Abstract
B-1 cells represent an innate-like early-developing B cell population, whose existence as an independent lymphocyte subset has been questioned in the past. Recent molecular and lineage tracing studies have not only confirmed their unique origins and differentiation paths, they have also provided a rationale for their distinctive functionalities compared to conventional B cells. This review summarizes our current understanding of B-1 cell development, and the activation events that regulate B-1 cell responses to self and foreign antigens. We discuss the unresolved question to what extent BCR engagement, i.e. antigen-specificity versus innate signaling contribute to B-1 cell’s participation in tissue homeostasis and immune defense as providers of “natural” and antigen-induced antibody responses, and as cytokine-producing immune regulators.
Introduction
The discovery of CD5+ (or Ly-1+) B-1 cells in mice in the early 1980’s was followed by scientific inquiry that initially focused on the B-1 cell’s developmental origins, their phenotypic resemblance to human CLL, and remarkable ability to continuously generate broadly self-reactive IgM antibodies in the steady-state, even in mice held under germfree conditions. We now understand B-1 cells to be mainly of fetal origin, selected during development for their ability to recognize self-antigens, and prevented from causing autoimmune disease through the expression of CD5, identified as an inhibitory component of the BCR complex. More recent studies have begun to reveal a protective and immune-regulatory role for B-1 cells in immune defense against pathogens. Because understanding the development of B-1 cells is critical for understanding the regulation of their functions, this review will first provide a brief summary of B-1 cell development, and then describes our current understanding of B-1 cell’s contributions to immunity against infectious agents. As we discuss below, a challenge for the field remains gaining a more complete understanding of the mechanisms by which these self-reactive B-1 cells are regulated to contribute to immune host defense without causing autoimmune disease.
B-1 cells development and maintenance
Adoptive cell transfer studies initially showed that B-1 cells develop early in ontogeny, including prenatally from precursors residing in the embryonic splanchnopleura and in the fetal liver, as well as shortly after birth in bone marrow and spleen (reviewed in [1]). A series of recent studies have revealed the molecular basis for the ontogenically-restricted development of B-1 cells. A first critical step was the identification of distinct B cell precursors in fetal liver and bone marrow that developed into either only B-1 or B-2 cells [2]. Then, comparing gene expression by these distinct precursors, Lin 28b was identified as the master regulator of the genetic program that controls fetal but not adult hematopoiesis, including the development of B-1 cells [3,4]. These studies are significant, as they identified B-1 cells independent of any phenotypic markers as distinct, fetal-derived lymphocyte populations that develop in multiple waves throughout early ontogeny [5]. Follow-up studies, consistent with the earlier adoptive transfer studies, confirmed that de novo B-1 cell development from the defined B-1 cell precursors cedes a few weeks after birth due to precursor-intrinsic changes [6] that correlate with the loss of Lin 28b expression [3,4]. Studies with lethally-irradiated mice suggest that bone marrow B-1 precursors may be reactivated to a limited extend during lymphopenia and/or severe stress [7,8].
For maturation into the peripheral B cell pools, B-1 cells require a positive selection step. Thwarting one of the major dogmas of immunology, central tolerance induction, which predicts the removal of all strongly self-reactive B cells, Hayakawa and colleagues demonstrated the presence of the self-antigen Thy-1 to be required for the development and/or expansion of Thy-1 specific B-1 cells [9]. The data not only explain the emergence of a B cell population that is self-reactive, they also explain why numerous genetic manipulations that alter the BCR-complex, or its downstream signaling cascade, usually also affect B-1 cell development (reviewed in [10]).
Self-reactive B cells must be regulated to avoid inappropriate activation. For B-1 cells this is likely achieved through the expression of CD5, a surface-expressed molecule, found mostly on T cells, that helped to first identify B-1 cells as distinct from conventional B cells [11]. CD5 is part of the antigen-receptor complex and acts as an inhibitor of both, TCR and BCR signaling. The level of CD5 expression by T cells correlated with the strength of TCR-signaling during positive selection of thymocytes [12]. On B cells, CD5 expression was identified not only on B-1 cells but also on anergic conventional B cells [13]. Consistent with a prominent inhibitory role for CD5 in BCR-stimulation, B-1 cells cannot proliferate in response to anti-IgM stimulation, unless taken from CD5-deficient mice, or otherwise lacking CD5 expression [14]. Thus, the induction of CD5 following positive selection of the B-1 cells appears to ensure suppression of responsiveness to BCR-mediated signals, i.e. responsiveness to self-antigens. Any regulation of CD5 surface expression therefore would be expected to greatly affect B-1 cell responsiveness.
Once de novo development of B-1 cells cedes, B-1 cell pools are maintained predominantly through self-renewal (reviewed in [15]), a process that is largely unexplored. Given the dramatic and ongoing shifts in the BCR-repertoire of B-1 cells during the first 6 months of life, even in germfree mice [16], it is likely that interaction of the BCR with self-antigen contribute to this process. This is supported by findings that B-1 cells specific for phosphotidylcholine (PtC), encoding specificity for dead and dying cells, strongly clonally expand over the first 6 months of life [16,17]. Thus, although CD5+ B-1 cell appear unresponsive to BCR-mediated stimulation, certain triggers must exist that can overcome their response block.
Tissue distribution and natural antibody production by B-1 cells
In steady state the highest frequencies, albeit not absolute numbers, of B-1 cells are found in the pleural and peritoneal cavities. Their presence at those sites is regulated by the local production of CXCL13 by macrophages [18,19]. Other tissues, including secondary and tertiary lymphoid tissue sites such as spleen, bone marrow, lymph node, pericardium, and mucosal associated lymphoid tissue, contain B-1 cell frequencies that are mostly below 1% [20–26].
In the body cavities, B-1 cells are identified easily as CD19hi, B220lo, CD11bpos, CD23neg, CD43+ and either CD5+ or CD5lo/− [27]. In response to insults, such as infections, body cavity B-1 cells are rapidly activated to migrate to lymph tissues where they differentiate to cytokine and antibody-secreting cells. Those cells rapidly lose CD11b expression [28], making them difficult to differentiate from activated B-2 cells at those sites. Most natural IgM production, however, occurs in bone marrow and spleen. These sites contain two types of spontaneous IgM-secreting B-1 cells: cells of similar phenotype than found in the body cavities, albeit lacking CD11b expression, and B-1-derived, Blimp-1+ CD138+ and mostly CD5- plasma cells [29]. Natural IgM is produced at similar amounts in mice held under SPF and germ- and antigen-free conditions [29,30], but is altered in mice with changes to BCR-signaling and lack of Blimp-1 expression [29,31,32]. Whether the repertoire of natural IgM antibodies is shaped by microbiota or other non-microbial antigens such as components of food, however, has not been studied systematically. Natural serum IgM levels are higher in female compared to male mice, and recent studies suggested estrogen underlying this difference [33]. Thus, existing data indicate a role for self-antigen and sex-hormone-mediated B-1 cell activation and differentiation in the regulation of protective IgM.
The presence of B-1 cell-derived, constitutively-produced, self-reactive natural IgM serves as an important “first line of defense”, providing immune protection against a wide variety of pathogens, and reducing the risk of sepsis (Table 1 [33–38]). Knockout mice lacking the ability to secrete IgM (sIgM−/− mice) showed increased mortality following cecal ligation and puncture induced peritonitis [39], intranasal infection with influenza virus [35] and infection with the fungal pathogen Cryptococcus neoformans [40]. Conversely, reconstitution of mice deficient in CD5+ B-1 cells or sIgM production by B-1 cells were rescued from Salmonella LPS-induced endotoxemia and influenza infection via passive transfer of serum IgM from non-immune mice [41,42]. Thus, while B-1 cells are selected for the binding to self-antigens, the antibodies produced by these cells appear to broadly bind to many pathogens. This suggests that natural IgM, similar to TLR and other innate receptors, binds conserved structures (reviewed in [43]). The mechanisms of immune protection from natural IgM seem to be linked, at least in part, to their exquisite ability to activate the classical complement cascade. This was highlighted recently in two studies, one involving the role of IgM in protection from enteropathogenic E. coli [33], the other demonstrating a role for complement-mediated lysis in inhibiting tumor-growth [44].
Table 1:
B-1 cells and B-1 cell derived antibodies protect from a variety of pathogens
| Pathogen | B-1 Cell Response | References |
|---|---|---|
| Bacteria | ||
| Sepsis | Natural IgM protects against endotoxemia. CD5+ B-1 derived IL-10 decreases mortality and multi-organ dysfunction. B-1 cell-derived GM-CSF and IL-3 regulate immunity to bacterial sepsis. | [39,41,57–59,65] |
| Borrelia hermsii | CD5- B-1 cells develop long lasting memory and produce Borrelia-binding IgM. | [36,52,66,67] |
| Enteropathogenic E. coli (EPEC) | Natural IgM protects from EPEC infection via complement-mediated lysis of bacteria. Enhanced levels in females over males explains their increased resistance | [33] |
| Fransicella tularensis | F. tularensis LPS specific CD5+ B-1 cell populations expand and develop memory following exposure. | [50,54,68] |
| Listeria monocytogenes | Natural IgM protects against bacterial systemic dissemination. | [34,69] |
| Mycobacterium spp. | CD5+ B-1 cells differentiation into CD138+ antibody secreting cells in response to mycobacterium lipids in vitro. | [69] |
| Salmonella spp. | Natural IgM is protective. CD5- B-1 cells develop memory and secrete increased amounts antigen-specific protective antibodies following challenge. | [37,70] |
| Staphylococcus aureus | IL-10 production by CD5+ B-1 cells protects against bacterial dissemination. | [60] |
| Streptococcus pneumoniae | CD5+ B-1 cells produce natural antibodies to S. pneumoniae. CD5- B-1 cells produce antigen-specific antibodies and develop protective memory following vaccination with S. pneumoniae antigen. | [38,53,71–73] |
| Fungi | ||
| Cryptococcus neoformans | Natural IgM protects against death and dissemination to pulmonary infection. | [40] |
| Altenaria alternate | Accumulation of B-1 cells in fat associated lymphoid clusters in the mediastinum and increase local production of IgM | [26] |
| Viruses | ||
| Influenza Virus | Natural IgM neutralizes virus early after infection. CD5+ B-1 cells migrate to MedLN and increase local production of polyreactive and influenza-binding IgM. | [24,28,35,61,64,74,75] |
| Lymphocytic Choriomengitis Virus (LCMV) | Natural IgM can bind to LCMV | [34] |
| Vesicular Stomatitis Virus (VSV) | Natural IgM neutralizes multiple strains of VSV. Natural IgM improves survival and prevents systemic dissemination. | [34] |
| Parasites | ||
| Litomosoides sigmodontis | Accumulation in fat-associated lymphoid tissue; increased production of IgM | [26] |
| Leishmania spp | IL-10 production by splenic B-1 cells impairs macrophage responses to visceral leishmaniasis. B-1 cells reduce parasite burden in subcutaneous infection. | [76,77] |
B-1 cells also provide class-switched antibodies, specifically IgA and IgG3 [25,29]. Unlike production of natural IgM, however, mucosal IgA production by B-1 cells is dependent on host microbiota, to which these antibodies can bind [33,45]. Thus, in the strictest sense these antibodies are not “natural” antibodies, as they rely on foreign antigen for their production. Yet, what they have in common with natural IgM is their critical role in maintaining homeostasis between the host and its environment. For example, IgA acts primarily on mucosal surfaces to provide steady-state immune control of microbiota while avoiding tissue damage, as it cannot bind to and activate complement. Natural antibodies also seem to shape the development of a functional immune system in offspring following their passive transfer from the mother [33,45]. The mechanisms regulating the production of class-switched B-1 cell-derived antibodies, generally found to be induced independent of T cells, remain to be identified. However, recent studies suggest that they require TLR-mediated signals [45], raising questions about the relationship between B-1 cell activation and the antigen-specificity of their responses.
B-1 cell responses to infections
In addition to the provision of steady-state levels natural IgM, B-1 cells also actively respond with induced IgM production to infections to a variety of pathogens, including bacteria, viruses, fungi, and parasites (Table 1). For example, introduction of intestinal bacteria, or LPS, into the peritoneal cavity resulted in MyD88-dependent changes in surface expression of integrins, including CD9, and the subsequent migration of B-1 cells from the body cavity and their accumulation in omentum, mesenteric lymphoid tissues and spleen [46]. Nematode infection of the pleural cavity resulted in the accumulation of B-1 cells in local mediastinal fat-associated lymphoid tissues [26], and following influenza infection, pleural cavity B-1 cells accumulated in the draining mediastinal lymph nodes [28].
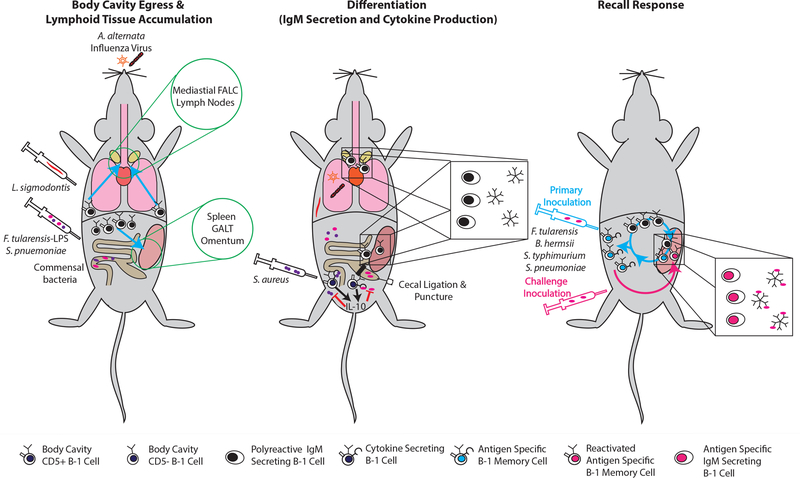
The mechanisms controlling B-1 cell activation during infections are incompletely resolved. Existing data suggest that distinct innate signals control a stepwise B-1 cell activation process: i) egress from body cavities, ii) accumulation in lymphoid tissue and iii) differentiation to antibody secreting cells (Figure 1). Egress may be regulated via chemokine/chemokine receptor expression, as LPS injection-induced egress of B-1 cells from the peritoneal cavity correlated with an up-regulation of CXCR4 and increased responsiveness of B-1 cells to CXCL12 [47]. Accumulation of CD5+ B-1 cells in the draining lymph nodes after influenza infection was shown to be facilitated by, and depend on, the Type I IFN-induced conformational changes of CD11b on B-1 cells to a high-affinity state [28]. Yet, the observed enhanced migration of CD5+ B-1 cells from the pleural cavity following influenza infection was independent of either Type I IFN or CD11b [28].
Figure 1. Outcomes of B-1 cell activation.
Pleural cavity B-1 cells respond to microbial insults by migration to regional lymphoid tissue such as mediastinal lymph nodes and mediastinal fat-associated lymphocyte tissue, whereas peritoneal cavity B-1 cells will migrate to spleen, gut-associated lymphoid tissue and/or omentum. Although B-1 cells circulate continuously in and out of the body cavities, their migration seems enhanced after an insult (left panel). Following arrival in the lymphoid tissues B-1 cells were shown to differentiate into IgM-secreting cells. B-1 cells in the cavities may also spontaneously secrete cytokines, specifically IL-10, or GM-CSF and IL-3 following their arrival in lypmphoid tissues (middle panel). Weeks to months after foreign antigen exposure a higher frequency of antigen-binding B-1 cells accumulate in body cavities, which are otherwise phenotypically indistinguishable from other B-1 cells. Following adoptive transfer of body cavity cells, these cell populations have been shown to provide increased immune protection, i.e. a memory function. It is unresolved whether antigen-stimulated B-1 cells have an enhanced ability to differentiate to antibody-secreting cells, or whether their higher frequencies alone can provide enhanced protection during challenge (right panel).
Once B-1 cells accumulate in regional lymph tissues, B-1 cell differentiation may be regulated at least in part by cytokine production. Recently this was shown for nematode infection, were the differentiation to IgM-producing cells depended on the local production of IL-5 [26], consistent with other studies [48,49]. Identifying the critical innate regulators of B-1 cell activation during infection is of importance, as it could help with their recruitment to vaccine responses.
Antigen-specific B-1 cell responses to infections
While chemokines and other innate signals are clearly critical for the regulation of B-1 cell responses, the question arises whether B-1 cells, most expressing CD5, can respond also in a BCR-mediated, antigen-specific manner. Emerging in vivo data provide some evidence of antigen-induced proliferation and possibly clonal expansion in response to encounter with foreign antigens. For example, using labeled antigen and identifying Francisella tularensis LPS-specific B-1 cells by their ability to bind such antigen, it was shown that administration of the non-mitogenic LPS resulted in increased frequencies of LPS-binding B-1 cells in spleen and peritoneal cavity [50]. Neonatal, but not adult, intranasal exposure to cockroach allergens induced the local production of anti-alpha 1,3 glucan IgA antibodies that protected from induction of allergic reactions following challenge [51]. That latter study is significant, as it provides support for the concept that the neonatal B-1 cell repertoire is not fixed, but is modulated significantly by subsequent microbial stimulation. It is currently unresolved, however, why the window for changing allergy resistance appears so short (reportedly to be about 9 days in the neonates used), while sequencing approaches suggested repertoire shaping of CD5+ B-1 cells to occur for at least 6 months after birth [16,17,51]. Further support for antigen-specific responses by B-1 cells comes from reports of antigen-specific memory B-1 cell induction to various pathogens [52,53]. These memory B-1 cells were identified in the body cavities some time following antigen exposure by their enhanced ability to provide recall responses (Figure 1) [52,54].
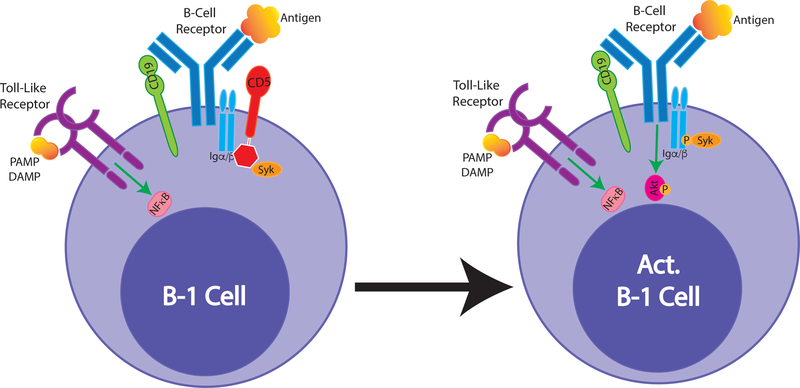
The occurrence of antigen-specific B-1 cell responses is puzzling, given the in vitro data that have shown a clear inability of CD5+ B-1 cells to respond to BCR-mediated stimulation. One potential explanation, suggested a number of years ago, is that only B-1 cells that lack expression of the inhibitor CD5 (so-called B-1b), can respond to antigenic stimulation [53,55]. However, only 25 – 30 % of body cavity B-1 cells express low/no CD5. Furthermore, studies with F. tularensis LPS identified CD5+ B-1 cells as responders [54], as did studies on influenza [24]. Interestingly, it was shown that the activation of F. tularensis specific memory B-1 cells into IgM-secreting cells required both antigen and TLR-mediated stimulation [54], consistent with studies on anti-microbial B-1 cell responses, which were shown also to require TLR signaling [45]. Thus, innate signaling may act as co-stimulator to enhance or enables antigen-specific B-1 cell responses. Our ongoing work supports such conclusions. We recently showed that stimulation with TLR-agonists, but not with anti-IgM, led to the loss of CD5 and the reorganization of the BCR-complex on CD5+ B-1 cells, thus potentially “licensing” B-1 cells for responses to antigen (Savage H.P et al, submitted). Furthermore, using proximal ligation assays we found that BCR-complex organization before and after stimulation differed significantly from that of B-2 cells. This is consistent with previous reports on differences between BCR stimulation by B-1 and B-2, most recently a study that analyzed the distinct roles of the Src-family kinase regulator CD148 in antigen-specific BCR signaling [56] (Figure 2).
Figure 2. BCR complex reorganization following B-1 cell stimulation.
B-1 cells express surface IgM- but little to no IgD-BCR. In the steady state their IgM-BCR closely associates with CD19 and with CD5. This complex is unable to signal via Igα/β following BCR-antigen stimulatik, however, B-1 cells vigoriously signal strongly following stimulation via TLR (left). Activation of B-1 cells via TLR alters their BCR complex, resulting in loss of CD5-mediated inhibition and BCR-downstream signaling (right). IgM-BCR complex reorganization may explain antigen-specific responses by self-reactive B-1 cells.
B-1 cells are important for effective innate and adaptive responses to pathogens
Given the role of B-1 cell-derived natural IgM and IgA as first lines of defense, it seems surprising that following their activation body cavity B-1 cells migrate to lymphoid tissues, rather than to mucosal surfaces, where they could strengthen the immune barrier. The data suggest that B-1 cells may have additional regulatory functions that go beyond simply providing pathogen-reactive antibodies.
Indeed, activated B-1 cells have been shown recently to secrete not only protective natural IgM during sepsis that can help the early control of bacterial dissemination, but also cytokines such as IL-3 and GM-CSF, which seem to play opposing roles in the control of inflammatory responses [39,41,57,58] and IL-10, which can control the extent of inflammation [59,60]. Little information exists about the interactions of B-1 cells with other cell types and the regulatory circuits that induce cytokine production by B-1 cells. However, for IL-10 production by B-1 cells, both in vitro and during sepsis in vivo, the induction of IL-10 required direct TLR4-mediated signaling [59], while in a Staphylococcus aureus peritonitis model, B-1 cell-derived protective IL-10 was dependent on TLR-2 [60]. Thus, B-1 cells represent one of a growing number of innate-like lymphocytes that respond to and in turn control the inflammatory process by generating cytokines.
B-1 cells do however, also generate increased amounts of IgM in secondary lymphoid tissues. This locally- secreted IgM was shown not to alter natural IgM titers in the serum following influenza infection [61], indicating that IgM acts locally in the lymph nodes. IgM could act in multiple distinct ways. Due to its large size, the secreted IgM may be prevented from leaving the lymph tissue environment, thereby strengthening the barrier by containing pathogens in the draining lymph nodes, inhibiting their disseminiation. Furthermore, earlier studies with mice lacking the ability to secrete IgM due to deletion of the µs splice region [35,62–64] showed that memory B cell as well as antigen-specific IgG responses required IgM secretion for their optimal induction. A recent study expanded these findings by demonstrating that the lack of the IgM Fc-receptor (FcµR) on B cells resulted in similar reductions in IgG responses, and reduced numbers of antigen-specific B cells in the draining lymph nodes, as seen also in mice lacking secreted IgM. Thus, existing data suggest that B-1 cells migration to local lymph tissue can support maximal conventional B cell responses by secreting IgM [31]. While the mechanism by which IgM-FcµR interactions enhance IgG responses remains to be identified, the data raise the interesting possibility that natural and polyreactive IgM facilitates the rapid uptake of antigen to B cells for antigen-processing and presentation, or may bring antigens in close contact with the BCR on the surface of the cell.
Conclusions
Recent studies have clarified the distinct molecular mechanism regulating the development of B-1 cells, supporting early conclusion about the distinct fetal/neonatal origin of B-1 cells that are selected to express a largely self-reactive BCR repertoire and then silenced to this antigen via expression of CD5. In-depths sequencing approaches, however, have revealed a much stronger shaping of the B-1 cell repertoire independent of microbial interactions over the first few months of life than previously anticipated. Thus, suggesting that B-1 cells remain responsive to BCR-stimulation in vivo, despite their inability to respond with proliferation to BCR-mediated stimulation in vitro. In addition, increasingly B-1 cells are shown to engage in antigen-driven responses to infections, but the mechanisms of their activation remain obscure. Existing data point to the stepwise activation of body cavity B-1 cells via various innate signals that result in their migration from the cavities to lymph tissues, where they can differentiate into antibody and cytokine-producing cells. To what extent and how BCR-mediated antigen-specific responses regulate these cells remain a future target of investigation.
Highlights.
Unique developmental paths generate self-reactive B-1 constraint in BCR responses
Extensive repertoire shaping of B-1 cells for months after birth
Accumulating evidence for antigen-specific activation of B-1 cells after infection
Innate signals may alter antigen-responsiveness of B-1 cells
Acknowledgement
Work cited from this laboratory is currently supported through NIH/NIAID grants U19-AI109962 and R01-AI117890. F.L.S. is the recipient of a NIH T-32 fellowship (T32 OD011147).
REFERENCES
- 1.Kantor AB, Herzenberg LA: Origin of murine B cell lineages. Annu Rev Immunol 1993, 11:501–538. [DOI] [PubMed] [Google Scholar]
- 2.Montecino-Rodriguez E, Leathers H, Dorshkind K: Identification of a B-1 B cell-specified progenitor. Nat Immunol 2006, 7:293–301. [DOI] [PubMed] [Google Scholar]
- 3.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA: Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 2012, 335:1195–1200.• First identification of Lin28b as a driver of fetal-derived B-1 cell development
- 4.Zhou Y, Li YS, Bandi SR, Tang L, Shinton SA, Hayakawa K, Hardy RR: Lin28b promotes fetal B lymphopoiesis through the transcription factor Arid3a. J Exp Med 2015, 212:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montecino-Rodriguez E, Dorshkind K: B-1 B cell development in the fetus and adult. Immunity 2012, 36:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber CL, Montecino-Rodriguez E, Dorshkind K: Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc Natl Acad Sci U S A 2011, 108:13700–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duber S, Hafner M, Krey M, Lienenklaus S, Roy B, Hobeika E, Reth M, Buch T, Waisman A, Kretschmer K, et al. : Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood 2009, 114:4960–4967. [DOI] [PubMed] [Google Scholar]
- 8.Holodick NE, Repetny K, Zhong X, Rothstein TL: Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol 2009, 39:2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayakawa K, Asano M, Shinton SA, Gui M, Allman D, Stewart CL, Silver J, Hardy RR: Positive selection of natural autoreactive B cells. Science 1999, 285:113–116. [DOI] [PubMed] [Google Scholar]
- 10.Kreslavsky T, Wong JB, Fischer M, Skok JA, Busslinger M: Control of B-1a cell development by instructive BCR signaling. Curr Opin Immunol 2018, 51:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA: The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med 1983, 157:202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE: CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med 1998, 188:2301–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hippen KL, Tze LE, Behrens TW: CD5 maintains tolerance in anergic B cells. J Exp Med 2000, 191:883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bikah G, Carey J, Ciallella JR, Tarakhovsky A, Bondada S: CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science 1996, 274:1906–1909. [DOI] [PubMed] [Google Scholar]
- 15.Baumgarth N: The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 2011, 11:34–46. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y, Wang C, Yang Q, Kantor AB, Chu H, Ghosn EE, Qin G, Mazmanian SK, Han J, Herzenberg LA: Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife 2015, 4:e09083.• This study provides a comprehensive repertoire analysis of CD5+ B-1 cells, indicating their continued repertoire shaping after birth
- 17.Prohaska TA, Que X, Diehl CJ, Hendrikx S, Chang MW, Jepsen K, Glass CK, Benner C, Witztum JL: Massively Parallel Sequencing of Peritoneal and Splenic B Cell Repertoires Highlights Unique Properties of B-1 Cell Antibodies. J Immunol 2018, 200:1702–1717.• This study provides a comprehensive repertoire analysis of CD5+ and CD5- B-1 cells
- 18.Ansel KM, Harris RB, Cyster JG: CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 2002, 16:67–76. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe N, Ikuta K, Fagarasan S, Yazumi S, Chiba T, Honjo T: Migration and differentiation of autoreactive B-1 cells induced by activated gamma/delta T cells in antierythrocyte immunoglobulin transgenic mice. J Exp Med 2000, 192:1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Tung JW, Ghosn EE, Herzenberg LA, Herzenberg LA: Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci U S A 2007, 104:4542–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawahara T, Ohdan H, Zhao G, Yang YG, Sykes M: Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol 2003, 171:5406–5414. [DOI] [PubMed] [Google Scholar]
- 22.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA: Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med 1985, 161:1554–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi YS, Dieter JA, Rothaeusler K, Luo Z, Baumgarth N: B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol 2012, 42:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi YS, Baumgarth N: Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med 2008, 205:3053–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroese FG, Butcher EC, Stall AM, Lalor PA, Adams S, Herzenberg LA: Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol 1989, 1:75–84. [DOI] [PubMed] [Google Scholar]
- 26.Jackson-Jones LH, Duncan SM, Magalhaes MS, Campbell SM, Maizels RM, McSorley HJ, Allen JE, Benezech C: Fat-associated lymphoid clusters control local IgM secretion during pleural infection and lung inflammation. Nat Commun 2016, 7:12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells SM, Kantor AB, Stall AM: CD43 (S7) expression identifies peripheral B cell subsets. J Immunol 1994, 153:5503–5515. [PubMed] [Google Scholar]
- 28.Waffarn EE, Hastey CJ, Dixit N, Soo Choi Y, Cherry S, Kalinke U, Simon SI, Baumgarth N: Infection-induced type I interferons activate CD11b on B-1 cells for subsequent lymph node accumulation. Nat Commun 2015, 6:8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savage HP, Yenson VM, Sawhney SS, Mousseau BJ, Lund FE, Baumgarth N: Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. J Exp Med 2017, 214:2777–2794.• Identification of B-1 cells and B-1-derived plasma cells in spleen and bone marrow, as major providers of natural IgM. Not all B-1 cells required Blimp-1 expression for antibody secretion
- 30.Hooijkaas H, Bos N, Benner R, Pleasants JR, Wostmann BS: Immunoglobulin isotypes and antibody specificity repertoire of “spontaneously” occurring (“background”) immunoglobulin-secreting cells in germfree mice fed chemically defined ultrafiltered “antigen-free” diet. Adv Exp Med Biol 1985, 186:131–138. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen TTT, Graf BA, Randall TD, Baumgarth N: sIgM-FcmuR Interactions Regulate Early B Cell Activation and Plasma Cell Development after Influenza Virus Infection. J Immunol 2017, 199:1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savitsky D, Calame K: B-1 B lymphocytes require Blimp-1 for immunoglobulin secretion. J Exp Med 2006, 203:2305–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng Z, Surewaard BGJ, Wong CHY, Guettler C, Petri B, Burkhard R, Wyss M, Le Moual H, Devinney R, Thompson GC, et al. : Sex-hormone-driven innate antibodies protect females and infants against EPEC infection. Nat Immunol 2018, 19:1100–1111.• Identifies a mechanism for increased protective natural IgM in female mice
- 34.Ochsenbein AF, Fehr T, Lutz C, Suter M, Brombacher F, Hengartner H, Zinkernagel RM: Control of early viral and bacterial distribution and disease by natural antibodies. Science 1999, 286:2156–2159. [DOI] [PubMed] [Google Scholar]
- 35.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J: B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med 2000, 192:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alugupalli KR, Gerstein RM, Chen J, Szomolanyi-Tsuda E, Woodland RT, Leong JM: The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol 2003, 170:3819–3827. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien AD, Scher I, Campbell GH, MacDermott RP, Formal SB: Susceptibility of CBA/N mice to infection with Salmonella typhimurium: influence of the X-linked gene controlling B lymphocyte function. J Immunol 1979, 123:720–724. [PubMed] [Google Scholar]
- 38.Briles DE, Forman C, Hudak S, Claflin JL: Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med 1982, 156:1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J: A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med 1998, 188:2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Subramaniam KS, Datta K, Quintero E, Manix C, Marks MS, Pirofski LA: The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol 2010, 184:5755–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reid RR, Prodeus AP, Khan W, Hsu T, Rosen FS, Carroll MC: Endotoxin shock in antibody-deficient mice: unraveling the role of natural antibody and complement in the clearance of lipopolysaccharide. J Immunol 1997, 159:970–975. [PubMed] [Google Scholar]
- 42.Baumgarth N, Chen J, Herman OC, Jager GC, Herzenberg LA: The role of B-1 and B-2 cells in immune protection from influenza virus infection. Curr Top Microbiol Immunol 2000, 252:163–169. [DOI] [PubMed] [Google Scholar]
- 43.Baumgarth N, Tung JW, Herzenberg LA: Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol 2005, 26:347–362. [DOI] [PubMed] [Google Scholar]
- 44.Haro MA, Dyevoich AM, Phipps JP, Haas KM: Activation of B-1 cells promotes tumor cell killing in the peritoneal cavity. Cancer Res 2018. [DOI] [PMC free article] [PubMed]
- 45.Koch MA, Reiner GL, Lugo KA, Kreuk LS, Stanbery AG, Ansaldo E, Seher TD, Ludington WB, Barton GM: Maternal IgG and IgA Antibodies Dampen Mucosal T Helper Cell Responses in Early Life. Cell 2016, 165:827–841.• Explores the immunoregulatory role of maternal IgG and IgA in neonatal immunity
- 46.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S: Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med 2006, 203:2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moon H, Lee JG, Shin SH, Kim TJ: LPS-induced migration of peritoneal B-1 cells is associated with upregulation of CXCR4 and increased migratory sensitivity to CXCL12. J Korean Med Sci 2012, 27:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nisitani S, Tsubata T, Murakami M, Honjo T: Administration of interleukin-5 or −10 activates peritoneal B-1 cells and induces autoimmune hemolytic anemia in anti-erythrocyte autoantibody-transgenic mice. Eur J Immunol 1995, 25:3047–3052. [DOI] [PubMed] [Google Scholar]
- 49.McKay JT, Haro MA, Daly CA, Yammani RD, Pang B, Swords WE, Haas KM: PD-L2 Regulates B-1 Cell Antibody Production against Phosphorylcholine through an IL-5-Dependent Mechanism. J Immunol 2017, 199:2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole LE, Yang Y, Elkins KL, Fernandez ET, Qureshi N, Shlomchik MJ, Herzenberg LA, Herzenberg LA, Vogel SN: Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc Natl Acad Sci U S A 2009, 106:4343–4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel PS, King RG, Kearney JF: Pulmonary alpha-1,3-Glucan-Specific IgA-Secreting B Cells Suppress the Development of Cockroach Allergy. J Immunol 2016, 197:3175–3187.• Study suggests that antigen shapes the natural Ig repertoire early but not later in life, providing protection from allergic reactions
- 52.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM: B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 2004, 21:379–390. [DOI] [PubMed] [Google Scholar]
- 53.Haas KM, Poe JC, Steeber DA, Tedder TF: B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 2005, 23:7–18. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y, Ghosn EE, Cole LE, Obukhanych TV, Sadate-Ngatchou P, Vogel SN, Herzenberg LA, Herzenberg LA: Antigen-specific memory in B-1a and its relationship to natural immunity. Proc Natl Acad Sci U S A 2012, 109:5388–5393.• Study indicates that innate signals are required for subsequent antigen-mediated (memory) B-1 cell activation
- 55.Alugupalli KR, Gerstein RM: Divide and conquer: division of labor by B-1 B cells. Immunity 2005, 23:1–2. [DOI] [PubMed] [Google Scholar]
- 56.Skrzypczynska KM, Zhu JW, Weiss A: Positive Regulation of Lyn Kinase by CD148 Is Required for B Cell Receptor Signaling in B1 but Not B2 B Cells. Immunity 2016, 45:1232–1244.Study identifies BCR signaling differences between B-1 and B-2-expressed BCR
- 57.Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, et al. : Innate response activator B cells protect against microbial sepsis. Science 2012, 335:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber GF, Chousterman BG, He S, Fenn AM, Nairz M, Anzai A, Brenner T, Uhle F, Iwamoto Y, Robbins CS, et al. : Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science 2015, 347:1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aziz M, Holodick NE, Rothstein TL, Wang P: B-1a Cells Protect Mice from Sepsis: Critical Role of CREB. J Immunol 2017, 199:750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leech JM, Lacey KA, Mulcahy ME, Medina E, McLoughlin RM: IL-10 Plays Opposing Roles during Staphylococcus aureus Systemic and Localized Infections. J Immunol 2017, 198:2352–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA: Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci U S A 1999, 96:2250–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J: Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol 1998, 160:4776–4787. [PubMed] [Google Scholar]
- 63.Ehrenstein MR, O’Keefe TL, Davies SL, Neuberger MS: Targeted gene disruption reveals a role for natural secretory IgM in the maturation of the primary immune response. Proc Natl Acad Sci U S A 1998, 95:10089–10093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez Gonzalez S, Jayasekera JP, Carroll MC: Complement and natural antibody are required in the long-term memory response to influenza virus. Vaccine 2008, 26 Suppl 8:I86–93. [DOI] [PubMed] [Google Scholar]
- 65.Barbeiro DF, Barbeiro HV, Faintuch J, Ariga SK, Mariano M, Popi AF, de Souza HP, Velasco IT, Soriano FG: B-1 cells temper endotoxemic inflammatory responses. Immunobiology 2011, 216:302–308. [DOI] [PubMed] [Google Scholar]
- 66.Colombo MJ, Alugupalli KR: Complement factor H-binding protein, a putative virulence determinant of Borrelia hermsii, is an antigenic target for protective B1b lymphocytes. J Immunol 2008, 180:4858–4864. [DOI] [PubMed] [Google Scholar]
- 67.Colombo MJ, Abraham D, Shibuya A, Alugupalli KR: B1b lymphocyte-derived antibodies control Borrelia hermsii independent of Fcalpha/mu receptor and in the absence of host cell contact. Immunol Res 2011, 51:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crane DD, Griffin AJ, Wehrly TD, Bosio CM: B1a cells enhance susceptibility to infection with virulent Francisella tularensis via modulation of NK/NKT cell responses. J Immunol 2013, 190:2756–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ordonez C, Savage HP, Tarajia M, Rivera R, Weeks-Galindo C, Sambrano D, Riley L, Fernandez PL, Baumgarth N, Goodridge A: Both B-1a and B-1b cells exposed to Mycobacterium tuberculosis lipids differentiate into IgM antibody-secreting cells. Immunology 2018. [DOI] [PMC free article] [PubMed]
- 70.Wijburg OL, Uren TK, Simpfendorfer K, Johansen FE, Brandtzaeg P, Strugnell RA: Innate secretory antibodies protect against natural Salmonella typhimurium infection. J Exp Med 2006, 203:21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Binder CJ, Horkko S, Dewan A, Chang MK, Kieu EP, Goodyear CS, Shaw PX, Palinski W, Witztum JL, Silverman GJ: Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med 2003, 9:736–743. [DOI] [PubMed] [Google Scholar]
- 72.Martin F, Oliver AM, Kearney JF: Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 2001, 14:617–629. [DOI] [PubMed] [Google Scholar]
- 73.Cotte C, Szczepanek SM: Peritoneal B-1b and B-2 B-cells confer long-term protection from pneumococcal serotype 3 infection after vaccination with Prevnar-13 and are defective in sickle cell disease mice. Vaccine 2017, 35:3520–3522. [DOI] [PubMed] [Google Scholar]
- 74.Jayasekera JP, Moseman EA, Carroll MC: Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol 2007, 81:3487–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harada Y, Muramatsu M, Shibata T, Honjo T, Kuroda K: Unmutated immunoglobulin M can protect mice from death by influenza virus infection. J Exp Med 2003, 197:1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arcanjo AF, Nico D, de Castro GMM, da Silva Fontes Y, Saltarelli P, Decote-Ricardo D, Nunes MP, Ferreira-Pereira A, Palatnik-de-Sousa CB, Freire-de-Lima CG, et al. : Dependency of B-1 Cells in the Maintenance of Splenic Interleukin-10 Producing Cells and Impairment of Macrophage Resistance in Visceral Leishmaniasis. Front Microbiol 2017, 8:978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonzaga W, Geraldo MM, Vivanco BC, Popi AF, Mariano M, Batista WL, Xander P: Evaluation of Experimental Infection with L. ( L.) Amazonensis in X-Linked Immunodeficient Mice. J Parasitol 2017, 103:708–717. [DOI] [PubMed] [Google Scholar]