Abstract
Background
Both attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) are neurodevelopmental disorders with high prevalence. They are often comorbid and both exhibit abnormalities in sustained attention, yet common and distinct neural patterns of ASD and ADHD remain unidentified.
Aims
To investigate shared and distinct functional connectivity patterns in a relatively large sample of boys (7–15 years old) with ADHD, ASD and typical development (TD) matched by age, gender, and IQ.
Method
We applied machine learning techniques to investigate patterns of surface-based brain resting state connectivity in 86 boys with ASD, 83 boys with ADHD, and 125 boys with TD.
Results
We observed increased functional connectivity within the limbic and somatomotor networks in boys with ASD compared to boys with TD. We also observed increased functional connectivity within the limbic, visual, default mode, somatomotor, dorsal attention, frontoparietal, and ventral attention networks in boys with ADHD compared to boys with ASD. In addition, using a machine learning approach, we were able discriminate TD from ASD, TD from ADHD, and ASD from ADHD with accuracy rates of 76.3%, 84.1%, and 79.1%, respectively.
Conclusion
Our results may shed new light on the underlying mechanisms of ASD and ADHD and facilitate the development of new diagnostic methods for these disorders.
Introduction
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder characterized by inattention and abnormal hyperactivity and impulsivity, affecting nearly 6% of children 1. Autism spectrum disorder (ASD) is another highly prevalent neurodevelopmental disorder characterized by difficulties in social communication and social interaction 2. ADHD and ASD are often comorbid 3,4, with about 30% of ASD patients having comorbid ADHD characterized by age-inappropriate inattention, impulsiveness, and hyperactivity. Neuroimaging studies have shown that both disorders are associated with abnormal resting state functional brain connectivity 5–12. For instance, studies have suggested that compared with typical development (TD), 1) children with ADHD showed disrupted functional connectivity patterns in brain regions involved in attention and sensory processing 7,8 and 2) children with ASD displayed increased resting state functional connectivity in the posterior cingulate cortex and salience network, and the strength of functional connectivity was linked to severity of social interaction deficits 5,9,12. These studies have significantly enhanced the neurophysiological understanding of ADHD and ASD. Nevertheless, the mechanisms underlying the comorbidity and distinction between the two disorders remain unclear. In this study, taking advantage of the Autism Brain Imaging Data Exchange (ABIDE) and ADHD200 data set (http://fcon_1000.projects.nitrc.org/indi/adhd200/), we investigated shared and distinct functional connectivity patterns in a relatively large sample of boys (7–15 years old) with ADHD, ASD, and TD matched by age, gender, and IQ. For better functional alignment across subjects, the FreeSurfer image analysis suite was applied to generate a cortical surface for each subject 13. We hypothesized that 1) children with ASD and ADHD would be associated with altered functional connectivity compared to children with TD, and the altered patterns may be associated with symptoms of ASD and ADHD; and 2) machine learning techniques could be used to identify distinct and common functional connectivity features for both ASD and ADHD.
MATERIALS AND METHODS
Subject sample
We used an independent sample of individuals with ASD and individuals with TD from the Autism Brain Imaging Data Exchange (ABIDE) and individuals with ADHD from the ADHD200 data set (http://fcon_1000.projects.nitrc.org/indi/adhd200/). The inclusion criteria were as follows: i) full scale IQ (F-IQ) scores >80; ii) aged between 7 and 15 years to minimize potential developmental effects 14; iii) scanned in a 3T MRI scanner to increase between-site reliability 15; iv) right-handed; v) diagnosis of ASD based on DSM-IV-TR and assessed with the Autism Diagnostic Observation Schedule (ADOS), the Autism Diagnostic Interview–Revised (ADI-R), or both; vi) children with ASD do not have comorbid ADHD and children with ADHD do not have comorbid ASD; vii) diagnosis of ADHD based on DSM-IV-TR without Axis I disorders. In total, 294 participants fit the above criteria and were included in the present study. Fifty-five children with ASD (65%), sixty-one children with ADHD (73%), and all children with TD were psychotropic medication-naıve. Among children with ADHD, 44 met criteria for combined type ADHD, 3 met criteria for hyperactive/impulsive type ADHD, and 36 met criteria for predominantly inattentive type ADHD. Each site was required to confirm that their local Institutional Review Board (IRB) or ethics committee had approved both the initial data collection and sharing the datasets. Details of site-specific protocols, informed consent, and ethical approval at the time of the scan for each data set can be found at http://fcon_1000.projects.nitrc.org.
Data preprocessing
Anatomical image data were processed using FreeSurfer, version 5.3.0 software package (http://surfer.nmr.mgh.harvard.edu/) 16. To increase anatomical validation cross individuals with ADHD, ASD, and TD, FreeSurfer was used for segmentation of subcortical structures and automatic tessellation of the cortical surface because cortical surface variability is considerably improved by segmentation. The preprocessing of anatomical data was as follows: 1) motion correction and non-uniformity correction, 2) automatic Talairach transformation, 3) intensity normalization, 4) skull strip and segmentation of the subcortical white matter and gray matter, 5) tessellation of the white matter and gray matter, 6) surface smoothing and inflation, 7) topology correction, and 8) parcellation. Automated segmentation and parcellation results were reviewed for quality and corrected by two trained experts (authors MJ and WS) as necessary.
Resting-state fMRI data sets were processed with the CONN functional connectivity toolbox (http://www.nitrc.org/projects/conn) 17. Preprocessing involved 1) realignment to the mean image, 2) removal of volumes with a mean intensity >1.5% of the mean global signal or 0.5mm/TR framewise displacement to reduce the effect of head movement, 3) CompCor correction to reduce physiological and other noise artifacts 18, 4) entering segmented CSF and white matter as confounding regressors at the subject- level in FreeSurfer, and 5) band-pass filtering of the functional image (0.01–0.08 Hz).
ROIs and Connectivity analysis
We used 162 ROIs adopted from the Desikan–Killiany parcellation atlas by FreeSurfer 19 (Supplementary Table 1, Supplementary Figure 1). Mean time series were obtained for each subject by averaging the fMRI time series over all voxels in each of the 162 ROIs. Functional connectivity was estimated based on these regional mean time series by calculating the pairwise Pearson correlation coefficient between all possible (162×161/2=13041) ROI pairs. A symmetric connectivity matrix was constructed to represent these connections. Correlation coefficients were Fisher z-transformed to increase normality for statistical analyses.
Classification Analysis: Discriminating TD, ASD, and ADHD
To investigate diagnostic features between ADHD and ASD, a feature selection approach combining a univariate t-test and multivariate support vector machine- recursive feature elimination (SVM-RFE) was performed 20. To avoid the risk of overfitting, all analyses were performed using 10-fold cross validation21.
In the first step, we analyzed group-level differences of features between groups. Significant differences for each pair of ROIs were assessed using a mass univariate two sample t-test with a threshold of P < 0.001 and false discovery rate (FDR) correction. Features showing significant difference were retained for the remaining analyses. Our logic was that these features would be the most likely to contribute to the discrimination between groups.
In the second step, we used SVM-RFE to select the features with the most discriminative power for the classifier itself. SVM-RFE was used to train the classification model and obtain weights for each feature. The features were ranked according to the absolute values of weights, and the lowest ranking feature was discarded. Then the classification model was trained using the new feature set (i.e. without the discarded feature). This procedure was repeatedly performed until the feature set was empty. We conducted a full backward elimination procedure to further select the features with the highest classification accuracy. Since we used a 10-fold cross validation strategy to estimate the performance of the classifiers and feature ranking and each iteration was based on a slightly different dataset, the selected feature sets differed slightly from iteration to iteration.
To determine the most discriminative features, a consensus discrimination map which aggregated features selected in all cross validation iterations was used. Regional weight, which represents the contribution of each feature for discriminating different groups, was denoted by the number of ROI occurrences in the consensus discrimination map 22. The discriminative power of each feature was denoted by the average of its classification weights across all iterations. We conducted linear regression analyses of group-level differences to select features based on SVM-RFE and core symptom severity in ASD (total SRS scores), adjusting for data collection site, FIQ, and age using SPSS. Regression analyses were corrected for multiple comparisons using FDR correction (p<0.05).
Features with the most discriminative power were fed to an SVM with a linear kernel, which was implemented using LIBSVM. The classification (ASD vs. TD; ADHD vs. TD; ADHD vs. ASD) was also based on 10-fold cross validation, and the performance of the classifier was evaluated by accuracy, sensitivity, and specificity. Nonparametric permutation tests (1000 times) were used to estimate the statistical significance of the observed classification accuracy. We randomly permuted the class labels of the data prior to training. Cross validation was then performed on the permuted dataset and the procedure was repeated 1000 times. If the classifier trained on real class labels had an accuracy exceeding the 95% confidence interval generated from the accuracies of the classifiers trained on randomly relabeled class labels, this classifier was considered to be well-performing.
RESULTS
Demographic and Clinical Characteristics
294 subjects (86 boys with ASD, 83 boys with ADHD, and 125 boys with TD) were included in the study. There were no significant differences between the three groups for FIQ (p=0.51) and age (p=0.26). Demographic and clinical characteristics for all participants included in the analyses are presented in Table 1. There was a significant difference between ASD and TD groups for SRS total scores (p<0.001).
Table 1.
Demographic and Clinical Characteristics
| Measure | ASD n = 86 |
TD n = 125 |
ADHD n = 83 |
Group Comparisons | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F value | P value | |
| Age (years) | 11.4 | 2.1 | 10.9 | 1.6 | 11.2 | 1.8 | 1.32 | 0.267 |
| Full IQ | 110.4 | 13.2 | 111.7 | 9.7 | 109.9 | 13.1 | 0.67 | 0.512 |
| ADI-R | ||||||||
| Social | 18.9 | 4.9 | 0 | |||||
| Non-verbal | 8.34 | 3.1 | ||||||
| Verbal | 14.9 | 4.3 | ||||||
| SRS total scores | 91.8 | 25.7 | 19.2 | 12.4 | t = 26.3 | < 0.0001 | ||
Abbreviations: ADHD: attention deficit hyperactivity disorder; ASD: autism spectrum disorder; LR: likelihood ratios; TD: typically developed
Diagnostic features of ROI-to-ROI functional connectivity analysis
Comparison of the TD and ASD groups showed 72 ROI-to-ROI increased connectivities in the ASD group compared to the TD group (Figure 1). The ASD group showed increased connectivity in brain areas associated with the limbic, visual, default mode, somatomotor, dorsal attention, frontoparietal, and ventral attention networks. The TD group only showed 9 ROI-to-ROI increased connectivities in brain areas associated with the visual, default mode, dorsal attention, frontoparietal, and ventral attention networks. The classification accuracy of discriminating TD from ASD was 76.3% (p < 0.001; see Table 2).
Figure 1.
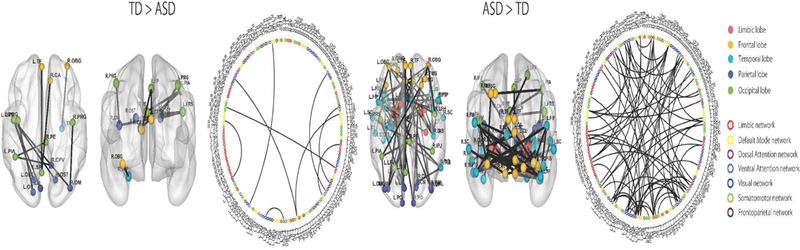
ROI-to-ROI functional connectivity analysis between TD and ASD. The ASD group showed predominantly higher connectivity than the TD group. Colored circles indicate brain regions. Colored lines indicate networks.
Table 2.
Results of machine learning analysis
| Method | Accuracy | Sensitibity | Specificity | Postive LR |
Negative LR |
|---|---|---|---|---|---|
| (%) | (%) | (%) | |||
| TD_ASD | 76.3 | 79.2 | 63.9 | 2.19 | 0.33 |
| TD_ADHD | 84.1 | 88.8 | 76.1 | 3.72 | 0.15 |
| ASD_ADHD | 79.3 | 75.6 | 83.1 | 4.47 | 0.29 |
Abbreviations: ADHD: attention deficit hyperactivity disorder; ASD: autism spectrum disorder; LR: likelihood ratios; TD: typically developed
Comparison of the TD and ADHD groups showed 8 ROI-to-ROI increased connectivities in the ADHD group compared to the TD group (Figure 2). Specifically, the ADHD group showed increased connectivity in brain areas associated with the limbic, ventral attention, visual, and default mode networks. In addition, the TD group showed 2 ROI-to-ROI increased connectivities in brain areas associated with the default mode and visual networks. The classification accuracy of discriminating TD from ADHD was 84.1% (p < 0.001; see Table 2).
Figure 2.
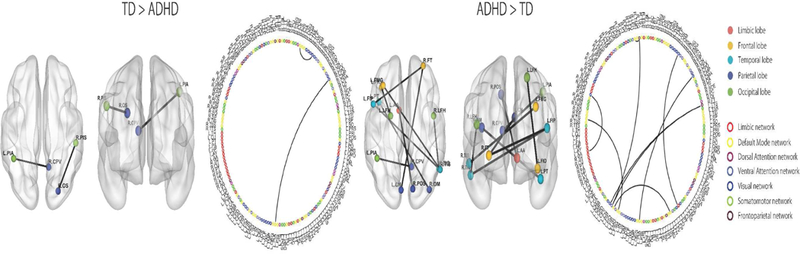
ROI-to-ROI functional connectivity analysis between TD and ADHD. The ADHD group showed predominantly higher connectivity than the TD group. Colored circles indicate brain regions. Colored lines indicate networks.
Comparison of the ASD and ADHD groups showed 9 ROI-to-ROI increased connectivities in the ADHD group compared to the ASD group (Supplementary Figure 2). Specifically, the ADHD group showed increased functional connectivity between brain regions associated with the limbic, visual, default mode, somatomotor, dorsal attention, frontoparietal, ventral attention networks. In addition, we found that the ASD group showed increased functional connectivity in the left middle occipital sulcus and right precentral sulcus associated with the somatomotor and dorsal attention networks. The classification accuracy of discriminating ADHD from ASD was 79.3% (p < 0.001; see Table 2).
Association between functional connectivity and core symptom severity
Regression analysis showed a positive association between increased connectivity in the ASD group compared to the TD group and SRS scores, specifically 1) with the right insula and post transverse collateral sulcus functional connectivity (p = 0.0004, p = 0.01 FDR corrected; r = 0.376) and 2) with the right orbital gyrus and right horizontal ramus of the lateral sulcus functional connectivity (p = 0.0076, p = 0.025 FDR corrected; r = 0.309) (Supplementary Figure 3, Supplementary Table 2).
Discussion
In this study, we investigated shared and distinct patterns of dysconnectivity in boys with ASD and ADHD. We found that 1) children with ASD showed increased functional connectivity compared to children with TD, and children with ADHD showed increased functional connectivity compared to children with ASD and children with TD; and 2) machine learning approaches can discriminate ASD and ADHD with accuracies of 76.3% (ASD from TD), 84.1% (ADHD from TD) and 79.6% (ADHD from ASD). Our results may deepen our understanding of the neurophysiological mechanisms underlying the comorbidity and distinction between ADHD and ASD.
Our findings of increased functional connectivity patterns within the limbic regions and the somatomotor network in ASD compared to TD are consistent with previous studies 12. For instance, Cerliani and colleagues found increased functional connectivity in the limbic area and sensory-motor area in male ASD patients12. Likewise, the DSM-5 manual also includes abnormal responses to sensory stimulation as a diagnostic criterion of ASD. Our results suggest that increased functional connectivity in the limbic and sensory-motor areas might reflect this abnormal response. The finding of increased functional connectivity patterns in children with ADHD compared to TD is also consistent with previous studies of ADHD 6. Studies have suggested that the default network and limbic area are associated with regulation of attention 23, self-cognition 24, and external cognition 25. We thus speculate that increased functional connectivity may underlie impairments in “sharing attention with other people,” which is a core symptom of ADHD.
We also found increased functional connectivity in the frontal lobe, temporal lobe, occipital lobe, parietal lobes, frontoparietal network, and ventral attention network in the ADHD group compared to the TD group. Rubia et al. found that temporal lobe and parietal lobe dysfunction in boys with ADHD during an attention allocation task is associated with symptoms of ADHD 26. We believe that abnormal functional connectivity in the temporal lobe and parietal lobe might disrupt or delay maturation of the regulation of attention in ADHD.
Nonetheless, our findings are inconsistent with other studies indicating that the ADHD cohort showed decreased functional connectivity in the posterior cingulate cortex 7 and increased functional connectivity within frontal regions of the executive control network8 as compared to the TD cohort. These conflicting findings may be due to inconsistent methodologies or variability in the ADHD sample population. For instance, Kyeong et al.7 used graph theory analysis to estimate degree centrality in the stratifying ADHD subgroups with mild symptom ADHD and severe symptom ADHD. Francx et al. 8 used independent component analysis to detect components or networks in the persistent ADHD subgroups and remittent ADHD subgroups. These discrepancies illustrate the importance of methodology and clinical subgroup differences during the interpretation of neuroimaging study findings.
We found that children with ASD showed increased functional connectivity between the left middle occipital sulcus and right precentral sulcus compared to ADHD. The occipital lobe and parietal lobe are involved in communication processing, including emotion perception 27 and face discrimination 28, as well as the pathophysiology of autism 29. A previous study indicated that ASD involves a different cognitive process during social interactions 30. We speculate that this may be due to the increased functional connectivity between the occipital and parietal lobes compared to ADHD and TD. These results may provide an explanation for the altered communication processing at the neural level in individuals with ASD.
Our machine-learning algorithms confirmed common classification features between ASD and ADHD in the limbic, ventral attention, visual, and default mode networks. This finding is consistent with previous studies that found abnormal functional connectivity in these networks in both ASD and ADHD5,6,31. Recent studies indicate that ADHD may be associated with difficulties in social interaction32. Symptoms of ADHD (e.g., attention deficits, impulse control, hyper-activity) are also frequently observed in ASD, demonstrating that the two disorders share some common manifestations. Taken together, the common classification features between ASD and ADHD may reflect shared neural mechanisms and clinical manifestations in the two disorders.
Translational neuroimaging studies have provided a basis for identifying neurophysiological features of ASD and showed potential clinical utility 33. Specifically, advanced machine learning techniques have been introduced to extract meaningful features from neuroimaging data and subsequently make an objective diagnosis for ASD. Anderson and colleagues used univariate t-tests to exclude irrelevant FCs and achieved an accuracy of 79%. Nielsen et al. used a leave-one-out classifier with a general linear model on the multisite ABIDE dataset and obtained accuracies of up to 60% for different sites.
More recently, Yahata and his colleagues developed a machine learning algorithm combining L1-regularized sparse canonical correlation analysis and sparse logistic regression for selecting a subset of FCs to obtain a classification accuracy of around 85% in a Japanese dataset, but generalization for independent cohorts using two independent validation cohorts obtained from the ABIDE dataset showed lower accuracy (75%). In the present study, we combined univariate t-tests and multivariate SVM-RFE to identify the most discriminative features between ASD, ADHD, and TD using 7 independent cohorts and obtained accuracies of 76.3% between TD and ASD, 84.1% between TD and ADHD, and 79.1% between ASD and ADHD. Our results demonstrated that a classifier developed using surface-based functional connectivity also showed high classification for ASD and ADHD across other independent cohorts.
We found that the increased functional connectivity in the right insula and right orbital cortex was associated with SRS scores. Functional and structural imaging studies of ASD have identified abnormalities in the insula, explaining the emotion dysregulation and social avoidance symptoms of ASD34,35. The orbital cortex, a critical brain region in social cognition, has been associated with high levels of autistic traits36. Taken together, these results suggest that atypical connectivity in the insula and orbital cortex are related to emotion dysregulation and social cognition.
There are several limitations in this study. First, our analyses were performed on boys with ASD who did not have comorbid ADHD and boys with ADHD who did not have comorbid ASD. Yet, we did not have ADHD symptom scores of subjects with ASD or ASD scores of subjects with ADHD. We thus cannot exclude the possibility that the disorders were comorbid. Further research including both ASD and ADHD symptom scores is needed. Second, this study only included boys with TD, ASD, and ADHD; thus, the results may not be generalizable to girls. Future studies including both boys and girls with ASD and ADHD are needed.
In summary, we found that boys with ASD are associated with increased functional connectivity in the limbic area, while boys with ADHD are associated with increased functional connectivity in the frontal and temporal areas. Machine learning-derived classification methods hold the potential to uncover neuroimaging biomarkers for ASD and ADHD.
Supplementary Material
Acknowledgement
Jian Kong is supported by R01AT006364, R01 AT008563, R21AT008707, and R61AT009310 from NIH/NCCIH.
Footnotes
Conflict of Interest
JK has a disclosure to report (holding equity in a startup company (MNT)); all authors declare no conflict of interest.
Contributor Information
Minyoung Jung, Research Center for Child Mental Development, University of Fukui, Japan.
Yiheng Tu, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, USA.
Joel Park, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, USA.
Kristen Jorgenson, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, USA.
Courtney Lang, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, USA.
Wenwen Song, The First Affiliated Hospital of Zhejiang Chinese Medical University, China.
Jian Kong, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, USA..
REFERENCES
- 1.Moffitt TE, Houts R, Asherson P, Belsky DW, Corcoran DL, Hammerle M, et al. Is adult ADHD a childhood-onset neurodevelopmental disorder? Evidence from a four-decade longitudinal cohort study. Am J Psychiatry 2015; 172: 967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai M- C, Lombardo M V, Baron-Cohen S. Autism. Lancet 2014; 383: 896–910. [DOI] [PubMed] [Google Scholar]
- 3.Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric Disorders in Children With Autism Spectrum Disorders: Prevalence, Comorbidity, and Associated Factors in a Population-Derived Sample. J Am Acad Child Adolesc Psychiatry 2008; 47: 921–9. [DOI] [PubMed] [Google Scholar]
- 4.Park B, Hong J, Lee S- H, Park H. Functional Connectivity of Child and Adolescent Attention Deficit Hyperactivity Disorder Patients: Correlation with IQ. Front Hum Neurosci 2016; 10: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lynch CJ, Uddin LQ, Supekar K, Khouzam A, Phillips J, Menon V. Default mode network in childhood autism: posteromedial cortex heterogeneity and relationship with social deficits. Biol Psychiatry 2013; 74: 212–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Martino A, Zuo X- N, Kelly C, Grzadzinski R, Mennes M, Schvarcz A, et al. Shared and distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry 2013; 74: 623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kyeong S, Kim JJ, Kim E. Novel subgroups of attention-deficit/hyperactivity disorder identified by topological data analysis and their functional network modular organizations. PLoS One 2017; 12: e0182603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francx W, Oldehinkel M, Oosterlaan J, Heslenfeld D, Hartman CA, Hoekstra PJ, et al. The executive control network and symptomatic improvement in attention- deficit/hyperactivity disorder. Cortex 2015; 73: 62–72. [DOI] [PubMed] [Google Scholar]
- 9.Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE, et al. Brain Hyperconnectivity in Children with Autism and its Links to Social Deficits. Cell Rep 2013; 5: 738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christakou a Murphy CM, Chantiluke K, Cubillo a I, Smith a B, Giampietro V, et al. Disorder-specific functional abnormalities during sustained attention in youth with Attention Deficit Hyperactivity Disorder (ADHD) and with autism. Mol Psychiatry 2013; 18: 236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray S, Miller M, Karalunas S, Robertson C, Grayson DS, Cary RP, et al. Structural and functional connectivity of the human brain in autism spectrum disorders and attention-deficit/hyperactivity disorder: A rich club-organization study. Hum Brain Mapp 2014; 35: 6032–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerliani L, Mennes M, Thomas RM, Di Martino A, Thioux M, Keysers C. Increased Functional Connectivity Between Subcortical and Cortical Resting- State Networks in Autism Spectrum Disorder. JAMA Psychiatry 2015; 72: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, et al. Functional connectomics from resting-state fMRI. Trends Cogn. Sci 2013; 17: 666–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickstein DP, Pescosolido MF, Reidy BL, Galvan T, Kim KL, Seymour KE, et al. Developmental meta-analysis of the functional neural correlates of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry 2013; 52: 279–289.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman L, Stern H, Brown GG, Mathalon DH, Turner J, Glover GH, et al. Test-retest and between-site reliability in a multicenter fMRI study. Hum Brain Mapp 2008; 29: 958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 1999; 8: 272–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitfield-Gabrieli S, Nieto-Castanon A. CONN: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect 2012; 2: 125–41. [DOI] [PubMed] [Google Scholar]
- 18.Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using aCompCor. Neuroimage 2014; 96: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–80. [DOI] [PubMed] [Google Scholar]
- 20.Guyon I, Weston J, Barnhill S, Vapnik V. Gene selection for cancer classification using support vector machines. Mach Learn 2002; 46: 389–422. [Google Scholar]
- 21.Tu Y, Zhang Z, Tan A, Peng W, Hung YS, Moayedi M, et al. Alpha and gamma oscillation amplitudes synergistically predict the perception of forthcoming nociceptive stimuli. Hum Brain Mapp 2016; 37: 501–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng L- L, Shen H, Liu L, Wang L, Li B, Fang P, et al. Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain 2012; 135: 1498–507. [DOI] [PubMed] [Google Scholar]
- 23.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain 2014; 137: 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci 2008; 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- 25.Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci 2011; 31: 3217–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubia K, Smith AB, Brammer MJ, Taylor E. Temporal Lobe Dysfunction in Medication-Naive Boys With Attention-Deficit/Hyperactivity Disorder During Attention Allocation and Its Relation to Response Variability. Biol Psychiatry 2007; 62: 999–1006. [DOI] [PubMed] [Google Scholar]
- 27.Gschwind M, Pourtois G, Schwartz S, Van De Ville D, Vuilleumier P. White- matter connectivity between face-responsive regions in the human brain. Cereb Cortex 2012; 22: 1564–76. [DOI] [PubMed] [Google Scholar]
- 28.Jonas J, Rossion B, Krieg J, Koessler L, Colnat-Coulbois S, Vespignani H, et al. Intracerebral electrical stimulation of a face-selective area in the right inferior occipital cortex impairs individual face discrimination. Neuroimage 2014; 99: 487–97. [DOI] [PubMed] [Google Scholar]
- 29.Jung M, Tu Y, Lang CA, Ortiz A, Park J, Jorgenson K, et al. Decreased structural connectivity and resting-state brain activity in the lateral occipital cortex is associated with social communication deficits in boys with autism spectrum disorder. Neuroimage 2017. doi: 10.1016/j.neuroimage.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 30.Hubbard AL, Mcnealy K, Scott-Van Zeeland AA, Callan DE, Bookheimer SY, Dapretto M. Altered integration of speech and gesture in children with autism spectrum disorders. Brain Behav 2012; 2: 606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoekzema E, Carmona S, Ramos-Quiroga JA, Richarte Fernández V, Bosch R, Soliva JC, et al. An independent components and functional connectivity analysis of resting state FMRI data points to neural network dysregulation in adult ADHD. Hum Brain Mapp 2014; 35: 1261–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiatry 2011; 69: 847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yahata N, Morimoto J, Hashimoto R, Lisi G, Shibata K, Kawakubo Y, et al. A small number of abnormal brain connections predicts adult autism spectrum disorder. Nat Commun 2016; 7: 11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazefsky CA, Herrington J, Siegel M, Scarpa A, Maddox BB, Scahill L, et al. The role of emotion regulation in autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2013; 52: 679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kosaka H, Omori M, Munesue T, Ishitobi M, Matsumura Y, Takahashi T, et al. Smaller insula and inferior frontal volumes in young adults with pervasive developmental disorders. Neuroimage 2010; 50: 1357–63. [DOI] [PubMed] [Google Scholar]
- 36.Mayer JL. The Relationship Between Autistic Traits and Atypical Sensory Functioning in Neurotypical and ASD Adults: A Spectrum Approach. J Autism Dev Disord 2017; 47: 316–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


