Abstract
Generalized anxiety disorder (GAD) is characterized by a range of cognitive and affective disruptions, such as pathological worry. There is debate, however, about whether such disruptions are specifically linked to heightened responses to aversive stimuli, or due to overgeneralized threat monitoring leading to deficits in the ability to discriminate between aversive and non-aversive affective information. The present study capitalized on the temporal and functional specificity of scalp-recorded event-related potentials (ERPs) to examine this question by exploring two targeted neurocognitive responses in a group of adults diagnosed with GAD: 1) visual processing of angry (aversive) versus neutral (non-aversive) faces; and 2) response monitoring of incorrect (aversive) versus correct (non-aversive) responses. Electroencephalography was recorded while 15 adults with GAD and 15 age-matched controls viewed angry and neutral faces prior to individual trials of a flanker task. ERPs to faces were the P1, reflecting attention allocation, the early posterior negativity (EPN), reflecting early affective discrimination, and the N170, reflecting face-sensitive visual discrimination. The error-related negativity (ERN) and positivity (Pe) were generated to incorrect and correct responses. Results showed reduced discrimination between aversive and non-aversive faces and responses in the GAD relative to the control group during visual discrimination (N170) and later-emerging error monitoring (Pe). These effects were driven by exaggerated processing of non-aversive faces and responses, suggesting over-generalized threat monitoring. Implications for cognitive-affective models of GAD are discussed.
Generalized anxiety disorder (GAD) is among the most commonly-diagnosed anxiety disorders (Ballenger, Davidson, Lecrubier, Nutt, Borkovec, Rickels, et al., 2001) affecting an estimated 5.7% of the adult population in their lifetime and over 18 million individuals in the U.S. alone (Kessler et al., 2005). Recent theoretical models of GAD (Mennin, Heimberg, Turk, & Fresco, 2002, 2005; Salters-Pedneault, Roemer, Tull, Rucker, & Mennin, 2006) posit that overgeneralized threat monitoring, measured in terms of reduced ability to differentiate between aversive and non-aversive affective information, may be a key mechanism in the emergence and maintenance of GAD symptoms.
Reduced affective discrimination may be one of several, temporally-distinct stages of disrupted threat processing in GAD. For example, research on GAD using reaction time measures documents early-emerging exaggerated attention to threat-themed relative to neutral stimuli (Amir, Beard, Burns, & Bomyea, 2009), whereas research using neuroimaging techniques such as fMRI documents reduced discrimination between aversive and neutral images (e.g., enhanced bilateral dorsal amygdala activation to cues predicting both aversive and neutral images; Nitschke and colleagues, 2009). These two findings, one of early exaggerated response to aversive stimuli and the other of reduced discrimination during more elaborative processing, may reflect methdological differences in the level of analysis (brain or behavior) and in the time-scale of measurement. The use of a single measurement technique that can simultaneously capture multiple stages of response to affective stimuli is therefore a crucial next step in clarifying the nature of affective discrimination in GAD.
To this end, the present study utilized scalp-recorded event-related potentials (ERPs) to study the time course of enhanced processing of threat relative to neutral images (for review see Olofsson, Nordin, Sequeira, & Polich, 2008). We targeted a novel combination of ERPs reflecting multiple stages of both stimulus processing and response monitoring: early attention allocation (P1), affective discrimination (early posterior negativity), attention discrimination and configural processing (N170), and response monitoring at both early (error-related negativity or ERN) and later (error positivity or Pe) stages. This approach allowed us to directly test the hypothesis that GAD is associated with early exaggerated responses to aversive stimuli, followed by reduced discrimination between aversive and non-aversive stimuli at more elaborative stages of processing.
Consistent with this hypothesis, but focusing on stimulus processing alone, a prior study (Weinberg & Hajcak, 2011) documented that a group of adults diagnosed with GAD showed increased neural responses to threat-themed relative to neutral images at early stages of attention allocation (the P1), but reduced discrimination between the two at later, more elaborative stages of processing (the late positive potential or LPP). Indeed, it is well-documented that early-emerging ERPs can be used to index exaggerated early threat processing (Olofsson, Nordin, Sequeira, & Polich, 2008). For example, the P1, which signals early attention allocation and enhanced activity of the extrastriate visual cortex (Hillyard & Anllo-Vento, 1998; Smith, Cacioppo, Larsen, & Chartrand, 2003) is enhanced to threat-themed images in anxious relative to non-anxious groups (Holmes, Nielsen, & Green, 2008; X. Li, Li, & Luo, 2005). The early posterior negativity (EPN), which indexes very early affective processing (Luck & Kappenman, 2011; Schupp, Flaisch, Stockburger, & Junghöfer, 2006; Schupp, Stockburger, et al., 2006), is also enhanced to threat-themed images in anxious populations (e.g. Frenkel & Bar-Haim, 2011; Holmes, Nielsen, & Green, 2008).
The later-emerging N170 may be selectively sensitive to disruptions in the discrimination between threat and non-threat. Peaking around 170 to 270 ms, the N170 indexes attention discrimination and configural processing (Bentin, Allison, Puce, Perez, & McCarthy, 1996; Eimer, 2000; Rousselet, Husk, Bennett, & Sekuler, 2008), and is enhanced to faces, familiar images, and affectively-charged images relative to other images (Itier & Taylor, 2004). A recent study examined the N170 to threat as a moderator of the efficacy of attention bias modification training (ABMT) for anxiety (Dennis-Tiwary, Egan, Babkirk, & Denefrio, 2016). In moderately anxious adults, stress reactivity was reduced following ABMT versus placebo training, but this effect was disrupted when participants showed heightened N170 responses to neutral relative to aversive stimuli.
ERPs have also been used to identify disruptions in response monitoring in GAD. The error-related negativity (ERN/Ne) and error positivity (Pe) are enhanced following the commission of an error (Falkenstein, Hohnsbein, Hoormann, & Blanke, 1991; Gehring, Goss, Coles, & Meyer, 1993; van Veen & Carter, 2002). The ERN is a sharp negative deflection measured in anterior electrodes peaking within about 100 ms after an incorrect response, whereas the Pe is a slower positive wave which is maximal around 200–400 ms after an error. Both have neural generators in the anterior cingulate cortex (Brázdil et al., 2005; Holroyd et al., 1998; Pizzagalli et al., 2006; Stemmer et al., 2004; Yeung et al., 2004). While the ERN reflects the relatively rapid and automatic detection of an error response (Falkenstein, Hoormann, Christ, & Hohnsbein, 2000; Van Veen & Carter, 2002), the later-emerging Pe may reflect emotional and motivational appraisal of the error or more conscious detection of the error having been made (Endrass, Reuter, & Kathmann, 2007; Leuthold & Sommer, 1999; Nieuwenhuis et al., 2001; O’Connell et al., 2007; Overbeek, Niewenhuis, & Ridderinkhof, 2005). Consistent with findings that making an error is fundamentally aversive (Aarts, De Houwer, & Pourtois, 2012, 2013) several studies (Weinberg, Klein, & Hajcak, 2012; Weinberg, Kotov, & Proudfit, 2015; Weinberg, Olvet, & Hajcak, 2010) document that, relative to healthy controls, adults diagnosed with GAD alone (no comorbidities) show larger magnitude ERNs following the commission of an error.
Findings from other research groups, however, suggest that error-related brain activity in GAD may reflect broader abnormalities in response monitoring that extend to non-aversive correct responses and later stages of processing beyond the ERN (i.e. Xiao et al., 2011; Endgrass et al., 2010). In a study comparing multiple clinical anxiety groups, Xiao and colleagues (2011) found that while relative to controls, both OCD and GAD patients evidenced larger magnitude ERNs specific to error trials, only the OCD patients showed enhanced ERNs to error versus correct trials. Consistent with this, although in the distinct context of a go/no-go paradigm, Yu and colleagues (2015) documented that GAD was characterized by reduced discrimination between congruence and incongruence at the level of the N2 ERP (~250–300 ms), suggesting downstream disruptions in the ability to effectively recruit response monitoring resources.
Taken together, these studies suggest that GAD may be associated with overgeneralized response monitoring later in the processing stream in addition to exaggerated initial error monitoring. Consistent with this, Weinberg and colleagues (2010) documented enhanced Pe in adults diagnosed with GAD across both errors and correct trials. This further suggests that the later-emerging Pe may be a particularly sensitive index of reduced discrimination in GAD.
Such effects may be amplified in both aversive and ambiguous contexts. For example, Jackson, Nelson, & Proudfit (2015) document that the magnitude of the ERN is larger in unpredictable compared to predictable contexts. A similar study found that the ERN was also enhanced when unfavorable outcomes were unexpected compared to expected (Holroyd, Nieuwenhuis, Yeung, & Cohen, 2003). Hajcak, McDonald, & Simons (2003) further found enhanced and non-specific exaggerated response monitoring related to worry, a core symptom of GAD.
In summary, prior research on visual processing in GAD documents exaggerated responses to aversive stimuli, measured by ERPs such as the P1, and possible disruptions in more elaborated attention discrimination due to exaggerated processing of non-aversive relative to aversive information (e.g., the N170; O’Toole et al., 2013). Research on GAD and response monitoring further suggests exaggerated early processing of errors (ERN) and abnormalities in the discrimination between aversive errors and non-aversive correct responses at more elaborative stages of error processing (Pe) due to overgeneralized error-monitoring (enhanced processing of correct responses).
The Present Study
Participants were adults with a primary diagnosis of GAD and healthy, age-matched controls, who completed a flanker task while EEG was recorded. ERPs were generated to angry (aversive) and neutral (non-aversive) faces presented prior to each trial of the flanker task, and to incorrect (aversive) and correct (non-aversive) responses to the flanker task.
We tested the hypothesis that, for aversive versus non-aversive stimuli and responses, those with GAD, relative to healthy controls, will show: (1) exaggerated early attention allocation (P1), affective processing (EPN), and error monitoring (ERN), but (2) dampened visual discrimination (N170) and later stages of error monitoring (Pe) due to exaggerated responses to non-aversive information. Next, we tested the hypothesis that the GAD group will display selective sensitivity to aversive affective contexts such that they will show more errors and enhanced disruptions in error monitoring following angry faces, given prior research showing such effects (Jackson et al., 2015; MacNamara & Hajcak, 2010).
Method
Participants
Participants were recruited from an urban community and a large urban university in the northeast United States. The Structured Clinical Interview for DSM-IV Disorders (SCID-I/P; First, Spitzer, Gibbon, & Williams, 2007) was used to identify participants with elevated anxiety levels who met criteria for Generalized Anxiety Disorder (GAD).1 Graduate students in psychology and post-baccalaureate research assistants conducted interviews.2 Individuals who met criteria for a primary diagnosis of current GAD were included. If primary diagnosis of GAD was met, other comorbidities were included (e.g., social phobia, dysthymia, past but not current MDE) given evidence that GAD is highly comorbid with other anxiety and distress-related disorders (Ballenger et al, 2001). Participants diagnosed with current Major Depressive Episodes (MDE), suicidality, or psychotic symptoms were excluded.
A total of 40 adults participated in this study. Participants who committed fewer than 4 errors per experimental block were excluded from analysis. Nine people (6 Control; 3 GAD) were excluded based on this criterion.3 In addition, one participant’s symptoms were determined to be subclinical for GAD after review at the consensus meeting. The final sample consisted of 30 individuals (24 female), aged 18–34 (M = 22.33, SD = 4.99). Self-reported race/ethnicity was as follows: 4 Caucasian, 8 Hispanic, 14 Asian, 2 African American, 1 other, and 1 person chose not to answer. Fifteen participants met full criteria for a primary diagnosis of GAD (13 female; MAGE = 22.40, SD = 5.33) with race/ethnicity as follows: 4 Caucasian, 2 Hispanic, 7 Asian, 1 African American, and 1 person chose not to answer. Fifteen participants (11 female; MAGE = 22.27, SD = 4.82) comprised the age-matched control group with race/ethnicity as follows: 6 Hispanic, 7 Asian, 1 African American, and 1 other. Participants were compensated for their participation with either $50 or 3 research credits.
Among the 15 GAD participants, 4 met criteria for past MDE; as per inclusion criteria, none met criteria for current MDE at the time of participation. Seven GAD participants met criteria for additional current Axis I disorders (4 Specific Phobia; 2 Post Traumatic Stress Disorder; 2 Social Phobia; 2 Dysthymia; 1 Panic Disorder). Five of those seven GAD participants met criteria for multiple current diagnoses consistent with the clinical presentation of GAD (Ballenger et al., 2001). All GAD participants were medication free at the time of participation. Control participants did not meet criteria for any Axis I disorder at the time of participation.
In addition to the SCID, paper-based questionnaires were given to assess differences in self-reported levels of anxiety and worry for each group. These measures included the Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990) and the Generalized Anxiety Questionnaire (GAD-Q-IV; Newman, Zuellig, Kachin, Constantino, Przeworski, Erickson et al., 2002). Means and standard deviations for anxiety measures by group are presented in Table 1. Independent samples t-tests confirmed that the GAD group had higher scores for GADQ [t(28) = −6.02, p < .001] and PSWQ [t(28) = −3.63, p < .001], compared to the control group. Lastly, trait and state anxiety was assessed using the State-Trait Anxiety Inventory (STAI; Spielberger & Gorsuch, 1983). Independent samples t-tests also confirmed that the GAD group had higher scores for state [t(28) = −2.61, p = .014] and trait [t(28) = −2.94, p = .006] anxiety, compared to the control group.
Table 1.
Descriptive Statistics for Self-Reported Anxiety Symptoms (top) ERP Amplitudes (μV; middle) and Error Rates (bottom)
| GAD M(SD) | Control M(SD) | |||||||
| GAD-Q*** | 10.40 (1.20) | 5.26 (3.08) | ||||||
| PSW-Q*** | 63.20 (13.94) | 46.20 (11.58) | ||||||
| STAI State* | 46.87 (12.03) | 35.27 (12.30) | ||||||
| STAI Trait** | 52.73 (10.13) | 41.60 (10.59) | ||||||
| GAD M(SD) | Control M(SD) | |||||||
| P1 | N170 | EPN | P1 | N170 | EPN | |||
| Neutral | 4.21 (2.40) |
−1.45 (2.44) |
4.97 (1.46) |
4.68 (2.79) |
−0.77 (3.38) |
3.97 (3.26) |
||
| Angry | 4.40 (2.62) |
−1.47 (2.09) |
3.70 (1.48) |
4.66 (2.79) |
−1.44 (3.03) |
2.02 (3.26) |
||
| ERN | Pe | ERN | Pe | |||||
| Correct | −0.87 (1.56) | 3.06 (2.79) | −0.74 (3.08) | 0.82 (2.97) | ||||
| Incorrect | −3.77 (3.18) | 4.91 (3.88) | −3.48 (2.19) | 5.38 (3.35) | ||||
| GAD M(SD) | Control M(SD) | |||||||
| Error Rates M(SD) | Error Rates M(SD) | |||||||
| Congruent | Incongruent | Congruent | Incongruent | |||||
| No Face | 2.53(2.62) | 20.40(11.74) | 1.73(2.22) | 20.53(15.17) | ||||
| Neutral | 3.13(2.88) | 24.40(19.59) | 1.60(1.99) | 18.40(13.78) | ||||
| Angry | 4.93(4.51) | 25.53(17.31) | 1.60(2.23) | 18.60(11.66) | ||||
Note. All measures of self-reported anxiety symptoms were significantly different between groups [
p < .05;
p < .01;
p < .001].
For significant interactions reported below in the results section, we used G*Power (Faul, Erdfelder, Lang, & Buchner, 2007) to compute the achieved power range as 94% - 99 % power, given our alpha criteria (p < .05), sample size (N = 30), and medium to large effect sizes detected for behavioral measures of performance (ηp2 = .12) and ERP measures (η2 = .14 - .17).
Materials and Procedure
Following consent procedures and questionnaires, a trained research assistant conducted the SCID. Then, participants completed the modified flanker task that included emotional faces as primes, while EEG was continuously recorded. Following each block, state anxiety was assessed. Each participant spent approximately two and a half hours in the laboratory to complete the study.
Modified Flanker Task
A modified flanker task with both facial primes (angry and neutral) and no face trials was employed. This task requires the participant to identify the direction (right or left) of the central arrow that is flanked by either four arrows (two on each side) facing the same direction (congruent trial) or the opposite direction (incongruent trial) (Eriksen & Eriksen, 1974). We chose the flanker task because of its well-established properties (e.g., the flanker congruency effect on reaction times; Miller, 1991; Yantis & Johnston, 1990) and because, as an active rather than passive task, it allowed us to draw inferences regarding whether GAD-related differences in the allocation of processing resources have a downstream influence on performance efficiency, a question with functional and clinical relevance.
To create three distinct contexts (neutral/ambiguous, threat, control) one of two face types, or no faces (no stimuli), were presented prior to the flanker. The no face condition was essentially a classic flanker task. This design allowed us to examine whether processing of inter-trial threat and non-threat faces influenced cognitive performance. The task included a total of 3 blocks (in the order of no face, neutral face, angry face), with 480 trials per block. To avoid carryover effects from the angry condition, the order of experimental blocks was not counterbalanced. Two hundred and forty of the trials in each block displayed congruent flankers while 240 trials displayed incongruent flankers. Each trial consisted of five events: (1) face (neutral, angry for 500 ms, or no face); (2) fixation period (variable 100–300 ms); (3) flanker (congruent, incongruent; 100 ms); (4) response time up to 1700 ms and (5) inter trial interval (varied 1700–2300 ms, minus the reaction time for that trial). Error rate was recorded and used in subsequent analyses. During this task, state anxiety was assessed on the computer following each block (no face, neutral, angry) for a total of three times.
Emotional Face Stimuli
We used human faces as stimuli given the social significance and salience of faces, and the extensive use of face stimuli in studies of anxiety-related attention to and processing of threat (Frenkel & Bar-Haim, 2011; Vuilleumier & Pourtois, 2007). The emotional face stimuli were taken from the NimStim database of the Research Network on Early Experience and Brain Development (Tottenham, Tanaka, Leon, McCarry, Nurse, Hare et al., 2009). All photographs were approximately 177 × 228 pixels, and displayed in grayscale against a white background. The stimuli were equally divided between males and females. Faces of 16 actors portraying angry and neutral expressions were shown for a total of 32 face stimuli. Each face stimulus was shown 30 times.4
EEG Recording and Analysis
EEG activity was recorded continuously via 64 Ag/AgCl scalp electrodes embedded in an elasticized nylon cap (BioSemi; Amsterdam, NL). Electrodes in this system are arranged in accordance with the international 10/20 system. Eye movements were monitored by electro-oculogram (EOG) using four flat-type facial electrodes placed one cm above and below the left eye (vertical eye movements) and one cm to the outer corner of each eye (horizontal eye movements). Electrodes preamplified the EEG signal to improve the signal-to-noise-ratio. EEG was recorded at a sampling rate of 512 Hz. During EEG acquisition, the voltage from each electrode was referenced online with respect to the common mode sense active electrode and the driven right leg electrode, which produces a monopolar (nondifferential) channel. Offline data processing was conducted using Brain Vision Analyzer (Version 2.2, GmbH; Munich, DE). All data were re-referenced offline to an average reference and filtered with a high pass frequency of .1 Hz and a low pass frequency of 30 Hz.
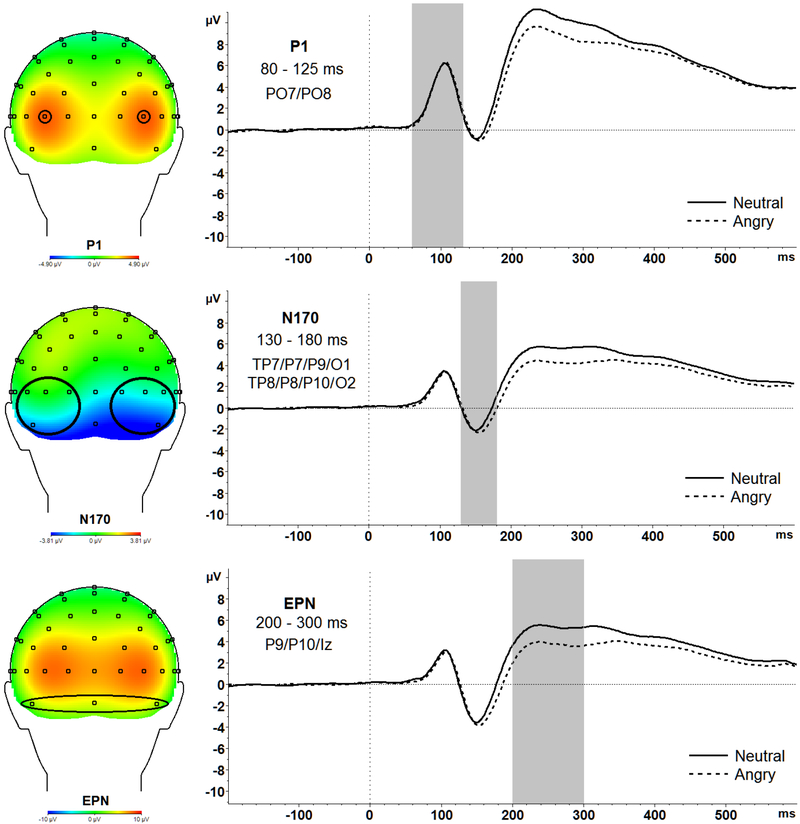
Stimulus-locked EEG to faces were segmented into epochs from 200 ms before stimulus presentation to 600 ms after stimulus onset, with a 200 ms (−200 ms to 0 ms prior to response onset) baseline correction. Following ocular correction (Gratton, Coles, & Donchin, 1983), artifacts were identified using the following criteria and removed from analyses: data with voltage steps greater than 50 μV, changes within a given segment greater than 300 μV, and activity lower than .5 μV per 100 ms. ERPs were quantified as follows, at electrodes where each ERP component was maximal5: the P1 was calculated as the average amplitude between 80 ms and 125 ms at PO7 and PO8, the N170 was calculated as the average amplitude between 130 ms and 180 ms at TP7, P7, P9, O1, TP8, P8, P10, and O2, the EPN was calculated as the average amplitude between 200 ms and 300 ms at P9, P10, and Iz (see Table 1 for ERP descriptive statistics and Figure 1 for scalp distributions).
Figure 1.
Topographic maps and waveforms of the entire sample averaged across both GAD and Control groups for P1 (top), N170 (middle), EPN (bottom) are presented above.
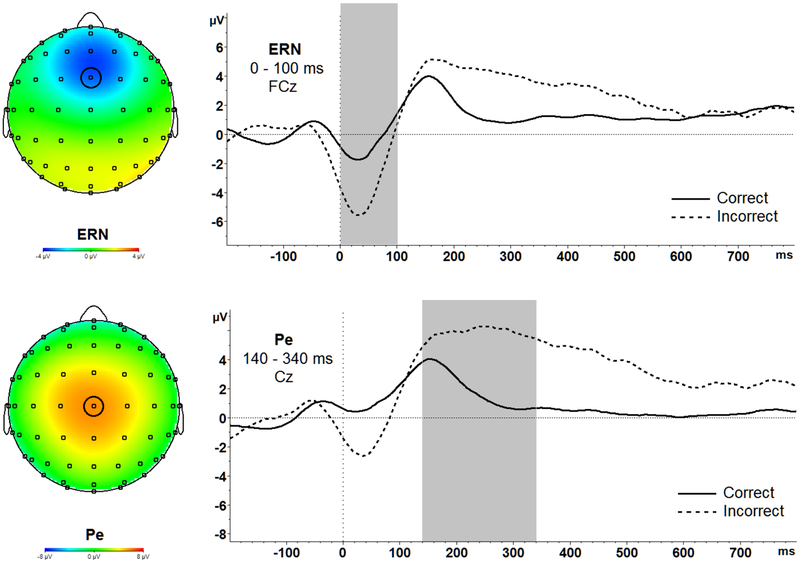
Response-locked data were segmented for each trial beginning at 200 ms before each response onset to 1000 ms after stimulus onset. The 200 ms window from −200 ms to 0 ms prior to response onset was used as the baseline. Following ocular correction (Gratton & Coles, 1983), artifacts were identified using the following criteria and removed from analyses: data with voltage steps greater than 50 μV, changes within a given segment greater than 300 μV, and activity lower than .5 μV per 100 ms. ERPs were quantified as follows, at electrodes where each ERP component was maximal: the ERN was calculated as the average amplitude between 0 ms and 100 ms at FCz (Figure 2); the Pe was calculated as the mean amplitude between 140 ms and 340 ms at Cz (Figure 2). Correct response negativity (CRN) and correct error positivity were also evaluated in the same time windows and electrodes respectively. Furthermore, to measure the difference in response monitoring on error compared to correct trials, difference scores for error minus correct trials were calculated in both the ERN/CRN and Pe/Pe on correct trials time windows, referred to as the ΔERN and ΔPe, respectively.
Figure 2.
Topographic maps and waveforms of the entire sample averaged across both GAD and Control groups and across all three conditions (no, neutral, angry) for ERN (top), and Pe (bottom) are presented above.
For each participant, an average ERN and Pe were calculated separately for error and correct incongruent trials and by face type (no, neutral, angry). Consistent with previous studies using a flanker task, results are based on incongruent trials only because almost all errors were made exclusively on incongruent trials (Santesso & Segalowitz, 2009; Segalowitz et al., 2010).
Artifact-free EEG trials were used to calculate ERPs for each individual, which were then grand-averaged across all participants for each condition. The average trial counts were as follows: P1 [Neutral: 474.50 (SD = 9.05); Angry: 470.80 (SD = 19.88)]; N170 [Neutral: 473.53 (SD = 9.80); Angry: 478.75 (SD = 1.77)]; EPN [Neutral: 476.17 (SD = 5.42); Angry: 478.83 (SD = 1.65)]; ERN correct [Neutral: 203.23 (SD = 27.73); Angry: 201.07 (SD = 24.84); No face: 209.4 (SD = 19.01)]; ERN incorrect [Neutral: 21.10 (SD = 16.79); Angry: 21.90 (SD = 14.87); No face: 20.37 (SD = 13.18)]; Pe correct [Neutral: 202.90 (SD = 27.78); Angry: 201.00 (SD = 24.67); No face: 209.50 (SD = 18.74)]; Pe incorrect [Neutral: 21.07 (SD = 16.73); Angry: 21.90 (SD = 14.87); No face: 20.40 (SD = 13.20)]. Because ERPs were stimulus-locked to the neutral and angry faces, the no-face trials were included in ERP analyses for response-locked components only. The GAD and control groups did not significantly differ in average trial count.
Results
All analyses were conducted using SPSS Version 21.0 (IBM Corp., Armonk, NY). Partial eta-squared (η2) is reported as a measure of effect size for all statistically significant findings using analysis of variance (ANOVA) and Cohen’s d as a measure of effect size for follow-up t-tests reported.
Descriptive Statistics
Task Accuracy.
Descriptive statistics for error rates in each condition by group are presented in Table 1. To confirm that participants made more errors on incongruent flanker trials, and to test whether the GAD group was selectively sensitive to the aversive affective context (angry faces), a 3 (Face Condition: no, neutral, angry) x 2 (Trial Type: congruent, incongruent) x 2 (Group: GAD, control) mixed-design factorial ANOVA was conducted for the number of errors. The main effect of congruency F(1,28) = 61.30, p < .001, partial ƞ2 = .69, confirmed that participants made more errors on incongruent (M = 21.31; SD = 13.97) compared to congruent trials (M = 2.59; SD = 2.48), t(29) = −7.93 p < .001, Cohen’s d = 1.86.
Further, the significant Condition x Group interaction, F(2,56) = 3.32, p = .044, partial ƞ2 = .12, showed that, as predicted, only in the GAD group, significantly more errors were made in the angry condition (M = 30.47; SD = 19.51) compared to the no face condition (M = 22.93; SD = 12.85), t(14) = −2.74, p = .02, Cohen’s d = .46. No other pairwise comparisons were significant within the GAD group (all ps > .207) and errors rates across conditions did not significantly differ between groups (all ps > .098). Therefore, while performance in the control group did not significantly change across blocks, the GAD group committed more errors when angry faces were present suggesting, as predicted, broad sensitivity to aversive affective context.
Exploratory Correlations among Target Variables.
To explore the functional implications of target ERPs, we conducted bivariate correlations between stimulus-locked ERPs (P1, N170, and EPN separately for neutral and angry conditions) and response-locked ERPs (ERN and Pe, separately for error and correct trials) ERPs (P1, N170, EPN, ERN, Pe) and total number of errors. A larger magnitude CRN across all emotion conditions was associated with committing fewer errors, r(30) = .54, p = .002. In contrast, a larger magnitude Pe on error trials [r(30) = .43, p = .019] and correct [r(30) = .51, p = .004] was associated with committing more errors.
Next, we conducted bivariate correlations between these same ERPs and self-report measures of GAD symptoms and trait anxiety (GAD-Q and STAI-trait).6 Larger magnitude Pe on correct trials was associated with greater trait anxiety r(30) = .44, p = .015. No other correlations reached significance.
ERP Analyses
Visual Stimulus Processing.
We conducted three 2 (Face Condition: neutral, angry) x 2 (Group: GAD, control) mixed-design factorial ANOVAs for each ERP as the dependent variable (P1, EPN, N170). We tested the hypothesis that those with GAD, relative to healthy controls, would show heightened early attention allocation (P1) and affective discrimination (EPN) to aversive angry relative to neutral faces, but reduced discrimination between angry and neutral faces at the level of the N170, the later due to enhanced responses to neutral faces.
P1.
There was no significant main effect of Face Condition F(1,28) = .448, p = .509, partial ƞ2 = .02 or interaction effect of Face Condition x Group F(1,28) = .618, p = .438, partial ƞ2 = .02 suggesting no group differences in early attention allocation to angry or neutral faces.
EPN.
There was a significant main effect of Face Condition F(1,28) = 39.53, p < .001, partial ƞ2 = .59. Pairwise comparisons revealed that EPN amplitudes were significantly enhanced (more negative) for angry (M = 2.86, SD = 2.63) compared to neutral (M = 4.47, SD = 2.53) faces across all participants, t(29) = 6.21, p < .001, Cohen’s d = .62. No other significant effects emerged. Thus, the EPN was enhanced to affectively-valenced relative to neutral faces as expected, but the GAD and control groups did not significantly differ.
N170.
There was a significant main effect of Face Condition, F(1,28) = 6.53, p = .016, partial ƞ2 = .19. Pairwise comparisons revealed that overall, N170 amplitudes were enhanced (more negative) for angry (M = −1.45, SD = 2.56) compared to neutral (M = −1.11, SD = 2.92) faces , t(29) = 2.37, p = .03, Cohen’s d = .12.
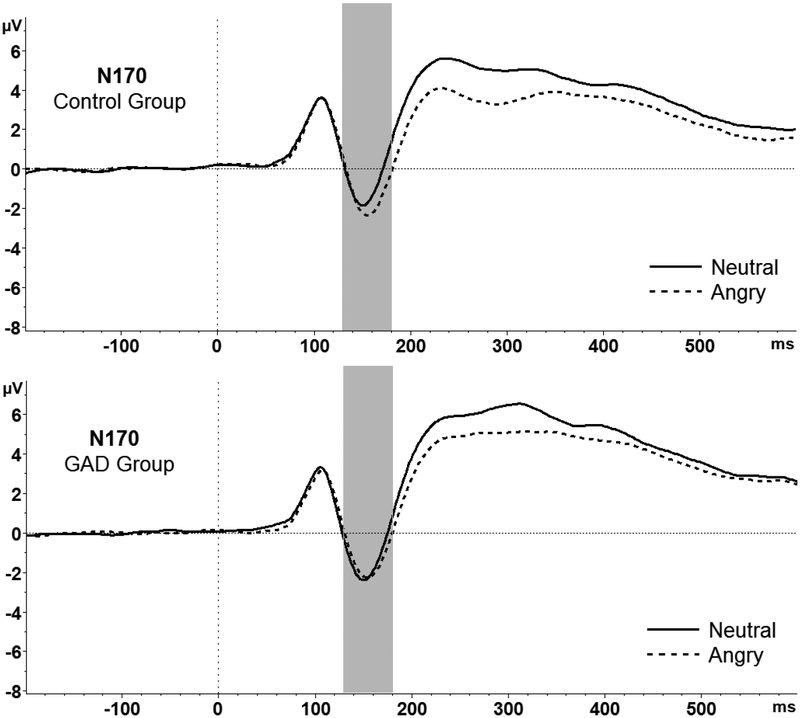
This main effect was qualified by a significant Face Condition x Group interaction, F(1,28) = 5.87, p = .022, ƞ2 = .17. Pairwise comparisons revealed that N170 amplitudes were significantly more negative for angry (M = −1.44, SD = 3.03) versus neutral (M = −.77, SD = 3.38) faces only in the control group, t(14) = 3.36, p = .005, Cohen’s d = .21. The difference between N170 amplitudes for angry (M = −1.47, SD = 2.09) and neutral (M = −1.45, SD = 2.44) faces was not significant for the GAD group (p = 0.92). Furthermore, the difference between the N170 for angry minus neutral faces (ΔN170) was significantly larger (more negative) in the control group (M = −.67, SD = .77) compared to the GAD group (M = −0.02, SD = 0.70), t(28) = −2.42, p = 0.02, Cohen’s d = .88 (see Figure 3). Although examination of group means shows that the magnitude of the N170 to neutral faces was twice as high in the GAD compared to control group, group differences did not reach significance, all p’s > .50.
Figure 3.
N170 amplitudes were significantly more negative to angry versus neutral faces in the control group (top); N170 amplitudes did not differ between face types in the GAD group (bottom).
Given that between-group variance was high, we further explored whether reduced discrimination between angry and neutral faces in the GAD versus control group was due to heightened N170 responses to neutral faces by conducting a 2-way (Group: GAD, control) ANCOVA with N170 amplitudes to neutral faces as the dependent variable, and N170 amplitudes to angry faces as the covariate. This allowed us to control for general magnitude and variability in the N170 response to better isolate potential group differences in N170 to the neutral faces. There was a main effect of Group, F (1, 27) = 6.45, p = 0.02, partial η2 = 0.19, such that individuals with GAD showed a larger magnitude N170 to neutral faces (M = −1.44, SD = 2.44) compared to controls (M = −.79, SD = 3.38), Cohen’s d = .22.
Error-monitoring.
We conducted two 3 (Face Condition: no, neutral, angry) x 2 (Correctness: error, correct) x 2 (Group: GAD, control) mixed-design factorial ANOVAs for ERN and Pe mean amplitudes7 and two (Face Condition: no, neutral, angry) x 2 (Group: GAD, control) mixed-design factorial ANOVAs for ERN-CRN (ΔERN) and PE incorrect – correct (ΔPe) differences scores. We tested the hypothesis that those with GAD, relative to healthy controls, would show heightened early error monitoring (ERN), but reduced elaborative error-monitoring (Pe), the latter due to enhanced responses on correct trials. We expected that effects would be amplified following aversive angry faces.
ERN.
As expected, there was a main effect of Correctness, F(1,28) = 25.45, p < .001, partial ƞ2 = .48. Overall, pairwise comparisons revealed that ERN amplitudes were significantly more negative on error (M = - 3.63, SD = 2.69) versus correct trials (M = −.81, SD = 2.40), t(29) = −5.13, p < .001, Cohen’s d = 1.11. Therefore, the presence of inter-trial emotional faces did not significantly disrupt the sensitivity of the ERN to errors across the sample as a whole.
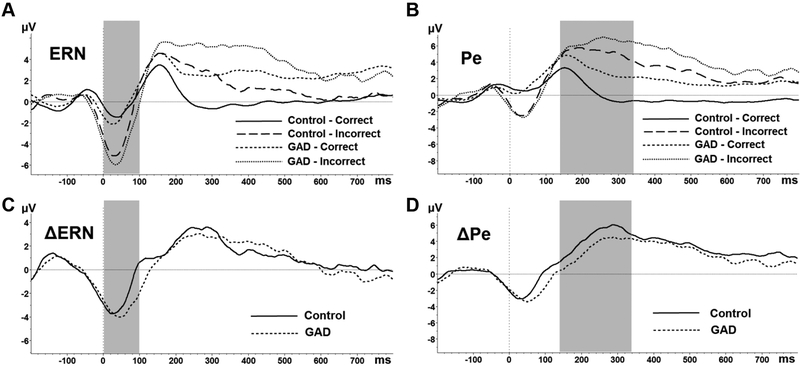
In addition, there was a main effect of Condition suggesting emotional sensitivity of the ERN, F(2, 56) = 5.10, p = .009, partial ƞ2 = .15. Overall, ERN amplitudes were significantly less negative for the angry (M = −1.77, SD = 2.11) compared to no face condition (M = −2.76, SD = 2.53), t(29) = −2.76, p = .010, Cohen’s d = .42. Contrary to predictions, however, ERN amplitudes and ΔERN did not significantly differ between the GAD and Control groups (see Figure 4; top left, bottom left).
Figure 4.
ERN (left) and Pe (right) waveforms are depicted separately by group (GAD; Control) and correct/incorrect responses (A; B). ΔERN and ΔPe difference waveforms are presented separately by group (C; D). Sections B and D represent significant group differences in Pe such that (B) individuals with GAD had significantly larger Pe amplitudes on correct responses compared to Controls and (D) the Pe difference between incorrect and correct responses was significantly smaller in the GAD group relative to Controls.
Pe.
There was a main effect of Correctness, F(1,28) = 25.65, p < .001, partial ƞ2 = .48. As expected, pairwise comparisons revealed that Pe amplitudes were significantly more positive on error (M = 5.15, SD = 3.17) versus correct trials (M = 1.94, SD = 3.05), t(29) = −4.78 p < .001, Cohen’s d = 1.03. Therefore, as for the ERN, the presence of inter-trial emotional faces did not significantly disrupt the sensitivity of the Pe to errors across the sample as a whole.
Also like the ERN, there was a main effect of Condition suggesting emotional sensitivity of the Pe, F(2,56) = 3.72, p = .031, partial ƞ2 = .12. Pairwise comparisons revealed that Pe amplitudes were significantly more positive for the neutral (M = 3.98, SD = 3.32) t(29) = −2.38, p = .024, Cohen’s d = .37, and angry conditions (M = 3.83, SD = 2.99) t(29) = −2.58, p = .015, Cohen’s d = .19, compared to the no face condition (M = 2.81, SD = 3.05).
Next, as predicted, there was a significant Correctness x Group interaction, F(1,28) = 4.58, p = .041 partial, ƞ2 = .14. Figure 4 (top right) depicts the Pe for incorrect and correct trials, averaged across all Condition types and separately for the GAD and Control groups. The interaction revealed that while Pe amplitudes were significantly more positive on errors compared to correct trials in both groups (both p’s <.05), the ΔPe (Pe incorrect – correct difference score averaged across conditions) was significantly reduced in the GAD (M = 1.85, SD = 3.32) compared to control group (M = 4.57, SD = 3.61), t(28) = 2.14, p = .041, Cohen’s d = .78 (Figure 4; bottom right). As predicted, this reduced discrimination between correct and incorrect trials was due to enhanced Pe amplitudes on correct trials in the GAD group (M = 3.06, SD = 2.79) compared to the control group (M = .82, SD = 2.97), t(28)= −2.13 p = .043, Cohen’s d = .78. Pe amplitudes on error trials (p = .72) did not significantly differ between the GAD and Control groups.
Discussion
The present study examined the time course of disruptions in the processing of aversive and non-aversive stimuli and responses in individuals diagnosed with GAD, relative to healthy controls. We targeted a novel combination of ERPs reflecting both early and elaborative stages of stimulus processing and response monitoring. Consistent with hypotheses, those in the GAD group showed reduced discrimination at the stimulus-sensitive stage of visual discrimination (N170) and elaborative stage of error monitoring (Pe). Follow-up analyses suggested heightened responsivity to neutral faces (N170) and enhanced processing of correct responses (Pe) might have driven these effects. However, we failed to find predicted initial exaggerated responses to aversive stimuli for the P1, EPN, and ERN. Taken together, results address both the temporal and functional course of GAD-related cognitive-affective disruptions and suggest that disruptions at elaborative stages of affective discrimination may represent a broad, clinically-significant psychopathological mechanism in GAD.
Evidence for reduced discrimination in GAD emerged at the level of the N170. For the control group, N170 amplitudes were more negative to angry versus neutral faces. This selective enhancement to threat was not significant for the GAD group, who instead showed similar amplitudes to both neutral and angry faces. In interpreting the findings for N170, it is important to note that while the N170 is face-sensitive, it is not face-specific. Indeed, it is thought that the N170, like activity of the fusiform gyrus which it is thought to reflect, more broadly signifies responses to stimuli with which one is highly familiar or has expertise (Maurer, Brandeis, & McCandliss, 2005; Scott, Tanaka, Sheinberg, & Curran, 2006; Tanaka & Curran, 2001). Thus, findings have implications beyond face processing, being relevant for more generalized allocation of neural resources during discrimination among salient stimuli. Indeed, the N170 has been used to examine anxiety-related individual differences in visual discrimination between threat and non-threat (e.g., Dennis-Tiwary et al., 2016). For example, enhanced N170 to angry versus neutral faces predicted increased broad symptoms of anxiety in children over a two-year period (O’Toole et al., 2013). Although this study implicates the N170 as an anxiety-sensitive response, the effect is inverted (greater N170 predicting greater broad spectrum anxiety in children over time). This suggests that reduced discrimination as measured by the N170 might be specific to GAD or might emerge in adulthood rather than childhood. It also highlights the need for future, lifespan developmental research that examines how the N170, as a potential biomarker, might change across development.
Indiscriminant processing is a costly and chronic expenditure of cognitive resources. This may be reflected in our finding that GAD participants showed significantly diminished accuracy by the last experimental block (inter-trial angry faces) compared to the first block in which no faces were seen. MacNamara & Hajcak (2010) document a similar behavioral pattern of increased errors in the presence of aversive stimuli in GAD relative to healthy controls, which was not discernable at the physiological level. Broadly, N170 results are consistent with recent neurobehavioral studies suggesting stimulus generalization to innocuous cues in GAD. For example, GAD participants compared to healthy controls show less discrimination between threat and safety cues indexed by increased ventral medial prefrontal cortex (vmPFC) activity (Cha, Greenberg, Carlson, DeDora, Hajcak & Mujica-Parodi, 2014). In another study, Lissek et al. (2014) found significant overgeneralization of conditioned fear to innocuous cues resembling the feared stimulus.
The current findings taken together with those cited above are consistent with theoretical conceptualizations of GAD that distinguish it from other anxiety disorders. Theories posit that GAD is best defined by broad deficits in emotion regulation which include a strained understanding of emotion and an intensified response to emotional experiences (Salters-Pedneault et al., 2006; Turk, Heimberg, Luterek, Mennin, & Fresco, 2005). Furthermore, a pattern of shallow or dampened discrimination might specifically contribute to GAD core symptoms of worry through a propensity to interpret a range of environmental cues as threatening. Neutral faces in particular may be interpreted as potentially negative or threatening given their ambiguity and lack of clear approach signals (Lee, Kang, Park, Kim, & An, 2008; Wieser & Brosch, 2012).
In terms of error monitoring, the GAD group showed smaller ΔPe (error minus correct) amplitudes driven by enhanced Pe amplitudes on correct trials, but no differences in the ERN. Consistent with this, Weinberg and colleagues (2010) documented enhanced Pe in adults diagnosed with GAD across both errors and correct trials. This provides some initial evidence that the later-emerging Pe may be a sensitive index of over-generalized threat monitoring in GAD. This finding, however, runs counter to several extant studies that show enhanced ΔERN (error minus correct; Weinberg et al., 2012, 2010) and ERN to error trials (Xiao et al., 2011) in GAD relative to healthy controls. Several interpretations of this inconsistency are possible. The introduction of an emotional context (faces) to the flanker task was an important difference between this and prior studies. The presence of a no-face condition, however, reduces this concern because ERN group differences would have been detectable.
Another difference with prior studies is that the present study included individuals who were diagnosed with GAD along with a range of other comorbid disorders, in contrast to studies excluding participants with comorbidities (MacNamara & Hajcak, 2010; Weinberg & Hajcak, 2011; Weinberg et al., 2010). In GAD, comorbidities are the rule rather than the exception (Fava, Rankin, Wright, Alpert, Nierenberg, Pava et al., 2000; MacNamara, Kotov, & Hajcak, 2016), and thus the present GAD sample with significant rates of comorbidity provides a valuable point of comparison for the literature. At the same time, these comorbidities might have introduced more variability in the ERN response, thus obfuscating group differences. Future research should examine whether patterns of reduced affective discrimination documented in the present study are specific to subgroups of those diagnosed with GAD.
The extant research has not strongly focused on abnormalities in the Pe in GAD. The central role of deliberative and top-down processes in GAD, like worry, however, suggest that more deliberative stages of error monitoring as measured by the Pe could be disrupted. This is consistent with neuroimaging studies highlighting GAD-related dysregulation of neural circuitry underlying aspects of cognitive control such as emotional conflict monitoring (Etkin et al., 2010; Etkin, Prater, Schatzberg, Menon, & Greicius, 2009), as well as and reduced ability to normalize overactive neural activity following a worry induction (Paulesu, Sambugaro, Torti, Danelli, Ferri, Scialfa et al., 2010).
The P1 and EPN did not significantly differ between the GAD and control group. This was somewhat surprising given previous research showing that anxiety is broadly associated with larger magnitude ERPs in response to aversive stimuli (Dennis-Tiwary et al., 2016; Eldar, Yankelevitch, Lamy, & Bar-Haim, 2010; Frenkel & Bar-Haim, 2011; Weinberg & Hajcak, 2011) and errors (Gehring, Himle, & Nisenson, 2000). In the case of the stimulus-locked ERPs, faces are low-arousal stimuli signifying mild threat (Phillips et al., 2000), and thus might not consistently tap anxiety-related differences in these rapid and early-emerging ERP responses, particularly when presented over numerous trials. Future research should include more complex, salient, high-arousal and ecologically-valid images, as well as un-ambiguous and genuinely neutral stimuli. In the case of the response-locked ERPs, performance on the present task was very high with error rates for all participants below 36% with nine participants having to be excluded due to the commission of too few errors. Thus, it may be valuable for future studies to select tasks with increasingly difficult attention demands to produce more frequent and subjectively aversive errors.
Several limitations should be noted. Importantly, the small sample size may have limited our ability to detect clinically-significant effects. While the study was adequately powered to detect medium to large effect sizes, it was not optimized for small effect sizes. One limitation of the present design is the absence of a second emotional face type for comparison, such as fearful or happy face stimuli. For example, Morel, George, Foucher, Chammat, & Dubal (2014) found an enhanced P1 to happy faces in trait anxious individuals when compared to controls. Thus, future versions of the task should include a happy face comparison block, as well as comparison with other threat-relevant and negatively-valenced faces, such as fearful faces. Although we did find a large main effect of face condition for the N170, past studies have found mixed sensitivity of the N170 to negative relative to neutral NimStim facial expressions (Smith, Weinberg, Moran, & Hajcak, 2013). Furthermore, the presentation of faces was, by design, not counterbalanced, with presentation of a block of neutral faces followed by a block of angry faces, to prevent “emotional contagion” effects of threat-themed stimuli (angry faces) on neutral stimuli. Future studies could directly test whether contagion occurs.
Despite limitations, the present study leveraged the excellent temporal resolution of ERPs to document reduced attention discrimination and error-monitoring at elaborative stages of processing, driven by exaggerated responding to non-aversive information. Further research is needed to understand the full range of clinically-relevant mechanisms involved in this “neutral stimulus reactivity effect,” such as the contribution of anticipatory contexts, and the failure of individuals with GAD to utilize adaptive strategies (e.g., attentional broadening, decentering, reappraisal) to promote identification of safety cues (Aldao & Mennin, 2012; Mennin & Fresco, 2014). Clarifying the nature of disrupted attention discrimination will also allow for the development of targeted and effective intervention strategies complementing current treatment approaches.
Footnotes
The SCID is a semi-structured interview that assesses the presence and severity of DSM-IV defined mental disorders.
All interviewers were trained over a 6-month period in diagnostic interviewing with the SCID. Reliability was determined via the clinician severity rating (CSR) from the Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV; DiNardo, Brown, & Barlow, 1994). The CSR is a 0–8 rating of the severity of symptoms and impairment associated with each diagnosis that was evaluated between the interviewer and an expert diagnostician (Ph.D.), who provided ratings following a dispositional case presentation. Reliability was examined in 60% (9/15) of participants. There was 100% agreement (k = 1.00, p < .001) such that CSR ratings were at or above the clinical level of severity (4). Clinical participants had a primary diagnosis of GAD according to the SCID and the highest CSR rating when comorbidity was present.
When possible, analyses were conducted with these additional participants and results remained the same.
Actor Numbers Used: 5,7,8,10,11,12,13,14,20,21,23,33,38,39,41,43
Electrodes and time windows were selected based on topographical maps generated from the combined sample (GAD and Control).
Multiple comparisons were corrected for using the Benjamini-Hochberg procedure (Benjamini & Hochberg, 1995) that ranks p values and accounts for the number of comparisons made. Corrections were applied separately for each family of ERPs (stimulus-locked and response-locked) with anxiety questionnaires. The raw p value is reported and was significant using a false discovery rate criterion of 0.25.
ERN was also examined as ERN-CRN difference scores (ΔERN) and at peak amplitudes. For peak amplitudes the same main effects of Correctness and Condition were replicated.
References
- Aarts K, De Houwer J, & Pourtois G (2012). Evidence for the automatic evaluation of self-generated actions. Cognition, 124(2), 117–127. [DOI] [PubMed] [Google Scholar]
- Aarts K, De Houwer J, & Pourtois G (2013). Erroneous and correct actions have a different affective valence: Evidence from ERPs. Emotion, 13(5), 960. [DOI] [PubMed] [Google Scholar]
- Aldao A, & Mennin DS (2012). Paradoxical cardiovascular effects of implementing adaptive emotion regulation strategies in generalized anxiety disorder. Behaviour Research and Therapy, 50(2), 122–130. [DOI] [PubMed] [Google Scholar]
- Amir N, Beard C, Burns M, & Bomyea J (2009). Attention modification program in individuals with generalized anxiety disorder. Journal of Abnormal Psychology, 118(1), 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Borkovec TD, Rickels K, … Wittchen H-U (2001). Consensus statement on generalized anxiety disorder from the International Consensus Group on Depression and Anxiety. The Journal of Clinical Psychiatry, (62 Suppl 11), 53–8. [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, & McCarthy G (1996). Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience, 8(6), 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J, Greenberg T, Carlson JM, DeDora DJ, Hajcak G, & Mujica-Parodi LR (2014). Circuit-Wide Structural and Functional Measures Predict Ventromedial Prefrontal Cortex Fear Generalization: Implications for Generalized Anxiety Disorder. Journal of Neuroscience, 34(11), 4043–4053. 10.1523/JNEUROSCI.3372-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis-Tiwary TA, Egan LJ, Babkirk S, & Denefrio S (2016). For whom the bell tolls: Neurocognitive individual differences in the acute stress-reduction effects of an attention bias modification game for anxiety. Behaviour Research and Therapy, 77, 105–117. 10.1016/j.brat.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimer M (2000). Event-related brain potentials distinguish processing stages involved in face perception and recognition. Clinical Neurophysiology, 111(4), 694–705. [DOI] [PubMed] [Google Scholar]
- Eldar S, Yankelevitch R, Lamy D, & Bar-Haim Y (2010). Enhanced neural reactivity and selective attention to threat in anxiety. Biological Psychology, 85(2), 252–257. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, & Eriksen CW (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics, 16(1), 143–149. 10.3758/BF03203267 [DOI] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, & Hohnsbein J (2000). ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology, 51(2–3), 87–107. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, & Buchner A (2007). G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Fava M, Rankin MA, Wright EC, Alpert JE, Nierenberg AA, Pava J, & Rosenbaum JF (2000). Anxiety disorders in major depression. Comprehensive Psychiatry, 41(2), 97–102. 10.1016/S0010-440X(00)90140-8 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (2007). SCID-I/P. Retrieved from http://scid5.org/revisions/pdf/score_sheet_patient_edition.pdf [DOI] [PubMed] [Google Scholar]
- Frenkel TI, & Bar-Haim Y (2011). Neural activation during the processing of ambiguous fearful facial expressions: An ERP study in anxious and nonanxious individuals. ResearchGate, 88(2–3), 188–195. 10.1016/j.biopsycho.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Himle J, & Nisenson LG (2000). Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science, 11(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, & Simons RF (2003). Anxiety and error-related brain activity. Biological Psychology, 64(1), 77–90. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Römmler J, Ehlis A-C, Heidrich A, & Fallgatter AJ (2004). Source localization (LORETA) of the error-related-negativity (ERN/Ne) and positivity (Pe). Cognitive Brain Research, 20(2), 294–299. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, & Anllo-Vento L (1998). Event-related brain potentials in the study of visual selective attention. Proceedings of the National Academy of Sciences, 95(3), 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Nielsen MK, & Green S (2008). Effects of anxiety on the processing of fearful and happy faces: An event-related potential study. Biological Psychology, 77(2), 159–173. 10.1016/j.biopsycho.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Nieuwenhuis S, Yeung N, & Cohen JD (2003). Errors in reward prediction are reflected in the event-related brain potential. Neuroreport, 14(18), 2481–2484. [DOI] [PubMed] [Google Scholar]
- Itier RJ, & Taylor MJ (2004). N170 or N1? Spatiotemporal differences between object and face processing using ERPs. Cerebral Cortex, 14(2), 132–142. [DOI] [PubMed] [Google Scholar]
- Jackson F, Nelson BD, & Proudfit GH (2015). In an uncertain world, errors are more aversive: Evidence from the error-related negativity. Emotion, 15(1), 12. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Lee E, Kang JI, Park IH, Kim J-J, & An SK (2008). Is a neutral face really evaluated as being emotionally neutral? Psychiatry Research, 157(1), 77–85. [DOI] [PubMed] [Google Scholar]
- Li W, Zinbarg RE, Boehm SG, & Paller KA (2008). Neural and Behavioral Evidence for Affective Priming from Unconsciously Perceived Emotional Facial Expressions and the Influence of Trait Anxiety. Journal of Cognitive Neuroscience, 20(1), 95–107. 10.1162/jocn.2008.20006 [DOI] [PubMed] [Google Scholar]
- Li X, Li X, & Luo Y-J (2005). Anxiety and attentional bias for threat: an event-related potential study. Neuroreport, 16(13), 1501–1505. [DOI] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, & Grillon C (2014). Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biological Psychiatry, 75(11), 909–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, & Kappenman ES (2011). The Oxford handbook of event-related potential components. Oxford university press; Retrieved from https://books-google-com.ezproxy.gc.cuny.edu/books?hl=en&lr=&id=gItoAgAAQBAJ&oi=fnd&pg=PP1&dq=oxford+handbook+of+ERP&ots=B-KSALLkRD&sig=ugvEFkPt6bQOhSe27KtT77J36V8 [Google Scholar]
- MacNamara A, & Hajcak G (2010). Distinct electrocortical and behavioral evidence for increased attention to threat in generalized anxiety disorder. Depression and Anxiety, 27(3), 234–243. [DOI] [PubMed] [Google Scholar]
- MacNamara A, Kotov R, & Hajcak G (2016). Diagnostic and Symptom-Based Predictors of Emotional Processing in Generalized Anxiety Disorder and Major Depressive Disorder: An Event-Related Potential Study. Cognitive Therapy and Research, 40(3), 275–289. 10.1007/s10608-015-9717-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer U, Brandeis D, & McCandliss BD (2005). Fast, visual specialization for reading in English revealed by the topography of the N170 ERP response. Behavioral and Brain Functions, 1(1), 13 10.1186/1744-9081-1-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennin DS, & Fresco DM (2014). Handbook of emotion regulation.
- Mennin Douglas S., Heimberg RG, Turk CL, & Fresco DM (2002). Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clinical Psychology: Science and Practice, 9(1), 85–90. [Google Scholar]
- Mennin Douglas S., Heimberg RG, Turk CL, & Fresco DM (2005). Preliminary evidence for an emotion dysregulation model of generalized anxiety disorder. Behaviour Research and Therapy, 43(10), 1281–1310. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, & Borkovec TD (1990). Development and validation of the penn state worry questionnaire. Behaviour Research and Therapy, 28(6), 487–495. [DOI] [PubMed] [Google Scholar]
- Miller J (1991). The flanker compatibility effect as a function of visual angle, attentional focus, visual transients, and perceptual load: A search for boundary conditions. Perception & Psychophysics, 49(3), 270–288. [DOI] [PubMed] [Google Scholar]
- Morel S, George N, Foucher A, Chammat M, & Dubal S (2014). ERP evidence for an early emotional bias towards happy faces in trait anxiety. Biological Psychology, 99, 183–192. [DOI] [PubMed] [Google Scholar]
- Newman MG, Zuellig AR, Kachin KE, Constantino MJ, Przeworski A, Erickson T, & Cashman-McGrath L (2002). Preliminary reliability and validity of the Generalized Anxiety Disorder Questionnaire-IV: A revised self-report diagnostic measure of generalized anxiety disorder. Behavior Therapy, 33(2), 215–233. [Google Scholar]
- Nitschke JB, Sarinopoulos I, Oathes DJ, Johnstone T, Whalen PJ, Davidson RJ, & Kalin NH (2009). Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. American Journal of Psychiatry, 166(3), 302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, & Polich J (2008). Affective picture processing: An integrative review of ERP findings. Biological Psychology, 77(3), 247–265. 10.1016/j.biopsycho.2007.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole LJ, DeCicco JM, Berthod S, & Dennis TA (2013). The N170 to Angry Faces Predicts Anxiety in Typically Developing Children Over a Two-Year Period. Developmental Neuropsychology, 38(5), 352–363. 10.1080/87565641.2013.802321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek TJ, Nieuwenhuis S, & Ridderinkhof KR (2005). Dissociable components of error processing: on the functional significance of the Pe vis-à-vis the ERN/Ne. Journal of Psychophysiology, 19(4), 319–329. [Google Scholar]
- Phillips M, Williams L, Young A, Andrew C, Bullmore E, Brammer M, … Gray J (2000). Differential neural responses to overt and covert presentations of facial expressions of fear and disgust. NeuroImage, 11(5), S232. [DOI] [PubMed] [Google Scholar]
- Rousselet GA, Husk JS, Bennett PJ, & Sekuler AB (2008). Time course and robustness of ERP object and face differences. Journal of Vision, 8(12), 3. [DOI] [PubMed] [Google Scholar]
- Salters-Pedneault K, Roemer L, Tull MT, Rucker L, & Mennin DS (2006). Evidence of broad deficits in emotion regulation associated with chronic worry and generalized anxiety disorder. Cognitive Therapy and Research, 30(4), 469–480. [Google Scholar]
- Santesso DL, & Segalowitz SJ (2009). The error-related negativity is related to risk taking and empathy in young men. Psychophysiology, 46(1), 143–152. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, & Junghöfer M (2006). Emotion and attention: event-related brain potential studies. Progress in Brain Research, 156, 31–51. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghöfer M, Weike AI, & Hamm AO (2006). Stimulus novelty and emotion perception: the near absence of habituation in the visual cortex. Neuroreport, 17(4), 365–369. [DOI] [PubMed] [Google Scholar]
- Scott LS, Tanaka JW, Sheinberg DL, & Curran T (2006). A Reevaluation of the Electrophysiological Correlates of Expert Object Processing. Journal of Cognitive Neuroscience, 18(9), 1453–1465. 10.1162/jocn.2006.18.9.1453 [DOI] [PubMed] [Google Scholar]
- Segalowitz SJ, Santesso DL, Murphy TI, Homan D, Chantziantoniou DK, & Khan S (2010). Retest reliability of medial frontal negativities during performance monitoring. Psychophysiology, 47(2), 260–270. [DOI] [PubMed] [Google Scholar]
- Smith E, Weinberg A, Moran T, & Hajcak G (2013). Electrocortical responses to NIMSTIM facial expressions of emotion. International Journal of Psychophysiology, 88(1), 17–25. [DOI] [PubMed] [Google Scholar]
- Smith NK, Cacioppo JT, Larsen JT, & Chartrand TL (2003). May I have your attention, please: Electrocortical responses to positive and negative stimuli. Neuropsychologia, 41(2), 171–183. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, & Gorsuch RL (1983). State-Trait Anxiety Inventory for Adults: Manual and Sample: Manual, Instrument and Scoring Guide. Consulting Psychologists Press. [Google Scholar]
- Tanaka JW, & Curran T (2001). A Neural Basis for Expert Object Recognition. Psychological Science, 12(1), 43–47. 10.1111/1467-9280.00308 [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, … Nelson C (2009). The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk CL, Heimberg RG, Luterek JA, Mennin DS, & Fresco DM (2005). Emotion Dysregulation in Generalized Anxiety Disorder: A Comparison with Social Anxiety Disorder. Cognitive Therapy and Research, 29(1), 89–106. 10.1007/s10608-005-1651-1 [DOI] [Google Scholar]
- Van Veen V, & Carter CS (2002). The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior, 77(4), 477–482. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, & Pourtois G (2007). Distributed and interactive brain mechanisms during emotion face perception: Evidence from functional neuroimaging. Neuropsychologia, 45(1), 174–194. 10.1016/j.neuropsychologia.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Weinberg A, & Hajcak G (2011). Electrocortical evidence for vigilance-avoidance in Generalized Anxiety Disorder. Psychophysiology, 48(6), 842–851. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, & Hajcak G (2012). Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of Abnormal Psychology, 121(4), 885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Kotov R, & Proudfit GH (2015). Neural indicators of error processing in generalized anxiety disorder, obsessive-compulsive disorder, and major depressive disorder. Journal of Abnormal Psychology, 124(1), 172. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Olvet DM, & Hajcak G (2010). Increased error-related brain activity in generalized anxiety disorder. Biological Psychology, 85(3), 472–480. 10.1016/j.biopsycho.2010.09.011 [DOI] [PubMed] [Google Scholar]
- Wieser MJ, & Brosch T (2012). Faces in Context: A Review and Systematization of Contextual Influences on Affective Face Processing. Frontiers in Psychology, 3 10.3389/fpsyg.2012.00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H-U (2002). Generalized anxiety disorder: prevalence, burden, and cost to society. Depression and Anxiety, 16(4), 162–171. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Wang J, Zhang M, Li H, Tang Y, Wang Y, … Fromson JA (2011). Error-related negativity abnormalities in generalized anxiety disorder and obsessive–compulsive disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 35(1), 265–272. [DOI] [PubMed] [Google Scholar]
- Yantis S, & Johnston JC (1990). On the locus of visual selection: evidence from focused attention tasks. Journal of Experimental Psychology: Human Perception and Performance, 16(1), 135. [DOI] [PubMed] [Google Scholar]