Abstract
There is growing evidence from small animal models that myeloid-derived suppressor cells (MDSC) can play a crucial role in inhibiting allograft rejection and in promoting transplant tolerance. Here, we identified CD3−CD20−HLA-DR−CD14+CD33+CD11b+ cells in peripheral blood of normal rhesus macaques. These putative, monocytic MDSC constituted 2.1 ± 1.7% of lin−HLA-DR− PBMC. Administration of granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte (G)-CSF increased their incidence to 5.3 ± 3.4%. The total number of MDSC that could be flow-sorted from a single whole rhesus leukapheresis product was 38 ± 13.106 (n=10 monkeys). Freshly-isolated or cryopreserved MDSC from mobilized monkeys incorporated in cultures of anti-CD3− and anti-CD28-stimulated autologous T cells, markedly suppressed CD4+ and CD8+ T cell proliferation and cytokine secretion (IFNγ, IL-17A). Moreover, these MDSC enhanced CD4+CD25hiFoxp3+ regulatory T cell (Treg) expansion, while inhibiting proliferation of activated memory T cells and increasing Treg relative to effector and terminally-differentiated memory T cells. Inhibition of arginase-1 (Arg-1), but not inducible nitric oxide synthase activity, partially reversed the inhibitory effect of the MDSC on CD8+ T cell proliferation. Thus, functional MDSC can be isolated from NHP for prospective use as therapeutic cellular vaccines in transplantation.
Introduction
Myeloid-derived suppressor cells (MDSC) are an emerging, heterogeneous, regulatory innate immune cell population that consists of immature myeloid cells and myeloid progenitors (1, 2). During cancer and acute and chronic inflammation, including transplantation, and also following vaccination, MDSC arise from the bone marrow (BM) and can potently inhibit T cell activation and proliferation (1, 3–5). Myelopoiesis-promoting growth factors, such as granulocyte-colony stimulating factor (G-CSF) (6), macrophage (M)-CSF (7) and GM-CSF (8, 9), act together with inflammatory cytokines to promote MDSC expansion and prevent their differentiation into terminally-differentiated dendritic cells (DC), macrophages or granulocytes (1). We have also shown that the endogenous myeloid growth factor, fms-like tyrosine kinase 3 ligand (Flt3L) (10) and histone deacetylase inhibition (11) promote MDSC expansion in mice.
Immunosuppressive MDSC expand in experimental models of skin (12), heart (13) and kidney transplantation (14, 15). Importantly, they are required for induction of organ transplant tolerance in rodents using co-stimulation blockade (13, 14) and for induction of chimerism and tolerance to heart grafts in mice using total lymphoid irradiation and anti-thymocyte serum (16). There is also evidence that mechanistic target of rapamycin (mTOR) inhibition prolongs heart allograft survival by inducing MDSC (17). Use of MDSC as cellular therapeutic agents in murine models has demonstrated their potent ability to promote islet allograft survival (18, 19) and to inhibit graft-versus-host disease following hematopoietic cell transplantation (20). MDSC suppress both CD4+ and CD8+ T cells (4, 21–23) and can induce antigen-specific CD8+ T cell tolerance in mice following their adoptive transfer (24). In a recent study of renal transplant patients, accumulation in peripheral blood of MDSC capable of suppressing CD4+ T cell proliferation ex vivo was correlated with forkhead box p3 (Foxp3)+ regulatory T cell (Treg) expansion (25). Compared to other tolerogenic myeloid cells (regulatory DC or regulatory macrophages) (2), MDSC are unique in being activated to suppress immunity by inflammatory signals. The latter include pro-inflammatory cytokines (20, 22, 23, 26) that are elevated after surgery, and Toll-like receptor ligands (27), making MDSC potentially well-suited for prospective use in transplantation as cellular therapeutic agents.
MDSC have been increasingly well-characterized in mice and humans (28, 29). Only limited information is available, however, about these cells in non-human primates (NHP). These species provide important preclinical models for testing innovative therapeutic strategies in transplantation, including cell-based therapy. Recently, Sui et al (30) have described increases in MDSC in vaccinated rhesus monkeys and the ability of these cells to suppress T cell responses in vitro. Here, we describe the in vivo mobilization of rhesus monocytic MDSCs in response to colony-stimulating factors and the ability of these MDSC, flow-sorted from fresh or cryopreserved leukapheresis products, to inhibit activated T cell responses and promote Treg/suppress memory T cell (Tmem) responses. We also report that the catabolic enzyme arginase-1 (Arg-1) contributes to their suppressive function. The findings demonstrate the feasibility of generating NHP monocytic MDSC for prospective in vivo testing as therapeutic cellular vaccines in transplantation.
Materials and Methods
Animals
Captive-bred, male Indian juvenile rhesus monkeys (Macaca mulatta; 5–7kg) were obtained from the NIAID-sponsored NHP colony (AlphaGenesis; Yemassee, SC). Before acceptance, they were screened for macaque viruses, namely herpes B, simian immunodeficiency virus, SRV, and STLV (BioReliance Corporation Rockville, MD). They were housed in environmentally-controlled rooms in an AAALAC-accredited facility, in strict conformance with the NIH Guide for Care and Use of Laboratory Animals. All procedures were carried out in accordance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ published by the NIH (publication 80–23, revised 1978) and approved by the University of Pittsburgh IACUC. Specific environment enrichment was provided.
Leukapheresis
Prior to leukapheresis, monkeys received recombinant human (rhu) GM-CSF (Leukine; Genzyme, Cambridge, MA; 10µg/kg/day for 4 days), followed by rhu G-CSF (Neupogen; Amgen, Thousand Oaks, CA; 10µg/kg/day for 4 days). Leukapheresis was performed as described (31, 32), using a NHP research-dedicated COBE® Spectra Apheresis System (Lakewood, CO). Isolated PBMC were cryopreserved in liquid N2 until needed for MDSC isolation or generation.
Abs and FACS staining procedure for flow cytometric analysis
The following anti-human/rhesus monkey cross-reactive Abs (Abs) were used to examine the frequency of monocytic MDSC, CD4+ and CD8+ naïve (Tn) and memory T cells (Tmem) and CD4+ Treg by multi-color fluorescence-activated cell staining (FACS) analysis: anti-CD3 (clone # SP34–2), −CD4 (L200), −CD8 (RPA-T8), −CD11b (ICRF44), −CD14 (M5E5), −CD62L (SK11), − CD45RA (5H9), −HLA-DR (G46–6) (all BD Pharmingen, San Diego, CA), anti-CD20 (2H7), − CD274 (=B7-H1; programmed death ligand-1 [PD-L1]) (29E.2A3) (both Biolegend, San Diego, CA), anti-CD33 (AC104.3E3), −FoxP3 (PCH101) (both Miltenyi Biotec, Auburn, CA) and anti-CD25 (BC96), (eBioscience, San Diego, CA). Ab cross-reactivity was confirmed by vendor technical data sheets and by the NHP Reagent Resource website (www.nhpreagents.org). Briefly, PBMC were suspended in cell staining buffer (CSB; 1x PBS/ 1% denatured FCS, Atlanta Biological). After addition of Abs, cells were incubated for 20 min at 4°C protected from light then washed with 2 ml cell staining buffer. After centrifugation, cell pellets were suspended in CSB and held on ice until analyzed. For Foxp3 intracellular staining, cells were processed after surface staining and washing, using eBioscience Foxp3 Staining Buffer Set according to the manufacturer’s instructions. To calculate absolute cell numbers, BD Pharmingen Count Bright Beads were used. Isotype-matched irrelevant Abs were used with all samples as controls. Flow cytometry was performed using a LSR Fortessa II cytometer (BD Sciences). Data acquired were analyzed with FlowJo software (Tree Star Inc., San Carlos, CA).
Cell isolation and phenotypic characterization of rhesus MDSC
PBMC were isolated by standard Ficoll density gradient centrifugation (GE Healthcare, Pittsburgh, PA) from freshly-drawn normal human or normal rhesus monkey heparinized peripheral blood or from cytokine-mobilized rhesus monkey leukapheresis products. Anti-CD2 and −CD20 microbeads (Miltenyi Biotec) were used to positively select and remove T and B cells respectively from the PBMC. The resulting cell population was FACS stained and the putative monocytic MDSC (CD3−CD20−HLA-DR−CD33+CD14+) were recovered via flow sorting using a LSR ARIA II cell sorter (BD Sciences). In addition, the flow-sorted MDSC were stained for CD11b and CD274 (B7-H1) and analyzed by flow cytometry. Data were acquired as described above.
In vitro generation of MDSCs
To ascertain whether the rhesus MDSC phenotype could be induced in vitro as described for differentiation of human monocytic MDSC (33), CD14+ microbead-isolated normal human or rhesus peripheral blood monocytes were cultured in 24-well plates (BD Biosciences; 5.105 cells well) with rhu GM-CSF and IL-4 (each 1000 U/ml) (RD Systems, Minneapolis, MN) in the presence or absence of prostaglandin (PG) E2 (1μM; Sigma-Aldrich) added at the start of cultures and at day 3 and day 5. Phenotypic analysis of the PGE2-conditioned cells was performed by flow cytometry on day 6 using cross-reactive anti-CD1a (SK9) (Becton Dickinson Immunocytometry Systems, San Jose, CA) and anti-CD209(=DC-SIGN (DCN46), BD Pharmingen) mAbs, in addition to anti-CD14 and anti-CD33.
MDSC suppression assay
The suppressive function of monocytic MDSC was evaluated by their ability to suppress the proliferation of autologous pan T cells. Untouched autologous T cells were isolated using a Rhesus Pan T cell isolation kit (Miltenyi Biotec), according to the manufacturer’s instructions. The T cells were labelled with Violet Proliferation Dye 450 (VPD450) (BD Pharmingen) according to the manufacturer’s instructions. VPD450-labelled T cells (1 × 105) were cultured in RPMI-1640 supplemented with 5% human AB serum (Gemini Bioproducts), penicillin/streptomycin (LONZA) and L-glutamine (CellGro) with (1 × 105) MDSC (1:1) or (5 × 104) MDSC (1:2) in 96-well, U-bottom plates. Microbead particles loaded with anti-monkey CD2, CD3 and CD28 Abs (Miltenyi Biotec T cell expansion kit) were used for T cell stimulation and added at 7.5 microbeads per T cell. The cultures were incubated for 4 days at 37°C in 5% CO2. T cell proliferation was determined by VPD450 dilution flow cytometric analysis. At the end of the culture period, T cells were harvested and the percentage and phenotype of proliferating T cells determined by FACS staining for naïve T cells (Tn), Tmem and Treg.
Cell bead array quantitation of cytokines
MDSC suppression assay culture supernatants were analyzed for the presence of IFN-γ, IL-2, IL-4, IL-10 and IL-17A using BD Cell Bead Array (CBA) flex sets and human soluble protein master buffer kit (BD Sciences) according to the manufacturer’s instructions. Samples were evaluated on a LSR Fortessa II cytometer (BD Sciences). Flow data were analyzed using FCAP Array software (BD Sciences).
Testing of Arg-1 and iNOS inhibitors on MDSC-mediated suppression
To test the role of specific mediators of MDSC function, inhibitors of Arg-1 (NorNOHA, N-hydroxy-nor-L-arginine) (Calbiochem) or inducible nitric oxide (NO) synthase (iNOS) (L-NMMA, Methyl-L-arginine) (Sigma) were added at the start of the suppression assay cultures (day 0). The inhibitors were used at a final concentration of 0.5mM. Control cultures were also set up in which each inhibitor was added to stimulated T cells in the absence of MDSC.
Statistical analysis
Results are expressed as means ±1 standard deviation (SD). Significances of differences between means were determined by paired or unpaired, two-tailed Student ‘t’ tests, as appropriate. P<0.05 was considered to be significant.
Results
Identification of putative monocytic MDSCs in peripheral blood of normal and hematopoietic growth factor-mobilized rhesus monkeys
Monocytic MDSC have been characterized previously in humans, including transplant patients (25, 34), and in rhesus macaques (30) by cell surface expression of CD14, the integrin CD11b, the sialic acid binding lectin CD33, and by low expression of the MHC class II surface receptor HLA-DR. We stained normal rhesus monkey peripheral blood PBMC (n=5) (and for comparison, normal adult human PBMC [n=4]) and determined the incidence of lin− (CD3−CD20−) HLA-DR-/lo, CD14+CD33+CD11b+ cells. As shown in Figure 1, the incidence of these putative MDSC in normal rhesus blood was 2.1 ± 1.7% (range 0.8–4.5%) of lin− HLA-DR−/lo cells, compared with 6.5 ± 3.3% (range 2.1–10.0%) in normal human blood. We also determined the incidence of MDSC in peripheral blood of monkeys that had been injected over the preceding 8 days with the hematopoietic growth factors GM-CSF and G-CSF (n=5). As also shown in Figure 1, the incidence of MDSC was increased in these monkeys to 5.3 ± 3.4% of lin−HLA-DR−/lo cells (range 2.2–11.1%).
Figure 1. Identification of putative monocytic MDSC defined as CD3−CD20−HLA-DR−CD14+CD33+CD11b+ cells in normal human and rhesus peripheral blood and in leukapheresis products of growth factor-mobilized monkeys.

(A), Representative phenotypic data from normal human and rhesus blood and from a rhesus monkey that was cytokine-mobilized for 8 days (4 days GM-CSF + 4 days G-CSF) prior to leukapheresis. PBMC were stained as described in the Materials and Methods. Data are representative of 4 normal humans, 5 normal monkeys and 5 mobilized leukapheresed monkeys. (B) means ±1SD for each group.
Yield of flow-sorted MDSC from normal and growth factor-mobilized rhesus monkey blood
We next examined the numbers of monocytic MDSC that could be flow-sorted from PBMC of normal monkeys and from leukapheresis products of GM-CSF+G-CSF-mobilized monkeys. As shown in Figure 2, much larger numbers of MDSC could be flow-sorted from leukapheresis products of growth factor-mobilized than from blood of normal controls where the incidences of putative MDSC (% PBMC) were 1.6 ± 0.8 (n=11) and 0.3 ± 0.3 (n=4), respectively, representing 5–6-fold enrichment. From a total of 11 leukapheresis products obtained from n=10 mobilized monkeys, we determined that a mean of 38±13.106 (range 12–56.106) flow-sorted MDSC could be purified. This, in principle, would allow a mean total of 7.5±3.106 MDSC/kg to be administered to a 5 kg cynomolgus monkey recipient.
Figure 2. Quantitation of monocytic MDSC in growth factor-mobilized rhesus monkey leukapheresis products.

(A) Recovery of MDSC from normal rhesus blood (n=4 monkeys). (B), Numbers of flow-sorted MDSC recovered from PBMC of 10 growth factor (GM-CSF + G-CSF)-mobilized donors. (C), means ±1SD and (D), fold-increase. *p<0.01
PGE2 does not induce a distinct MDSC phenotype from rhesus blood monocytes
Differentiation of human monocytes into CD1a+ DC can be prevented by their exposure to PGE2 at early stages of DC development (35). Moreover, Obermajer et al (33) have reported that culture of human monocytes with PGE2 induces CD1a−CD14+CD33+ MDSC (HLA-DR expression was not documented) with immunosuppressive function. To test whether we could induce MDSC similarly from human or rhesus blood monocytes, we cultured CD14 immunobead-selected cells with GM-CSF and IL-4 in the presence of PGE2. Cytokine-stimulated human monocytes exposed to PGE2 expressed very low levels of CD1a and were HLA-DR+ CD14+CD33+. By contrast, rhesus monocytes cultured under identical conditions, were CD1a+, although also like their human counterparts, HLA-DR+, CD14+ and CD33+ (data not shown). Thus, in our hands, PGE2 did not redirect GM-CSF− and IL-4-driven differentiation of human or rhesus DCs towards a distinct MDSC phenotype.
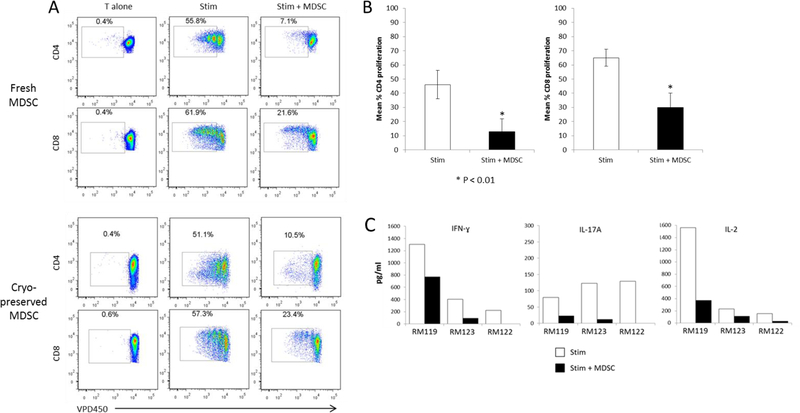
Freshly-isolated or cryopreserved MDSC from growth factor-mobilized monkeys markedly suppress T cell proliferative responses and IFNγ, IL-17A and IL-2 production
To ascertain the ability of rhesus monocytic MDSC to inhibit T cell proliferative responses, we added MDSC flow-sorted from the freshly-isolated or cryopreserved leukapheresis products of growth factor-mobilized monkeys to autologous T cells that were stimulated with anti-CD3 and anti-CD28 microbeads in 4 day cultures. As shown in Figure 3A & B, pronounced suppression of both CD4+ and CD8+ T cell proliferation was observed at MDSC:T cell ratios of 1:1 (approx. 80%) and 1:2 (approx. 60%). Addition of MDSC at the start of these cultures also profoundly reduced Th1 (IFNγ) and Th17 (IL-17A) cytokine and IL-2 levels in culture supernatants (Figure 3C). IL-4 or IL-10 secretion was not detected in either the absence or presence of MDSC.
Figure 3. Suppression of CD4+ and CD8+ T cell proliferation and proinflammatory cytokine production by flow-sorted, freshly-isolated or cryopreserved rhesus MDSC.
Monocytic MDSC (CD3−CD20−HLA-DR−CD14+CD33+CD11b+) were flow-sorted from freshly-isolated or cryopreserved leukapheresis products obtained from monkeys mobilized for 8 days (4 days GM-CSF + 4 days G-CSF) prior to leukapheresis. The MDSC were then co-cultured with VPD450-labeled autologous pan T cells (1 MDSC:2 T cells), with or without anti-CD3/anti-CD28 bead stimulation (1 bead:7.5 T cells) for 96 hr. (A), suppression of both CD4+ and CD8+ T cell proliferation by flow-sorted freshly-isolated and cryopreserved MDSC. Data are from one monkey (RM122) and are representative of 3 experiments performed with MDSC from 3 individual monkeys that gave similar results. (B), overall extent of suppression (means ±1SD) of CD4+ and CD8+ T cell proliferation by the rhesus MDSC sorted from cryopreserved leukapheresis products (C), Pronounced suppression of IFNγ, IL-17A and IL-2 production determined by CBA assay in culture supernatants at 96 hr. Data obtained with MDSC from 3 individual monkeys are shown.
Rhesus MDSC enhance Foxp3+ Treg and their incidence relative to Tmem
There is evidence that MDSC in cancer-bearing hosts can induce Treg (36, 37) and that HLA-DR−CD11b+CD33+ MDSC isolated by CD33 positive separation from peripheral blood 6 months after human renal transplantation, can expand CD4+Foxp3+ Treg in vitro (25). Moreover, monocytic suppressor cells that mediate heart transplant tolerance in mice promote the induction of Foxp3+ Treg in vivo (13). Thus, we examined the influence of rhesus MDSC on Treg in cultures of VPD450-labeled proliferating autologous T cells. As shown in Figure 4, addition of MDSC at the start of anti-CD3/CD28-stimulated T cell cultures significantly increased the incidence and absolute numbers of proliferating Foxp3+CD25hi T cells. Moreover, MDSC reduced the incidences of activated CD4+ and CD8+ CD45RA+CD62L− terminally-differentiated memory T cells (Temra) and the ratios of Treg to Tn, Temra and effector memory T cells (Tmem; CD62L+CD45RA+) (Figure 5).
Figure 4. Rhesus flow-sorted MDSC enhance the expansion of CD4+Foxp3+CD25hi Treg.

Flow-sorted rhesus monocytic MDSC from individual cryopreserved leukapheresis products were added to immunobead (anti-CD3/CD28)-stimulated (1 bead:7.5 T cells) VPD 450-labeled autologous pan T cells (1 MDSC:2T cells) at the start of 4-day cultures. (A), MDSC flow-sorted from both monkeys shown (RM121 and RM122) enhanced the incidence of proliferating Treg, determined at 4 days. Data obtained with MDSC from two representative monkeys are presented as percentages of proliferating CD4+ T cells. Numbers in quadrants denote percent positive cells. (B), Overall fold increase in Treg (n=4 experiments). Data are means ±1SD; †, p<0.05.
Figure 5. Rhesus MDSC inhibit the proliferation of activated T memory cell (Tmem) responses and enhance the ratio of Treg/Tmem.
Flow-sorted rhesus monocytic MDSC obtained from cryopreserved leukapheresis products were added to immunobead-stimulated (anti-CD3/CD28), CFSE-labeled autologous pan T cells (1MDSC:2 T cells) at the start of 96-hr cultures. (A) Representative data showing the incidences of CD62L+CD45RA+ (naïve; TN), CD62L+CD45RA− (central memory; TCM), CD62L−CD45RA− (effector memory; TEM) and CD62L−CD45RA+ (terminally-differentiated effector memory; TEMRA) cells among gated CD4+and CD8+ T cell populations at 96 hr. (B) Overall ratios of CD4+Foxp3+CD25hi Treg:CD4+ Tn, Temra or Tmem (n=4 experiments using MDSC from 4 separate monkeys). Data are means ±1SD; †, p<0.05
Rhesus MDSC exert their suppressive activity in part via arginase-1 activity.
There is evidence that monocytic MDSCs produce Arg-1 and NO via inducible NO synthase (iNOS), both of which have been implicated in suppression of T cell activation by MDSC (14, 38). To examine the role of these effector molecules, we co-cultured flow-sorted MDSC from mobilized monkeys with activated T cells in the presence of inhibitors of Arg-1 or iNOS. As shown in Figure 6, inhibition of Arg-1, but not iNOS partially reversed the inhibitory effect of rhesus MDSC on CD8+ T cells. No direct inhibitory effect of the inhibitors on responder T cell viability or proliferation was detected (data not shown).
Figure 6. Arginase-1 but not iNOS inhibition partially reverses the suppressive effect of rhesus MDSC on activated CD8+ T cell proliferation.
(A), Flow-sorted rhesus monocytic MDSC obtained from cryopreserved leukapheresis products were added to immunobead-stimulated (anti-CD3/CD28), VPD450-labeled autologous pan T cells (1 MDSC:2 T cells) at the start of 96 hr cultures, in the presence of either the arginase-1 inhibitor NorNOHA (0.5 mM) or the inducible nitric oxide synthase (iNOS) inhibitor LMMA. (B), Mean values (±1SD) obtained across multiple experiments performed with MDSC sorted from 2 individual monkeys.
Discussion
In this study, we have confirmed that, on the basis of their cell surface phenotype (lin−HLA-DR-CD14+CD33+CD11b+), monocytic MDSC can be identified in blood of normal rhesus macaques. These cells closely resemble MDSC described previously in normal human peripheral blood [lin−HLA-DR−CD11b+ (39) or lin−HLA-DR−CD14+CD33+ cells (34)]. As in humans, where monocytic MDSC represent approx. 5% of normal PBMC (25), we found that these immune regulatory myeloid cells represent a small proportion (2.1 ± 1.7%) of normal rhesus lin−HLA-DR−PBMC. However, we observed that their numbers or their frequency could be increased significantly in vivo in response to sequential GM− and G-CSF administration. This mobilization protocol has been used by us previously to mobilize hematopoietic cells in rhesus monkeys (31, 40) from which myeloid cells in apheresis products have been isolated. Since both G-CSF and GM-CSF have been reported to promote MDSC expansion either in humans or mice, we considered that use of both cytokines would be appropriate in our effort to expand these cells in monkeys. We do not know, however, whether the mobilization protocol we selected can be further optimized to enhance the yield of MDSC, eg by alterations in cytokine dosages or by concurrent versus sequential combination of G− and GM-CSF (41). The mobilized rhesus MDSC in the present study resemble circulating myeloid cells (lin−HLA-DR−CD14+CD33+CD11b+) that are upregulated in rhesus peripheral blood during vaccination and simian immunodeficiency virus infection and that suppress virus-specific CD8+ T cells (30). Moreover, elevated frequencies of MDSC have been detected in human renal transplant recipients compared with normal/baseline controls (25, 34).
We focused on monocytic MDSCs since, first, this subset is more suppressive than granulocytic MDSCs in mice (22, 42, 43); second, increased numbers of monocytic MDSC that prevent the initiation of adaptive immune responses have been described in mouse heart transplant tolerance (13) and third, monocytic MDSCs isolated from renal transplant patients are highly efficient in suppressing CD4 T cell proliferation in MLR and expanding Treg (25). Furthermore, there is evidence that, compared with granulocytic MDSC, human monocytic MDSC are relatively undamaged by cryopreservation (44) and most of the analyses we report were performed with freeze-thawed PBMC. This is in line with our long-term goal of isolating monocytic MDSCs from cryopreserved PBMC for adoptive transfer to allograft recipients.
Stem cell mobilization with G-CSF promotes the emergence of myeloid precursors (45) or regulatory monocytes (46) that inhibit alloimmunity. Many factors have been implicated in the expansion of MDSC, particularly in cancer, including the hematopoietic growth factors M-CSF, G-CSF, GM-CSF and stem cell factor, vascular endothelial growth factor, various cytokines (e.g. IL-6), and PGE2 (47). Monocytic MDSC are also induced in human bone marrow and PBMC after G-CSF stem cell mobilization (39, 48). Here, we show that MDSC flow-sorted from fresh or cryopreserved leukapheresis products of GM− and G-CSF-mobilized monkeys markedly suppress autologous, activated T cell proliferation, depressing both Th1 (IFNγ) and Th17 cell (IL-17A) cytokine and IL-2 production and enhancing the incidence of CD4+CD25hiFoxp3+Treg relative to naïve and both CD4+ and CD8+ Tmem. These findings are consistent with the ability of human monocytic MDSC isolated post-transplant to expand autologous Foxp3+ Treg in vitro and with the positive correlation between circulating monocytic MDSC and Treg in renal transplant patients (25).
We were unable to determine the suppressive function of flow-sorted normal (non-mobilized) monkey monocytic MDSCs because of the comparative paucity of these cells in the normal steady-state. Others however, have reported that MDSCs flow-sorted from normal human PBMC do not suppress alloactivated T cell proliferation (25). In transplant recipients, recent evidence supports a role of MDSC in regulation of alloimmunity and promotion of transplant tolerance in rodent models (13, 47, 49, 50). Recent reports also show that adoptive transfer of in vivo- or in vitro-induced MDSC can inhibit allograft rejection (12, 45, 51) or suppress graft-versus-host disease following hematopoietic stem cell transplantation (20). Our ability to flow-sort highly-purified rhesus monocytic MDSCs enhances the feasibility of testing these cells as therapeutic innate regulatory immune cells in clinically-relevant NHP models of hematopoietic cell or organ transplantation. Following growth factor mobilization of monkeys weighing 5–7 kg, we could obtain a total of 38 ± 13.106 MDSC from a single whole leukapheresis product. Based on our observations, sufficient cells could be sorted from each leukapheresis product of mobilized rhesus donors to administer approx. 2–10.106/kg MDSC to a prospective recipient, with potential for repeated growth factor administration, leukapheresis and MDSC sorting to acquire additional cells. While the number of adoptively-transferred rhesus MDSC that might be required to achieve a therapeutic effect is uncertain, similar numbers of regulatory macrophages (7.1–8.0.106/kg) have been infused into human renal allograft recipients (52) and a single administration of 2–10.106/kg regulatory myeloid dendritic cells has been shown to prolong rhesus monkey renal allograft survival (31).
Production of Arg-1 by MDSC appears to be a key mechanism underlying their suppressive function (28). In this study, we found that rhesus monocytic MDSC were more effective in inhibiting CD8+ compared with CD4+ T cell responses and indeed, CD8+ T cells have been recognized as important targets of MDSC (53) that interfere with their proliferation function in an L-arginine dependent manner (54). In preliminary studies (data not shown) we have observed rare, presumptive MDSC expressing Arg-1 (CD11b+Arg-1+) in transplant biopsies of renal allograft recipient monkeys experiencing improved graft survival (as the result of regulatory immune cell therapy), but not in control allografts rejected acutely at the same time post-transplant. Migration of murine MDSC from the bone marrow to heart allografts, where they appear to prevent the initiation of adaptive immunity and promote tolerance via Arg-1 and iNOS production and Treg development, has been implicated as a key mechanism underlying the induction of transplant tolerance (13). In addition, Dugast et al (14) have suggested that iNOS-expressing MDSCs that accumulate in tolerated rat renal allografts are required for the maintenance of tolerance. However, in this study, we found that the rhesus MDSC mobilized in vivo did not mediate their suppressive function via iNOS and expressed only low levels of B7-H1, that has been implicated in the immunosuppressive function of MDSCs in tumor-bearing mice (42). Other possible mediators of the suppressive action of rhesus monocytic MDSC are heme oxygenase-1, that has been shown to mediate suppression of murine alloreactive T cell responses (51), and indoleamine deoxygenase (55).
In conclusion, MDSC capable of regulating activated T cell responses can be readily flow-sorted from growth factor-mobilized NHP. Investigation of the in vivo fate and function of adoptively-transferred NHP MDSC and the role of these cells in regulation of allograft survival will be important aspects of future investigations.
Acknowledgments
The work was supported by National Institute of Health (NIH) grants U01 AI102456 (Opportunities Pool award) and U01 AI51698, part of the NIH NHP Transplantation Tolerance Cooperative Study Group and sponsored by the NIAID and NIDDK. We thank Lisa Mathews for skilled technical support.
Abbreviations:
- Arg-1
arginase-1
- MDSC
myeloid-derived suppressor cells
- NHP
non-human primate
- Tem
effector memory T cells
- Temra
terminally-differentiated memory T cells
- Tmem
memory T cells
- Tn
naïve T cells
- Treg
regulatory T cells
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9(3):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosborough BR, Raich-Regue D, Turnquist HR, Thomson AW. Regulatory myeloid cells in transplantation. Transplantation 2014;97(4):367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makarenkova VP, Bansal V, Matta BM, Perez LA, Ochoa JB. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J Immunol 2006;176(4):2085–2094. [DOI] [PubMed] [Google Scholar]
- 4.Marhaba R, Vitacolonna M, Hildebrand D, Baniyash M, Freyschmidt-Paul P, Zoller M. The importance of myeloid-derived suppressor cells in the regulation of autoimmune effector cells by a chronic contact eczema. J Immunol 2007;179(8):5071–5081. [DOI] [PubMed] [Google Scholar]
- 5.Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol 2003;74(2):186–196. [DOI] [PubMed] [Google Scholar]
- 6.Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 2008;111(12):5457–5466. [DOI] [PubMed] [Google Scholar]
- 7.Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res 2003;63(15):4441–4449. [PubMed] [Google Scholar]
- 8.Fu YX, Watson G, Jimenez JJ, Wang Y, Lopez DM. Expansion of immunoregulatory macrophages by granulocyte-macrophage colony-stimulating factor derived from a murine mammary tumor. Cancer Res 1990;50(2):227–234. [PubMed] [Google Scholar]
- 9.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol 1999;162(10):5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 10.Rosborough BR, Mathews LR, Matta BM, Liu Q, Raich-Regue D, Thomson AW et al. Cutting edge: Flt3 ligand mediates STAT3-independent expansion but STAT3-dependent activation of myeloid-derived suppressor cells. J Immunol 2014;192(8):3470–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosborough BR, Castellaneta A, Natarajan S, Thomson AW, Turnquist HR. Histone deacetylase inhibition facilitates GM-CSF-mediated expansion of myeloid-derived suppressor cells in vitro and in vivo. J Leukoc Biol 2012;91(5):701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Liang S, Wu J, Horuzsko A. Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation 2008;86(8):1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Invest 2010;120(7):2486–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol 2008;180(12):7898–7906. [DOI] [PubMed] [Google Scholar]
- 15.Dilek N, Poirier N, Usal C, Martinet B, Blancho G, Vanhove B. Control of transplant tolerance and intragraft regulatory T cell localization by myeloid-derived suppressor cells and CCL5. J Immunol 2012;188(9):4209–4216. [DOI] [PubMed] [Google Scholar]
- 16.Hongo D, Tang X, Baker J, Engleman EG, Strober S. Requirement for interactions of natural killer T cells and myeloid-derived suppressor cells for transplantation tolerance. Am J Transplant 2014;14(11):2467–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura T, Nakao T, Yoshimura N, Ashihara E. Rapamycin Prolongs Cardiac Allograft Survival in a Mouse Model by Inducing Myeloid-Derived Suppressor Cells. Am J Transplant 2015. [DOI] [PubMed]
- 18.Chou HS, Hsieh CC, Charles R, Wang L, Wagner T, Fung JJ et al. Myeloid-derived suppressor cells protect islet transplants by B7-H1 mediated enhancement of T regulatory cells. Transplantation 2012;93(3):272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arakawa Y, Qin J, Chou HS, Bhatt S, Wang L, Stuehr D et al. Cotransplantation with myeloid-derived suppressor cells protects cell transplants: a crucial role of inducible nitric oxide synthase. Transplantation 2014;97(7):740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood 2010;116(25):5738–5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin B, Ma G, Yen CY, Zhou Z, Wang GX, Divino CM et al. Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J Immunol 2010;185(10):5828–5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 2008;111(8):4233–4244. [DOI] [PubMed] [Google Scholar]
- 23.Zhu B, Bando Y, Xiao S, Yang K, Anderson AC, Kuchroo VK et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol 2007;179(8):5228–5237. [DOI] [PubMed] [Google Scholar]
- 24.Kusmartsev S, Nagaraj S, Gabrilovich DI. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol 2005;175(7):4583–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luan Y, Mosheir E, Menon MC, Wilson D, Woytovich C, Ochando J et al. Monocytic myeloid-derived suppressor cells accumulate in renal transplant patients and mediate CD4(+) Foxp3(+) Treg expansion. Am J Transplant 2013;13(12):3123–3131. [DOI] [PubMed] [Google Scholar]
- 26.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol 2003;170(1):270–278. [DOI] [PubMed] [Google Scholar]
- 27.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest 2010;120(2):457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol 2012;144(3):250–268. [DOI] [PubMed] [Google Scholar]
- 29.Boros P, Ochando JC, Chen SH, Bromberg JS. Myeloid-derived suppressor cells: natural regulators for transplant tolerance. Hum Immunol 2010;71(11):1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sui Y, Hogg A, Wang Y, Frey B, Yu H, Xia Z et al. Vaccine-induced myeloid cell population dampens protective immunity to SIV. J Clin Invest 2014;124(6):2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ezzelarab MB, Zahorchak AF, Lu L, Morelli AE, Chalasani G, Demetris AJ et al. Regulatory dendritic cell infusion prolongs kidney allograft survival in nonhuman primates. Am J Transplant 2013;13(8):1989–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pathiraja V, Matar AJ, Gusha A, Huang CA, Duran-Struuck R. Leukapheresis protocol for nonhuman primates weighing less than 10 kg. J Am Assoc Lab Anim Sci 2013;52(1):70–77. [PMC free article] [PubMed] [Google Scholar]
- 33.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 2011;118(20):5498–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hock BD, Mackenzie KA, Cross NB, Taylor KG, Currie MJ, Robinson BA et al. Renal transplant recipients have elevated frequencies of circulating myeloid-derived suppressor cells. Nephrol Dial Transplant 2012;27(1):402–410. [DOI] [PubMed] [Google Scholar]
- 35.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol 1997;159(1):28–35. [PubMed] [Google Scholar]
- 36.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008;135(1):234–243. [DOI] [PubMed] [Google Scholar]
- 37.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006;66(2):1123–1131. [DOI] [PubMed] [Google Scholar]
- 38.Raber PL, Thevenot P, Sierra R, Wyczechowska D, Halle D, Ramirez ME et al. Subpopulations of myeloid-derived suppressor cells impair T cell responses through independent nitric oxide-related pathways. Int J Cancer 2014;134(12):2853–2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luyckx A, Schouppe E, Rutgeerts O, Lenaerts C, Fevery S, Devos T et al. G-CSF stem cell mobilization in human donors induces polymorphonuclear and mononuclear myeloid-derived suppressor cells. Clin Immunol 2012;143(1):83–87. [DOI] [PubMed] [Google Scholar]
- 40.Zahorchak AF, Kean LS, Tokita D, Turnquist HR, Abe M, Finke J et al. Infusion of stably immature monocyte-derived dendritic cells plus CTLA4Ig modulates alloimmune reactivity in rhesus macaques. Transplantation 2007;84(2):196–206. [DOI] [PubMed] [Google Scholar]
- 41.Sohn SK, Kim JG, Seo KW, Chae YS, Jung JT, Suh JS et al. GM-CSF-based mobilization effect in normal healthy donors for allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant 2002;30(2):81–86. [DOI] [PubMed] [Google Scholar]
- 42.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008;181(8):5791–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol 2010;40(1):22–35. [DOI] [PubMed] [Google Scholar]
- 44.Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods 2012;381(1–2):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacDonald KP, Rowe V, Clouston AD, Welply JK, Kuns RD, Ferrara JL et al. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol 2005;174(4):1841–1850. [DOI] [PubMed] [Google Scholar]
- 46.D’Aveni M, Rossignol J, Coman T, Sivakumaran S, Henderson S, Manzo T et al. G-CSF mobilizes CD34+ regulatory monocytes that inhibit graft-versus-host disease. Sci Transl Med 2015;7(281):281ra242. [DOI] [PubMed] [Google Scholar]
- 47.Dilek N, Vuillefroy de Silly R, Blancho G, Vanhove B. Myeloid-derived suppressor cells: mechanisms of action and recent advances in their role in transplant tolerance. Front Immunol 2012;3:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lv M, Zhao XS, Hu Y, Chang YJ, Zhao XY, Kong Y et al. Monocytic and promyelocytic myeloid-derived suppressor cells may contribute to G-CSF-induced immune tolerance in haplo-identical allogeneic hematopoietic stem cell transplantation. Am J Hematol 2015;90(1):E9–E16. [DOI] [PubMed] [Google Scholar]
- 49.Wu T, Sun C, Chen Z, Zhen Y, Peng J, Qi Z et al. Smad3-deficient CD11b(+)Gr1(+) myeloid-derived suppressor cells prevent allograft rejection via the nitric oxide pathway. J Immunol 2012;189(10):4989–5000. [DOI] [PubMed] [Google Scholar]
- 50.Lees JR, Azimzadeh AM, Bromberg JS. Myeloid derived suppressor cells in transplantation. Curr Opin Immunol 2011;23(5):692–697. [DOI] [PubMed] [Google Scholar]
- 51.De Wilde V, Van Rompaey N, Hill M, Lebrun JF, Lemaitre P, Lhomme F et al. Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant 2009;9(9):2034–2047. [DOI] [PubMed] [Google Scholar]
- 52.Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M et al. Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol 2011;187(5):2072–2078. [DOI] [PubMed] [Google Scholar]
- 53.Schouppe E, Van Overmeire E, Laoui D, Keirsse J, Van Ginderachter JA. Modulation of CD8(+) T-cell activation events by monocytic and granulocytic myeloid-derived suppressor cells. Immunobiology 2013;218(11):1385–1391. [DOI] [PubMed] [Google Scholar]
- 54.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol 2011;11(7):802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu J, Du W, Yan F, Wang Y, Li H, Cao S et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol 2013;190(7):3783–3797. [DOI] [PubMed] [Google Scholar]