Abstract
Objective:
Endoplasmic reticulum (ER) stress and subsequent phosphorylation of eukaryotic initiation factor 2 alpha (eIF2α) by protein kinase R-like ER kinase (PERK) plays an important role in the development and chemoresistance of pancreatic ductal adenocarcinoma (PDAC). However the expression and significance of p-eIF2α and PERK in PDAC have not been examined.
Methods:
We examined p-eIF2α and PERK expression in 84 PDAC and paired normal pancreas samples by immunohistochemistry and Western blotting and correlated the results with clinicopathologic parameters and survival.
Results:
Mean PERK H score was 140.8 in PDAC compared to 82.1 in normal pancreas (P < 0.001). High p-eIF2α expression was present in 56% PDACs verse 7.6% normal pancreas (P < 0.001). High PERK and p-eIF2α expression correlated with shorter overall survival (P = 0.048 and P = 0.03 respectively). By multivariate analysis, high p-eIF2α (P = 0.01), positive margin (P = 0.002) and lymph node metastasis (P = 0.01) were independent prognosticators for survival.
Conclusions:
The expression levels of PERK and p-eIF2α are higher in PDAC than those in normal pancreas. High levels of PERK and p-eIF2α are predictors of shorter survival in PDAC patients, suggesting that PERK and eIF2α could be promising targets in PDAC.
Keywords: Pancreatic Cancer; PERK; p-eIF2α, Survival; Prognosis
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of cancer death in the United States with a 5-year survival rate of approximately 6%. The incidence of pancreatic cancer is estimated to rise over the next two decades, ranking third in the leading causes of cancer death in the US by 2030.1 In spite of the recent advances in surgical and medical oncology during the past 30 years, the clinical outcome and survival for patients with PDAC remains largely unchanged. Surgical resection remains the only hope for curative treatment for this highly aggressive malignancy.2 However, patients with PDAC are often asymptomatic in early stages of the disease and in part for this reason the majority of PDAC patients present with locally advanced/unresectable disease or metastasis. Therefore, molecular markers for early diagnosis and prognosis are urgently needed for patients with PDAC.
A range of stressful conditions may trigger disruptions of endoplasmic reticulum (ER) homeostasis, which leads to ER stress and activation of an unfolded protein response (UPR).3–5 Previous studies have shown that ER stress and UPR result in the phosphorylation of α subunit of the eukaryotic initiation factor 2α (eIF2α) at serine 51 by activated protein kinase RNA-like endoplasmic reticulum kinase (PERK). Phosphorylation of eIF2α blocks GDP to GTP recycling on eIF2B, markedly attenuates translation initiation and inhibits overall protein synthesis, which allows the tumor cells to adapt to stressful conditions by reducing energy expenditure for protein synthesis.3,6,7 On the other hand, phosphorylation of eIF2α facilitates preferential translation of specific mRNAs, including activating transcription factor 4 (ATF4) and ATF5, which upregulate the expression of genes involved in oxidative stress, metabolism, and nutrient uptake.3,6,7 Thus, phosphorylation of eIF2α plays a pivotal role in the recovery and survival of tumor cells from stress-induced damage and may contribute to the adaptations of tumor cells to hypoxic conditions and to chemoresistance. However, long-term phosphorylation of eIF2α due to chronic ER stress may also evoke a paradoxical response via the initiation of apoptotic cell death.3,6,7
The PERK-eIF2a pathway has been shown to play a role in pancreatic cancer and other types of human malignancies.8–11 Inhibition of PERK by GSK2656157, a small ATP-competitive inhibitor of PERK activity, results in inhibition of eIF2α phosphorylation and a dose-dependent inhibition of PDAC growth in a xenograft mouse model.8 Using a fusion protein, Fv2E-PERK, which is generated by fusing the PERK kinase domain to a protein module that binds a small dimerizer molecule, Lu et al showed that Fv2E-PERK activation led to up-regulation of numerous stress-induced genes and protected cells from the lethal effects of oxidants and ER stress.8–11 These results suggest that eIF2α phosphorylation can initiate cytoprotective effects independent of upstream stress-induced signals.10 Similar to these results, Ranganathan et al showed that inducible expression of Fv2E-PERK fusion protein increased the expression of p-eIF2α, and promoted G0-G1 arrest and survival in vitro.11 On the other hand, their study also found that activation of the PERK-eIF2a pathway led to inhibition of proliferation as measured by decreased expression of Ki67, phospho-histone H3, and cyclin D1/D3, and in vivo tumor growth of T-HEp3 squamous cell carcinoma cells and SW620 colon cancer cells through induction of quiescence.11 In contrast, high levels of phospho-PKR or p-eIF2α expression correlate significantly with longer survival compared to those with little or no p-PKR or p-eIF2alpha expression in patients with non-small cell carcinoma.9 Therefore the functions of PERK-eIF2a pathway in cancer remain unclear. Importantly, the expression and clinical significance of PERK and p-eIF2α in PDAC have not been examined. In this study, therefore we examined the expression of PERK and p-eIF2α protein in 84 PDAC samples and their match benign pancreas tissue using PDAC tissue microarrays and immunohistochemical staining. We also examined the expression of PERK and p-eIF2α in fresh-frozen tissue samples of PDAC and their matched normal pancreas by western blotting. Our results demonstrate that PERK and p-eIF2α could be valuable prognostic markers for patients with PDAC, and furthermore suggest that the PERK-eIF2α pathway may serve as a promising target for therapy in PDAC.
MATERIALS AND METHODS
Patient Population and Follow up
Eighty-four patients with PDAC, who had undergone upfront pancreaticoduodenectomy at the University of Texas M. D. Anderson Cancer Center between 1990 and 2012 were included in this study. There were 34 females and 50 males with an ages range from 42.2 to 84.8 years (median: 64.5 years). None of the patients received neoadjuvant chemotherapy or chemoradiation therapy. The pathologic stages of the patients were grouped according to the American Joint committee on Cancer (AJCC) Cancer Staging Manual, 8th edition12. There were 12, 61 and 11 patients with pT1, pT2, and pT3, respectively and 16, 32, and 36 patients with pN0, pN1 and pN2, respectively. Patient follow-up information was extracted from the prospectively maintained institutional pancreatic cancer database, verified by reviewing the patient medical record and, if necessary, updated by review of the U.S. Social Security Index. After surgery, disease recurrence or metastasis was based on the radiographic and clinical suspicion during the follow-up visits. This study was approved by the Institutional Review Board of our institution.
Tissue Microarrays and Immunohistochemical Analysis for p-eIF2α and PERK
The PDAC tissue microarrays (TMA) were constructed as described previously.13 Matched hematoxylin and eosin-stained (H & E) slides from each case were reviewed and screened for representative tumor regions and normal pancreas. For each patient, two 1.0 mm-cores of tumor tissue and one 1.0 mm-core of normal pancreas were represented in the PDAC TMAs.
Immunohistochemical staining for p-eIF2α and PERK was performed on 5-µm unstained sections from the tissue microarray blocks using a rabbit polyclonal antibody against PERK (1:2000) and p-eIF2α (1:50, Cell Signaling Technology, Danvers, Mass). Standard avidin-biotin immunohistochemical analysis of the sections was done according to the manufacturer’s recommendations (Vector Laboratories, Burlingame, Calif). The staining results for PERK and p-eIF2α were evaluated by two pathologists (J.Z. and H.W.). The H-score for PERK expression was calculated by multiplying the intensity score (score: 0-negative, 1-weak, 2-moderate, and 3-strong) and the percentage of tumor cells that were stained positively for PERK. For statistical analysis, the expression of PERK was categorized as low or high according to the median H score. Since all PDAC samples showed either negative or diffuse staining for p-eIF2α, the expression of p-eIF2α were graded based on the staining intensity alone as negative (0), weak (1), moderate (2) and strong (3) and was categorized as low (scores of 0 or 1) or high (score of 2 or 3).
Western Blot Analysis of p-eIF2α and PERK Expression in Paired Samples of PDAC and Normal Pancreas
PERK and p-eIF2α protein expression was analyzed by 10% SDS-PAGE, which was electroblotted onto PVDF membranes (Novex, San Diego, Calif), blocked in 5% skim milk in 1x TBS, and probed with PERK and p-eIF2α antibodies. Proteins were detected using an enhanced chemiluminescence kit (Amersham-Pharmacia Biotech, Piscataway, NJ).
Statistical Analysis
Chi-square analyses or Fisher exact tests were used to compare categorical data and One-sample t tests were used to compare continuous variables. The overall survival was calculated as the time from the date of diagnosis to the date of death or censored at the date of last follow-up if death did not occur. Survival curves were constructed using the Kaplan-Meier method, and the log-rank test was used to evaluate the statistical significance of differences. The prognostic significances of clinicopathologic parameters and the expression of PERK and p-eIF2α were determined using a univariate Cox regression analysis. Cox proportional hazards models were fitted for multivariate analysis using a backward stepwise procedure. Statistical analyses were performed using Statistical Package for Social Sciences software for Windows (SPSS version 22, IBM, Armonk N.Y). A 2-sided significance level of 0.05 was used for all statistical analyses.
RESULTS
PERK and p-eIF2α are Overexpressed in PDAC
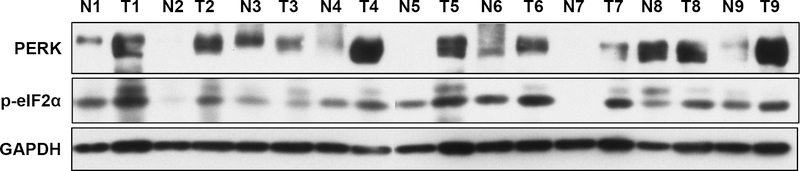
Representative micrographs illustrating expression of PERK and p-eIF2α are shown in Figures 1 and 2. The mean H score for PERK expression was 140.8 (standard deviation [SD], 75.4) in 84 PDAC samples compared to 82.1 (SD, 57.1) in 63 matched normal pancreas that were available for analysis (P < 0.001). High level of p-eIF2α expression was present in 47 of 84 (56%) PDACs, but only in 5 of 66 (7.6%) matched normal pancreas that were available for analysis (P < 0.001). The expression levels of PERK and p-eIF2α was significantly higher in PDAC samples than those in benign pancreas. Consistent with our immunohistochemical staining results, 8 of 9 human PDAC samples had higher expression levels of PERK and p-eIF2α than their matched normal pancreatic tissue by Western blots (Fig. 3).
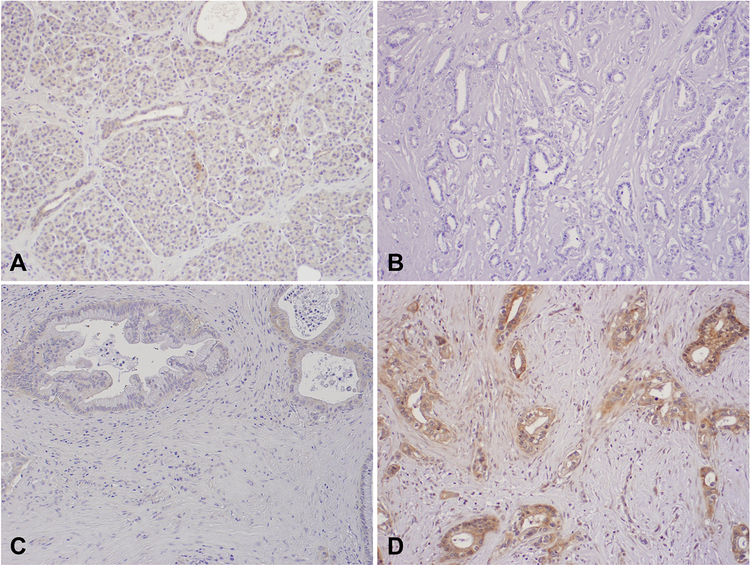
FIGURE 1.
Representative micrographs showing the expression of PERK in normal pancreas tissue (A) and different expression levels of PERK in PDAC samples: PERK-negative (B), PERK-Low (C) and PERK-High (D). Original magnification, 100×.
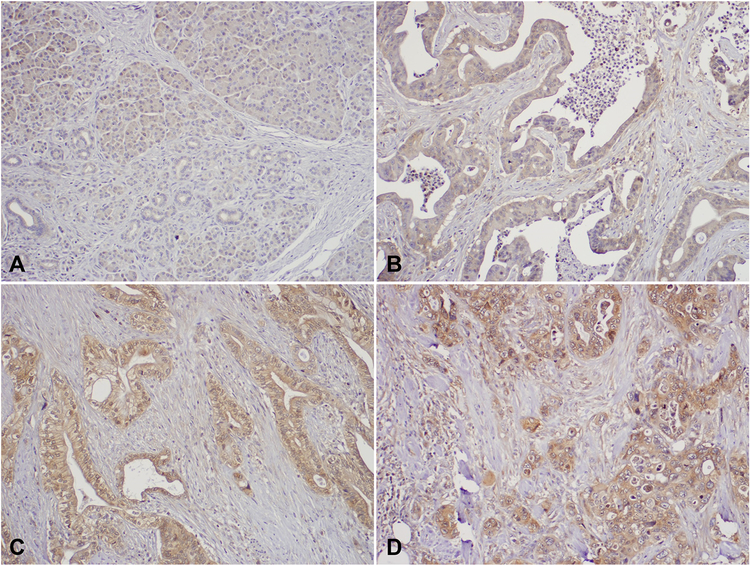
FIGURE 2.
Representative micrographs showing the expression of p-eIF2α in normal pancreas tissue (A) and different expression levels of p-eIF2α in PDAC samples: Intensity Score 1 (B), Intensity Score 2 (C), and Intensity Score 1 (D). Original magnification, 100×.
FIGURE 3.
Western blots showing the expression of PERK and p-eIF2α in nine matched pairs of benign pancreas tissue (N) and PDAC samples (T).
The Expression of PERK Correlates With p-eIF2α expression in PDAC
Among the 42 PDAC samples that had high level of PERK expression, 29 (69.0%) had high level of p-eIF2α expression. In comparison, only 18 (42.9%) of 42 PDAC samples that were PERK-low were p-eIF2α-high (P = 0.02, Table 1). This finding supports the function of PERK in the phosphorylation of p-eIF2α in pancreatic cancer.
TABLE 1.
Correlation Between the Expression of PERK and P-eIF2α in PDAC
| p-eIF2α Expression |
Total |
||
|---|---|---|---|
| Low | High | ||
| PERK expression | |||
| Low | 24 | 18 | 42 |
| High | 13 | 29 | 42 |
| Total | 37 | 47 | 84 |
χ2 = 5.85, P = 0.02
Correlations of PERK and p-eIF2α Expression With Clinicopathological Parameters and Survival
High level of p-eIF2α expression correlated with significantly higher risk of recurrence/metastasis after surgery. Among the 47 patients whose tumors were p-eIF2α-high, 38 (80.9%) had recurrence/metastasis compared to 54.1% (20/37) in those whose tumors were p-eIF2α-low (P = 0.02). There was no correlation between p-eIF2α expression and other clinicopathological parameters (P > 0.05, Table 2). We did not find correlation between PERK expression and clinical pathologic parameters (P > 0.05, Table 2).
TABLE 2.
Clinicopathological Correlation of PERK and P-eIF2α Expression in Patients With PDAC
| Characteristics | PERK-low (%) (n = 42) |
PERK-high (%) (n = 42) |
P | p-eIF2α-low (%) (n = 37) |
p-eIF2α-high (%) (n = 47) |
P |
|---|---|---|---|---|---|---|
| Sex | 0.66 | 0.18 | ||||
| Female | 16 (38.1) | 18 (42.9) | 12 (32.4) | 22 (46.8) | ||
| Male | 26 (61.9) | 24 (57.1) | 25 (67.6) | 25 (53.2) | ||
| Tumor differentiation | 0.35 | 0.46 | ||||
| Well-Moderate | 31 (73.8) | 27 (64.3) | 24 (64.9) | 34 (72.3) | ||
| Poor | 11 (26.2) | 15 (35.7) | 13 (35.1) | 13 (27.7) | ||
| pT (AJCC 8th edition) | 0.46 | 0.75 | ||||
| pT1 | 8 (19.1) | 4 (9.5) | 5 (13.5) | 7 (14.9) | ||
| pT2 | 29 (69.0) | 32 (76.2) | 26 (70.3) | 35 (74.5) | ||
| pT3 | 5 (11.9) | 6 (14.3) | 6 (16.2) | 5 (10.6) | ||
| pN (AJCC 8th edition) | 0.20 | 0.81 | ||||
| pN0 | 11 (26.2) | 5 (11.9) | 6 (16.2) | 10 (21.3) | ||
| pN1 | 16 (38.1) | 16 (38.1) | 14 (37.8) | 18 (38.3) | ||
| pN2 | 15 (35.7) | 21 (50.0) | 17 (46.0) | 19 (40.4) | ||
| Margin | 0.29 | 0.49 | ||||
| Negative | 39 (92.9) | 36 (85.7) | 34 (91.9) | 41 (87.2) | ||
| Positive | 3 (7.1) | 6 (14.3) | 3 (8.1) | 6 (12.8) | ||
| Recurrence/metastasis | 0.10 | 0.01 | ||||
| No | 17 (40.5) | 9 (21.4) | 17 (45.9) | 9 (19.1) | ||
| Yes | 25 (59.5) | 33 (78.6) | 20 (54.1) | 38 (80.9) |
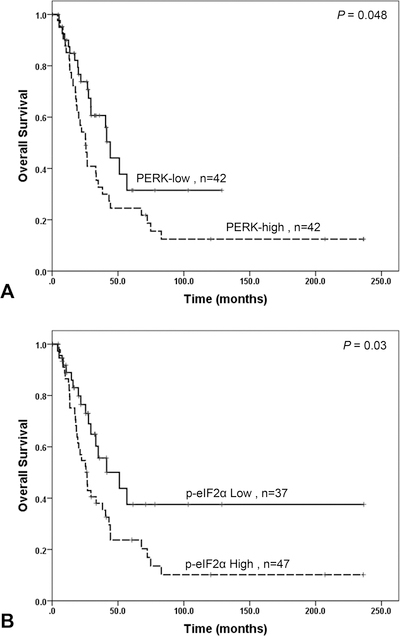
Expression of PERK showed significant correlation with overall survival. The median overall survival in patients whose tumors were PERK-high was 25.4 months (95% confidence interval [CI], 19.1–31.8 months) compared to 44.1 months (95% CI, 38.2–50.1 months) in those whose tumors were PERK-low (P = 0.048, Fig. 4A). High level of p-eIF2α expression was also associated with shorter overall survival in patients with PDAC. The median overall survival in patients whose tumor expressed high level of p-eIF2α was 26.5 months [95% CI, 20.5–32.5 months] compared to 51.0 months (95% CI, 22.8–79.3 months) for those whose tumor was p-eIF2α-low (P = 0.03, Fig. 4B and Table 3). By multivariate analysis, high level of p-eIF2α (P = 0.01), positive margin (P = 0.002) and lymph node metastasis (P = 0.01) were significant independent prognosticators for shorter overall survival. PERK expression was not a significant predictor of overall survival by multivariate analysis (P = 0.99, Table 4).
FIGURE 4.
Kaplan-Meier curves for overall survival stratified by the expression level of PERK (A) and the expression level of p-eIF2α (B) in 84 PDAC patients.
TABLE 3.
Univariate Cox Regression Analysis of Overall Survival
| Characteristics | No. Patients | Overall Survival | |
|---|---|---|---|
| HR (95% CI) | P | ||
| Sex | |||
| Female (reference) | 34 | 1.00 | |
| Male | 50 | 1.35 (0.78–2.34) | 0.28 |
| Age | 84 | 1.02 (0.99–1.06) | 0.15 |
| Tumor differentiation | |||
| Well-moderate (reference) | 58 | 1.00 | |
| Poor | 26 | 1.16 (0.64–2.12) | 0.63 |
| pT stage (AJCC 8th edition) | 0.09 | ||
| pT1 (reference) | 12 | 1.00 | |
| pT2 | 61 | 2.89 (1.13–7.42) | 0.03 |
| pT3 | 11 | 2.46 (0.77–7.86) | 0.13 |
| pN stage (AJCC 8th edition) | 0.02 | ||
| pN0 (reference) | 16 | 1.00 | |
| pN1 | 32 | 2.47 (0.93–6.55) | 0.07 |
| pN2 | 36 | 3.85 (1.45–10.19) | 0.007 |
| Margin | |||
| Negative (reference) | 75 | 1.00 | |
| Positive | 9 | 5.29 (2.26–12.4) | <0.001 |
| PERK expression | |||
| Low (reference) | 42 | 1.00 | |
| High | 42 | 1.77 (1.00–3.12) | 0.05 |
| p-eIF2α Expression | |||
| Low (reference) | 37 | 1.00 | |
| High | 47 | 1.94 (1.08–3.50) | 0.03 |
TABLE 4.
Multivariate Cox Regression Analysis of Overall Survival
| Characteristics | No. Patients | Overall Survival | |
|---|---|---|---|
| HR (95% CI) | P | ||
| pT stage (AJCC 8th edition) | 0.47 | ||
| pT1 (reference) | 12 | 1.00 | |
| pT2 | 61 | 1.75 (0.64–4.82) | 0.28 |
| pT3 | 11 | 1.32 (0.38–4.56) | 0.66 |
| pN stage (AJCC 8th edition) | 0.01 | ||
| pN0 (reference) | 16 | 1.00 | |
| pN1 | 32 | 2.46 (0.92–6.62) | 0.07 |
| pN2 | 36 | 4.25 (1.56–11.59) | 0.005 |
| Margin | |||
| Negative (reference) | 75 | 1.00 | |
| Positive | 9 | 3.96 (1.66–9.47) | 0.002 |
| PERK expression | |||
| Low (reference) | 42 | 1.00 | |
| High | 42 | 1.00 (0.51–1.94) | 0.99 |
| p-eIF2α Expression | |||
| Low (reference) | 37 | 1.00 | |
| High | 47 | 2.13 (1.17–3.89) | 0.01 |
DISCUSSION
Pancreatic ductal adenocarcinoma is characterized by extensive desmoplastic stroma and hypoxic tumor microenvironment.14–16 Hypoxia within the tumor microenvironment of PDAC may result in the disruption of ER homeostasis and the accumulation of unfolded and misfolded proteins, which activate PERK-eIF2α pathway and subsequently lead to the global inhibition of protein translation, metabolic reprogramming, cell adaptation to stress conditions and promote survival.3 The PERK-eIF2α signaling pathway has been shown to contribute to cancer formation, tumor growth, angiogenesis and tumor resistance to chemotherapy, in part through its adaptive and pro-survival effects via the p-eIF2α (S51)-dependent activation of NFκB, down regulation of cyclin D1 and activation of PI3 kinase-Akt pathway.7 A recent study by Atkins et al showed that pharmacological inhibition of PERK by a small molecular inhibitor, GSK2656157, inhibited in vivo growth of BXPC-3, HPAC and CAPAN2 pancreatic cancer cells in a dose-dependent manner and decreased tumor angiogenesis using xenograft tumor models.8 On the other hand, their study also showed toxicity of GSK2656157 on both the exocrine and endocrine pancreas8. Knockout of eIF2 kinase general control nonderepressible 2 (GCN2) predisposes mice to abnormal ER stress response, autophagy, and asparaginase-induced pancreatitis.17 This prompted us to examine the expression of PERK and p-eIF2α in human PDAC and matched normal pancreatic samples. Our study demonstrated that both PERK and p-eIF2α were expressed in normal exocrine and endocrine pancreas, but at significantly lower levels compared to matched PDAC samples. Our findings could explain, in part, the toxic effect of GSK2656157 on normal exocrine and endocrine pancreas, especially when a high dose of GSK2656157 was used to treat mice8. We observed a strong correlation between the expression of PERK and p-eIF2α in human PDAC samples by both immunohistochemistry and Western blot analysis. Our findings are consistent with previous studies in which expression of constitutively-active PERK leads the up-regulation of p-eIF2α and numerous downstream stress-induced genes in PDAC cells, and targeted inhibition of PERK by small molecular inhibitor results in a decrease in phosphorylation of eIF2α in PDAC cells.8,10 Expression of p-eIF2α has also been shown to be a key regulator controlling cell survival or death in response to oxidative stress through Akt activation in immortalized mouse embryonic fibroblasts (MEFs).18 The strong correlation between PERK expression and the expression of p-eIF2α observed in our study provided additional support for the critical functions of PERK-eIF2α signaling pathway in PDAC.
In this investigation, we found that high level of PERK and p-eIF2α expression in patients with PDAC correlated with poor survival, and that the expression of p-eIF2α was an independent prognostic factor for shorter survival in patients with PDAC. Consistent with our findings were several previous studies, which show that targeting p-eIF2α in cancer increases the efficacy of chemo- and radiation therapies.3,7,8,18–21 It was interesting that patients with NSCLC whose tumor had high level of p-PKR or p-eIF2α expression had a longer survival than those whose tumor had little or no p-PKR or p-eIF2α.9 It is possible that the differences in tumor microenvironment, cell stress signaling and biology between NSCLC and PDAC may affect the functions of phosphorylated eIF2α in these two tumors. Future studies are needed to further validate the functions and prognostic value of p-eIF2α in cancer.
In summary, our study showed that both PERK and p-eIF2α are overexpressed in PDAC. We demonstrated a significant correlation between PERK expression and the expression of p-eIF2α in PDAC. More importantly, high level of PERK expression and p-eIF2α expression in PDAC correlates with short patient survival. Therefore pharmacological inhibition of PERK-eIF2α signaling pathway may be a suitable approach to treat this lethal disease and/or to improve the treatment efficacy when used in combination with other chemotherapy drugs.
Footnotes
The authors declare no conflict of interest or funding.
REFERENCES
- 1.Are C, Chowdhury S, Ahmad H, et al. Predictive global trends in the incidence and mortality of pancreatic cancer based on geographic location, socio-economic status, and demographic shift. J Surg Oncol 2016;114:736–742. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73–85. [DOI] [PubMed] [Google Scholar]
- 3.Rozpedek W, Pytel D, Mucha B, et al. The Role of the PERK/eIF2alpha/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr Mol Med 2016;16:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 2005;74:739–789. [DOI] [PubMed] [Google Scholar]
- 5.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res 2005;569:29–63. [DOI] [PubMed] [Google Scholar]
- 6.Ron D Translational control in the endoplasmic reticulum stress response. J Clin Invest 2002;110:1383–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koromilas AE. Roles of the translation initiation factor eIF2alpha serine 51 phosphorylation in cancer formation and treatment. Biochim Biophys Acta 2015;1849:871–880. [DOI] [PubMed] [Google Scholar]
- 8.Atkins C, Liu Q, Minthorn E, et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res 2013;73:1993–2002. [DOI] [PubMed] [Google Scholar]
- 9.He Y, Correa AM, Raso MG, et al. The role of PKR/eIF2alpha signaling pathway in prognosis of non-small cell lung cancer. PLoS One 2011;6:e24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu PD, Jousse C, Marciniak SJ, et al. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J 2004;23:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranganathan AC, Ojha S, Kourtidis A, et al. Dual function of pancreatic endoplasmic reticulum kinase in tumor cell growth arrest and survival. Cancer Res 2008;68:3260–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amin MB, Edge S, Greene F, et al. , eds. AJCC Cancer Staging Manual 8th ed. New York, NY: Springer International Publishing; 2017. [Google Scholar]
- 13.Wang H, Zhang W, Fuller GN. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol 2002;12:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feig C, Gopinathan A, Neesse A, et al. The pancreas cancer microenvironment. Clin Cancer Res 2012;18:4266–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25:719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014;25:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phillipson-Weiner L, Mirek ET, Wang Y, et al. General control nonderepressible 2 deletion predisposes to asparaginase-associated pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol 2016;310:G1061–G1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajesh K, Krishnamoorthy J, Kazimierczak U, et al. Phosphorylation of the translation initiation factor eIF2alpha at serine 51 determines the cell fate decisions of Akt in response to oxidative stress. Cell Death Dis 2015;6:e1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozpedek W, Nowak A, Pytel D, et al. Molecular basis of human diseases and targeted therapy based on small-molecule inhibitors of ER stress-induced signaling pathways. Curr Mol Med 2017. [DOI] [PubMed] [Google Scholar]
- 20.Rajesh K, Papadakis AI, Kazimierczak U, et al. eIF2alpha phosphorylation bypasses premature senescence caused by oxidative stress and pro-oxidant antitumor therapies. Aging (Albany NY) 2013;5:884–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rouschop KM, Dubois LJ, Keulers TG, et al. PERK/eIF2alpha signaling protects therapy resistant hypoxic cells through induction of glutathione synthesis and protection against ROS. Proc Natl Acad Sci U S A 2013;110:4622–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]