Abstract
Microbial assemblages residing within and on animal gastric tissues contribute to various host beneficial processes that include diet accessibility and nutrient provisioning, and we sought to examine the degree to which intergenerational and community-acquired gut bacteria impact development in a tractable germ-free (GF) invertebrate model system. Coprophagy is a common behavior in cockroaches and termites that provides access to both nutrients and the primary means by which juveniles are inoculated with beneficial gut bacteria. This hypothesis was tested in the American cockroach (Periplaneta americana) by interfering with this means of acquiring gut bacteria, which resulted in GF insects that exhibited prolonged growth rates and gut tissue dysmorphias relative to wild-type (WT) P. americana. Conventionalization of GF P. americana via consumption of frass (feces) from conspecifics and siblings reared under non-sterile conditions resulted in colonization of P. americana gut tissues by a diverse microbial community and a significant (p < 0.05) recovery of WT level growth and hindgut tissue development phenotypes. These data suggest that coprophagy is essential for normal gut tissue and organismal development by introducing beneficial gut bacteria to P. americana, and that the GF P. americana model system is a useful system for examining how gut bacteria impact host outcomes.
Keywords: Germ-free insects, Gut microbiome, Development
Introduction
Host-associated bacteria are increasingly recognized as being inextricably involved in the health, development and evolution of their animal hosts, and the digestive tract is a major intersection for the host and its environment, and its gut bacterial consortia. In vertebrates, microbial ecosystems located within animal digestive tracts, hereafter referred to as “gut microbiota,” (Yasuda et al., 2015; Costea et al., 2018) can impact host health, behavior, dietary nutrient accessibility, infectious disease susceptibility, and disease morbidity (Sonnenburg & Bäckhed, 2016; Koppel, Maini Rekdal & Balskus, 2017; Parker et al., 2017; Foster, Rinaman & Cryan, 2017; Zhao & Elson, 2018). Invertebrates also harbor sparse-to-abundant multispecies microbial communities within gastric tissues that can be as specialized as those observed in vertebrates (Dillon & Dillon, 2004; Engel & Moran, 2013; Douglas, 2015), and the global distribution, trophic diversity, ecological contributions, and agricultural, environmental and human health impacts of insects have stimulated interest in insect-gut microbiota interactions. Although host diet accessibility and nutrient metabolism has been a major focus of insect host-gut microbiota work (Six, 2013; Engel & Moran, 2013; Brune & Dietrich, 2015; Peterson & Scharf, 2016; Kwong & Moran, 2016; Bonilla-Rosso & Engel, 2018), these microbial assemblages have also been shown to participate in zoonotic infections (Aksoy, 2018), mediate plant-insect interactions (Hansen & Moran, 2014; Casteel & Hansen, 2014; Shikano et al., 2017), shape host behavior (Keesey et al., 2017), and stimulate immune system functions (Ryu et al., 2008; Buchon, Broderick & Lemaitre, 2013) and host growth and development (Coon et al., 2014; Zheng et al., 2017). When the gut microbiota is comprised of, or dominated by, a single or few taxa, it is possible to link individual taxa to host benefits, as observed in Riptortus pedestris stink bugs (Burkholderia that confer pesticide resistance, (Kikuchi, Hosokawa & Fukatsu, 2007; Itoh et al., 2018)) and in honey bees (Gilliamella that degrades pollen constituents and Bifidobacterium asteroids that produce growth-promoting hormones, (Engel, Martinson & Moran, 2012; Kešnerová et al., 2017). Additionally, amenability of these insect models, as well as Drosophila fruit flies (Apidianakis & Rahme, 2011), to axenic/germ-free (GF) rearing has greatly facilitated efforts to assign discrete functions to specific gut taxa.
Among invertebrate model systems, cockroaches are also proving to be useful for exploring host-gut microbiota interactions. The gut microbiota of omnivorous cockroaches had been characterized primarily by cultivation and microscopy approaches (Bracke, Cruden & Markovetz, 1979; Cruden & Markovetz, 1980, 1987; Umunnabuike & Irokanulo, 1986; Dillon & Dillon, 2004; Thompson et al., 2012) until cultivation-independent community profiling (i.e., 16S rRNA gene amplicon sequencing) approaches became widely available. Tremendous phylogenetic diversity, spanning over 20 bacterial phyla (Mikaelyan et al., 2015), has been detected in cockroach guts, with members of the Bacteroidetes, Firmicutes, Fusobacteria, and Proteobacteria comprising the majority of the gut microbiota, and diet appears to exert a significant impact on presence and relative abundance of the gut microbiota (Schauer, Thompson & Brune, 2012; Krych et al., 2013; Sabree & Moran, 2014; Schauer et al., 2014; Dietrich, Köhler & Brune, 2014; Perez-Cobas et al., 2015; Mikaelyan et al., 2015; Tinker & Ottesen, 2016; Zheng et al., 2017). Additionally, cultivation under oxygen-limited conditions has coaxed new species of cockroach gut bacteria into cultivation (Tegtmeier et al., 2016a, 2018).
Cockroaches are amenable to axenic rearing and such studies have helped to shed light on some possible roles of gut bacteria in cockroach development and behavior. The majority of these studies have focused on the cockroach Blattella germanica, reporting successful but delayed development to adulthood, with normal fertility and viability of young (House, 1949; Clayton, 1959; Benschoter & Wrenn, 1972). Host aggregation, a common cockroach behavior, was found to be stimulated by gut bacterial products in B. germanica that were absent when B. germanica was treated with antibiotics (Wada-Katsumata et al., 2015). GF Shelfordella lateralis cockroaches have facilitated the determination of how oxygen impacts gut tissue colonization and metabolic activity of two bacterial strains isolated from S. lateralis (Tegtmeier et al., 2016b). Additionally, digestive tracts in GF S. lateralis exposed to environmental and animal-derived inocula were capable of enriching for bacteria, primarily members of the Bacteroidetes, Firmicutes, and Proteobacteria, that were closely related to gut residents typically found in S. lateralis gut tissues (Mikaelyan et al., 2016). Furthermore, a clear phylogenetic signal was detected between inoculum source (i.e., cockroach > termite > mice > soil) along with recapitulation of a gut microbiota composition typically observed in S. lateralis (Mikaelyan et al., 2016). Although these efforts have made great strides in illustrating how cockroaches and their gut microbiota collaborate, less is known about host physiological responses to gut microbiota colonization. Recent work has shown that consumption of feces (coprophagy) from conspecifics and nestmates is common in cockroaches (Nalepa, Bignell & Bandi, 2001; Kopanic et al., 2001), and is a means for the transfer of hindgut bacteria (Rosas et al., 2018). Additionally, coprophagy provides consumers with amino acids, lipids, carbohydrates, and micronutrients that remain in the diet post-digestion and from microbes that have colonized the fecal pellet during digestion or since its deposition in the environment (Nalepa, Bignell & Bandi, 2001). Cockroach nymphs exhibit the strongest responses to aggregation pheromones present in feces (Dambach, Stadler & Heidelbach, 1995), of which at least some of these chemical signals are produced by bacteria therein (Wada-Katsumata et al., 2015), suggesting that inoculation of early-stage nymphs with gut bacteria via coprophagy is important for normal development. This study seeks to detail the impact of coprophagy on Periplaneta americana physiology and development, and it is expected that physiological systems within the cockroach that are most heavily influenced by exposure to microbiota-enriched frass will highlight sites of host-microbiota interactions for further study.
Materials and Methods
Insects
Periplaneta americana nymphs, adults and ootheca were obtained from a live collection maintained in the Insectary at the Ohio State University Biological Sciences Greenhouse (Columbus, Ohio).
Ootheca treatment
Ootheca were manually detached from gravid P. americana females and surface sterilized by three rounds of cleaning using a detergent scrub (1% Alconox detergent), dilute bleach (0.08% sodium hypochlorite), and then an enzymatic lysis buffer (lysozyme 10 mg/ml, EDTA, Tris-HCl and Triton X-100), each step followed by three rinses with sterile MilliQ water (MQW) to remove antiseptic solution and induce intermittent hypo-osmotic shock on surface-clinging bacteria (Schwinghamer, 1980; Salema et al., 1982). An aliquot of each third MQW rinse was reserved to monitor effectiveness of previous cleanings to reduce bacterial load. Aseptic ootheca were incubated individually in sterile microcentrifuge tubes capped with sterile cotton at 31 °C for approximately 30 days until hatching, which yielded up to 16 GF nymphs per oothecum. No decreased oothecum viability due to washing treatments was observed.
Rearing germ-free P. americana
Cohorts of three to four GF first instar nymphs were aseptically-transferred to sterile rearing chambers of approximately 16 cm3, stocked with gamma-irradiated (aseptic) rat chow and aseptic water, which was renewed weekly. Rearing chambers were passively ventilated with air filtered through a 0.22 µm membrane.
Rearing conventionalized P. americana
To introduce bacteria native to P. americana, cohorts of three to four GF first instars were exposed to frass taken from a lab-maintained colony of non-sterile or “wild-type” (WT) P. americana in lieu of food for 3 days. Subsequently, conventionalized (Conv) first instar nymphs were housed under the same conditions as GF insects except for that sterility was not maintained. As each generation of P. americana typically acquires their gut microbial community through conspecific and nestmate coprophagy (Nalepa, Bignell & Bandi, 2001; Bell, Roth & Nalepa, 2007), the conventionalization approach used reflects the normal route of gut microbe acquisition.
Rearing wild-type P. americana
One-day old first instar insects from 10 ootheca were deposited in an aquarium containing 10 adult male cockroaches from a non-sterile mixed generation colony maintained in the lab and provided with gamma-irradiated rat chow and access to MQW ad libitum; nymphs from this colony were designated “WT”. WT hatchlings were free to interact with adult cockroaches and their frass to facilitate coprophagy and subsequent acquisition of normal gut microbiota. As cannibalism of deceased nestmates is also a putative mechanism for gut microbiota acquisition, late-stage nymphs from the non-sterile colony were sacrificed and deposited in the WT colony. WT insects did not experience spatial constraints or undergo the oothecum sterilization procedure as compared to GF and Conv insects and were exposed to unfiltered air.
Quality control
Quality control measures were employed throughout experiments to ensure maintenance of GF status and to confirm colonization of Conv individuals. Oothecum rinse water, collected between each antiseptic treatment, was plated in triplicate on Luria-Bertani agar (LBA) plates (incubated at 31 °C for up to 1 week) to detect cultivable contaminants flushed from ootheca, and to provide confidence that ootheca are aseptic as they enter incubation. Ootheca shedding no contaminants at final rinses were considered to be aseptic and used for subsequent experiments. At the time of hatching and installation into habitats, one first instar nymph from each oothecum, or the oothecum itself, was sacrificed and homogenized in sterile 1% PBS and plated on LBA in triplicate to confirm aseptic status. At the termination of insect growth, and just prior to insect dissection, frass was collected from rearing chambers, suspended in 1% PBS, and plated to confirm that habitats remained GF throughout the duration of the experiment. Bi-monthly sampling of GF-reared individuals for contamination was performed by homogenizing individuals at various instars and plating homogenate on LBA. During sterile treatment method development, diagnostic PCR with universal 16S primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1391R (5′-GACGGGCGGTGTGTRCA-3′) was performed on DNA extracted from homogenates prepared from GF insects and their frass to detect non-cultivable contaminants of the gut or habitat. Additionally, microscopic examination of 4′,6-diamidino-2-phenylindole (DAPI)-stained frass, gut contents, and gut thin-sections were performed to further evaluate the effectiveness of the GF, and conventionalization protocols.
Instar duration measurement
Germ-free and Conv nymphs raised in cohorts of three to four insects were monitored individually for molting activity, and dates of instar transitions of individuals within each cohort were recorded. Aquarium rearing of WT insects in cohorts of dozens of individuals prevented tracking of individual insects and corresponding molt date and developmental times within the WT treatment. Insects dissected at subsequent instars for gut morphological measurements resulted in decreasing sample sizes at later instars, and non-parametric statistical methods were used when making comparisons across instars. Targeting early instar insects constrains the experiment to a relatively short timeframe, as rearing to adulthood is prohibitively long (6–12 months for WT insects), with GF P. americana progression to adulthood uncertain.
Morphological measurements
Nymphs were collected as they molted to third, fourth, and fifth instars, and duration (days) of these instars were recorded. Previous work has shown that the cumulative effects of bacterial colonization, or lack thereof, in the host could be observed in fourth and fifth instars and thus all experimental measures in this study were taken from fifth instar individuals (Bracke, Cruden & Markovetz, 1978; Gijzen & Barugahare, 1992). Nymphs at designated life stages were dissected approximately 5 days after molting, at peak of feeding within instar (Valles, Strong & Koehler, 1996), to minimize variability in gut morphology associated with instar transitions. Eight morphological metrics were collected in millimeters (mm), unless otherwise noted: body length, body width, body mass (grams, g), whole gut length, foregut length, midgut length, hindgut length, and gut mass (g). FIJI image analysis package (ImageJ) was used to perform measurements of the full length of dissected guts (esophagus to anus) and their corresponding carcasses from digital images of these tissues taken immediately following dissection. Results of statistical tests performed on comparisons of morphological measurements are reported in Table S1. Additionally, gut compartments and bodies were traced and lengths measured, with measurements calibrated to a scale in each photo. Mass measurements were also collected for whole GF, Conv, and WT individuals prior to dissection and of their dissected digestive tracts using a microbalance. Additionally, qualitative observations of gut texture, color, opacity, and segmentation were collected from dissected fifth instar GF, Conv, and WT individuals.
Phenotype analysis
All statistics were performed in R using vegan and FSA packages (Ogle, Wheeler & Dinno, 2018; R Development Core Team, 2013; Oksanen et al., 2015). Morphological measurements at instar rather than calendar age yielded differences in sample size, as insects aged out of instar classes at different rates; non-parametric statistics were utilized to accommodate sample size variation. Instar duration, and morphological measurements were analyzed for statistical differences using a Mann–Whitney Test for pairwise-comparisons. Kruskal–Wallis and Dunn Tests were performed for multiple comparisons, with Bonferroni correction-adjusted p-values. Principal component analysis (PCA) was performed to simultaneously examine multiple morphological variables across treatments. The function “Varpart” within the vegan R package was used to partition variance among morphological variables and the PCA was constrained to the three variables representing 93% of the variation. Subsequently, multi-response permutation procedure (MRPP) was used to assess the significance of the observed differences between treatment centroids.
Results
Germ-free insects exhibited reduced width and prolonged instar duration
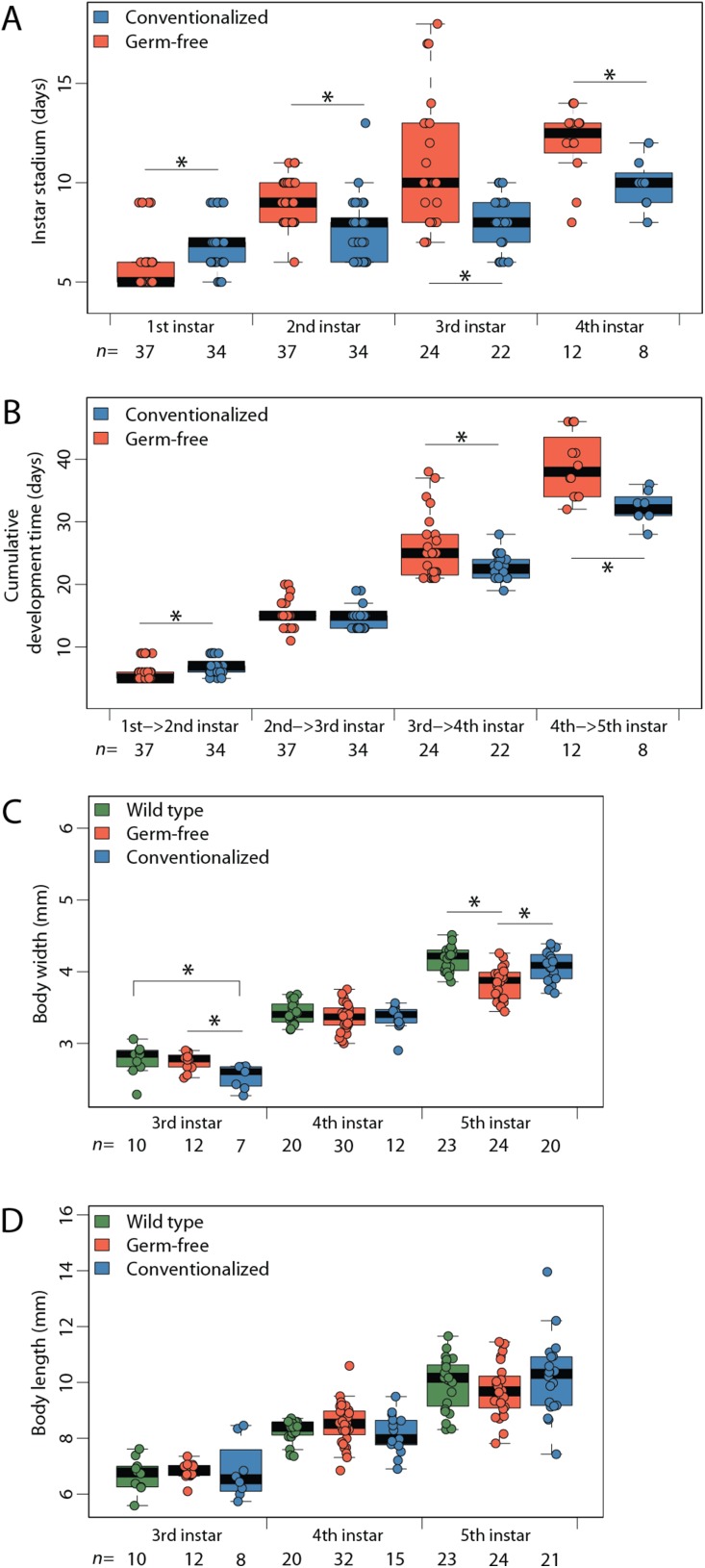
Germ-free (n = 37) P. americana individuals remained in each instar an average of two days longer than Conv (n = 34) individuals (Fig. 1A), which resulted in longer times between molts in GF insects and prolonged developmental periods (Fig. 1B) being observed. While Conv insects molted to fifth instar after an average of 32.3 days, GF insects required an average of 38.9 days to reach the same life stage. While stadium duration of WT insects was not obtained for every instar because of the complexity of tracking individual insects in a large cohort, average age at fifth instar was 30.2 days (Fig. S1). GF insects also exhibited the lowest average body width (3.84 mm) when compared to WT (4.18 mm) and Conv (4.08 mm) insects (Fig. 1C). Body lengths of GF and Conv individuals were not significantly different at third, fourth, or fifth instars (Fig. 1D), and body mass did not differ between treatments (Fig. S2).
Figure 1. Maturation rate and body morphology.
Limiting access to gut bacteria via coprophagy prolongs development in Periplanata americana. (A) Duration (stadium) of individuals within each life stage; (B) Cumulative development time; (C) Body width measurements; (D) Body length measurements. Asterisks indicate significant (p < 0.05) differences given a Dunn test. n, number of individuals measured per sample.
Conventionalization of germ-free P. americana recovers normal hindgut tissue morphology and development
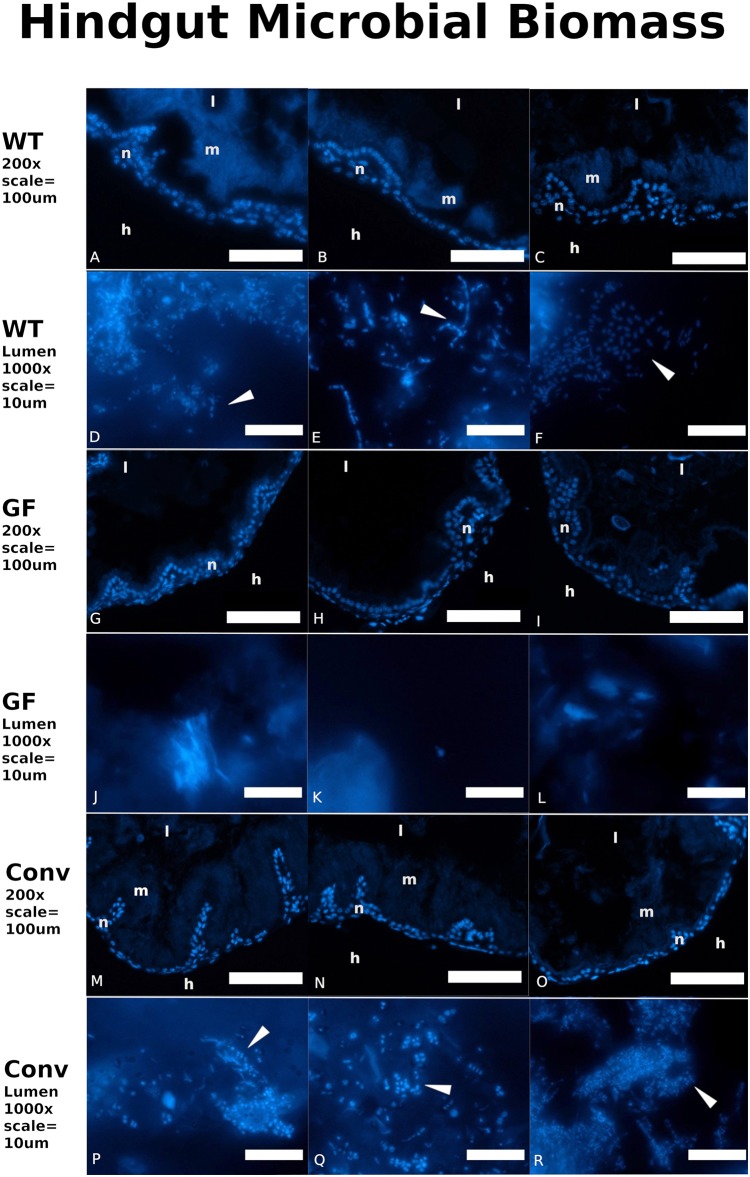
Distinct visual contrasts between fifth instar WT (n = 23) (Fig. 2A; Figs. S3A–S3C) and GF (n = 32) (Fig. 2B; Figs. S3D–S3F) hindgut tissues were consistently evident. WT P. americana hindguts are characterized by numerous lateral folds along the length of the hindgut that are visible as an undulating gut margin with lateral creases (Fig. 2D). All inspected WT hindguts were opaque, turgid in texture, and generally filled with mustard-yellow hindgut contents (Fig. 2A; Figs. S3A–S3C). Conv hindguts (Fig. 2C; Figs. S3G–S3I) were similar to those from WT insects in that they were turgid in texture and more opaque than GF hindguts (Fig. 2B; Figs. S3D–S3F), and Conv hindguts exhibited frequent lateral folds and coloration characteristic of WT insects. Further, dissection of Conv revealed mustard-yellow contents, similar to those observed in WT digestive tracts, and this appears to be a mix of digestate and bacterial biomass (note the fluorescence of material in gut lumen in Fig. 3) lodged in hindgut folds (Fig. 3). DAPI stained hindgut tissue thin sections reveal a dense mat of bacterial biomass adjacent to the host gut epithelial lining and planktonic bacterial growth deep within the lumen of WT and Conv insects (Fig. 3). In contrast, GF hindguts were uniformly flaccid, translucent, and displayed less frequent and less pronounced lateral folding (Fig. 2B; Figs. S2D–S2F). Additionally, gut contents were comprised of partially digested diet and no evidence of bacteria were observed (Fig. 3). No amplicon was observed after diagnostic PCR conducted on GF frass DNA extract (Fig. S4) using bacteria-specific primers. Despite thorough cleaning of fat bodies from guts at the time of dissection, the fat body specific endosymbiont Blattabacterium was detected in diagnostic PCR reactions of gut tissue DNA extracts from GF insects, negating the utility of bacteria-specific primers for contamination detection in gut homogenates by diagnostic PCR.
Figure 2. Qualitative comparisons of hindgut morphology in fifth instar Periplaneta americana.
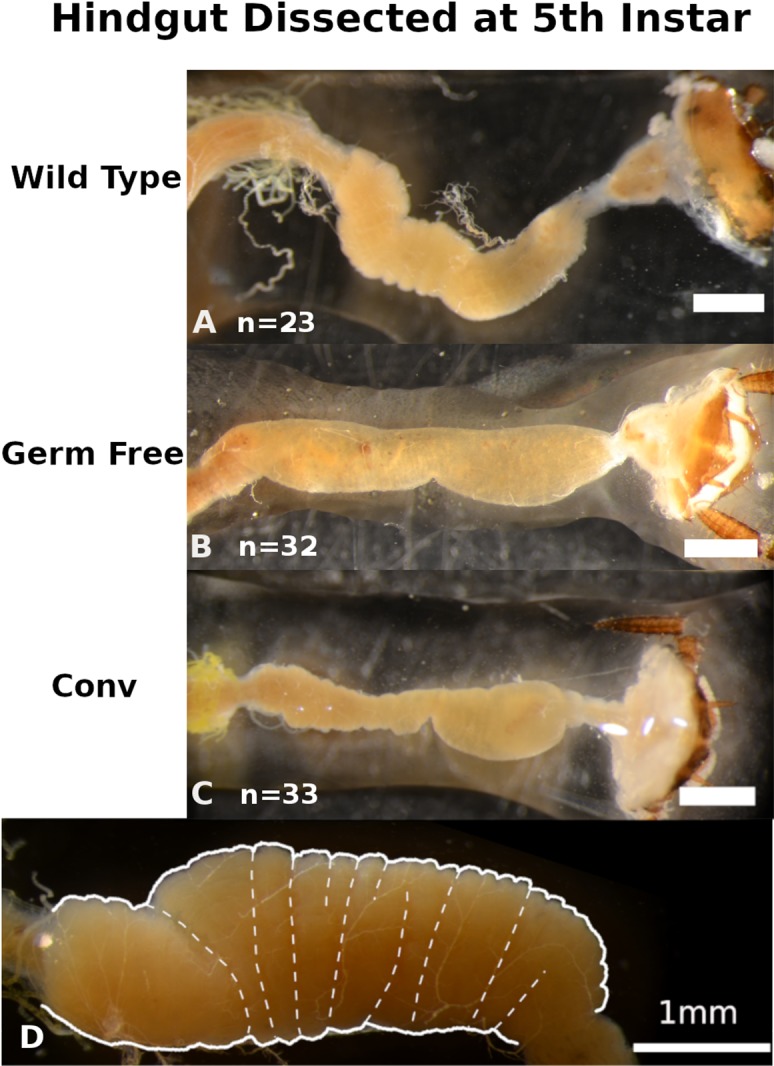
Exemplars from three wild-type (A), germ-free (B), and conventionalized (Conv.; C) P. americana individuals are presented. A magnified hindgut from a wild-type P. americana (D) is provided to highlight normal morphological features, including undulating gut margin highlighted by a thick white line and gut segmentation highlighted with thin dashed lines. Scale represents one mm.
Figure 3. Conventionalized Periplanata americana hindguts are enriched with microbial biomass.
Wild-type and conventionalized Periplanata americana hindguts are enriched with microbial biomass that aggregates adjacent to the epithelium, while germ-free hindguts lack microbial biomass. 4′,6-diamidino-2-phenylindole (DAPI) stained thin-sections of hindguts from wild-type (WT) (A–F) (n = 3), germ-free (GF) (G–L) (n = 3) and conventionalized (Conv) (M–R) (n = 3) P. americana were viewed under epifluorescence to visualize DNA associated with epithelium and luminal contents. h, Hemocoel; l, lumen; n, gut epithelial cell nuclei; m, microbial biomass; arrows—fluorescence consistent with bacterial morphology.
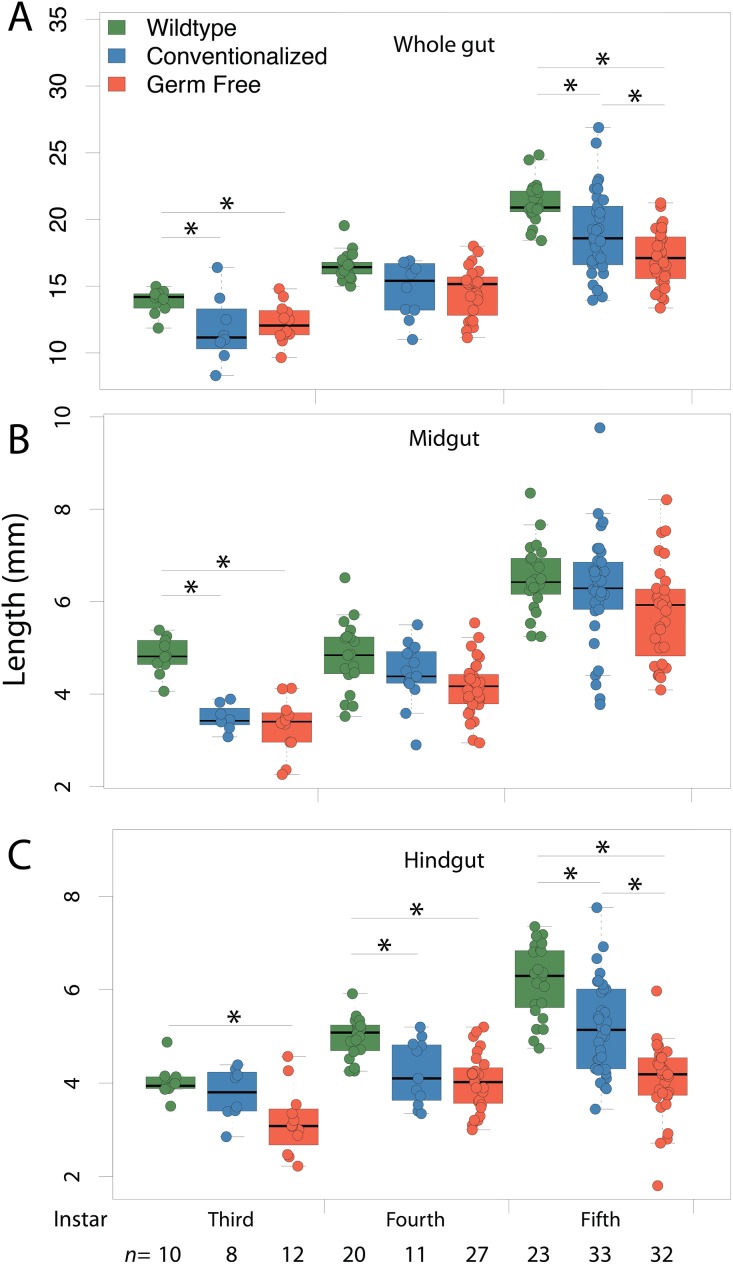
Average length of the complete fore-to-hindgut tissues was shorter in GF insects (17.13 mm) than in both Conv (18.96 mm) and WT (21.24 mm) insects (Fig. 4A; Dunn Test: GF-Conv p = 0.0244, Conv-WT p = 0.0028, GF-WT p = 0.0054). When fore- (Fig. S5) and mid-gut (Fig. 4B) sections were examined separately, average lengths did not differ significantly between any treatments at any instar, except for between GF (3.30 mm) and either WT (4.83 mm) or Conv (3.48 mm) third instar midguts (Dunn Test: WT-GF p = 0.0058, Conv-GF p = 0.0455). Conversely, hindgut length was significantly reduced in GF insects at third, fourth, and fifth instars relative to either Conv or WT insects (Fig. 4C). Finally, significant differences in gut mass were only detected at fourth instar between GF and WT insects (Fig. S6), with respective masses of 5.2 and 6.7 mg (Dunn Test: WT-GF p < 0.0070), but variability was high within treatments.
Figure 4. Gut morphology.
Conventionalization of germ-free Periplaneta americana partially recovers wild-type gut length morphology as individuals age. Whole gut (A) midgut (B) and hindgut (C) lengths were measured in germ-free (GF), conventionalized (Conv) and Wild-Type (WT) individuals at third, fourth and fifth instars, and, at the sixth instar for WT and Conv individuals. Asterisks indicate significant (p < 0.05) differences given a Dunn test. n, number of individuals measured per sample.
Wild-type, conventionalized, and germ-free insects bear morphological signatures that define a gradient across treatments
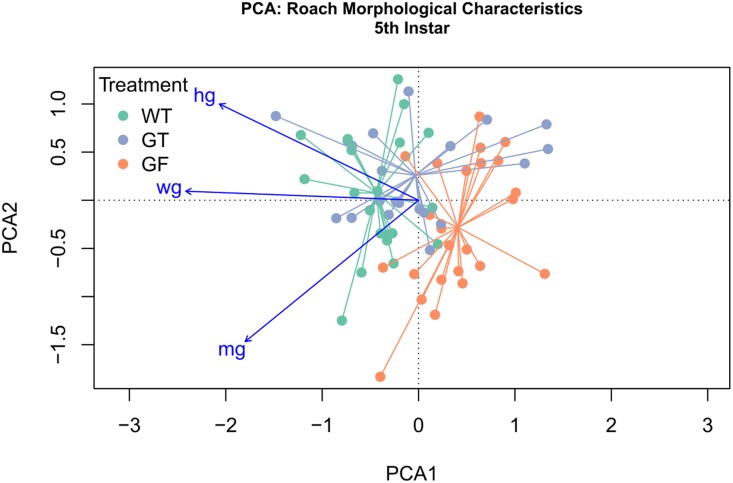
Of eight morphological variables examined in this study whole gut length, midgut length, and hindgut length explained 91.4% of variance across treatments (Fig. S7). PCA was performed to examine the relationship among treatments at each instar in reference to these three response variables. Ordination was conducted on the first two principal components, representing 84.5% and 6.9% of variance explained, respectively (Fig. 5). Treatment centroids were significantly different (MRPP: p < 0.001), with the Conv centroid being intermediate to that of GF and WT centroid. All vector loadings associated with morphological variables were significant, with strong linearity (p < 0.001, whole gut length r2 = 0.99, midgut length r2 = 0.91, hindgut length r2 = 0.89). Loading vectors for hindgut length and whole gut length demonstrate a gradient of low values in proximity to the GF centroid to high values toward the WT centroid. The vector for midgut length appears to follow a gradient associated with within-instar variation, as midgut length varies more within instar than across treatments. A second PCA was performed on data from all instars and treatments, simultaneously, with 93.1% and 1.9% of variance explained by the first two axes, yielding greater linearity of loading vectors but reduced separation of centroids (Fig. S8). With multiple instars represented within each treatment, vector loadings previously associated with within-instar variation (i.e., midgut length) realign to describe an across-treatment gradient, implying that variation in midgut length is better explained by treatment than instar or within-instar effects.
Figure 5. Principal Component Analysis (PCA) of morphological characteristics at fifth instar.
PCA and ordination was performed, constrained by the three variables explaining the greatest variation, specifically, whole gut length (wg), hindgut length (hg), and midgut length (mg). The first component explained 84.5% of variation, with 6.9% and 2.2% explained by the second and third components. Treatment clusters displayed the least overlap at instar 5 and are situated with Conventionalized (Conv) treatment sandwiched between germ-free (GF) treatments and Wild-Type (WT). Distance between treatment centroids was 0.22 between the WT and GF centroids, 0.15 between the Conv and GF centroids, and 0.12 between the Conv and WT centroids. All vector loadings are significant (p < 0.000999) with r2 values of 0.99 (wg), 0.91 (hg), and 0.89 (mg). Vector length is proportional to fit.
Discussion
We demonstrated that GF P. americana exposed to frass from lab-reared, WT conspecifics partially restored instar duration periods and digestive tissue development, particularly in hindgut tissues, to levels observed in WT insects, and these findings suggest a relationship between exposure to gut microbiota in cockroach frass and host digestive tissue and developmental outcomes. P. americana and other cockroaches typically live in dense multigenerational aggregates that afford them easy access to waste, discarded exoskeletons, and dead bodies, and the microbes therein, of conspecifics (Mira, 2000; Nalepa, Bignell & Bandi, 2001; Kopanic et al., 2001; Rychtář et al., 2014). These social and dietary behaviors are common in cockroaches and reflect a reliable means for the transmission of gut microbes that contribute to host fitness (Nalepa, Bignell & Bandi, 2001; Sabree & Moran, 2014). We hypothesized that P. americana relies upon community-acquired gut microbes for normal development, and preventing their exposure to these microbes (as in GF insects) would result in growth and developmental defects. GF insect growth stalled in fourth and fifth instars, as no insects progressed to sixth instar within the timeframe of the experiment and some insects remained in fifth instar indefinitely. Future work may determine whether germ free P. americana progress to adulthood. GF insects conventionalized by a single exposure to conspecific frass early in life (i.e., Conv insects) exhibited a partial, but significant, remediation of instar duration (Figs. 1A–1C) and gut tissue development (Figs. 2A–2D, 3 and 4A–4C). Interestingly, Conv insects exhibited an initial delay in growth and maturation at first instar, compared to both WT and GF insects (Figs. 1A–1D). This was likely due to the 3 day exposure to frass, without food, to enforce frass consumption. Yet, the cumulative benefit of microbial colonization allowed the Conv insects to surpass the GF and approach growth and maturation levels observed in WT insects by fifth instar. Given these data, it was surmised that the observed positive growth and developmental responses to frass exposure and subsequent gut colonization were cumulative due to persistent colonization of the cockroach gut rather than a burst of growth at time of exposure. It is notable that Blattabacterium was not removed as part of making GF insects and was present in GF, Conv, and WT insects, yet its contributions alone did not support normal host development in the GF insects.
The partial recovery of WT growth and development observed may be due to Conv insects having received only a single exposure to conspecific frass, instead of multiple exposures, during development. Specifically, Conv insects were restricted to a single exposure to WT frass during their first instar and were housed in small cohorts (n < 4) for the duration of the experiment (i.e., through fifth instar) and each experienced at least four moltings. Molting represents a potential bottleneck event during which portions of the gut lining, along with substantial gut contents and associated bacteria, are shed (Engel & Moran, 2013). If the exuvium and gut lining are not rapidly consumed by insects there is potential for loss of gut microbes, especially oxygen-sensitive taxa (e.g., some members of the Bacteroidetes and Firmicutes), that may not easily be horizontally reacquired through coprophagy within a relatively tiny cohort of insects of the same life-stage experiencing the same experimental conditions. Under normal conditions, developing insects living in multigenerational, population dense communities would have several opportunities to reacquire gut bacteria lost as a result of molting by coprophagy. As these experimental conditions were meant to severely limit exposure to microbes present in frass, further work is planned to determine minimum frass exposure frequency for nymphs to achieve WT growth and development.
An additional explanation for the observed results is that the absence of gut microbes in GF insects may have limited the host’s access to dietary nutrients that are typically liberated by members of the gut microbiota, and thus prolonged their development due to inadequate access to nutrients. Cockroaches and other insects that consume a diet comprised primarily of plant biomass that is rich in recalcitrant polysaccharides like pectin, cellulose, and hemicellulose have a characteristically enlarged hindgut colonized with diverse microbes that serves as an anaerobic digester for these biopolymers (Bignell, 1977, 2016; Breznak, 1982; Kane & Breznak, 1991; Kane, 1997; Bell, Roth & Nalepa, 2007; Watanabe & Tokuda, 2010). Exposure to conspecific frass partially recovered WT levels of gut tissue development and instar duration, which may indicate the colonization of some endemic taxa that facilitate dietary nutrient accessibility. It was notable that despite little size and weight difference at equivalent instar, the gastrointestinal tract developed differentially in GF and Conv insects, especially in the hindgut where the highest numbers of bacteria have been found. The hindgut is a major site of microbial activity along the cockroach alimentary tract, with the greatest numbers of bacteria consistently identified in this compartment through methods as diverse as microscopy, cultivation, and 16S sequencing (Bignell, 1977; Bracke, Cruden & Markovetz, 1979; Schauer, Thompson & Brune, 2012; Schauer et al., 2014). As such, it is unsurprising to find significant divergence in growth phenotype between GF and either WT or Conv insects in this compartment in the absence of microbial influence. Additionally, the microbial and biochemical composition of the frass is likely more similar to that of the hindgut than the midgut, which may explain why the remediative value of the frass was more pronounced in these tissues.
Gut microbes have been shown to mediate nutritional and immune factors and influence physiological systems (Engel & Moran, 2013), and numerous avenues to microbial promotion of host growth have been identified in other model organisms, and are likely functioning in P. americana. Efforts to link gut microbiota to host health and development have been spurred by the tremendous progress being made to detail the membership and composition of these communities. Model systems, especially GF animals, provide invaluable platforms for linking microbes to specific host outcomes. P. americana is a valuable addition to available invertebrate model systems because it harbors species of many bacterial taxonomic groups typically found in some vertebrates, including humans, is trophically omnivorous, and can be reared GF without antibiotics and, given this study, responds positively when exposed to conspecific frass. The relatively low rearing costs and high fecundity add to the amenability of the GF P. americana model system, which further ensures that many of the questions raised by results obtained in this study can easily be experimentally pursued. Further efforts to scrutinize how conspecific frass and its biochemical and microbial components are linked to host growth and development are underway and may reveal details of host-microbe interactions that may be generalizable to other animals.
Conclusion
A single inoculation of germ free P. americana with conspecific frass was sufficient to yield persistent gut colonization by microbiota, significantly decrease instar duration and speed maturation to fifth instar, while significantly increasing hindgut length. Future work should examine whether inoculation frequency further impacts development.
Supplemental Information
This file includes three bar charts summarizing various body mass, foregut length, and gut mass comparisons between germ-free, wild-type, and conventionalized P. americana. Additionally, a Venn diagram describing variation attributed to each morphological parameter and a Principal Components Analysis of treatment groups. Finally, a table summarizing the results of statistical comparisons of the treatment groups.
Raw data includes morphological measurements such as body mass (bm), body width (bw), body length (bl), gut mass (gm), whole gut length (wg), foregut length (fg), midgut length (mg), and hindgut length (hg) along with categorical variables associated with treatment, oothecum cohort, and instar.
Raw data includes duration of days spent in each instar in the column durval and cumulative days to each molt in the column moltval along with categorical variables associated with instar and treatment.
Contains R scripts for PCA at each instar and a PCA at all instars along with code for ordinations.
File contains R code for generating instar duration boxplots and time to 4th molt plots.
File includes R code for generating boxplots to summarize morphological data.
Acknowledgments
We thank George Keeney in the OSU Insectary and the anonymous reviewers for their comments on the manuscript. Thanks to Sema Osman for assistance with insect rearing.
Funding Statement
This work was supported by National Science Foundation IOS-1656786. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Benjamin C. Jahnes conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Madeline Herrmann performed the experiments.
Zakee L. Sabree conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
References
- Aksoy (2018).Aksoy S. Insect gut microbiota: accessories to the bite. Cell Host & Microbe. 2018;23(1):8–9. doi: 10.1016/j.chom.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Apidianakis & Rahme (2011).Apidianakis Y, Rahme LG. Drosophila melanogaster as a model for human intestinal infection and pathology. Disease Models & Mechanisms. 2011;4(1):21–30. doi: 10.1242/dmm.003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, Roth & Nalepa (2007).Bell WJ, Roth LM, Nalepa CA. Cockroaches: ecology, behavior, and natural history. Baltimore: The Johns Hopkins University Press; 2007. p. 248. [Google Scholar]
- Benschoter & Wrenn (1972).Benschoter CA, Wrenn RT. Germfree techniques for establishment and maintenance of a colony of aseptic German cockroaches. Annals of the Entomological Society of America. 1972;65(3):641–644. doi: 10.1093/aesa/65.3.641. [DOI] [Google Scholar]
- Bignell (1977).Bignell DE. Some observations on the distribution of gut flora in the American Cockroach, Periplaneta americana. Journal of Invertebrate Pathology. 1977;29(3):338–343. doi: 10.1016/S0022-2011(77)80040-2. [DOI] [Google Scholar]
- Bignell (2016).Bignell DE. The role of symbionts in the evolution of termites and their rise to ecological dominance in the tropics. Advances in Environmental Microbiology. 2016;2:121–172. doi: 10.1007/978-3-319-28068-4_6. [DOI] [Google Scholar]
- Bonilla-Rosso & Engel (2018).Bonilla-Rosso G, Engel P. Functional roles and metabolic niches in the honey bee gut microbiota. Current Opinion in Microbiology. 2018;43:69–76. doi: 10.1016/j.mib.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Bracke, Cruden & Markovetz (1978).Bracke JW, Cruden DL, Markovetz AJ. Effect of metronidazole on the intestinal microflora of the american cockroach, Periplaneta americana L. Antimicrobial Agents and Chemotherapy. 1978;13(1):115–120. doi: 10.1128/AAC.13.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracke, Cruden & Markovetz (1979).Bracke JW, Cruden DL, Markovetz AJ. Intestinal microbial flora of the of the american cockroach, Periplaneta americana L. Applied and Environmental Microbiology. 1979;38(5):945–955. doi: 10.1128/aem.38.5.945-955.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breznak (1982).Breznak JA. Intestinal microbiota of termites and other xylophagous insects. Annual Review of Microbiology. 1982;36(1):323–343. doi: 10.1146/annurev.mi.36.100182.001543. [DOI] [PubMed] [Google Scholar]
- Brune & Dietrich (2015).Brune A, Dietrich C. The gut microbiota of termites: digesting the diversity in the light of ecology and evolution. Annual Review of Microbiology. 2015;69(1):145–166. doi: 10.1146/annurev-micro-092412-155715. [DOI] [PubMed] [Google Scholar]
- Buchon, Broderick & Lemaitre (2013).Buchon N, Broderick NA, Lemaitre B. Gut homeostasis in a microbial world: insights from Drosophila melanogaster. Nature Reviews Microbiology. 2013;11(9):615–626. doi: 10.1038/nrmicro3074. [DOI] [PubMed] [Google Scholar]
- Casteel & Hansen (2014).Casteel CL, Hansen AK. Evaluating insect-microbiomes at the plant-insect interface. Journal of Chemical Ecology. 2014;40(7):836–847. doi: 10.1007/s10886-014-0475-4. [DOI] [PubMed] [Google Scholar]
- Clayton (1959).Clayton RB. A simplified method for the culture of Blattella germanica under aseptic conditions. Nature. 1959;184(4693):1166–1167. doi: 10.1038/1841166a0. [DOI] [Google Scholar]
- Coon et al. (2014).Coon KL, Vogel KJ, Brown MR, Strand MR. Mosquitoes rely on their gut microbiota for development. Molecular Ecology. 2014;23(11):2727–2739. doi: 10.1111/mec.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costea et al. (2018).Costea PI, Hildebrand F, Manimozhiyan A, Bäckhed F, Blaser MJ, Bushman FD, De Vos WM, Ehrlich SD, Fraser CM, Hattori M, Huttenhower C, Jeffery IB, Knights D, Lewis JD, Ley RE, Ochman H, O’Toole PW, Quince C, Relman DA, Shanahan F, Sunagawa S, Wang J, Weinstock GM, Wu GD, Zeller G, Zhao L, Raes J, Knight R, Bork P. Enterotypes in the landscape of gut microbial community composition. Nature Microbiology. 2018;3(1):8–16. doi: 10.1038/s41564-017-0072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden & Markovetz (1980).Cruden DL, Markovetz AJ. A thick-walled organism isolated from the cockroach gut by using a spent medium technique. Applied and Environmental Microbiology. 1980;39(1):261–264. doi: 10.1128/aem.39.1.261-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruden & Markovetz (1987).Cruden DL, Markovetz AJ. Microbial ecology of the cockroach gut. Annual Review of Microbiology. 1987;41(1):617–643. doi: 10.1146/annurev.mi.41.100187.003153. [DOI] [PubMed] [Google Scholar]
- Dambach, Stadler & Heidelbach (1995).Dambach M, Stadler A, Heidelbach J. Development of aggregation behavior in the German cockroach, Blattella germanica (Dictyoptera: Blattellidae) Entomologia Generalis. 1995;19(3):129–141. doi: 10.1127/entom.gen/19/1995/129. [DOI] [Google Scholar]
- Dietrich, Köhler & Brune (2014).Dietrich C, Köhler T, Brune A. The cockroach origin of the termite gut microbiota: patterns in bacterial community structure reflect major evolutionary events. Applied and Environmental Microbiology. 2014;80(7):2261–2269. doi: 10.1128/AEM.04206-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon & Dillon (2004).Dillon RJ, Dillon VM. The gut bacteria of insects: nonpathogenic interactions. Annual Reviews in Entomology. 2004;49(1):71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- Douglas (2015).Douglas AE. Multiorganismal insects: diversity and function of resident microorganisms. Annual Review of Entomology. 2015;60(1):17–34. doi: 10.1146/annurev-ento-010814-020822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel, Martinson & Moran (2012).Engel P, Martinson VG, Moran NA. Functional diversity within the simple gut microbiota of the honey bee. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(27):11002–11007. doi: 10.1073/pnas.1202970109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel & Moran (2013).Engel P, Moran NA. The gut microbiota of insects—diversity in structure and function. FEMS Microbiology Reviews. 2013;37(5):699–735. doi: 10.1111/1574-6976.12025. [DOI] [PubMed] [Google Scholar]
- Foster, Rinaman & Cryan (2017).Foster JA, Rinaman L, Cryan JF. Stress and the gut-brain axis: regulation by the microbiome. Neurobiology of Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijzen & Barugahare (1992).Gijzen HJ, Barugahare M. Contribution of anaerobic protozoa and methanogens to hindgut metabolic activities of the American cockroach, Periplaneta americana. Applied and Environmental Microbiology. 1992;58(8):2565–2570. doi: 10.1128/aem.58.8.2565-2570.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen & Moran (2014).Hansen AK, Moran NA. The impact of microbial symbionts on host plant utilization by herbivorous insects. Molecular Ecology. 2014;23(6):1473–1496. doi: 10.1111/mec.12421. [DOI] [PubMed] [Google Scholar]
- House (1949).House HL. Nutritional Studies with Blattella germanica (L.) Reared Under Aseptic Conditions: II. A Chemically Defined Diet. The Canadian Entomologist. 1949;81(5):105–112. doi: 10.4039/Ent81105-5. [DOI] [Google Scholar]
- Itoh et al. (2018).Itoh H, Hori T, Sato Y, Nagayama A, Tago K, Hayatsu M, Kikuchi Y. Infection dynamics of insecticide-degrading symbionts from soil to insects in response to insecticide spraying. ISME Journal. 2018;12(3):909–920. doi: 10.1038/s41396-017-0021-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane (1997).Kane MD. Microbial fermentation in insect guts. In: Mackie RI, White BA, editors. Gastrointestinal Microbiology. Boston: Springer; 1997. pp. 231–265. [Google Scholar]
- Kane & Breznak (1991).Kane MD, Breznak JA. Effect of host diet on production of organic acids and methane by cockroach gut bacteria. Applied and Environmental Microbiology. 1991;57(9):2628–2634. doi: 10.1128/aem.57.9.2628-2634.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesey et al. (2017).Keesey IW, Koerte S, Khallaf MA, Retzke T, Guillou A, Grosse-Wilde E, Buchon N, Knaden M, Hansson BS. Pathogenic bacteria enhance dispersal through alteration of Drosophila social communication. Nature Communications. 2017;8(1):265. doi: 10.1038/s41467-017-00334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kešnerová et al. (2017).Kešnerová L, Mars RAT, Ellegaard KM, Troilo M, Sauer U, Engel P. Disentangling metabolic functions of bacteria in the honey bee gut. PLOS Biology. 2017;15(12):e2003467. doi: 10.1371/journal.pbio.2003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi, Hosokawa & Fukatsu (2007).Kikuchi Y, Hosokawa T, Fukatsu T. Insect-microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Applied and Environmental Microbiology. 2007;73(13):4308–4316. doi: 10.1128/AEM.00067-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopanic et al. (2001).Kopanic RJ, Holbrook GL, Sevala V, Schal C. An adaptive benefit of facultative coprophagy in the German cockroach Blattella germanica. Ecological Entomology. 2001;26(2):154–162. doi: 10.1046/j.1365-2311.2001.00316.x. [DOI] [Google Scholar]
- Koppel, Maini Rekdal & Balskus (2017).Koppel N, Maini Rekdal V, Balskus EP. Chemical transformation of xenobiotics by the human gut microbiota. Science. 2017;356(6344):eaag2770. doi: 10.1126/science.aag2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krych et al. (2013).Krych L, Hansen CHF, Hansen AK, Van Den Berg FWJ, Nielsen DS. Quantitatively different, yet qualitatively alike: a meta-analysis of the mouse core gut microbiome with a view towards the human gut microbiome. PLOS ONE. 2013;8(5):e62578. doi: 10.1371/journal.pone.0062578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong & Moran (2016).Kwong WK, Moran NA. Gut microbial communities of social bees. Nature Reviews Microbiology. 2016;14(6):374–384. doi: 10.1038/nrmicro.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaelyan et al. (2015).Mikaelyan A, Köhler T, Lampert N, Rohland J, Boga H, Meuser K, Brune A. Classifying the bacterial gut microbiota of termites and cockroaches: a curated phylogenetic reference database (DictDb) Systematic and Applied Microbiology. 2015;38(7):472–482. doi: 10.1016/j.syapm.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Mikaelyan et al. (2016).Mikaelyan A, Thompson CL, Hofer MJ, Brunea A. Deterministic assembly of complex bacterial communities in guts of germ-free cockroaches. Applied and Environmental Microbiology. 2016;82(4):1256–1263. doi: 10.1128/AEM.03700-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira (2000).Mira A. Exuviae eating: a nitrogen meal? Journal of Insect Physiology. 2000;46(4):605–610. doi: 10.1016/S0022-1910(99)00146-8. [DOI] [PubMed] [Google Scholar]
- Nalepa, Bignell & Bandi (2001).Nalepa CA, Bignell DE, Bandi C. Detritivory, coprophagy, and the evolution of digestive mutualisms in Dictyoptera. Insectes Sociaux. 2001;48(3):194–201. doi: 10.1007/PL00001767. [DOI] [Google Scholar]
- Ogle, Wheeler & Dinno (2018).Ogle DH, Wheeler P, Dinno A. R package version 0.8.22https://github.com/droglenc/FSA FSA: Fisheries Stock Analysis. 2018
- Oksanen et al. (2015).Oksanen J, Blanchet F, Friendly M, Kindt R. vegan: community ecology package. Ordination methods, diversity analysis and other functions for community and vegetation ecologists. Version 2.3-3https://CRAN.R-project.org/package=vegan 2015
- Parker et al. (2017).Parker A, Lawson MAE, Vaux L, Pin C. Host-microbe interaction in the gastrointestinal tract. Environmental Microbiology. 2017;20(7):2337–2353. doi: 10.1111/1462-2920.13926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Cobas et al. (2015).Perez-Cobas EA, Maiques E, Angelova A, Carrasco P, Moya A, Latorre A. Diet shapes the gut microbiota of the omnivorous cockroach Blattella germanica. FEMS Microbiology Ecology. 2015;91(4):1–14. doi: 10.1093/femsec/fiv022. [DOI] [PubMed] [Google Scholar]
- Peterson & Scharf (2016).Peterson BF, Scharf ME. Lower termite associations with microbes: synergy, protection, and interplay. Frontiers in Microbiology. 2016;7(e6577):422. doi: 10.3389/fmicb.2016.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team (2013).R Development Core Team . R: a language and environment for statistical computing. Vienna: The R Foundation for Statistical Computing; 2013. [3 May 2016]. [Google Scholar]
- Rosas et al. (2018).Rosas T, García-Ferris C, Domínguez-Santos R, Llop P, Latorre A, Moya A. Rifampicin treatment of Blattella germanica evidences a fecal transmission route of their gut microbiota. FEMS Microbiology Ecology. 2018;94(2):293. doi: 10.1093/femsec/fiy002. [DOI] [PubMed] [Google Scholar]
- Rychtář et al. (2014).Rychtář J, Frynta D, Tomek J, Varadinováí Z, Brom C. Waste recycling can promote group living: a cockroach case study. Letters in Biomathematics. 2014;1(1):17–22. doi: 10.1080/23737867.2014.11414467. [DOI] [Google Scholar]
- Ryu et al. (2008).Ryu J-H, Kim S-H, Lee H-Y, Bai JY, Nam Y-D, Bae J-W, Lee DG, Shin SC, Ha E-M, Lee W-J. Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science. 2008;319(5864):777–782. doi: 10.1126/science.1149357. [DOI] [PubMed] [Google Scholar]
- Sabree & Moran (2014).Sabree ZL, Moran NA. Host-specific assemblages typify gut microbial communities of related insect species. SpringerPlus. 2014;3(1):138. doi: 10.1186/2193-1801-3-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salema et al. (1982).Salema MP, Parker CA, Kidby DK, Chatel DL, Armitage TM. Rupture of nodule bacteria on drying and rehydration. Soil Biology and Biochemistry. 1982;14(1):15–22. doi: 10.1016/0038-0717(82)90071-2. [DOI] [Google Scholar]
- Schauer, Thompson & Brune (2012).Schauer C, Thompson CL, Brune A. The bacterial community in the gut of the cockroach Shelfordella lateralis reflects the close evolutionary relatedness of cockroaches and termites. Applied and Environmental Microbiology. 2012;78(8):2758–2767. doi: 10.1128/AEM.07788-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer et al. (2014).Schauer C, Thompson C, Brune A, Li S, Wu Z, Korb J. Pyrotag sequencing of the gut microbiota of the cockroach Shelfordella lateralis reveals a highly dynamic core but only limited effects of diet on community Structure. PLOS ONE. 2014;9(1):e85861. doi: 10.1371/journal.pone.0085861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwinghamer (1980).Schwinghamer EA. A method for improved lysis of some gram-negative bacteria. FEMS Microbiology Letters. 1980;7(2):157–162. doi: 10.1111/j.1574-6941.1980.tb01597.x. [DOI] [Google Scholar]
- Shikano et al. (2017).Shikano I, Rosa C, Tan C-W, Felton GW. Tritrophic interactions: microbe-mediated plant effects on insect herbivores. Annual Review of Phytopathology. 2017;55(1):313–331. doi: 10.1146/annurev-phyto-080516-035319. [DOI] [PubMed] [Google Scholar]
- Six (2013).Six DL. The bark beetle holobiont: why microbes matter. Journal of Chemical Ecology. 2013;39(7):989–1002. doi: 10.1007/s10886-013-0318-8. [DOI] [PubMed] [Google Scholar]
- Sonnenburg & Bäckhed (2016).Sonnenburg JL, Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeier et al. (2018).Tegtmeier D, Belitz A, Radek R, Heimerl T, Brune A. Ereboglobus luteus gen. nov. sp. nov. from cockroach guts, and new insights into the oxygen relationship of the genera Opitutus and Didymococcus (Verrucomicrobia: Opitutaceae) Systematic and Applied Microbiology. 2018;41(2):101–112. doi: 10.1016/j.syapm.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Tegtmeier et al. (2016a).Tegtmeier D, Riese C, Geissinger O, Radek R, Brune A. Breznakia blatticola gen. nov. sp. nov. and Breznakia pachnodae sp. nov., two fermenting bacteria isolated from insect guts, and emended description of the family Erysipelotrichaceae. Systematic and Applied Microbiology. 2016a;39(5):319–329. doi: 10.1016/J.SYAPM.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Tegtmeier et al. (2016b).Tegtmeier D, Thompson CL, Schauer C, Brune A. Oxygen affects colonization and metabolic activities of gut bacteria in a gnotobiotic cockroach model. Applied and Environmental Microbiology. 2016b;82(4):1080–1089. doi: 10.1128/AEM.03130-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson et al. (2012).Thompson CL, Vier R, Mikaelyan A, Wienemann T, Brune A. “Candidatus Arthromitus” revised: segmented filamentous bacteria in arthropod guts are members of Lachnospiraceae. Environmental Microbiology. 2012;14(6):1454–1465. doi: 10.1111/j.1462-2920.2012.02731.x. [DOI] [PubMed] [Google Scholar]
- Tinker & Ottesen (2016).Tinker KA, Ottesen EA. The core gut microbiome of the American cockroach, Periplaneta americana, is stable and resilient to dietary shifts. Applied and Environmental Microbiology. 2016;82(22):6603–6610. doi: 10.1128/AEM.01837-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umunnabuike & Irokanulo (1986).Umunnabuike AC, Irokanulo EA. Isolation of Campylobacter subsp. jejuni from Oriental and American cockroaches caught in kitchens and poultry houses in Vom, Nigeria. International Journal of Zoonoses. 1986;13(3):180–186. [PubMed] [Google Scholar]
- Valles, Strong & Koehler (1996).Valles SM, Strong CA, Koehler PG. Inter- and intra-instar food consumption in the German cockroach, Blattella germanica. Entomologia Experimentalis et Applicata. 1996;79(2):171–178. doi: 10.1111/j.1570-7458.1996.tb00823.x. [DOI] [Google Scholar]
- Wada-Katsumata et al. (2015).Wada-Katsumata A, Zurek L, Nalyanya G, Roelofs WL, Zhang A, Schal C. Gut bacteria mediate aggregation in the German cockroach. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(51):15678–15683. doi: 10.1073/pnas.1504031112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe & Tokuda (2010).Watanabe H, Tokuda G. Cellulolytic systems in insects. Annual Review of Entomology. 2010;55(1):609–632. doi: 10.1146/annurev-ento-112408-085319. [DOI] [PubMed] [Google Scholar]
- Yasuda et al. (2015).Yasuda K, Oh K, Ren B, Tickle TL, Franzosa EA, Wachtman LM, Miller AD, Westmoreland SV, Mansfield KG, Vallender EJ, Miller GM, Rowlett JK, Gevers D, Huttenhower C, Morgan XC. Biogeography of the intestinal mucosal and lumenal microbiome in the rhesus macaque. Cell Host & Microbe. 2015;17(3):385–391. doi: 10.1016/j.chom.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao & Elson (2018).Zhao Q, Elson CO. Adaptive immune education by gut microbiota antigens. Immunology. 2018;154(1):28–37. doi: 10.1111/imm.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. (2017).Zheng H, Powell JE, Steele MI, Dietrich C, Moran NA. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(18):4775–4780. doi: 10.1073/pnas.1701819114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file includes three bar charts summarizing various body mass, foregut length, and gut mass comparisons between germ-free, wild-type, and conventionalized P. americana. Additionally, a Venn diagram describing variation attributed to each morphological parameter and a Principal Components Analysis of treatment groups. Finally, a table summarizing the results of statistical comparisons of the treatment groups.
Raw data includes morphological measurements such as body mass (bm), body width (bw), body length (bl), gut mass (gm), whole gut length (wg), foregut length (fg), midgut length (mg), and hindgut length (hg) along with categorical variables associated with treatment, oothecum cohort, and instar.
Raw data includes duration of days spent in each instar in the column durval and cumulative days to each molt in the column moltval along with categorical variables associated with instar and treatment.
Contains R scripts for PCA at each instar and a PCA at all instars along with code for ordinations.
File contains R code for generating instar duration boxplots and time to 4th molt plots.
File includes R code for generating boxplots to summarize morphological data.
Data Availability Statement
The following information was supplied regarding data availability:
Raw measurements are available in Data S1 and Data S2. Supplementary Files Code S1, Code S2, and Code S3 include the R code used to generate figures and statistics.