Abstract
Background:
Ruminant feed containing animal byproduct proteins (ABPs) is prohibited in many countries due to its risk of transmitting prion diseases (PD). In most cases the entire herd is sacrificed, which causes great harm to the producer countries by preventing their exportation of ruminant derived-products.
Methods:
We used stable isotope ratio mass spectrometry (IRMS) of carbon (13C/12C) and nitrogen (15N/14N) to trace the animal protein in the blood of 15 buffaloes (Bubalus bubalis) divided into three experimental groups: 1 - received only vegetable protein (VP) during 117 days; 2 - received animal and vegetable protein (AVP); and 3 - received animal and vegetable protein with animal protein subsequently removed (AVPR). Groups 2 and 3 received diets containing 13.7% bovine meat and bone meal (MBM) added to a vegetable diet (from days 21-117 in the AVP group and until day 47 in the AVPR group, when MBM was removed).
Results:
On the 36th day, differences were detectable in the feeding profile (p <0.01) among the three experimental groups, which remained for a further 49 days (85th day). The AVPR group showed isotopic rate reversibility on the 110th day by presenting values similar to those in the control group (VP) (p> 0.05), indicating that it took 63 days to eliminate MBM in this group. Total atoms exchange (> 95%) of 13C and 15N was observed through incorporation of the diet into the AVP and AVPR groups.
Conclusions:
IRMS is an accurate and sensitive technique for tracing the feeding profile of ruminants through blood analysis, thus enabling investigation of ABP use.
Keywords: prion diseases, monitoring, bovine spongiform, encephalopathy, stable isotopes
Background
Prion diseases (PD) are a fatal group of neurodegenerative disorders that include Creutzfeldt-Jakob disease (CJD), kuru, scrapie, chronic wasting disease, and bovine spongiform encephalopathy (BSE) [1-4]. Use of animal byproduct proteins (ABPs) in ruminant feed is prohibited in most countries due to the risk of PD transmission [5,6].
The lack of methods that specifically identify the use of mammalian processed animal proteins (PAPs) to feed ruminants led to the introduction of a ban of PAPs for all farmed animals [7] by amending Regulation 999/2001 [8], through Commission Regulation 1234/2003 [9].
Except for the consumption of ABPs, epidemiological studies have failed to identify other specific risk factors for prion diseases. However, experiments have demonstrated BSE and scrapie transmission by blood transfusion [10,11]. Thus, PD monitoring becomes crucial and is dependent on accurate diagnosis, which still represents a unique challenge in the development of novel assays to explore the prion protein complex [3].
Furthermore, diagnostic tests for PD are costly and can be performed only when the animal shows symptoms. In most cases the entire herd is sacrificed, which causes great harm to the producers and their countries by banning them from exporting their products. Therefore, the search for accurate methods to detect previous animal protein intake is essential, since they can imply how and where the animals were raised, denoting prohibited and/or fraudulent practices, thereby ensuring the quality of the meat consumed [4].
Methods such as DNA Hybridization, "Enzyme Linked Immuno Sorbent Assay (ELISA), and Polymerase Chain Reaction (PCR) were employed to identify ABPs in diets supplied to ruminants [12,13]. However, these techniques are not able to detect the presence of animal protein in final products such as meat, blood, eggs, milk, serum samples, and others.
Accurate methods are necessary to identify previous animal protein intake because they can show how and where the animals were raised, determine whether there is a risk for human consumption and, finally, select animals suitable for exploitation by the pharmaceutical industry [4,14-16].
Recently, the measurement of δ13C and δ15N using isotope-ratio mass spectrometry (IRMS) has been used successfully in the processes of disease control, authentication and certification of animal products [17-19], traceability [4,20,21] and evaluation of conventional and organic production systems for beef [17,22,23].
Traceability investigation through IRMS starts at its main food source, which includes plants of C3 photosynthetic cycle (e.g. rice, wheat, barley, alfalfa, peanut, cotton, etc.), with an average δ13C isotopic enrichment relative to 13C of = -28 ‰, and plants of C4 photosynthetic cycle (e.g., sugarcane, corn, tropical grasses, etc.), with a mean δ13C value of -12 ‰ ([24,25]. Stable isotopes are used as dietary markers because they are rapidly replaced in tissues by dietary isotopes [26]. It was possible to detect bovine meat-and-bone meal (MBM) in egg yolk and egg albumen of laying birds, even with the inclusion of other ingredients such as vegetables and yeasts [4,27,28].
This study was designed to detect previous ABP intake by determining the stable isotope ratios for carbon (13C/12C) and nitrogen (15N/14N) in ruminant blood in order to prevent the feeding of humans with meat susceptible to prion contamination, and to select animals that would provide biological samples to pharmaceutical companies.
Material and methods
Animals and experimental diets
Fifteen Murrah buffaloes (Bubalus bubalis), aged one year and weighing approximately 200 kg, were fed vegetable-based diet for 20 days to achieve homogenization of 13C and 15N isotopic values.
Thus animals were divided into three experimental groups: one control group (Vegetable Protein: VP; n = 4), which continued with the starter diet (vegetable-based diet only) for the 117 days of the experiment (days 0-117); and two treated groups (Animal and Vegetable Protein: AVP, n = 6/group; and Animal and Vegetable Protein Removal: AVPR, n = 5/group) that from the 21th day were fed diets containing 13.7% bovine meat and bone meal (MBM; Mondelli® commercial feed - humidity, 8%; crude protein, 45% and ether extract, 6%). The AVPR group was fed until the 47th day (day 21-47), when the MBM was removed.
All the animals were microchipped and received Coast-cross hay (88.96% dry matter with 92.12% organic matter, 11.45% crude protein, 1.75% ether extract, 30.76% crude fiber, 48.16% nitrogen-free extract, and 7.88% ash) and water ad libitum throughout experimental period.
Ethics statement
This study was conducted in accordance with the Ethical Principles in Animal Research of the Brazilian College of Animal Experimentation and was approved by the Ethics Committee for Animal Experimentation (protocol nº 78/ 2009) of the College of Veterinary Medicine and Animal Husbandry (FMVZ-UNESP), Brazil. Animal welfare was respected throughout experimental period, in accordance with the “five freedoms” defined by the Farm Animal Welfare Council.
At the end of the experiment, the animals from the animal and vegetable protein (AVP) and the animal and vegetable protein removal (AVPR) groups were euthanized following the approved ethical protocol.
Serum preparation and δ 13 C and δ 15 N analyses by stable isotope-ratio mass spectrometry (IRMS)
Blood samples were drawn from the animals’ jugular veins twice a week, from 8:00 am to 10:00 am, collected in 10 mL Vacutainer® tubes of 10 mL and centrifuged at 1,000xg for 30 min at 4ºC. Then 1 mL of serum was lyophilized and stored in microtubes at -20ºC. Lyophilized serum weights of approximately 50-70 µg for 13C and 500-600 µg for 15N were placed in tin capsules for analysis of 13C and 15N. Subsequently, the capsules were stored in Elisa microplates and maintained at 4ºC until the time of the isotopic analysis.
Isotopic ratios of 13C/12C and 15N/14N were measured in the mass spectrometer Delta V Advantage Isotope Ratio MS (Thermo Scientific®). Isotopic ratio values were expressed as delta per thousand (δ) relative to the international standards Pee Dee Belemnite (PDB) for 13C and atmospheric air nitrogen for 15N, according to equation 1 [25,29]:
| (1) |
Where: δ13C = relative enrichment of the 13C/12C ratio in the sample relative to the PDB standard; R = isotopic ratio (13C/12C) of the sample and standard.
Percentage of exchanged atoms
The percentage of exchanged atoms in serum was determined according to equation 2 [30]:
| (2) |
Where:
F represents the fraction of exchanged atoms, reliable when more than 95%; t is the total time of experiment; k is the turnover.
Data analysis
The data were subjected to univariate statistical analysis (ANOVA and complemented with Tukey's test), for each day of collection. The period that presented the same characteristic was grouped, forming three groups: one that did not present difference between the treatments; a second that differed from the control group; and a third that differed from the treatment using animal protein. Each response group was applied to principal components analysis (PCA) and discriminate analysis. Values were considered significant when p <0.05.
For evaluation of diet incorporation behavior as a function of time, data were adjusted by linear and nonlinear (polynomial and first order exponential) functions.
Results and discussion
Homogenization of 13C and 15N isotopic values was possible only by administering the vegetable-based diet during the first 20 days to the three experimental groups despite not observing the total exchange of atoms (<95%) in this period (Figures 1 and 2). It was attributable to an isotopic ratio from an animal origin diet outcome based on the Brachiaria genus inserted in a system of feeding the photosynthetic cycle C4 plants (where δ13C varies from -9 ‰ to -16 ‰) and evidenced by a δ13C value reduction of 2.5 ‰ in this period (δ13C = -12 ‰ to -14.5 ‰), but still encompassing C4 values (Figure 1).
Figure 1. Animal feed δ13C measurement. A: adaptation period; B: period in which the AVP and AVPR groups received diet with animal protein; C: period in which the AVPR group received a strictly vegetable diet.
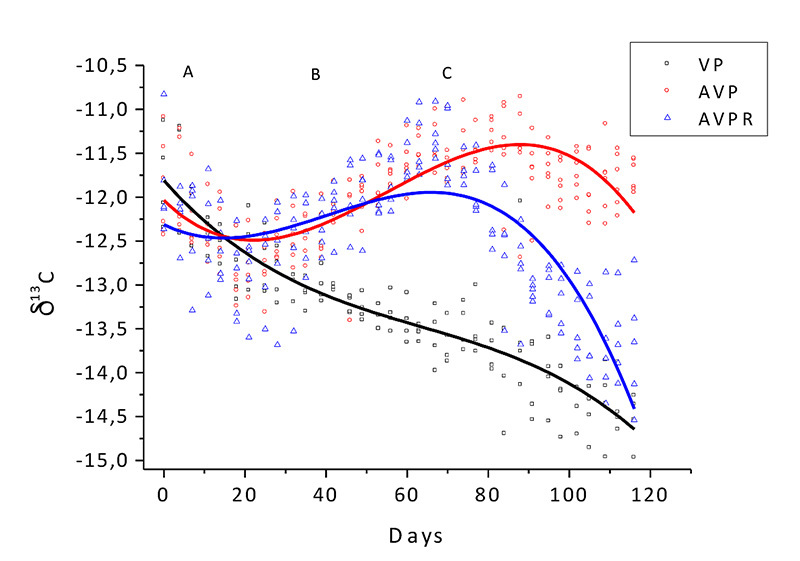
Figure 2. Animal feed δ15N measurement. A: adaptation period; B: period in which the AVP and AVPR groups received diet with animal protein; C: period in which the AVPR group received a strictly vegetable diet.
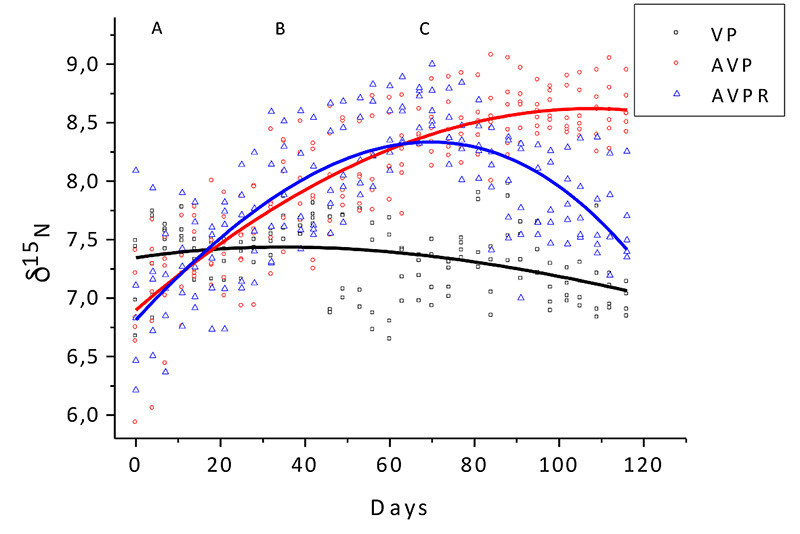
However, the isotopic value of 15N did not change significantly during the adaptation period (Figure 2), indicating that both source and experimental diets were similar. The 15N/14N isotopic ratio does not depend on the photosynthetic cycle, but rather on the fixation mode due to the incorporation of nitrogenous compounds of the soil. The 15N values can suffer interference from soil type, leguminous plants in the diet and animal metabolic factors [31].
After the adaptation period, a variation in the δ13C and δ15N isotope values was observed in animals’ serum due to MBM inclusion in the diet remaining in the range of plant values of the C4 photosynthetic cycle (Figure 1). This finding verified the isotopic analysis signature of experimental diet that presents a higher percentage of C4 plants than C3, in addition to MBM, which according to Denadai et al. [27,28], shows a C4 isotopic value of -12.82.
The δ13C and δ15N variation among the experimental groups was detected in buffalo sera after 26 days of diet containing animal protein (p <0.01). It was observed for another 35 days in the AVPR group and maintained until the end of the experiment in the AVP group (p <0.01) (Figures 1 to 5).
Figure 5. Principal component analysis of δ13C and δ15N values from experimental day 88 to 116.
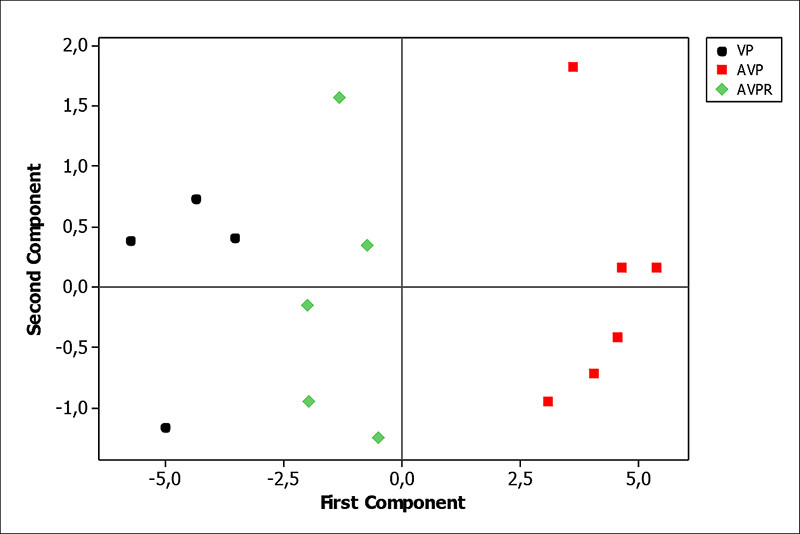
AVPR did not differ from PV at the 88th day but both groups differed from AVP, which demonstrates that when the strictly vegetable one replaces the animal protein diet, the isotopic signature of the animal protein diet is maintained until 41 days. Discrimination among groups had been most influenced by the 13C isotope (Figure 3, 4 and 5).
Figure 3. Principal component analysis of δ13C and δ15N values from experimental day zero to 46.
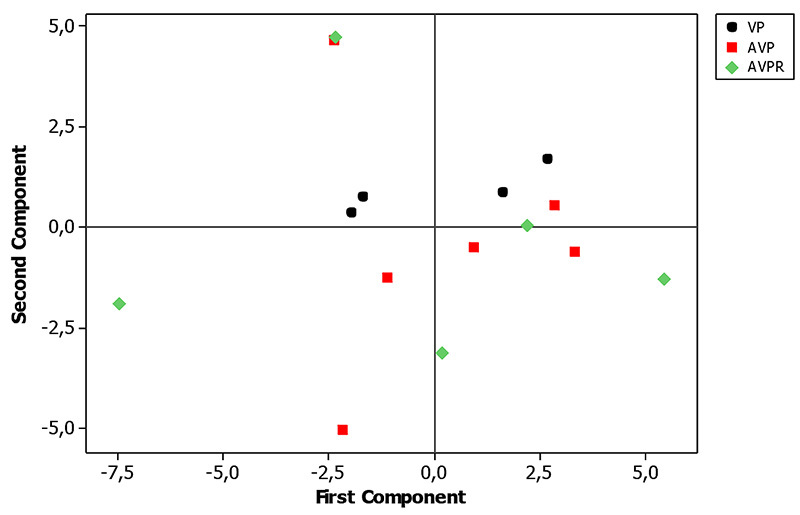
Figure 4. Principal component analysis of δ13C and δ15N values during experimental days 49 to 84.
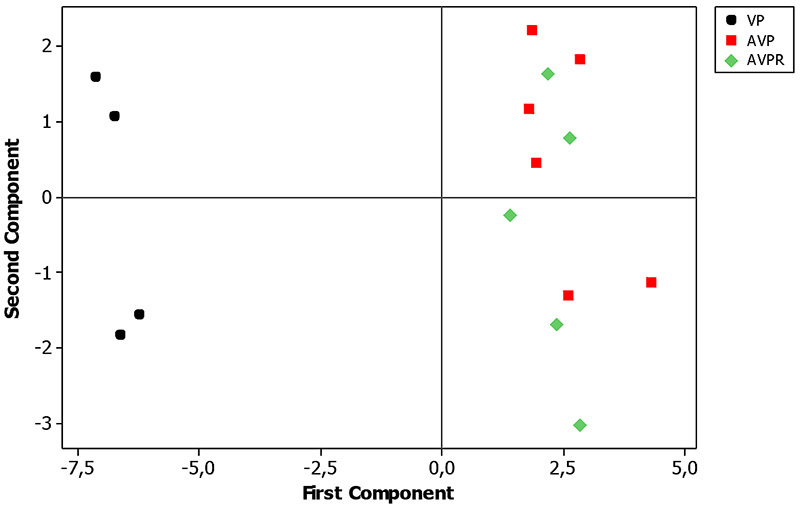
Total cell turnover or maximum incorporation of the diet, with MBM as a function of time in the AVPR group, for both 13C and 15N, occurred within approximately 60 days (Figures 1 and 2). AVPR and VP (control) groups presented similar isotopic values for the food profile at the 110th day (Figure 1 and 2), showing that during this period the MBM diet was fully eliminated in the AVPR group.
PCA analysis revealed the stability of 13C and 15N isotopic values when compared with the behavior of diet incorporation as a function of time in the AVPR group, revealing stability of 13C and 15N isotopic values (Figures 1, 2 and 5).
The percentage of 13C atoms exchanged for incorporating the MBM diet in buffalo serum was 98.9% in AVP and 98.0% in AVPR, whereas that of 15N was 97.7% in AVP and 97.5% in AVPR. For diet elimination in the AVPR group, the atom percentage exchanged was 98.5% for 13C and 99.7% for 15N (Table 1). These results reveal reliability of the data because they present a small margin of error, which guarantees precision in the detection of animal protein in buffalo serum.
Table 1. Percentages of 13C and 15N atoms exchanged during incorporation and elimination of animal protein diet in the AVP and AVPR groups.
| 13 C atoms exchanged (%) | 15 N atoms exchanged (%) | |||
|---|---|---|---|---|
| AVP | AVPR | AVP | AVPR | |
| Serum isotopic incorporation | 98.9 | 98.0 | 97.7 | 97.5 |
| Serum isotopic elimination | - | 98.5 | - | 99.7 |
AVP: Animal and Vegetable Protein group; AVPR: Animal and Vegetable Protein Removal group.
An animal protein diet have already been successfully traced in other end products, where the MBM isotopic values found for laying egg yolk and egg albumen, was -17.40 ‰ and -17.24 ‰, respectively [27,28].
In the present study, the MBM diet, administered for 27 days, presented maximum incorporation at approximately 60 days, showing elimination at 63 days. Silva et al., determined the time periods for the incorporation and elimination of the diet by the detection of MBM using IRMS in sheep serum and plasma, followed by the evaluation of the turnover. It was observed that MBM took 54 days to be incorporated and 53 days to be eliminated from sheep serum, which demonstrates that traceability of biological samples by this technique, might reveal the animal protein diet [4].
The factors considered - namely age, growth rate, and body mass of the animals may influence the rates of incorporation and elimination of a diet [24,32], which could explain the longer time observed for buffalo serum samples. Future studies to evaluate turnover of 13C and 15N in different tissues of ruminants should be investigated.
Conclusion
Zoonoses must be dealt with at the interface between human public health and veterinary public health. Prion diseases have caused not only great economic loss in the cattle industry of European countries but also provoked great public concern and put millions of people at risk and caused more than 160 human deaths [33].
Banning of supplemental feeding is generally considered a primary and necessary control strategy in an attempt to limit the transmission and spread of prion diseases [34]. Thus, the current study is particularly important because it shows, for the first time, that IRMS is sensitive and accurate for tracking the feeding profile of ruminants through blood analysis, thereby enabling investigation of the use of ABPs in ruminant feed worldwide.
Abbreviations
Not applicable.
Acknowledgements
The authors are grateful to the Center for Stable Isotopes (CIE) Bioscience Institute and Prof. Carlos Ducatti (in memoriam).
Footnotes
Availability of data and materials: Not applicable.
Funding: This work was supported IN PART by Coordination of Improvement of Higher Level Personnel (CAPES) Grant 23038.000823/2011-74 and São Paulo Research Foundation (FAPESP) Grant 2008/57411-4, Brazil. Moreover, this publication was supported in part by the Coordination for the Improvement of Higher Education Personnel (CAPES) through “Programa Editoração CAPES” - call No. 3/2016, grant No. 0722/2017, record No. 88881.142062/2017-01 and by the National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personnel (CAPES) through “Programa Editorial CNPq/CAPES” call No. 18/2018, grant No. 404770/2018-5.
Ethics approval: This study was conducted in accordance with the Ethical Principles in Animal Research of the Brazilian College of Animal Experimentation and was approved by the Ethics Committee for Animal Experimentation (protocol nº 78/ 2009) of the College of Veterinary Medicine and Animal Husbandry (FMVZ-UNESP), Brazil. Animal welfare was respected throughout experimental period, in accordance with the “five freedoms” defined by the Farm Animal Welfare Council.
Consent for publication: Not applicable.
References
- Bencsik A, Baron T. Histopathological studies of ‘‘CH1641-like’’ scrapie sources versus classical scrapie and BSE transmitted to ovine transgenic mice (TgOvPrP4) PLoS One. 2011;6(7):e22105. doi: 10.1371/journal.pone.0022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marruchella G, Ligios C, Di Guardo G. Age, scrapie status, PrP genotype and follicular dendritic cells in ovine ileal Peyer’s patches. Res Vet Sci. 2012;93(2):853–856. doi: 10.1016/j.rvsc.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Sobrova P, Ryvolova M, Adam V, Kizek R. Capillary electromigration based techniques in diagnostics of prion protein caused diseases. Electrophoresis. 2012;33(24):3644–3652. doi: 10.1002/elps.201200208. [DOI] [PubMed] [Google Scholar]
- da Silva DAF, Biscola NP, dos Santos LD, Sartori MMP, Denadai JC, da Silva ET, et al. Detecting animal by-product intake using stable isotope ratio mass spectrometry (IRMS) Vet J. 2016;217:119–125. doi: 10.1016/j.tvjl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Europe Commission Regulation EU Nº 56/2013. [7 may 2018];OJEU. 2013 21(3):1. http://www.eurl.craw.eu/img/page/Legislation/56-2013_EN.pdf. [Google Scholar]
- US Government Publishing Office Title 21, Chapter I, Subchapter E, Part 589 - Substances prohibited from use in animal food or feed. 2018. [7 may 2018]. http://www.ecfr.gov/cgi-bin/text-idx?SID=d39395bd805dd6dfc837d2a79ae27a18&mc=true&node=pt21.6.589&rgn=div5.
- Prado M, Berben G, Fumière O, van Duijn G, Mensinga-Kruize J, Reaney S, et al. Detection of ruminant meat and bone meals in animal feed by real-time polymerase chain reaction: result of an interlaboratory study. J Agric Food Chem. 2007;55(18):7495–7501. doi: 10.1021/jf0707583. [DOI] [PubMed] [Google Scholar]
- European Parliament Regulation (EC) Nº 999/2001 of the European Parliament and of the Council laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. OJEU. 2001;147:1–40. [Google Scholar]
- Europe Commission Regulation EC Nº 1234/2003 of 10 July 2003 amending Annexes I, IV and XI to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Regulation (EC) Nº 1326/2001 as regards transmissible spongiform encephalopathies and animal feeding. OJEU. 2003;173:6–14. [Google Scholar]
- Hunter N, Foster J, Chong A, McCutcheon S, Parnham D, Eaton S, et al. Transmission of prion diseases by blood transfusion. 11J Gen Virol. 2002;83:2897–2905. doi: 10.1099/0022-1317-83-11-2897. [DOI] [PubMed] [Google Scholar]
- Houston F, McCutcheon S, Goldmann W, Chong A, Foster J, Sisó S, et al. Prion diseases are efficiently transmitted by blood transfusion in sheep. Blood. 2008;112(12):4739–4745. doi: 10.1182/blood-2008-04-152520. [DOI] [PubMed] [Google Scholar]
- Prado M, Casqueiro J, Iglesias Y, Cepeda A, Barros-Velázquez J. Application of a polymerase chain reaction (PCR) method as a complementary tool to microscopic analysis for the detection of bones and other animal tissues in home-made animal meals. J Sci Food Agric. 2004;84(6):505–512. [Google Scholar]
- Ofori JA, Hsieh YH. Sandwich enzyme-linked immunosorbent assay for the detection of bovine blood in animal feed. J Agric Food Chem. 2007;55(15):5919–5924. doi: 10.1021/jf070034r. [DOI] [PubMed] [Google Scholar]
- Abbade LPF, Barraviera SRCS, Silvares MRC, Ferreira RS, Jr, Carneiro MTR, Medolago NB, et al. A new fibrin sealant derived from snake venom candidate to treat chronic venous ulcers. J Am Acad Dermatol. 2015;72(5):AB271 [Google Scholar]
- Biscola NP, Cartarozzi LP, Ulian-Benitz S, Barbizan R, Castro MV, Spejo AB, et al. Multiple uses of fibrin sealant for nervous system treatment following injury and disease. J Venom Anim Toxins incl Trop Dis. 2017;23:13. doi: 10.1186/s40409-017-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira RS, Jr , de Barros LC, Abbade LPF, Barraviera SRCS, Silvares MRC, de Pontes LG, et al. Heterologous fibrin sealant derived from snake venom: from bench to the bedside - an overview. J Venom Anim Toxins incl Trop Dis. 2017;23:21. doi: 10.1186/s40409-017-0109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahar B, Schmidt O, Moloney AP, Scrimgeour CM, Begley IS, Monahan FJ. Seasonal variation in the C, N and S stable isotope composition of retail organic and conventional Irish beef. Food Chem. 2008;106(3):1299–1305. [Google Scholar]
- Chesson LA, Podlesak DW, Thompson AH, Cerling TE, Ehleringer JR. Variation of hydrogen, carbon, nitrogen and oxygen stable isotope ratios in an American diet: fast food meals. J Agric Food Chem. 2008;56(11):4084–4091. doi: 10.1021/jf0733618. [DOI] [PubMed] [Google Scholar]
- Heaton K, Kelly SD, Hoogewerff J, Woolfe M. Verifying the geographical origin of beef: the application of multi-element isotope and trace element analysis. Food Chem. 2008;107(1):506–515. [Google Scholar]
- Cruz VC, Araújo PC, Sartori JR, Pezzato AC, Denadai JC, Polycarpo GV, et al. Poultry offal meal in chicken: traceability using the technique of carbon (13C/12C)-and nitrogen (15N/14N)-stable isotopes. Poult Sci. 2012;91(2):478–486. doi: 10.3382/ps.2011-01512. [DOI] [PubMed] [Google Scholar]
- Fossato da Silva DA, Biscola NP, Souza RMF, Caetano DA, Denadai JC, Sartori MMP, et al. Carbon-13 and Nitrogen-15 turnover in serum of bubaline donors of biological material for medical use. Toxicon. 2012;60(2):117 [Google Scholar]
- Schmidt O, Quilter JM, Bahar B, Moloney AP, Scrimgeour CM, Begley IS, et al. Inferring the origin and dietary history of beef from C, N and S stable isotope ratio analysis. Food Chem. 2005;91(3):545–549. [Google Scholar]
- Osorio MT, Moloney AP, Schmidt O, Monahan FJ. Beef authentication and retrospective dietary verification using stable isotope ratio analysis of bovine muscle and tail hair. J Agric Food Chem. 2011;59(7):3295–3305. doi: 10.1021/jf1040959. [DOI] [PubMed] [Google Scholar]
- Ducatti C, Martins CL, Arrigoni MB, Martins MB, Vieira LC, Jr , Denadai JC. Use of stable isotopes in ruminants. Braz J Anim Sci. 2011;40:68–75. [Google Scholar]
- Martinez MG, Ducatti C, Silva ET, Sant’Anna SS, Sartori MMP, Barraviera B. Does the rattle of Crotalus durissus terrificus reveal its dietary history? J Venom Anim Toxins incl Trop Dis. 2014;20(1):53. doi: 10.1186/1678-9199-20-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson KA, Clark RG. Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor. 1992;94(1):181–188. [Google Scholar]
- Denadai JC, Ducatti C, Pezzato AC, Carrijo AS, Caldara FR, Oliveira RP. Studies on carbon-13 turnover in eggs and blood of commercial layers. Braz J Poult Sci. 2006;8(4):251–256. [Google Scholar]
- Denadai JC, Ducatti C, Sartori JR, Pezzato AC, Móri C, Gottmann R, et al. The traceability of animal meals in layer diets as detected by stable carbon and nitrogen isotope analyses of eggs. Braz J Poult Sci. 2008;10(3):89–194. [Google Scholar]
- Ducatti C, Sartori MMP, Denadai JC, Costa VE, Pelícia VC, Macari M. Modelling and determination of metabolic pools by stable carbon isotopes in the avian duodenal mucosa and albumen. J Anim Physiol Anim Nutr. 2016;100:77–84. doi: 10.1111/jpn.12339. [DOI] [PubMed] [Google Scholar]
- Cerling TE, Ayliffe LK, Dearing MD, Ehleringer JR, Passey BH, Podlesak DW, et al. Determining biological tissue turnover using stable isotopes: the reaction progress variable. Oecologia. 2007;151(2):175–189. doi: 10.1007/s00442-006-0571-4. [DOI] [PubMed] [Google Scholar]
- Piasentier E, Valusso R, Camin F, Versini G. Stable isotope ratio analysis for authentication of lamb meat. Meat Sci. 2003;64(3):239–247. doi: 10.1016/S0309-1740(02)00183-3. [DOI] [PubMed] [Google Scholar]
- Zazzo A, Moloney AP, Monahan FJ, Scrimgeour CM, Schmidt O. Effect of age and food intake on dietary carbon turnover recorded in sheep wool. Rapid Commun Mass Spectrom. 2008;22(18):2937–2945. doi: 10.1002/rcm.3693. [DOI] [PubMed] [Google Scholar]
- Murphy FA. Emerging zoonoses: the challenge for public health and biodefense. Prev Vet Med. 2008;86(3-4):216–223. doi: 10.1016/j.prevetmed.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen A, van Beest FM, Brook RK. Impacts of wildlife baiting and supplemental feeding on infectious disease transmission risk: a synthesis of knowledge. Prev Vet Med. 2014;113(4):356–363. doi: 10.1016/j.prevetmed.2013.11.010. [DOI] [PubMed] [Google Scholar]


