Abstract
Background:
Physical training signals cardiac hypertrophy through PI3K as an upstream and Hand2 gene as a downstream agent. The present study aimed to find the role of PI3K and Hand2 gene in myocardial hypertrophy following interval and endurance training (ET).
Materials and Methods:
Twenty-four adult Wistar male rats (210–250 g) randomly divided into control, sham, high-intensity interval training (HIIT), and ET group. Swimming time in ET increased incrementally 30–75 min, whereas in HIIT, load/body weight, and time/rest ratio increased within 12 weeks. Heart morphometry, including left ventricle end systolic (LVESV) and Diastolic (LVEDV) volume, LV posterior wall (LVPW), stroke volume (SV), ejection fraction (EF), fraction shortening (%FS), pure heart weight (HW) and left ventricle weight (LVW), and PI3K and Hand2 gene expression were measured.
Results:
HW and LVW were significantly more than control after ET (P < 0.05) and HIIT (P < 0.05). Both of the training groups demonstrated significantly thicker LVPW (P < 0.05), SV (P < 0.05), and %FS (P < 0.05). Furthermore, PI3K concentration and Hand2 expression significantly increased in ET (P < 0.001; P < 0.001, respectively) and HIIT (P < 0.05; P < 0.001, respectively) compared to control.
Conclusion:
It can be concluded that this training protocol caused physiological hypertrophy in both of ET and HIIT groups, whereas HIIT can be more beneficial because of shorter training time.
Keywords: Endurance training, gene expression, high-intensity interval training, physiological hypertrophy, rat
INTRODUCTION
Chronic exercise training induces left ventricular (LV) hypertrophy known as physiological hypertrophy.[1,2] This adaptation compensates hemodynamic overload following increased venous return, LV posterior wall thickness and LV end diastolic volume[3] during physical activity associated with sarcomeres added in series and in parallel to lengthen the cardiac cell.[4] In general, these cardiac mass changes are supposed to increase LV stroke volume (SV), percent of ejection fraction (EF%), and cardiac output.[5]
Correspondingly, physiological hypertrophy is induced by regulatory triggers such as kinases activation that signal downstream processes. Alteration of these signaling pathways may enhance the reprogramming of cardiac gene factors which lead to cardiac gene expression and myocyte growth.[6] In this regard, it is widely accepted that PI3K signaling through Akt is an important regulator of membrane trafficking, adhesion, actin rearrangement, cell growth, and cell survival.[7,8] Interestingly, PI3K/Akt pathway upregulates crucial cardiomyocytes hypertrophic-inducing transcriptional activation of several genes, such as Hand2 and Gata4.[9,10]
Considering this signaling pathway, several studies reported that constitutive activation of PI3K increases heart weight/body weight (BW) ratio without any pathological sign.[7,11] Meanwhile, there are some evidences verifying that PI3K knock-out mice had smaller heart with decreased cardiac function.[7] In the downstream, the regulation of Hand2 (Heart and neural crest derivatives expressed 2) gene is a crucial trigger for encoding Hand2 protein. This gene in synergy with Gata4 upregulates cardiac-specific promoters[9] that results in cardiac cell proliferation, remodeling, and cardiac development,[9,10,12] while blocking Hand2 gene causes severe cardiovascular developmental malformations.[13,14,15] Taking the above findings into consideration, the synergy between an upstream (PI3K) and a downstream regulator (Hand2) of cardiac hypertrophy after different training regimens seems interesting. Although a few studies reported the accompany of PI3K in cardiac hypertrophy following endurance training (ET),[7,16] little is known about its role in cardiac remodeling after high-intensity interval training (HIIT). For the downstream, to the best of our knowledge, there is only one published scientific-based training method by Fathi and Gharakhanlou which reported increase in Hand2 gene expression after ET,[17] and no data exist about this mediating gene regarding to HIIT discipline. HIIT is characterized by repeated sessions of short-time intermittent efforts performed at relatively high intensities. This training type is a potent strategy to induce cardiac remodeling that might be resemble to changes associated with ET.[18] However, few existed studies focused on the benefits of HIIT due to shorter time, higher stimulation, and rapid phenotypic changes on cardiovascular rehabilitation[1] and athletic performance[16,18] compared to ET.[7,16]
Given the presumable changes in cardiac remodeling upregulated by HIIT or ET, the present study tested the hypothesis that 12 weeks of brief, intense interval, or continuous swimming training would regulate PI3K concentration and Hand2 gene expression and causes physiological hypertrophy of the cardiac tissue in rats.
MATERIALS AND METHODS
Experimental approach to the problem
Twenty-four adult male Wistar rats (weighing 210–250 g) obtained from Pasteur Institute of Iran, hospitalized and trained at the Exercise Physiology Laboratory-Shahid Rajaee Teacher Training University, in collective cages (three rats per cage) at 22°C ± 2°C and 12/12 h light/dark period, whereas they had free access to water and standard chewing rodent food ad libitum. After randomly dividing into (ET, n = 6), (HIIT, n = 6), Sham (SH, n = 6), and Control (C, n = 6), they accustomed to swimming (Animal Pool, Danesh. Salar. Co, Tehran, Iran) with moderate stable current generated by circulating water pump (to avoid rats to stay on floatability) for 5 days before the main protocol starts. Swimming training executed in individual chambers (60 cm height × 30 cm diameter) with water temperature at 32°C ± 1°C while the surface of the walls of tank was clear and smooth. The study was approved by Ethics Committee of Iran University of Medical Sciences (IUMS.95.06.10.04) and conformed to the Declaration of Helsinki.
Procedure
Training protocol
The swimming training was conducted for 12 weeks, 5 days per week (rest at Monday and Friday) between 11.00 a.m. and 15.00 p.m. The load was attached to the animal lower back while individually adjusted every weekend according to animal body mass. The progression of load, time of interval (ratio of rest to workout), and time of continuous training were adopted from da Rocha et al. research protocols.[19] Regarding to load selection, Gobatto et al. reported that lactate threshold was achieved by bearing a weight in 5%–6% of rats’ body mass.[20] Therefore, the continuous protocol in this study was done without any external load and interval protocol was considered as high-intensity training (incremental load between 5% and 16% of individual body mass). Table 1 shows swimming training protocols. Sham group was put on the water for 3 min without any artificial current on all of the training days, whereas control stayed at the cages in animal house all the period of training.
Table 1.
12-week swimming training protocol in endurance training and high-intensity interval training
| ET | HIIT | |||||
|---|---|---|---|---|---|---|
| Week | Swimming time (min) | Week | Swimming time (s) | Rest time (s) | Repetitions | Percentage body weight load (g) |
| 1 | 30 | 1 | 60 | 60 | 5 | 2–5 |
| 2 | 45 | 2 | 60 | 60 | 5 | 7 |
| 3 | 60 | 3 | 60 | 60 | 5 | 8 |
| 4 | 60 | 4 | 60 | 60 | 5 | 10 |
| 5 | 60 | 5 | 20 | 10 | 14 | 13 |
| 6 | 60 | 6 | 20 | 10 | 14 | 14 |
| 7 | 60 | 7 | 20 | 10 | 14 | 15 |
| 8 | 60 | 8 | 20 | 10 | 14 | 16 |
| 9 | 60 | 9 | 20 | 10 | 14 | 16 |
| 10 | 60 | 10 | 20 | 10 | 14 | 16 |
| 11 | 75 | 11 | 20 | 10 | 16 | 16 |
| 12 | 75 | 12 | 20 | 10 | 16 | 16 |
ET=Endurance training; HIIT=High-intensity interval training
Tissue sampling and heart structure
Forty-eight hours after finishing the last training session, animals were anesthetized using ketamine/xylasine (5 and 50 ml/kg, respectively). Rats were sacrificed after measuring the length of body from the mouth to the root of the tail. Three milliliters of blood sample was donated from LV and cardiac tissue was harvested and weighed after washing completely with ddH2O to assess heart weight (HW) and heart weight/HW/BW. Immediately, the atriums and right ventricle were trimmed off and the LV weighed, snap frozen in liquid nitrogen (in RNAase free tubes) and stored at − 80°C until further use to assess LV weight (LVW), LVW/BW and LVW to body surface area (LVW/BSA). To count BSA, we used the procedure mentioned with Farriol et al. for rats.[21]
RNA extraction, cDNA synthesis, and real time-polymerase chain reaction
Tissue samples were weighed (40–50 mg) and the total RNA was isolated according to the AccuZol™ manufacturer's instructions (BIONEER) from LV and resolved in 50 μl RNase-free water. The 260/280 nm absorbance ratio was used to determine the quality of RNA samples in a NanoDrop 2000c(r) Spectrophotometer (Thermo Scientific, Wilmington, DE, EUA). Samples with ratios <1.7 were not included in the study. cDNA for Hand2 as Target gene and β-actin as reference gene were then synthesized using 2X real time-polymerase chain reaction (RT-PCR) Pre-Mix (Taq, Biofact) protocol. Then, 1 μg of synthesized cDNA utilized for SYBR Green-based RT-PCR through using two-step procedure of 2X RT-PCR Master Mix (Biofact) protocol. In this protocol, 5.00 μℓ Master Mix, 3.5 μℓ Deps water, and 0.5 μℓ Hand2 Primer (0.25 Forward and 0.25 Reverse) gathered with 1.00 μℓ template cDNA. Thermocycling parameters were as follows: One cycle at 95°C for 10 min, one cycle at 95°C for 20 s, and one cycle at 58°C for 45 s followed by 40 amplification cycles at 95°C for 30 s. Values from β-actin were used to loading normalization for each sample. Relative change in expression was determined using the ΔΔCt method relative to gene expression values for control (β-actin).
Primer pairs that were used are as follows: Hand2 Forward GACACCAAACTCTCCAAA and Reverse TTCTTCCTCTTCTCCTCT; β -actin Forward GGAGAAGATTTGGCACCACAC and Reverse GGATGGCTACGTACATGGCTG.
PI3K concentration assay
PI3K enzymatic concentration was determined using a competitive ELISA kit (CK-E 30310, Hangzhou East Biopharm Co). The blood that was taken directly from LV (3 ml) was replaced gently into EDTA Glass tube and centrifuged to separate plasma from blood cells. Extracted plasma (~ 1.5 ml) was assayed for PI3k concentration by ELISA, according to the manufacturer's instructions.
Echocardiogram recording and analyzing
Rat echocardiography was performed 24 h after last training session as previously described.[22] Briefly, following the induction of anesthesia for echocardiography (Ketamine 5 ml/kg-Xylasine 50 ml/kg), the anterior chest was shaved and rats were placed in the left lateral decubitus position. A rectal temperature probe was placed and the body temperature was carefully maintained between 37°C and 37.5°C with a heating pad throughout the study.
Conventional M-mode and two-dimensional echocardiography images (GE-Vingmed Ultrasound, USA) were obtained in the parasternal long-and short-axis views with a 10-MHz probe. A representative cross-sectional echocardiogram is shown in Figure 1. Using these recordings, left ventricle end diastolic volume (LVEDV), SV, EF%, fractional shortening (FS%), LV posterior wall thickness in systole (LVPWs), and diastole (LVPWd) were measured. One operator, blinded to genotype and treatment, performed all echocardiograms using dedicated software (EchoPac v113; GE Healthcare).
Figure 1.
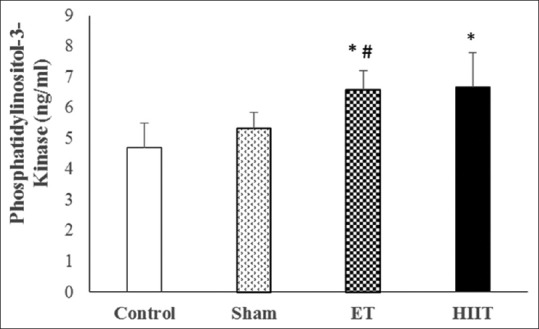
Changes of phosphatidylinositol-3-kinase concentration in trained (high-intensity interval training and endurance training) and untrained (Sham and Control) rats (values are means ± standard deviation). *P ≤ 0.001 compared with Control group; #P ≤ 0.05 compared with sham group
Statistical analysis
Data were expressed as mean ± standard deviation. The normality of data was assessed using the Shapiro–Wilk test. To compare the cardiac performance characteristics, PI3K concentration and Hand2 gene expression values between ET, HIIT, Sham and C groups, one-way analysis of variance utilized followed by the Tukey honestly significant difference post hoc test for pair-wise comparison. The statistical significance level was set at P ≤ 0.05 for all comparisons.
RESULTS
The Shapiro–Wilk test approved the normality of the data distribution. Swimming training was performed in ET and HIIT for 12 weeks. PI3K concentration, Hand2 gene expression, echocardiography characteristics, BW, HW, LV, and relative weights of the heart and LV were assessed.
PI3K concentration
The findings approve that PI3K in ET (6.58 ng/ml; P = 0.00) and HIIT (6.67 ng/ml; P = 0.00) is significantly different form control (4.70 ng/ml). Furthermore, there was a statistically significant difference between Sham (5.32 ng/ml) and HIIT (P = 0.04) but not ET. However, there was no statistically significant difference between HIIT and ET [Figure 1].
Relative gene expression changes
The differences between Hand2 gene expression of HIIT and ET (Hand2 Change folds in HIIT 6.76; P = 0.00 and ET 6.15; P = 0.01) with Control (change fold 1) were statistically significant. Furthermore, there were significant differences between HIIT (P = 0.01) and ET (P = 0.00) with Sham (Hand2 change fold 1.05). However, the difference between HIIT and ET was not significant [Figure 2].
Figure 2.
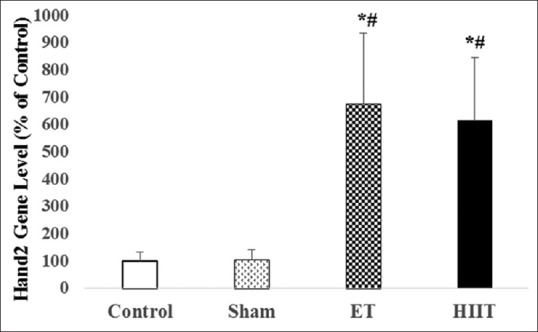
Expression of Hand2 in the left ventricle of trained (high-intensity interval training or endurance training) and untrained (Sham and Control) rats (values are means ± standard deviation). *P ≤ 0.001 compared with control group; #P ≤ 0.001 compared with sham group
Heart and left ventricular structural changes
As shown in Figure 3a, there were insignificant changes in BW between Control (335 g) in comparison with Sham (310 g), ET (308 g), and HIIT (298 g). HW was significantly different between ET (P = 0.01) and HIIT (P = 0.01) with Control. Furthermore, the difference between ET (P = 0.04) and HIIT (P = 0.03) with Sham was significant. LVW revealed significant differences between HIIT (P < 0.01) and ET (P < 0.04) with Sham, whereas control was significantly different only with HIIT (P = 0.01). The normalized LVW/BW was significantly different between HIIT (P = 0.00) and ET (P = 0.01) with control. These differences were significant between HIIT (P = 0.01) and ET (P = 0.01) with Sham. Furthermore, the normalized ratio of LVW/BSA showed statistically significant differences between HIIT (P = 0.02) and ET (P = 0.02) with Control. These differences were significant between HIIT (P = 0.03) and ET (P = 0.04) with Sham [Figure 3b].
Figure 3.
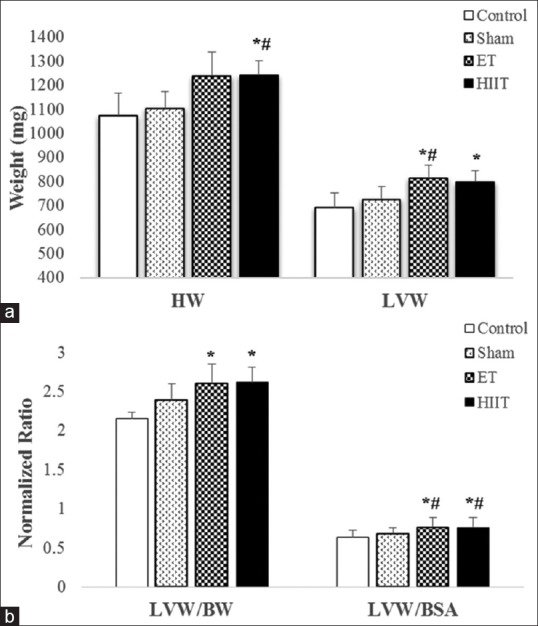
(a) Heart weight and left ventricle weight of rats in high-intensity interval training, endurance training, Sham or Control groups; (b) left ventricle weight to body weight ratio and left ventricle weight to body surface area ratio of rats in high intensity interval training, endurance training, Sham and Control groups (values are means ± standard deviation). *P ≤ 0.05 compared with Control group; #P ≤ 0.05 compared with sham group
Cardiac functional changes
Echocardiography was performed in anesthetized rats. The results are summarized in Table 2. Both of the training groups demonstrated significantly thicker LVPWs in comparison to Control (HIIT: P = 0.00, ET: P = 0.01) and Sham (HIIT: P = 0.00, ET: P = 0.02). Furthermore, there were significant differences in LVEV of systolic (HIIT: P = 0.02; ET: P = 0.01) and diastolic (HIIT: P = 0.01; ET: P = 0.03) phases in both swimming-trained groups in comparison to Control. Furthermore, LVEDV was significantly different between HIIT (0.736 ml; P = 0.01) and ET (0.535 ml; P = 0.03) in comparison to Control (0.368 ml).
Table 2.
Observation of echocardiographic characteristics between groups (values are means±standard deviation)
| Observation | Mean±SD | |||
|---|---|---|---|---|
| Control | Sham | Endurance | HIIT | |
| LVEDV | 0.380±0.073 | 0.368±0.120* | 0.736±0.147† | 0.545±0.196*,‡ |
| LVPWs | 0.240±0.020 | 0.251±0.011 | 0.280±0.012† | 0.286±0.022† |
| LVPWd | 0.152±0.004 | 0.163±0.013 | 0.160±0.008 | 0.168±0.011 |
| SV | 0.284±0.055 | 0.303±0.077 | 0.536±0.143† | 0.524±0.119* |
| EF (%) | 82.83±0.205 | 82.26±3.32 | 86.16±0.74 | 81.03±4.09 |
| FS (%) | 44.38±0.163 | 45.10±4.65 | 51.45±3.46* | 51.04±4.88* |
*Significant difference with control (P≤0.05), †Significant difference with sham (P≤0.05), ‡Significant difference with ET (P≤0.05). SD=Standard deviation; HIIT=High-intensity interval training; SV=Stroke volume; EF=Ejection fraction; FS=Fraction shortening; LV=Left ventricle; LVEDV=LV end diastolic volume; LVPWs=LV posterior walls; LVPWd=LV posterior wall
SV was significantly elevated throughout ET (0.536 ml) and HIIT (0.524 ml) rather than Control (0.284 ml) (HIIT: P = 0.04, ET: P = 0.04). However, EF was not significantly increased. Furthermore, FS% was significantly higher in HIIT (51.04%) and ET (51.45%) than Control (44.38%) (HIIT: P = 0.04, ET: P = 0.03).
DISCUSSION
This study was designed to determine if ET and HIIT mediate PI3K and Hand2 gene expression that accompany physiological cardiac hypertrophy. Increases in HW, LVW, and LVW/BW after both of the training schedules approved physiologic hypertrophy of the heart. This adaptation regulates with different intrinsic kinase activations together with a wide range of the gene expressions. In this regard, PI3K is assumed to be a key factor for programming this hypertrophy. Furthermore, significant changes in LVEDV, LV posterior wall (LVPW), SV, and FS% approved the functional improvement due to physiologic hypertrophy. These observations provided the rationale for our hypothesis that both of the training schedules are efficient for triggering the cardiac hypertrophy.
The increases in LVEDd together with greater SV and FS% in ET group seems to be made by more blood return to the heart because of muscle vasomotor pump and Frank-Starling Mechanism which is an adaptive response to continuous hypertrophic stimulations.[3] It is in accordance with studies that noted ET causes physiological hypertrophy in the heart specially in LV.[2,23,24]
To certify this, findings from echocardiography showed thicker LVPWs, increased LVEDV, higher SV, FS%, and EF in ET rather than control and Sham. In line with these findings, a prior study revealed that swimming, cycling, rowing, and running increased LV wall thickness, SV, and dilatation of LV.[5]
In addition, we showed that LVEDV is significantly higher in HIIT than ET. It is not in accordance with the biggest LVEDV in ET[24,25] and decreased LVEDV after interval training.[4] Meanwhile, several studies declared that exercise training does not significantly change structural and functional characters same as LVDd, LVDs, and FS in adult rats,[22,25,26,27] whereas increased EF after both of endurance and interval running trainings was reported in a study.[4] Similarly, several studies reported that exercise training had no significant effect on systolic septal wall thickness, diastolic septal wall thickness, systolic posterior wall thickness, and diastolic posterior wall thickness,[27,28] whereas others found greater LVPW in athletes than controls.[24] Different heredities of physical activities (running on treadmill or ground vs. swimming) might be the reason of these discrepancies.
In HIIT trained rats, similar adaptations seem to establish stronger and thicker septal wall of the heart. Reports of Wolfe et al. (1978) and West (1998) for increased LVEDV, more rapid LV diastolic filling and enhanced systolic contractility in athletes compared to nonathletes confirm these findings.[29,30] Contrarily, Adler et al. and Vinereanu et al. reported no change in systolic and diastolic function after HIIT between athletes and nonathletes. They presumed that this is related to enhanced early relaxation of myocardia in athletes.[31,32]
In addition to mentioned findings of this study, EF% in ET and HIIT was not significantly more than Control and Sham. This finding seems to be in contrast with higher LVPW, LVED, and SV in both training groups. It might be as a cause of anesthetization. As mentioned in Rottman et al., the systolic phase is more influenced from anesthetizes than diastolic phase.[33] This reverse influence may cause similar %EF in trained and untrained rats.
Other main findings of present study about kinases concentration and Hand2 gene expression showed significant increases after both of physical trainings. In this line, previous evidences approved the role of PI3K and Hand2 gene on cardiomyocytes growth.[8,9,13,14]
For example, in endurance swimming trained mice, McMullen and Jennings reported 32% increase in HW/BW in nontransgenic mice and 39% increase in cnPI3K mice after 4 weeks of training.[23] Furthermore, Shioi et al. showed significant structural and functional increases in cardiac PI3K activity, HW/BW and LVPWd but not FS% and LV diameters in caPI3K transgenic mice.[11] Contrarily, PI3K activity was decreased in dnPI3K transgenic mice and HW/BW decreased by 20%, PI3K decreased by 70% and cardiac function was devastated.[34] In a study by Lu et al. (2015) that compared HIIT and ET, although the amount of PI3K was less in training groups compared to myocardial infarction (MI), but FS% and EF% were increased in ET and HIIT compared to MI.[16] Even though cardiac structural characteristics of mentioned study were in line with the present findings, EF% and FS% increased more significantly in HIIT than ET. The difference between the participants of this study and this mentioned article (healthy rats vs. MI rats) assumes to be the reason of different results for PI3K.
To the best of our knowledge, this is the first study which investigates the effect of HIIT on Hand2 gene expression. In the field of exercise biology, only one research study has been investigated the effects of treadmill running (ET) on Hand2 Expression who found significant increase in Hand2 gene, HW and LVW in trained than untrained rats.[17] Considering the role of this gene on cardiac gene programming, remodeling and development,[9,13,14] it can be concluded that 6-fold increased expression in training groups after significant increase in PI3K might led to cardiac development known as “athlete's heart.”[2] Accordingly, present findings confirm several lines of evidences attesting that Hand2 acts as a key regulator gene associated with a large number of genes which are functioning during heart development and cardiomyocytes cushion[9,35] and blocking this gene causes cardiovascular developmental malformations.[13,14,15]
CONCLUSION
Collectively, the present results provided the rationale for our hypothesis that physical training in both types of endurance (alactic) and high-intensity interval (lactic) swimming regimens led to increased PI3K concentration and Hand2 gene expression which are crucial for cardiac hypertrophy. These considerations approved by the differences in heart and LVWs and echocardiography characters as well. Although mostly same results between different training schedules approved the benefits of both training regimens on physiological hypertrophy, the benefits of HIIT due to shorter time (~234 min comparing to ET executed for ~ 3525 min) and same advantage are debatable.[18] Accumulation of lactate during HIIT[20] assume to create similar responses after this training regimen compared to ET. Based on this rationale, we recommend HIIT as a more beneficial training regimen than ET.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We are thankful of constructive and scientific assistance of Dr. Golaab and Dr. Barati who devoted those time to promote the setting of gene expression procedure. All procedures were approved by Research Ethics Committee of Iran University of Medical Sciences (IUMS,95,06,10,04) in accordance with the declaration of Helsinki for animals.
REFERENCES
- 1.Haram PM, Kemi OJ, Lee SJ, Bendheim MØ, Al-Share QY, Waldum HL, et al. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res. 2009;81:723–32. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weeks KL, McMullen JR. The athlete's heart vs. the failing heart: Can signaling explain the two distinct outcomes? Physiology (Bethesda) 2011;26:97–105. doi: 10.1152/physiol.00043.2010. [DOI] [PubMed] [Google Scholar]
- 3.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336–44. doi: 10.1161/01.cir.101.3.336. [DOI] [PubMed] [Google Scholar]
- 4.Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: A randomized study. Circulation. 2007;115:3086–94. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 5.Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991;324:295–301. doi: 10.1056/NEJM199101313240504. [DOI] [PubMed] [Google Scholar]
- 6.Zhou H, Dickson ME, Kim MS, Bassel-Duby R, Olson EN. Akt1/protein kinase B enhances transcriptional reprogramming of fibroblasts to functional cardiomyocytes. Proc Natl Acad Sci U S A. 2015;112:11864–9. doi: 10.1073/pnas.1516237112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A. 2003;100:12355–60. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toker A, Cantley LC. Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature. 1997;387:673–6. doi: 10.1038/42648. [DOI] [PubMed] [Google Scholar]
- 9.Dai YS, Cserjesi P, Markham BE, Molkentin JD. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J Biol Chem. 2002;277:24390–8. doi: 10.1074/jbc.M202490200. [DOI] [PubMed] [Google Scholar]
- 10.Laurent F, Girdziusaite A, Gamart J, Barozzi I, Osterwalder M, Akiyama JA, et al. HAND2 target gene regulatory networks control atrioventricular canal and cardiac valve development. Cell Rep. 2017;19:1602–13. doi: 10.1016/j.celrep.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, et al. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–48. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akazawa H, Komuro I. Roles of cardiac transcription factors in cardiac hypertrophy. Circ Res. 2003;92:1079–88. doi: 10.1161/01.RES.0000072977.86706.23. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16:154–60. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- 14.Thattaliyath BD, Livi CB, Steinhelper ME, Toney GM, Firulli AB. HAND1 and HAND2 are expressed in the adult-rodent heart and are modulated during cardiac hypertrophy. Biochem Biophys Res Commun. 2002;297:870–5. doi: 10.1016/s0006-291x(02)02297-0. [DOI] [PubMed] [Google Scholar]
- 15.Vincentz JW, Firulli BA, Lin A, Spicer DB, Howard MJ, Firulli AB. Twist1 controls a cell-specification switch governing cell fate decisions within the cardiac neural crest. PLoS Genet. 2013;9:e1003405. doi: 10.1371/journal.pgen.1003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu K, Wang L, Wang C, Yang Y, Hu D, Ding R, et al. Effects of high-intensity interval versus continuous moderate-intensity aerobic exercise on apoptosis, oxidative stress and metabolism of the infarcted myocardium in a rat model. Mol Med Rep. 2015;12:2374–82. doi: 10.3892/mmr.2015.3669. [DOI] [PubMed] [Google Scholar]
- 17.Fathi M, Gharakhanlou R. The effect of endurance activity on left ventricle Hand2 gene expression in wistar male rat. Exerc Physiol. 2015;7:57–68. [Google Scholar]
- 18.Sheykhlouvand M, Khalili E, Agha-Alinejad H, Gharaat M. Hormonal and physiological adaptations to high-intensity interval training in professional male canoe polo athletes. J Strength Cond Res. 2016;30:859–66. doi: 10.1519/JSC.0000000000001161. [DOI] [PubMed] [Google Scholar]
- 19.da Rocha GL, Crisp AH, de Oliveira MR, da Silva CA, Silva JO, Duarte AC, et al. Effect of high intensity interval and continuous swimming training on body mass adiposity level and serum parameters in high-fat diet fed rats. ScientificWorldJournal. 2016;2016:2194120. doi: 10.1155/2016/2194120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gobatto CA, de Mello MA, Sibuya CY, de Azevedo JR, dos Santos LA, Kokubun E. Maximal lactate steady state in rats submitted to swimming exercise. Comp Biochem Physiol A Mol Integr Physiol. 2001;130:21–7. doi: 10.1016/s1095-6433(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 21.Farriol M, Rossell J, Schwar S. Body surface area in Sprague-Dawley rats. J Anim Physiol Anim Nut. 1997;77:61–5. [Google Scholar]
- 22.Dayan A, Feinberg MS, Holbova R, Deshet N, Scheinowitz M. Swimming exercise training prior to acute myocardial infarction attenuates left ventricular remodeling and improves left ventricular function in rats. Ann Clin Lab Sci. 2005;35:73–8. [PubMed] [Google Scholar]
- 23.McMullen JR, Jennings GL. Differences between pathological and physiological cardiac hypertrophy: Novel therapeutic strategies to treat heart failure. Clin Exp Pharmacol Physiol. 2007;34:255–62. doi: 10.1111/j.1440-1681.2007.04585.x. [DOI] [PubMed] [Google Scholar]
- 24.Venckunas T, Lionikas A, Marcinkeviciene JE, Raugaliene R, Alekrinskis A, Stasiulis A. Echocardiographic parameters in athletes of different sports. J Sports Sci Med. 2008;7:151–6. [PMC free article] [PubMed] [Google Scholar]
- 25.Hayward R, Lien CY. Echocardiographic evaluation of cardiac structure and function during exercise training in the developing sprague-dawley rat. J Am Assoc Lab Anim Sci. 2011;50:454–61. [PMC free article] [PubMed] [Google Scholar]
- 26.Groban L, Jobe H, Lin M, Houle T, Kitzman DA, Sonntag W. Effects of short-term treadmill exercise training or growth hormone supplementation on diastolic function and exercise tolerance in old rats. J Gerontol A Biol Sci Med Sci. 2008;63:911–20. doi: 10.1093/gerona/63.9.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wisløff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2001;280:H1301–10. doi: 10.1152/ajpheart.2001.280.3.H1301. [DOI] [PubMed] [Google Scholar]
- 28.Hydock DS, Lien CY, Schneider CM, Hayward R. Effects of voluntary wheel running on cardiac function and myosin heavy chain in chemically gonadectomized rats. Am J Physiol Heart Circ Physiol. 2007;293:H3254–64. doi: 10.1152/ajpheart.00801.2007. [DOI] [PubMed] [Google Scholar]
- 29.West JB. Left ventricular filling pressures during exercise: A cardiological blind spot? Chest. 1998;113:1695–7. doi: 10.1378/chest.113.6.1695. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe LA, Cunningham DA, Davis GM, Rosenfeld H. Relationship between maximal oxygen uptake and left ventricular function in exercise. J Appl Physiol Respir Environ Exerc Physiol. 1978;44:44–9. doi: 10.1152/jappl.1978.44.1.44. [DOI] [PubMed] [Google Scholar]
- 31.Adler Y, Fisman EZ, Koren-Morag N, Tanne D, Shemesh J, Lasry E, et al. Left ventricular diastolic function in trained male weight lifters at rest and during isometric exercise. Am J Cardiol. 2008;102:97–101. doi: 10.1016/j.amjcard.2008.02.105. [DOI] [PubMed] [Google Scholar]
- 32.Vinereanu D, Florescu N, Sculthorpe N, Tweddel AC, Stephens MR, Fraser AG. Left ventricular long-axis diastolic function is augmented in the hearts of endurance-trained compared with strength-trained athletes. Clin Sci (Lond) 2002;103:249–57. doi: 10.1042/cs1030249. [DOI] [PubMed] [Google Scholar]
- 33.Rottman JN, Ni G, Khoo M, Wang Z, Zhang W, Anderson ME. Temporal changes in ventricular function assessed echocardiographically in conscious and anesthetized mice. J Am Soc Echocardiogr. 2003;16:1150–7. doi: 10.1067/S0894-7317(03)00471-1. [DOI] [PubMed] [Google Scholar]
- 34.Hildick-Smith DJ, Shapiro LM. Echocardiographic differentiation of pathological and physiological left ventricular hypertrophy. Heart. 2001;85:615–9. doi: 10.1136/heart.85.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holler KL, Hendershot TJ, Troy SE, Vincentz JW, Firulli AB, Howard MJ. Targeted deletion of Hand2 in cardiac neural crest-derived cells influences cardiac gene expression and outflow tract development. Dev Biol. 2010;341:291–304. doi: 10.1016/j.ydbio.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


