and neck (24.0% vs 5.8%). In contrast, cSCCs were more numerous on the lower limbs of women (25.1% vs 4.9%).
Table 1. Patient Demographics of First Primary cSCC and First Metastatic cSCCa.
| Patient Demographics | First Primary cSCC (n = 76 977) | First Metastatic cSCC (n = 1566) | ||
|---|---|---|---|---|
| Male (n = 48 254) | Female (n = 28 723) | Male (n = 1216) | Female (n = 350) | |
| Age at diagnosis, median (IQR), y | 78 (71-84) | 80 (71-87) | 80 (72-86) | 84 (75-88) |
| Age bands, No. (%) | ||||
| <50 | 808 (1.7) | 613 (2.1) | 13 (1.1) | <5 |
| 50-59 | 2117 (4.4) | 1491 (5.2) | 42 (3.5) | 8 (2.3) |
| 60-69 | 7352 (15.2) | 3930 (13.7) | 164 (13.5) | 35 (10.0) |
| 70-79 | 16 158 (33.5) | 7735 (26.9) | 349 (28.7) | 86 (24.6) |
| 80-89 | 17 822 (36.9) | 10 385 (36.2) | 496 (40.8) | 144 (41.1) |
| ≥90 | 3997 (8.3) | 4569 (15.9) | 152 (12.5) | 74 (21.1) |
| Ethnicity, No. (%) | ||||
| White | 43 283 (89.7) | 25 226 (87.8) | 1153 (94.8) | 332 (94.9) |
| Mixed | 29 (0.1) | 22 (0.1) | <5 | 0 |
| Indian or other southeast Asian | 73 (0.2) | 50 (0.2) | 12 (1.0) | <5 |
| Afro-Caribbean or other black | 42 (0.1) | 38 (0.1) | <5 | 0 |
| Chinese | 7 (0.01) | 8 (0.03) | 0 | 0 |
| Other | 169 (0.4) | 96 (0.3) | <5 | 0 |
| Unknown | 4651 (9.6) | 3283 (11.4) | 45 (3.7) | 16 (4.6) |
| Deprivation quintile, No. (%)b | ||||
| 1 | 13 227 (27.4) | 7317 (25.5) | 310 (25.5) | 70 (20.0) |
| 2 | 12 336 (25.6) | 7090 (24.7) | 296 (24.3) | 86 (24.6) |
| 3 | 10 331 (21.4) | 6333 (22.0) | 251 (20.6) | 76 (21.7) |
| 4 | 7569 (15.7) | 4799 (16.7) | 191 (15.7) | 61 (17.4) |
| 5 | 4791 (9.9) | 3184 (11.1) | 168 (13.8) | 57 (16.3) |
Abbreviations: cSCC, cutaneous squamous cell carcinoma; IQR, interquartile range.
Data were acquired from the National Cancer Registration and Analysis Service in England from January 1, 2013, through December 31, 2015. Percentages have been rounded and may not total 100. Cells with fewer than 5 patients are not counted owing to patient identifiability.
One indicates least deprived; 5, most deprived.
Metastatic cSCC
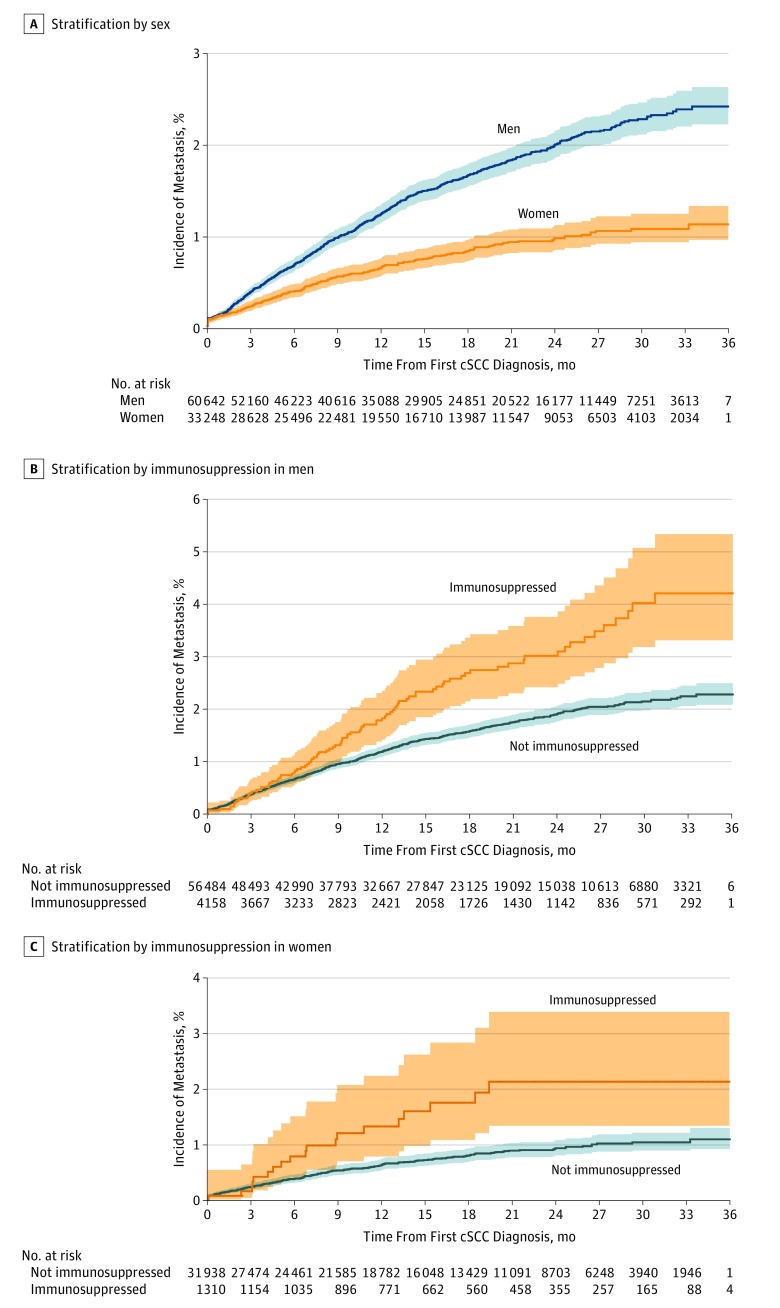
In 2013 through 2015, a total of 1566 patients living in England had a first diagnosis of mcSCC. The median age at diagnosis was 80 years (IQR, 72-86 years) in male patients and 84 years (IQR, 75-88 years) (Table 1). Of the 1566 patients with a first mcSCC, the primary cSCC was diagnosed before 2013 for 471 (30.1%) and in 2013 through 2015 for 1076 (68.7%) (Table 2). When the cSCC was diagnosed in 2013 through 2015, the origin of the mcSCC was the first registered primary cSCC in 836 patients and a nonfirst primary cSCC for 240 patients. For 19 patients with mcSCC (1.2%), the date of primary cSCC diagnosis was unknown. Most mcSCCs (1335 [85.2%]) were diagnosed within 2 years of the primary cSCC. In a minority of patients with cSCC (102 [6.5%]), the detection of mcSCC preceded or was made at the time of the diagnosis of the primary cSCC. The site of the metastasis was the head and neck lymph nodes or parotid for 1152 patients with mcSCC (73.6%). After excluding mcSCC in which the presumed primary source of mcSCC occurred before 2013, the cumulative risk of occurrence of mcSCC was 2.1% (1.1% in women and 2.4% in men) in 2013 through 2015 after a median follow-up of 15.2 months (range, 0-36 months) (Figure).
Table 2. Main Characteristics of 1566 Patients With Metastatic cSCCa.
| Characteristic | Patient Data |
|---|---|
| Year primary cSCC diagnosed | |
| Before 2011 | 70 |
| 2011 | 103 |
| 2012 | 298 |
| 2013 | 471 |
| 2014 | 409 |
| 2015 | 196 |
| Unknown | 19 |
| Year metastasis diagnosed | |
| 2013 | 479 |
| 2014 | 520 |
| 2015 | 567 |
| Site of primary tumor | |
| Lip (cutaneous) | 54 |
| Eyelid, including canthus | 36 |
| Ear | 324 |
| Face | 430 |
| Scalp or neck | 308 |
| Trunk, including perianal | 115 |
| Upper limb, including shoulder | 136 |
| Lower limb, including hip | 158 |
| Skin NOS | 5 |
| Site of first nodal or distant metastasis (ie, each patient counted once) | |
| Neck or parotid | 1155 |
| Axilla | 219 |
| Groin | 175 |
| Otherb | 7 |
| Distant | 10 |
| Distant metastasis overall | 37 |
| Time from diagnosis of primary tumor to diagnosis of metastasis, median (IQR), dc | 262 (−93 to 3553) |
| Time to diagnosis of metastasis, No. (%)d | |
| <2 y | 1335 (85.2) |
| <3 y | 1455 (92.9) |
| Metastasis diagnosed before or on day of diagnosis of primary tumor, No. (%) | 102 (6.5) |
Abbreviations: cSCC, cutaneous squamous cell carcinoma; IQR, interquartile range; NOS, not otherwise specified.
Data were acquired from the National Cancer Registration and Analysis Service in England from January 1, 2013, through December 31, 2015.
Includes orbital, popliteal, or epitrochlear lymph nodes.
Excludes those with unknown date of primary diagnosis (n = 19).
Unknown date of primary diagnosis (n = 19) was assumed to be at least 3 years, given that historical data were less accurate.
Figure. Kaplan-Meier Curves for Risk of Occurrence of Metastatic Cutaneous Squamous Cell Carcinoma (cSCC) in Patients Diagnosed With Primary cSCC.
Data were acquired from the National Cancer Registration and Analysis Service in England. Time from diagnosis was measured from January 1, 2013, through December 31, 2015. Shaded areas indicate 95% CI.
The male to female ratio of mcSCC site distribution was consistent with the ratio found for the site distribution of cSCCs except for the eyelid (1.3 and 6.3, respectively), a result that could be due to the small number of mcSCCs associated with that site. In both sexes, the rates of mcSCC were highest for cSCC diagnosed on the ear (crude risk, 1.67 and 2.05, respectively) and the lip (crude risk, 1.67 and 2.05, respectively), plus the eyelid in men (crude risk, 1.89). The mcSCC rates were greater in men for all body sites except for the scalp, neck, and ears, where rates were similar in both sexes, suggesting that the likelihood of mcSCC from cSCC diagnosed on these sites are the same in both sexes (Table 3). We found no association between deprivation and the site from which mcSCC arose. Overall, the rate of mcSCC per 1000 patients with cSCC was 13.9 in men and 7.0 in women, giving a risk 1.98 (95% CI, 1.72-2.29) times greater in men than in women.
Table 3. Site-Specific Crude Risk of Metastasis by Site of Primary cSCC .
| Site of Primary Tumora | Nonmetastatic cSCCb | Metastatic cSCC | Crude Risk of mcSCC by Primary Sitec | |||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients (n = 92 814) | M:F Ratio | No. of Patients | M:F Ratio | |||||
| Male | Female | Male | Female | Male | Female | |||
| Lip (cutaneous) | 882 | 740 | 1.2 | 23 | 11 | 2.1 | 1.67 | 2.05 |
| Eyelid, including canthus | 643 | 511 | 1.3 | 19 | 3 | 6.3 | 1.89 | 0.81 |
| Ear | 8518 | 370 | 23.0 | 214 | 10 | 21.4 | 1.60 | 3.73 |
| Face | 14 691 | 9807 | 1.5 | 230 | 71 | 3.2 | 1.00 | 1.00 |
| Scalp or neck | 13 193 | 1805 | 7.3 | 205 | 23 | 8.9 | 0.99 | 1.76 |
| Trunk, including perianal | 3393 | 2317 | 1.5 | 62 | 18 | 3.4 | 1.17 | 1.07 |
| Upper limb, including shoulder | 7735 | 5884 | 1.3 | 54 | 34 | 1.6 | 0.45 | 0.80 |
| Lower limb, including hip | 2587 | 7856 | 0.3 | 36 | 63 | 0.6 | 0.89 | 1.11 |
| Skin NOS | 8157 | 3725 | 2.2 | 0 | 0 | NA | 0.00 | 0.00 |
| All sites | 59 799 | 33 015 | 1.8 | 843 | 233 | 3.6 | 0.90 | 0.97 |
Abbreviations: cSCC, cutaneous squamous cell carcinoma; mcSCC, metastatic cSCC; M:F, male to female; NA, not applicable; NOS, not otherwise specified.
Includes primary cSCC if metastatic or if first cSCC was diagnosed from January 1, 2013, through December 31, 2015. Excludes patients who developed metastases from primary tumors before 2013 (n = 93 890).
Includes primary tumors that had not metastasized during the 2013-2015 follow-up period.
Risk by site of primary tumor was compared with that for the face.
The risk of mcSCC was highest in patients who were aged 80 to 89 years (HR, 1.23; 95% CI, 1,07-1.43) and 90 years or older (HR, 1.35; 95% CI, 1.09-1.66), male (HR, 1.79; 95% CI, 1.52-2.10), and within the highest level of deprivation (HR, 1.64; 95% CI, 1.35-2.00), who had cSCC on the lip (HR, 1.85; 95% CI, 1.29-2.63), eyelid (HR, 1.54; 95% CI, 1.00-2.38), and ear (HR, 1.70; 95% CI, 1.42-2.03), and with immunosuppression (HR, 1.99; 95% CI, 1.64-2.42) (Table 4). Restricting the analyses to the 76 977 patients with the first primary cSCC obtained did not change conclusions.
Table 4. Cox Proportional Multivariate Hazards Regression Analysis of the 93 890 Patients Who Were Diagnosed With a Primary cSCCa.
| Patient Variable | Primary cSCC Diagnosed in 2013-2015 | Risk of Metastasis by Variable | Risk of Death, Including Site | Risk of Death Excluding Site, Including Risk of Metastasis | ||||
|---|---|---|---|---|---|---|---|---|
| Nonmetastatic (n = 92 814) | Metastatic (n = 1076) | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Metastasis (yes vs no) | 0 | 1076 | NA | NA | NA | NA | 2.44 (2.21-2.67) | <.001 |
| Age, y | ||||||||
| <50 | 1484 | 13 | 0.71 (0.41-1.24) | .23 | 0.35 (0.25-0.47) | <.001 | 0.34 (0.25-0.46) | <.001 |
| 50-59 | 3941 | 36 | 0.82 (0.58-1.16) | .26 | 0.47 (0.39-0.55) | <.001 | 0.46 (0.39-0.55) | <.001 |
| 60-69 | 12 585 | 142 | 0.99 (0.81-1.21) | .90 | 0.57 (0.52-0.63) | <.001 | 0.57 (0.52-0.62) | <.001 |
| 70-79 | 28 206 | 317 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| 80-89 | 35 330 | 443 | 1.23 (1.07-1.43) | .005 | 2.50 (2.38-2.62) | <.001 | 2.51 (2.39-2.64) | <.001 |
| ≥90 | 11 268 | 125 | 1.35 (1.09-1.66) | .005 | 5.56 (5.26-5.87) | <.001 | 5.62 (5.32-5.93) | <.001 |
| Sex | ||||||||
| Male | 59 799 | 843 | 1.79 (1.52-2.10) | <.001 | 1.21 (1.16-1.27) | <.001 | 1.28 (1.23-1.33) | <.001 |
| Female | 33 015 | 233 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| Deprivation quintileb | ||||||||
| 1 | 24 937 | 262 | 1 [Reference] | NA | 1 [Reference] | NA | 1 [Reference] | NA |
| 2 | 23 481 | 263 | 1.07 (0.90-1.27) | .42 | 1.09 (1.04-1.15) | .001 | 1.09 (1.04-1.15) | .001 |
| 3 | 20 070 | 218 | 1.04 (0.87-1.25) | .63 | 1.16 (1.09-1.22) | <.001 | 1.16 (1.10-1.22) | <.001 |
| 4 | 14 777 | 174 | 1.14 (0.94-1.38) | .19 | 1.29 (1.22-1.37) | <.001 | 1.29 (1.22-1.36) | <.001 |
| 5 | 9549 | 159 | 1.64 (1.35-2.00) | <.001 | 1.41 (1.33-1.50) | <.001 | 1.40 (1.31-1.49) | <.001 |
| Sitec | ||||||||
| Lip | 1622 | 34 | 1.85 (1.29-2.63) | .001 | 0.90 (0.76-1.06) | .19 | NA | NA |
| Eyelid | 1154 | 22 | 1.54 (1.00-2.38) | .049 | 0.97 (0.82-1.15) | .73 | NA | NA |
| Ear | 8888 | 224 | 1.70 (1.42-2.03) | <.001 | 1.03 (0.96-1.10) | .42 | NA | NA |
| Face | 24 498 | 301 | 1 [Reference] | NA | 1 [Reference] | NA | NA | NA |
| Scalp or neck | 14 998 | 228 | 1.08 (0.91-1.29) | .38 | 1.14 (1.08-1.21) | <.001 | NA | NA |
| Trunk | 5710 | 80 | 1.18 (0.92-1.51) | .19 | 1.03 (0.94-1.12) | .55 | NA | NA |
| Upper limb | 13 619 | 88 | 0.54 (0.43-0.69) | <.001 | 0.92 (0.86-0.97) | .005 | NA | NA |
| Lower limb | 10 443 | 99 | 0.96 (0.76-1.21) | .72 | 0.87 (0.81-0.93) | <.001 | NA | NA |
| Other or unknown | 11 882 | 0 | NA | NA | 1.06 (1.01-1.13) | .03 | NA | NA |
| Immunosuppression before primary cSCC diagnosis | 5355 | 113 | 1.99 (1.64-2.42) | <.001 | 2.15 (2.02-2.29) | <.001 | 2.14 (2.01-2.28) | <.001 |
Abbreviations: cSCC, cutaneous squamous cell carcinoma; HR, hazard ratio; NA, not applicable.
Data were acquired from the National Cancer Registration and Analysis Service in England from January 1, 2013, through December 31, 2015. All models include all variables in columns. Survival calculations started on the date of the first cSCC diagnosis in 2013 to 2015.
One indicates least deprived; 5, most deprived.
The site is the skin area of first cSCC or primary cSCC if metastatic.
Survival of Patients With cSCC
Until the end of 2016, 13 453 deaths due to all causes were observed among the 76 977 patients diagnosed with a first primary cSCC in 2013 through 2015. The 3-year survival was 65% among men and 68% among women. In the 836 patients who subsequently developed mcSCC, the 3-year survival was 46% in men and 29% in women. Comparatively, expected 3-year survival of an 80-year old in England in 2013 through 2015 would be 76% for men and 82% for women.16
Multivariate Cox proportional hazards regression analyses examined the influence of factors on the risk of mcSCC and of death due to all causes among the 93 890 patients with cSCC (Table 4). The risk of short-term death due to all causes was highest in age bands 80 to 89 years (HR, 2.51; 95% CI, 2.39-2.64) and 90 years or older (HR, 5.62; 95% CI, 5.32-5.93), those with a diagnosis of mcSCC (HR, 2.44; 95% CI, 2.21-2.67), and those with the highest level of deprivation (HR, 1.40; 95% CI, 1.31-1.49). The occurrence of mcSCC and a history of immunosuppression were associated with a 2-fold short-term increase in risk of death (HR, 2.14; 95% CI, 2.01-2.28) (Table 4). Again, restricting the analyses to the 76 977 patients with a first primary cSCC obtained did not change conclusions.
Discussion
To our knowledge, this study has resulted in the first national incidence report of mcSCC in light-skinned populations owing to the modernization of national English cancer registration data collection and the largest cohort of mcSCC studied to date. A UK Translational Research Network in Dermatology electronic Delphi exercise to assess the research needs of health care professionals in the United Kingdom17 identified cSCC as a research priority because of the limited research progress over the years. These observational data form the basis for evaluating prevention and early detection efforts, planning health care activities, and determining the characteristics of patients with cSCC more likely to develop mcSCC.
Study Strengths
The main strength of this study relates to the use of a novel data source consolidating national databases since 2013. Before 2013, cancer registration was performed at the regional level. Moreover, the national database became automated for all keratinocyte cancers, which allows more complete data collection. Indeed, the manual registration of all cancers meant that keratinocyte cancers were often neglected because these cancers were not a priority for registration data. In addition, the national automated registry has greatly facilitated the retrieval of pathology reports, which led to exhaustive identification of patients with mcSCC.
Cutaneous SCC
The age-standardized rate of first primary cSCC was 55.7 per 100 000 PY in 2013 through 2015. In the systematic review of Lomas et al,5 age-standardized rates of cSCC in England for the period 2000 through 2006 ranged from 18.4 to 33.0 per 100 000 PY. If the substantial differences in incidence were due to increases over time, then an annual increase of about 7% would be assumed.5 Improvements in data collection and registration may account for some of the difference in incidence rates.
Primary sites of cSCC differ between men and women, presumably owing to varying exposure to UV radiation provided as a result of male pattern baldness and cultural preferences (ie, shorter hair for men, women wearing dresses or skirts). This difference results in men being more likely to develop cSCC on the ear and scalp and women more likely on a lower limb. However, the most common site in men and women for cSCC remains the face.
Similar to previous studies, we show that cSCCs are significantly associated with lower deprivation quintiles.18 This finding is likely to be in part the result of the expense of foreign travel and therefore higher cumulative UV exposure in the generations affected.
Metastatic cSCC
During a median follow-up of 15.2 months, 1.1% of female and 2.4% of male patients developed mcSCC (2.1% overall). This finding is in line with previous observations in a single-center study (4% during a median of 43 months) and a small UK population-based study (1.6% during a median follow-up of 79 months).10 The lower incidence in the population-based study may be owing to lower capture rates emphasizing the importance of a national cancer registry.
When including all patients diagnosed with mcSCC in 2013 through 2015 (ie, including primary tumors diagnosed before 2013), we found that 92.9% of cSCCs that metastasized did so within 3 years of the primary cSCC diagnosis, as shown in previous studies.19 In contrast to cSCC incidence, the risk of mcSCC and death is highest in the most deprived quintile. This survival deprivation gap18 in which, despite the tumors being more common in the least deprived patients, mortality is highest in the most deprived quintiles, possibly reflects inequalities in health care and educational attainment.
The propensity of cSCC to metastasize is greater in men than in women for all anatomical sites, except the ear, scalp, neck, and upper limb. Lip and ear lesions have frequently been found to be higher-risk tumors in previous studies.19,20,21 It is unclear whether lip lesions may result from additional risk factors other than cumulative UV radiation, including smoking, alcohol intake, or human papillomavirus infection, which could account for its more aggressive behavior.22 The apparent sex contrast in metastatic rate that we have found may warrant further investigation.
Implications for Future Policy and Practice
Increasing cSCC incidence is presumed to result from an aging population and increased cumulative UV radiation exposure resulting from easier access to travel abroad and tanning trends. Owing to their frequency, the health care burden of cSCC is substantial, with high-risk patients requiring at least 2 to 5 years of clinical follow-up after treatment and patients often developing multiple tumors.23 With poor 3-year survival once cSCC has metastasized, earlier identification of these high-risk patients and improved treatment options become clear priorities.
Limitations
Our study was limited to 3 years and did not identify patients with multiple cSCC in 2013 through 2015. The 5-year risk of a subsequent cSCC is estimated to be 37%.24 Previous studies have shown that when counting all keratinocyte cancers as opposed to the first registered tumor, an additional 30% to 50% of tumors are counted.25,26 Identification of patients living in England with more than 1 primary cSCC in 2013 through 2015 would have required the review of medical files for nearly 100 000 patients. With more complete registration of keratinocyte cancers from 2013 onward, the number of first cSCC may be overestimated, when patients may have previously had unregistered cSCC.
Primary cSCC affecting perianal sites have different pathophysiological features, with human papillomavirus infection thought to be a pivotal cause rather than cumulative UV radiation.27 Unfortunately, we were unable to identify perianal tumors from the laboratory coding, which categorizes perianal tumors as truncal; however, this number should represent a minority of truncal cSCC.27
The estimation of mcSCC occurrences may be underestimated. No ICD-10 code is specific to mcSCC. Hence, analysis of several different data sources as well as clinical interpretation of pathology reports were required. Clinical information was limited to that written on the pathology report by the physician and pathologist, as well as linked hospital data (Cancer Outcomes and Services Dataset and Hospital Episode Statistics). Moreover, reliance on histologic data means that cSCCs and mcSCC managed without histologic confirmation are excluded, which may exclude older, frailer patients.
Immunosuppression is likely to have been underestimated owing to reliance on hospital diagnosis and operation coding. Also, causes of immunosuppression, such as long-term treatment with immunosuppressive drugs and anticancer immunotherapies, were not captured.
Conclusions
Although only a small proportion of patients with cSCC develop mcSCC, the high mortality of mcSCC results in most patients with cSCC undergoing close clinical surveillance for many years. Owing to the high frequency of cSCC, this surveillance has a large influence on health care services. The availability of a nationwide population-based cancer registration allows the capture of all patients diagnosed with a cSCC or an mcSCC in England, an epidemiologic tool likely to greatly enhance interpretation of the quality and cost-efficiency of preventive, screening, staging, and treatment activities.
eFigure. Flowchart of Patient Cohorts From the National Cancer Registration and Analysis Service, England 2013-2015
References
- 1.Burton KA, Ashack KA, Khachemoune A. Cutaneous squamous cell carcinoma: a review of high-risk and metastatic disease. Am J Clin Dermatol. 2016;17(5):491-508. doi: 10.1007/s40257-016-0207-3 [DOI] [PubMed] [Google Scholar]
- 2.Hollestein LM, de Vries E, Nijsten T. Trends of cutaneous squamous cell carcinoma in the Netherlands: increased incidence rates, but stable relative survival and mortality 1989-2008. Eur J Cancer. 2012;48(13):2046-2053. doi: 10.1016/j.ejca.2012.01.003 [DOI] [PubMed] [Google Scholar]
- 3.Trakatelli M, Ulrich C, del Marmol V, Euvrard S, Stockfleth E, Abeni D. Epidemiology of nonmelanoma skin cancer (NMSC) in Europe: accurate and comparable data are needed for effective public health monitoring and interventions [published correction appears in Br J Dermatol. 2007;157(3):634]. Br J Dermatol. 2007;156(suppl 3):1-7. doi: 10.1111/j.1365-2133.2007.07861.x [DOI] [PubMed] [Google Scholar]
- 4.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(suppl 61):1-6. doi: 10.1046/j.1365-2133.146.s61.2.x [DOI] [PubMed] [Google Scholar]
- 5.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069-1080. doi: 10.1111/j.1365-2133.2012.10830.x [DOI] [PubMed] [Google Scholar]
- 6.Karia PS, Jambusaria-Pahlajani A, Harrington DP, Murphy GF, Qureshi AA, Schmults CD. Evaluation of American Joint Committee on Cancer, International Union Against Cancer, and Brigham and Women’s Hospital tumor staging for cutaneous squamous cell carcinoma. J Clin Oncol. 2014;32(4):327-334. doi: 10.1200/JCO.2012.48.5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151(10):1081-1086. doi: 10.1001/jamadermatol.2015.1187 [DOI] [PubMed] [Google Scholar]
- 8.Pandeya N, Olsen CM, Whiteman DC. The incidence and multiplicity rates of keratinocyte cancers in Australia. Med J Aust. 2017;207(8):339-343. doi: 10.5694/mja17.00284 [DOI] [PubMed] [Google Scholar]
- 9.Perera E, Gnaneswaran N, Staines C, Win AK, Sinclair R. Incidence and prevalence of non-melanoma skin cancer in Australia: a systematic review. Australas J Dermatol. 2015;56(4):258-267. doi: 10.1111/ajd.12282 [DOI] [PubMed] [Google Scholar]
- 10.Nelson TG, Ashton RE. Low incidence of metastasis and recurrence from cutaneous squamous cell carcinoma found in a UK population: do we need to adjust our thinking on this rare but potentially fatal event? J Surg Oncol. 2017;116(6):783-788. doi: 10.1002/jso.24707 [DOI] [PubMed] [Google Scholar]
- 11.Farasat S, Yu SS, Neel VA, et al. . A new American Joint Committee on Cancer staging system for cutaneous squamous cell carcinoma: creation and rationale for inclusion of tumor (T) characteristics. J Am Acad Dermatol. 2011;64(6):1051-1059. doi: 10.1016/j.jaad.2010.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisemann N, Jansen L, Castro FA, et al. ; GEKID Cancer Survival Working Group . Survival with nonmelanoma skin cancer in Germany. Br J Dermatol. 2016;174(4):778-785. doi: 10.1111/bjd.14352 [DOI] [PubMed] [Google Scholar]
- 13.Dal H, Boldemann C, Lindelöf B. Trends during a half century in relative squamous cell carcinoma distribution by body site in the Swedish population: support for accumulated sun exposure as the main risk factor. J Dermatol. 2008;35(2):55-62. doi: 10.1111/j.1346-8138.2008.00416.x [DOI] [PubMed] [Google Scholar]
- 14.National Archives. National Health Service Act 2006. http://www.legislation.gov.uk/ukpga/2006/41/contents. Accessed August 3, 2017.
- 15.Ministry of Housing, Communities & Local Government Official statistics: English indices of deprivation 2015. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2015. September 30, 2015. Accessed October 9, 2017.
- 16.Office for National Statistics. National life tables: England. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/lifeexpectancies/datasets/nationallifetablesenglandreferencetables. September 25, 2018. Accessed September 9, 2017.
- 17.Healy E, Brown SJ, Langan SM, Nicholls SG, Shams K, Reynolds NJ. Identification of translational dermatology research priorities in the UK: results of an electronic Delphi exercise. Br J Dermatol. 2015;173(5):1191-1198. doi: 10.1111/bjd.14022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doherty VR, Brewster DH, Jensen S, Gorman D. Trends in skin cancer incidence by socioeconomic position in Scotland, 1978-2004. Br J Cancer. 2010;102(11):1661-1664. doi: 10.1038/sj.bjc.6605678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brantsch KD, Meisner C, Schönfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9(8):713-720. doi: 10.1016/S1470-2045(08)70178-5 [DOI] [PubMed] [Google Scholar]
- 20.Rowe DE, Carroll RJ, Day CL Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip: implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976-990. doi: 10.1016/0190-9622(92)70144-5 [DOI] [PubMed] [Google Scholar]
- 21.Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152(4):419-428. doi: 10.1001/jamadermatol.2015.4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Visscher JG, van der Waal I. Etiology of cancer of the lip: a review. Int J Oral Maxillofac Surg. 1998;27(3):199-203. doi: 10.1016/S0901-5027(98)80010-6 [DOI] [PubMed] [Google Scholar]
- 23.Motley RJ, Preston PW, Lawrence CM; British Association of Dermatologists Multi-professional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. http://www.bad.org.uk/library-media%5Cdocuments%5CSCC_2009.pdf. Updated December 11, 2009. Accessed April 1, 2018.
- 24.Flohil SC, van der Leest RJ, Arends LR, de Vries E, Nijsten T. Risk of subsequent cutaneous malignancy in patients with prior keratinocyte carcinoma: a systematic review and meta-analysis. Eur J Cancer. 2013;49(10):2365-2375. doi: 10.1016/j.ejca.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 25.de Vries E, Micallef R, Brewster DH, et al. ; EPIDERM Group . Population-based estimates of the occurrence of multiple vs first primary basal cell carcinomas in 4 European regions. Arch Dermatol. 2012;148(3):347-354. doi: 10.1001/archdermatol.2011.2244 [DOI] [PubMed] [Google Scholar]
- 26.Lucke TW, Hole DJ, Mackie RM. An audit of the completeness of non-melanoma skin cancer registration in greater Glasgow. Br J Dermatol. 1997;137(5):761-763. doi: 10.1111/j.1365-2133.1997.tb01114.x [DOI] [PubMed] [Google Scholar]
- 27.Dawson H, Serra S. Tumours and inflammatory lesions of the anal canal and perianal skin revisited: an update and practical approach. J Clin Pathol. 2015;68(12):971-981. doi: 10.1136/jclinpath-2015-203056 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flowchart of Patient Cohorts From the National Cancer Registration and Analysis Service, England 2013-2015