Abstract
Objective:
Chronic pancreatitis is the consequence of multiple episodes of recurrent acute pancreatitis (RAP). We hypothesized that apigenin can minimize the sequelae of RAP by limiting acinar cells’ pro-inflammatory signaling pathways.
Methods:
AR42J acinar cells were treated in vitro with transforming growth factor beta (TGF-β), apigenin, and other inhibitors. Dual luciferase reporter assay measured parathyroid hormone related protein (PTHrP) promoter activity. MAPK/ERK pathway activity was assessed by immunoblotting and in vivo by immunohistochemistry with a cerulein-induced RAP mouse model. Nuclear factor kappa B (NF-κB) nuclear localization was analyzed in vitro in cells stimulated with tumor necrosis factor alpha (TNF-α). Primary acini were isolated and treated with cerulein; interleukin 6 (IL-6) mRNA were measured comparing PTHrP wild-type and knockout mice.
Results:
Apigenin and PD98059 each downregulated TGF-β stimulation of PTHrP P3 promoter activity. In a RAP mouse model, apigenin reduced pERK nuclear localization in acinar cells and preserved acinar cell architecture. Apigenin suppressed TNF-α mediated signaling by decreasing NF-κB nuclear localization and decreased IL-6 mRNA levels via a PTHrP-dependent mechanism.
Conclusions:
Apigenin reduced inflammatory responses in experimental models of RAP. The mechanisms mediating the actions of apigenin, in part, are due to attenuation of PTHrP and TGF-β pro-inflammatory signaling.
Keywords: recurrent acute pancreatitis, acinar cells, apigenin, parathyroid hormone related protein (PTHrP)
INTRODUCTION
Current clinical, experimental and genetic evidence support the hypothesis that chronic pancreatitis is result of recurrent acute pancreatitis (RAP).1–3 Common etiologies of pancreatitis include alcohol and gallstones.4 Irrespective of the cause, it is known that acinar cell injury initiates a cascade of pancreatic autodigestion and inflammation which involves acinar cell release of inflammatory mediators, activation of pancreatic stellate cells (PSCs) and recruitment of various immune cells.5,6 Most patients can fully recover from a single bout of acute pancreatitis.5 However, for a subset of high-risk patients, recurrent tissue injury can result in the persistent pro-inflammatory state and aberrant tissue repair (i.e., acinar cell depletion and fibrosis) that characterizes a clinical diagnosis of chronic pancreatitis.6
Previously, work by Bhatia et al7,8 identified parathyroid hormone related protein (PTHrP) as an important pro-inflammatory and pro-fibrotic mediator of pancreatitis. Immunohistochemical staining of pancreatic tissue from animal models of both acute and chronic pancreatitis, as well as patients with chronic pancreatitis, have demonstrated upregulation of PTHrP expression within acinar cells and PSCs.7,8 Human pancreatic acini, islets of Langerhans, and PSCs, also express the parathyroid hormone 1 receptor (PTH1R), which bind PTHrP with high affinity.7,9 In mice, acinar cell-specific disruption of the PTHrP gene (Pthlh) significantly reduced the pancreatic injury induced by either bile duct ligation or treatment with the cholecystokinin receptor agonist, cerulein.8
Recent clinical efforts towards a mechanistic understanding of chronic pancreatitis10 suggest that pharmacologic disruption of RAP could be an effective therapeutic strategy to slow or stop disease progression. To begin testing this concept, we have identified the naturally-occurring polyphenol apigenin (4’,5,7-trihydroxyflavone) as a potential lead compound. In oncological research, apigenin has been shown to exert anti-inflammatory, anti-oxidant, anti-proliferative, and pro-apoptotic properties.11 Our laboratory has reported that apigenin inhibited pancreatic stellate cell (PSC) viability, stimulated PSC apoptosis, reduced pancreatic fibrosis in response to repeated bouts of cerulein-induced pancreatitis.12 At the molecular level, we showed that apigenin inhibited PTHrP-induced production of inflammatory cytokines and extracellular matrix proteins by PSCs.12
The aim of this study was to determine whether apigenin exerts direct effects on acinar cells to limit the sequelae of RAP. We report that apigenin inhibited the PTHrP promoter in acinar cells; the effects were mimicked by treatment with the ERK1/2 inhibitor, PD98059. Also, apigenin inhibited cerulein-induced IL-6 expression in acinar cells isolated from wild-type mice, but not by acinar cells from PTHrP knockout mice. Our data reveal that apigenin inhibits acinar cell damage in a PTHrP-dependent manner.
MATERIALS AND METHODS
Cell Line
The AR42J acinar cell line was purchased from the American Type Culture Collection (ATCC CRL-1492, Manassas, Va), grown in RPMI with 10% FBS, and maintained at 37°C in a humidified 95% O2/5% CO2 atmosphere.
Reagents
Apigenin (95% purity) and methylcellulose (MC) were obtained from Sigma-Aldrich (St. Louis, Mo), Cerulein (CR) peptide was purchased from Bachem (Torrance, Calif), and PTHrP (1–36) from PolyPeptide Laboratories (San Diego, Calif). Transforming growth factor beta (TGF-β) and tumor necrosis factor alpha (TNF-α) were obtained from R&D Systems (Minneapolis, Minn). PD98059 and LY294002 were purchased from Life Technologies (Carlsbad, Calif) and Selleck Chemicals (Houston, Texas), respectively.
Primary Acinar Cell Isolation
Animal protocols were approved by the University of Texas Medical Branch Institutional Animal Care and Use Committee. PTHrP gene disruption was induced in 6–8-week-old male and female PTHrPflox/flox; Cre+ mice by intraperitoneal injection of 100 μl/mouse of tamoxifen (20 mg/ml; Cayman Chemical, Ann Arbor, Mich) once daily for 5 days, as previously described.8 Control mice were of the same genetic background were injected with the vehicle, corn oil, following an identical schedule. One week after completion of the tamoxifen or control, the mice were anesthetized and sacrificed per protocol.
The protocol for primary acinar cell isolation was published previously.7,8 Briefly, the pancreata from 4–5 mice were harvested and placed in an isolation buffer [PBS with Ca2+ and Mg2+, 0.1% BSA, and 10 μg/ml STI], finely minced, and digested with collagenase type IV, 1 mg/ml, using continuous brisk trituration for 15 minutes at 37°C. Enzymatic inactivation was achieved by a 1:2 dilution with cold isolation buffer. The cells were washed three times with cold isolation buffer and filtered through a 100 μm mesh followed by re-suspension in 10 mL of DMEM with 10% FBS and 0.025% soybean trypsin inhibitor. The cells were seeded into a laminin-coated six-well plate and allowed to attach for 24 hours before initiating treatment.
Quantitative Polymerase Chain Reaction (qPCR)
Total RNA was isolated using the RNAqueous (Ambion; Austin, Texas) and reverse transcribed to cDNA using the Applied Biosystems cDNA synthesis kit (Foster City, Calif) as previously described.7,8 The primers used were for mouse IL-6 (forward TGGAGTCACAGAAGGAGTGGCTAAG and reverse TCTGACCACAGTGAGGAATGTCCAC) and actin (forward TCACCCACACTGTGCCCATCTACGA and reverse GGATGCCACAGGATTCCATACCCA). The threshold cycle (CT) value for each gene was normalized to that of β-actin; relative expression levels were calculated using n-fold change = 2^ (-ΔΔCT), where ΔΔCT = ΔCT (target sample) ΔCT — (control).
Luciferase Reporter Assay
The PTHrP-P3 plasmid, containing the 140 bp upstream of the P3 TATA box, was cloned into the pGL-2 vector and obtained from Cataisson et al13 The AR42J cells were transfected with the PTHrP plasmid or empty vector (control), and co-transfected with a Renilla luciferase construct via electroporation.8 After experimental treatments, cell lysates were prepared following the Dual-Luciferase Reporter (Promega; Madison, Wis). Luciferase activity was quantitated, in triplicate, using a Synergy 2luminometer (BioTek, Winooski, Vt). Readings for the empty vector were subtracted from their corresponding luciferase values. The firefly luciferase activity was normalized to Renilla luciferase activity and the fold differences were plotted as the firefly/Renilla ratio.
Western Blot Analysis
In-well cell lysis was performed on ice with lysis buffer (Cell Signaling Technology, Inc., Billerica, Mass) per manufacturer instructions. Equal amounts of protein were separated on 10–12% tris-glycine polyacrylamide mini-gels (Thermo Fisher Scientific, Inc., Waltham, Mass) and transferred to polyvinylidene fluoride membranes. Membranes was blocked with 5% BSA in Tris-buffered saline and 0.02% Tween-20 (TBST) and subjected to overnight incubation with primary antibody for pERK or total ERK (1:1000 dilution; Cell Signaling) at 4°C. After washing with TBST three times, the membrane was incubated with HRP-conjugated secondary antibody (1:5000 dilution; Santa Cruz Biotechnology, Dallas, Texas) for 1 hour at 25°C. Immunoreactive bands were detected with Enhanced chemiluminescence (ECL) SuperSignal West Pico and Femto substrates (Thermo Fisher Scientific, Inc.) Densitometry was performed using ImageJ software.
In Vivo Model of RAP
Male and female mice of C57BL/6 or C57/129P2 background were purchased from Harlan Laboratories (Indianapolis, Ind) and Jackson Laboratory (Bar Harbor, Maine). Under an IACUC-approved protocol, RAP was induced by intraperitoneal injections of cerulein (50 μg/kg, 5 hourly injections/day, 3 days/week) for 4 weeks.12,14 Control mice received the vehicle (PBS) following the same schedule. After the first week of the RAP protocol, apigenin (50 μg/mouse) or vehicle (0.5% methylcellulose + 0.025% Tween20) was administered via oral gavage 6 days/wk for the remaining 3 weeks. After sacrifice, pancreata was harvested and processed for histology at the end of the experiment.
Immunohistochemistry
Fresh pancreata was fixed in 10% formalin, paraffin-embedded and sectioned (5 μm). Briefly, the slides were deparaffinized and subjected to antigen retrieval solution (10 mM sodium citrate, pH 6.0) for 20 minutes at 98°C. Endogenous peroxidases were quenched with 3% H2O2. Non-specific binding was blocked using an avidin/biotin blocking kit (Thermo Fisher Scientific, Inc.). The sections were incubated with p-ERK primary antibody (1:200) for 1 hour. The sections were washed in TBS-T and incubated with biotinylated goat anti-rabbit antibody (1:200; Vector Laboratories, Inc.; Burlingame, Calif), washed, and incubated with horseradish peroxidase streptavidin. Positive staining was visualized with diaminobenzidine (DAB; Vector Laboratories) and sections were counterstained hematoxylin. The sections were dehydrated through a graded ethanol series, cleared in xylene, and glass coverslips were applied with Permount (Thermo Fisher Scientific, Inc.). Images were captured at 400x using an Olympus BX51 microscope coupled to a DP71 Olympus digital camera using PictureFrame program Version 2.3 (Optronics, Goleta, Calif). The same acquisition exposure-time conditions were used to capture images from the different treatment groups. The percentage of positively stained nuclei was evaluated with the ImmunoRatio software.15
Immunocytochemistry
AR42J cells (5 × 104) were seeded onto collagen (15 μg/mL) coated coverslips. The next day, the cells were washed twice with ice-cold PBS, fixed with 4% paraformaldehyde and permeabilized with 0.3% Triton X-100 for 10 minutes at 25°C. Nonspecific binding was blocked by incubating cells in a 1% BSA/PBS for 20 minutes. The cells were incubated with NF-κB p65 Ab (1:100; Santa Cruz Biotechnology) for 1 hour. After washing the cells in PBS, they were incubated with anti-rabbit Alexa Fluor 488 antibody (1:200; Life Technologies). The coverslips were attached to slides using Vectashield mounting medium with DAPI (Vector Lab). Image analysis was performed using ImageJ. The corrected total cell fluorescence (CTCF) was calculated using the formula CTCF = integrated density – (area of selected cell x mean fluorescence of background readings). An average of 25 representative nuclei was analyzed per treatment group. The nuclear fluorescence was graphed as the mean CTCF ± standard error of the mean.
Statistical Analysis
Graphs were generated using GraphPad Prism 5 (La Jolla, Calif.), and the results were expressed as mean ± standard error of the mean. Quantile-to-quantile (Q-Q) plots were generated to determine whether data was normally distributed using SPSS, Version 20 (IBM, Armonk, NY) Parametric and nonparametric data were evaluated using one-way or two-way ANOVA with post hoc Mann-Whitney U tests or using Kruskal-Wallis test with post hoc Tukey-Kramer multiple comparisons tests, respectively. Statistical significance was set as P < 0.05.
RESULTS
Apigenin Downregulated TGF-β-mediated PTHrP Promoter Activity
After induction of pancreatitis, TGF-β is secreted by inflammatory cells and injured acinar cells.16 TGF-β activates quiescent pancreatic stellate cells (PSCs) into myofibroblast-like cells; activated PSCs further secrete TGF-β and produce excessive ECM proteins, leading to pancreatic fibrosis.17,18 The pro-fibrogenic role of TGF-β is mediated by Smad2/3-dependent and Smad-independent pathways.19,20
TGF-β upregulates PTHrP in both acinar cells and PSCs.21 Human PTHrP is synthesized from a single gene with nine exons. Three isoforms of the protein can be generated by the expression of three distinct promoters and alternative splicing mechanisms. The P3 promoter which contains putative Smad sites is the most widely used sequence in both normal and tumor tissue.22 There is 90% homology between the human P3 promoter and the single PTHrP TATA promoter observed in rats and mice.23 We have previously shown that TGF-β increases transcriptional activity from the PTHrP promoter P3 region in a Smad3-dependent manner in rat acinar AR42J cells.21
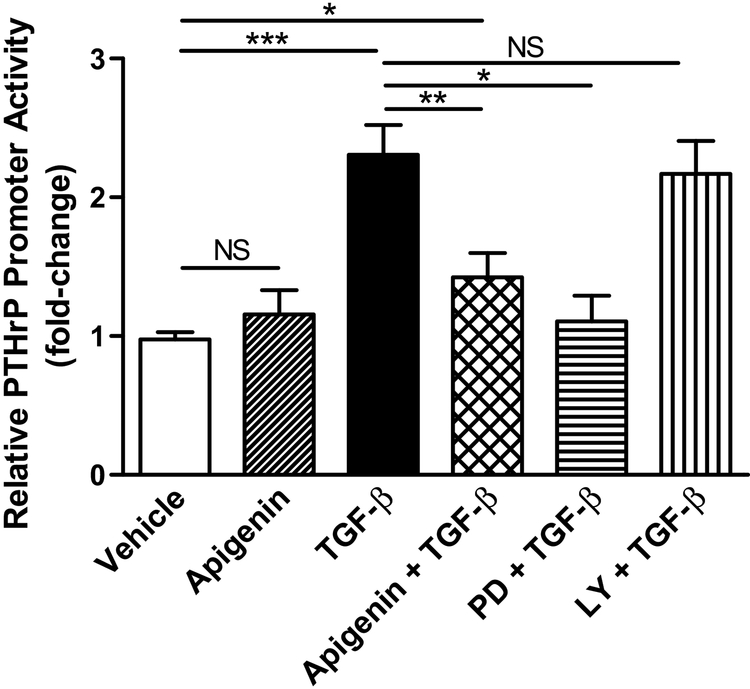
TGF-β was used as the stimulant since it is a well-known crucial growth factor in the regenerative response of RAP, secreted by both acinar cells and activated pancreatic stellate cells. The P3 promoter has an EBS 1 site that binds Ets-1 and Smad 3, which are components of the classical TGF-β signaling pathway.22 To assess apigenin’s effect on PTHrP P3 promoter activity, AR42J acinar cells were co-transfected with a PTHrP-P3 promoter construct and a dual-luciferase reporter gene. AR42J cells treated with vehicle alone or apigenin demonstrate similar PTHrP promoter (basal) activity (Fig. 1). As expected, TGF-β stimulated PTHrP P3 transcriptional activity (P < 0.001); the increased activity was significantly inhibited by apigenin (P < 0.01), as well as with the MAPK kinase inhibitor PD98059. In contrast, inhibition of the PI3K/AKT pathway with LY294002 did not significantly decrease TGF-β induced changes in reporter activity.
FIGURE 1.
Apigenin limited TGF-β stimulation of PTHrP P3 promoter activity. AR42J cells were pre-treated with inhibitors PD98059 (PD, 10 μM) and LY294002 (LY, 25 μM) for 30 minutes. Cells were treated with vehicle (DMSO), apigenin (50 μM), and/or TGF-β (1 ng/mL) for 4 hours. Promoter activity was measured with the Dual-Luciferase Reporter Assay System. The data graphed was a combination of 3 independent assays. One-way ANOVA, P < 0.001; Tukey’s test P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), non-significant (NS).
Apigenin Reduced Acinar Cell pERK In Vitro and In Vivo
Previously, apigenin was reported to be an inhibitor of the ERK pathway in breast, prostate, thyroid, colon, and pancreatic cancer cell lines.11,24,25 Because PD98059 significantly inhibited TGF-β stimulated PTHrP P3 transcriptional activity similar to apigenin (Fig. 1), and cerulein is a activator of the MAPK pathway,26,27 we further evaluated the effect of apigenin on the MAPK/ERK pathway.
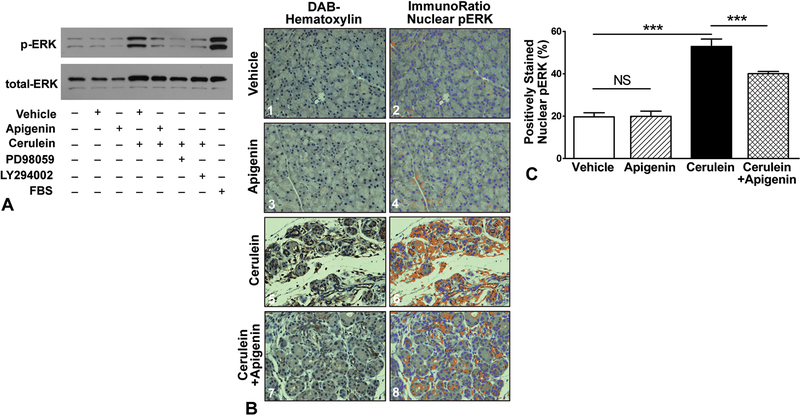
AR42J acinar cells were pre-treated with vehicle, apigenin, or the inhibitors PD98059 and LY294002 followed by stimulation with cerulein for 5 minutes. As shown in Figure 2A, minimal pERK expression was observed in the negative control, vehicle and apigenin treated conditions. As expected, cerulein and addition of FBS (positive control) significantly increased pERK band intensity at 42/44 kDa. Apigenin, PD98059 and LY294002 treatment all downregulated pERK expression at this single time point.
FIGURE 2.
Apigenin significantly reduced cerulein-induced pERK activation. A, AR42J cells were pre-treated with vehicle (DMSO) or apigenin (50 μM) for 1 hour. Inhibitors PD98059 (PD, 10 μM) and LY294002 (LY, 25 μM) pre-treatment was for 30 minutes. Cells were stimulated with cerulein (0.1 μM) for 5 minutes. A representative immunoblot for pERK and total ERK is shown provided. B, Mice were subjected to 4 weeks of cerulein-induced recurrent acute pancreatitis. After the first week, apigenin therapy was initiated (2.5 mg/kg, oral gavage, once daily, 6 days/week) and continued for the duration of the experiment. Pancreata were harvested and immunostained for pERK; hematoxylin was used as a counterstain. Representative 400× microscopic images of each group are shown: (B1,2) vehicles (PBS IP, 0.5% MC + 0.025% Tween20 by oral gavage); (B3,4) apigenin (+PBS); (B5,6) cerulein. (+apigenin’s vehicle); and (B7,8) cerulein + apigenin. Image analysis was performed with ImmunoRatio. C, The percent area of positively stained nuclei (co-localization) is graphed. One-way ANOVA, P < 0.001; Tukey’s tests P < 0.001 (***) or non-significant (NS).
To evaluate the effect of apigenin on the MAPK pathway in vivo, serial supraoptimal doses of cerulein have been shown to recapitulate the biochemical, morphologic, and pathophysiologic features of human pancreatitis.28,29 Mice were subjected to cerulein-induced recurrent acute pancreatitis (RAP) one week prior to the initiation of apigenin therapy, simulating a clinically relevant scenario. Apigenin was administered at a dose of 2.5 mg/kg, given daily by oral gavage, 6 days/week. The repeated induction of pancreatitis was continued with apigenin therapy for an additional 3 weeks. ImmunoRatio image analysis was used to quantify the percentage of acinar cell nuclei staining positive for pERK after immunohistochemical staining (representative images per treatment group are shown in Fig. 2B, C).
Activation of the MAPK pathway involves a phosphorylation cascade of sequential serine/threonine kinases, which transduces signals from cell surface receptor-ligand interactions to cytoplasmic and nuclear targets. The downstream effector, pERK, translocates to the nucleus and phosphorylate additional transcription factors required for the expression of genes involved in cell growth, differentiation, apoptosis, and inflammation. As shown in Figures 2B1–B4, basal pERK expression appeared to be mostly cytoplasmic among the vehicle and apigenin groups. Cerulein treatment increased pancreatic translocation of pERK to the nucleus (Figs. 2B5–B6). Daily oral apigenin therapy not only reduced MAPK pathway activation (P < 0.001; Figs. 2B7–B8, C) but also preserved pancreatic acinar cell architecture despite induction of RAP. When compared to the histologic damage induced by cerulein, apigenin treatment limited acinar cell atrophy, interstitial edema, inflammatory infiltrate, and stromal fibrosis (Fig. 2B).
Apigenin Reduced NF-κB Pathway Activation and IL-6 Expression in Acinar Cells
It is well-known that during acute pancreatitis, an early event is that acinar cells produce, release, and respond to TNF-α, a mediator of the acute inflammatory response.30 TNF-α-stimulated activation of transcription factor NF-ĸB mediates the upregulation of many genes in the pro-inflammatory cascade during pancreatitis.31–34 In quiescent acinar cells, cytoplasmic NF-κB (p65/p50 subunits) is inactivated by its complex with the inhibitor of NF-κB (IκB). When growth factors such as TNF-α activate IκB kinase (IKK), phosphorylated ubiquitination of IκB releases NF-κB from the inhibitory complex. Free p65/p60 translocates to the nucleus, where the transcription factors interact with gene targets.35 Thus, nuclear localization of NF-κB serves as a measure of pathway activation. Immunostaining of pancreatic tissue from patients with chronic pancreatitis show significant acinar cell nuclear p65 positivity of acini.36
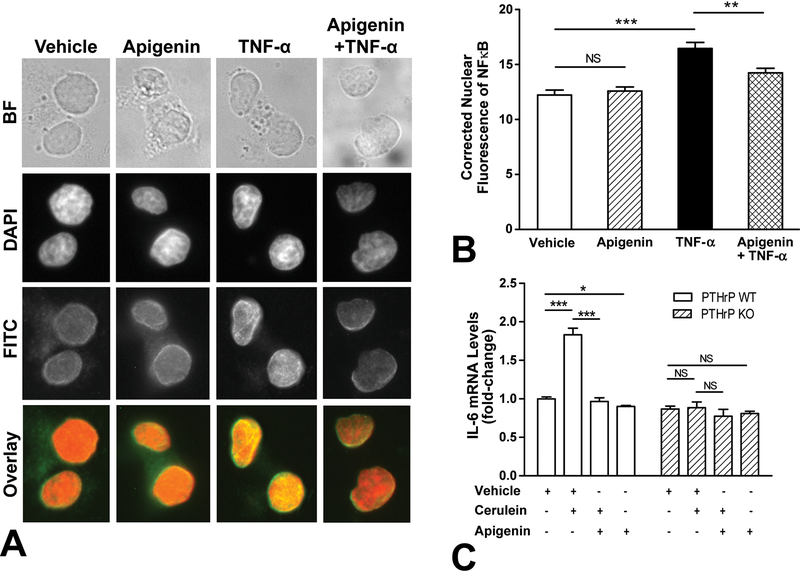
To investigate whether apigenin modulated TNF-α activation of NF-ĸB in acinar cells, AR42J cells were pre-treated with apigenin followed by stimulation with TNF-α. As shown in Figure 3A, TNF-α caused nuclear nuclear translocation of NF-κB (yellow), whereas treatment with vehicle or apigenin alone did not (orange). Pre-treatment with apigenin significantly decreased nuclear p65 staining (P < 0.01) (Figs. 3A, B).
FIGURE 3.
Apigenin decreased IL-6 mRNA levels, an example of the NF-κB pathway. A-B, AR42J cells were pre-treated with vehicle (DMSO) or apigenin (50 μM) for 1 hour followed by stimulation with TNF-α (10 ng/mL) for 30 minutes. Nuclear p65 staining was quantified and graphed; a minimum of 25 nuclei were analyzed per group, and the assay was independently replicated twice. A one-way ANOVA with post-hoc Tukey’s test revealed P < 0.01 (**), P < 0.001 (***), and non-significant (NS). B, Primary acini were isolated from PTHrP WT (fed with corn oil) and PTHrP KO (Tamoxifen) mice and treated ex vivo with vehicle (DMSO), cerulein (10−7 M), and/or apigenin (50 μM) for 4 hours. IL-6 levels were measured by qPCR. The data graphed was a combination of 3 independent assays. One-way ANOVA with Tukey’s test revealed P < 0.05 (*), P < 0.001 (***), and non-significant (NS).
The inflammatory mediator interleukin-6 (IL-6) has been shown to be regulated in a NF-ĸB-dependent manner in rat acinar cells.32 Importantly, serum IL-6 levels correlate with the clinical severity of pancreatitis.37,38 Our group has previously shown that conditional knockout of the Pthrp gene in mouse acinar cells decrease cerulein-stimulated8 or TGF-β-induced IL-6 secretion.21 Using the same tamoxifen-inducible Cre-lox recombination system to induce an acinar cell-specific PTHrP gene knockout, we sought to determine whether apigenin would decrease acinar cell elaboration of IL-6. Mice of the same background were treated with the vehicle of tamoxifen (corn oil) only; absent cre recombinase activity, these wild-type control mice retained their PTHrP gene. Primary acini harvested from murine pancreata were treated ex vivo with vehicle (DMSO), cerulein (10−7 M), and/or apigenin (50 μM) for 4 hours. Cerulein significantly stimulated IL-6 mRNA levels in WT mice with functional PTHrP, and this pro-inflammatory response was inhibited by apigenin (P < 0.001) (Fig. 3C). Compared to the vehicle, apigenin treatment reduced basal IL-6 levels (P < 0.05). In contrast, among acini derived from the PTHrP knockout mice, neither cerulein, apigenin, nor both stimulated IL-6 mRNA levels compared to vehicle. Taken together with the findings that apigenin downregulated PTHrP promoter activity (Fig. 1), these results implicate apigenin acting through a PTHrP-dependent mechanism.
DISCUSSION
The Sentinel Acute Pancreatitis Event model provides a framework for understanding pancreatitis as a continuum of pathology rather than discrete, isolated events.5,6,39 During acute pancreatitis, acinar cell injury incites an inflammatory response through cytokine generation, activation of resident macrophages and mast cells, and recruitment of lymphocytes and monocytes. Premature intrapancreatic zymogen activation leads to parenchymal autodigestion, further amplifying the inflammatory response. As part of physiologic wound-healing mechanisms, PSCs respond by generating inflammatory mediators, and also secreting extracellular matrix proteins such as collagen and fibronectin, which remodel the stromal compartment.40 The pancreas is able to recover from an isolated bout of acute pancreatitis. However, recurrent injury interferes with the physiologic repair mechanisms and recovery, shifting pancreatic homeostasis toward a pro-inflammatory, pro-fibrotic state.5,6 RAP results in progressive acinar cell atrophy, inflammation, and stromal fibrosis. Cumulative pancreatic organ damage accumulates, leading to a scarred, exhausted gland as seen in chronic pancreatitis. Our data demonstrates that during the early phase of RAP, treatment with apigenin may help attenuate disease progression.
Apigenin, a plant-derived polyphenolic flavanoid, is a promising lead compound in the development of a pharmacologic agent for RAP. While chamomile tea contains the highest dietary concentration of apigenin, other dietary sources of apigenin include citrus fruits, grains, green-leafy vegetables like celery, and herbs such as parsley, peppermint and thyme. Apigenin has been shown to exhibit multiple properties including anti-inflammatory, anti-oxidant, anti-proliferative, and pro-apoptotic activity,11 all of which are beneficial if applied to decrease the severity of pancreatitis. In review of the literature, only one other study tested apigenin in the setting of pancreatitis. Lampropoulos and colleagues41 used a ductal ligation model of acute pancreatitis in rats and report that a one-time 5 mg oral dose of apigenin minimized the histologic severity of pancreatitis and myeloperoxidase (MPO) activity, a measure of neutrophil inflammatory response. In a followup study by the same group, Basios et al42 evaluated pro-inflammatory cytokines (TNF-α, IL-6) and MPO activity in rat lung tissue at 6 hours, and up to 72 hours after induction of acute pancreatitis. They showed that the severity scores of lung tissue were significantly decreased in the apigenin treated rats compared to control at these early time points, indicating that the anti-inflammatory effects of apigenin can be beneficial systemically. However, the molecular mechanism(s) of how apigenin can attenuate pancreatitis severity is not known.
Using murine model of RAP, we found that apigenin induced a protective phenotype, preserving pancreatic architecture and limiting inflammatory infiltrates.12,14 Our previous in vitro studies revealed that apigenin and its analogs inhibited PSC viability and induced PSC apoptosis in a time- and dose-dependent manner. Additionally, apigenin treatment of PSCs attenuated PTHrP-induced transcription of ECM proteins (collagen type 1α1 and fibronectin), cell proliferation marker PCNA (proliferating cell nuclear antigen), TGF-β1, and pro-inflammatory cytokines IL-6 and IL-8.12 The findings in PSCs illustrate a potential mechanism by which apigenin significantly reduced stromal fibrosis in the CR-induced mouse model of RAP.12,14 This current study is the first to examine the molecular pathways through which apigenin may directly reduce acinar cell injury.
PTHrP is recognized as a pro-inflammatory and fibrogenic mediator in pancreatitis.7,8,43 Under basal conditions, PTHrP is predominately expressed by pancreatic islets; however, acini and PSCs have been shown to over-express PTHrP during experimental models of acute and chronic pancreatitis.7 We show that apigenin exerts its anti-inflammatory effects, in part, by downregulating acinar cell PTHrP-dependent IL-6 transcription. Importantly, apigenin reduced IL-6 mRNA levels significantly below that of the vehicle (P <0.05, Fig. 3C), suggesting that apigenin may be lowering the ‘threshold’ of cerulein-mediated activation intracellular signaling pathways which result in the generation of inflammatory mediators. Using rat acinar cells, we also show that apigenin reduced TNF-α-stimulated NF-κB translocation (Fig. 3A, B). In a study by Wu et al,44 apigenin treatment of AsPc-1, a pancreatic cancer cell line, resulted in both reduced basal and TNF-α-stimulated NF-κB DNA binding, transcriptional activity, IκB phosphorylation, and nuclear p65/p50 nuclear translocation. Wu’s findings support the concept that apigenin lowers the threshold for activation of pro-inflammatory intracellular pathways.
Both apigenin and the MAPK kinase inhibitor PD98059 suppressed TGF-β stimulation of PTHrP P3 activity. To further investigate apigenin-mediated inhibition the MAPK/ERK pathway, we pre-treated AR42J cells with 50 μM of apigenin but this experimental condition was insufficient to significantly inhibit pERK activation by CR. Using a mouse model of RAP, assessment of MAPK pathway activation revealed that apigenin significantly reduced pERK nuclear localization. The MAPK pathway is a critical link between extracellular stimuli, such as CR or CCK, intracellular response, and regulation of cell proliferation, differentiation, and apoptosis. Signal transduction is transferred through the sequential phosphorylation of a kinase cascade, classically leading to pERK translocation from the cytoplasm to the nucleus, where it can phosphorylate transcription factors.45 In quiescent cells, ERK is largely anchored in the cytoplasm; upon activation, pERK transiently enters the nucleus and returns to the cytoplasm due to an inherent nuclear export sequence at its N-terminus.46,47 Thus, the positive pERK nuclear staining correlated with MAPK activation by RAP, and apigenin inhibition of this response correlated with a reduction in pancreatic injury. Together, these data suggest an indirect link between the MAPK pathway and apigenin’s transcriptional regulation of PTHrP. Regulation of promoter activity is mediated by the binding and cooperation of multiple transcription factors (Ets-1, Sp1, and Smads) and co-activators like CREB-binding protein (CBP)/p300.22 For example, Ets-1 is activated by both TGF-β and MAPK,22 a potential mechanistic target for apigenin. Future studies are needed to directly assess the effect of apigenin on the binding of transcription factors to the PTHrP promoter.
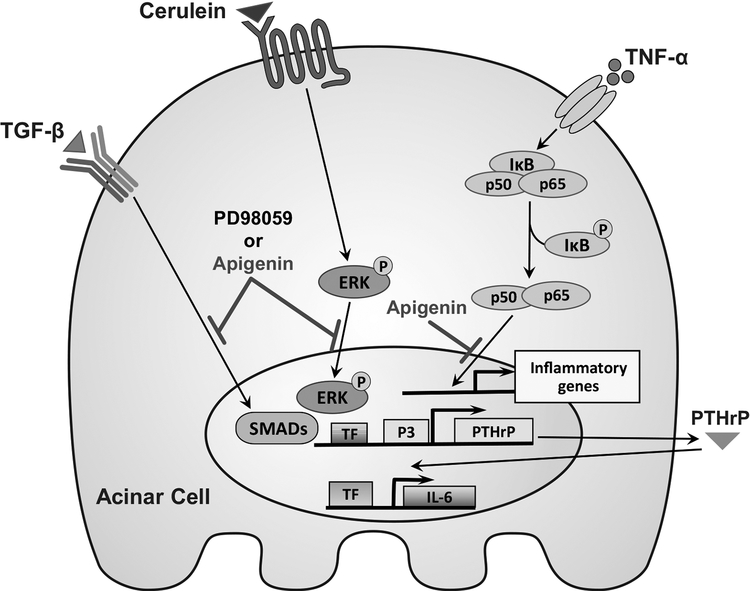
Thus, apigenin is most likely acting through multiple signaling pathways and cell types to limit pancreatic injury in RAP, summarized in Figure 4. Apigenin treatment of primary acini decreases IL-6 mRNA levels in a PTHrP-dependent manner. Experimental activation of acinar cell secretory pathways was induced by multiple stimuli including TNF-α, TGF-β, and CR, the CCK analog. Apigenin inhibited TNF-α induced p65 nuclear translocation, reduced TGF-β stimulation of P3 promoter activity, and limited CR-induced MAPK pathway activation in vivo. Apigenin and apigenin analogs14 show promise as novel therapeutics for the treatment of RAP by inhibiting pro-inflammatory and pro-fibrotic mediators. Additional mechanistic knowledge gained from the study of PTHrP-mediated acinar and PSC response to RAP will provide more targets for drug design and analog optimization.
FIGURE 4.
Schematic of apigenin’s mechanisms of action in acini and PSCs. Downregulation by apigenin is indicated.
ACKNOWLEDGMENTS
We appreciate the work of Eileen Figueroa, Steve Schuenke, and David Chavarria with the Dept. of Surgery for their assistance in manuscript preparation.
Conflicts of Interest and Source of Funding: The authors declare no conflicts of interest. This research was supported by NIH P01 DK035608 (MRH), K08 CA125209 (CC), and T32 DK763920 (MRH).
REFERENCES
- 1.Comfort MW, Gambill EE, Baggenstoss AH. Chronic relapsing pancreatitis; a study of 29 cases without associated disease of the biliary or gastrointestinal tract. Gastroenterology. 1946;6:376–408. [PubMed] [Google Scholar]
- 2.Gorry MC, Gabbaizedeh D, Furey W, et al. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology. 1997;113:1063–1068. [DOI] [PubMed] [Google Scholar]
- 3.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. [DOI] [PubMed] [Google Scholar]
- 4.Schneider A, Lohr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol. 2007;42:101–119. [DOI] [PubMed] [Google Scholar]
- 5.Schneider A, Whitcomb DC. Hereditary pancreatitis: a model for inflammatory diseases of the pancreas. Best Pract Res Clin Gastroenterol. 2002;16:347–363. [DOI] [PubMed] [Google Scholar]
- 6.Whitcomb DC. Hereditary pancreatitis: new insights into acute and chronic pancreatitis. Gut. 1999;45:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatia V, Kim SO, Aronson JF, et al. Role of parathyroid hormone-related protein in the pro-inflammatory and pro-fibrogenic response associated with acute pancreatitis. Regul Pept. 2012;175:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia V, Rastellini C, Han S, et al. Acinar cell-specific knockout of the Pthrp gene decreases the pro-inflammatory and pro-fibrotic response in pancreatitis. Am J Physiol: Gastrointest Liver Physiol 2014;307:G533–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasavada RC, Cavaliere C, D’Ercole AJ, et al. Overexpression of parathyroid hormone-related protein in the pancreatic islets of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J Biol Chem. 1996;271:1200–1208. [DOI] [PubMed] [Google Scholar]
- 10.Whitcomb DC, Shimosegawa T, Chari ST, et al. International consensus statements on early chronic Pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with The International Association of Pancreatology, American Pancreatic Association, Japan Pancreas Society, PancreasFest Working Group and European Pancreatic Club. Pancreatology. 2018: 10.1016/j.pan.2018.1005.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mrazek AA, Porro LJ, Bhatia V, et al. Apigenin inhibits pancreatic stellate cell activity in pancreatitis. J Surg Res. 2015;196:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cataisson C, Gordon J, Roussiere M, et al. Ets-1 activates parathyroid hormone-related protein gene expression in tumorigenic breast epithelial cells. Mol Cell Endocrinol. 2002;204:155–168. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Mrazek AA, Wang X, et al. Design, synthesis, and characterization of novel apigenin analogues that suppress pancreatic stellate cell proliferation in vitro and associated pancreatic fibrosis in vivo. Bioorg Med Chem. 2014;22:3393–3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isola J, Tuominen VJ. ImmunoRatio. 2015; 1.0c. Available at: http://153.1.200.58:8080/immunoratio/. Accessed May 28, 2018.
- 16.Yu JH, Kim KH, Kim H. SOCS 3 and PPAR-gamma ligands inhibit the expression of IL-6 and TGF-beta1 by regulating JAK2/STAT3 signaling in pancreas. Int J Biochem Cell Biol. 2008;40:677–688. [DOI] [PubMed] [Google Scholar]
- 17.Gao X, Cao Y, Yang W, et al. BMP2 inhibits TGF-beta-induced pancreatic stellate cell activation and extracellular matrix formation. Am J Physiol: Gastrointest Liver Physiol 2013;304:G804–G813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogelmann R, Ruf D, Wagner M, et al. Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-beta1 transgenic mouse model. Am J Physiol Gastrointest Liver Physiol. 2001;280:G164–G172. [DOI] [PubMed] [Google Scholar]
- 19.Aoki H, Ohnishi H, Hama K, et al. Autocrine loop between TGF-beta1 and IL-1beta through Smad3- and ERK-dependent pathways in rat pancreatic stellate cells. Am J Physiol Cell Physiol. 2006;290:C1100–C1108. [DOI] [PubMed] [Google Scholar]
- 20.Aoki H, Ohnishi H, Hama K, et al. Existence of autocrine loop between interleukin-6 and transforming growth factor-beta1 in activated rat pancreatic stellate cells. J Cell Biochem. 2006;99:221–228. [DOI] [PubMed] [Google Scholar]
- 21.Bhatia V, Cao Y, Ko TC, et al. Parathyroid hormone-related protein interacts with the transforming growth factor-beta/bone morphogenetic protein-2/gremlin signaling pathway to regulate proinflammatory and profibrotic mediators in pancreatic acinar and stellate cells. Pancreas. 2016;45:659–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard V, Rosol TJ, Foley J. PTHrP gene expression in cancer: do all paths lead to Ets? Crit Rev Eukaryot Gene Expr. 2005;15:115–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karperien M, Farih-Sips H, Hendriks JA, et al. Identification of a retinoic acid-inducible element in the murine PTH/PTHrP (parathyroid hormone/parathyroid hormone-related peptide) receptor gene. Mol Endocrinol. 1999;13:1183–1196. [DOI] [PubMed] [Google Scholar]
- 24.Van Dross R, Xue Y, Knudson A, et al. The chemopreventive bioflavonoid apigenin modulates signal transduction pathways in keratinocyte and colon carcinoma cell lines. J Nutr. 2003;133:3800S–3804S. [DOI] [PubMed] [Google Scholar]
- 25.Pham H, Chen M, Takahashi H, et al. Apigenin inhibits NNK-induced focal adhesion kinase activation in pancreatic cancer cells. Pancreas. 2012;41:1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiological Reviews. 2006;86:805–847. [DOI] [PubMed] [Google Scholar]
- 27.Dabrowski A Exocrine pancreas; molecular basis for intracellular signaling, damage and protection--Polish experience. J Physiol Pharmacol. 2003;54 Suppl 3:167–181. [PubMed] [Google Scholar]
- 28.Aghdassi AA, Mayerle J, Christochowitz S, et al. Animal models for investigating chronic pancreatitis. Fibrogenesis Tissue Repair. 2011;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neuschwander-Tetri BA, Burton FR, Presti ME, et al. Repetitive self-limited acute pancreatitis induces pancreatic fibrogenesis in the mouse. Dig Dis Sci. 2000;45:665–674. [DOI] [PubMed] [Google Scholar]
- 30.Gukovskaya AS, Gukovsky I, Zaninovic V, et al. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gukovsky I, Gukovskaya A. Nuclear factor-kappaB in pancreatitis: Jack-of-all-trades, but which one is more important? Gastroenterology. 2013;144:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gukovsky I, Gukovskaya AS, Blinman TA, et al. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–1414. [DOI] [PubMed] [Google Scholar]
- 33.Rakonczay Z Jr, Hegyi P, Takacs T, et al. The role of NF-kappaB activation in the pathogenesis of acute pancreatitis. Gut. 2008;57:259–267. [DOI] [PubMed] [Google Scholar]
- 34.Steinle AU, Weidenbach H, Wagner M, et al. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116:420–430. [DOI] [PubMed] [Google Scholar]
- 35.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nature Reviews Drug Discovery. 2004;3:17–26. [DOI] [PubMed] [Google Scholar]
- 36.Sah RP, Dudeja V, Dawra RK, et al. Cerulein-induced chronic pancreatitis does not require intra-acinar activation of trypsinogen in mice. Gastroenterology. 2013;144:1076–1085.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain S, Midha S, Mahapatra SJ, et al. Interleukin-6 significantly improves predictive value of systemic inflammatory response syndrome for predicting severe acute pancreatitis. Pancreatology. 2018. May 14 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 38.Leser HG, Gross V, Scheibenbogen C, et al. Elevation of serum interleukin-6 concentration precedes acute-phase response and reflects severity in acute pancreatitis. Gastroenterology. 1991;101:782–785. [DOI] [PubMed] [Google Scholar]
- 39.Whitcomb DC. Genetic polymorphisms in alcoholic pancreatitis. Dig Dis. 2005;23:247–254. [DOI] [PubMed] [Google Scholar]
- 40.Masamune A, Watanabe T, Kikuta K, et al. Roles of pancreatic stellate cells in pancreatic inflammation and fibrosis. Clin Gastroenterol Hepatol. 2009;7:S48–54. [DOI] [PubMed] [Google Scholar]
- 41.Lampropoulos P, Lambropoulou M, Papalois A, et al. The role of apigenin in an experimental model of acute pancreatitis. J Surg Res. 2013;183:129–137. [DOI] [PubMed] [Google Scholar]
- 42.Basios N, Lampropoulos P, Papalois A, et al. Apigenin attenuates inflammation in experimentally induced acute pancreatitis-associated lung injury. J Invest Surg. 2016;29:121–127. [DOI] [PubMed] [Google Scholar]
- 43.Falzon M, Bhatia V. Role of parathyroid hormone-related protein signaling in chronic pancreatitis. Cancers (Basel). 2015;7:1091–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu D-G, Yu P, Li JW, et al. Apigenin potentiates the growth inhibitory effects by IKK-beta-mediated NF-kappaB activation in pancreatic cancer cells. Toxicol Let. 2014;224:157–164. [DOI] [PubMed] [Google Scholar]
- 45.Kondoh K, Torii S, Nishida E. Control of MAP kinase signaling to the nucleus. Chromosoma. 2005;114:86–91. [DOI] [PubMed] [Google Scholar]
- 46.Fukuda M, Gotoh I, Gotoh Y, et al. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem. 1996;271:20024–20028. [DOI] [PubMed] [Google Scholar]
- 47.Adachi M, Fukuda M, Nishida E. Nuclear export of MAP kinase (ERK) involves a MAP kinase kinase (MEK)-dependent active transport mechanism. J Cell Biol. 2000;148:849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]