Abstract
Keratinocytes undergo significant structural remodeling during epidermal differentiation, including a broad transformation of the proteome coupled with a reduction in total cellular biomass. This suggests that intracellular digestion of proteins and organelles is necessary for keratinocyte differentiation. Here, we use both genetic and pharmacologic approaches to demonstrate that autophagy and lysosomal functions are required for keratinocyte differentiation in organotypic human skin. Lysosomal activity was required for mTOR signaling and mitochondrial oxidative metabolism. In turn, mitochondrial reactive oxygen species (mtROS), produced as a natural byproduct of oxidative phosphorylation, were necessary for keratinocyte differentiation. Finally, treatment with exogenous ROS rescued the differentiation defect in lysosome-inhibited keratinocytes. These findings highlight a reciprocal relationship between lysosomes and mitochondria, in which lysosomes support mitochondrial metabolism and the associated production of mtROS. The mtROS released to the cytoplasm in suprabasal keratinocytes triggers autophagy and lysosome-mediated degradation necessary for epidermal differentiation. As defective lysosome dependent autophagy is associated with common skin diseases including psoriasis and atopic dermatitis, a better understanding of the role of lysosomes in epidermal homeostasis may guide future therapeutic strategies.
INTRODUCTION
Epidermal differentiation is a highly orchestrated process that involves activation of key transcription factors, the expression of hallmark proteins, and assembly of structural complexes within the suprabasal layers of the epidermis (Bao et al., 2017, Eckhart et al., 2013, Fuchs, 2008, Mistry et al., 2012, Sen et al., 2012, Sen et al., 2010, Simpson et al., 2011). The processes by which differentiating keratinocytes eliminate intracellular material and clear organelles are less well understood. Cells organize bulk intracellular digestion via autophagy, in which material is sequestered in autophagic vesicles and degraded by fusion with lysosomes (Luzio et al., 2007). During differentiation, keratinocytes eliminate their nuclei and other organelles, suggesting that autophagy and lysosome-mediated degradation are involved in the maintenance of epidermal architecture. Consistent with this, previous microscopy-based investigations have described the accumulation of lysosomal bodies in progressive layers of keratinizing epithelium and visualized lysosome-sequestered organelles (Eckhart et al., 2013, Lavker and Matoltsy, 1970, Morioka et al., 1999). However, the role of specific autophagy related genes and related signaling pathways in epidermal homeostasis is unclear, as the few skin-specific deletions of core autophagy genes have yielded conflicting phenotypes in mice. Keratinocyte-specific deletion of autophagy related genes, Atg5 and Atg7, was not associated with significant histological abnormalities, which would imply that autophagy is dispensable for mouse epidermal homeostasis (Rossiter et al., 2013, Sukseree et al., 2013). However, subsequent investigation described a marked differentiation defect in Atg7−/− mouse skin allografts in which tissues were acanthotic and hyperkeratotic, with reduced granulation, and downregulated expression of differentiation proteins including loricrin, filaggrin, and involucrin (Yoshihara et al., 2015). These inconsistencies, and the suggestion of functional redundancy between proteins that coordinate formation of autophagic vesicles (Rebecca and Amaravadi, 2016), have left the role of autophagy in epidermal maintenance unclear, and functional studies in human skin are especially lacking. We used complementary genetic and pharmacologic approaches to inhibit autophagy or lysosomal function directly in engineered organotypic (OTC) human skin, and discovered that reciprocal signaling between lysosomes and mitochondria is necessary for human epidermal differentiation.
RESULTS
Lysosomal activity is required for epidermal differentiation.
We first evaluated the effects of lysosome inhibition in cultured primary human keratinocytes. Proliferating keratinocytes continually degrade intracellular material to maintain normal cellular components and cellular health. When lysosomal activity was inhibited with Lys05, a potent chloroquine-derivative that accumulates within, and deacidifies lysosomes (Supplementary Figure S1a) (Amaravadi and Winkler, 2012, Ma et al., 2014, McAfee et al., 2012, Pellegrini et al., 2014), lysosome-mediated degradation was inhibited, resulting in accumulation of proteins associated with autophagic vesicles, including p62, and lipidated forms of LC3A and LC3B (Figure 1a). Despite this degradative block, Lys05 was nontoxic, and keratinocytes continued to proliferate, albeit at a slightly decreased rate (Figure 1b). Lys05 inhibited lysosomes specifically, as the compound did not interfere with proteasome-mediated protein degradation (Supplementary Figure S1b).
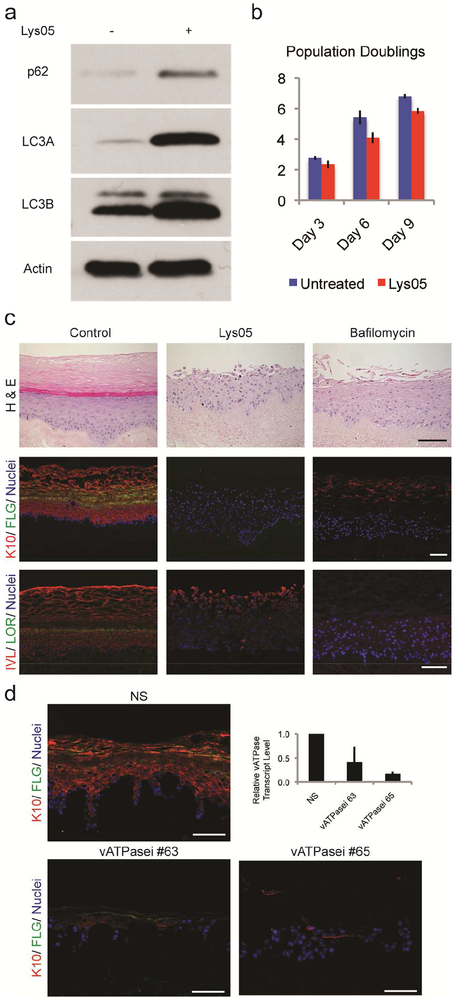
Figure 1. Lysosomes are necessary for epidermal differentiation.
(a) 2 μM Lys05, for 48-hours, blocked lysosome-mediated degradation of autophagosomes, resulting in the retention of p62, LC3AII, and LC3BII. (b) Over the 9-day time course of this experiment Lys05-treated keratinocytes proliferated at nearly the same rate as control keratinocytes (Log2 of means ± s.d.). (c) Control versus Lys05 or bafilomycin-A1 treated organotypic cultures. Lysosomal inhibition results in undifferentiated tissue lacking normal epidermal architecture (H+E), including the expression of keratin 10 (red), filaggrin (green), involucrin (red), and loricrin (green), nuclei (blue). (d) Nonsilencing control (NS) and vATPase shRNA knockdown (vATPasei) organotypic cultures highlighting keratin-10 (red), filaggrin (green), and nuclei (blue). vATPase gene knockdown was confirmed by qPCR. All scale bars = 100μM.
To determine whether lysosomal function is required for epidermal differentiation, we used Lys05 to inhibit lysosomes in three-dimensional organotypic human skin. In control tissues, keratinocytes stratified and differentiated normally as evidenced by the coordinated expression of early differentiation markers keratin-10 and involucrin, and late differentiation markers filaggrin and loricrin (Figure 1c). In contrast, Lys05 completely blocked epidermal differentiation. This undifferentiated phenotype was also observed in tissues exposed to bafilomycin-A1, a drug structurally unrelated to Lys05 that inhibits lysosomal functions by directly inhibiting the vacuolar-type H+-ATPase responsible for lysosome acidification (Zhang et al., 1994) (Figure 1c, Supplementary Figure S2a). While Lys05 and bafilomycin-A1 OTCs failed to express differentiation proteins in suprabasal layers, normal expression of keratin-5 was maintained. Lys05 did not inhibit keratinocyte proliferation, which remain appropriately restricted to the basal layer (Supplementary Figure S2b, c).
To validate the findings obtained using the pharmacologic lysosome inhibitors, we next inhibited lysosome function by genetically depleting the V0C-subunit of the Vacuolar-type H+-ATPase (the bafilomycin-A1 target) using two different shRNAs. The vATPasei tissues largely recapitulated the phenotype seen with pharmacologic lysosome inhibition. Tissues were poorly organized, with severely diminished expression of terminal differentiation proteins (Figure 1d). Epidermal tissues with these severe differentiation defects are not capable of forming tissue in vivo as xenografts. Together, these pharmacologic and genetic data indicate that functional lysosomes are critical for both early and late epidermal differentiation.
Autophagy promotes the lysosome-mediated degradation essential for epidermal differentiation.
To specifically investigate the role of autophagy in keratinocyte differentiation, we next examined the effects of two small molecule inhibitors of early autophagy, SBI-0206965 (SBI) and Spautin-1, in organotypic skin. Through distinct mechanisms, both drugs inhibit the assembly of the autophagosome. SBI blocks the kinase activity of ULK1, diminishing the phospho-regulated capacity of beclin-1 to engage with its binding partners (Egan et al., 2015) and Spautin-1 inhibits the USP10 and USP13 hydrolases that remove ubiquitin from beclin-1 (Liu et al., 2011). When autophagy was blocked by either SBI or Spautin-1, OTCs were undifferentiated, parakeratotic, and lacked keratin-10 and filaggrin expression (Figure 2a, Supplementary Figure S3).
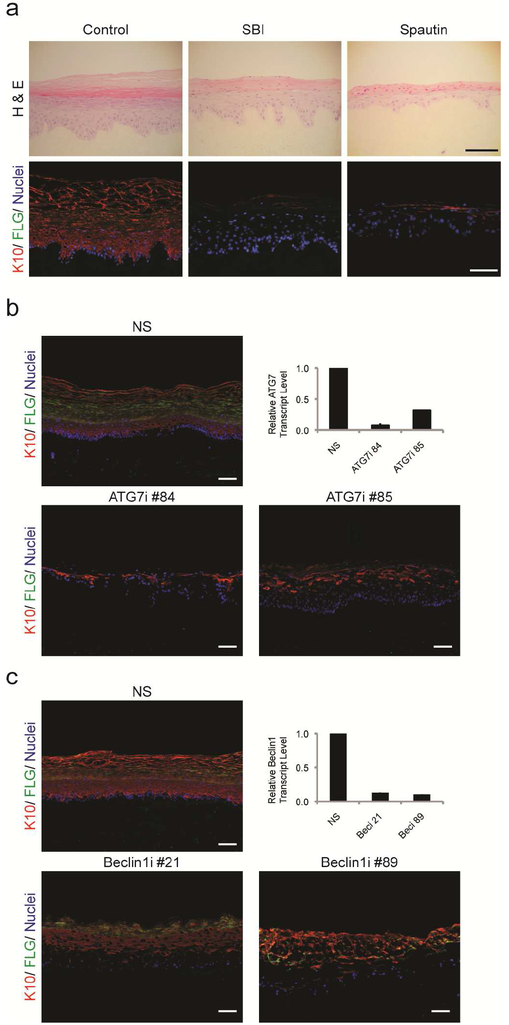
Figure 2: Epidermal differentiation requires autophagy.
(a) Control, SBI, and Spautin-1 organotypic cultures examined by H & E and immunofluorescence. Inhibition of autophagy blocked epidermal differentiation, including the expression of Keratin 10 (red) and filaggrin (green). Nuclei are (blue). (b) ATG7 depletion inhibited differentiation as demonstrated by loss of keratin-10 (red) and filaggrin (green). ATG7 knockdown was confirmed by qPCR. (c) Cryosections of NS and beclin-1i (shRNA knockdown) organotypic cultures highlighting keratin-10 (red), filaggrin (green), and nuclei (blue). Knockdowns of beclin-1 were confirmed by qPCR. All scale bars = 100μM.
To validate the phenotypes we observed with SBI and Spautin-1, we genetically targeted two key autophagy proteins ATG7 and beclin-1, using shRNAs. Knockdown of ATG7 severely diminished the expressions of keratin-1 and filaggrin (Figure 2b) and corroborated earlier work implicating autophagy in epidermal differentiation (Yoshihara et al., 2015). Similarly, knockdown of beclin-1 markedly delayed differentiation in organotypic epidermis (Figure 2c). Therefore, blocking the early autophagic events in the degradation pathway largely recapitulated the epidermal phenotype observed with direct lysosome inhibition.
Lysosomes support the mTOR-signaling important for protein translation and oxidative mitochondrial metabolism.
In addition to their role in intracellular degradation, lysosomes also support mechanistic Target of Rapamycin (mTOR) complex 1, a cell-signaling hub seated at lysosomal membranes that contributes to the metabolic balance between anabolism and catabolism (Laplante and Sabatini, 2012). Lysosomal acidification influences the localization and activity of mTORC1 (Hu et al., 2016, Zoncu et al., 2011) and a skin-specific deletion of MTOR results in epidermal barrier defects, indicating the importance of mTOR signaling in skin morphogenesis (Ding et al., 2016). The mTOR complex1 phosphorylates S6K1, which stimulates translation through activation of S6 Ribosomal Protein. In normal human OTC skin, phospho-S6 was localized throughout the suprabasal layers. In contrast, phospho-S6 was markedly decreased in both the undifferentiated Lys05 and bafilomycin-A1 treated tissues, indicative of defective mTOR-pS6K-pS6 signaling (Figure 3a). The differential activation of S6 ribosomal protein throughout epidermal layers suggests that it is involved in translating proteins upregulated during differentiation, and its relative inactivation with Lys05 or Bafilomycin treatment indicates this signaling is dependent upon lysosomal function.
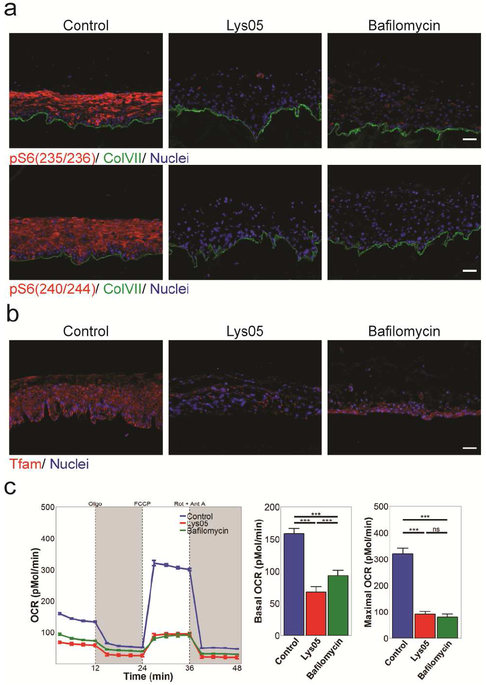
Figure 3: Lysosomes support the mTOR-signaling that promotes protein translation and mitochondrial metabolism.
(a-b) Lysosomal inhibition lead to loss of both pS6 and TFAM. (a) pS6 (red), ColVII (green), and nuclei (blue) or (b) for TFAM (red) and nuclei (blue). All scale bars = 100μM. (c) Seahorse analysis shows reduced baseline and maximal oxygen consumption in keratinocytes treated with Lys05 or bafilomycin-A1.
The mTOR complex1 also phosphorylates eukaryotic translation initiation factor 4E (eIF4E)-binding proteins (4E-BPs), enabling translation of TFAM, an important mitochondrial regulator required to transcribe the mitochondrial genes needed for oxidative phosphorylation (Kasashima et al., 2011, Morita et al., 2013, Morita et al., 2015). Consistent with this, TFAM protein was diminished in Lys05 and bafilomycin-A1 OTCs (Figure 3b). We measured oxygen consumption rates to verify that TFAM depletion was associated with compromised mitochondrial oxidative metabolism. Both Lys05 and bafilomycin-A1 markedly decreased baseline and maximal oxygen consumption in keratinocytes (Figure 3c). Taken together these data indicate that functional lysosomes support mTOR signaling that promotes both translation and mitochondrial oxidative metabolism in keratinocytes.
Lysosome to mitochondria signaling is required for production of the reactive oxygen species necessary for keratinocyte differentiation.
Differentiating keratinocytes experience an elevated level of cytoplasmic calcium, which triggers opening of the mitochondrial permeability transition pore, and compromises the outer mitochondrial membrane allowing mtROS and cytochrome-c to leak into cytoplasm (Carafoli, 2010, Contreras et al., 2010, Santo-Domingo and Demaurex, 2010, Tamiji et al., 2005). Since lysosomal inhibition in keratinocytes lead to diminished mitochondrial function, we hypothesized that the differentiation block in Lys05 and bafilomycin-A1 OTCs was related to mtROS deficiency, despite the presence of high levels of extracellular calcium.
To determine if leaked mtROS are necessary for human epidermal differentiation, we exposed OTCs to the ROS scavenger N-acetylcysteine (NAC). NAC treated tissues were completely undifferentiated, and lacked expression of both keratin-10 and filaggrin (Figure 4a, Supplementary Figure S4b). They were also parakeratotic, retaining nuclei through the stratum corneum. Other antioxidants (EUK134 and TEMPOL) similarly inhibited calcium-induced keratinocyte differentiation (Supplementary Figure S4a) (Hamanaka et al., 2013). A similar phenotype was observed when we targeted mitochondrial metabolism directly with dideoxycytidine (DDC) (Figure 4a, Supplementary Figure S4b). DDC interferes with mtDNA replication and inhibits oxidative phosphorylation and associated mtROS (Supplementary Figure S4c, d) (Piechota et al., 2006). This indicates that ROS, and specifically mtROS, are required for keratinocyte differentiation. To confirm that Lys05 and bafilomycin-A1 OTCs lacked ROS-stimulated signaling, we examined NRF2 expression. NRF2 regulates the expression of antioxidant proteins, and accumulates in cells experiencing oxidative stress (Jaiswal, 2004). NRF2 was abundant throughout control OTCs, but was markedly diminished in tissues with inhibited lysosomes (Figure 4b). Together, these data indicate that the reduced mitochondrial metabolism, resultant from lysosomal inhibition, was insufficient to produce the normal mtROS required for epidermal differentiation.
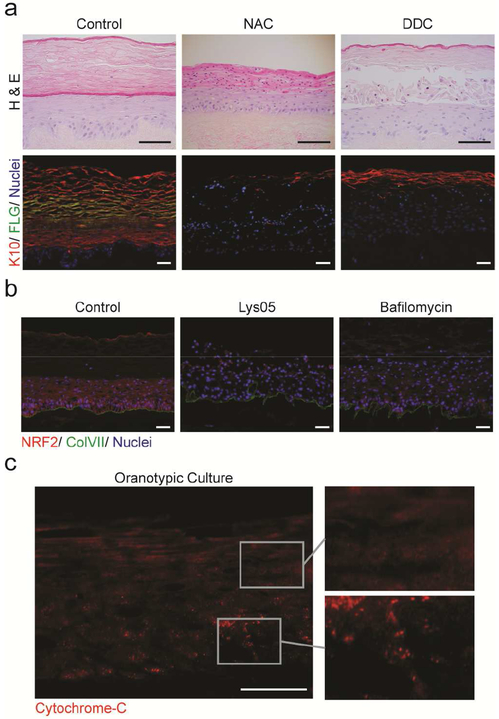
Figure 4: Reactive Oxygen Species are critical for epidermal architecture.
(a) The ROS-scavenger N-acetylcysteine (NAC) or mitochondrial inhibitor dideoxycytidine (DDC) inhibit epidermal differentiation indicated by keratin-10 (red), filaggrin (green) and nuclei (blue). (b) Lysosomal inhibition diminished ROS, as demonstrated by the expression of NRF2 (red), with ColVII (green), and nuclei (blue). (c) Diffuse localization of cytochrome-c (red) in suprabasal layers of OTC reflects mitochondrial depolarization. All scale bars = 100μM.
To determine whether the pattern of mitochondrial leak paralleled the calcium/differentiation gradient in epidermis (Menon et al., 1985), we visualized cytochrome-c, a protein involved in oxidative phosphorylation in mitochondria and in the initiation of cell death programs when released to the cytosol. In basal and early spinous layers of organotypic skin, cytochrome-c was localized as distinct puncta, indicating that it was contained within intact mitochondrial bodies. In contrast, the pattern became progressively cytoplasmic and diffuse in upper spinous and granular layers (Figure 4c) indicative of mitochondrial leak. A similar pattern was detected in native human skin (Supplementary Figure S4e). These data support previous studies proposing that mitochondrial leak is a normal event in the terminal differentiation of keratinocytes (Harada et al., 1998, Hornig-Do et al., 2007, Tamiji et al., 2005). Mitochondrial depolarization and cytoplasmic release of cytochrome-c did not induce canonical apoptosis in this context. Though differentiation and apoptosis have overlapping features, the cornification of epidermal keratinocytes is a specialized programmed cell death distinct from apoptosis (Takahashi et al., 2000) (Supplementary Figure S4f, g).
Exogenous ROS restores differentiation in epidermis with inhibited lysosomes.
Though Lys05 and bafilomycin-A1 treated OTCs were exposed to elevated levels of extracellular calcium, the calcium signal was insufficient to drive differentiation. We then questioned whether exogenous ROS would rescue differentiation in OTCs lacking mtROS as a consequence of inhibited lysosomes. Reintroduction of ROS, in the form of a 0.005% NaOCl in the media (Leung et al., 2013), largely restored differentiation and normal epidermal architecture to both Lys05 and bafilomycin-A1 treated OTCs (Figure 5a, and Supplementary Figure S5a, b). As expected, exogenous ROS did not fully restore the ribosomal protein S6 signaling (Supplementary Figure S5c), consistent with an epidermal differentiation model in which mtROS is downstream of mTOR activity regulated by lysosomes. Importantly, NaOCl did not inhibit the efficacy of the lysosome inhibiting compounds Lys05 and bafilomycin-A1 (Supplementary Figure S5d), nor adversely affect otherwise untreated OTCs (Figure 5a). Neither lysosome inhibition nor exogenous ROS affected localization or calcium-dependent phosphorylation of p-PKC∂1.
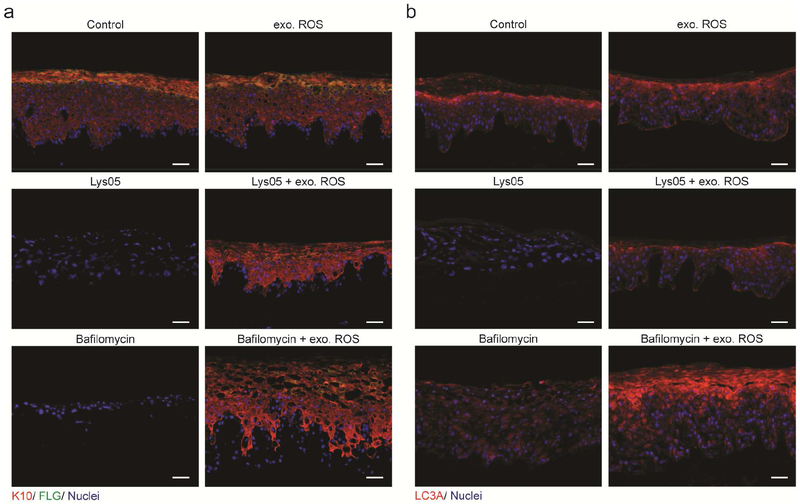
Figure 5: Exogenous ROS restores differentiation in epidermis with inhibited lysosomes.
(a) Exogenous ROS restored differentiation to Lys05 or bafilomycin-A1 OTCs as demonstrated by immunofluorescent probing for keratin-10 (red) and filaggrin (green), with nuclei (blue). (b) Exogenous ROS rescued LC3A (red) localization in Lys05 and bafilomycin-A1 OTCs. All scale bars = 100μM.
In contrast to antioxidants that suppress autophagy, oxidative agents activate autophagy as a cytoprotective measure to sequester damaged cellular components (Filomeni et al., 2015, Harr and Distelhorst, 2010). We tested the hypothesis that exogenous ROS restored differentiation by reengaging autophagy by visualizing the autophagy marker LC3A in OTC skin. In control OTCs, punctate LC3A signal indicated that autophagic vesicles accumulated in a gradient pattern concentrating in upper epidermal layers. In contrast, drug-induced lysosomal inhibition disrupted autophagy. Lys05 resulted in complete loss of LC3A, whereas bafilomycin-A1 treatment resulted in retention of diffuse LC3A signal throughout organotypic skin. In both settings, exogenous ROS restored the normal distribution of LC3A (Figure 5b) suggesting that autophagosomes were competent to form in response to exogenous ROS, even when inhibited lysosomes were incapable of degrading associated autophagic cargo. In contrast, exogenous ROS were not sufficient to rescue the differentiation defect induced by the autophagy-blocking drug Spautin-1 (Supplementary Figure S5f). This demonstrates that the activation of autophagy, and not ROS itself, is required for expression and proper assembly of keratinocyte differentiation proteins.
DISCUSSION
Epidermal architecture is largely regulated by calcium, with extracellular calcium acting as the key element required for intercellular adhesion, and also to trigger release of internal calcium stores that induce signaling and transcription events required for keratinocyte differentiation (Bikle et al., 2012, Tu and Bikle, 2013). In normal skin, calcium increases in concentration in the upper differentiating layers (Menon et al., 1985). Keratinocyte differentiation is also associated with detachment from the basement membrane (Tennenbaum et al., 1996). However, lysosome-inhibited keratinocytes resist differentiation despite elevated extracellular calcium and basement membrane detachment. This indicates that calcium is necessary, but not sufficient, for normal epidermal differentiation.
Here, we establish that reciprocal signaling between lysosomes and mitochondria is required for the maintenance of the basal layer, and for normal epidermal differentiation (Supplementary Figure S6). We propose a keratinocyte differentiation cascade model in which extracellular calcium induces internal stores of calcium to spill into cytoplasm, triggering release of mtROS, that then induces autophagy to promote cell death by cornification (Gosselin et al., 2009). Since basal keratinocytes do not begin to spill mtROS until cells detach from the basement membrane, we propose that mtROS-induced autophagy may be required for the translation of calcium-induced transcripts. Our finding that ROS rescued the lysosome-inhibited differentiation defect, and associated loss of mtROS, is consistent with work from others showing that exogenous ROS restored differentiation capability in keratinocytes lacking components necessary for maintenance of the mitochondrial electron transport chain (Bhaduri et al., 2015, Hamanaka et al., 2013). When we restored differentiation in lysosome-inhibited tissues by exogenous ROS, the basal layer remained appropriately undifferentiated. This precludes the notion that the basement membrane microenvironment protects the basal cells from differentiation solely by blocking mtROS spill, and suggests that expression of endogenous antioxidants helps prevent premature differentiation of the basal layer. These studies demonstrate that epidermal tissues spatially regulate calcium and ROS signaling to maintain the homeostatic balance between keratinocyte proliferation and differentiation.
The discovery that lysosomes regulate the signaling and degradation necessary for normal epidermal differentiation may be relevant to common skin disorders associated with differentiation defects. Our work, and that of others (Akinduro et al., 2016), demonstrated that disrupted autophagy compromises nucleophagy in differentiating keratinocytes, linking defects in autophagy to the parakeratosis in psoriatic skin. Consistent with this, polymorphisms in autophagy related genes are associated with psoriasis (Douroudis et al., 2012). The main component of the protective barrier is ceramide, a lipid assembled from precursors by acid sphingomyelinase, an enzyme that is only active within lysosomes. Lysosomal dysfunction that interferes with the normal biosynthesis or trafficking of lipids may contribute to the pathology of atopic dermatitis (Elias and Wakefield, 2014, Raymond et al., 2008), which is characterized by disrupted epidermal architecture leading to barrier function defects. Our work advances the understanding of human epidermal differentiation and demonstrates that lysosomal pathways may be effective targets for therapeutic intervention in dermatological conditions where differentiation and barrier function are compromised.
MATERIALS AND METHODS
Cell culture
All experiments were conducted using primary keratinocytes. Cells were isolated from normal human skin by previously described methods (Lazarov et al., 2002, Ridky et al., 2010). Primary cells were obtained through the SBDRC core from deidentified discarded material though an IRB approved protocol. Keratinocytes were cultured in a 1:1 mixture of Gibco Keratinocytes-SFM medium + L-glutamine + EGF + BPE and Gibco Cascade Biologics 154 medium with 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA). Transduced keratinocytes were fully puromycin selected before the commencement of each experiment. Lys05 (Gift from R. Amavaradi) was used at 2 μM, Bafilomycin-A1 (Sigma, St. Louis, MO) was at 10 nM. SBI-0206965 and Spautin-1 (Sigma, St. Louis, MO) were used at 2 μM, DDC (Abcam, Cambridge, MA) was used at 20 μM, N-acetylcysteine (Sigma, St. Louis, MO) was used at 10mM, EUK134 (Sigma, St. Louis, MO) was used 50 μM at and TEMPOL (Sigma, St. Louis, MO) was used at 1 mM.
Proliferation assay
Cells were originally plated at 10,000 cells per cm2. At the indicated time points, cells were trypsinized and manually counted. Results are the log2 of the means of three technical replicates (± s.d.) from three biological replicates.
Lentiviral Production and Transduction
293T were cultured in Dulbecco’s Modified Eagles Medium (DMEM) supplemented with 5% FBS containing Antibiotic/Antimycotic. Lentiviral shRNA (OpenBiosystems, Lafayette, CO) particles were generated according to Thermoscientific specifications and as described previously (Chudnovsky et al., 2005, Hansen and Johansen, 2011, Lazarov et al., 2002, Ridky et al., 2010) . For the production of viral particles, lentiviral constructs were co-transfected with viral packaging plasmids pCMVΔR8.91 and pUC-MDG into 293T cells using Fugene 6 Transfection Reagent (Promega, Fitchburg, WI).
Quantitative RT/PCR
RNA was extracted from cells and tissues according to the RNeasy Mini Kit protocol (Qiagen, Valencia, CA), and reverse transcribed to cDNA using the High Capacity RNA-to mM. cDNA kit (Applied Biosystems, Grand Island, NY). Quantitative PCR of resulting cDNA was conducted using Power SYBR Green Master Mix (Applied Biosystems, Grand Island, NY) and gene-specific primers, with three technical replicates from at least two individual donors, on a ViiA 7 Real-Time PCR System (Life Technologies, Grand Island, NY). Relative expression was determined using the 2-[delta][delta] Ct method. Results are the average from at least two individual donors (± s.d.).
Western Analysis
Cells from monolayer cultures were lysed in RIPA Buffer containing 1X protease inhibitors and 1X phosphatase inhibitors (Roche, Branchburg, NJ), and extracts analyzed by SDS-PAGE and western blotting. Primary antibodies used in this study include Actin, LC3A, and c-myc (Cell Signaling, Danvers, MA), and LC3B (Abcam, Cambridge, MA), and p62 (Santa Cruz, Dallas, TX). After incubation with the appropriate secondary antibodies, proteins were detected using either ECL Western Blotting Analysis System (GE Healthcare, Lafayette, CO) or Luminata Crescendo Western HRP Substrate (Millipore, Billerica, MA).
Organotypic cultures
Organotypic skin cultures were established using parental or genetically engineered keratinocytes. For each culture, between 8.0 × 105 and 1.0 × 106 keratinocytes were suspended in 80 μL KGM or high calcium (1.2uM CaCl2) growth media, and seeded onto devitalized human dermis, according to previously established methods (Chudnovsky et al., 2005, Ridky et al., 2010). Unless otherwise indicated, small molecule and other chemical treatments were begun at seeding. OTCs were maintained at 37 °C at an air-liquid interface for 8-12 days. To quantify differentiation, the ratio of the area in pixels of keratin-10 positive epidermis to area in pixels of total epidermis was measured as a percentage in ImageJ. This analysis was based previously reported methods (Billings et al., 2015, Natale et al., 2018). Results are the mean of at least 3 technical replicates across at least three biologic replicates from individual donors (± s.d.)
Seahorse Analysis
Pretreated keratinocytes were seeded into XF96-well plates (Agilent) at 50,000 cells/well and incubated overnight. Prior to measurements, samples were washed and incubated in Seahorse media (Agilent, Santa Clara, CA) supplemented with 0.5 mM D-glucose (Sigma, St. Louis, MO). The mitostress kit (Agilent, Santa Clara, CA) was prepared per manufacturer instructions by loading 1.5 μM oligomycin 1.5 μM FCCP, and 1 μM rotenone/antimycin A into injection ports. Measurements were made using an XF96 Extracellular Flux Analyzer (Agilent, Santa Clara, CA) and results processed with Wave v2.2.0 software. These results are the average of 7 technical replicates and are representative of analysis from three individual donors.
Immunofluorescence microscopy
Whole mount cryosections were prepared for immunofluorescence microscopy as previously described (Ridky et al., 2010). In short, slides were fixed in 4% paraformaldehyde or −20°C methanol, permeabilized as required and blocked with 10% horse serum/PBS, followed by incubation with primary antibodies and secondary antibodies conjugated to fluorophores. Slides were mounted with Prolong Gold Antifade Reagent with DAPI (Life Technologies, Grand Island, NY). The primary antibodies used in this study were keratin-5, keratin-10, loricrin, and filaggrin (Covance, Conshohocken, PA), Collagen VII (Millipore, Billerica, MA), involucrin (Sigma, St. Louis, MO), phospho-S6 (235/236), phospho-S6 (240/244), cleaved caspase-3, cleaved caspase-8, LC3A (Cell Signaling, Danvers, MA), p-PKC∂1 (Cell Signaling, Danvers, MA), TFAM (gift from C. Cameron, Penn State University), cytochrome c (BD Pharmingen, San Diego, CA), TRF2 (R & D Systems, Minneapolis, MN) and ki67 (ThermoScientific, Fremont, CA).
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the University of Pennsylvania Skin Biology and Diseases Resource-based Center, funded by 1P30AR069589-01 (Millar) for primary cell procurement, H & E staining, and technical assistance. We also acknowledge Thomas Leung, Christopher Natale, and Kelly Dunlevy for critical presubmission manuscript review. This work was supported by The National Institutes of Health (NIH) (R01CA163566, T.W.R.); CLM was partially supported by an NIH/NIAMS training grant (T32AR007465).
Abbreviations:
- (OTC)
organotypic culture
- (mtROS)
mitochondrial reactive oxygen species
- (ATG)
autophagy-related gene
- (NS)
non-silencing
- (ROS)
reactive oxygen species
- (NAC)
N-acetylcysteine
- (DDC)
dideoxycytidine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
R. Amavaradi is an inventor on the Lys05 patent. The remaining authors state no conflict of interest.
REFERENCES
- Akinduro O, Sully K, Patel A, Robinson DJ, Chikh A, McPhail G, et al. Constitutive Autophagy and Nucleophagy during Epidermal Differentiation. Journal of Investigative Dermatology 2016. [DOI] [PubMed] [Google Scholar]
- Amaravadi RK, Winkler JD. Lys05: a new lysosomal autophagy inhibitor. Autophagy 2012;8(9):1383–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Siprashvili Z, Zarnegar BJ, Shenoy RM, Rios EJ, Nady N, et al. CSNK1a1 Regulates PRMT1 to Maintain the Progenitor State in Self-Renewing Somatic Tissue. Developmental cell 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaduri A, Ungewickell A, Boxer LD, Lopez-Pajares V, Zarnegar BJ, Khavari PA. Network Analysis Identifies Mitochondrial Regulation of Epidermal Differentiation by MPZL3 and FDXR. Developmental cell 2015;35(4):444–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle DD, Xie Z, Tu CL. Calcium regulation of keratinocyte differentiation. Expert Rev Endocrinol Metab 2012;7(4):461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings PC, Sanzari JK, Kennedy AR, Cengel KA, Seykora JT. Comparative analysis of colorimetric staining in skin using open-source software. Exp Dermatol 2015;24(2):157–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E The fateful encounter of mitochondria with calcium: how did it happen? Biochimica et Biophysica Acta (BBA)-Bioenergetics 2010;1797(6):595–606. [DOI] [PubMed] [Google Scholar]
- Chudnovsky Y, Adams AE, Robbins PB, Lin Q, Khavari PA. Use of human tissue to assess the oncogenic activity of melanoma-associated mutations. Nature genetics 2005;37(7):745–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta 2010;1797(6–7):607–18. [DOI] [PubMed] [Google Scholar]
- Ding X, Bloch W, Iden S, Rüegg MA, Hall MN, Leptin M, et al. mTORCl and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nature communications 2016;7:13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douroudis K, Kingo K, Traks T, Reimann E, Raud K, Ratsep R, et al. Polymorphisms in the ATG16L1 gene are associated with psoriasis vulgaris. Acta Derm Venereol 2012;92(1):85–7. [DOI] [PubMed] [Google Scholar]
- Eckhart L, Lippens S, Tschachler E, Declercq W. Cell death by cornification. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 2013;1833(12):3471–80. [DOI] [PubMed] [Google Scholar]
- Egan DF, Chun MG, Vamos M, Zou H, Rong J, Miller CJ, et al. Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Molecular cell 2015;59(2):285–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Wakefield JS. Mechanisms of abnormal lamellar body secretion and the dysfunctional skin barrier in patients with atopic dermatitis. J Allergy Clin Immunol 2014;134(4):781–91 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ 2015;22(3):377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E Skin stem cells: rising to the surface. The Journal of cell biology 2008;180(2):273–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin K, Deruy E, Martien S, Vercamer C, Bouali F, Dujardin T, et al. Senescent keratinocytes die by autophagic programmed cell death. Am J Pathol 2009;174(2):423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka RB, Glasauer A, Hoover P, Yang S, Blatt H, Mullen AR, et al. Mitochondrial reactive oxygen species promote epidermal differentiation and hair follicle development. Sci Signal 2013;6(261):ra8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TE, Johansen T. Following autophagy step by step. BMC biology 2011;9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H, Mitsuyasu T, Seta Y, Maruoka Y, Toyoshima K, Yasumoto S. Overexpression of bcl-2 protein inhibits terminal differentiation of oral keratinocytes in vitro. J Oral Pathol Med 1998;27(1):11–7. [DOI] [PubMed] [Google Scholar]
- Harr MW, Distelhorst CW. Apoptosis and Autophagy: Decoding Calcium Signals that Mediate Life or Death. Cold Spring Harbor Perspectives in Biology 2010;2(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornig-Do HT, von Kleist-Retzow JC, Lanz K, Wickenhauser C, Kudin AP, Kunz WS, et al. Human epidermal keratinocytes accumulate superoxide due to low activity of Mn-SOD, leading to mitochondrial functional impairment. J Invest Dermatol 2007;127(5):1084–93. [DOI] [PubMed] [Google Scholar]
- Hu Y, Carraro-Lacroix LR, Wang A, Owen C, Bajenova E, Corey PN, et al. Lysosomal pH Plays a Key Role in Regulation of mTOR Activity in Osteoclasts. J Cell Biochem 2016;117(2):413–25. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med 2004;36(10): 1199–207. [DOI] [PubMed] [Google Scholar]
- Kasashima K, Sumitani M, Endo H. Human mitochondrial transcription factor A is required for the segregation of mitochondrial DNA in cultured cells. Exp Cell Res 2011;317(2):210–20. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR Signaling. Cold Spring Harb Perspect Biol 2012;4(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavker RM, Matoltsy AG. Formation of horny cells: the fate of cell organelles and differentiation products in ruminal epithelium. The Journal of cell biology 1970;44(3):501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov M, Kubo Y, Cai T, Dajee M, Tarutani M, Lin Q, et al. CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nat Med 2002;8(10): 1105–14. [DOI] [PubMed] [Google Scholar]
- Leung TH, Zhang LF, Wang J, Ning S, Knox SJ, Kim SK. Topical hypochlorite ameliorates NF-kappaB-mediated skin diseases in mice. J Clin Invest 2013;123(12):5361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell 2011;147(1):223–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat Rev Mol Cell Biol 2007;8(8):622–32. [DOI] [PubMed] [Google Scholar]
- Ma XH, Piao SF, Dey S, McAfee Q, Karakousis G, Villanueva J, et al. Targeting ER stress-induced autophagy overcomes BRAF inhibitor resistance in melanoma. J Clin Invest 2014;124(3):1406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee Q, Zhang Z, Samanta A, Levi SM, Ma XH, Piao S, et al. Autophagy inhibitor Lys05 has single-agent antitumor activity and reproduces the phenotype of a genetic autophagy deficiency. Proc Natl Acad Sci U S A 2012;109(21):8253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol 1985;84(6):508–12. [DOI] [PubMed] [Google Scholar]
- Mistry DS, Chen Y, Sen GL. Progenitor function in self-renewing human epidermis is maintained by the exosome. Cell Stem Cell 2012;11(1): 127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka K, Takano-Ohmuro H, Sameshima M, Ueno T, Kominami E, Sakuraba H, et al. Extinction of organelles in differentiating epidermis. Acta histochemica et cytochemica 1999;32(6):465–76. [Google Scholar]
- Morita M, Gravel SP, Chenard V, Sikstrom K, Zheng L, Alain T, et al. mTORCl controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab 2013;18(5):698–711. [DOI] [PubMed] [Google Scholar]
- Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St-Pierre J, et al. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell cycle (Georgetown, Tex) 2015;14(4):473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natale CA, Li J, Zhang J, Dahal A, Dentchev T, Stanger BZ, et al. Activation of G protein-coupled estrogen receptor signaling inhibits melanoma and improves response to immune checkpoint blockade. Elife 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini P, Strambi A, Zipoli C, Hagg-Olofsson M, Buoncervello M, Linder S, et al. Acidic extracellular pH neutralizes the autophagy-inhibiting activity of chloroquine: implications for cancer therapies. Autophagy 2014;10(4):562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechota J, Szczesny R, Wolanin K, Chlebowski A, Bartnik E. Nuclear and mitochondrial genome responses in HeLa cells treated with inhibitors of mitochondrial DNA expression. Acta Biochim Pol 2006;53(3):485–95. [PubMed] [Google Scholar]
- Raymond A-A, de Peredo AG, Stella A, Ishida-Yamamoto A, Bouyssie D, Serre G, et al. Lamellar Bodies of Human Epidermis Proteomics Characterization by High Throughput Mass Spectrometry and Possible Involvement of CLIP-170 in their Trafficking/Secretion. Molecular & Cellular Proteomics 2008;7(11):2151–75. [DOI] [PubMed] [Google Scholar]
- Rebecca VW, Amaravadi RK. Emerging strategies to effectively target autophagy in cancer. Oncogene 2016;35(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridky TW, Chow JM, Wong DJ, Khavari PA. Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat Med 2010;16(12): 1450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossiter H, König U, Barresi C, Buchberger M, Ghannadan M, Zhang C-F, et al. Epidermal keratinocytes form a functional skin barrier in the absence of Atg7 dependent autophagy. Journal of dermatological science 2013;71(1):67–75. [DOI] [PubMed] [Google Scholar]
- Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim Biophys Acta 2010;1797(6-7) :907–12. [DOI] [PubMed] [Google Scholar]
- Sen GL, Boxer LD, Webster DE, Bussat RT, Qu K, Zarnegar BJ, et al. ZNF750 is a p63 target gene that induces KLF4 to drive terminal epidermal differentiation. Developmental cell 2012;22(3):669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen GL, Reuter JA, Webster DE, Zhu L, Khavari PA. DNMT1 maintains progenitor function in self-renewing somatic tissue. Nature 2010;463(7280):563–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol 2011;12(9):565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukseree S, Rossiter H, Mildner M, Pammer J, Buchberger M, Gruber F, et al. Targeted deletion of Atg5 reveals differential roles of autophagy in keratin K5-expressing epithelia. Biochem Biophys Res Commun 2013;430(2):689–94. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Aoki N, Nakamura S, Asano K, Ishida-Yamamoto A, Iizuka H. Cornified cell envelope formation is distinct from apoptosis in epidermal keratinocytes. Journal of dermatological science 2000;23(3):161–9. [DOI] [PubMed] [Google Scholar]
- Tamiji S, Beauvillain JC, Mortier L, Jouy N, Tual M, Delaporte E, et al. Induction of apoptosis-like mitochondrial impairment triggers antioxidant and Bcl-2-dependent keratinocyte differentiation. J Invest Dermatol 2005;125(4):647–58. [DOI] [PubMed] [Google Scholar]
- Tennenbaum T, Li L, Belanger AJ, De Luca LM, Yuspa SH. Selective changes in laminin adhesion and alpha 6 beta 4 integrin regulation are associated with the initial steps in keratinocyte maturation. Cell Growth Differ 1996;7(5):615–28. [PubMed] [Google Scholar]
- Tu CL, Bikle DD. Role of the calcium-sensing receptor in calcium regulation of epidermal differentiation and function. Best Pract Res Clin Endocrinol Metab 2013;27(3):415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara N, Ueno T, Takagi A, Trejo JAO, Haruna K, Suga Y, et al. The significant role of autophagy in the granular layer in normal skin differentiation and hair growth. Archives of dermatological research 2015;307(2):159–69. [DOI] [PubMed] [Google Scholar]
- Zhang J, Feng Y, Forgac M. Proton conduction and bafilomycin binding by the V0 domain of the coated vesicle V-ATPase. J Biol Chem 1994;269(38):23518–23. [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011;334(6056):678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.