Abstract
Objectives:
Aim of this study was to evaluate the impact of epidural analgesia on postoperative length of stay, expeditious discharge, and pain relief after pancreaticoduodenectomy and distal pancreatectomy.
Methods:
Retrospective reviews of 2014–2015 American College of Surgeons National Surgical Quality Improvement Program databases and our institutional pancreas surgery database were conducted.
Results:
On univariate analysis, epidural analgesia was associated with statistically significant longer lengths of stay for both pancreaticoduodenectomy and distal pancreatectomy. On comparative analysis at mode length of stay, discharged before vs. after 7 days for pancreaticoduodenectomy and 6 days for distal pancreatectomy, epidural analgesia was a significant predictor for the longer groups for both procedures on multivariable analysis (pancreaticoduodenectomy: odds ratio 1.465; P < 0.001, distal pancreatectomy: odds ratio 1.471; P = 0.004). On review of our institution’s pancreas surgery database, patient-reported pain scores were significantly lower in the epidural analgesia groups than intravenous narcotics groups on the day of surgery only for both pancreaticoduodenectomy and distal pancreatectomy.
Conclusions:
Epidural analgesia was associated with longer length of stay with a most pronounced effect on early discharge after surgery for patients undergoing open pancreaticoduodenectomy and distal pancreatectomy. It only resulted in superior pain control on the day of surgery.
Keywords: epidural analgesia, pancreatic surgery, length of hospital stay, early discharge, pain control
Introduction
Epidural catheters are increasingly utilized for patients undergoing pancreatic surgery.1,2 This trend is largely due to the numerous direct benefits associated with epidural analgesia (EA) and the avoidance of adverse effects observed with alternative pain management approaches with intravenous narcotics, often administered with patient-controlled analgesia (PCA) pumps.2,3,4 A majority of prospective randomized studies reveal that EA yields better pain relief after major abdominal surgeries.3,5,6 There is also evidence that postoperative EA improves disease-specific survival in patients after cancer resection.7
The upward trend of EA utilization can also be attributed to Enhanced Recovery After Surgery (ERAS) programs. Initiated in 2005, ERAS protocols include a bundle of recommendations in the perioperative period that aim to facilitate recovery, decrease metabolic stress response, limit postoperative organ dysfunction, and reduce postoperative hospital length of stay (LOS).8 Many centers have developed and utilized ERAS protocols in patients undergoing pancreatic surgery, and use of EA is a core component of most pancreatic surgery ERAS protocols.9
However, there is inconsistent evidence on the impact of EA on LOS after pancreatic surgery.1,2,6,10,11 At our institution, it has been noted that epidural analgesia increases the complexity of care prior to discharge, including removal of epidural catheter and appropriate timing of prophylactic anticoagulation in anticipation of catheter removal, removal of Foley catheter, and spontaneous urinary void after Foley removal. A delay with any of these steps can hinder timely discharge. Therefore, we hypothesized that EA increases LOS in patients with an uncomplicated postoperative course who are candidates for early discharge.
Using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) clinical database, which incorporates data from hundreds of hospitals across the United States, we examined the impact of EA on LOS following open pancreaticoduodenectomy (PD) and distal pancreatectomy (DP), and performed a detailed multivariable analysis to assess the effect of EA on early discharge. In addition, we utilized our own institutional prospectively maintained pancreas surgery database to compare the effectiveness of EA and intravenous narcotics via PCA (IV-PCA) on postoperative pain control.
MATERIALS AND METHODS
Study Design and Patient Population
This study was exempt from Institutional Review Board approval since all data were de-identified and HIPAA-compliant. The ACS-NSQIP databases compile clinical data on patients undergoing surgical procedures at participating institutions. Data collected by NSQIP-trained clinical reviewers include demographics, preoperative risk factors, procedural details, and postoperative complications up to 30 days after surgery. The data are audited by the ACS to ensure accurate reporting of variables. We queried the NSQIP databases, specifically the general participant use data file (PUF) and the pancreatectomy-targeted PUF, to identify patients undergoing PD (Current Procedural Terminology (CPT) codes 48150 and 48153) and DP (CPT code 48140) between January 1, 2014 and December 31, 2015. Cases were matched between the two databases using unique NSQIP identification numbers; cases that had entries in only one of the PUFs were excluded. Cases with missing variables were also excluded from analysis. Patients were divided into two groups based on whether they received EA or not. Demographic data on sex, age, ASA classification, malignant pathology, preoperative albumin level, postoperative complications and LOS were compared between these two groups by procedure-type. Impact of factors that may affect LOS, including EA and postoperative complications, were examined by univariate and multivariable analyses. In addition, the proportions of patients with EA in short vs. long LOS groups were reviewed at multiple cutoff timepoints. Cutoff timepoints of 7 days for PD and 6 days for DP were used to distinguish short vs long LOS for detailed analyses, as these were the most common days of discharge for each procedure in the NSQIP database. A multivariable analysis was performed to determine the impact of EA on LOS with confounders specific to pancreatic surgery that may also impact LOS. These included pneumonia, pulmonary embolism or deep vein thrombosis (PE/DVT), urinary tract infection, pancreatic fistula, delayed gastric emptying, and surgical site infection (SSI).
To assess the efficacy of EA on pain control during the postoperative course, a retrospective review of the prospectively maintained University of California at Los Angeles (UCLA) pancreatic surgery database was conducted. Medical records of patients who underwent open PD and DP during June 2013 to December 2016 were queried for the collection of demographic and clinical data as well as patient-reported pain scores. Intensity of pain was measured using an 11-point numeric rating scale (0–10: 0 = no pain, 10 = worst pain) and collected at least 6 times per day by nursing staff. The mean pain score was determined for each patient on the day of surgery (POD0) and each postoperative day (POD). Patients were removed from the EA group on the day the epidural catheter was removed.
Statistical Analysis
Chi-square test for categorical variables and Mann-Whitney test for continuous variables were used for univariate comparisons of patient characteristics, perioperative details, and pain scores. Multiple regression analysis was used to test the effect of EA and pancreatectomy-related complications on LOS as a continuous variable. Multivariable binary logistic regression was used to examine the effect of EA on short vs. long LOS while controlling for other variables that may affect LOS. Analyses were performed using IBM SPSS Statistics 24 (Armonk, NY). All tests were 2-sided and P ≤0.05 was considered statistically significant.
RESULTS
Epidural Analgesia and Postoperative Length of Stay
A total of 8098 patients who underwent an open pancreatic surgery were identified in the 2014 and 2015 NSQIP databases. Of these patients, 6185 patients underwent PD and 1913 underwent DP (Table 1). The frequencies at which patients were administered EA were 23.2% for PD and 19.4% for DP.
TABLE 1.
Demographics Stratified by EA and Non-EA in Patients Undergoing Open Pancreatic Surgery From 2014–2015 NSQIP Database
| PD (n = 6185) | DP (n = 1913) | ||||||
|---|---|---|---|---|---|---|---|
| EA (n = 1437) | Non-EA (n = 4748) | P | EA (n = 372) | Non-EA (n = 1541) | P | ||
| Sex, male, n (%) | 775 (53.9) | 2563 (54.0) | 0.974 | 173 (46.5) | 710 (46.1) | 0.881 | |
| Age, median (IQR), y | 66 (57–73) | 65 (57–72) | 0.480 | 63 (53–70) | 63 (53–71) | 0.977 | |
| ASA classification, median (IQR) | 3 (2–3) | 3 (3–3) | 0.002 | 3 (2–3) | 3 (2–3) | 0.467 | |
| Malignant pathology, n (%) | 988 (68.8) | 3374 (71.1) | 0.093 | 124 (33.3) | 569 (36.9) | 0.196 | |
| Albumin, median (IQR), g/dL | 3.8 (3.4–4.2) | 3.8 (3.4–4.2) | 0.813 | 4.0 (3.7–4.3) | 4.0 (3.6–4.3) | 0.678 | |
| Pneumonia, n (%) | 53 (3.7) | 211 (4.4) | 0.214 | 11 (3.0) | 73 (4.7) | 0.133 | |
| PE/DVT, n (%) | 52 (3.6) | 163 (3.4) | 0.736 | 22 (5.9) | 49 (3.2) | 0.012 | |
| Urinary tract infection, n (%) | 56 (3.9) | 149 (3.1) | 0.159 | 11 (3.0) | 50 (3.2) | 0.777 | |
| Pancreatic fistula, n (%) | 275 (19.1) | 825 (17.4) | 0.126 | 87 (23.4) | 277 (18.0) | 0.017 | |
| Delayed gastric emptying, n (%) | 251 (17.5) | 789 (16.6) | 0.451 | 21 (5.6) | 87 (5.6) | 1.000 | |
| Wound infection, n (%) | |||||||
| Superficial incisional SSI | 141 (9.8) | 415 (8.7) | 0.213 | 21 (5.6) | 60 (3.9) | 0.132 | |
| Deep incisional SSI | 37 (2.6) | 110 (2.3) | 0.574 | 4 (1.1) | 26 (1.7) | 0.394 | |
| Organ space SSI | 191 (13.3) | 652 (13.7) | 0.670 | 44 (11.8) | 180 (11.7) | 0.937 | |
| Length of stay, median (IQR), d | 9 (7–14) | 9 (7–13) | 0.007 | 7 (5–9) | 6 (5–9) | 0.001 | |
Bold font indicates a statistically significant P-value of less than or equal to 0.05.
EA indicates epidural analgesia; NSQIP, American College of Surgeons National Surgical Quality Improvement Program; PD, pancreaticoduodenectomy; DP, distal pancreatectomy; IQR, interquartile range; ASA, American Society of Anesthesiologists; PE/DVT, pulmonary embolism or deep vein thrombosis; SSI, surgical site infection.
The clinical, operative, and postoperative characteristics of patients with and without EA by procedure-type are listed in Table 1. There were relatively few differences between the EA groups and the non-EA groups. Patients undergoing PD with EA had a statistically but not clinically significant lower ASA score (P = 0.002); those undergoing DP with EA had higher rates of PE/DVT and pancreatic fistula than patients without EA (P = 0.012, 0.017 respectively). Epidural analgesia was associated with a statistically but not clinically significant longer median LOS for PD (P = 0.007), while median LOS of EA group was one day longer (7 vs 6) for patients undergoing DP (P = 0.001).
We next examined EA in the context of factors previously shown to increase LOS after pancreatic surgery. In addition to EA, pneumonia, PE/DVT, urinary tract infection, pancreatic fistula, delayed gastric emptying, and SSI were all associated with significantly longer LOS for both PD and DP (Table 2). On multiple regression analyses, all factors except for EA were again significant for PD, while pneumonia, PE/DVT, urinary tract infection, pancreatic fistula, and delayed gastric emptying were significant for DP.
TABLE 2.
Mann-Whitney and Multiple Regression Analyses of Factors That Affect Postoperative LOS in Patients Undergoing PD and DP in 2014–2015 NSQIP Database
| Yes | No | P | B | SE B | β | P | ||
|---|---|---|---|---|---|---|---|---|
| PD (n = 6185)* | ||||||||
| EA | 9 (1437) | 9 (4748) | 0.007 | 0.118 | 0.224 | 0.006 | 0.598 | |
| Pneumonia | 18 (264) | 8 (5921) | <0.001 | 7.419 | 0.473 | 0.17 | <0.001 | |
| PE/DVT | 15 (215) | 8 (5970) | <0.001 | 5.425 | 0.520 | 0.113 | <0.001 | |
| Urinary tract infection | 13 (205) | 8 (5980) | <0.001 | 2.090 | 0.529 | 0.042 | <0.001 | |
| Pancreatic fistula | 14 (1100) | 8 (5085) | <0.001 | 4.402 | 0.256 | 0.191 | <0.001 | |
| Delayed gastric emptying | 17 (1040) | 8 (5145) | <0.001 | 8.623 | 0.260 | 0.366 | <0.001 | |
| Superficial incisional SSI | 10 (556) | 8 (5629) | <0.001 | 1.057 | 0.332 | 0.034 | 0.001 | |
| Deep incisional SSI | 13 (147) | 9 (6038) | <0.001 | 2.126 | 0.628 | 0.037 | 0.001 | |
| DP (n = 1913)† | ||||||||
| EA | 7 (372) | 6 (1541) | 0.001 | 0.585 | 0.420 | 0.029 | 0.163 | |
| Pneumonia | 15 (84) | 6 (1829) | <0.001 | 11.029 | 0.817 | 0.279 | <0.001 | |
| PE/DVT) | 8 (71) | 6 (1842) | <0.001 | 1.847 | 0.882 | 0.043 | 0.036 | |
| Urinary tract infection | 9 (61) | 6 (1852) | <0.001 | 2.851 | 0.945 | 0.062 | 0.003 | |
| Pancreatic fistula | 7 (364) | 6 (1549) | <0.001 | 1.912 | 0.431 | 0.093 | <0.001 | |
| Delayed gastric emptying | 15 (108) | 6 (1805) | <0.001 | 9.446 | 0.732 | 0.269 | <0.001 | |
| Superficial incisional SSI | 8 (81) | 6 (1832) | 0.001 | 0.902 | 0.824 | 0.022 | 0.274 | |
| Deep incisional SSI | 8 (30) | 6 (1883) | 0.035 | 1.792 | 1.337 | 0.027 | 0.180 | |
Data presented as median (n).
R2 = 0.291
R2 = 0.206
LOS indicates length of stay; PD, pancreaticoduodenectomy; DP, distal pancreatectomy; NSQIP, American College of Surgeons National Surgical Quality Improvement Program; B, unstandardized beta; SE B, standard error for unstandardized beta; β, standardized beta; EA, epidural analgesia; PE/DVT, pulmonary embolism/deep vein thrombosis; SSI, surgical site infection.
Epidural Analgesia and Early Discharge
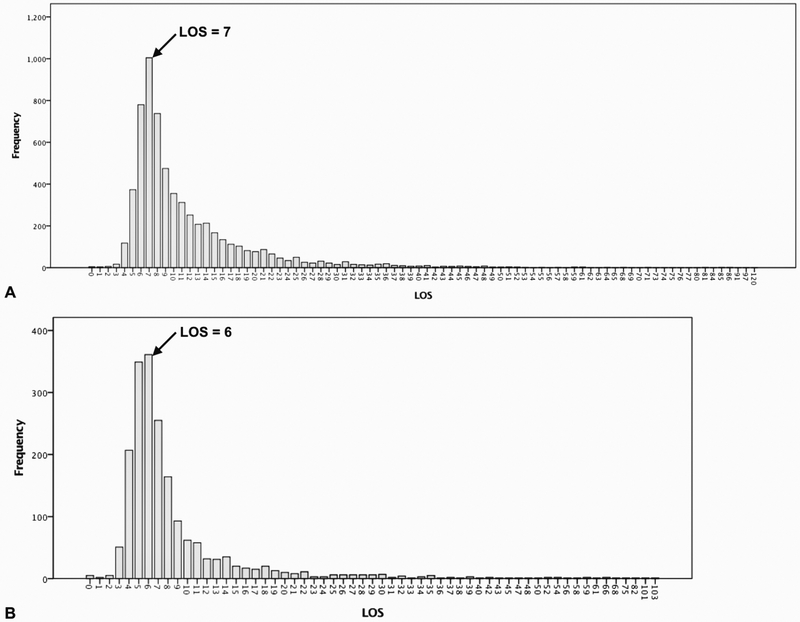
Even though EA was not significant on multivariable analysis with LOS as a continuous variable, we expanded our analysis of EA and LOS. We hypothesized that the impact of EA on LOS was greatest in patients with an uncomplicated postoperative course who were amenable for early discharge. This would not be captured by the analysis in Table 2 using LOS as a continuous variable due to the numerous postoperative complications that can extend LOS and contribute to its highly skewed distribution with a long tail extending to the right in both PD and DP (Fig. 1).
FIGURE 1.
Distribution of postoperative length of stay (LOS) after (A) pancreaticoduodenectomy and (B) distal pancreatectomy in 2014–2015 NSQIP database.
As depicted in Table 3, we next examined the impact of EA on early discharge after PD and DP. This was done by comparing the proportion of patients with EA who stayed longer vs. shorter than defined LOS cutoff variables. The proportion of patients with EA was significantly higher in the patients who were discharged after the LOS cutoff values, particularly at the earlier time points. As expected, it decreased as the LOS cutoffs increased (Table 3).
TABLE 3.
Proportions of Patients With EA in Short Versus Long LOS Groups at Different LOS Cutoff Values for PD and DP From 2014–2015 NSQIP
| PD (n = 6185) | DP (n = 1913) | |||
|---|---|---|---|---|
| EA (</≥), % | P | EA (</≥), % | P | |
| LOS </≥5 days | 16.7/23.4 | 0.030 | 12.2/20.6 | 0.001 |
| LOS </≥6 days | 17.2/23.8 | <0.001 | 15.5/21.3 | 0.001 |
| LOS </≥7 days | 18.1/24.7 | <0.001 | 16.5/22.5 | 0.001 |
| LOS </≥8 days | 21.5/24.2 | 0.008 | 17.7/22.6 | 0.007 |
| LOS </≥9 days | 22.8/23.6 | 0.231 | 18.3/22.6 | 0.022 |
| LOS </≥10 days | 22.7/23.9 | 0.157 | 19.0/21.1 | 0.177 |
Bold font indicates mode LOS cutoff for each procedure
PD, pancreaticoduodenectomy; DP, distal pancreatectomy.
Cutoff timepoints of 7 days for PDs and 6 days for DPs were selected for detailed analyses, as they were the most frequent LOS for these procedures and generally represent an efficient and complication-free postoperative course (Fig. 1). Notably, 22.6% (n = 1395) of patients undergoing PD were discharged in less than seven days, and 32.4% (n = 619) of patients undergoing DP were discharged in less than six days. There were significantly more patients who received EAs in the longer LOS groups for both procedures (PD: 24.7% vs. 18.1%, P < 0.001, DP: 21.3% vs. 15.5%, P = 0.001) (Table 4).
TABLE 4.
Univariate Comparisons and Multivariable Binary Logistic Regressions by Short Versus Long LOS Groups in Patients Undergoing PD and DP From 2014–2015 NSQIP Database
| PD (n = 6185) | LOS≥7 days | |||||
|---|---|---|---|---|---|---|
| LOS <7 days, n (%) (n = 1395) |
LOS ≥7 days, n (%) (n = 4790) |
P | OR (95% CI) | P | ||
| EA | 253 (18.1) | 1184 (24.7) | <0.001 | 1.465 (1.254–1.712) | <0.001 | |
| Pneumonia | 21 (1.5) | 243 (5.1) | <0.001 | 2.390 (1.504–3.798) | <0.001 | |
| PE/DVT | 18 (1.3) | 197 (4.1) | <0.001 | 2.172 (1.316–3.584) | 0.002 | |
| Urinary tract infection | 18 (1.3) | 187 (3.9) | <0.001 | 2.527 (1.536–4.156) | <0.001 | |
| Pancreatic fistula | 84 (6.0) | 1016 (21.2) | <0.001 | 3.288 (2.598–4.160) | <0.001 | |
| Delayed gastric emptying | 63 (4.5) | 977 (20.4) | <0.001 | 4.255 (3.260–5.552) | <0.001 | |
| Superficial incisional SSI | 75 (5.4) | 481 (10.0) | <0.001 | 1.699 (1.312–2.199) | <0.001 | |
| Deep incisional SSI | 17 (1.2) | 130 (2.7) | 0.001 | |||
| LOS <6 days, n (%) (n = 619) |
LOS ≥6 days, n (%) (n = 1294) |
P | OR (95% CI) | P | ||
| EA | 96 (15.5) | 276 (21.3) | 0.001 | 1.471 (1.134–1.909) | 0.004 | |
| Pneumonia | 2 (0.3) | 82 (6.3) | <0.001 | 17.68 (4.311–72.512) | <0.001 | |
| PE/DVT | 13 (2.1) | 58 (4.5) | 0.005 | 1.763 (0.942–3.298) | 0.076 | |
| Urinary tract infection | 11 (1.8) | 50 (3.9) | 0.009 | 1.848 (0.937–3.643) | 0.076 | |
| Pancreatic fistula | 78 (12.6) | 286 (22.1) | <0.001 | 1.650 (1.247–2.183) | <0.001 | |
| Delayed gastric emptying | 8 (1.3) | 100 (7.7) | <0.001 | 4.962 (2.375–10.365) | <0.001 | |
| Superficial incisional SSI | 18 (2.9) | 63 (4.9) | 0.028 | 1.459 (0.843–2.528) | 0.177 | |
| Deep incisional SSI | 8 (1.3) | 22 (1.7) | 0.324 | |||
OR indicates odds ratio; CI, confidence interval.
As there were also more pancreatectomy-relevant complications in the EA (Table 1) and longer LOS (Table 4) groups, a multivariable analysis was performed using the LOS cutoffs of 7 days for PD and 6 days for DP. Despite controlling for complications, EA use remained significant for both procedure-types (PD: Odds ratio (OR), 1.465, P < 0.001; DP: OR, 1.471, P = 0.004) (Table 4).
Epidural Analgesia and Pain Control
We next examined the impact of EA on pain control after PD and DP. As the NSQIP database does not include patient-reported pain scores, we utilized our institution’s pancreas surgery database. At UCLA, 80% of patients undergoing open PD and 57% of patients undergoing open DP were administered EA from June 2013 to December 2016. The EA composition at our institution includes 0.1% bupivacaine with 10 mcg/cc hydromorphone, and they are routinely started in the operating room during surgery. Of 210 patients who underwent PD, 20 consecutive patients who received EA and 20 with IV-PCA containing either morphine or hydromorphone were included to generate representative cohorts; all patients undergoing DP during this timeframe were included (EA: n = 24, IV-PCA: n = 18) (Table 5). There were no significant differences in the clinical, operative or postoperative characteristics, including length of stay, between patients receiving EA vs IV-PCA in both PD and DP groups.
TABLE 5.
Demographics Stratified by EA and PCA in Patients Undergoing PD and DP From 2013–2016 UCLA Database
| PD (n = 210) | DP (n = 4 2) | ||||||
|---|---|---|---|---|---|---|---|
| EA (n = 167) | PCA (n = 43) | P | EA (n = 24) | PCA (n = 18) | P | ||
| Pneumonia, n (%) | 5 (3.0) | 1 (2.3) | 1.000 | 0 | 1 (5.6) | 0.429 | |
| PE/DVT, n (%) | 5 (3.0) | 3 (7.0) | 0.441 | 1 (4.2) | 0 | 1.000 | |
| Urinary tract infection, n (%) | 11 (6.6) | 3 (7.0) | 1.000 | 0 | 2 (11.1) | 0.178 | |
| Pancreatic fistula, n (%) | 35 (21.0) | 11 (25.6) | 0.513 | 7 (29.2) | 3 (16.7) | 0.565 | |
| Delayed gastric emptying, n (%) | 19 (11.4) | 8 (18.6) | 0.207 | 0 | 0 | ||
| Wound infection, n (%) | |||||||
| Superficial incisional SSI | 22 (13.2) | 6 (14.0) | 0.893 | 3 (12.5) | 1 (5.6) | 0.820 | |
| Deep incisional SSI | 6 (3.6) | 1 (2.3) | 1.000 | 1 (4.2) | 0 | 1.000 | |
| Length of stay, median (IQR), d | 9 (7–12) | 9 (7–13) | 0.648 | 6 (5–8) | 6 (5–9) | 0.990 | |
PCA, intravenous patient-controlled analgesia; PD, pancreaticoduodenectomy; DP, distal pancreatectomy.
The rates at which EAs were taken out during the postoperative course are shown (Table 6); all were left in through POD4 for PD and POD2 for DP. Patients were routinely transitioned to oral narcotic-based pain medications with intravenous narcotics for breakthrough pain after epidural catheters were removed. Median pain scores were significantly lower on POD0 for patients receiving EA in the PD (1.39 vs 5.17, P = 0.008) and DP (1.68 vs 4.25, P = 0.031) groups compared to those receiving IV-PCA (Table 6). However, the difference in pain scores diminished after POD0.
TABLE 6.
Pain Score Comparisons Between EA and PCA in Patients Undergoing PD and DP From 2013–2016 UCLA Database
| POD0 | POD1 | POD2 | POD3 | POD4 | POD5 | POD6 | POD7 | |
|---|---|---|---|---|---|---|---|---|
| PD | ||||||||
| Remaining EA, % | 100 | 100 | 100 | 100 | 100 | 85 | 55 | 20 |
| Pain-EA (n = 20) | 1.39 | 2.28 | 1.45 | 0.88 | 1.00 | 1.67 | 0.60 | – |
| Median (IQR) | (0.67–3.43) | (1.04–3.17) | (0.14–2.60) | (0.00–2.25) | (0.10–2.37) | (0.00–2.41) | (0.00–3.00) | |
| Pain-PCA (n = 20) | 5.17 | 3.16 | 2.14 | 2.00 | 1.54 | 0.96 | 1.83 | – |
| Median (IQR) | (1.38–6.58) | (1.38–5.11) | (0.00–3.65) | (1.08–3.13) | (0.09–2.94) | (0.00–2.00) | (0.26–3.67) | |
| P | 0.008 | 0.223 | 0.521 | 0.066 | 0.576 | 0.975 | 0.419 | – |
| DP | ||||||||
| Remaining EA, % | 100 | 100 | 100 | 95.8 | 58.3 | 37.5 | 8.3 | 0 |
| Pain-EA (n = 24) | 1.68 | 2.13 | 1.00 | 0.80 | 0.60 | 2.00 | – | – |
| Median (IQR) | (0.00–3.56) | (0.79–3.67) | (0.29–3.13) | (0.17–2.00) | (0.00–3.97) | (0.29–2.85) | ||
| Pain-PCA (n = 18) | 4.25 | 3.04 | 2.17 | 1.10 | 1.27 | 2.67 | – | – |
| Median (IQR) | (1.34–6.88) | (1.14–4.94) | (0.63–4.71) | (0.09–3.70) | (0.31–3.83) | (1.50–3.75) | ||
| P | 0.031 | 0.309 | 0.252 | 0.335 | 0.527 | 0.232 | – | – |
Bold font indicates a statistically significant P-value of less than or equal to 0.05.
DISCUSSION
Epidural analgesia is increasingly utilized for patients undergoing pancreatic surgery and is a component of pancreatectomy-specific ERAS protocols.1,2,9 However, there is conflicting evidence in the literature on whether EA shortens or lengthens postoperative LOS.1,2,6,10,11 Therefore, we utilized the NSQIP clinical databases to determine the impact of EAs on LOS after PD and DP. Based on our clinical observations, we hypothesized that EA hinders timely discharge in complication-free patients who are candidates for early discharge. To assess the impact of EA on pain control, we also queried a prospectively maintained UCLA pancreatic surgery database to compare postoperative pain scores for patients with EA vs IV-PCA.
Utilization of EA for patients undergoing pancreatic surgery appears to be increasing in the United States. Two previous studies using the National Inpatient Sample (NIS), one from 2009 and another from 2008 to 2011, found an EA incidence of 11% for PDs2 and 9.1% for all pancreatectomy cases,1 respectively. As the NIS is an administrative database, utilization was determined from ICD-9 codes. Using the 2014–2015 NSQIP dataset, we observed that EA was used in 23.2% of PDs and 20.1% of all pancreatectomy cases. Although there are fundamental differences between NIS and NSQIP databases (administrative vs. clinical),1,2 the data collectively suggest that the use of EA in pancreatic surgery is increasing over time. This is further supported by the fact that EA is a key component of ERAS protocols that were initiated for pancreatic surgery in 2012.9 In light of these trends, a thorough evaluation of the impact of EA on LOS and expeditious discharge is necessary.
With a few exceptions,11,12,13 the majority of the literature suggests that EA reduces postoperative morbidity after major abdominal and pancreatic surgery.1,2,10 Most notably, patients with EA experience fewer respiratory, cardiovascular, and thromboembolic complications, and they are less likely to develop a paralytic ileus. Therefore, one would predict that EA should reduce postoperative LOS. Indeed, a study utilizing the 2009 NIS found that EA reduced LOS after PD from a mean of 15.7 to 13 days (P < 0.001).2 Similarly, a separate study using a propensity matching analysis from the 2008–2011 NIS determined that EA reduced LOS for patients undergoing pancreatectomy.1 However, there is also evidence to the contrary. A study also utilizing the NIS database from 2000 to 2012 found LOS was statistically longer for patients with EA undergoing hepatopancreatic surgeries but did not reach clinical significance after propensity score matching.10 Similarly, a single-institution analysis found that there were higher complication rates and a trend towards longer LOS in patients undergoing PDs who were administered EA.11 Our results appear to support the latter studies, as LOS for patients undergoing PD and DP were significantly longer in those with EA from a large NSQIP database on univariate analysis (Table 1). Moreover, there were significantly greater percentages of patients with EA in the long LOS groups at multiple LOS cutoffs for both PD and DP (Table 3).
We further hypothesized that the biggest impact of EAs on LOS would be at timepoints reflective of complication-free discharges, the goal of ERAS programs. Our hypothesis was rooted in the observations that EAs are often continued for five to seven days and there is more to do for patients with EA prior to discharge. For example, once the decision to remove the epidural catheter is made, prophylactic anticoagulation must be timed appropriately before anesthesia team can assess the patient and remove the catheter. Also, Foley catheters are used routinely with EA, as epidural catheters are placed in the thoracic region for abdominal and pancreatic surgeries, which can potentially lead to bladder paralysis, overdistension, and urinary retention. Many patients require longer time to spontaneously void after removal of epidural and Foley catheters and are kept in hospital longer until they are able to spontaneously void, and some patients require re-insertion of Foley catheter. Delays in any of the steps involved with EA removal can hinder expeditious discharge.
The results of this study confirmed our suspicions that EA can impede timely postoperative discharges. Despite controlling for pancreatectomy-relevant complications, we found that EA was associated with longer LOS (≥ 7 days for PD and ≥ 6 days for DP) on multivariable analysis (Table 4). These results are consistent with a previous study that showed EA duration of less than or equal to three days was independently associated with postoperative LOS less than or equal to five days in patients undergoing PD.14
It is generally believed that EA yields superior pain control in the early postoperative period than IV-PCA. Meta-analyses of patients undergoing surgery3 and open abdominal surgery8 have supported this point. However, the duration of the benefit for specific procedure types is less well-defined. A randomized controlled trial comparing EA vs IV-PCA showed better pain control with EA in the first 48 hours after hepatopancreatobiliary surgery, but this study did not assess further timepoints.6 On the contrary, an independent single-institution study of patients undergoing pancreatic and gastric surgery found no significant difference in pain control between EA and non-EA groups from POD0 to POD3.12 To assess the time-course of the benefit conferred by EA after open pancreatic surgery, we extracted all of the patient-reported pain scores from the medical records of a representative cohort in our institutional database. We found that the mean pain scores on POD0 were significantly lower, both clinically and statistically, in patients with EA for both PD and DP. However, this effect diminished after POD0.
There are limitations to our study. There are biases inherent to the retrospective nature of reviewing the NSQIP database. There is no detailed information in NSQIP about the composition, administration method (continuous vs. patient-controlled), or duration of EA. The NSQIP database does not include patient-reported pain scores; therefore, we had to review a smaller cohort of patients from our own institution to address the time-course of benefit of EA after surgery. There were no differences in LOS for PD or DP in the UCLA database, and it therefore could not be used to examine additional potential impacts on LOS not reported in NSQIP (e.g. postoperative day of Foley catheter removal, perioperative intravenous fluid administration, etc.). There are also other factors that can impact LOS after surgery, such as socioeconomic status, patient anxiety, and discharge disposition, that were not reported in the databases. A prospective study is necessary to further evaluate the findings of this study.
CONCLUSION
To our knowledge, this is the first time that the effect of EA on LOS and early discharge was examined for PD and DP using a national clinical database. Our results reveal that EA was associated with statistically increased LOS and lower rates of early discharge in patients undergoing open pancreatic surgeries. On reviewing our institutional database with patient-reported pain scores, EA only offered superior pain control on the day of surgery and did not provide additional pain relief over IV-PCA after POD0. Therefore, we suggest an earlier transition from EA to opioids, provided pain is well-controlled and the patient appears to be on course for an uncomplicated recovery.
Grant support:
NIH T32 Training grant T32DK07180 (SS Kim)
Footnotes
Disclosure: The authors declare no financial conflicts of interest to disclose.
REFERENCES
- 1.Sanford DE, Hawkins WG, Fields RC. Improved peri-operative outcomes with epidural analgesia in patients undergoing a pancreatectomy: a nationwide analysis. HPB (Oxford). 2015;17:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amini A, Patanwala AE, Maegawa FB, et al. Effect of epidural analgesia on postoperative complications following pancreaticoduodenectomy. Am J Surg. 2012;204:1000–1004. [DOI] [PubMed] [Google Scholar]
- 3.Wu CL, Cohen SR, Richman JM, et al. Efficacy of postoperative patient-controlled and continuous infusion epidural analgesia versus intravenous patient-controlled analgesia with opioids: a meta-analysis. Anesthesiology. 2005;103:1079–1088. [DOI] [PubMed] [Google Scholar]
- 4.Cho JS, Kim HI, Lee KY, et al. Comparison of the effects of patient-controlled epidural and intravenous analgesia on postoperative bowel function after laparoscopic gastrectomy: a prospective randomized study. Surg Endosc. 2017;31:4688–4696. [DOI] [PubMed] [Google Scholar]
- 5.Jayr C, Beaussier M, Gustafsson U, et al. Continuous epidural infusion of ropivacaine for postoperative analgesia after major abdominal surgery: comparative study with i.v. PCA morphine. Br J Anaesth. 1998;81:887–892. [DOI] [PubMed] [Google Scholar]
- 6.Aloia TA, Kim BJ, Segraves-Chun YS, et al. A randomized controlled trial of postoperative thoracic epidural analgesia versus intravenous patient-controlled analgesia after major hepatopancreatobiliary surgery. Ann Surg. 2017;266:545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105:106–115. [DOI] [PubMed] [Google Scholar]
- 8.Hughes MJ, Ventham NT, McNally S, et al. Analgesia after open abdominal surgery in the setting of enhanced recovery surgery: a systematic review and meta-analysis. JAMA Surg. 2014;149:1224–1230. [DOI] [PubMed] [Google Scholar]
- 9.Pecorelli N, Nobile S, Partelli S, et al. Enhanced recovery pathways in pancreatic surgery: State of the art. World J Gastroenterol. 2016;22:6456–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amini N, Kim Y, Hyder O, et al. A nationwide analysis of the use and outcomes of perioperative epidural analgesia in patients undergoing hepatic and pancreatic surgery. Am J Surg. 2015;210:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pratt WB, Steinbrook RA, Maithel SK, et al. Epidural analgesia for pancreatoduodenectomy: a critical appraisal. J Gastrointest Surg. 2008;12:1207–1220. [DOI] [PubMed] [Google Scholar]
- 12.Shah DR, Brown E, Russo JE, et al. Negligible effect of perioperative epidural analgesia among patients undergoing elective gastric and pancreatic resections. J Gastrointest Surg. 2013;17:660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axelrod TM, Mendez BM, Abood GJ, et al. Peri-operative epidural may not be the preferred form of analgesia in select patients undergoing pancreaticoduodenectomy. J Surg Oncol. 2015;111:306–310. [DOI] [PubMed] [Google Scholar]
- 14.Lee GC, Fong ZV, Ferrone CR, et al. High performing whipple patients: factors associated with short length of stay after open pancreaticoduodenectomy. J Gastrointest Surg. 2014;18:1760–1769. [DOI] [PubMed] [Google Scholar]