Abstract
Rationale:
Endothelial barrier function depends on the proper localization and function of the adherens junction protein VE-cadherin. Previous studies have suggested a functional relationship between integrin-mediated adhesion complexes and VE-cadherin yet the underlying molecular links are unclear. Binding of the cytoskeletal adaptor protein talin to the β integrin cytoplasmic domain is a key final step in regulating the affinity of integrins for extracellular ligands (activation) but the role of integrin activation in VE-cadherin mediated endothelial barrier function is unknown.
Objective:
To test the requirement of talin-dependent activation of β1 integrin in VE-cadherin organization and endothelial cell barrier function.
Methods and Results:
Endothelial cell-specific deletion of talin in adult mice resulted in impaired stability of intestinal microvascular blood vessels, hemorrhage and death. Talin-deficient endothelium showed altered VE-cadherin organization at endothelial cell-cell junctions in vivo. shRNA-mediated knockdown of talin1 expression in cultured endothelial cells led to increased radial actin stress fibers, increased adherens junction width and increased endothelial monolayer permeability measured by electrical cell-substrate impedance sensing. Restoring β1 integrin activation in talin-deficient cells with a β1 integrin activating antibody normalized both VE-cadherin organization and endothelial cell barrier function. In addition, VE-cadherin organization was normalized by re-expression of talin or integrin activating talin head domain but not a talin head domain mutant that is selectively deficient in activating integrins.
Conclusions:
Talin-dependent activation of endothelial cell β1 integrin stabilizes VE-cadherin at endothelial junctions and promotes endothelial barrier function.
Subject Terms: Basic Science Research, Cell Signaling/Signal Transduction, Endothelium/Vascular Type/Nitric Oxide, Vascular Biology
Keywords: Integrin, talin, adhesion molecule, endothelium, endothelial cell, endothelium function, vascular permeability
INTRODUCTION
Endothelial cells (ECs) line the luminal blood vessel surface forming a barrier that separates the blood from surrounding tissues. EC barrier function is tightly regulated, dynamic and plays a central role in human health and disease. The EC barrier is maintained in part by adherens junctions (AJs), comprised of VE-cadherin and associated cytoplasmic interacting proteins1-3. In response to permeability inducing hormones and autocoids such as vascular endothelial growth factor and thrombin, AJs remodel and the endothelium becomes more permeable. These changes in vascular permeability play an important role in leukocyte transmigration and tissue fluid homeostasis4-6.
The plasticity of AJs in response to extracellular cues depends upon connections to the actin cytoskeleton. VE-cadherin is linked indirectly to actin through its interaction with the β-catenin and α-catenin complex. 7 Mice expressing a VE-cadherin-α-catenin fusion which is retained at AJs were protected from VEGF-induced permeability clearly demonstrating the functional significance of VE-cadherin junctional stability in vivo. 8 Actomyosin-dependent contraction of ECs regulates the endothelial barrier and the reorganization of junctional VE-cadherin pools in response to the altered actin cytoskeleton tension induces the appearance of tensile AJs referred to as focal adherens junctions (FAJs). 9, 10 Like AJs, integrin containing adhesion complexes are linked indirectly to the actin cytoskeleton through interactions with cytoskeleton adaptor proteins11 . Indeed, a functional relationship been AJs and integrins is well-established but the underlying molecular mechanisms are yet unclear12.
Integrins are heterodimeric adhesion receptors comprised of α and β-subunits which bind, among other ligands, extracellular matrix components important in mediated cell adhesion to the basement membrane.13 Quiescent endothelium expresses at least seven classes of integrin with β1 integrin, one of the best studied in endothelium, dictating specificity for fibronectin (α5β1), collagen (α1β1, α2β1) and laminin (α3β1, α6β1). 14 Concomitant endothelial-specific deletion of α5 and αv leads to cardiovascular defects and embryonic lethality by E14.5.15 EC-specific deletion of β1 integrin during development resulted in embryonic lethality between E9.5-E.10.5 characterized by vascular patterning defects and vessel malformations16 whereas inducible genetic deletion of β1 integrin in ECs or postnatal pharmacological blockade of β1 integrin resulted in impaired lumen formation and defects in EC apical-basal polarity during new vessel growth.17 More recently, EC-specific genetic deletion of β1 integrin in mice supported a role for β1 integrin expression in stabilizing VE-cadherin at cell-cell junctions.18 Collectively, these data indicate that the expression of β1 integrin in ECs is critical for normal vascular development and blood vessel stability.
An important property of integrins is the modulation of affinity for extracellular ligands, a process termed integrin activation or “inside-out integrin signaling”. A key final step in activating integrins is binding of the N-terminal head domain of the cytoskeletal protein talin to the β integrin cytoplasmic domain. 19-22 Whereas many of the molecular and structural details of how talin binding activates integrins21 and the biological significance of talin-dependent integrin activation have been clearly demonstrated in hematopoietic cells23-27, the requirement for talin-dependent integrin activation in established blood vessels has not been tested. EC-specific deletion of Tln1 in mice causes embryonic lethality due to defects in angiogenesis resulting in extensive vascular hemorrhaging and lethality by E9.5 28 supporting a clear role of talin in embryonic developmental angiogenesis.
Here, we analyzed mice in which we have genetically deleted Tln1 selectively in the endothelium of established blood vessels of adult mice using an inducible conditional Cre/loxP recombination approach. Interestingly, our findings indicate the importance of EC talin1 in the stability and barrier function of the intestinal microvasculature. Furthermore, we present both in vivo and in vitro data that support a role for talin in VE-cadherin organization and show that talin-dependent activation of β1 integrin is a key node in this pathway required for AJ stability and integrity of the endothelium.
METHODS
The authors declare that all supporting data are available within the article and its online-only Data Supplement.
Mice.
To delete talin1 postnatally in endothelial cells, Tln1f/f;Cdh5-CreERT2+/− 29, 30 (a gift from Ralf Adams, Max Planck Institute) male mice were crossed with Tln1f/f female mice to generate Tln1f/f;Cdh5-CreERT2−/− (Tln1 CTRL) and Tln1f/f;Cdh5-CreERT2+/− (Tln1 EC-KO) offspring. Studies using the tdTomato reporter were done by comparing mice with genotype Tln1f/f;Cdh5-CreERT2+/−;Rosa26-tdTomato+/− with Tln1f/wt;Cdh5-CreERT2+/−;Rosa26-tdTomato+/− mice. Adult mice, 8-10 week old mice were treated with tamoxifen (Cayman Chemicals) dissolved in corn oil via intraperitoneal injection (2mg/mouse/day) for 3 consecutive days. For retinal angiogenesis studies, pups received tamoxifen (dissolved in corn oil) via intragastric injection (50μg/mouse/day) for 3 consecutive days starting on postnatal day 2. Similar ratios of males and female mice were used for experiments and experimenters were blinded to the genotypes of mice until all data was collected. In control experiments to test the effects of tamoxifen versus corn oil on survival, mice were randomly assigned to treatment groups. Experimental procedures were approved by the Emory University Institutional Animal Care and Use Committee.
Statistical analysis.
All statistical tests were performed using Prism Software 8.0. The specific test that was used to analyze individual experiments is noted in the figure legends but briefly for comparison of parametric data from two groups, an unpaired t-test was used. Data sets analyzed with parametric statistical tests were tested for a normal distribution using a Shapiro-Wilk test. For comparison of vessel leak in mouse organs, we performed multiple unpaired t-tests as only two groups (CTRL vs EC-KO) were compared. Where noted in the legends, we performed one-way analysis of variance with either a Dunnet’s, Tukey or_Sidak’s Multiple comparison test. The number of animals and experimental repeats is listed in individual legends.
Detailed methods can be found in the Online Data Supplement.
RESULTS
Endothelial-specific deletion of talin1 in established blood vessels causes intestinal vascular hemorrhage and death.
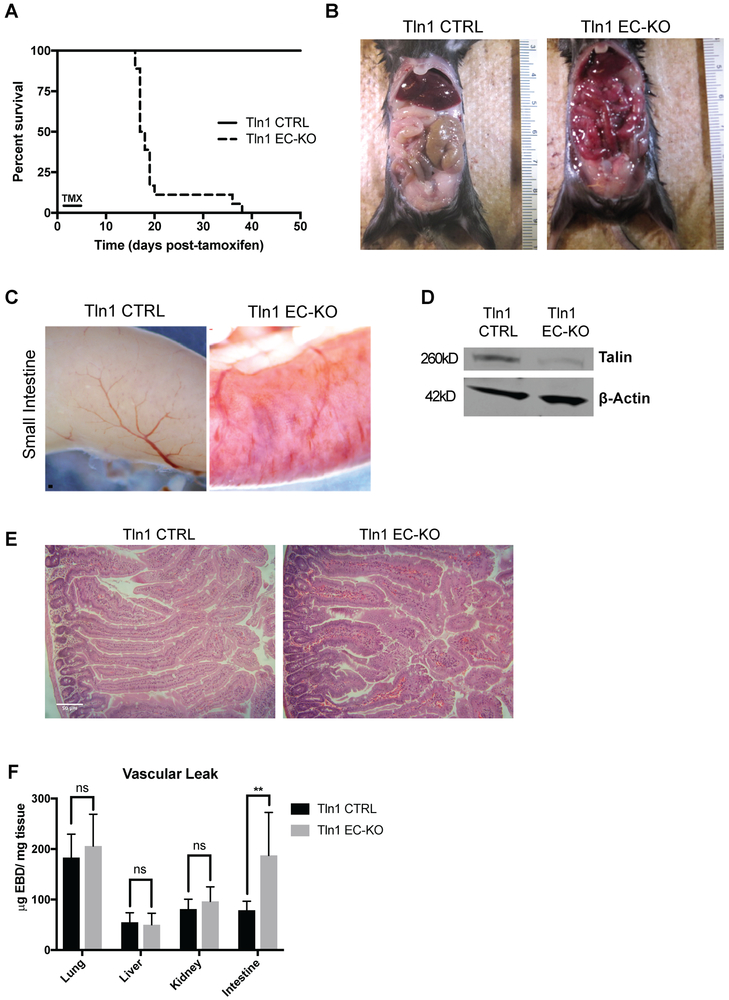
To test the contribution of talin-dependent integrin activation in endothelial cells in the maintenance and stability of mature vasculature, we generated endothelial cell (EC)-specific talin1 knock-out mice utilizing Tln1 floxed mice 26, 27 expressing a tamoxifen-inducible Cre driven by the VE-cadherin (Cdh5) promoter30. Adult Tln1f/f;Cdh5-CreERT2−/− (referred to as Tln1 CTRL) and Tln1f/f;Cdh5-CreERT2+/− (Tln1 EC-KO) mice were administered tamoxifen at 8-10 weeks of age. Strikingly, Tln1 EC-KO mice developed sudden-onset morbidity, including inactivity and hunched posture, starting approximately 14 days after tamoxifen treatment and died 16-21 days after tamoxifen treatment (Fig 1A). Neither Tln1 EC-KO mice treated with corn oil vehicle nor Tln1f/f;Cdh5-CreERT2−/− mice treated with tamoxifen showed adverse effects or reduced survival (data not shown and Fig 1A). Gross examination of Tln1 EC-KO adult mice 16 days after deletion of talin1 revealed bloody intestines whereas abnormalities in other organs were not observed (Fig 1B and 1C). Talin protein expression was reduced by 82% in ECs isolated from lungs of Tln1 EC-KO mice treated with tamoxifen in vitro (Fig 1D). In light of the observed extravascular red blood cells in the intestinal capillaries of Tln1 EC-KO mice visualized by Hematoxylin and eosin staining (Fig 1E), we measured the leak of circulating Evan’s Blue Dye (EBD), a well-established in vivo assay to assess vascular permeability31. Two hours after intravenous injection of EBD, which binds tightly to serum albumin, Tln1 EC-KO mice showed approximately a 2.5 fold increase in EBD content in the small intestines compared to Tln1 CTRL mice indicating impaired endothelial barrier function in the intestines of Tln1 EC-KO mice (Fig 1F). We also deleted Tln1 utilizing a second EC-specific, tamoxifen-inducible PDGFβ-CreERT2 mouse line32. Tamoxifen treatment of Tln1f/f,PDGFβ-CreERT+/− mice resulted in similar intestinal bleeding and death as when Tln1 was deleted with Cdh5-CreERT2 (Online Figure I A-C). The possibility of Cre-mediated recombination in hematopoietic cells in Cdh5-CreERT2 and PDGFβ-CreERT2 mice was examined with a Rosa26-flox-stop-flox-TdTomato (TdTom) Cre reporter mouse (Jackson Labs #007914). Flow cytometry of peripheral blood isolated from tamoxifen-treated PDGFβ-CreERT2+/−;TdTom mice showed TdTomato expression in approximately 10% of platelets consistent with the previous characterization of these mice32. In contrast, TdTomato expression was not detected in any peripheral hematopoietic cells isolated from tamoxifen-treated Cdh5-CreERT2+/−;TdTom mice (Online Figure I D). Since talin expression in platelets is essential for hemostasis26, 27, we utilized Cdh5-CreERT2 to delete EC talin1 in all subsequent experiments.
Figure 1: Endothelial cell-specific deletion of talin1 in established blood vessels causes intestinal vascular hemorrhage and death.
A-C. Adult Tln1 EC-KO (Tln1fl/fl;Cdh5creERT2+/−) and Tln1 CTRL (Tln1fl/fl;Cdh5creERT2−/−) mice were administered tamoxifen once a day for 3 consecutive days via intraperitoneal injection. A. Survival of Tln1 EC-KO and Tln1 CTRL mice following tamoxifen treatment (n=15, Tln1 CTRL; n=14, Tln1 EC-KO). B. Pictures of exposed peritoneum of adult Tln1 EC-KO and CTRL mice sacrificed 16 days after tamoxifen treatment. C. Macroscopic images of intestinal vascular hemorrhage in Tln1 EC-KO adult mice 16 days after tamoxifen treatment. D. Western blot analysis of talin expression in mouse lung endothelial cell cultures isolated from Tln1 CTRL or Tln1 EC-KO mice. Cultures were treated with 4-hydroxy-tamoxifen for 4 days and protein lysates subjected to Western blotting with talin and β-actin antibodies. (n=2). E. Hematoxylin/eosin staining of small intestine isolated from Tln1 CTRL and Tln1 EC-KO mice 16 days after tamoxifen treatment showing the presence of extravascular red blood cells in Tln1 EC-KO villi. (n=3; scale=50 μm). F. Measurements of Evan’s Blue Dye (EBD) in lung, liver, intestine, brain and kidney of Tln1 CTRL and Tln1 EC-KO mice 2 hours after intravenous injection. (n=12, Tln1 CTRL; n=10, Tln1 EC-KO; **p = 0.0013 two-tailed unpaired t-test).
Endothelial Talin1 is required for intestine vascular barrier function.
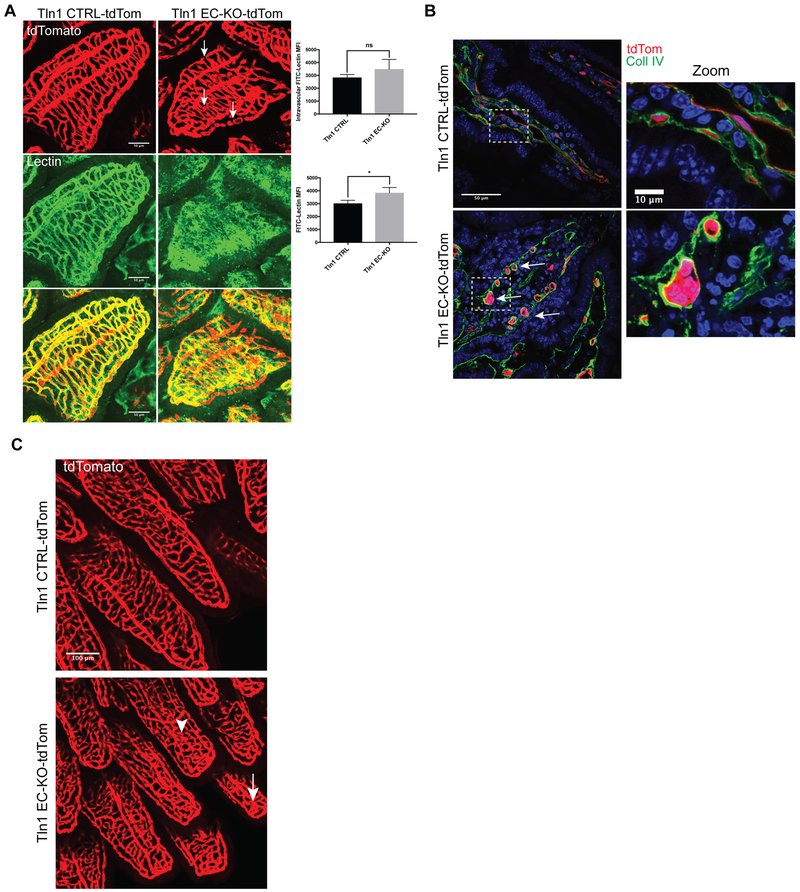
To visualize vascular morphology in cre-recombined cells, the above-described TdTomato Cre-reporter was bred into Tln1 EC-KO and CTRL mouse lines to create Tln1wt/f;Cdh5-CreERT2+/−,TdTomato+ (Tln1 CTRL-TdTom) and Tln1f/f;Cdh5-CreERT2+/−,TdTomato+ (Tln1 EC-KO-TdTom) mice. Intravascular labeling of the endothelium with FITC-lectin for 30 minutes revealed extravascular accumulation of FITC-lectin in surrounding intestinal tissue in Tln1 EC-KO-TdTom mice 16 days after tamoxifen suggestive of vascular leak despite comparable intravascular FITC-lectin labeling (Fig 2A). Confocal microscopic analysis of Tln1 EC-KO-TdTom villi revealed disorganized villi capillary beds with the appearance of round cyst-like malformations composed of multiple ECs (Fig 2B) that were not observed in Tln1 CTRL-TdTom littermates. We examined the vasculature of whole-mounted segments of small intestine from adult mice as early as 12 days after tamoxifen injection and observed morphological defects in the microvasculature of Tln1 EC-KO-TdTom mice, characterized by small cyst-like structures and widening of the villi capillaries (Fig 2C). Importantly, TdTomato was expressed in the blood vessels of all organs examined including the brain, liver and heart, of Tln1 CTRL-TdTom and Tln1 EC-KO-TdTom mice indicating efficient activation of Cre after tamoxifen treatment (Online Figure II). Deletion of Tln1 transcript in intestinal ECs was confirmed by reverse transcription and real-time PCR analysis of RNA isolated from FACS-sorted intestinal ECs (Online Figure III). Together, the foregoing data support an important function of talin in the maintenance and stability of intestinal microvasculature.
Figure 2: Endothelial talin is required for maintenance of intestinal vascular integrity and barrier function.
A. TdTomato and FITC-lectin were visualized in the villi of mice 16 days after tamoxifen injection. Mice were injected intravenously with FITC-Lectin 30 minutes prior to sacrifice. (n=3;scale=50 μm). Total FITC-Lectin fluorescence and intravascular lectin levels were quantitated indicating increased extravascular leak in Tln1 EC-KO-tdTom mice relative to Tln1 CTRL-tdTom (n=3 mice/group; *p=0.039 two-tailed unpaired t-test) B. Confocal microscopic analysis of cryosections of intestine showing tdTomoto fluorescence and collagen IV immunofluorescence. Inset shows a zoomed region demonstrating endothelial cell rounding (white arrows) and detachment from neighboring cells in the intestinal villi of Tln1 EC-KO-tdTom mice. (n=3; scale=50 μm; zoom scale=10 μm). C. TdTomato fluorescence showing disorganized capillaries and cyst-like structures (white arrows) in Tln1 EC-KO-tdTom intestinal wall and villi 12 days after tamoxifen injections. (n=3; scale=100 μm).
Reduced β1 integrin activation and disorganized adherens junctions in established vessels of Talin1 EC-KO mice.
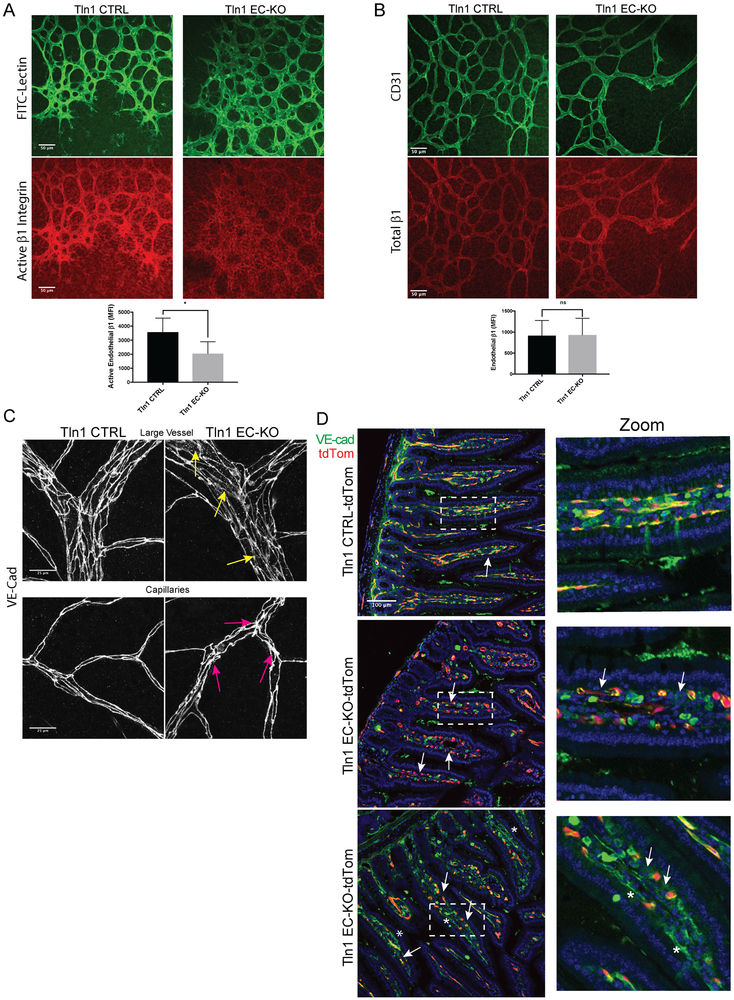
Consistent with the established role of talin as a key regulator of integrin activation, immunofluorescence analysis of retinas of P7 Tln1 EC-KO and CTRL neonates with a β1 integrin activation-sensitive antibody indicated a significant reduction in active β1 integrin in Tln1 EC-KO endothelium (Fig 3A). Importantly, total β1 integrin expression in the retina appeared similar between groups (Fig 3B). Furthermore, similar levels of β1 integrin surface expression were observed in acutely isolated lung ECs from adult Tln1 EC-KO and CTRL mice 15-days after tamoxifen treatment (Online Figure IV A). Endothelial barrier function depends on VE-cadherin (VE-Cad)1, 2. Recent work highlighting the requirement of endothelial β1-integrin in maintaining vessel stability by regulating VE-cadherin localization18 suggested that VE-Cad localization might be altered in the endothelium of Tln1 EC-KO mice. Whole-mount staining of retinal vasculature from adult Tln1 EC-KO and CTRL mice 15 days after tamoxifen treatment revealed disorganized capillary cell-cell junctions and increased intracellular VE-Cad staining relative to Tln1 CTRL mice (Fig 3C). Interestingly, intestinal capillary junctions visualized by immunofluorescence of VE-Cad were discontinuous with ECs detached from neighboring ECs (Fig 3D). Analysis of zonula occludens-1 (ZO-1), a component of tight junctions, similarly showed altered organization in P7 Tln1 EC-KO retinas (Online Figure V). Together, these data indicate that talin expression is necessary for β1 integrin activation in ECs in vivo and suggest an important mechanistic link between talin-dependent β1 integrin activation and the regulation of cell-cell junction organization in ECs.
Figure 3: Reduced β1 integrin activation and disorganized adherens junctions in established vessels of Talin1 EC-KO mice.
A-B. Immunofluorescence analysis of active β1 integrin with the activation-sensitive antibody 9EG7 (A) or total β1 integrin with an activation insensitive β1 integrin antibody HMb1-1 (n=3-4; *p=0.03 two-tailed unpaired t-test) (B) in whole mounted retinas from Tln1 EC-KO and Tln1 CTRL mice. Neonates were treated with tamoxifen on P1-3 and sacrificed on postnatal day 7 (P7) at which time retina whole mounts were prepared for staining. (n=3-4; ns=not significant unpaired t-test; scale=50 μm). C. Altered junctional thickness (magenta arrows) and localization of VE-Cadherin (yellow arrows) in retinal vessels and capillaries of Tln1 EC-KO mice 16 days after tamoxifen injections compared to Tln1 CTRL mice. (n=3; scale=25 μm). D. VE-cadherin immunofluorescence of intestine cryosections showing disrupted cell-cell junctions in villi (white arrows) of Tln1 EC-KO-tdTom mice 16 days after tamoxifen treatment. Changes in cell-cell junctions appear cell autonomous as junctions between non-recombined ECs in Tln1 EC-KO-tdTom villi (asterisks) are intact. (n=3; scale=100 μm).
Disorganized cell-cell junctions in talin-deficient endothelial cells.
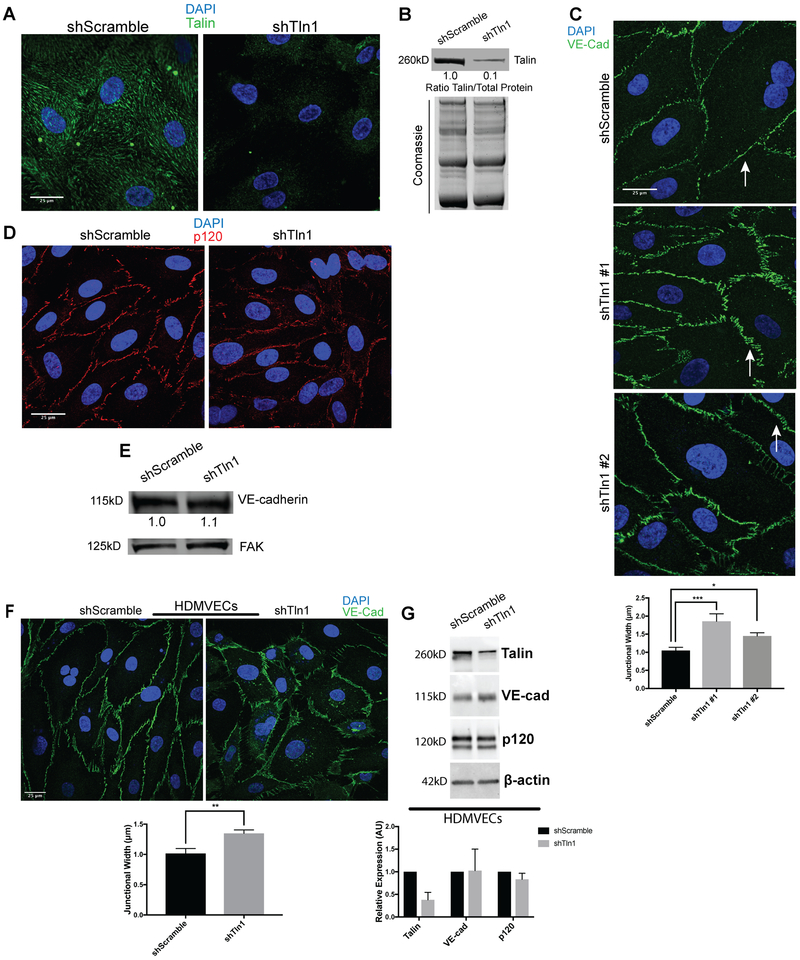
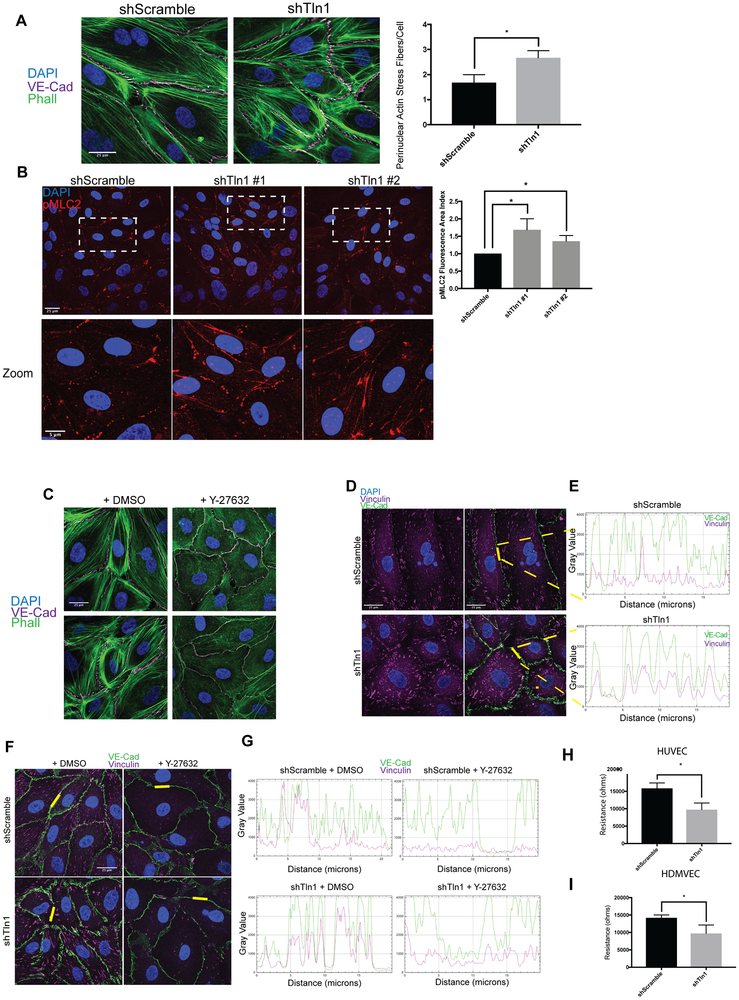
To further investigate the mechanisms by which EC talin1 contributes to cell-cell junction stability, we deleted talin1 using short hairpin RNAs in human umbilical vein endothelial cells (HUVECs) as measured by immunofluorescence and western blot analysis (Fig 4A and 4B). Deletion of talin1 did not alter surface expression of the major endothelial integrins as measured by flow cytometry (Online Figure IV B). Talin-deficient cells exhibited a striking difference in the junctional organization of VE-cadherin (Fig 4C) and ZO-1 (Online Figure V) with significantly wider cell junctions in shTln1 cells relative to shScramble control cells. p120 staining at the cell-cell junctions was discontinuous and diffuse in shTln1 cells consistent with altered AJ organization (Fig 4D). Altered junctional organization in talin-deficient ECs was not accompanied by any consistent detectable changes to VE-cadherin or p120 protein expression relative to shScramble cells (Fig 4E, Online Figure VI B). Changes to junctional organization in talin-deficient ECs were also evident in human dermal blood microvascular endothelial cells (HDMVECs). Deletion of talin1 in HDMVECs increased junctional width (Fig 4F) with no consequence to the total expression of adherens junction components VE-cadherin and p120 (Fig 4G). Because of the junctional alterations exhibited in talin-deficient ECs, we speculated that increased cell contraction18 might be responsible for changes in junctional morphology of talin-deficient ECs. Indeed, talin-deficient HUVECs and HDMVECs displayed increased actin stress fiber formation relative to shScramble cells (Fig 5A and Online Figure VI D). Phosphorylation of myosin light chain was also increased in talin-deficient HUVECs, consistent with increased contractility in the absence of talin (Figure 5B). Pharmacological inhibition of Rho-Kinase in talin-deficient HUVECs mitigated the alterations in VE-Cad organization and actin stress fiber formation (Fig 5C). These findings indicate that talin stabilizes endothelial AJs at least in part by suppressing_actin-myosin contractility.
Figure 4: Increased width of adherens junctions formed by talin-deficient endothelial cells.
A. HUVECs were infected with talin1 shRNA (shTln1) or scramble sequence shRNA (shScramble) lentivirus. Immunostaining for talin1/2 72 hours after shRNA infection shows efficient knockdown of talin protein in shTln1 cells (n=5; scale=25 μm). B. Western blot analysis and quantitation of talin1 protein levels relative to total protein in shScramble and shTln1 treated HUVECs (n=5). C. Max-intensity projections depicting Immunofluorescence of VE-Cadherin in cells infected with two different shTln1 lentiviruses. shTln1 HUVECs shows disorganized cell-cell junctions and increased junctional width (white arrows) compared to shScramble HUVECs. (n=3; ***p=0.0007, *p=0.0225 one-way ANOVA with Dunnett’s multiple comparisons test). D. Immunofluorescence of p120-catenin in shTln1 and shScramble HUVECs. (n=3). E. Western blot analysis of VE-cadherin protein levels in shScramble and shTln1 HUVECs show similar levels of expression (n=6). F. Human dermal microvascular cells (HDMVECs) treated with shTln1 lentivirus exhibit increased junctional width relative to shScramble treated cells (n=3; scale=25 μm; **p=0.0042 two-tailed unpaired t-test). G. Western blot analysis and quantitation of talin1/VE-cad/p120 protein levels in shScramble and shTln1 HDMVECs. Efficient deletion of talin1 does not appear to affect relative expression of VE-cad or p120 protein (n=3).
Figure 5: Increased cell contraction and tensile adherens junctions in talin-deficient endothelial cell.
A. Phalloidin and VE-cadherin immunofluorescence on shScramble and shTln1 HUVECs. The number of perinuclear stress fibers per cell were quantified as described in methods. (n=3; scale=25 μm *p=0.016 two-tailed unpaired t-test) B. Max-intensity immunofluorescence projections of HUVECs stained with anti-pMLC2 antibody indicate increased pMLC2+ cell area in shTln1#1 and shTln1#2 cells relative to shScramble (n=2-4; scale=25 μm; *p=0.0105, *p=0.0405 one-way ANOVA with Kruskal Wallis multiple comparisons test) C. Inhibition of cytoskeletal contraction by treating cells with Rho-associated kinase inhibitor Y-27632 (50nM, 12 hours) reduces junctional disorganization and FAJ formation in talin-deficient HUVECs. (n=3; scale=25 μm). D. Co-localization of VE-Cadherin (magenta) and Vinculin (green) at cell-cell junctions is increased in shTln1 cells compared to shScramble HUVECs (scale=25 μm). E. Intensity profile plot of VE-Cadherin and vinculin immunofluorescence shown in D. (n=4) F. Inhibition of ROCK-mediated cellular contraction (Y-27623) reduces vinculin localization at cell-cell junctions of shTln1 HUVECs relative to vehicle treated cells (n=3; scale=25 μm). G. Intensity profile plot of VE-cadherin and vinculin immunofluorescence depicted in G (n=3). H. HUVEC monolayer resistance measured using electrical cell impedance sensing (ECIS) of shTln1 infected monolayers is reduced relative to shScramble (n=3; *p=0.0131; two-tailed unpaired t-test). I. HDMVEC monolayer resistance measured by ECIS of shTln1 infected monolayers is reduced relative to shScramble (n=3; *p=0.0399; two-tailed unpaired t-test).
Reduced barrier function of talin-deficient endothelial cells.
To test whether altered cell-cell junctions of talin-deficient ECs was due to increased cell-cell junctional tension, we performed immunofluorescence co-localization of VE-cadherin and vinculin to identify tensile AJs. Previous work has described changes in junctional VE-cad organization in response to increased actin cytoskeleton tension demarcated by co-localization of vinculin and VE-cadherin at cell-cell contacts9, 10. Deletion of talin1 in HUVECs resulted in pronounced co-localization of VE-cadherin and increased junctional pools of vinculin (Fig 5D-E) as measured by line-scanning of cell junctions. Interestingly, the appearance of tensile VE-cadherin+/vinculin+ cell-cell contacts could be reversed by Rho Kinase inhibition supporting the role of increased contraction in the change in tensile junction formation (Fig 5F-G). To test whether the increased appearance of tensile cell-cell contacts and wider junctions of shTln1 cells coincided with altered EC barrier function, we performed Electric Cell-Substrate Impedance Sensing (ECIS Z0; Applied Biophysics) experiments to assess basal impedance of EC monolayers in response to talin depletion. shTln1 HUVECs exhibited a 39% reduction in endothelial monolayer impedance compared to shScramble-treated control cells (Fig 5H). Defects in monolayer resistance were similarly observed in shTln1 HDMVECs relative to control cells (Fig 5I). Together, these results are consistent with the observation of leaky blood vessels in Tln1 EC-KO mice and indicates that talin1 is required for EC barrier function in vitro and in vivo.
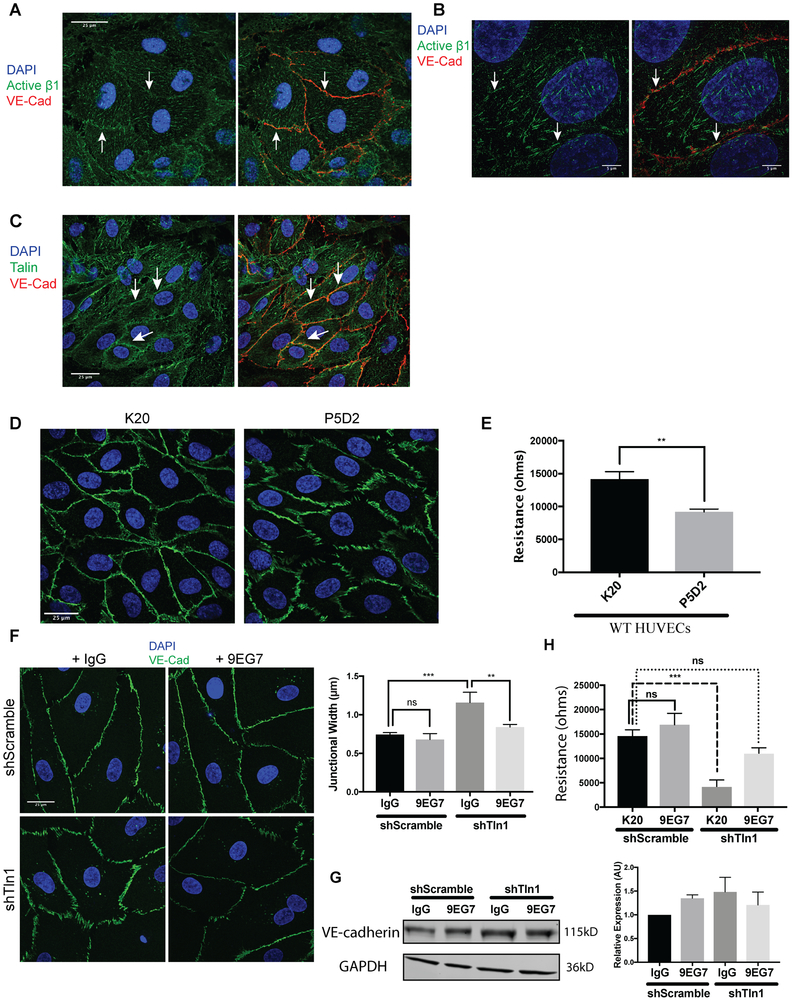
β1 integrin localizes to cell junctions and β1 activation is required for junctional stability.
The expression of β1-integrin in endothelial cells has been shown to promote VE-cadherin stability 18. Interestingly, a pool of β1 integrin has previously been reported to localize to EC junctions33. To investigate whether active β1 integrin contributes to AJ stability we first examined the localization of active β1 integrin by immunofluorescence with an activation-sensitive β1 integrin antibody in HUVECs. As expected, active β1 integrin localized to focal adhesions (Fig 6A). In addition, we observed a pool of active β1 integrin at VE-cadherin-containing cell-cell junctions (Fig 6B). We confirmed these findings using higher resolution 3D structure illuminated microscopy (3D-SIM) (Fig 6B). In addition, a pool of talin was localized at cell-cell junctions (Fig 6C). Treatment of HUVECs with the ligand blocking β1 integrin antibody P5D2 induced VE-cadherin disorganization and reduced endothelial barrier function compared to cells treated with the non-function altering β1 integrin antibody K20. (Fig 6D-E). To test whether impaired β1 integrin activation contributed to the altered VE-cadherin junction organization we observed in talin-deficient HUVECs, we treated shTln1 and shScramble HUVECs with either β1 integrin activating antibody (9EG7) or nonimmune IgG. 9EG7 treatment largely reversed the increased AJ width of talin-deficient HUVECs (Fig 6F). Treatment of shTln1 and shScramble HUVECs with 9EG7 did not alter total protein expression of VE-cadherin relative to control groups treated with rat isotype IgG (Fig 6G). Functionally, treatment of talin-deficient HUVECs with 9EG7, but not the non-function altering β1 integrin antibody K20, rescued EC barrier function up to 6 hours after treatment (Fig 6H). Together, these results indicate that talin-dependent activation of β1 integrin is required for maintaining VE-cadherin organization and EC barrier function.
Figure 6: Talin-dependent β1-integrin activation is required for endothelial barrier function.
A. Z-stack projections of immunofluorescence using an activation-sensitive β1 integrin antibody (9EG7) (green) and VE-Cadherin antibody (red) on HUVECs showing the appearance of a subpopulation of β1 integrin near AJs of confluent HUVECs. White arrows highlight cell-cell border regions enriched for β1 integrin. (n=3; scale=25 μm). B. Super resolution 3D structured illumination (3D-SIM) immunostaining of active β1 integrin and VE-cadherin in HUVECs highlight a subset of β1 integrin (white arrows) at VE-cadherin-positive junctions (n=3; scale=5 μm). C. Immunofluorescence on HUVECs with antibodies against talin (green) and VE-cadherin (red). A subset of talin localized to cell-cell contacts (white arrows) in addition to the expected pool of talin at focal adhesions (n=3; scale=25 μm). D. Treatment of HUVEC monolayers with a β1 integrin blocking antibody (P5D2) alters cell-cell junction organization relative to cells treated with a non-function altering β1 integrin antibody (K20). (n=3; scale=25 μm). E. HUVEC monolayers treated with a β1 integrin blocking antibody (P5D2) exhibit reduced barrier function as measured by electrical cell-substrate impedance sensing (4000 Hz) relative to HUVECs treated with a non-function altering β1 integrin antibody (K20). Measurements were made 3 hours after antibody incubation and remained stable for up to 6 hours post-treatment. (n=3; p=0.0019 unpaired t-test). F. Junctional width measured by VE-Cadherin immunofluorescence (green) is normalized by antibody-mediated β1 integrin activation (9EG7) in shTln1 HUVECs relative to talin-deficient HUVECs treated with a non-function altering antibody (K20). (n=3; scale=25 μm; *** p=0.0006, ** p=0.0036 ordinary one-way ANOVA with Sidak’s multiple comparisons test). G. VE-cadherin protein expression measured by western blot in shScramble and shTln1 HUVECs treated with 5 μg/mL Rat Isotype IgG or β1 integrin activating antibody 9EG7 for 12 hours. (n=2; ns; One-way anova with a Tukey multiple comparisons test) H. Electrical resistance of shScramble and shTln1 HUVECs treated with either the activating β1 integrin antibody 9EG7 or the non-function altering β1 antibody K20. (n=3; *** p=0.0002 one-way ANOVA with a Tukey multiple comparisons test).
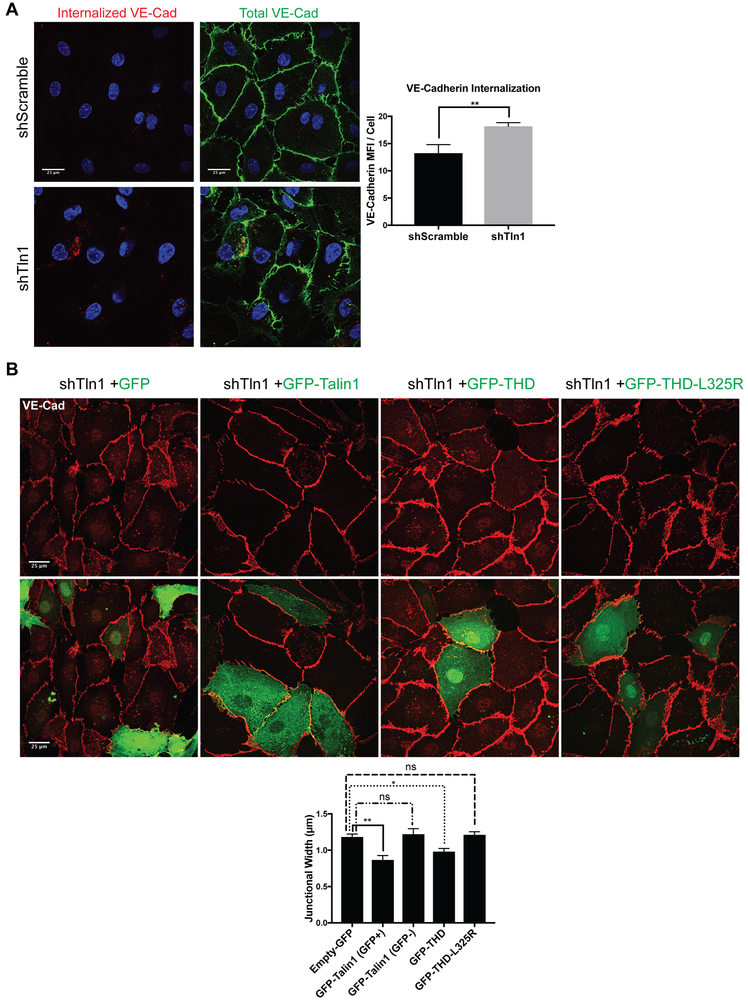
Talin-dependent β1 integrin activation stabilizes VE-cadherin.
To test whether changes in talin-dependent β1 integrin activation altered turnover of VE-cadherin, we performed an antibody internalization assay34, 35 to visualize VE-cadherin internalization in talin-deficient HUVECs. Whereas total pools of VE-cadherin appeared similar, shTln1 cells exhibited a 37% increase in internalized VE-cadherin (Fig 7A). The increased VE-cadherin width in talin-deficient ECs was abrogated by transfecting shTln1 cells with GFP-talin but not GFP (Fig 7B). Reconstitution of HUVECs with GFP-tagged talin head domain (GFP-THD) which activates integrins36 but lacks most actin binding sites appears to partially rescue junctional width while expression of GFP-THD-L325R, capable of binding to, but not activating integrins36 failed to normalize AJ organization compared to GFP-Tln1 (Fig 7B). These data reveal a critical role of talin-dependent integrin activation in regulating the junctional organization of VE-cadherin: when β1 integrin is activated, either by expression of talin or by treating talin-deficient cells with β1 integrin activating antibodies, cell-cell junctions are stabilized. Importantly, changes to junctional organization in response to stimulation of β1 integrin activation or blockade of β1 integrin correspond functionally with altered monolayer barrier function.
Figure 7: Talin-dependent integrin activation is indispensable for endothelial adherens junction organization.
A. Antibody internalization assay in which mouse VE-Cadherin (red) antibody was incubated with either shTln1 or shScramble HUVECs for 30min prior to fixation after which excess surface-bound antibody was removed by acid washing as described in Methods. After fixation/permeabilization, total levels of VE-Cadherin (green) were visualized using a rabbit VE-Cadherin antibody. (n=3; scale= 25 μm; **p=0.0077 two-tailed unpaired t-test). B. shTln1 HUVECs were transfected with either Empty-GFP, GFP-Talin1, GFP-THD or GFP-THD-L325R after which junctional width was assessed as described above and in Methods. GFP-Talin1 (GFP+) and GFP-Talin1 (GFP-) cells junctional widths were quantitated as an internal control for transfected groups. (n=3; scale=25 μm; *** p=0.0007, ** p=0.0099, ns=not significant, one-way ANOVA with a Tukey multiple comparisons test.
DISCUSSION
Here we tested the requirement for EC talin in the maintenance of established blood vessels. Deletion of EC talin in adult mice results in vascular leak and lethal intestinal hemorrhage 16-21 days after Tln1 deletion. EC-specific talin1 knockout mice exhibit altered cell-cell junction organization and intestinal EC detachment from adjacent ECs. Mechanistically, depletion of talin with Tln1 shRNA in HUVECs resulted in cell-cell junction remodeling, increased cytoskeletal contraction and increased junctional width. Loss of talin promoted the appearance of tensile Focal Adherens Junctions (FAJs) suggesting increased junctional tension. Activation of β1-integrin rescued junctional VE-cadherin organization while β1-integrin blockade in WT HUVECs phenocopied shRNA-mediated talin depletion suggesting a critical role for talin-dependent integrin activation in maintaining cell-cell junctions. Functionally, deletion of talin in HUVECs increased VE-cadherin internalization and reduced EC barrier electrical resistance. Defects in cell-cell junction organization and EC barrier function in talin-deficient ECs were rescued by treating cells with a β1 activating antibody. Furthermore, reconstitution of talin-deficient HUVECs with either full length talin1 or an integrin-activating talin1 head-domain normalized VE-cadherin organization. Collectively, these studies reveal an important role of talin-dependent β1-integrin activation in the maintenance of vascular barrier function.
Our observation that inducible genetic deletion of talin1 in ECs of adult mice causes defects predominantly in the intestinal microvasculature is striking. Similar phenotypes were observed utilizing two widely-used tamoxifen-inducible EC-specific Cre mice limiting the chances that the phenotype could be attributed to deletion of talin in non-ECs. While EC turnover appears to be similar across various established vascular beds 37, the concept of vascular heterogeneity and organ-specific EC function is well-supported38-40. For example, the unique structures and functions of the blood brain barrier (BBB)41, 42, blood retinal barrier (BRB)43, 44 and the gut vascular barrier (GVB)45 each play context-dependent roles in regulating paracellular permeability.2, 3, 46 The endothelium of the BBB lacks fenestrations and contains continuous intercellular tight/adherens junctions.41 In contrast, the endothelium of the outer BRB and of the GVB are characterized by fenestrated capillaries and increased permeability to components of the blood, immune cells and, in the case of the GVB, microbiota5, 45. Furthermore, compelling evidence suggests that fenestrated microvasculature and endocrine organ vasculature are preferentially dependent on VEGF-VEGFR2 signaling as pharmacological VEGF inhibition reduces fenestrations and vessel growth47, 48. The reasons for Tln1 EC-KO mice developing defects predominantly in the intestinal microvasculature is not clear. Integrin signaling has been shown to promote VEGFR2 function49-51. It is therefore plausible that VEGFR2 activity may be reduced in the endothelium of Tln1 EC-KO mice. If the fenestrated52 capillaries of the intestine are more dependent on VEGF signaling than other vascular beds, this could contribute to the observed dysfunction of the intestinal microvasculature of Tln1 EC-KO mice.
A key regulator of vascular permeability is the AJ molecule, VE-cadherin2, 7. As cell-matrix and cell-cell adhesions are linked through their independent interactions with the actin cytoskeleton53, 54, their downstream signaling pathways converge at a number of molecular hubs which act downstream of mechanosensory stimuli.55 Small GTPases, cytoskeletal adaptors and mechanosensitive proteins such as vinculin play critical roles in establishing and maintaining both cell-cell and cell-matrix adhesions. Our observation that talin-deficient HUVECs exhibit altered vinculin localization to cell-cell borders (Fig 5D) is reminiscent of recent work which showed thrombin-induced cell contraction promotes weakening of the endothelial barrier and the appearance of FAJs. 9 FAJs are demarcated by VE-Cad-vinculin co-localization at cell-cell contacts. As talin1 contains several vinculin-binding sites 56, 57 and the actin cytoskeleton is disorganized in talin-deficient ECs (Fig 5A), loss of EC talin may promote the association of vinculin with the AJ complex by promoting vinculin-α-catenin binding and thereby altering cellular tension. Interestingly, the altered organization of tight junction component ZO-1 would suggest that changes in cellular tension likely affect the junctional stability of other adhesive structures, an observation that warrants further future investigation (Online Figure V).
Together, our in vitro and in vivo results provide evidence for a link between inside-out integrin activation and the maintenance of the endothelial barrier in the intestinal microvasculature. Early studies established the importance of endothelial α5β1 integrin in barrier function as antibody-mediated blockade of α5β1, but not αvβ3 integrin, weakened barrier function in vitro 33. Subsequent studies have revealed the importance of β1 integrin during developmental angiogenesis as it dictates endothelial cell polarity17 and functions in the stabilization of established and maturing vessels 18. Together, these studies highlight the requirement of EC β1 integrin expression in the growth, maintenance and stability of blood vessels. Our results here build on these concepts by demonstrating that talin-dependent integrin activation controls the organization of cell-cell junctions in the intestinal microvasculature. Our in vitro results, utilizing both pharmacological and genetic approaches, indicate that it is the capacity of talin to activate integrins, not mechanically linking integrins to the actin cytoskeleton, that contributes to VE-cadherin organization. Importantly, this finding suggests the exciting possibility that pharmacological manipulation of integrin activation may represent a novel therapeutic strategy for modifying the endothelial barrier in the context of a variety of human diseases.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Integrins play an important role in endothelial cell (EC) function and talin is an important regulator of integrin affinity modulation.
VE-cadherin at EC-EC junctions is critical for EC barrier function.
β1 integrin expression is required for the establishment and stability of VE-cadherin at EC-EC junctions.
What New Information Does This Article Contribute?
Talin expression is required for EC β1 integrin activation in mice.
Loss of talin in ECs results in increased cell contractility and altered VE-cadherin organization.
β1 integrin activation plays an important role in VE-cadherin organization and EC barrier function.
A functional interaction between integrin-containing cell-matrix adhesions and VE-cadherin-containing adherens junctions has been described but the molecular mechanisms underlying this relationship is unclear. Indeed, the stability of VE-cadherin has recently been shown to depend on the expression of β1 integrin in ECs. In the current study, we tested the hypothesis that VE-cadherin organization and EC barrier function depends on integrin affinity modulation (activation) by deleting a key regulator of integrin activation, talin, in ECs in vitro and in vivo. Inducible-deletion of EC talin1 in adult mice caused increased intestinal vascular leak and death within 3 weeks of talin deletion. In vitro, talin-deficient ECs showed increased cell contraction associated with altered intercellular junctions and increased EC barrier function. VE-cadherin disorganization and reduced EC barrier function in talin-deficient ECs was dependent on reduced activation of β1 integrin. These results reveal a role for β1 integrin activation in VE-cadherin organization and promoting EC barrier function that may inform novel strategies to therapeutically modulate EC barrier function.
ACKNOWLEDGEMENTS
The authors thank the following investigators for generously providing mice used in these studies: David Critchley and Susan Monkley (Tln1fl/fl mice), Ralf Adams (Cdh5-CreERT2 mice), and Marcus Fruttiger (PDGFβ-CreERT2 mice). We are also grateful for support from Children's Healthcare of Atlanta and Emory University Pediatric Flow Cytometry Core and the Emory University Integrated Cellular Imaging Core.
SOURCES OF FUNDING
This work was supported in part by National Institutes of Health (NIH) National Heart, Lung, and Blood Institute grant HL117061 (B.G.P.), F31HL136194(F.E.P.) and AR050501 (A.P.K).
Nonstandard Abbreviations and Acronyms:
- EC
endothelial cell
- AJ
adherens junction
- FAJ
focal adhesion junction
- ECIS
electrical cell-substrate impedance sensing
- EBD
Evan’s blue dye
- HUVEC
human umbilical vein endothelial cell
- HDMVEC
human dermal blood microvascular endothelial cell
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA and Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A. 1999;96:9815–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dejana E and Vestweber D. The role of VE-cadherin in vascular morphogenesis and permeability control. Prog Mol Biol Transl Sci. 2013;116:119–44. [DOI] [PubMed] [Google Scholar]
- 3.Lampugnani MG, Dejana E and Giampietro C. Vascular Endothelial (VE)-Cadherin, Endothelial Adherens Junctions, and Vascular Disease. Cold Spring Harb Perspect Biol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komarova YA, Kruse K, Mehta D and Malik AB. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ Res. 2017;120:179–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spadoni I, Fornasa G and Rescigno M. Organ-specific protection mediated by cooperation between vascular and epithelial barriers. Nat Rev Immunol. 2017;17:761–773. [DOI] [PubMed] [Google Scholar]
- 6.Shechter R, London A and Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. 2013;13:206–18. [DOI] [PubMed] [Google Scholar]
- 7.Giannotta M, Trani M and Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Developmental Cell. 2013;26:441–54. [DOI] [PubMed] [Google Scholar]
- 8.Schulte D, Kuppers V, Dartsch N, Broermann A, Li H, Zarbock A, Kamenyeva O, Kiefer F, Khandoga A, Massberg S and Vestweber D. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 2011;30:4157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huveneers S, Oldenburg J, Spanjaard E, van der Krogt G, Grigoriev I, Akhmanova A, Rehmann H and de Rooij J. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J Cell Biol. 2012;196:641–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dorland YL, Malinova TS, van Stalborch AM, Grieve AG, van Geemen D, Jansen NS, de Kreuk BJ, Nawaz K, Kole J, Geerts D, Musters RJ, de Rooij J, Hordijk PL and Huveneers S. The F-BAR protein pacsin2 inhibits asymmetric VE-cadherin internalization from tensile adherens junctions. Nat Commun. 2016;7:12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morse EM, Brahme NN and Calderwood DA. Integrin cytoplasmic tail interactions. Biochemistry. 2014;53:810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mui KL, Chen CS and Assoian RK. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J Cell Sci. 2016;129:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hynes RO, Lively JC, McCarty JH, Taverna D, Francis SE, Hodivala-Dilke K and Xiao Q. The diverse roles of integrins and their ligands in angiogenesis. Cold Spring Harbor symposia on quantitative biology. 2002;67:143–53. [DOI] [PubMed] [Google Scholar]
- 14.Stupack DG and Cheresh DA. ECM remodeling regulates angiogenesis: endothelial integrins look for new ligands. Sci STKE. 2002;2002:pe7. [DOI] [PubMed] [Google Scholar]
- 15.van der Flier A, Badu-Nkansah K, Whittaker CA, Crowley D, Bronson RT, Lacy-Hulbert A and Hynes RO. Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development. 2010;137:2439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei L, Liu D, Huang Y, Jovin I, Shai SY, Kyriakides T, Ross RS and Giordano FJ. Endothelial expression of beta1 integrin is required for embryonic vascular patterning and postnatal vascular remodeling. Molecular and Cellular Biology. 2008;28:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, Fassler R, Mellman I, Lane TF and Iruela-Arispe ML. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Developmental Cell. 2010;18:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto H, Ehling M, Kato K, Kanai K, van Lessen M, Frye M, Zeuschner D, Nakayama M, Vestweber D and Adams RH. Integrin beta1 controls VE-cadherin localization and blood vessel stability. Nat Commun. 2015;6:6429. [DOI] [PubMed] [Google Scholar]
- 19.Calderwood DA, Zent R, Grant R, Rees DJ, Hynes RO and Ginsberg MH. The Talin head domain binds to integrin beta subunit cytoplasmic tails and regulates integrin activation. J Biol Chem. 1999;274:28071–4. [DOI] [PubMed] [Google Scholar]
- 20.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH and Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–6. [DOI] [PubMed] [Google Scholar]
- 21.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH and Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–82. [DOI] [PubMed] [Google Scholar]
- 22.Ye F, Kim C and Ginsberg MH. Molecular mechanism of inside-out integrin regulation. J Thromb Haemost. 2011;9 Suppl 1:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefanini L, Ye F, Snider AK, Sarabakhsh K, Piatt R, Paul DS, Bergmeier W and Petrich BG. A talin mutant that impairs talin-integrin binding in platelets decelerates alphaIIbbeta3 activation without pathological bleeding. Blood. 2014;123:2722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klann JE, Remedios KA, Kim SH, Metz PJ, Lopez J, Mack LA, Zheng Y, Ginsberg MH, Petrich BG and Chang JT. Talin Plays a Critical Role in the Maintenance of the Regulatory T Cell Pool. J Immunol. 2017;198:4639–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yago T, Petrich BG, Zhang N, Liu Z, Shao B, Ginsberg MH and McEver RP. Blocking neutrophil integrin activation prevents ischemia-reperfusion injury. J Exp Med. 2015;212:1267–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrich BG, Marchese P, Ruggeri ZM, Spiess S, Weichert RA, Ye F, Tiedt R, Skoda RC, Monkley SJ, Critchley DR and Ginsberg MH. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204:3103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nieswandt B, Moser M, Pleines I, Varga-Szabo D, Monkley S, Critchley D and Fassler R. Loss of talin1 in platelets abrogates integrin activation, platelet aggregation, and thrombus formation in vitro and in vivo. J Exp Med. 2007;204:3113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monkley SJ, Kostourou V, Spence L, Petrich B, Coleman S, Ginsberg MH, Pritchard CA and Critchley DR. Endothelial cell talin1 is essential for embryonic angiogenesis. Developmental Biology. 2011;349:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitulescu ME, Schmidt I, Benedito R and Adams RH. Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nature Protocols. 2010;5:1518–34. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Nakayama M, Pitulescu ME, Schmidt TS, Bochenek ML, Sakakibara A, Adams S, Davy A, Deutsch U, Luthi U, Barberis A, Benjamin LE, Makinen T, Nobes CD and Adams RH. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483–6. [DOI] [PubMed] [Google Scholar]
- 31.Lakshmikanthan S, Sobczak M, Chun C, Henschel A, Dargatz J, Ramchandran R and Chrzanowska-Wodnicka M. Rap1 promotes VEGFR2 activation and angiogenesis by a mechanism involving integrin alphavbeta(3). Blood. 2011;118:2015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Claxton S, Kostourou V, Jadeja S, Chambon P, Hodivala-Dilke K and Fruttiger M. Efficient, inducible Cre-recombinase activation in vascular endothelium. genesis. 2008;46:74–80. [DOI] [PubMed] [Google Scholar]
- 33.Lampugnani MG, Resnati M, Dejana E and Marchisio PC. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991;112:479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, Vincent PA and Kowalczyk AP. p120-catenin binding masks an endocytic signal conserved in classical cadherins. J Cell Biol. 2012;199:365–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiasson CM, Wittich KB, Vincent PA, Faundez V and Kowalczyk AP. p120-catenin inhibits VE-cadherin internalization through a Rho-independent mechanism. Mol Biol Cell. 2009;20:1970–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kopp PM, Bate N, Hansen TM, Brindle NP, Praekelt U, Debrand E, Coleman S, Mazzeo D, Goult BT, Gingras AR, Pritchard CA, Critchley DR and Monkley SJ. Studies on the morphology and spreading of human endothelial cells define key inter- and intramolecular interactions for talin1. European Journal of Cell Biology. 2010;89:661–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tannock IF and Hayashi S. The proliferation of capillary endothelial cells. Cancer Res. 1972;32:77–82. [PubMed] [Google Scholar]
- 38.Aird WC. Vascular bed-specific hemostasis: role of endothelium in sepsis pathogenesis. Crit Care Med. 2001;29:S28–34; discussion S34-5. [DOI] [PubMed] [Google Scholar]
- 39.Aird WC. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–43. [PubMed] [Google Scholar]
- 40.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. 2012;2:a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obermeier B, Daneman R and Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reese TS and Karnovsky MJ. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaur C, Foulds WS and Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008;27:622–47. [DOI] [PubMed] [Google Scholar]
- 44.Kim JH, Kim JH, Yu YS, Kim DH and Kim KW. Recruitment of pericytes and astrocytes is closely related to the formation of tight junction in developing retinal vessels. J Neurosci Res. 2009;87:653–9. [DOI] [PubMed] [Google Scholar]
- 45.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, Penna G, Dejana E and Rescigno M. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830–4. [DOI] [PubMed] [Google Scholar]
- 46.Giannotta M, Trani M and Dejana E. VE-cadherin and endothelial adherens junctions: active guardians of vascular integrity. Dev Cell. 2013;26:441–54. [DOI] [PubMed] [Google Scholar]
- 47.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O’Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ and McDonald DM. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol. 2006;290:H560–76. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y, Zhang Y, Cao Z, Ji H, Yang X, Iwamoto H, Wahlberg E, Lanne T, Sun B and Cao Y. Anti-VEGF- and anti-VEGF receptor-induced vascular alteration in mouse healthy tissues. Proc Natl Acad Sci U S A. 2013;110:12018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahabeleshwar GH and Byzova TV. Vascular integrin signaling. Methods Enzymol. 2008;443:199–226. [DOI] [PubMed] [Google Scholar]
- 50.Mahabeleshwar GH, Feng W, Phillips DR and Byzova TV. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203:2495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Plow EF, Meller J and Byzova TV. Integrin function in vascular biology: a view from 2013. Curr Opin Hematol. 2014;21:241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stan RV, Tse D, Deharvengt SJ, Smits NC, Xu Y, Luciano MR, McGarry CL, Buitendijk M, Nemani KV, Elgueta R, Kobayashi T, Shipman SL, Moodie KL, Daghlian CP, Ernst PA, Lee HK, Suriawinata AA, Schned AR, Longnecker DS, Fiering SN, Noelle RJ, Gimi B, Shworak NW and Carriere C. The diaphragms of fenestrated endothelia: gatekeepers of vascular permeability and blood composition. Dev Cell. 2012;23:1203–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parsons JT, Horwitz AR and Schwartz MA. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat Rev Mol Cell Biol. 2010;11:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bershadsky AD, Balaban NQ and Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–95. [DOI] [PubMed] [Google Scholar]
- 55.Mui KL, Chen CS and Assoian RK. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. Journal of Cell Science. 2016;129:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Calderwood DA, Campbell ID and Critchley DR. Talins and kindlins: partners in integrin-mediated adhesion. Nat Rev Mol Cell Biol. 2013;14:503–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goult BT, Zacharchenko T, Bate N, Tsang R, Hey F, Gingras AR, Elliott PR, Roberts GC, Ballestrem C, Critchley DR and Barsukov IL. RIAM and vinculin binding to talin are mutually exclusive and regulate adhesion assembly and turnover. J Biol Chem. 2013;288:8238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.