Summary
Long-acting antiretrovirals can improve therapy and prevention for HIV-1 infection. Current long-acting cabotegravir (CAB LAP) can be administered every other month. Previously, we demonstrated that a myristoylated CAB prodrug encased in poloxamer 407 provided extended plasma drug concentrations. We now demonstrate that this first-generation nanoformulated prodrug can sustain plasma CAB concentrations above the PA-IC90 for four months in rhesus macaques. A 2.5-fold extension in CAB half-life and a 1.6-fold increase in area under the concentration-time curve were observed compared to CAB LAP.
Keywords: Long-acting antiretrovirals, LASER ART, cabotegravir, pharmacokinetics, rhesus macaques
Control of human immunodeficiency virus (HIV-1) infection requires lifelong combination antiretroviral therapy [1, 2]. Adherence to any treatment regimen is a common limitation with consequent emergence of viral mutations and comorbid events [3-7]. The development of long acting parenteral antiretroviral drugs presents an important means to improve regimen adherence with secondary benefits in viral prevention. This can be achieved through the deployment of such medicines for pre-exposure prophylaxis[8] as well as the fact that efficacious treatment prevents viral transmission [9]. Cabotegravir (CAB), a novel integrase strand transfer inhibitor, is being developed as a long acting parenteral formulation (CAB LAP) with a half-life of up to 52 days for every month or every other month dosing [10-12]. CAB is effective against a variety of HIV clades and against vaginal transmission of and intravenous challenge with simian and simian-human immunodeficiency virus in non-human primates [13-17]. The combination of CAB and rilpivirine (RPV) present a two-drug regimen for both treatment and prevention against HIV infection [18, 19]. However, limitations for its use include multiple 2 ml intramuscular injection volumes required to deliver an effective dose of 800 mg, injection site reactions and the requirements for health care providers to provide administration education and follow on [11, 18]. To meet needs for reduced volumes and longer therapeutic durations we have developed medicinal chemistry and nanoformulation approaches to improve prodrugs that facilitate what we have coined as long acting slow effective release antiretroviral therapy (LASER ART) that allows the formation of hydrophobic and lipophilic drug nanocrystals stabilized by polymer excipients [20-22]. Myristoyl ester modified cabotegravir (MCAB) and encasement of the prodrug into poloxamer 407 (P407)-stabilized nanocrystals (NMCAB) defines LASER ART and leads to an extended CAB plasma apparent half-life of 278 hours compared to 71 hours for CAB LAP in mice [22]. Intramuscular administration of 45 mg/kg CAB equivalents as NMCAB provided plasma drug levels above 4 times the protein-adjusted 90% inhibitory concentration (4 × PA-IC90, 660 ng/mL) for 56 days in mice and above the 1X PA-IC90 for 89 days in rhesus macaques [22].
While early screens assessed drug pharmacokinetics to 3 months the prior published report lacked a complete pharmacokinetic analysis of the drug’s apparent half-life. Plasma drug decay was not previously defined. As any assessment of long acting antiretroviral drugs needs to include a complete follow on to where plasma drug levels fall below the 50% effective concentration (EC50) values we have extended analyses of both prodrug and native drug levels in NMCAB treated rhesus macaques to 36.6 weeks. To emphasize the importance of our observations, responses were compared to those from data previously published following a single intramuscular dose of 50 mg/kg CAB LAP.[14]. Myristoylated CAB was synthesized and stabilized into P407-coated NMCAB nanocrystals prepared by high pressure homogenization (Avestin EmulsiFlex-C3, Avestin Inc, Ottawa, Ontario, Canada) [22] using good laboratory practice protocols in the Nebraska Nanomedicine Production Plant [20]. Particle size (351.1 nm), polydispersity index (0.18) and zeta potential (−26.8 mV) were determined by dynamic light scattering (Malvern Zetasizer NanoZSP, Malvern Instruments, Westborough, MA, USA). MCAB concentration in the nanoformulation (131.3 mg/ml) was quantified using ultraperformance liquid chromatography tandem mass spectrometry (UPLC-MS/MS; Waters Acquity XevoTQ-S micro system; Waters Corp., Milford, MA, USA) as described [22]. Endotoxin content (Lonza Limulus Amebocyte Lysate Pyrogent-500, Lonza, Walkersville, MD, USA) was < 5 EU/kg. Two rhesus macaques were injected intramuscularly with 45 mg CAB equivalents/kg as NMCAB. Blood was collected over 8 months for cell counts, metabolic profile and drug analyses. Complete blood counts and metabolic panels were performed by the Nebraska Medical Center Pathology and Microbiology Laboratory. Plasma CAB concentrations were determined by UPLC-MS/MS with current drug quantitation sensitivities of 0.5 ng/ml [22].
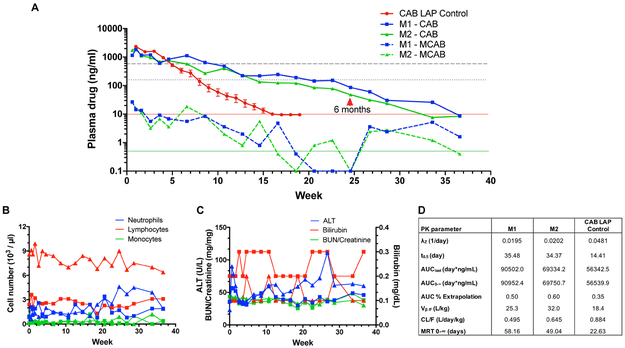
As illustrated in Fig. 1A plasma CAB concentrations remained above the 1X PA-IC90 (166 ng/ml) for 12.7 weeks in both animals given NMCAB. In contrast in the prior report of SIV-infected rhesus macaques treated with 50 mg/kg CAB-LAP [14], the average plasma CAB concentrations dropped below the 1X PA-IC90 by 8 weeks and were below the limit of quantification (10 ng/ml) by 16 weeks. In contrast, in our current studies, plasma CAB concentrations in rhesus macaques that received NMCAB remained at or above 10 ng/ml for 33 weeks, and remained above our assay limit of quantitation (0.5 ng/mL) for the study duration. CAB apparent plasma terminal half-life was increased 2.5-fold from 14 days for CAB-LAP to 34-36 days for NMCAB (Fig. 1D). The longer CAB half-life was reflected in the increase in area under the curve, lower clearance (Cl/F) and higher volume of distribution (V/F) for CAB in animals receiving NMCAB compared to those treated with CAB-LAP. In both animals treated with NMCAB plasma MCAB concentrations peaked at 27 ng/ml at 4 days after treatment, dropped to less than 10 ng/ml at 18 days, then remained at the limit of detection (0.5 ng/mL) for the study duration. CBCs and metabolic panels in animals treated with NMCAB remained unchanged from pre-treatment values throughout the study (Fig. 1B and 1C).
Fig. 1. Plasma CAB concentrations, pharmacokinetics, metabolic and hematologic profiles following intramuscular injection of NMCAB or CAB-LAP.
(A) Plasma CAB concentrations were determined over 36.6 weeks after treatment of two rhesus macaques (M1 and M2) intramuscularly with 45 mg CAB equivalents/kg as NMCAB. Data for CAB LAP Control was extracted from [14] with permission, and averages and standard error of the means were determined. CAB LAP was given as a single 50 mg/kg intramuscular injection to SIV-infected rhesus macaques (N=12). The 1X PA-IC90 and 4X PA-IC90 for CAB in patients are indicated by the small-dashed and large-dashed gray lines, respectively. The solid red line indicates the limit of CAB quantitation for the CAB LAP Control study (10 ng/ml). For our analyses in the current study, CAB and MCAB limits of quantitation (0.5 ng/ml each) are indicated by the solid green line. CAB concentrations = solid lines; MCAB concentrations = dashed lines (B) White blood cell counts following NMCAB administration to 2 rhesus macaques. Blood was collected into EDTA tubes and cell counts assessed by manual differentiation. Data from each animal is presented. Square symbols: M1; Triangle symbols: M2. (C) Alanine aminotransferase (ALT), total bilirubin, blood urea nitrogen (BUN) and creatinine were determined in plasma following intramuscular NMCAB administration to 2 rhesus macaques. Blood was collected into EDTA tubes and plasma separated by centrifugation at 3,000 g for 10 min. Data from each animal is presented. Square symbols: M1; Triangle symbols: M2. (D) Plasma pharmacokinetic (PK) parameters for CAB were determined using noncompartmental analysis in animals treated with NMCAB (M1 and M2) or CAB LAP (control). λZ, individual estimate of the terminal elimination rate constant; AUClast, AUC 0 h to last time point; AUC0–∞, AUC from 0 h to infinity; Vβ, volume of distribution at β phase; CL, clearance; MRT, mean residence time.
These results demonstrate that LASER ART may be used to extend the apparent half-life of CAB with either a decrease in injection volume or extended dosing intervals to 3 months or longer. Further enhancements of drug lipophilicity and hydrophobicity are currently under investigation to further extend dosing intervals beyond six-months. Importantly, lack of observed injection site and systemic reactions indicates that the minimal use of excipients may improve patient acceptance to these regimens. These improvements in chemical composition and administration of CAB could enable improved treatment and preventative measures for HIV-1 infection.
Acknowledgements
The authors are grateful to Dr. David Ho, Aaron Diamond AIDS Research Center, The Rockefeller University, New York, NY, for permission to use and replot the CAB LAP data from his previously-published work. The authors thank Ms. Bhagya Laxmi Dyavar Shetty for her expert technical assistance in UPLC-MS/MS analyses of formulation and plasma drug concentrations. We acknowledge the assistance of the UNMC Comparative Medicine staff, the Nebraska Medicine Pathology/Microbiology Laboratory, and the UNMC Flow Cytometry Core facility. This research was supported by ViiV Healthcare and by National Institutes of Health grants R01 MH104147, P01 DA028555, R01 NS36126, P01 NS31492, 2R01 NS034239, P01 MH64570, P01 NS43985, P30 MH062261, P30 AI078498, and R01 AG043540.
Footnotes
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Lewden C, Chene G, Morlat P, Raffi F, Dupon M, Dellamonica P, et al. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr 2007; 46:72–77. [DOI] [PubMed] [Google Scholar]
- 2.Vittinghoff E, Scheer S, O’Malley P, Colfax G, Holmberg SD, Buchbinder SP. Combination antiretroviral therapy and recent declines in AIDS incidence and mortality. J Infect Dis 1999; 179:717–720. [DOI] [PubMed] [Google Scholar]
- 3.Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV-1 drug resistance and resistance testing. Infect Genet Evol 2016; 46:292–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shubber Z, Mills EJ, Nachega JB, Vreeman R, Freitas M, Bock P, et al. Patient-Reported Barriers to Adherence to Antiretroviral Therapy: A Systematic Review and Meta-Analysis. PLoS Med 2016; 13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treisman GJ, Soudry O. Neuropsychiatric Effects of HIV Antiviral Medications. Drug Saf 2016; 39:945–957. [DOI] [PubMed] [Google Scholar]
- 6.Troya J, Bascunana J. Safety and Tolerability: Current Challenges to Antiretroviral Therapy for the Long-Term Management of HIV Infection. AIDS Rev 2016; 18:127–137. [PubMed] [Google Scholar]
- 7.Utrillo L, Vidal F, Puig T, Domingo P. Switching antiretroviral regimes for the treatment of HIV: safety implications. Expert Opin Drug Saf 2016; 15:1349–1360. [DOI] [PubMed] [Google Scholar]
- 8.Spreen WR, Margolis DA, Pottage JC Jr., Long-acting injectable antiretrovirals for HIV treatment and prevention. Curr Opin HIV AIDS 2013; 8:565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365:493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, Eron JJ, Yazdanpanah Y, Podzamczer D, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390:1499–1510. [DOI] [PubMed] [Google Scholar]
- 11.Spreen W, Williams P, Margolis D, Ford SL, Crauwels H, Lou Y, et al. Pharmacokinetics, safety, and tolerability with repeat doses of GSK1265744 and rilpivirine (TMC278) long-acting nanosuspensions in healthy adults. J Acquir Immune Defic Syndr 2014; 67:487–492. [DOI] [PubMed] [Google Scholar]
- 12.Trezza C, Ford SL, Spreen W, Pan R, Piscitelli S. Formulation and pharmacology of long-acting cabotegravir. Curr Opin HIV AIDS 2015; 10:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmon SL, Mohri H, Spreen W, Markowitz M. GSK1265744 demonstrates robust in vitro activity against various clades of HIV-1. J Acquir Immune Defic Syndr 2015; 68:e39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews CD, Spreen WR, Mohri H, Moss L, Ford S, Gettie A, et al. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science 2014; 343:1151–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews CD, Yueh YL, Spreen WR, St Bernard L, Boente-Carrera M, Rodriguez K, et al. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med 2015; 7:270ra274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews CD, Bernard LS, Poon AY, Mohri H, Gettie N, Spreen WR, et al. Cabotegravir long acting injection protects macaques against intravenous challenge with SIVmac251. Aids 2017; 31:461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radzio J, Spreen W, Yueh YL, Mitchell J, Jenkins L, Garcia-Lerma JG, et al. The long-acting integrase inhibitor GSK744 protects macaques from repeated intravaginal SHIV challenge. Sci Transl Med 2015; 7:270ra275. [DOI] [PubMed] [Google Scholar]
- 18.Markowitz M, Frank I, Grant RM, Mayer KH, Elion R, Goldstein D, et al. Safety and tolerability of long-acting cabotegravir injections in HIV-uninfected men (ECLAIR): a multicentre, double-blind, randomised, placebo-controlled, phase 2a trial. Lancet HIV 2017; 4:e331–e340. [DOI] [PubMed] [Google Scholar]
- 19.Landovitz R, Li S, Grinsztejn B, Dawood H, Liu A, Magnus M, et al. Safety, tolerability and pharmacokinetics of longacting injectable cabotegravir in low-risk HIV-uninfected women and men: HPTN 077. In: 9th IAS Conference on HIV Science (IAS 2017) Paris, France; 2017. pp. 23–26. [Google Scholar]
- 20.McMillan J, Szlachetka A, Slack L, Sillman B, Lamberty B, Morsey B, et al. Pharmacokinetics of a long-acting nanoformulated dolutegravir prodrug in rhesus macaques. Antimicrob Agents Chemother 2018; 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sillman B, Bade AN, Dash PK, Bhargavan B, Kocher T, Mathews S, et al. Creation of a long-acting nanoformulated dolutegravir. Nat Commun 2018; 9:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou T, Su H, Dash P, Lin Z, Dyavar Shetty BL, Kocher T, et al. Creation of a nanoformulated cabotegravir prodrug with improved antiretroviral profiles. Biomaterials 2018; 151:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]