Abstract
Numerous health benefits are attributed to the n-3 long-chain PUFA (n-3 LCPUFA); EPA and DHA. A systematic literature review was conducted to investigate factors, other than diet, that are associated with the n-3 LCPUFA levels. The inclusion criteria were papers written in English, carried out in adult non-pregnant humans, n-3 LCPUFA measured in blood or tissue, data from cross-sectional studies, or baseline data from intervention studies. The search revealed 5076 unique articles of which seventy were included in the qualitative synthesis. Three main groups of factors potentially associated with n-3 LCPUFA levels were identified: (1) unmodifiable factors (sex, genetics, age), (2) modifiable factors (body size, physical activity, alcohol, smoking) and (3) bioavailability factors (chemically bound form of supplements, krill oil v. fish oil, and conversion of plant-derived α-linolenic acid (ALA) to n-3 LCPUFA). Results showed that factors positively associated with n-3 LCPUFA levels were age, female sex (women younger than 50 years), wine consumption and the TAG form. Factors negatively associated with n-3 LCPUFA levels were genetics, BMI (if erythrocyte EPA and DHA levels are <5·6 %) and smoking. The evidence for girth, physical activity and krill oil v. fish oil associated with n-3 LCPUFA levels is inconclusive. There is also evidence that higher ALA consumption leads to increased levels of EPA but not DHA. In conclusion, sex, age, BMI, alcohol consumption, smoking and the form of n-3 LCPUFA are all factors that need to be taken into account in n-3 LCPUFA research.
Key words: Fatty acid status, Effects, Determinants, Healthy adults, Review studies, Measurement, Implications
n-3 Long-chain PUFA (n-3 LCPUFA) are fatty acids with twenty or more carbons, and they are the elongation and desaturation products of the essential fatty acid α-linolenic acid (ALA, 18 : 3n-3). Whilst there are emerging health benefits of docosapentaenoic acid (DPA, 22 : 5n-3)( 1 ), the vast majority of health benefits have been attributed to the n-3 LCPUFA EPA (20 : 5n-3) and DHA (22 : 6n-3)( 2 ).
n-3 LCPUFA have been shown to be important for neurological development in very early pregnancy( 3 ), during later pregnancy and lactation( 4 ) and cardiovascular health( 5 , 6 ) and there is also emerging evidence for mental health( 7 ). Several mechanisms have been suggested( 8 ), such as their structural role in the cell membrane influencing signal transduction, stimulating neuronal growth, influencing neurotransmitter release and facilitating glucose uptake from the endothelial cells into the brain. n-3 LCPUFA are also important precursors of the eicosanoids, resulting in reduced blood clotting and increased blood flow( 8 ). DHA is a precursor of docosanoids such as resolvins and maresins, resulting in anti-inflammatory effects( 9 ) and neuroprotectins which protect neurons( 8 ).
The aforementioned potential health benefits have been observed from a wide variety of evidence including epidemiological, observational studies and randomised controlled trials. However, many studies have failed to measure the n-3 LCPUFA in blood or tissue, and this may severely limit the interpretations of the results as these n-3 LCPUFA might be influenced by many factors besides intake.
It is well established that diet and supplementation with n-3 LCPUFA have the largest impact on n-3 LCPUFA levels( 10 ); however, research has indicated that factors other than diet also play a role( 11 ). As researchers may not be aware of the many non-dietary factors associated with the n-3 LCPUFA levels per se, and the way these can influence the study outcomes, the aims of the present paper are to (1) report the results of a systematic literature review of the well-described non-dietary factors that are associated with the n-3 LCPUFA levels, (2) identify important non-dietary factors that should be considered in future studies and (3) discuss whether measuring n-3 LCPUFA levels is necessary in research that assesses the health benefits of n-3 LCPUFA.
Methods
Search strategy
A Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) systematic literature search was conducted using four different electronic databases (ProQuest, Medline, Web of Science and Cochrane). The search was conducted in June 2017 and covered all years up to June 2017. Appropriate truncation and relevant indexing terms were used. Search terms were related to (1) n-3 LCPUFA (e.g. n-3 fatty acids, EPA, DHA) and (2) factors or determinants associated with/influencing n-3 LCPUFA levels (e.g. sex, age, genetics, body size, physical activity, alcohol and smoking). An outline of the search strategy is available in online Supplementary Table S1.
Comparison of n-3 long-chain PUFA levels across studies
For the present review ‘n-3 LCPUFA levels’ are used as an umbrella term to describe the n-3 fatty acids with twenty or more carbon atoms in any blood or tissue fractions measured. We do not focus on DPA as it appears to have a poor association with diet in epidemiological studies (see Fig. 1(c) in Sullivan et al. ( 12 )). Please note that various comparable terminologies exist in the literature, including the Holman index; the Lands highly unsaturated fatty acids( 13 ); long-chain n-3 PUFA( 14 ) and the Harris, von Schacky (HS)-n-3 index( 15 ).
Fig. 1.
Flow diagram of systematic literature review search. The flow diagram outlines the identification, screening, eligibility and inclusion process of the systematic literature search. n-3 LCPUFA, n-3 long-chain PUFA.
For comparison between different studies, we used the available n-3 LCPUFA data and re-calculated them into erythrocyte EPA and DHA levels using the equations developed by Stark et al. ( 16 ) where applicable.
Inclusion and exclusion criteria
The search results were screened based on the titles and abstracts. Titles and abstracts which suggested the study identified one or more factors that are associated with the n-3 LCPUFA levels were selected and screened for eligibility. Research studies met the inclusion criteria if (1) they were written in the English language, (2) they were conducted in humans, (3) the participants were at least 18 years of age, (4) the participants were not pregnant, (5) the n-3 LCPUFA levels were reported (EPA or DHA or both) and (6) they were cross-sectional studies or were intervention studies that included baseline data; the results from the effects of n-3 LCPUFA intervention studies were excluded (except for the factor ‘bioavailability’, because intervention studies are the only way to determine this). In addition, (7) relevant previous review publications were included if they focused on factors associated with/influencing the n-3 LCPUFA levels. In that case, only additional publications published after the release date of the review publications on this respective factor were included. Publications that did not meet these criteria based on abstract review were excluded, and those that did were read in detail to confirm their inclusion. Further studies were then obtained through hand searching the reference lists of these articles and applying the above eligibility criteria. Quality checks were performed and consensus on scores agreed on by all authors, using either the National Heart Lung and Blood Institute ‘Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies’ (at https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools)( 17 ) or the Effective Public Health Practice Project ‘Quality Assessment Tool For Quantitative Studies’ (at https://merst.ca/wp-content/uploads/2018/02/quality-assessment-tool_2010.pdf)( 18 ), depending on the type of study.
Review of the literature
Eligible articles were categorised into groups, according to the factors that they covered. The groups were unmodifiable factors – ‘sex’, ‘genetics’ and ‘age’; modifiable factors – ‘body size’, ‘physical activity’, ‘alcohol’ and ‘smoking’; and bioavailability factors – ‘chemically bound form of supplement’, ‘krill oil v. fish oil’ and ‘conversion of plant-derived ALA to n-3 LCPUFA’. Some articles covered more than one factor and were therefore included in each group that they represented.
Four review articles were identified, wherein one evaluated the association of sex with n-3 LCPUFA levels( 19 ) and three reviewed the bioavailability factors( 20 – 22 ). We therefore did not execute a full systematic review of the factors sex and bioavailability.
Results
The search returned 10 275 articles and after removal of duplicates 5076 articles remained. The flow diagram (Fig. 1) outlines the number of articles included after the screening and eligibility criteria were applied.
Sex
A previous systematic literature review( 19 ) demonstrated differences in plasma DHA (expressed as weight/weight percentage of total plasma fatty acids) between sexes; namely, women had 0·12 % of total plasma fatty acids and 0·20 % of plasma phospholipids (PL) higher than men (P=0·002 and P<0·00001, respectively)( 19 ). In participants aged 13–50 years, the DHA values were significantly higher in women (0·16 % of total plasma fatty acids) compared with men; whereas the DHA values did not differ when aged over 50 years( 19 ). In high fish intake groups, sex differences in DHA did not exist; however, in low fish intake groups, the DHA was significantly higher in women (0·24 % of total plasma fatty acids)( 19 ).
Since the publication of the systematic literature review( 19 ), one large study( 23 ) showed that women from teens to aged 40 years had lower erythrocyte EPA and DPA compared with men, but women from teens to age 30 years had higher erythrocyte DHA levels compared with men.
Heritability
One study( 10 ) identified that heritability (meaning the fraction of phenotype variability that can be attributed to genetic variation) explains 24 % of the variance of the n-3 LCPUFA levels, see online Supplementary Table S2.
Genetics
Our systematic literature search revealed sixteen papers on the association of the factor ‘genetics’ and n-3 LCPUFA levels, as described subsequently.
Fatty acid desaturase
Nine studies were found, which looked at the relationship between fatty acid desaturase (FADS) genotypes and n-3 LCPUFA levels( 24 – 32 ). A minor allele carrier of a FADS SNP was negatively associated with plasma EPA in six studies( 24 – 26 , 29 , 31 , 32 ) and a negative association with DHA in three of those studies( 24 , 25 , 32 ). Three studies( 27 , 28 , 30 ) found no association between FADS minor allele carriers and plasma EPA or DHA. In essence, minor allele carriers for FADS1 and FADS2 resulted in decreased plasma levels of γ-linolenic acid (GLA, 18 : 3n-6), arachidonic acid (AA, 20 : 4n-6) and 20 : 5n-3 (EPA) (Table 1). The comparison of the major allele to the minor allele (homozygous or heterozygous plus homozygous) for FADS1 and FADS2 and their effects on plasma fatty acid levels are shown in Table 1.
Table 1.
Comparison of major allele with minor allele (homozygous or heterozygous plus homozygous) for fatty acid desaturase (FADS)1 and FADS2 on fatty acid levels (Percentage increase or percentage decrease)*,†
| rs174561 (FADS1) | rs174537 (C10orf10, FADS1) | rs174583 (FADS2) | rs3834458 (FADS2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fatty acid | Gillingham( 26 ) | Al-Hilal( 24 ) | Tanaka (InCHIANTI)( 31 ) | Tanaka (GOLDN)( 31 ) | Al-Hilal( 24 ) | FADS1 (average) | Gillingham( 26 ) | Baylin( 32 ) | Al-Hilal( 24 ) | FADS2 (average) |
| 18 : 2n-6 | NS | ↑ 3 | ↑ 7 | ↑ 13 | NS | ↑ 5 | NS | NS | NS | NS |
| 18 : 3n-6 | ↓ 61 | ↓ 19 | NR | NR | ↓ 19 | ↓ 33 | ↓ 62 | ↓ 53 | ↓ 20 | ↓ 45 |
| 20 : 2n-6 | NR | NR | ↓ 25 | NR | NR | ↓ 25‡ | NR | ↑ 19 | NR | ↑ 19‡ |
| 20 : 3n-6 | NS | ↑ 11 | NR | NR | ↑ 11 | ↑ 7 | NS | NR | ↑ 10 | ↑ 5 |
| 20 : 4n-6 | ↓ 37 | ↓ 18 | ↓ 27 | ↓ 12 | ↓ 18 | ↓ 22 | ↓ 37 | ↓ 39 | ↓ 18 | ↓ 31 |
| 18 : 3n-3 | NS | ↑ 8 | ↑ 10 | ↑ 14 | NS | ↑ 6 | NS | NS | ↑ 10 | ↑ 3 |
| 20 : 3n-3 | NR | NR | NR | NR | NR | NR | NR | ↑ 90 | NR | ↑ 90‡ |
| 20 : 5n-3 | ↓ 50 | ↓ 17 | ↓ 22 | ↓ 14 | ↓ 16 | ↓ 24 | ↓ 52 | ↓ 34 | ↓ 13 | ↓ 33 |
| 22 : 5n-3 | NS | ↓ 9 | NR | ↓ 7 | ↓ 10 | ↓ 7 | NS | NR | ↓ 9 | ↓ 5 |
| 22 : 6n-3 | NS | ↓ 11 | NS | NS | ↓ 10 | ↓ 4 | NS | ↓ 15 | ↓ 10 | ↓ 8 |
InCHIANTI, a study involving people in Chianti in Italy; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network Study in the USA; NR, not reported.
NS, for the calculation of the average, NS was taken as being 0.
The data in Table 1 are the fatty acid data taken from the publications that reported fatty acid data and then expressed as percentage increase or percentage decrease in the fatty acid compared with the major allele (no mutation). This is a rough estimate of the magnitude of effect and therefore needs to be interpreted with caution.
Limited data available as only one study reported this result.
Elongation of very-long-chain fatty acid 2
Three studies were identified that looked at the relationship between elongation of very-long-chain fatty acid (ELOVL)2 and EPA, DPA and DHA plasma levels( 26 , 31 , 33 ). Two studies( 31 , 33 ) observed lower plasma DHA levels in minor allele carriers, whereas one of them( 31 ) saw higher EPA levels in minor allele carriers. Another study( 26 ) found no association of ELOVL2 rs953413 and plasma fatty acids (Table 2). The comparison of the major allele to the minor allele (homozygous or heterozygous plus homozygous) for ELOVL2 and their effects on plasma n-3 LCPUFA are shown in Table 2.
Table 2.
Comparison of major allele with minor allele (homozygous or heterozygous plus homozygous) for ELOVL2 on fatty acid levels (Percentage increase or percentage decrease)*,†
| rs953413 (ELOVL2) | rs3734398 (ELOVL2) | rs2236212 (ELOVL2) | |||||
|---|---|---|---|---|---|---|---|
| Fatty acid | Gillingham( 26 ) | Alsaleh( 33 ) | Tanaka-InCHIANTI( 31 ) | Tanaka-GOLDN( 31 ) | Alsaleh( 33 ) | Alsaleh( 33 ) | ELOVL2 average |
| 20 : 5n-3 | NS | NS | ↑ 14 | NS | NS | NS | ↑ 2 |
| 22 : 5n-3 | NS | NS | NR | ↓ 1·1 | NS | NS | ↓ 1 |
| 22 : 6n-3 | NS | ↓ 7 | ↓ 8 | ↓ 7 | ↓ 6 | ↓ 6 | ↓ 6 |
ELOVL2, elongation of very-long-chain fatty acid 2; InCHIANTI, a study involving people in Chianti in Italy; GOLDN, Genetics of Lipid Lowering Drugs and Diet Network Study in the USA; NR, not reported.
NS, for the calculation of the average, NS was taken as being 0.
The data in Table 2 are the fatty acid data taken from the publications that reported fatty acid data and then expressed as percentage increase or percentage decrease in the fatty acid compared with the major allele (no mutation). This is a rough estimate of the magnitude of effect and therefore needs to be interpreted with caution.
ApoE4
Only five studies were available on ApoE4, as shown in Supplementary Table S2, and the results did not suggest a strong relationship between ApoE4 and EPA or DHA plasma levels( 34 – 38 ). One study( 34 ) found ApoE4 carriers had higher plasma TAG EPA and DHA than non-carriers at baseline, but baseline EPA and DHA levels were not shown to be different in any of the participant groups in the additional four papers reviewed( 35 – 38 ) (online Supplementary Table S2).
Age
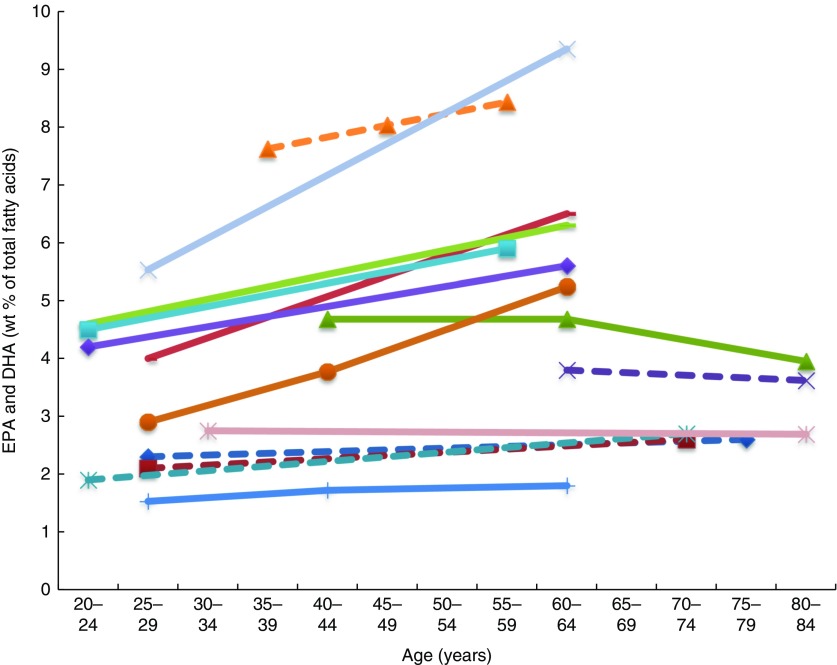
Twenty-six articles, which looked at ‘age’ as a factor associated with n-3 LCPUFA levels, were included( 10 , 23 , 39 – 62 ). Most publications reported plasma EPA and DHA and only four studies reported erythrocyte levels of EPA and DHA( 23 , 39 , 53 , 57 ). Twenty-four found a positive association, one found no association( 57 ), and another( 54 ) an inverse association between age 40–60 and age 61–82 years and n-3 LCPUFA (specifically DHA levels in elderly women) (Figs. 2 and 3). See online Supplementary Table S3 for detailed information on the range of age groups and the relevant outcomes for each of the twenty-six studies reviewed.
Fig. 2.
Plasma EPA levels and DHA (wt% of total fatty acids) of different age groups from studies reviewed in this systematic literature review. Each line represents a different study (population) and each symbol on a line represents an age group. Full line = significant difference between age groups measured in that study. Broken line = no significant difference between age groups measured in that study.  , Plourde et al.(
43
) NS;
, Plourde et al.(
43
) NS;  , Vandal et al.(
42
) NS;
, Vandal et al.(
42
) NS;  , Sfar et al. (women)(
54
);
, Sfar et al. (women)(
54
);  , Sfar et al. (men)(
54
) NS;
, Sfar et al. (men)(
54
) NS;  , Fortier et al.(
40
) NS;
, Fortier et al.(
40
) NS;  , Dewailly et al.(
58
);
, Dewailly et al.(
58
);  , Dewailly et al.(
59
);
, Dewailly et al.(
59
);  , Rees et al. g1(
41
);
, Rees et al. g1(
41
);  , Rees et al. g2(
41
);
, Rees et al. g2(
41
);  , Rees et al. g3(
41
);
, Rees et al. g3(
41
);  , Rees et al. g4(
41
);
, Rees et al. g4(
41
);  , Kuriki et al.(
46
) NS;
, Kuriki et al.(
46
) NS;  , Dewailly et al.(
61
);
, Dewailly et al.(
61
);  , Babin et al.(
57
) NS.
, Babin et al.(
57
) NS.
Fig. 3.
Erythrocyte EPA levels and DHA (wt% of total fatty acids) of different age groups from studies reviewed in this systematic literature review. Each line represents a different study (population) and each symbol on a line represents an age group. Full line=significant difference between age groups measured in that study. Broken line=no significant difference between age groups measured in that study.  , Kawabata et al.
(
53
) (women subjects) NS;
, Kawabata et al.
(
53
) (women subjects) NS;  , Kawabata et al.
(
53
) (male subjects);
, Kawabata et al.
(
53
) (male subjects);  , Babin et al.
(
57
) NS;
, Babin et al.
(
57
) NS;  , Walker et al.
(
39
) NS.
, Walker et al.
(
39
) NS.
Plasma EPA and DHA levels are positively associated with age in the majority of studies. Erythrocyte EPA and DHA levels only tended to be positively associated with age in adults( 39 , 53 ), with only statistical significance shown in men( 53 ). One study did not show associations with increased age( 57 ). One large study( 23 ) showed that the net effect on erythrocyte EPA and DHA was an overall 7 % increase per decade up to 70 years of age and not much change after that.
Body size
Of the fourteen studies that looked for associations between the factor ‘body size’ and n-3 LCPUFA levels( 10 , 11 , 47 , 49 , 52 , 58 – 61 , 63 – 67 ), eight studies used ‘BMI’( 11 , 47 , 49 , 52 , 60 , 63 – 65 ), three used ‘girth’( 58 , 59 , 61 ) and three studies( 10 , 66 , 67 ) used both to compare the weight-based association and n-3 LCPUFA. Despite the strong correlations, we chose to report BMI and girth in relation to n-3 LCPUFA separately, because they provide different information about the participants’ fat distribution and require very different methodology for measurement.
BMI
Overall, of the eleven cross-sectional studies that investigated the association of BMI and n-3 LCPUFA levels, five identified negative associations( 10 , 49 , 64 , 66 , 67 ), whereas six found no association( 11 , 47 , 52 , 60 , 63 , 65 ).
As shown in Table 3, it appears that there is no association when erythrocyte EPA and DHA is >7 % of total fatty acids and that there is a negative association when it is lower than 5·6 % of the total fatty acids (Table 3). An exception to this is the study by Block et al. ( 52 ) in which the mean erythrocyte EPA and DHA was 4·3 % and no association was found, wherein this population group comprised mostly overweight and obese (Table 3).
Table 3.
Overview of studies investigating associations between BMI and n-3 long-chain PUFA levels presented in order of decreasing erythrocyte EPA and DHA
| Reference | Subjects (n) | BMI range and (mean) | Erythrocyte EPA and DHA*: range and (mean) | Association |
|---|---|---|---|---|
| Ogura( 47 ) | 75 | 16·5–37·8 kg/m2 (26·2 kg/m2) | Range not given (9·6 %) | No |
| Makhoul( 65 ) | 330 | Range not given (28·1 kg/m2) 71 % overweight or obese | Erythrocyte EPA range: 0·2–9·6 % Erythrocyte DHA range: 1·6–10·3 % EPA+DHA range not given (9·6 %) | No |
| Itomura( 60 ) | 456 | 16–38 kg/m2 (22·5 kg/m2) | 3·9–12·9 % (8·5 %) | No |
| Sala-Vila( 11 ) | 198 | 21·2–38·5 kg/m2 (29·2 kg/m2) | Interquartile range: 6·1–8·1 % (7·1 %) | No |
| Kuriki( 63 ) | 106 | Range not given (female mean 21·5 kg/m2) (male mean 22·3 kg/m2) | Range not given (7·1 %†) | No |
| Harris( 10 ) | 3196 | Range not given (28·4 kg/m2) | Range not given (5·6 %) | Negative |
| Howe( 67 ) | 476 | 18–59 kg/m2 (female mean 34 kg/m2) (male mean 31·4 kg/m2) | Range not given (female 5·3 %) (male 5·1 %) | Negative |
| Sands( 49 ) | 163 | 18–47 kg/m2 (26·2 kg/m2) | 1·7–12·4 % (4·9 %) | Negative |
| Block( 52 ) | 704 | Range not given (28 kg/m2) Study reports most participants were overweight or obese | Range not given (4·3 %) | No |
| Cazzola( 64 ) | 100 | Range and mean not given | 4·2–6·3 % (3·7 %) | Negative |
| Micallef( 66 ) | 124 | 20–40 kg/m2 (mean not given) Twenty-one subjects between 20 and 24·9 kg/m2 Forty subjects between 25 and 29·9 kg/m2 Sixty-three subjects between 30 and 40 kg/m2 | Range and mean not given | Negative |
Plasma levels were converted to erythrocyte EPA+DHA using the Stark et al. ( 16 ) equation.
Girth
Six studies were identified dealing with girth and n-3 LCPUFA levels. Three studies( 58 , 59 , 61 ) showed positive associations. Three studies( 10 , 66 , 67 ) found inverse associations; one of them( 66 ) found this only in the obese group, another study( 67 ) found this only in females, whereas the third study( 10 ) found that 1sd increase (14·7 cm) in girth was associated with 2 % lower n-3 LCPUFA status.
Given the small number of studies available for review and the differing results between studies, the relationship between girth and n-3 LCPUFA levels remains inconclusive.
Physical activity
Many studies observed associations between exercise and n-3 LCPUFA levels; however, not all studies included EPA and DHA in their analyses( 68 ). Therefore, only eight studies were included( 11 , 60 , 63 , 69 – 73 ) in this review. Studies that investigated the effect of acute exercise were excluded.
One cross-sectional study that compared muscle fatty acids in male endurance athletes (mean training time of 74 (SD 24) min/d) with sedentary men (no regular physical activity) showed that DHA was approximately 30 % higher in male endurance athletes( 70 ).
Two studies found a positive association( 11 , 71 ), two studies found a negative association( 60 , 69 ), whilst four studies( 63 , 70 , 72 , 73 ) found no association between n-3 LCPUFA levels and physical activity (Table 4).
Table 4.
Cross-sectional studies looking at differences in n-3 long-chain PUFA levels at different physical activity levels
| Reference | Sex | Subjects (n) | Exercise | Biomarkers reported | Findings |
|---|---|---|---|---|---|
| Sala-Vila( 11 ) | Women and men | 198 | Not specified | Whole blood | Physical activity positively ↑ associated with EPA and DHA |
| Kamada( 71 ) | Men | 6 long-distance runners 9 sprinters 10 sedentary controls | Athletes v. controls | Erythrocytes | EPA higher ↑ in long-distance runners compared with controls No difference in DHA levels between groups |
| Itomura( 60 ) | Men and women | 456 | Not specified | Erythrocytes | Physical activity negatively ↓ associated with EPA and DHA |
| Sumikawa( 69 ) | Sex not specified | 12 rowers 9 sedentary controls | Athletes v. controls | Erythrocyte PC PS | Athletes had lower ↓ DHA composition in PS No difference in PC DHA levels EPA not measured |
| Andersson( 70 ) | Men | 15 endurance athletes 16 sedentary controls | Endurance athletes v. untrained | Serum | No differences in EPA or DHA between groups |
| Arsic( 72 ) | Women | 15 waterpolo players 19 footballers 14 sedentary controls | Athletes v. controls | Erythrocytes and plasma | No differences in EPA or DHA between groups |
| Kuriki( 63 ) | Women and men | 84 | Not specified | Plasma | Physical activity was not associated with EPA and DHA |
| Tepsic( 73 ) | Men | 23 basketballers 24 footballers 16 sedentary controls | Athletes v. sedentary controls | Erythrocytes and plasma | No differences in EPA or DHA between groups |
PC, phosphatidylcholine; PS, phosphatidylserine.
Alcohol intake
Twelve papers were suitable for inclusion when looking at the association between alcohol and n-3 LCPUFA levels( 11 , 58 , 59 , 61 , 63 , 74 – 80 ). Six papers identified positive associations( 59 , 74 – 76 , 78 , 79 ); three found no association( 11 , 63 , 77 ) and four studies found negative associations( 58 , 61 , 76 , 80 ). One study( 76 ) is mentioned twice as they found erythrocyte PL EPA to be positively associated, whereas DHA was negatively associated.
Wine and n-3 long-chain PUFA levels
Six papers found positive associations with wine and n-3 LCPUFA levels, and there were no studies showing negative or no associations. Three studies had cohorts that of mostly (>88 %) wine drinkers( 74 , 78 , 79 ), one study separated wine from beer or spirits and found that drinking ‘only’ wine was positively associated with n-3 LCPUFA levels but drinking ‘only’ beer or spirits was not( 75 ); and two studies( 59 , 76 ) did not mention the type of alcohol consumed but consisted of participants living in locations (France and Quebec) where wine is the most regularly consumed alcoholic beverage( 74 , 81 ). Fig. 4 shows the increases in plasma EPA or DHA associated with increasing wine intake among three studies that had sufficient data( 74 , 75 , 78 ). The optimal amount of wine consumption seems to plateau between two to three glasses per d (Fig. 4). In addition, it was found that erythrocyte PL EPA was positively associated (β=0·182, P=0·011) with alcohol intake in a French cohort, whereas DHA was negatively associated (β=–0·218, P=0·01)( 76 ) and one study( 79 ) found significantly higher DHA in phosphatidylethanolamine among wine drinkers compared to non-drinkers (P<0·05) but no differences in EPA. Whilst five studies found increases in EPA with increasing alcohol (mostly wine) intake( 59 , 74 , 75 , 78 , 79 ), Simonetti et al. ( 79 ) and Di Giuseppe et al.( 75 ) were the only studies to find a positive association between wine intake and plasma DHA.
Fig. 4.
EPA and DHA levels in alcohol abstainers v. wine drinkers. Bar graph presents plasma EPA and DHA (percentage of total fatty acids) for de Lorgeril( 74 ) and di Giuseppe( 75 ) studies and EPA and DHA concentration in HDL phosphatidylcholines for the Perret study( 78 ). *P<0·05. †DHA intake was approximately 3× lower in subjects who drank >3 drinks per d compared with other subjects. a,bBars in the same study with unlike letters have significantly different fatty acid levels. ALA, α-linolenic acid.
Alcohol type not further specified
Two studies( 11 , 77 ) found no association between alcohol and n-3 LCPUFA levels, whereas one study( 63 ) identified a positive association in females only. Three studies found negative associations with alcohol intake and EPA and/or DHA levels (Fig. 5)( 58 , 61 , 80 ). None of these studies reported the type of alcohol consumed by participants, and relevant intake surveys or research to indicate the types of drinks most commonly consumed by these populations have not been reported.
Fig. 5.
EPA and DHA levels at different alcohol intake. di Giuseppe( 75 ) shows EPA and DHA levels for beer and spirit drinkers. Alcohol type was not reported for the four other studies shown in the bar graph. Bar graph presents plasma EPA and DHA (percentage of total fatty acids) for Dewailly( 58 , 59 , 61 ) and di Giuseppe( 75 ) studies and DHA concentration in HDL phosphatidylcholines for the Alling study( 80 ). a,b Bars in the same study with unlike letters are significantly different from each other.
Smoking
Twelve studies were identified( 10 , 11 , 49 , 52 , 58 – 61 , 63 , 77 , 82 , 83 ) of which eight found a negative association between smoking and n-3 LCPUFA levels( 10 , 11 , 52 , 58 , 59 , 77 , 82 , 83 ) and four studies found no association( 49 , 60 , 61 , 63 ).
Of the twelve studies, four studies( 58 , 59 , 61 , 83 ) provided numerical data on FA levels of smokers and non-smokers. Using the Stark et al. ( 16 ) equation or the already available data( 10 , 52 , 83 ), erythrocyte EPA and DHA levels in smokers and non-smokers were determined and ranged from 6 to 17 % lower in smokers compared with non-smokers. Three studies had no numerical data( 11 , 77 , 82 ), but each also reported lower n-3 LCPUFA levels among smokers compared with non-smokers.
Bioavailability factors
Chemically bound form of n-3 supplement
One study( 22 ) reviewed the different factors associated with the bioavailability of n-3 LCPUFA. This review and another subsequently published study( 84 ) showed that the chemically bound form TAG are more bioavailable than ethyl ester (EE) forms and that there is no enough evidence to suggest that PL (like krill oil) are more bioavailable than the TAG form from fish oil. They also showed that matrix effects such as sufficient amounts of fat in the meal have the greatest bioavailability effect of up to three times higher( 85 ). It appears that some studies from the Schuchardt review( 22 ) showed that the galenic form (i.e. microencapsulation, emulsification) had an effect on increased bioavailability (e.g. up to 4-fold( 86 )) of emulsification and microencapsulation compared with oil, whilst others showed no effect.
Krill oil v. fish oil
A review( 20 ) identified fourteen articles comparing krill oil and fish oil, and they found that some studies showed increased bioavailability with krill oil v. fish oil, but other studies did not show any difference and concluded that more studies are needed. Following the publishing of this review, two more clinical trials have been published. One study( 87 ) found no difference in bioavailability of fish oil (TAG-rich or EE-rich) and krill oil supplements when identical doses were used in a 4-week intervention. Another study examined the amount of PL in krill oil( 88 ) and showed no difference between krill oil with high PL content and krill oil with low PL content in plasma n-3 LCPUFA levels. However, the high PL supplement significantly increased erythrocyte EPA, EPA+DHA and n-3 PUFA concentrations compared with the low PL supplement( 88 ).
Conversion of plant-derived n-3, α-linolenic acid to n-3 long-chain PUFA
The International Society for the Study of Fatty Acids and Lipids (ISSFAL) statement five (http://www.issfal.org/statement-5) concludes that ‘With no other changes in diet, improvement of blood DHA status can be achieved with dietary supplements of preformed DHA, but not with supplementation of ALA, EPA, or other precursors’. Furthermore, a comprehensive review on the metabolism of ALA and stearidonic acid (SDA, 18 : 4n-3)( 21 ) suggests that each 1 g increase in ALA intake results in approximately 10 % relative increase in EPA plasma PL content, whereas no change occurs in plasma PL DHA content. With high intake of EPA and DHA, however, the metabolism of ALA to EPA and DHA appears to become down-regulated( 21 ). High intakes of linoleic acid (LA, 18 : 2n-6) can also impact the metabolism of ALA to its longer chain metabolites. Furthermore, increased LA intake have been shown to decrease the metabolism of ALA to EPA( 21 ).
It was also demonstrated( 21 ) that SDA intake between 0·25 and 2 g/d can increase plasma EPA anywhere from 19 to 190 %. No superior ability was noted for SDA to increase the DHA levels, and some studies actually noted a decrease in DHA levels when participants consumed SDA( 21 ).
Discussion
Besides dietary intake, many factors affect n-3 LCPUFA levels. Generally women have higher plasma DHA compared with men( 19 , 23 ), and this appears to be independent of diet( 89 ). Women also have increased levels of EPA derived from ALA( 90 ) which is believed to be indicative of increased synthesis( 91 , 92 ). The sex differences can be explained by (1) decreased rates of ALA β-oxidation( 91 , 92 ), therefore making more ALA available for metabolism to DHA; (2) women having more DHA in their adipose tissue( 62 ) and therefore can mobilise more DHA (but it is still not known whether this occurs in non-pregnant women); (3) the fasting state wherein NEFA are released from adipose tissue; and women have increased NEFA compared with men( 89 ), and this is likely due to increased adipose tissue stores; (4) the total fractional excursions of EPA, DPA and DHA in plasma phosphatidylcholine were greater in younger women (74 %) compared with men (59·6 %)( 93 ) and (5) the influence of different sex hormones on the n-3 pathway( 94 ), which is likely due to the up-regulation mechanism of oestrogen on the desaturase–elongase n-3 pathway and a possible down-regulation by testosterone( 94 ). This may partially explain why women >50 years of age have DHA levels that are comparable with men. Increased requirements during pregnancy and lactation could provide a biological explanation to why higher DHA levels have been observed in women( 21 ). Certainly, in very early pregnancy, the requirement to increase maternal circulating DHA at the time of the neural tube closure is likely due to increased synthesis of DHA from ALA as well as an increase in the mobilisation of DHA from maternal adipose and other tissues( 3 ). Later pregnancy shows maternal erythrocyte DHA levels being 38 % higher in the third trimester (3·85 %) compared with the post-partum levels (2·79 %)( 95 ), demonstrating that the magnitude of effect of increased DHA in pregnancy is much higher than the differences seen in non-pregnant women v. men.
In terms of genetics, when comparing the baseline cross-sectional n-3 LCPUFA levels between major and minor allele carriers for FADS1 and FADS2, we deduced that there was decreased enzyme activity at the first Δ-6 desaturase and Δ-5 desaturase in the minor allele carriers (Fig. 6) and therefore this resulted in decreased levels of EPA, GLA and AA. However, as the FADS1 and FADS2 genotypes are strongly associated, controlling for one FADS1 or FADS2 would be sufficient. Similarly, when comparing baseline cross-sectional n-3 LCPUFA levels between major and minor allele for ELOVL2, we deduced that there was decreased enzyme activity between EPA and DHA in the minor allele carriers (Fig. 6), which explains the reduced DHA levels seen with these mutations. These findings are supported by a meta-analysis( 96 ). Dietary supplementation with pre-formed EPA and DHA (1·8 g/d) may overcome these decreased enzyme activities as EPA and DHA in minor allele carriers were 26–30 and 8–9 % higher, respectively, than non-carriers( 33 ). Therefore, in supplementation trials with pre-formed EPA and DHA, it may not be necessary to measure the minor allele SNP. Furthermore, heritability explains 24 % of the variance of n-3 LCPUFA levels( 10 ), which is more relevant than genetics alone.
Fig. 6.
Mammalian PUFA synthesis pathway showing the n-3 PUFA pathway and the n-6 PUFA pathway, including the enzymes responsible for the elongation and desaturation steps. ELOVL, elongation of very long-chain fatty acid; DPA, docosapentaenoic acid.
It appears that higher plasma EPA and DHA levels are associated with increased age, that is, up until 70 years of age( 10 , 23 , 39 – 41 , 44 – 53 , 55 , 56 , 58 – 62 ). Based on studies that reported no differences in DHA levels between elderly and young groups( 42 , 43 ), those studies that included elderly participants of over 70 years of age( 42 , 43 , 57 ) and women aged 40–82 years showed lower levels of plasma EPA and DHA in the 60- to 82-year-olds compared with the 40- to 60-year-olds( 54 ), which could be explained by the 60- to 82-year-old women being post-menopausal and therefore likely to have lower oestrogen levels and hence lower synthesis of DHA from ALA.
Negative associations between n-3 LCPUFA levels and BMI have been found in participants with erythrocyte EPA and DHA of 5·6 % or lower (Table 3). No associations have been found with higher than 7 % erythrocyte EPA and DHA and BMI. With the contradictory evidence in terms of girth and n-3 LCPUFA levels and taking into account that the majority of populations’ erythrocyte EPA and DHA is likely to be lower than 5·6 % of the total fatty acids, future studies should take BMI into account in their analyses.
The following different mechanisms have been suggested for the negative associations occasionally observed: (1) higher susceptibility to peroxidation in overweight and obese individuals, compared with normal-weight individuals( 64 , 97 , 98 ), (2) individuals with higher BMI may be more likely to consume lower intakes of n-3 LCPUFA( 63 ), although the opposite was found( 49 ), (3) alternatively, a relationship between weight and dose might exist for n-3 LCPUFA( 99 ), as supported by a study showing a three-unit rise in BMI is associated with a decrease in the n-3 LCPUFA status by 0·3 units, independent of fish intake( 49 ).
There is no conclusive evidence on whether an association exists between physical activity and n-3 LCPUFA, though several potential underlying mechanisms have been suggested( 72 , 73 , 100 , 101 ), and more research is warranted. Potential associations might depend on type, duration and intensity of physical activity, as higher DHA in skeletal muscle was observed in endurance athletes compared with sedentary controls( 70 ) but not in participants who followed a low-intensity exercise programme for 6 weeks( 102 ). Differences in fatty acid composition between athletes from different sports have also been found( 72 , 73 ).
The association between alcohol consumption and n-3 LCPUFA levels is either negative or neutral, except for wine consumption where there is a positive association( 59 , 74 – 76 , 78 , 79 ), in particular for EPA( 59 , 74 – 76 , 78 ). Studies that did not demonstrate the type of alcohol consumed showed conflicting results between papers( 11 , 58 , 61 , 63 , 77 , 80 ); the majority of these showed negative associations with alcohol intake( 58 , 61 , 76 , 80 ). Mechanisms for the negative associations between alcohol intake and n-3 LCPUFA are still not fully understood in humans; however, animal and in vitro studies propose lipid peroxidation and changes in desaturase activities( 103 – 105 ). Different findings observed between the studies might be due to the differences in amounts of alcohol consumed, the regularity with which alcohol is consumed and whether participants consumed more quantity of alcohol for prolonged periods( 106 ).
The positive associations between wine drinking and n-3 LCPUFA seem to partly contradict the mechanisms discussed above. This poses the question whether components in wine other than alcohol might be responsible for the positive associations observed. This warrants further research but could explain why one study( 75 ) saw no association for beer or spirits and n-3 LCPUFA but a positive association between wine and n-3 LCPUFA. Diet is the main contributor of n-3 LCPUFA levels( 10 ); and therefore, differences in dietary intake between drinkers and non-drinkers could also influence associations, though this is not uniformly supported by the literature( 11 , 61 , 63 , 74 , 107 ). Any research on n-3 LCPUFA levels should capture not only alcohol consumption but also the type and amount of alcohol.
Smoking is associated with a lower erythrocyte EPA and DHA (from 6 to 17 % lower) and thus smoking is a factor that needs to be controlled for in research studies. A plausible reason for lower erythrocyte EPA and DHA in smokers compared with non-smokers could be diet( 108 – 110 ), though others suggest the involvement of non-dietary factors, as they found this negative association regardless of the dietary intake( 10 , 52 , 77 ). It has been suggested that the pro-oxidative state caused by smoking degrades PUFA( 111 ); n-3 LCPUFA oxidation has been shown to be increased in smokers. Of the four studies that showed no association between smoking and n-3 LCPUFA, three consisted of cohorts with high n-3 LCPUFA intakes, providing mean annual daily intake of EPA and DHA of 1293 mg( 60 ), 2115 mg( 61 ) and 885 mg/d( 63 ), and one study had a very low number (n 13 of 163) of smokers in their cohort( 49 ). It could be that the high intake of n-3 LCPUFA negates the effect of smoking on erythrocyte EPA and DHA. It should be noted, however, that in the Spanish cohort( 11 ) the erythrocyte EPA and DHA were very similar to the cohort of Nunavik Inuit( 61 ), thereby showing a negative association between smoking and erythrocyte EPA and DHA.
The major contributor to increased bioavailability of n-3 LCPUFA appears to be fat in the meal when supplements are being taken. The biological plausibility is that the fat in the meal stimulates the release of pancreatic lipase necessary for fat digestion( 112 ). The increased bioavailability of the emulsified forms compared with the larger oil droplets supports that these emulsified forms are more readily available for pancreatic lipase. The limited evidence suggests that the EE form of n-3 LCPUFA is less bioavailable compared with the TAG form. Compared to low PL krill oil, high PL krill oil resulted in higher erythrocyte EPA and DHA( 88 ), which has been demonstrated in only one study. There is no definitive evidence to support that krill oil or fish oil is superior to the other in terms of bioavailability as studies to date (1) were underpowered( 22 ), (2) used different doses of EPA and DHA( 20 ) and (3) involved short duration of supplementation, that is, for 4 weeks( 87 ), which was not enough, given the mean erythrocyte lifespan of 115 d( 113 ). One cross-over study( 22 ) noted the high standard deviations, even though each person was their own control, thereby contribute to the lack of definitive evidence.
Supplementation with ALA increases plasma EPA but not DHA, and high intake of LA reduces the conversion of ALA to EPA( 21 ). Limited evidence suggests that SDA supplementation increases plasma EPA to a greater extent than supplementation with ALA, but SDA supplementation does not increase plasma DHA levels( 21 ). More research is warranted on SDA.
Given all the non-dietary factors that are associated with n-3 LCPUFA levels as discussed above and summarised in Table 5 below (but keep in mind that fish, seafood and n-3 supplement consumption have the biggest influence), it is difficult to stipulate how these non-dietary factors relate to each other, as these factors are associated with n-3 LCPUFA levels and do not show cause and effect. Furthermore, no scientific evidence is available that shows the relationship between these factors; however, one could speculate about these relationships. The global data of the prevalence of smoking and drinking alcohol are higher among males compared with females( 114 – 116 ). Therefore, future investigators should consider when studying males, who smoke more than women do and drink more alcohol than wine, that their n-3 LCPUFA levels would likely be lower than that in females who smoke less and drink wine rather than beer/spirits( 114 – 119 ). Furthermore, the prevalence of overweight and obesity is high in many western countries( 120 , 121 ); and taken together with the low n-3 LCPUFA levels globally( 122 ), the negative association between BMI and n-3 LCPUFA is also of concern. Overall, given the potential lower levels of n-3 LCPUFA in males, they are likely to see a benefit through n-3 LCPUFA supplementation. Conversely, given the n-3 LCPUFA levels may be higher in females compared with males, researchers need to be careful not to reach the potential ceiling effect. Given that n-3 LCPUFA levels are positively associated with age, researchers need to carefully consider the age range within studies. The best advice would be to measure the n-3 LCPUFA levels in all types of research including at baseline and post-supplementation in clinical trials.
Table 5.
Summary of factors affecting n-3 long-chain PUFA (LCPUFA) levels
| Factor | Conclusion 1 and quantification | Conclusion 2 and quantification | Conclusion 3 and quantification | Should take factor into account yes or no |
|---|---|---|---|---|
| Sex | DHA in all women is 0·12 % of total plasma fatty acids higher than men | DHA in women aged 13–50 years is 0·16 % of total plasma fatty acids higher than men | In low fish consumers, DHA in women is 0·24 % of total plasma fatty acids higher than men | Yes |
| Genetics | Mutations in FADS1 and FADS2 generally result in decreased levels of EPA, GLA and AA | Supplementation of EPA and DHA at doses of 1·8 g/d seemed to overcome the genetic differences, and therefore in supplementation trials, measuring these mutations may not be necessary | No in supplementation trials | |
| Age | Plasma EPA and DHA is positively associated with age (approximately 38 % increase from age 20 to 79 years) | Erythrocyte EPA and DHA tended to be positively associated with age (approximately 19 % increase from age 20 to 75 years) | Yes | |
| BMI | n-3 LCPUFA levels is negatively associated with erythrocyte EPA and DHA of 5·6 % or lower | No association with erythrocyte EPA and DHA >7 % | Yes | |
| Girth | Inconclusive | No | ||
| Physical activity | Inconclusive | No | ||
| Alcohol | No or negative association between alcohol consumption and n-3 LCPUFA levels (except for wine) | Positive association between wine drinking and n-3 LCPUFA levels | The optimal amount of wine consumption seems to plateau between two and three glasses per d | Yes, but specified according to type and amount of alcohol |
| Smoking | Smoking is negatively associated with erythrocyte EPA and DHA | High intakes of n-3 LCPUFA seem to overcome this at least partially | Smoking is associated with a 6–17 % lower erythrocyte EPA and DHA | Yes |
| Bioavailability chemically-bound form | TAG form is better than EE form | Yes | ||
| Bioavailability krill oil v. fish oil | Inconclusive | No | ||
| Bioavailability ALA conversion to n-3 LCPUFA | ALA is converted to EPA but not DHA | Higher conversion of ALA to DHA in women compared with men |
FADS, fatty acid desaturase; GLA, γ-linolenic acid; AA, arachidonic acid; EE, ethyl ester; ALA, α-linolenic acid.
Whilst the focus of this systematic review was not to assess the effect of n-3 LCPUFA interventions, a few points regarding the importance of measuring n-3 LCPUFA levels pre- and post-supplementation are warranted. Assessing only dietary or supplemental intake of n-3 LCPUFA is not good enough to really demonstrate the efficacy of n-3 LCPUFA supplementation. For example, a study investigating the effect of 1·4 g/d of n-3 LCPUFA supplementation for 6 months in young people at ultra-high risk of psychotic disorders failed to show the efficacy of n-3 LCPUFA; but this was most likely due to the lack of compliance as more than half of the participants were non-compliant, and a limitation of this study was the lack of measuring the blood n-3 LCPUFA levels( 123 ). Another study measured blood n-3 LCPUFA levels pre- and post-supplementation where the participants consumed 1 g/d of n-3 LCPUFA through the consumption of n-3 LCPUFA-enriched foods, and this resulted in an increase in n-3 LCPUFA erythrocyte levels from 4 to 7·1 % of total erythrocyte fatty acids( 124 ). This increase in n-3 LCPUFA was associated with improvements in arterial compliance and chronic inflammation as assessed by serum C-reactive protein( 124 ), demonstrating the importance of measuring n-3 LCPUFA levels pre- and post-supplementation in terms of not only compliance but also assessing the effect of n-3 LCPUFA in relation to health outcomes. Furthermore, we recently reviewed the trials investigating the effect of n-3 LCPUFA supplementation in cardiac mortality and demonstrated that the dose of n-3 LCPUFA is important, but also ensuring that the study populations’ n-3 LCPUFA levels are not too high at baseline in order to alleviate a potential ceiling effect( 125 ). More recently two large clinical trials have been published, where the study using high dose (4 g/d, Reduction of Cardiovascular Events with IcosapentEthyl-Intervention Trial (REDUCE-IT)) showed efficacy in cardiovascular risk reduction( 126 ), and another study using a lower dose (1 g/d, VITamin D and omegA-3 triaL (VITAL)) did not show efficacy in the prevention of cardiovascular disease and cancer( 127 ). These trials further highlight the importance of dose of n-3 LCPUFA in clinical trials. Moreover, blood analyses of n-3 LCPUFA pre- and post-supplementation will (1) ensure the baseline levels are not too high to potentially reach a ceiling effect and (2) after supplementation show compliance to n-3 LCPUFA as well as being able to attribute the health outcomes to the effect of n-3 LCPUFA supplementation. Therefore, it is recommended that research into the health benefits of n-3 LCPUFA should include blood analyses of n-3 LCPUFA pre- and post-supplementation.
In conclusion, as summarised in Table 5 those scientifically supported factors that are associated with the n-3 LCPUFA levels must be considered in future (design of) studies. It is recommended that blood or tissue n-3 LCPUFA levels are measured in all types of research (including cross-sectional, cohort and clinical research), which assesses the health benefits of n-3 LCPUFA. Furthermore, in randomised controlled trials, n-3 LCPUFA levels should be measured pre- and post-supplementation. It is beyond the scope of this review to recommend which tissue or fraction of blood to measure, but there are a couple of good reviews available on this topic( 128 , 129 ).
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0007114519000138.
click here to view supplementary material
Acknowledgements
The authors acknowledge ISSFAL for providing a grant for author R. E. to conduct the literature search for this systematic review.
R. H. M. d. G. and B. J. M. designed the study; R. E. conducted the literature search; all authors performed quality checks, analysed the data and interpreted it. R. H. M. d. G. and B. J. M. wrote the paper and share primary responsibility for final content.
The authors declare that there are no conflicts of interest.
References
- 1. Hino A, Adachi H, Toyomasu K, et al. (2004) Very long chain n-3 fatty acids intake and carotid atherosclerosis: an epidemiological study evaluated by ultrasonography. Atherosclerosis 176, 145–149. [DOI] [PubMed] [Google Scholar]
- 2. Calder PC & Yaqoob P (2009) Omega-3 long chain polyunsaturated fatty acids and human health outcomes. Biofactors 35, 266–272. [DOI] [PubMed] [Google Scholar]
- 3. Meyer BJ, Onyiaodike CC, Brown EA, et al. (2016) Maternal plasma DHA levels increase prior to 29 days post-LH surge in women undergoing frozen embryo transfer: a prospective, observational study of human pregnancy. J Clin Endocrinol Metab 101, 1745–1753. [DOI] [PubMed] [Google Scholar]
- 4. Makrides M & Gibson RA (2000) Long-chain polyunsaturated fatty acid requirements during pregnancy and lactation. Am J Clin Nutr 71, 307S–311S. [DOI] [PubMed] [Google Scholar]
- 5. GISSI Prevezione Investigators (1999) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet 354, 447–455. [PubMed] [Google Scholar]
- 6. Yokoyama M, Origasa H, Matsuzaki M, et al. (2007) Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 369, 1090–1098. [DOI] [PubMed] [Google Scholar]
- 7. Sinn N, Milte C & Howe PR (2010) Oiling the brain: a review of randomized controlled trials of omega-3 fatty acids in psychopathology across the lifespan. Nutrients 2, 128–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parletta N, Milte CM & Meyer BJ (2013) Nutritional modulation of cognitive function and mental health. J Nutr Biochem 24, 725–743. [DOI] [PubMed] [Google Scholar]
- 9. Duvall MG & Levy BD (2016) DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur J Pharmacol 785, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris WS, Pottala JV, Lacey SM, et al. (2012) Clinical correlates and heritability of erythrocyte eicosapentaenoic and docosahexaenoic acid content in the Framingham Heart Study. Atherosclerosis 225, 425–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sala-Vila A, Harris WS, Cofán M, et al. (2011) Determinants of the omega-3 index in a Mediterranean population at increased risk for CHD. Br J Nutr 106, 425–431. [DOI] [PubMed] [Google Scholar]
- 12. Sullivan BL, Williams PG & Meyer BJ (2006) Biomarker validation of a long-chain omega-3 polyunsaturated fatty acid food frequency questionnaire. Lipids 41, 845–850. [DOI] [PubMed] [Google Scholar]
- 13. Lands WE (1992) Biochemistry and physiology of n-3 fatty acids. FASEB 6, 2530–2536. [DOI] [PubMed] [Google Scholar]
- 14. Meyer BJ (2011) Are we consuming enough long chain omega-3 polyunsaturated fatty acids for optimal health? Prostaglandins Leukot Essent Fatty Acids 85, 275–280. [DOI] [PubMed] [Google Scholar]
- 15. Harris WS & Von Schacky C (2004) The omega-3 index: a new risk factor for death from coronary heart disease? Prev Med 39, 212–220. [DOI] [PubMed] [Google Scholar]
- 16. Stark KD, Aristizabal Henao JJ, Metherel AH, et al. (2016) Translating plasma and whole blood fatty acid compositional data into the sum of eicosapentaenoic and docosahexaenoic acid in erythrocytes. Prostaglandins Leukot Essent Fatty Acids 104, 1–10. [DOI] [PubMed] [Google Scholar]
- 17. National Heart, Lung and Blood Institute, National Institutes of Health (2017) Quality assessment tool for observational cohort and cross-sectional studies. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed June 2017).
- 18. Effective Public Health Practice Project (1998) Quality Assessment Tool For Quantitative Studies. Hamilton, ON: Effective Public Health Practice Project; https://merst.ca/ephpp/ (accessed June 2017). [Google Scholar]
- 19. Lohner S, Fekete K, Marosvölgyi T, et al. (2013) Gender differences in the long-chain polyunsaturated fatty acid status: systematic review of 51 publications. Ann Nutr Metab 62, 98–112. [DOI] [PubMed] [Google Scholar]
- 20. Ulven SM & Holven KB (2015) Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc Health Risk Manag 11, 511–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baker EJ, Miles EA, Burdge GC, et al. (2016) Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog Lipid Res 64, 30–56. [DOI] [PubMed] [Google Scholar]
- 22. Schuchardt JP & Hahn A (2013) Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot Essent Fatty Acids 89, 1–8. [DOI] [PubMed] [Google Scholar]
- 23. Harris WS, Pottala JV, Varvel SA, et al. (2013) Erythrocyte omega-3 fatty acids increase and linoleic acid decreases with age: observations from 160,000 patients. Prostaglandins Leukot Essent Fatty Acids 88, 257–263. [DOI] [PubMed] [Google Scholar]
- 24. Al-Hilal M, Alsaleh A, Maniou Z, et al. (2013) Genetic variation at the FADS1–FADS2 gene locus influences delta-5 desaturase activity and LC-PUFA proportions after fish oil supplement. J Lipid Res 54, 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mathias RA, Vergara C, Gao L, et al. (2010) FADS genetic variants and omega-6 polyunsaturated fatty acid metabolism in a homogeneous island population. J Lipid Res 51, 2766–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gillingham LG, Harding SV, Rideout TC, et al. (2013) Dietary oils and FADS1–FADS2 genetic variants modulate [13C]alpha-linolenic acid metabolism and plasma fatty acid composition. Am J Clin Nutr 97, 195–207. [DOI] [PubMed] [Google Scholar]
- 27. Tintle NL, Pottala JV, Lacey S, et al. (2015) A genome–wide association study of saturated, mono- and polyunsaturated red blood cell fatty acids in the Framingham Heart Offspring Study. Prostaglandins Leukot Essent Fatty Acids 94, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malerba G, Schaeffer L, Xumerle L, et al. (2008) SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids 43, 289–299. [DOI] [PubMed] [Google Scholar]
- 29. Schaeffer L, Gohlke H, Muller M, et al. (2006) Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet 15, 1745–1756. [DOI] [PubMed] [Google Scholar]
- 30. Rzehak P, Heinrich J, Klopp N, et al. (2009) Evidence for an association between genetic variants of the fatty acid desaturase 1 fatty acid desaturase 2 (FADS1 FADS2) gene cluster and the fatty acid composition of erythrocyte membranes. Br J Nutr 101, 20–26. [DOI] [PubMed] [Google Scholar]
- 31. Tanaka T, Shen J, Abecasis GR, et al. (2009) Genome–wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet 5, e1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baylin A, Ruiz-Naraez E, Kraft P, et al. (2007) Alpha-linolenic acid, delta 6 desaturase gene polymorphism, and the risk of myocardial infarction. Am J Clin Nutr 85, 554–560. [DOI] [PubMed] [Google Scholar]
- 33. Alsaleh A, Maniou Z, Lewis F, et al. (2014) ELOVL2 gene polymorphisms are associated with increases in plasma eicosapentaenoic and docosahexaenoic acid proportions after fish oil supplement. Genes Nutr 9, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plourde M, Vohl MC, Vandal M, et al. (2009) Plasma n-3 fatty acid response to an n-3 fatty acid supplement is modulated by apoE epsilon4 but not by the common PPAR-alpha L162V polymorphism in men. Br J Nutr 102, 1121–1124. [DOI] [PubMed] [Google Scholar]
- 35. Chouinard-Watkins R, Rioux-Perreault C, Fortier M, et al. (2013) Disturbance in uniformly C-13-labelled DHA metabolism in elderly human subjects carrying the apoE epsilon 4 allele. Br J Nutr 110, 1751–1759. [DOI] [PubMed] [Google Scholar]
- 36. Yassine HN, Rawat V, Mack WJ, et al. (2016) The effect of APOE genotype on the delivery of DHA to cerebrospinal fluid in Alzheimer’s disease. Alzheimers Res Ther 8, 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whalley L, Deary I, Starr J, et al. (2008) n-3 Fatty acid erythrocyte membrane content, APOE varepsilon4, and cognitive variation: an observational follow-up study in late adulthood. Am J Clin Nutr 87, 449–454. [DOI] [PubMed] [Google Scholar]
- 38. Samieri C, Feart C, Proust-Lima C, et al. (2011) Omega-3 fatty acids and cognitive decline: modulation by ApoE epsilon 4 allele and depression. Neurobiol Aging 32, 2317.e13–2317.e22. [DOI] [PubMed] [Google Scholar]
- 39. Walker CG, Browning LM, Mander AP, et al. (2014) Age and sex differences in the incorporation of EPA and DHA into plasma fractions, cells and adipose tissue in humans. Br J Nutr 111, 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fortier M, Tremblay-Mercier J, Plourde M, et al. (2010) Higher plasma n-3 fatty acid status in the moderately healthy elderly in southern Quebec: higher fish intake or aging-related change in n-3 fatty acid metabolism? Prostaglandins Leukot Essent Fatty Acids 82, 277–280. [DOI] [PubMed] [Google Scholar]
- 41. Rees D, Miles E, Banerjee T, et al. (2006) Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr 83, 331–342. [DOI] [PubMed] [Google Scholar]
- 42. Vandal M, Freemantle E, Tremblay-Mercier J, et al. (2008) Plasma omega-3 fatty acid response to a fish oil supplement in the healthy elderly. Lipids 43, 1085–1089. [DOI] [PubMed] [Google Scholar]
- 43. Plourde M, Chouinard-Watkins R, Vandal M, et al. (2011) Plasma incorporation, apparent retroconversion and [beta]-oxidation of 13C-docosahexaenoic acid in the elderly. J Nutr Metab 8, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hennebelle M, Courchesne-Loyer A, St-Pierre V, et al. (2016) Preliminary evaluation of a differential effect of an [alpha]-linolenate-rich supplement on ketogenesis and plasma [omega]-3 fatty acids in young and older adults. Nutrition 32, 1211–1216. [DOI] [PubMed] [Google Scholar]
- 45. Bjerve KS, Fougner KJ, Midthjell K, et al. (1989) n-3 fatty-acids in old-age. J Intern Med 225, 191–196. [DOI] [PubMed] [Google Scholar]
- 46. Kuriki K, Nagaya T, Imaeda N, et al. (2002) Discrepancies in dietary intakes and plasma concentrations of fatty acids according to age among Japanese female dietitians. Eur J Clin Nutr 56, 524–531. [DOI] [PubMed] [Google Scholar]
- 47. Ogura T, Takada H, Okuno M, et al. (2010) Fatty acid composition of plasma, erythrocytes and adipose: their correlations and effects of age and sex. Lipids 45, 137–144. [DOI] [PubMed] [Google Scholar]
- 48. Otsuka R, Kato Y, Imai T, et al. (2013) Higher serum EPA or DHA, and lower ARA compositions with age independent fatty acid intake in Japanese aged 40 to 79. Lipids 48, 719–727. [DOI] [PubMed] [Google Scholar]
- 49. Sands SA, Reid KJ, Windsor SL, et al. (2005) The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids 40, 343–347. [DOI] [PubMed] [Google Scholar]
- 50. de Groot RH, van Boxtel MP, Schiepers OJ, et al. (2009) Age dependence of plasma phospholipid fatty acid levels: potential role of linoleic acid in the age-associated increase in docosahexaenoic acid and eicosapentaenoic acid concentrations. Br J Nutr 102, 1058–1064. [DOI] [PubMed] [Google Scholar]
- 51. Bolton-Smith C, Woodward M & Tavendale R (1997) Evidence for age-related differences in the fatty acid composition of human adipose tissue, independent of diet. Eur J Clin Nutr 51, 619–624. [DOI] [PubMed] [Google Scholar]
- 52. Block RC, Harris WS & Pottala JV (2008) Determinants of blood cell omega-3 fatty acid content. Open Biomark J 1, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kawabata T, Hirota S, Hirayama T, et al. (2011) Age-related changes of dietary intake and blood eicosapentaenoic acid, docosahexaenoic acid, and arachidonic acid levels in Japanese men and women. Prostaglandins Leukot Essent Fatty Acids 84, 131–137. [DOI] [PubMed] [Google Scholar]
- 54. Sfar S, Laporte F, Braham H, et al. (2010) Influence of dietary habits, age and gender on plasma fatty acids levels in a population of healthy Tunisian subjects. Exp Gerontol 45, 719–725. [DOI] [PubMed] [Google Scholar]
- 55. Crowe FL, Skeaff CM, Green TJ, et al. (2008) Serum n-3 long-chain PUFA differ by sex and age in a population-based survey of New Zealand adolescents and adults. Br J Nutr 99, 168–174. [DOI] [PubMed] [Google Scholar]
- 56. Saga LC, Liland KH, Leistad RB, et al. (2012) Relating fatty acid composition in human fingertip blood to age, gender, nationality and n-3 supplementation in the Scandinavian population. Int J Food Sci Nutr 63, 790–795. [DOI] [PubMed] [Google Scholar]
- 57. Babin F, Abderrazik M, Favier F, et al. (1999) Differences between polyunsaturated fatty acid status of non-institutionalised elderly women and younger controls: a bioconversion defect can be suspected. Eur J Clin Nutr 53, 591–596. [DOI] [PubMed] [Google Scholar]
- 58. Dewailly E, Blanchet C, Lemieux S, et al. (2002) Cardiovascular disease risk factors and n-3 fatty acid status in the adult population of James Bay Cree. Am J Clin Nutr 76, 85–92. [DOI] [PubMed] [Google Scholar]
- 59. Dewailly E, Blanchet C, Gingras S, et al. (2001) Relations between n-3 fatty acid status and cardiovascular disease risk factors among Quebecers. Am J Clin Nutr 74, 603–611. [DOI] [PubMed] [Google Scholar]
- 60. Itomura M, Fujioka S, Hamazaki K, et al. (2008) Factors influencing EPA plus DHA levels in red blood cells in Japan. In vivo 22, 131–135. [PubMed] [Google Scholar]
- 61. Dewailly E, Blanchet C, Lemieux S, et al. (2001) n-3 Fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. Am J Clin Nutr 74, 464–473. [DOI] [PubMed] [Google Scholar]
- 62. Tavendale R, Lee AJ, Smith WC, et al. (1992) Adipose tissue fatty acids in Scottish men and women: results from the Scottish Heart Health Study. Atherosclerosis 94, 161–169. [DOI] [PubMed] [Google Scholar]
- 63. Kuriki K, Nagaya T, Tokudome Y, et al. (2003) Plasma concentrations of (n-3) highly unsaturated fatty acids are good biomarkers of relative dietary fatty acid intakes: a cross-sectional study. J Nutr 133, 3643–3650. [DOI] [PubMed] [Google Scholar]
- 64. Cazzola R, Rondanelli M, Russo-Volpe S, et al. (2004) Decreased membrane fluidity and altered susceptibility to peroxidation and lipid composition in overweight and obese female erythrocytes. J Lipid Res 45, 1846–1851. [DOI] [PubMed] [Google Scholar]
- 65. Makhoul Z, Kristal AR, Gulati R, et al. (2011) Associations of obesity with triglycerides and C-reactive protein are attenuated in adults with high red blood cell eicosapentaenoic and docosahexaenoic acids. Eur J Clin Nutr 65, 808–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Micallef M, Munro I, Phang M, et al. (2009) Plasma n-3 polyunsaturated fatty acids are negatively associated with obesity. Br J Nutr 102, 1370–1374. [DOI] [PubMed] [Google Scholar]
- 67. Howe PR, Buckley JD, Murphy KJ, et al. (2014) Relationship between erythrocyte omega-3 content and obesity is gender dependent. Nutrients 6, 1850–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nikolaidis M & Mougios V (2004) Effects of exercise on the fatty-acid composition of blood and tissue lipids. Sports Med 34, 1051–1076. [DOI] [PubMed] [Google Scholar]
- 69. Sumikawa K, Mu Z, Inoue T, et al. (1993) Changes in erythrocyte membrane phospholipid composition induced by physical training and physical exercise. Eur J Appl Physiol 67, 132–137. [DOI] [PubMed] [Google Scholar]
- 70. Andersson A, Sjodin A, Hedman A, et al. (2000) Fatty acid profile of skeletal muscle phospholipids in trained and untrained young men. Am J Physiol Endocrinol Metab 279, E744–E751. [DOI] [PubMed] [Google Scholar]
- 71. Kamada T, Tokuda S, Aozaki S, et al. (1993) Higher levels of erythrocyte membrane fluidity in sprinters and long-distance runners. J Appl Physiol 74, 354–358. [DOI] [PubMed] [Google Scholar]
- 72. Arsic A, Vucic V, Tepsic J, et al. (2012) Altered plasma and erythrocyte phospholipid fatty acid profile in elite female water polo and football players. Appl Physiol Nutr Metab 37, 40–47. [DOI] [PubMed] [Google Scholar]
- 73. Tepsic J, Vucic V, Arsic A, et al. (2009) Plasma and erythrocyte phospholipid fatty acid profile in professional basketball and football players. Eur J Appl Physiol 107, 359–365. [DOI] [PubMed] [Google Scholar]
- 74. de Lorgeril M, Salen P, Martin J-L, et al. (2008) Interactions of wine drinking with omega-3 fatty acids in patients with coronary heart disease: a fish-like effect of moderate wine drinking. Am Heart J 155, 175–181. [DOI] [PubMed] [Google Scholar]
- 75. di Giuseppe R, de Lorgeril M, Salen P, et al. (2009) Alcohol consumption and n-3 polyunsaturated fatty acids in healthy men and women from 3 European populations. Am J Clin Nutr 89, 354–362. [DOI] [PubMed] [Google Scholar]
- 76. Theret N, Bard JM, Nuttens MC, et al. (1993) The relationship between the phospholipid fatty acid composition of red blood cells, plasma lipids, and apolipoproteins. Metabolism 42, 562–568. [DOI] [PubMed] [Google Scholar]
- 77. Simon JA, Fong J, Bernert J, et al. (1996) Relation of smoking and alcohol consumption to serum fatty acids. Am J Epidemiol 144, 325–334. [DOI] [PubMed] [Google Scholar]
- 78. Perret B, Ruidavets JB, Vieu C, et al. (2002) Alcohol consumption is associated with enrichment of high-density lipoprotein particles in polyunsaturated lipids and increased cholesterol esterification rate. Alcohol Clin Exp Res 26, 1134–1140. [DOI] [PubMed] [Google Scholar]
- 79. Simonetti P, Brusamolino A, Pellegrini N, et al. (1995) Evaluation of the effect of alcohol consumption on erythrocyte lipids and vitamins in a healthy population. Alcohol Clin Exp Res 19, 517–522. [DOI] [PubMed] [Google Scholar]
- 80. Alling C, Gustavsson L, Kristensson-Aas A, et al. (1984) Changes in fatty acid composition of major glycerophospholipids in erythrocyte membranes from chronic alcoholics during withdrawal. Scand J Clin Lab Invest 44, 283–289. [DOI] [PubMed] [Google Scholar]
- 81. Educ-Alcool (2012) Quebecers and alcohol. www.educalcool.qc.ca (accessed December 2017).
- 82. Leng GC, Smith FB, Fowkes FGR, et al. (1994) Relationship between plasma essential fatty-acids and smoking, serum-lipids, blood-pressure and hemostatic and rheological factors. Prostaglandins Leukot Essent Fatty Acids 51, 101–108. [DOI] [PubMed] [Google Scholar]
- 83. Hibbeln JR, Makino KK, Martin CE, et al. (2003) Smoking, gender, and dietary influences on erythrocyte essential fatty acid composition among patients with schizophrenia or schizoaffective disorder. Biol Psychiatry 53, 431–441. [DOI] [PubMed] [Google Scholar]
- 84. West AL, Burdge GC & Calder PC (2016) Lipid structure does not modify incorporation of EPA and DHA into blood lipids in healthy adults: a randomised-controlled trial. Br J Nutr 116, 788–797. [DOI] [PubMed] [Google Scholar]
- 85. Lawson LD & Hughes BG (1988) Absorption of eicosapentaenoic acid and docosahexaenoic acid from fish oil triacylglycerols or fish oil ethyl esters co-ingested with a high-fat meal. Biochem Biophys Res Commun 156, 960–963. [DOI] [PubMed] [Google Scholar]
- 86. Garaiova I, Guschina IA, Plummer SF, et al. (2007) A randomised cross-over trial in healthy adults indicating improved absorption of omega-3 fatty acids by pre-emulsification. Nutr J 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yurko-Mauro K, Kralovec J, Bailey-Hall E, et al. (2015) Similar eicosapentaenoic acid and docosahexaenoic acid plasma levels achieved with fish oil or krill oil in a randomized double-blind four-week bioavailability study. Lipids Health Dis 14, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ramprasath V, Eyal I, Zchut S, et al. (2015) Supplementation of krill oil with high phospholipid content increases sum of EPA and DHA in erythrocytes compared with low phospholipid krill oil. Lipids Health Dis 14, 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bakewell L, Burdge GC & Calder PC (2006) Polyunsaturated fatty acid concentrations in young men and women consuming their habitual diets. Br J Nutr 96, 93–99. [DOI] [PubMed] [Google Scholar]
- 90. Childs C, Kew S, Finnegan Y, et al. (2014) Increased dietary alpha-linolenic acid has sex-specific effects upon eicosapentaenoic acid status in humans: re-examination of data from a randomised, placebo-controlled, parallel study. Nutr J 13, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Burdge GC, Jones AE & Wootton SA (2002) Eicosapentaenoic and docosapentaenoic acids are the principal products of alpha-linolenic acid metabolism in young men. Br J Nutr 88, 355–363. [DOI] [PubMed] [Google Scholar]
- 92. Burdge GC & Wootton SA (2002) Conversion of alpha-linolenic acid to eicosapentaenoic, docosapentaenoic and docosahexaenoic acids in young women. Br J Nutr 88, 411–420. [DOI] [PubMed] [Google Scholar]
- 93. Emken EA, Adlof RO & Gulley RM (1994) Dietary linoleic acid influences desaturation and acylation of deuterium-labeled linoleic and linolenic acids in young adult males. Biochim Biophys Acta 1213, 277–288. [DOI] [PubMed] [Google Scholar]
- 94. Childs CE, Romeu-Nadal M, Burdge GC, et al. (2008) Gender differences in the n-3 fatty acid content of tissues. Proc Nutr Soc 67, 19–27. [DOI] [PubMed] [Google Scholar]
- 95. Stewart F, Rodie VA, Ramsay JE, et al. (2007) Longitudinal assessment of erythrocyte fatty acid composition throughout pregnancy and post partum. Lipids 42, 335–344. [DOI] [PubMed] [Google Scholar]
- 96. Lemaitre RN, Tanaka T, Tang W, et al. (2011) Genetic loci associated with plasma phospholipid n-3 fatty acids: a meta-analysis of genome–wide association studies from the CHARGE Consortium. Plos Genetics 7, e1002193–e1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Olusi SO (2002) Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int J Obes 26, 1159–1164. [DOI] [PubMed] [Google Scholar]
- 98. Vasilaki AT & McMilan DC (2011) Lipid peroxidation. In Encyclopedia of Cancer [M Schwab, editor]. Berlin, Heidelberg: Springer. [Google Scholar]
- 99. Flock M, Skulas-Ray A, Harris W, et al. (2013) Determinants of erythrocyte omega-3 fatty acid content in response to fish oil supplementation: a dose–response randomized controlled trial. J Am Heart Assoc 2, e000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Horowitz JF & Klein S (2000) Lipid metabolism during endurance exercise. Am J Clin Nutr 72, 558S–563S. [DOI] [PubMed] [Google Scholar]
- 101. von Schacky C, Kemper M, Haslbauer R, et al. (2014) Low omega-3 index in 106 German elite winter endurance athletes: a pilot study. Int J Sport Nutr Exerc Metab 24, 559–564. [DOI] [PubMed] [Google Scholar]
- 102. Andersson A, Sjodin A, Olsson R, et al. (1998) Effects of physical exercise on phospholipid fatty acid composition in skeletal muscle. Am J Physiol 274, E432–E438. [DOI] [PubMed] [Google Scholar]
- 103. Pawlosky RJ, Bacher J & Salen N (2001) Ethanol consumption alters electroretinograms and depletes neural tissues of docosahexaenoic acid in rhesus monkeys: nutritional consequences of a low n-3 fatty acid diet. Alcohol Clin Exp Res 25, 1758–1765. [PubMed] [Google Scholar]
- 104. Pawlosky RJ, Flynn BM & Salem NJ (1997) The effects of low dietary levels of polyunsaturates on alcohol-induced liver disease in rhesus monkeys. Hepatology 26, 1386–1392. [DOI] [PubMed] [Google Scholar]
- 105. Narce M, Poisson JP, Bellenger J, et al. (2001) Effect of ethanol on polyunsaturated fatty acid biosynthesis in hepatocytes from spontaneously hypertensive rats. Alcohol Clin Exp Res 25, 1231–1237. [PubMed] [Google Scholar]
- 106. Pawlosky RJ & Salem N Jr (2004) Perspectives on alcohol consumption: liver polyunsaturated fatty acids and essential fatty acid metabolism. Alcohol 34, 27–33. [DOI] [PubMed] [Google Scholar]
- 107. Christensen JH, Skou HA, Fog L, et al. (2001) Marine n-3 fatty acids, wine intake, and heart rate variability in patients referred for coronary angiography. Circulation 103, 651–657. [DOI] [PubMed] [Google Scholar]
- 108. Thompson RL, Pyke SDM, Scott EA, et al. (1993) Cigarette smoking, polyunsaturated fats, and coronary heart disease. Ann N Y Acad Sci 686, 130–138. [DOI] [PubMed] [Google Scholar]
- 109. Thompson RL, Pyke SDM, Scott EA, et al. (1995) Dietary change after smoking cessation: a prospective study. Br J Nutr 74, 27–38. [DOI] [PubMed] [Google Scholar]
- 110. D’Avanzo B, La Vecchia C, Braga C, et al. (1997) Nutrient intake according to education, smoking, and alcohol in Italian women. Nutr Cancer 28, 46–51. [DOI] [PubMed] [Google Scholar]
- 111. Pryor W (1997) Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Health Perspect 105, 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lowe ME (1997) Molecular mechanisms of rat and human pancreatic triglyceride lipases. J Nutr 127, 549–557. [DOI] [PubMed] [Google Scholar]
- 113. Franco RS (2012) Measurement of red cell lifespan and aging. Transfus Med Hemother 39, 302–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tobacco in Australia (2018) Prevalence of smoking adults. https://www.tobaccoinaustralia.org.au/chapter-1-prevalence/1-3-prevalence-of-smoking-adults (accessed December 2018).
- 115. Our World in Data (2018) Smoking. https://ourworldindata.org/alcohol-consumption (accessed December 2018).
- 116. Our World in Data (2018) Alcohol consumption. https://ourworldindata.org/smoking (accessed December 2018).
- 117. Wine Market Council (2017) Wine market council wine consumer segmentation slide handbook. http://winemarketcouncil.com/wp-content/uploads/2017/10/2017_WMC_Wine_Consumer_Segmentation_Slide_Handbook2.pdf (accessed December 2018).
- 118. Makela P, Gmel G, Grittner U, et al. (2006) Drinking patterns and their gender differences in europe. Alcohol Alcohol 41, i8–i18. [DOI] [PubMed] [Google Scholar]
- 119. Australian Institute of Health and Welfare (2010) Drinking Patterns in Australia, 2001–2007. Canberra: AIHW; Cat. no. PHE 133. [Google Scholar]
- 120. World Health Organization (2017) IER: global BMI 2016 female. http://gamapserver.who.int/mapLibrary/Files/Maps/Global_BMI_2016_Female.png (accessed December 2018).
- 121. World Health Organization (2017) IER: global BMI 2016 male. http://gamapserver.who.int/mapLibrary/Files/Maps/Global_BMI_2016_Male.png (accessed December 2018).
- 122. Stark KD, Van Elswyk ME, Higgins MR, et al. (2016) Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog Lipid Res 63, 132–152. [DOI] [PubMed] [Google Scholar]
- 123. McGorry PD, Nelson B, Markulev C, et al. (2017) Effect of omega-3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: the NEURAPRO randomized clinical trial. JAMA Psychiatry 74, 19–27. [DOI] [PubMed] [Google Scholar]
- 124. Murphy KJ, Meyer BJ, Mori TA, et al. (2007) Impact of foods enriched with n-3 long-chain polyunsaturated fatty acids on erythrocyte n-3 levels and cardiovascular risk factors. Br J Nutr 97, 749–757. [DOI] [PubMed] [Google Scholar]
- 125. Meyer BJ & Groot RHM (2017) Effects of omega-3 long chain polyunsaturated fatty acid supplementation on cardiovascular mortality: the importance of the dose of DHA. Nutrients 9, 1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bhatt DL, Steg PG, Miller M, et al. (2019) Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 380, 11–22. [DOI] [PubMed] [Google Scholar]
- 127. Manson JE, Cook NR, Lee IM, et al. (2019) Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 380, 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Brenna JT, Plourde M, Stark KD, et al. (2018) Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. Am J Clin Nutr 108, 211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Browning LM, Walker CG, Mander AP, et al. (2012) Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am J Clin Nutr 96, 748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0007114519000138.
click here to view supplementary material