Abstract
Temporal and spatial regulation of membrane-trafficking events is crucial to both membrane identity and overall cell polarity. Small GTPases of the Rab, Ral and Rho protein families have been implicated as important regulators of vesicle docking and fusion events. This review focuses on how these GTPases interact with the exocyst complex, which is a multi-subunit tethering complex involved in the regulation of cell-surface transport and cell polarity. The Rab and Ral GTPases are thought to function in exocyst assembly and vesicle-tethering processes, whereas the Rho family GTPases seem to function in the local activation of the exocyst complex to facilitate downstream vesicle-fusion events. The localized activation of the exocyst by Rho GTPases is likely to have an important role in spatial regulation of exocytosis.
Introduction
The ability of cells to direct specific membrane and protein components to defined places on the cell surface is fundamental to the establishment and maintenance of cell polarity. The mechanism by which proteins and lipids are delivered to the cell surface is through transport, docking and fusion of secretory vesicles with the plasma membrane. In polarized cells, the location of these transport events is highly regulated, but the precise mechanism of regulation is still poorly understood. A protein complex, the function of which seems to be closely linked to polarized cell-surface delivery events in several cell types, is known as the exocyst complex. This complex has been reported to be involved in the tethering, docking and fusion of post-Golgi vesicles with the plasma membrane. It is composed of eight subunits that are conserved from yeast to mammalian cells: Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84. Recent structural studies have indicated that these proteins are primarily composed of structurally similar helical bundles that seem to associate through an extensive network of interactions within the complex [1,2]. The exocyst complex also seems to be distantly related to vesicle-tethering complexes that function at other stages of membrane trafficking such as the COG (conserved oligomeric Golgi) and GARP (Golgi-associated retrograde protein) complexes [3,4]. Although it is clear that the exocyst complex has an important role in regulating exocytosis, little is known about the mechanism by which it promotes exocytosis or cell polarity. Information from several model systems has demonstrated that the exocyst complex is regulated by several small GTPases. In this review, we focus on how Rab, Ral and Rho small GTPases regulate exocytosis through both the localization and function of the exocyst complex on the plasma membrane.
Rab GTPases: conserved regulators of vesicle tethering to target membranes
Rab proteins comprise one of the most abundant families within the Ras superfamily of small GTPases. There are 11 Rab proteins in yeast and >60 in mammalian cells [5]. Rab proteins have been reported to regulate different membrane trafficking and signaling pathways through their interaction with various effectors. Like other small GTPases in the Ras superfamily, Rab proteins cycle between a GTP-bound active form and a GDP-bound inactive form [6] and interact with downstream effectors through their active conformation. Sec4 is a Rab family small GTPase in the yeast Saccharomyces cerevisiae, which was first identified in a screen for mutants with secretory defects [7]. Electron microscopy and invertase secretion assay [8] have shown that the sec4–8 mutant, which contains a substitution of glycine to aspartic acid at position 147, accumulates post-Golgi vesicles. Immunofluorescence and subcellular-fractionation experiments have demonstrated that Sec4 resides on secretory vesicles and on the plasma membrane [9]. Genetic and cell-biological evidence has demonstrated that duplication of Sec4 suppresses the loss of Sec15 function and that the polarized localization pattern of the Sec15 protein is lost in sec4–8 mutants [10], thereby indicating that Sec15 might represent an effector of Sec4. However, the first evidence that Sec15 might encode a direct downstream target of Sec4 came in the late 1990s when researchers [11] found that Sec4 interacted with the Sec15 component of the exocyst component by using a yeast two-hybrid assay. Importantly, this interaction seemed to be GTP-dependent; mutant alleles of Sec4 predicted to be in the GDP form failed to interact, whereas GTP-locked mutants showed an increase in interaction, as measured in the two-hybrid system. This interaction was supported by immunoprecipitation experiments demonstrating that, following chemical cross-linking, Sec4 could be co-immunoprecipitated with Sec15. Further analyses using mutant forms of Sec4 have indicated that this interaction was specific to Sec4 but not to other closely related yeast Rab proteins, such as Ypt1 and Ypt51. Making chimeric proteins between the effector domain of Sec4 and Ypt1 has indicated that the effector domain is responsible for interacting with Sec15 because Sec4 with the effector domain of Ypt1 failed to interact with Sec15 [11].
Recently, it has been reported that the yeast lethal giant larvae (lgl) family protein Sro7 is likely to represent a second direct effector for Sec4 [12]. Sro7 has previously been identified as a binding partner for the plasma-membrane t-SNARE (target membrane-associated soluble N-ethylmaleimide-sensitive factor-attachment protein receptor) Sec9, and loss of this protein and its paralog, Sro77, results in severe post-Golgi secretory defects similar to those seen in sec4 and sec9 mutant cells [13]. Consistent with the idea that Sro7 acts in parallel with the exocyst as a downstream effector of Sec4 [12], overexpression of Sro7 suppresses defects associated both with mutations in the exocyst components [13] and in Sec4 [12–14]. Biochemical experiments support the notion that Sro7 is a direct effector of Sec4, in that purified Sro7 has been found to bind specifically to Sec4 preloaded with GTP, but not to Sec4 preloaded with GDP. Further characterization of the interaction of Sec9 with Sro7 has indicated that this is likely to be a highly regulated and transient event in vivo. In particular, the interaction with the essential SNAP-25 (synaptosomal-associated protein of 25 kDa) domain of Sec9 has been found to be stimulated by release of an autoinhibitory interaction within the Sro7 protein [15]. This led to the model that Sro7 regulates SNARE assembly events in response to an upstream binding event, which triggers the localized presentation of the Sec9 SNARE domain to its cognate t- and v-SNAREs. If, in fact, Sec4 is part of this ‘trigger’, then it would help to coordinate the timing of the SNARE assembly with the arrival of the Sec4-bound vesicle. Two other proteins involved in polarized exocytosis in yeast, the Exo84 component of the exocyst, and the type-V myosin, Myo2, have also been shown to interact with Sro7 and might contribute to the final ‘triggering’ of Sro7-dependent SNARE assembly [14,16].
More recently, a homologous interaction between Rab11 and mammalian Sec15 has been described [17,18]. This supports the idea that Rab GTPase regulation of exocyst function during tethering probably represents an ancestral and, therefore, central regulatory interaction (Figure 1).
Figure 1.
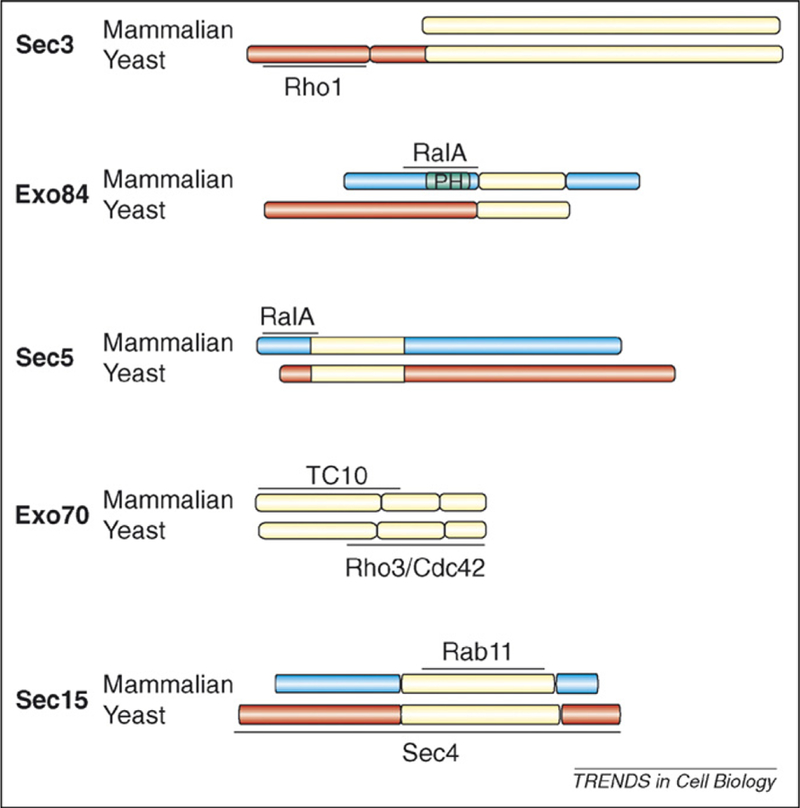
Alignment of mammalian and yeast exocyst subunits that interact with Rho, Ral and Rab small GTPases. Regions of each subunit that are conserved between yeast and mammals are shown in yellow. Regions lacking obvious sequence similarity are blue in mammalian cells and red in yeast.
Ral GTPases: metazoan regulators of exocyst assembly
Ral GTPases are, evolutionarily, recent additions to the small GTPase family found only in animal cells [19]. They have been implicated in the regulation of a diverse array of cellular processes, including oncogenic transformation, endocytosis [20] and actin-cytoskeleton dynamics [21,22]. Ral proteins have also been shown to associate with secretory granules and synaptic vesicles [23]. Recently, it was reported that Ral small GTPases directly interact with Exo84 and Sec5, components of the exocyst complex, which, in mammalian cells, have been implicated in the targeting of Golgi-derived vesicles to the basolateral membrane of polarized epithelial cells and to the growth cones of differentiating PC12 cells [24].
Sec5 as an effector for Ral small GTPases was first identified in a yeast two-hybrid screen searching for novel downstream targets using an activated form of RalA as bait. An effector domain mutation of RalA, which fails to interact with Sec5, results in mis-sorting of basolateral membrane proteins (e.g. the epidermal growth factor receptor) to the apical surface of polarized epithelial cells [22], indicating that RalA is required for appropriate basolateral-membrane-protein-targeting. RalA interacts with Sec5 in a GTP-dependent manner, and truncation studies have indicated that the N-terminal domain (1–120 amino acids) of Sec5 is necessary for interacting with RalA. The effector domain mutant of RalA72L49E, which does not interact with Sec5 and Exo84, fails to promote delivery of E-cadherin to the basolateral surface of MDCK (Madin– Darby canine kidney) cells. This indicates that exocyst binding is crucial for the RalA GTPase to promote exocytic function. Interestingly, RalA72L49N, another Ral effector mutant that retains the ability to bind to Sec5 and Exo84, also fails to enhance basolateral membrane delivery, indicating that exocyst binding is necessary, but not sufficient, for RalA to enhance secretion [25]. Knockdown of RalA by siRNA results in disassembly or destabilization of the exocyst complex, indicating that Ral might regulate exocytosis by facilitating the proper assembly of the exocyst complex (Figure 2). Overexpression of the constitutively active Ral (Ral23V) results in mislocalization of basolateral membrane proteins, indicating that Ral function also requires the cycling between the GTP-bound state and the GDP-bound state. However, studies using RalA72L, another form of Ral, predicted to be ‘locked’ in the active GTP-bound state, have found that this mutant enhances trafficking to the basolateral membrane. It is not clear if these different results are due to differences in the RalA mutants used or differences in the basolateral trafficking assays. Exo84 is another effector of RalA that has been identified through a similar two-hybrid screen, with the activated form of RalA as bait. A structural analysis of the RalA–Exo84 interaction has indicated that the binding site on Exo84 is located at residues 228–234, which represents a conserved motif AxxNx(K/R)D, and is retained in all metazoan members of the Exo84 family [24].
Figure 2.
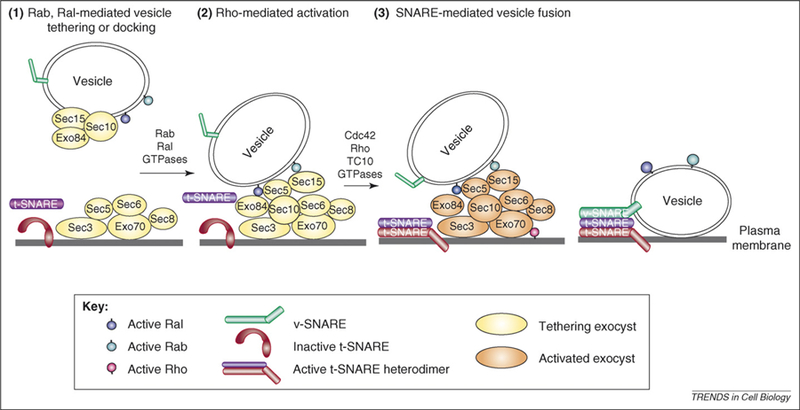
A three-step model for vesicle docking, exocyst activation and vesicle fusion regulated by small GTPases. (1) The initial vesicle-docking or tethering event is regulated by Rab and Ral GTPases, perhaps by promoting exocyst assembly. The association of particular exocyst subunits with the vesicle or plasma membrane in this diagram is speculative. There is evidence that exocyst assembly is regulated by Ral and this function, like that of Rab GTPases, is first required for vesicle tethering rather than fusion [67]. Fluorescence recovery after photobleaching (FRAP) studies in yeast have indicated that all of the exocyst subunits except Sec3 are likely to be delivered to sites of polarized growth through vesicle-mediated events [68]. (2) This is followed by local activation of the exocyst complex by Rho3, Cdc42 or TC10 family GTPases in their active GTP-bound state. Exocyst activation results in a stimulation of downstream fusion activity, probably by promoting assembly of active t-SNARE heterodimers. (3) The presence of active t-SNARE dimers results in SNARE-mediated fusion of the secretory vesicles at the site of exocyst activation.
RalB shares 88% identity to RalA in its first 162 amino acids. Although both proteins contain binding sequences for the exocyst components within this region, activated RalB binds to the exocyst components much less efficiently than active RalA. In addition to the difference in binding to the exocyst components, RalA and RalB also display distinct localization patterns owing to the C-terminal variable domain. Immunofluorescence studies in MDCK cells have indicated that RalA is predominantly localized on the plasma membrane at the cell–cell junctions, with diffuse punctuate staining throughout the cytoplasm. Antibody staining of RalA72L-induced MDCK cells not only have increased staining pattern on the plasma membrane, but also reveal intense perinuclear staining. This perinuclear staining disappears when the effector domain mutants (RalA72L49N or RalA72L49E) are introduced to the cell, indicating that this staining pattern is likely to be functionally relevant to the role of RalA in basolateral trafficking. RalB, however, has a denser punctuate intracellular-staining pattern and little RalB is observed on the plasma membrane. This difference in the localization pattern is consistent with the observation that RalA but not RalB, is important for basolateral membrane-targeting in polarized epithelial cells [25].
In addition to its role in regulating polarized membrane trafficking in epithelial cells, a more recent study [26] has discovered a role for the interaction between RalA and the exocyst complex in insulin-dependent Glut4 (glucose transporter 4) translocation in adipocytes. To identify proteins that might be involved in vesicle–exocyst recognition, researchers have screened for vesicle-localized GTPases in adipocytes by pull-down experiments. RalA, but not RalB, Arf6 or Rab11 specifically precipitate the exocyst components, including Sec5, Sec8, Exo84 and Exo70, in a GTP-dependent manner in both 3T3L1 adipocytes and primary mouse adipocytes [26]. RalA is activated upon insulin stimulation in a dose-dependent manner. Overexpression of the dominant-negative (GDP-mutant) form of RalA blocks the insulin-stimulated Glut4 translocation and its subsequent fusion with the plasma membrane, indicating that RalA has an important role in Glut4 trafficking. In adipocytes, RalA has also been found to be associated with the unconventional myosin, Myo1c, indicating that Ral could have a role in recruiting a vesicle motor and in docking the Glut4-containing vesicles to the membrane by enabling formation of a stable tethering-complex [26].
Rho GTPases: yeast as a model for polarity
The Rho family of small GTPases are regulators of many biological processes including cell polarization, morphogenesis, cell growth and development [27]. The function of the Rho family small GTPases in spatial regulation of exocytosis has been most extensively examined in the yeast, S. cerevisae. Yeast is an excellent model for studying polarized secretion owing to the highly polarized nature of its growth pattern and the extensive genetic and cell biological tools available to analyze membrane trafficking and cytoskeletal structures within these cells [28]. Yeast has six Rho proteins, Rho1–5, and Cdc42. Among these six proteins, Rho1, Rho3 and Cdc42 have been the most carefully studied and have each been implicated in regulation of polarized exocytosis.
Rho1: important for the localization of the Sec3 component of the exocyst complex
The Rho1 GTPase is essential to many biological processes in yeast and is thought to be a regulator of a variety of downstream effectors, including protein kinase C (PKC)1 [29,30], the formin family protein, Bni1 [31], and the cell-wall β-glucan synthases, Fks1 and Fks2 [32]. Studies in different rho1 mutants have revealed that Rho1 has an important role in regulating the localization of the Sec3 component of the exocyst complex [33]. This was found to be owing to a GTP-dependent interaction between Rho1 and the non-essential N-terminal domain of Sec3 (Figure 1). However, in strains in which the sole source of Sec3 lacks the N-terminal Rho1-interaction domain, the remaining exocyst subunits were polarized normally and secretion was also normal [33,34]. This demonstrates that the remaining components of the exocyst complex must be polarized by a distinct pathway that is independent of both the N-terminal domain of Sec3 and Rho1. Although this interaction is not essential, Sec3 mutants lacking this domain exhibit synthetic genetic interactions with a secretory deficient allele of Cdc42 and other late acting secretory mutants [34]. This is consistent with the notion that this domain of Sec3 functions to increase the local concentration of Sec3 at sites of growth, although much lower amounts are sufficient to promote full secretory function under most circumstances.
Rho3: a direct regulator of exocytosis
The first evidence for the participation of a Rho GTPase in exocytic function came from two genetic screens. The first screen focused on genes that, when overexpressed, rescued the extremely slow growth phenotype associated with loss of Rho3 [35,36]. This screen isolated several genes, including BEM1, CDC42, and two genes later identified as coding for the yeast Rab GTPase and SEC4 and its effector, SRO7 [35–37]. A second screen identified RHO3 itself as a potent suppressor of a cold-sensitive allele in SEC4 [38,39]. Further characterization demonstrated that Rho3 was the only one of the five RHO genes in yeast that could function as a suppressor for the sec4–P48 mutant [39]. Rho3 also suppressed both the sec15–1 and the sec8–9 mutants, both of which are components of the exocyst, indicating that Rho3 has an important role in regulating exocytosis through the exocyst complex.
An analysis of a cold-sensitive effector domain mutant of Rho3, rho3–V51, was particularly informative [39]. This mutant demonstrated a profound secretory defect and accumulation of post-Golgi vesicles following a shift to the restrictive temperature. However, unlike other rho3 mutants examined, the polarization of the actin cytoskeleton was found to be normal at both permissive and non-permissive conditions. This was the first evidence for a direct role for Rho3 in exocytosis independent of the cytoskeleton. Biochemical and yeast two-hybrid analyses demonstrated that the rho3–V51 mutation blocked the ability of otherwise activated forms of Rho3 to bind to the Exo70 subunit of the exocyst. This indicates that Exo70 is likely to be the immediate target of Rho3 regulation of exocytic function. However, unlike the effect of Rho1 on Sec3 localization, this mutation was found to exert its effects on exocytosis independent of any detectable effects on Exo70 or exocyst localization [34].
Cdc42: a cell-cycle-specific regulator of exocytosis
Cell division cycle 42 (Cdc42) is a member of the Rho GTPase family that has an important role in coordinating several events necessary for polarized growth in yeast cells [40]. The identification of a novel temperature-sensitive mutant, cdc42–6, has led to the characterization of a new role for Cdc42 function in exocytosis [41]. This mutant displays properties that are distinct from previously described alleles of Cdc42, in that both actin polarity and budding seemed to be normal. Genetic analyses have demonstrated that cdc42–6 is likely to be defective for a pathway closely linked to that of rho3-V51, because both mutants are suppressed by a common set of genes including SEC4, SRO7, and SEC9. In addition, increased gene dosage of CDC42 has been found to suppress rho3 mutant growth defects, and increased dosage of RHO3 has been found to suppress cdc42–6 growth defects, indicating that these two Rho GTPases probably function to regulate a common effector pathway. Furthermore, the synthetic lethality observed in crosses of the cdc42–6 and rho3–V51 mutants has provided strong evidence that the effector pathways of these two GTPases functionally overlap.
An analysis of the secretory capacity of the cdc42–6 mutant has revealed a severe defect in the secretion of Bgl2, an abundant periplasmic enzyme involved in cellwall remodeling and the accumulation of 80–100 nm post-Golgi vesicles by electronic microscopy. Interestingly, this mutant has shown no defect in secretion of invertase, which is thought to be carried by a separate class of vesicles from that used to transport Bgl2 to the cell surface [42]. Surprisingly, electron microscopy studies on cdc42–6 cells have demonstrated that only cells with small buds accumulate post-Golgi vesicles, whereas larger budded cells show no abnormal numbers of vesicles. Consistent with the idea that the exocytic defect in the cdc42–6 mutant is specifically associated with early bud emergence, Bgl2 secretory defects have been found to mirror the time of appearance of small buds when secretion assays were conducted on synchronized populations of cells. Similarly to rho3–V51, the cdc42–6 mutation has been found to exert its effects on exocytosis independent of any detectable effects on Exo70 or exocyst localization [34,41].
TC10: a Cdc42 family GTPase involved in glucose-transporter trafficking
Insulin stimulation results in a dramatic translocation of the GLUT4 protein to the plasma membrane via a dynamic membrane-trafficking system, including vesicle sorting, budding, trafficking, tethering, docking and fusion of the GLUT4-containing post-Golgi vesicles. Extensive efforts have been made to identify the mechanism by which plasma-membrane translocation of GLUT4 occurs upon insulin stimulation. Recently, it has been reported that the Rho family small GTPase TC10 has a crucial role in regulating this signaling pathway. To search for potential effectors of TC10 that have a role in insulin-stimulated glucose transport, researchers screened a yeast two-hybrid cDNA library derived from 3T3L1 adipocytes with a constitutively active (GTP) form of human TC10α [43]. This screen has identified Exo70 as a potential downstream target for TC10. This interaction is specific to the GTP-bound form of TC10 and is not observed with GTP-bound forms of other small GTPases such as Rac and Cdc42. A dominant-negative form of Exo70 blocks the effects of insulin on Glut4 transport to the surface in 3T3 L1 adipocytes [43]. Interestingly, dominant–negative TC10 in the presence of insulin results in Glut4-containing vesicles appearing close to the cell surface. This indicates that the function of TC10 and the exocyst on Glut4 surface transport is at the level of Glut4 vesicle fusion rather than delivery or docking events [43] (Figure 2).
Rho and Cdc42 regulation of the exocyst is distinct from Rab regulation
Although many GTPases seem to work as signal-transduction agents, other GTPases are thought to control the specificity and timing of macromolecular recognition events. Examples of the latter include elongation factor Tu (EFTu) [44–46], signal recognition particle (SRP) and SRP-receptor complexes [47]. In the latter two examples, GTP hydrolysis and cycling through the GDP-bound, nucleotide-free, and GTP-bound states are crucial for these GTPases to carry out their biological function [44,47]. By contrast, GTPases that function as signal transducers can do so without any need for GTP hydrolysis per se. A simple test of this distinction is to examine the effect of GTP-hydrolysis-deficient mutants on the biological activity of the protein. Such mutations are predicted to lead to heightened activity or gain-of-function effects on signaling GTPases, but are expected to lead to loss-of-function effects on cycling or non-signaling GTPases.
Extension of this analysis to Rab and Rho GTPase function in exocytosis leads to some interesting differences in behavior, even in situations in which the target ‘effector’ complex is shared by these different GTPases. The hydrolysis mutant form of the yeast Rab, Sec4, is known to enhance the interaction with the Sec15 component of the exocyst. However, when introduced as the sole source of Sec4, the mutant behaves as a recessive loss-of-function allele, which is cold-sensitive and lethal when combined with other late-acting secretory mutants [48]. Similar recessive loss-of-function phenotypes have been observed with a GTP-hydrolysis mutant in Ypt1, a Rab involved in endoplasmic reticulum (ER)-to-Golgi transport [49]. By contrast, GTP-hydrolysis-deficient forms of Rho3, which stimulate the interaction with the Exo70 component of the exocyst, are fully functional as the only source of Rho3 and behave as gain-of-function alleles, strongly suppressing several late-acting secretory mutants [34]. Similarly, GTP-hydrolysis-deficient forms of Cdc42 also seem to be functional in promoting secretory function when expressed at low levels, although they are toxic to other pathways when expressed at higher levels [34]. Taken together, these data implicate Rho3 andCdc42 regulation of the exocyst as a pathway similar to other signaling GTPases such as Ras, whereas the function of Sec4 in regulating the exocyst is similar to recognition or cycling GTPases such as the SRP or EFTu.
The exocyst as a landmark or an activated machine? Local activation versus local recruitment models
Signaling GTPases regulate their effectors by one of two general mechanisms. The first mechanism involves regulation of the subcellular location of the downstream effector. In this mode, the binding of the GTPase to its effector helps to localize and concentrate the effector at a particular place within the cell. This would then stimulate a signaling event by placing the effector within close proximity to its downstream signaling partner. A good example of this mode of GTPase function is the Ras GTPase, which helps to promote oncogenic transformation through GTP-dependent interaction with the Raf kinase. The initial activation event in the Ras–Raf pathway involves recruitment of the Raf kinase from the cytosol to the plasma membrane by membrane-bound Ras [50,51]. This initial recruitment event then propagates other subsequent activation and signaling events [52] by placing activated Raf kinase in close proximity to its both its activator, Ras, and downstream targets in the mitogen-activated protein (MAP)-kinase cascade to promote sustained activation and signaling [53,54].
A second mechanism of regulation by signaling GTPases involves regulation of activity rather than the location of the downstream effector. In this mode, the binding of the GTPase to the effector induces a conformational change that either directly or indirectly stimulates an associated enzymatic activity. A good example of direct regulation is the formin family of actin-nucleating enzymes, the members of which normally reside in an inactive ‘autoinhibited’ conformation owing to the association of the DID (diaphanous inhibitory domain) and DAD (diaphanous autoregulatory domain) within the protein [55,56] (Figure 3). The closed conformation is inactive owing to the inaccessibility of the catalytic domain to its substrate (actin monomers) in this structure. The binding of the Cdc42 or Rho to the GTPase-binding domain (GBD) adjacent to the DID disrupts the inhibitory interaction, resulting in an opening of the structure and enabling its associated catalytic activity to be ‘active’.
Figure 3.
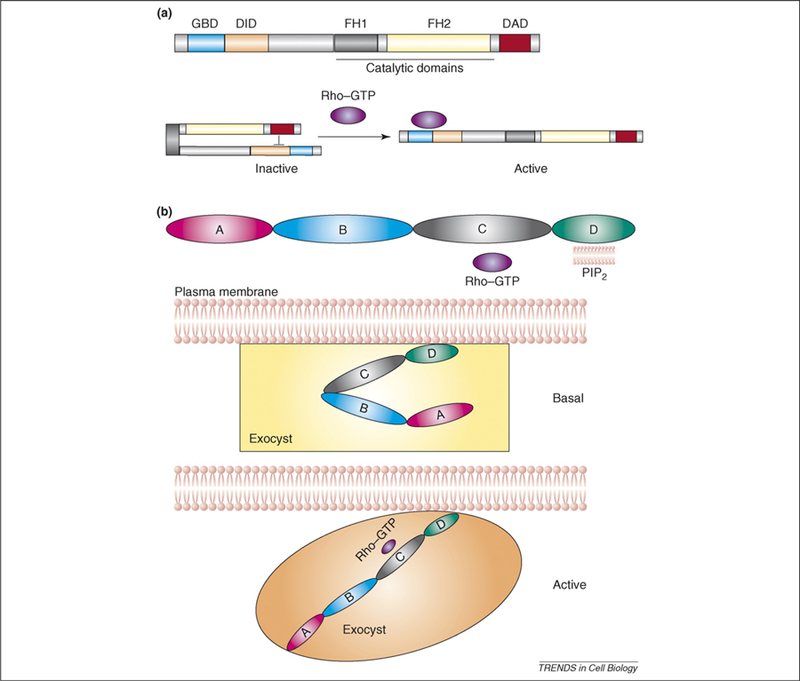
A model for activation of the exocyst complex by Rho family GTPases. (a) Domain organization and molecular regulation of formins. In the absence of Rho GTP, formins are maintained in an inactive state by an autoinhibitory interaction between the DAD and DID domains, which is relieved by association of an active, GTP-bound Rho GTPases with the GBD domain. This interaction enables DID to adopt a structural conformation that induces release of the DAD domain and leads to the activation of the formin protein. (b) Domain organization and model for molecular regulation of Exo70 and the exocyst complex by Rho GTPase. The D domain of Exo70 interacts with phospholipids containing phosphatidylinositol (4,5)-bisphosphate (PIP2) and the C domain is necessary for the interaction with Rho family small GTPases. In the absence of Rho GTP, Exo70, along with other components of the exocyst complex, remain in the inactive or basal activity state. Upon interaction of Exo70 with Rho GTP, Exo70 adopts an alternative conformation that leads to the activation of the exocyst complex. The activation in this case could be the result of disrupting an inhibitor interaction between Exo70 and another subunit of the exocyst complex or a direct change in the conformation of Exo70 itself, which then leads to a change in the overall structure of the complex.
There are aspects of Rho regulation of the exocyst in yeast that fit each of these models. An example of the first would be the recruitment of the yeast Sec3 by binding of its N terminus to Rho1 [33] (and, to a lesser degree, to Cdc42 [57]). Although this interaction is not necessary for the function of Sec3 in promoting efficient exocytosis and growth [33,34], it is important for enabling Sec3 to efficiently localize to sites of polarized growth. Thus the interaction of the N terminus of Sec3 with Rho1 is an example of Rho regulation by recruitment, similar to the Ras–Raf example.
In contrast to the Rho1 recruitment of Sec3 model, regulation of exocytosis by Rho3 and Cdc42 seems to be independent of any effect on the localization of the exocytic machinery. The analysis of the loss-of-function mutants rho3–V51 and cdc42–6 clearly demonstrates that these GTPases have a direct and crucial regulatory function on this process [34,39,41]. A simple explanation for these results is that Rho3 and Cdc42 regulation occurs through localized activation of the exocytic apparatus (probably through the Exo70 component of the exocyst; see later). This activation can be imagined to be a slight variation from the ‘relief of autoinihibition’ mechanism used by Rho GTPases to modulate effector function of the formins, PAK (p21-activated) family kinases and WASP (Wiskott–Aldrich syndrome protein) [58,59]. The major difference is that Exo70 seems to function primarily as part of a larger multiprotein complex. In this way, the inhibitory interactions disrupted by Rho GTPase binding might disrupt a protein–protein interaction within the exocyst complex rather than within the Exo70 protein itself (Figure 3). The result of the binding, however, would be quite similar: the exocyst would go from being in a form that has basal function to an ‘activated’ complex that would support increased rates of docking and fusion events while in this state. In this view, the exocyst complex functions not as a static scaffold for enabling vesicles to dock with the plasma membrane but, rather, as a dynamic machine with real catalytic function that can be modulated to control the rate at which vesicle docking and fusion with a specific site in the membrane can occur.
Consistent with the model that the Rho proteins are mostly responsible for activating the exocyst at the site of polarized growth but not localizing the exocyst complex, there are new results showing that the interaction between the exocyst components and phospholipids might be important for mediating the targeting of the exocyst to the plasma membrane. Both Exo70 and Sec3 have been shown to interact with phospholipids [60,61]. However, the interactions are mediated through two different domains. Sec3 interacts with phosphatidylinositol (4,5)-bisphosphate (PIP2) through its N terminus [60], whereas Exo70 interacts with PIP2 through its C-terminal domain [61], which is the most conserved domain on Exo70 among different species [62]. Interactions with Rho family small GTPases and with phospholipids are both required for proper localization and final activation of the exocyst complex [60–62].
What, more precisely, might this machine be catalyzing? There are many possibilities, but an attractive target for this catalysis is that the formation of active t-SNARE complexes on the plasma membrane. It is likely that the majority of t-SNAREs are present on the plasma membrane in an uncomplexed form [38,63]. Biochemical and kinetic analyses have also made it clear that the formation of the t-SNARE dimers of Sec9 and Sso is likely to be an extremely slow and inefficient process [64,65]. However, evidence for stable interactions between intact exocyst complex and SNARE proteins has not been detected [38]. Recently, it has been found that recombinant forms of the Sec6 protein, in the absence of the other exocyst subunits, show high-affinity interactions with the t-SNARE Sec9 [66]. This points toward the possibility that, within the exocyst complex, Sec6 may transiently interact with Sec9 as a means of regulating t-SNARE assembly and vesicle fusion. This transient interaction with Sec9 would be regulated by the functional state of the exocyst complex that probably involves both Rho and Cdc42 ‘throttling’ and a requirement for Sec4–GTP binding and release as the final triggering event. Clearly, an important area for future work will be to clarify the molecular mechanism of how exocyst activation is transmitted onto the downstream SNARE-dependent fusion events.
Concluding remarks
Work over the past decade has shown small GTPases to be crucial regulators of both cell polarity and membrane-trafficking events in the cell. The multisubunit protein complex known as the exocyst complex is an important target for coordination of trafficking and cell-polarization decisions. Several different subunits of the exocyst have evolved mechanisms by which regulatory signals from small GTPases act on specific aspects of exocyst function. These signals seem to be directed at one of two stages of exocyst activity. The first point of regulation is in the vesicle-tethering or -docking stage, where the exocyst helps to proof-read the correct vesicle-target-membrane combination. The second stage is the vesicle fusion reaction, where it is likely that the exocyst regulates localized SNARE assembly. By acting at these two steps, members of the Rab, Ral, and Rho GTPase families are able to regulate the fidelity of these events at the same time as they modulate the temporal and spatial nature of cell-surface delivery. Future work will help to unravel the details of how these regulatory interactions function mechanistically to spatially and temporally regulate exocytosis in both polarized and unpolarized cells.
Acknowledgements
We thank James Gardner for helpful comments on the manuscript. This work was supported by grants from the National Institutes of Health (GM54712) and The G. Harold and Leila Y. Mathers Charitable Foundation.
References
- 1.Munson M and Novick P (2006) The exocyst defrocked, a framework of rods revealed. Nat. Struct. Mol. Biol 13, 577–581 [DOI] [PubMed] [Google Scholar]
- 2.Hamburger ZA et al. (2006) Crystal structure of the S. cerevisiae exocyst component Exo70p. J. Mol. Biol 356, 9–21 [DOI] [PubMed] [Google Scholar]
- 3.Conibear E et al. (2003) Vps51p mediates the association of the GARP (Vps52/53/54) complex with the late Golgi t-SNARE Tlg1p. Mol. Biol. Cell 14, 1610–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whyte JR and Munro S (2001) The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev. Cell 1, 527–537 [DOI] [PubMed] [Google Scholar]
- 5.Barrowman J and Novick P (2003) Three Yips for Rab recruitment. Nat. Cell Biol 5, 955–956 [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer SR (1994) Rab GTPases: master regulators of membrane trafficking. Curr. Opin. Cell Biol 6, 522–526 [DOI] [PubMed] [Google Scholar]
- 7.Novick P et al. (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205–215 [DOI] [PubMed] [Google Scholar]
- 8.Salminen A and Novick PJ (1987) A ras-like protein is required for a post-Golgi event in yeast secretion. Cell 49, 527–538 [DOI] [PubMed] [Google Scholar]
- 9.Goud B et al. (1988) A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell 53, 753–768 [DOI] [PubMed] [Google Scholar]
- 10.Salminen A and Novick PJ (1989) The Sec15 protein responds to the function of the GTP binding protein, Sec4, to control vesicular traffic in yeast. J. Cell Biol 109, 1023–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo W et al. (1999) The exocyst is an effector for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J 18, 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grosshans BL et al. (2006) The yeast lgl family member Sro7p is an effector of the secretory Rab GTPase Sec4p. J. Cell Biol 172, 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lehman K et al. (1999) Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J. Cell Biol 146, 125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X et al. (2005) Lethal giant larvae proteins interact with the exocyst complex and are involved in polarized exocytosis. J. Cell Biol 170, 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattendorf DA et al. (2007) Structure of the yeast polarity protein Sro7 reveals a SNARE regulatory mechanism. Nature 446, 567–571 [DOI] [PubMed] [Google Scholar]
- 16.Gangar A et al. (2005) Structurally conserved interaction of Lgl family with SNAREs is critical to their cellular function. Curr. Biol 15, 1136–1142 [DOI] [PubMed] [Google Scholar]
- 17.Zhang XM et al. (2004) Sec15 is an effector for the Rab11 GTPase in mammalian cells. J. Biol. Chem 279, 43027–43034 [DOI] [PubMed] [Google Scholar]
- 18.Wu S et al. (2005) Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat. Struct. Mol. Biol 12, 879–885 [DOI] [PubMed] [Google Scholar]
- 19.Camonis JH and White MA (2005) Ral GTPases: corrupting the exocyst in cancer cells. Trends Cell Biol 15, 327–332 [DOI] [PubMed] [Google Scholar]
- 20.Jullien-Flores V et al. (2000) RLIP76, an effector of the GTPase Ral, interacts with the AP2 complex: involvement of the Ral pathway in receptor endocytosis. J. Cell Sci 113, 2837–2844 [DOI] [PubMed] [Google Scholar]
- 21.Ohta Y et al. (1999) The small GTPase RalA targets filamin to induce filopodia. Proc. Natl. Acad. Sci. U. S. A 96, 2122–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moskalenko S et al. (2002) The exocyst is a Ral effector complex. Nat. Cell Biol 4, 66–72 [DOI] [PubMed] [Google Scholar]
- 23.Vitale N et al. (2005) The Small GTPase RalA controls exocytosis of large dense core secretory granules by interacting with ARF6-dependent phospholipase D1. J. Biol. Chem 280, 29921–29928 [DOI] [PubMed] [Google Scholar]
- 24.Jin R et al. (2005) Exo84 and Sec5 are competitive regulatory Sec6/8 effectors to the RalA GTPase. EMBO J 24, 2064–2074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shipitsin M and Feig LA (2004) RalA but not RalB enhances polarized delivery of membrane proteins to the basolateral surface of epithelial cells. Mol. Cell. Biol 24, 5746–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen XW et al. (2007) Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev. Cell 13, 391–404 [DOI] [PubMed] [Google Scholar]
- 27.Symons M and Rusk N (2003) Control of vesicular trafficking by Rho GTPases. Curr. Biol 13, R409–R418 [DOI] [PubMed] [Google Scholar]
- 28.Brennwald P and Rossi G (2007) Spatial regulation of exocytosis and cell polarity: yeast as a model for animal cells. FEBS Lett 581, 2119–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nonaka H et al. (1995) A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J 14, 5931–5938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helliwell SB et al. (1998) The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton. Curr. Biol 8, 1211–1214 [DOI] [PubMed] [Google Scholar]
- 31.Tolliday N et al. (2002) Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol 12, 1864–1870 [DOI] [PubMed] [Google Scholar]
- 32.Mazur P and Baginsky W (1996) In vitro activity of 1,3-b-D-glucan synthase requires the GTP-binding protein Rho1. J. Biol. Chem 271, 14604–14609 [DOI] [PubMed] [Google Scholar]
- 33.Guo W et al. (2001) Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol 3, 353–360 [DOI] [PubMed] [Google Scholar]
- 34.Roumanie O et al. (2005) Rho GTPase regulation of exocytosis in yeast is independent of GTP hydrolysis and polarization of the exocyst complex. J. Cell Biol 170, 583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui Y and Toh EA (1992) Yeast RHO3 and RHO4 ras superfamily genes are necessary for bud growth, and their defect is suppressed by a high dose of bud formation genes CDC42 and BEM1. Mol. Cell. Biol 12, 5690–5699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsui Y and Toh-e A (1992) Isolation and characterization of two novel ras superfamily genes in Saccharomyces cerevisiae. Gene 114, 43–49 [DOI] [PubMed] [Google Scholar]
- 37.Imai J et al. (1996) Genetic analysis of the Saccharomyces cerevisiae RHO3 gene, encoding a rho-type small GTPase, provides evidence for a role in bud formation. Genetics 142, 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brennwald P et al. (1994) Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell 79, 245–258 [DOI] [PubMed] [Google Scholar]
- 39.Adamo JE et al. (1999) The Rho GTPase Rho3 has a direct role in exocytosis that is distinct from its role in actin polarity. Mol. Biol. Cell 10, 4121–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park HO and Bi E (2007) Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol. Mol. Biol. Rev 71, 48–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adamo JE et al. (2001) Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J. Cell Biol 155, 581–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harsay E and Bretscher A (1995) Parallel secretory pathways to the cell surface in yeast. J. Cell Biol 131, 297–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue M et al. (2003) The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 422, 629–633 [DOI] [PubMed] [Google Scholar]
- 44.Rodnina MV et al. (1995) Elongation factor Tu, a GTPase triggered by codon recognition on the ribosome: mechanism and GTP consumption. Biochem. Cell Biol 73, 1221–1227 [DOI] [PubMed] [Google Scholar]
- 45.Kaziro Y et al. (1991) Structure and function of signal-transducing GTP-binding proteins. Annu. Rev. Biochem 60, 349–400 [DOI] [PubMed] [Google Scholar]
- 46.Bourne HR (1988) Do GTPases direct membrane traffic in secretion? Cell 53, 669–671 [DOI] [PubMed] [Google Scholar]
- 47.Shan SO et al. (2007) Conformational changes in the GTPase modules of the signal reception particle and its receptor drive initiation of protein translocation. J. Cell Biol 178, 611–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walworth NC et al. (1992) Hydrolysis of GTP by Sec4 protein plays an important role in vesicular transport and is stimulated by a GTPase-activating protein in Saccharomyces cerevisiae. Mol. Cell. Biol 12, 2017–2028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson CJ et al. (1998) GTP hydrolysis is not important for Ypt1 GTPase function in vesicular transport. Mol. Cell. Biol 18, 827–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stokoe D et al. (1994) Activation of Raf as a result of recruitment to the plasma membrane. Science 264, 1463–1467 [DOI] [PubMed] [Google Scholar]
- 51.Leevers SJ et al. (1994) Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 369, 411–414 [DOI] [PubMed] [Google Scholar]
- 52.Mineo C et al. (1997) Physical association with ras enhances activation of membrane-bound raf (RafCAAX). J. Biol. Chem 272, 10345–10348 [DOI] [PubMed] [Google Scholar]
- 53.Morrison DK and Davis RJ (2003) Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol 19, 91–118 [DOI] [PubMed] [Google Scholar]
- 54.Raman M et al. (2007) Differential regulation and properties of MAPKs. Oncogene 26, 3100–3112 [DOI] [PubMed] [Google Scholar]
- 55.Goode BL and Eck MJ (2007) Mechanism and function of formins in the control of actin assembly. Annu. Rev. Biochem 76, 593–627 [DOI] [PubMed] [Google Scholar]
- 56.Lu J et al. (2007) Structure of the FH2 domain of Daam1: implications for formin regulation of actin assembly. J. Mol. Biol 369, 1258–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X et al. (2001) Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem 276, 46745–46750 [DOI] [PubMed] [Google Scholar]
- 58.Higgs HN and Pollard TD (2001) Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu. Rev. Biochem 70, 649–676 [DOI] [PubMed] [Google Scholar]
- 59.Prehoda KE et al. (2000) Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science 290, 801–806 [DOI] [PubMed] [Google Scholar]
- 60.Zhang X et al. (2008) Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J. Cell Biol 180, 145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He B et al. (2007) Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J 26, 4053–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu J et al. (2007) Phosphatidylinositol 4, 5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol. Biol. Cell 18, 4483–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grote E et al. (2000) Ordering the final events in yeast exocytosis. J. Cell Biol 151, 439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rossi G et al. (1997) Analysis of a yeast SNARE complex reveals remarkable similarity to the neuronal SNARE complex and a novel function for the C terminus of the SNAP-25 homolog, Sec9. J. Biol. Chem 272, 16610–16617 [DOI] [PubMed] [Google Scholar]
- 65.Nicholson KL et al. (1998) Regulation of SNARE complex assembly by an N-terminal domain of the t-SNARE Sso1p. Nat. Struct. Biol 5, 793–802 [DOI] [PubMed] [Google Scholar]
- 66.Sivaram MV et al. (2005) Dimerization of the exocyst protein Sec6p and its interaction with the t-SNARE Sec9p. Biochemistry 44, 6302–6311 [DOI] [PubMed] [Google Scholar]
- 67.Moskalenko S et al. (2003) Ral GTPases regulate exocyst assembly through dual subunit interactions. J. Biol. Chem 278, 51743–51748 [DOI] [PubMed] [Google Scholar]
- 68.Boyd C et al. (2004) Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J. Cell Biol 167, 889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]


