Abstract
Objective:
Obesity and diabetes are associated with an increased liver cancer risk. However, most studies have examined all primary liver cancers or hepatocellular carcinoma, with few studies evaluating intrahepatic cholangiocarcinoma (ICC), the second most common type of liver cancer. Thus, we examined the association between obesity and diabetes and ICC risk in a pooled analysis and conducted a systematic review/meta-analysis of the literature.
Design:
For the pooled analysis, we utilized the Liver Cancer Pooling Project, a consortium of 13 US-based, prospective cohort studies with data from 1,541,143 individuals (ICC cases n=414). In our systematic review, we identified 14 additional studies. We then conducted a meta-analysis, combining the results from LCPP with results from the 5 prospective studies identified through September 2017.
Results:
In the LCPP, obesity and diabetes were associated with a 62% [Hazard Ratio (HR)=1.62, 95% Confidence Interval (CI): 1.24–2.12] and an 81% (HR=1.81, 95%CI: 1.33–2.46) increased ICC risk, respectively. In the meta-analysis of prospectively ascertained cohorts and nested case-control studies, obesity was associated with a 49% increased ICC risk [Relative Risk (RR)=1.49, 95%CI: 1.32–1.70; n=4 studies; I2=0%]. Diabetes was associated with a 53% increased ICC risk (RR=1.53, 95%CI: 1.31–1.78; n=6 studies). While we noted heterogeneity between studies (I2=67%) for diabetes, results were consistent in subgroup analyses. Results from hospital-based case-control studies (n=9) were mostly consistent, but these studies are potentially subject to reverse causation.
Conclusions:
These findings suggest that obesity and diabetes are associated with increased ICC risk, highlighting similar etiologies of hepatocellular carcinoma and intrahepatic cholangiocarcinoma. However, additional prospective studies are needed to verify these associations.
INTRODUCTION
Obesity is an established risk factor for liver cancer (1), and diabetes (principally, type 2 diabetes mellitus) has been consistently associated with an increased risk of liver cancer (2). However, the majority of studies to date have examined all primary liver cancers combined or the dominant type of liver cancer, hepatocellular carcinoma, which accounts for approximately 75% of all primary liver cancers in the United States (US). The second most common type of liver cancer, intrahepatic cholangiocarcinoma (ICC), accounts for only 12% of primary liver cancers (3). Due to its rarity, few studies have examined associations of obesity or diabetes with risk of ICC.
ICC is known to be associated with preexisting medical conditions, including Caroli disease, primary sclerosing cholangitis, and inflammatory bowel disease (4). However, chronic infection with hepatitis B or C virus (HBV/HCV), excess alcohol consumption, and obesity/diabetes can trigger oxidative stress and hepatic and systemic inflammation, which may also lead to the development of ICC (5).
The incidence of ICC has been rapidly increasing in the US since the mid-1980s (6), approximately ten years after the beginning of the obesity epidemic (7). However, no prospective cohort studies in the US have investigated the association between obesity or diabetes and risk of ICC. To date, three prospective cohort studies have examined the association between obesity or diabetes and liver cancer in the US (8–10), but have either examined only hepatocellular carcinoma (8) or all primary liver cancer (9, 10). Additionally, several studies on the association between obesity or diabetes and ICC have been conducted within the US Surveillance, Epidemiology, and End Results (SEER)-Medicare database (11, 12). However, the prevalence of obesity in the SEER-Medicare database is based on Medicare insurance claims, thus it is known to be underestimated which can result in attenuated associations (13).
Thus, we conducted a pooled analysis of individual-level data from 13 US-based cohort studies to investigate the associations of obesity, as measured by body mass index (BMI, kg/m2), and diabetes with ICC risk. To further quantify these associations, we conducted a systematic review and meta-analysis of published studies examining the associations between obesity, diabetes, and ICC risk.
METHODS
Pooled Analysis
Study Population.
As described previously (14), all US-based, prospective cohort studies that are members of the National Cancer Institute’s (NCI) Cohort Consortium were invited to participate in the Liver Cancer Pooling Project (LCPP). All 14 participating studies contributed data on BMI and diabetes: NIH-AARP Diet and Health Study (AARP), Agricultural Health Study (AHS), United States Radiologic Technologists (USRT) Study, The Breast Cancer Detection Demonstration Project (BCDDP), Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO), Women’s Health Study (WHS), Physicians’ Health Study (PHS), Health Professionals Follow-Up Study (HPFS), New York University Women’s Health Study (NYUWHS), Cancer Prevention Study–II Nutrition Cohort (CPS-II), Iowa Women’s Health Study (IWHS), Black Women’s Health Study (BWHS), Women’s Health Initiative (WHI), and Nurses’ Health Study (NHS). In total, 1,584,703 individuals were included in the LCPP. PHS did not accrue any ICC outcomes and was excluded from the current study (n=29,033). We also excluded individuals with a diabetes diagnosis prior to age 30 (to avoid potential misclassification of type 1 diabetes, n=1,315), individuals with a body mass index (BMI) <15 kg/m2 (n=368), individuals missing age at study entry (n=9,813), and individuals missing follow-up time (n=693).
Outcomes.
Incident primary liver cancer (defined as International Classification of Diseases, 10th edition [ICD-10] diagnostic code C22) was ascertained by linkage to state cancer registries or medical/pathology record review. Cases were classified as ICC using ICD-O-3 morphology codes of 8032–8033, 8041, 8050, 8070–8071, 8140–8141, 8160, 8260, 8480, 8481, 8490, and 8560. Liver cancers other than ICC (n=1,718) and cases missing histology information (n=620) were excluded from the analysis. The current study included 414 ICC cases and 1,540,729 non-cases.
Exposures.
Height and weight were self-reported, with the exception of WHI, where participants’ height and weight were measured by study staff. Two studies (i.e., NHS and HPFS) validated the self-reported weight against weight measured by study staff and found 98% agreement (15). BMI at study baseline, calculated as weight in kilograms (kg) divided by height in meters (m) squared (kg/m2), was categorized according to World Health Organization definitions: underweight (15–<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), and obese (≥30.0) (16).
All parent studies assessed self-reported diabetes. Several studies (i.e., CPS-II, WHI, and NHS) additionally validated the self-reported diabetes via medical record review and found 83–98% agreement between self-reported and recorded prevalent diabetes (17–19).
Statistical Analysis.
Data were harmonized, as described above, and pooled for analysis. Cox proportional hazards regression analysis was used to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the associations of BMI and diabetes with ICC; follow-up time was used as the underlying time metric. Follow-up of the analytic cohort occurred from time at baseline until an event (i.e., incident ICC) or right-censoring (i.e., death, loss to follow-up, or last date of follow-up), whichever occurred first. The proportional hazards assumption was tested using an interaction between BMI or diabetes with log(time), as a continuous variable, in models that included confounders; no violations were observed (p>0.05).
Based on existing literature, potential confounders (20) including age at enrollment, race, sex, alcohol consumption, and cigarette smoking, were included in all models. Education and non-steroidal anti-inflammatory drug use were also examined as potential confounders, but adjustment for these parameters did not substantially change the HR for the main exposures (i.e., <10% change in log(HR)) and they were not included in the final models. All models were additionally adjusted for parent cohort. Effect measure modification by sex was assessed, and we examined the interaction between BMI and diabetes. Departures from the null were assessed using log-likelihood ratio tests. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). This study was approved by Institutional Review Boards of all participating institutions.
Nested Case-Control Study of HBV/HCV.
Serum samples for determination of HBV and HCV status were available from a subset of participants. To determine HBV status, hepatitis B surface antigen (HBsAg) was assayed using the Bio-Rad GS HBsAg 3.0 enzyme immunoassay (Bio-Rad Laboratories, Redmond, WA, USA). To determine HCV status, antibody to hepatitis C virus (anti-HCV) was assessed using the Ortho HCV Version 3.0 ELISA test system (Ortho-Clinical Diagnostics, Inc.).
Meta-Analysis
Search strategy and inclusion criteria.
A systematic literature search was conducted using MEDLINE (via PubMed), Excerpta Medica database (EMBASE), Scopus, and Web of Science through September 5, 2017, with no specified start date or language restrictions. This systematic review was conducted in accordance with PRISMA guidelines (21).
Broad search terms included: body mass index, diabetes, intrahepatic or bile duct, and cancer (e.g., neoplasm, carcinoma, tumor) (for full list, see Supplemental Table S1). Search terms were agreed upon by three co-authors (JLP, JET, KAM), in consultation with a reference librarian, and the database search was run by one co-author (JET). Criteria for eligible studies included: peer-reviewed publications with reported data on BMI or diabetes, a minimum of 20 adult human cases of primary ICC, and reported effect estimates and corresponding 95% CIs, or enough information to calculate these statistics. We preferentially selected covariate-adjusted estimates, as we assumed these were less confounded. However, we utilized unadjusted estimates or calculated estimates from counts when necessary. Review articles and editorials were eligible for inclusion if they contained original data. Abstracts were excluded. Primary and secondary references were searched to identify additional studies. If multiple publications presented data from the same population, only the publication with the largest sample size was included. Articles written in languages other than English were translated and reviewed. Because articles were excluded based on multiple criteria, we assigned a primary exclusion criterion from the following: study not conducted in humans (e.g., in vitro or animal study), not liver cancer, hepatocellular carcinoma only, all primary liver cancer only presented as a composite outcome, not primary tumor, not invasive cancer (e.g., in situ only), study population included only children (i.e., less than 18 years old), letter to the editor or review (without original data), case report, abstract only, histology unknown, less than 20 cases, and book/proceedings.
Data extraction.
For studies meeting our inclusion criteria, the following data were extracted: first author, publication year, study type (case-control or cohort/nested case-control), study country, dates of study enrollment and follow-up, sample size, source of case ascertainment (e.g., linkage to cancer registry or hospital database), source of exposure information (medical records or self-report), mean/median age at diagnosis, effect estimates, and adjustment variables. Exposures and outcomes were classified based on how these were defined in the original reports. For example, two studies (22, 23) conducted in Asia defined obesity according to the Asian-Pacific criterion (i.e., BMI ≥25 kg/m2) (24). Data extraction was performed for each article by two independent reviewers (JLP, JET) using a standardized data extraction form, and discrepancies were resolved by consensus.
Statistical analysis.
We meta-analyzed study-specific effect estimates using a random-effects model. Between-study heterogeneity was assessed using a χ2 test based on the Q statistic and the I2 statistic and its 95% uncertainty interval (UI) (25). As the 95% UI is unstable in meta-analyses with few included studies (25), we did not report these for meta-analyses of ≤3 studies. An I2 of 0% indicates no heterogeneity, whereas larger values indicate increasing heterogeneity between studies (26). To evaluate publication bias, funnel plots were visually inspected for asymmetry and quantitatively assessed using Begg and Mazumdar’s rank correlation test (27) and Egger’s linear regression test (28). To determine the influence of individual studies, we excluded one study at a time and re-estimated the summary effect estimates. We conducted subgroup analyses by source of exposure information (medical records or reported), study country (US, Europe, Asia), study design (cohort/nested case-control, hospital-based case-control), median age at diagnosis (≤65, >65), and adjustment status (minimally adjusted, fully adjusted), which were specified a priori. Meta-analyses were performed using STATA version 14 for Windows (StataCorp LP, College Station, TX). All p-values are two-sided.
RESULTS
Pooled Analysis
For the 13 participating LCPP cohorts, study characteristics and population size are shown in Table 1. Demographic characteristics of the ICC cases and non-cases in the LCPP are shown in Table 2. Compared with non-cases, individuals who developed ICC were more likely to be older (median baseline age of cases vs. non-cases: 64 years vs. 60 years), male (54% vs. 40%), heavy drinkers (quartile 4 of alcohol consumption: 23% vs. 17%), and current/former smokers (64% vs. 53%).
Table 1.
Cohorts Participating in the Liver Cancer Pooling Project.
| Cohort (Reference) | Location | Recruitment/Follow-up | Cohort Characteristics | Baseline Sample Size | Baseline BMI (kg/m2), Mean (SD) | Baseline BMI ≥30 kg/m2, % | Baseline Diabetes, % |
|---|---|---|---|---|---|---|---|
| Total Cohort | |||||||
| ICC | |||||||
| NIH-AARP Diet and Health Study (53) | multiple states | 1995/2008 | AARP members | 564,518 | 27.1 (4.7) | 21.3 | 9.3 |
| 235 | 27.6 (4.8) | 27.2 | 14.0 | ||||
| Agricultural Health Study (54) | North Carolina, Iowa | 1999/2008 | Farmers and spouses | 89,245 | 27.0 (4.6) | 16.4 | 3.2 |
| 6 | 28.6 (2.5) | 16.7 | 33.3 | ||||
| United States Radiologic Technologists Study (55) | multiple states | 1994/2008 | U.S. radiologic technologists | 72,382 | 25.7 (4.9) | 16.0 | 3.2 |
| 1 | – | – | – | ||||
| The Breast Cancer Demonstration Project (56) | multiple states | 1987/1999 | Breast cancer screening program | 51,523 | 25.1 (4.5) | 11.7 | 4.8 |
| 6 | 23.7 (2.5) | 0.0 | 0.0 | ||||
| Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (57) | multiple states | 1993/2009 | Trial of cancer screening modalities | 149,677 | 27.3 (4.8) | 23.6 | 7.7 |
| 42 | 27.0 (3.4) | 14.3 | 4.8 | ||||
| Women’s Health Study (58) | multiple states | 1993/2010 | Trial of low-dose aspirin and vitamin E in prevention of CVD | 39,769 | 26.0 (5.0) | 18.2 | 2.3 |
| 5 | 26.9 (6.0) | 40.0 | 0.0 | ||||
| Health Professionals Follow-Up Study (59) | multiple states | 1986/2010 | Male health professionals, aged 40–75 | 51,358 | 25.5 (3.2) | 8.0 | 3.6 |
| 14 | 25.3 (2.4) | 14.3 | 7.1 | ||||
| NYU Women’s Health Study (60) | New York | 1985/2010 | Study of hormones and breast cancer | 14,225 | 24.9 (4.5) | 12.5 | 1.7 |
| 3 | 25.6 (1.9) | 0.0 | 0.0 | ||||
| Cancer Prevention Study II (61) | U.S., nationwide | 1992/2007 | General population | 160,367 | 26.0 (4.3) | 14.9 | 7.5 |
| 31 | 27.4 (4.2) | 25.8 | 12.9 | ||||
| Iowa Women’s Health Study (62) | Iowa | 1986/2007 | Postmenopausal women, ages 55–69 years | 28,493 | 26.9 (4.9) | 22.5 | 6.2 |
| 10 | 27.6 (5.0) | 30.0 | 10.0 | ||||
| Black Women’s Health Study (63) | multiple states | 1995/2009 | General population, self-identified as black or African American | 56,771 | 27.7 (6.2) | 28.7 | 3.3 |
| 1 | – | – | – | ||||
| Women’s Health Initiative (64) | multiple states | 1993/variable | Postmenopausal women, ages 50–79 years | 160,677 | 27.8 (5.6) | 29.3 | 5.0 |
| 46 | 29.9 (6.5) | 34.8 | 13.0 | ||||
| Nurses’ Health Study (65) | multiple states | 1980/2010 | Married, female nurses, aged 30–55 | 102,138 | 24.4 (4.5) | 10.8 | 2.4 |
| 14 | 25.9 (4.0) | 21.4 | 7.1 | ||||
| TOTAL | 1,541,143 | 26.7 (4.9) | 19.8 | 6.5 | |||
| 414 | 27.6 (4.8) | 25.4 | 12.1 | ||||
Table 2.
Characteristics of participants in the Liver Cancer Pooling Project.
| Non-Cases (N=1,540,729) |
ICC (N=414) |
|||
|---|---|---|---|---|
| N | (%) | N | (%) | |
| Age at Entry | ||||
| <50 | 249,630 | (16.2) | 11 | (2.7) |
| 50–59 | 500,908 | (32.5) | 103 | (24.9) |
| 60–69 | 665,350 | (43.2) | 251 | (60.6) |
| ≥70 | 124,841 | (8.1) | 49 | (11.8) |
| Sex | ||||
| Male | 610,965 | (39.7) | 225 | (54.4) |
| Female | 929,764 | (60.3) | 189 | (45.6) |
| Race | ||||
| White | 1,353,684 | (87.9) | 368 | (88.9) |
| Black | 113,205 | (7.4) | 14 | (3.4) |
| Asian/Pacific Islander | 17,874 | (1.2) | 7 | (1.7) |
| American Indian/Alaskan Native | 3,341 | (0.2) | 3 | (0.7) |
| Other | 35,581 | (2.3) | 18 | (4.4) |
| Missing | 17,044 | (1.1) | 4 | (1.0) |
| Alcohol, drinks/day | ||||
| Non-drinker | 403,355 | (26.2) | 98 | (23.7) |
| Quartile 1 | 287,953 | (18.7) | 79 | (19.1) |
| Quartile 2 | 248,559 | (16.1) | 60 | (14.5) |
| Quartile 3 | 257,832 | (16.7) | 63 | (15.2) |
| Quartile 4 | 263,681 | (17.1) | 93 | (22.5) |
| Missing | 79,349 | (5.2) | 21 | (5.1) |
| Cigarette Smoking | ||||
| Never smoker | 683,208 | (44.3) | 139 | (33.6) |
| Former smoker | 629,453 | (40.9) | 217 | (52.4) |
| Current smoker | 191,912 | (12.5) | 49 | (11.8) |
| Missing | 36,156 | (2.4) | 9 | (2.2) |
Relative to a normal BMI, obesity was associated with a 62% increased risk of ICC (HR=1.62, 95% CI: 1.24–2.12; Table 3). This association was minimally attenuated with further adjustment for diabetes (HR=1.54, 95% CI: 1.18–2.02). Continuous BMI per 5 kg/m2 was associated with a 20% increased risk of ICC, which was consistent in both sexes (Pinteration=0.8). Being overweight was associated with a 23% increased risk of ICC (HR=1.23, 95% CI: 0.97–1.55). However, this increased risk was confined to women (HR=1.62, 95% CI: 1.15–2.30) and not found in men (HR=1.00, 95% CI: 0.72–1.37).
Table 3.
Adjusteda Hazard Ratios (HR) and 95% Confidence Intervals (Cl) for Associations Between Body Mass Index (BMI) and Intrahepatic Cholangiocarcinoma Risk, Liver Cancer Pooling Project.
| All |
Men |
Women |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | Non-case N | Case N | HR (95% Cl) | Diabetes Adjusted HR (95% Cl) | Non-case N | Case N | HR (95% Cl) | Diabetes Adjusted HR (95% Cl) | Non-case N | Case N | HR (95% Cl) | Diabetes Adjusted HR (95% Cl) |
| <18.5 | 15,873 | 1 | – | – | 2,946 | 1 | – | – | 12,927 | 0 | – | – |
| 18.5-<25.0 | 590,033 | 122 | 1.00 | 1.00 | 178,897 | 63 | 1.00 | 1.00 | 411,136 | 59 | 1.00 | 1.00 |
| 25.0-<30.0 | 570,003 | 170 | 1.23 (0.97–1.55) | 1.21 (0.95–1.53) | 286,644 | 98 | 1.00 (0.72–1.37) | 0.99 (0.72–1.36) | 283,359 | 72 | 1.62(1.15–2.30) | 1.59 (1.12–2.26) |
| ≥30.0 | 305,490 | 105 | 1.62 (1.24–2.12) | 1.54 (1.18–2.02) | 114,116 | 56 | 1.51 (1.05–2.18) | 1.44 (1.00–2.09) | 191,374 | 49 | 1.74(1.18–2.57) | 1.63 (1.10–2.43) |
| Per 5 kg/m2b | 1,465,526 | 397 | 1.20 (1.08–1.33) | 1.17 (1.06–1.30) | 579,657 | 217 | 1.20(1.02–1.42) | 1.17 (0.99–1.39) | 885,869 | 180 | 1.21(1.06–1.39) | 1.18 (1.03–1.35) |
| P trend c | 0.0006 | 0.003 | 0.03 | 0.06 | 0.005 | 0.02 | ||||||
| Pinteractiond | 0.8 | 0.7 | ||||||||||
Adjusted for age (continuous), race (white, black, other), sex, alcohol consumption (nondrinker and ≤.08,1.09–3.58, 3.59–13.54, >13.54 g/day), cigarette smoking (never, former, current), and study (AARP, AHS, USRT, BCDDP, PLCO, WHS, PHS, HPFS, NYU, CPS-II, IWHS, BWHS, WHI, and NHS).
Continuous BMI models exclude participants <18.5 kg/m2.
Ptrend from Wald tests.
P interaction with sex from log-likelihood ratio tests.
Self-reported diabetes at study baseline was associated with an 81% increased risk of ICC (HR=1.81, 95% CI: 1.33–2.46; Table 4) compared to no diabetes, which was minimally attenuated with further adjustment for BMI (HR=1.70, 95% CI: 1.24–2.31). While the HR was higher in women (HR=1.95, 95% CI: 1.18–3.22) than men (HR=1.58, 95% CI: 1.06–2.34), there was no evidence of interaction by sex (Pinteration=0.7). Similarly, there was no evidence of an interaction between diabetes and BMI (Pinteration=0.9; Supplemental Table S2).
Table 4.
Adjusteda Hazard Ratios (HR) and 95% Confidence Intervals (Cl) for Associations Between Diabetes and Intrahepatic Cholangiocarcinoma Risk, Liver Cancer Pooling Project.
| All |
Men |
Women |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-case N | Case N | HR (95% Cl) | BMI Adjusted HR (95% Cl) | Non-case N | Case N | HR (95% Cl) | BMI Adjusted HR (95% Cl) | Non-case N | Case N | HR (95% Cl) | BMI Adjusted HR (95% Cl) | |
| No Diabetes | 1,422,795 | 361 | 1.00 | 1.00 | 554,287 | 194 | 1.00 | 1.00 | 868,508 | 167 | 1.00 | 1.00 |
| Diabetes | 100,281 | 50 | 1.81(1.33–2.46) | 1.70 (1.24–2.31) | 52,912 | 31 | 1.58 (1.06–2.34) | 1.47 (0.98–2.19) | 47,369 | 19 | 1.95 (1.18–3.22) | 1.78 (1.07–2.96) |
| Pinteractionb | 0.7 | 0.8 | ||||||||||
Adjusted for age (continuous), race (white, black, other), sex, alcohol consumption (nondrinker and ≤1.08,1.09–3.58, 3.59–13.54, >13.54 g/day), cigarette smoking (never, former, current), and study (AARP, AHS, USRT, BCDDP, PLCO, WHS, PHS, HPFS, NYU, CPS-II, IWHS, BWHS, WHI, and NHS).
Pinteraction with sex from log-likelihood ratio tests.
Among the ICC cases tested (n=74), 1 (1.4%) was positive for anti-HCV and 1 (1.4%) was positive for HBsAg. Among the matched controls (n=178), 5 (2.8%) were positive for anti-HCV and 3 (1.7%) were positive for HBsAg. In the current study, there was no association between HCV or HBV status and BMI or diabetes (data not shown), which suggests that HCV and HBV are not confounders of the association between obesity or diabetes and ICC.
Systematic Review and Study Characteristics
In our systematic search, we identified 803 unique published papers (Figure 1). Of these, 14 (1.7%) studies were eligible and included in this systematic review and meta-analysis. Of the remaining 789 publications, 779 (98.7%) were excluded based on various exclusion criteria and 10 (1.3%) were identified as updated reports from a single study population (i.e., duplicate study populations). The primary reasons for exclusion included the following: the outcome studied was not liver cancer (n=310 publications) and the paper was a case report only (n=187 publications).
Figure 1.
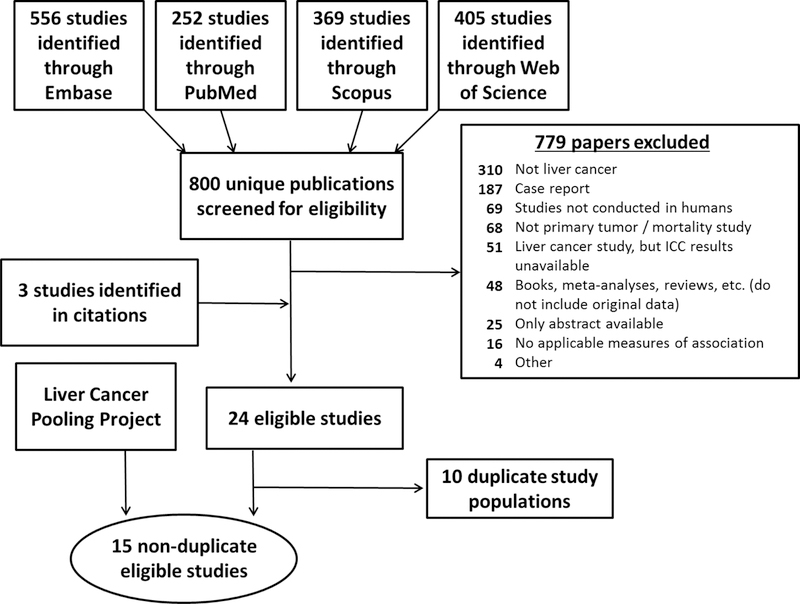
Flow chart for systematic review of the association between obesity/diabetes and intrahepatic cholangiocarcinoma.
Individual study characteristics of the 14 studies identified in our systematic review are summarized in Table 5. All studies included information on the diagnosis of diabetes, but only 7 studies, including LCPP, ascertained height and weight (11, 22, 23, 29–31). Exposure information was primarily assessed through medical records or ICD codes; only two studies, including LCPP, assessed the exposure information through self-report (32). For our main analyses, we restricted to cohort and nested case-control studies with prospectively collected exposure information, due to potential issues with reverse causation. Of the studies identified in the systematic review, LCPP and one other study (32) were cohorts; 4 were nested case-control studies (11, 30, 31, 33); and 9 were hospital-based case-control studies (22, 23, 29, 34–39). Thus, a total of 6 studies are included in the main meta-analysis.
Table 5.
Characteristics of studies identified in the systematic review of obesity/diabetes in association with intrahepatic cholangiocarcinoma (N=14).
| Author, Year | Country | Case Ascertainment | Exposure source | Obesity or Diabetes | Years of Recruitment/Follow-up | ICC N | Adjustment for Covariates |
|---|---|---|---|---|---|---|---|
|
Cohort |
|||||||
| Stepien et al., 2016 | Europe | Linkage to regional cancer registries (Denmark, Italy, Netherlands, Norway, Spain, Sweken, and UK); health insurance records, contact with cancer/pathology registries, or active follow-up (France, Germany, and Greece) | Self-Report | Diabetes | 1992–2010 | 66 | Crude |
|
Nested Case-Control |
|||||||
| Huang et al., 2017 | Taiwan | Linkage to Taiwan Cancer Registry | Medical Records | Diabetes | 2003–2009 | 4,695 | Conditioned on Age, Sex, Date of cohort entry, Date of last observation; Hemochromatosis, Alcoholic liver disease. Chronic non-alcoholic liver disease, HBV, HCV, Chronic pancreatitis. Inflammatory bowel disease. Peptic ulcer. Gastroesophageal reflux disease. Cardiovascular disease. Hyperlipidemia, Medication use |
| Petrick et al., 2017 | USA | Linkage to SEER 18 cancer registries | Medical Records | Both | 2000–2011 | 2,092 | Age, Sex, Race, Geographic region. State buy-in status |
| Welzel et al., 2007a | USA | Linkage to SEER 11 cancer registries | Medical Records | Both | 1993–1999 | 535 | Age, Sex, Race, Geographic region. State buy-in status |
| Welzel et al., 2007b | Denmark | Linkage to the Danish Cancer Registry | Medical Records | Both | 1978–1991 | 764 | Conditioned on Age at diagnosis, Sex, Year of birth |
|
Hospital-based Case-Control |
|||||||
| Choi et al., 2016 | USA | Hospital database search (Mayo Clinic Life Sciences System database)/medical records review | Medical Records | Both | 2000–2014 | 1,169 | Propensity score adjustment for Age, Sex, Race, Obesity/Diabetes, Hypertension, Stroke, Coronary artery disease. Peripheral vascular disease, Atrial fibrillation, Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, Primary sclerosing cholangitis, Cirrhosis, Inflammatory bowel disease, and Smoking status |
| Kinoshita et al., 2016 | Hospital database search/medical records review | Medical Records | Both | 1995–2014 | 34 | Obesity: Age, Non-alcoholic steatohepatitis, Albumin, Gamma- glutamyl transpeptidase; Diabetes: Crude | |
| Lee et al., 2015 | South Korea | Hospital database search/medical records review | Medical Records | Both | 2007–2013 | 83 | Obesity and Diabetes: conditioned on Age, Sex, Date of diagnosis; Diabetes: additionally adjusted for Cholecystolithiasis, Choledocholithiasis, Hepatolithiasis, HBV infection, HCV infection |
| Lee et al., 2008 | South Korea | Hospital database search/medical records review | Medical Records | Diabetes | 2000–2004 | 622 | Age, Sex, Date of admission, HBV, Clonorchis sinesis, Hepatolithiasis, Choledochal cyst. Liver cirrhosis. Alcohol |
| Peng et al., 2011 | China | Hospital database search/medical records review | Medical Records | Diabetes | 2002–2009 | 98 | Crude |
| Shaib et al., 2007 | USA | Hospital database search (M.D. Anderson Patients Informatics database)/medical records review | Medical Records | Diabetes | 1992–2002 | 83 | Age, Sex, Ethnicity |
| Wu et al., 2012 | China | Cases identified using PUMCH Patient Information Database, with further medical record confirmation | Medical Records | Diabetes | 1998–2010 | 102 | Crude |
| Yamamoto et al., 2004 | Japan | Hospital database search/medical records review | Medical Records | Diabetes | 1991–2002 | 50 | Age, Sex, Operation date, HCV infection, Transfusion, Hypertension, Liver status. Total bilirubin, Alanine aminotransferase, Albumin, Platelet count |
| Zhou et al., 2008 | China | Hospital database search/medical records review | Medical Records | Diabetes | 2004–2006 | 312 | Conditioned on Age, Sex, Date of hospital admission; HBV, HCV, Hypertension, Hepatolithiasis, Cigarette smoking, Alcohol use |
Meta-analysis
Obesity, relative to normal BMI, was associated with a 49% increased risk of ICC [Relative Risk (RR)=1.49, 95% CI: 1.32–1.70, n=4 studies; Table 6 and Figure 2] in prospective studies. There was no evidence of heterogeneity between studies (I2=0.0%, 95% UI: 0.0%–85.9%, p=0.7). Results were similar we stratified by study design (i.e., cohort vs. nested case-control). When we excluded one study at time, our results were robust (Supplemental Figure S1). Publication bias was not indicated by the Begg and Mazumdar’s (p=0.5) or Egger’s tests (p=0.09), but the funnel plot did appear asymmetrical (Supplementary Figure S2).
Table 6.
Meta-analysis of the Associations Between Obesity/Diabetes and Intrahepatic Cholangiocarcinoma Risk by Random-effects Models in Cohort and Nested Case-Control Studies.
| Obesity | Studies N | RR (95% Cl) | P-value | Heterogeneity |
|
|---|---|---|---|---|---|
| I2 (95% Ul) | P-value | ||||
| Overall | 4 | 1.49 (1.32–1.70) | <0.001 | 0.0 (0.0–85.9) | 0.7 |
| Source of Exposure | |||||
| Medical records | 3 | 1.46 (1.27–1.69) | <0.001 | 0.0 ( – ) | 0.6 |
| Self-report | 1 | 1.62 (1.23–2.12) | <0.001 | – | – |
| Study Location | |||||
| United States | 3 | 1.49 (1.31–1.69) | <0.001 | 0.0 ( – ) | 0.6 |
| Asia | 0 | – | – | – | – |
| Europe | 1 | 2.05 (0.75–5.59) | 0.2 | – | – |
| Study Design | |||||
| Cohort | 1 | 1.62 (1.23–2.12) | <0.001 | – | – |
| Nested Case-Control | 3 | 1.46 (1.27–1.69) | <0.001 | 0.0 ( – ) | 0.6 |
| Mean/Median Age at Diagnosis | |||||
| ≤65 | 0 | – | – | – | – |
| >65 | 4 | 1.49 (1.32–1.70) | <0.001 | 0.0 (0.0–85.9) | 0.7 |
| Adjustment Set | |||||
| Fully Adjusted | 1 | 1.62 (1.23–2.12) | <0.001 | – | – |
| Minimally Adjusted | 3 | 1.46 (1.27–1.69) | <0.001 | 0.0 ( – ) | 0.60 |
|
Diabetes | |||||
| Overall | 6 | 1.53 (1.31–1.78) | <0.001 | 67.3 (22.1–86.2) | 0.009 |
| Source of Exposure | |||||
| Medical records | 4 | 1.49 (1.25–1.78) | <0.001 | 78.2 (41.3–91.9) | 0.003 |
| Self-report | 2 | 1.79 (1.32–2.41) | <0.001 | 0.0 ( – ) | 0.7 |
| Study Location | |||||
| United States | 3 | 1.65 (1.47–1.86) | <0.001 | 38.0 ( – ) | 0.2 |
| Asia | 1 | 1.22 (1.07–1.40) | 0.004 | – | – |
| Europe | 2 | 1.42 (0.81–2.47) | 0.2 | 0.0 ( – ) | 0.9 |
| Study Design | |||||
| Cohort | 2 | 1.45 (0.99–2.13) | 0.06 | 81.1 ( – ) | 0.02 |
| Nested Case-Control | 4 | 1.59 (1.47–1.72) | <0.001 | 0.0 (0.0–92.4) | 0.4 |
| Mean/Median Age at Diagnosis | |||||
| ≤65 | 0 | – | – | – | – |
| >65 | 6 | 1.53 (1.31–1.78) | <0.001 | 67.3 (22.1–86.2) | 0.009 |
| Adjustment Set | |||||
| Fully Adjusted | 2 | 1.45 (0.99–2.13) | 0.06 | 81.1 ( – ) | 0.02 |
| Minimally Adjusted | 4 | 1.59 (1.47–1.72) | <0.001 | 0.0 (0.0–92.4) | 0.4 |
Figure 2.

Meta-analysis of the association between obesity and intrahepatic cholangiocarcinoma risk by random-effects model in cohort and nested case-control studies (N=4 studies).
A history of diabetes, relative to no diabetes, was associated with a 53% increased risk of ICC (RR=1.53, 95% CI: 1.31–1.78, n=6 studies; Table 6 and Figure 3) in prospective studies. There was moderate heterogeneity between studies (I2=67.3%, 95% UI: 22.1%–86.2%, p=0.009). Some of the heterogeneity reported may be explained by source of exposure (i.e., medical records vs. self-report) and study design (i.e., cohort vs. nested case-control), but the estimated RR was similar across all stratifications. For example, the association between diabetes and ICC risk was similar for diabetes assessment from medical records and self-report (RR=1.49, 95% CI: 1.25–1.78, I2=78.2% and RR=1.79, 95% CI: 1.32–2.41, I2=0.0%, respectively). The association between diabetes and ICC risk was also similar between cohort (RR=1.45, 95% CI: 0.99–2.13, I2=81.1%) and nested case-control studies (RR=1.59, 95% CI: 1.47–1.72, I2=0.0%). Additionally, when we excluded one study at a time, the summary relative risks did not materially change (Supplemental Figure S3). Publication bias was unlikely, as assessed by the Begg and Mazumdar’s (p=0.9) and Egger’s tests (p=0.9), and the funnel plot showed little evidence of asymmetry (Supplementary Figure S4).
Figure 3.
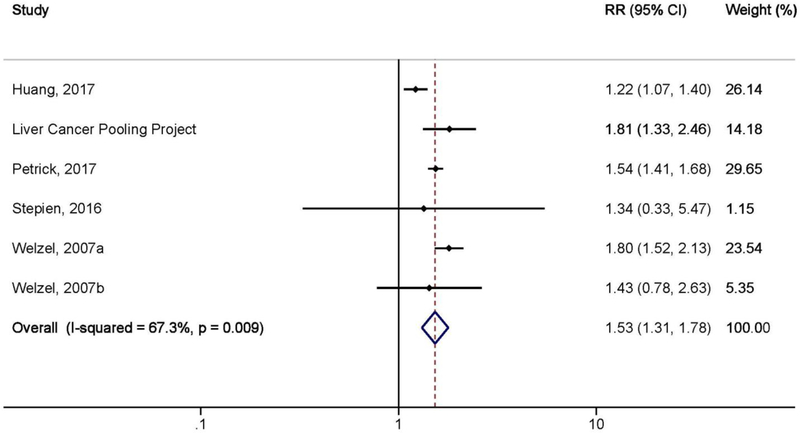
Meta-analysis of the association between diabetes and intrahepatic cholangiocarcinoma risk by random-effects model in cohort and nested case-control studies (N=6 studies).
With the exception of one outlier (29), the results from the hospital-based case-control studies were consistent (Supplemental Tables S3–S4, Figures S5–S10), although the association with diabetes was stronger (Supplemental Tables S3–S4, Figure S10).
DISCUSSION
In our pooled analysis of 13 US-based, prospective cohort studies, each 5 kg/m2 increase in BMI was associated with a 20% increased risk of ICC, and being obese was associated with a 62% increased risk of ICC. In a meta-analysis with 3 additional cohort/nested case-control studies, obesity was associated with 49% increased risk of ICC, and we observed little evidence of heterogeneity between these 4 studies (I2=0%). Diabetes was associated with an 81% increased risk of ICC in our pooled analysis. In the meta-analysis with 5 additional cohort/nested case-control studies, diabetes was associated with a 53% increased ICC risk; we observed heterogeneity between studies (I2=67%), but results were consistent in our subgroup analyses.
In our main meta-analysis, we report results from only the prospective studies, as the hospital-based case-control studies were susceptible to reverse causation. The study by Choi et al. was a notable outlier of the obesity-ICC association, in that the study reported a significant inverse association between obesity and ICC risk (RR=0.77, 95% CI: 0.64–0.92) (29). This study was a hospital-based case-control study conducted at the Mayo Clinic in Minnesota, and BMI was abstracted from medical records at time of ICC diagnosis. Thus, there was potential for reverse causation, as ICC cases were likely to have had cancer-related cachexia (29). The other two hospital-based case-control studies that examined the association between obesity and ICC risk reported non-significant or null associations (22, 23), which were also likely affected by cancer-related cachexia. However, these two studies were conducted in Japan (22) and South Korea (23) and utilized the Asian-Pacific criterion to define obesity, which is BMI ≥25 kg/m2 (24). In the systematic review of the diabetes-ICC association, only the study by Peng et al. reported a decreased relative risk (albeit non-significant) for the association between diabetes and ICC (39). This study was conducted by the First Affiliated Hospital and the Affiliated Tumor Hospital of Guangxi Medical University in China and reported only the unadjusted association between diabetes and ICC, which was likely confounded.
Recent meta-analyses have evaluated the association between obesity (1) or diabetes (2) and liver cancer risk. However, these reports have included all primary liver cancers and have not been able to evaluate the associations between obesity or diabetes and risk of ICC. To date, two meta-analyses have evaluated the associations between obesity/diabetes and ICC risk, but both have included duplicate or overlapping study populations in their analyses (40, 41). In the current study, we did not include overlapping study populations. Additionally, one of the meta-analyses included all cholangiocarcinomas (41) rather than just ICC.
Excess adiposity can cause chronic, low-grade systemic inflammation through decreased adiponectin levels and increased levels of TNF-α, IL-6, leptin, free fatty acids, and TLR4 (42). Many of these molecules have not been specifically examined in relation to ICC, but leptin, which is a hormone secreted by adipose tissue, has been shown to stimulate growth and migration and prevent apoptosis of ICC cells in vitro (43). Systemic inflammation is believed to contribute to metabolic dysregulation, including onset of insulin resistance and subsequent diabetes (44). Individuals with excess adiposity and/or diabetes may develop nonalcoholic fatty liver disease (NAFLD), which can progress to non-alcoholic steatohepatitis, fibrosis, and cirrhosis, possibly culminating in ICC development (42).
This study has several limitations. In the pooled analysis of LCPP, data on exposures and some potential confounders from almost all included cohorts were based on self-report. Self-report on weight is height is potentially problematic because individuals tend to under-report weight and over-report height (45). However, self-reported weight and height (15, 46) and self-reported diabetes (17–19) have been shown to have good agreement with study staff measurement or medical records, respectively. Additionally, HBV/HCV status was not available for all individuals, and information on primary sclerosing cholangitis, biliary cysts, Thorotrast exposure, liver fluke infections, non-alcoholic steatohepatitis, and cirrhosis were not available for any individuals. In the nested case-control study conducted within LCPP, there was no association between HBV or HCV and obesity or diabetes. Thus, we would not expect HBV or HCV to confound the associations between obesity or diabetes and ICC. While anti-HCV likely overestimates the number of chronic HCV carriers, we did not perform an RNA test on anti-HCV(+) individuals because there were few anti-HCV(+) individuals to justify using additional serum, particularly for cases. Further, as this study was conducted using prospectively conducted cancer cohorts, information on risk factors specific to ICC (e.g., primary sclerosing cholangitis, biliary cysts, Thorotrast exposure, liver fluke infections, non-alcoholic steatohepatitis, and cirrhosis) is not available. These cohorts are designed to capture as much information as possible for every cancer type, making it impossible to include questions relevant to rare cancer types. From a methodologic perspective, these factors would need to be associated with both ICC and BMI or diabetes, and not an intermediate between BMI or diabetes and ICC, to be considered a confounder. While primary sclerosing cholangitis, biliary cysts, Thorotrast exposure, and liver fluke infections are a known risk factors for ICC, there is little to no evidence to suggest that they are associated with BMI or diabetes. Additionally, non-alcoholic steatohepatitis and cirrhosis are potential intermediate steps between obesity or diabetes and ICC risk (47). Thus, lack of information on these factors should not bias our estimates of the association between BMI or diabetes and ICC risk. We were unable to adjust for diabetes medication use, as most of the included cohorts did not assess this information. In the meta-analysis, studies that relied on self-report may also have suffered from measurement error, and studies that relied on medical records were limited to what was recorded for patient care and/or billing purposes. Thus, there is likely to be measurement error in these studies, as well as incomplete adjustment for potential confounding. Only LCPP utilized self-report for obesity. In the meta-analysis of diabetes, results were similar between studies that relied on self-report (n=2 studies) or medical records (n=4 studies). For the meta-analysis, we extracted minimally or unadjusted estimates or calculated estimates from provided counts for the majority of included studies. While these associations are likely to be confounded by known risk factors, such as age, sex, smoking, and alcohol consumption, the results were similar between studies where we had “fully” versus minimally adjusted estimates. Finally, while tests of publication bias were non-significant, we cannot rule this out, especially for obesity, where the funnel plot appeared asymmetrical.
The pooled analysis of LCPP is one of the largest studies to date of prospectively ascertained ICC, which was drawn from over 1.54 million individuals participating in 13 US-based, prospective cohort studies. This large sample size allowed us to evaluate the association between obesity/diabetes and ICC risk by sex. By conducting a systematic review and meta-analysis of studies examining the associations between obesity/diabetes and ICC risk, we were able to further quantify these associations. In the LCPP, participants are primarily of European ancestry. Thus, the systematic review and meta-analysis allowed us to additionally examine associations in Asian populations. This analysis is important as BMI is lower in Asian countries than Western countries (46), but the prevalence of obesity and diabetes is increasing (48, 49).
In conclusion, findings from this pooled investigation, systematic review, and meta-analysis suggest that obesity and diabetes are associated with increased risks of ICC. As rates of ICC are increasing in most populations (50), it is critical to understand the underlying etiology, especially as prevalence of obesity (51) and diabetes (52) is also increasing. This may help to identify primary prevention measures for ICC.
Supplementary Material
What is current knowledge?
Primary liver cancer consists of two main types: hepatocellular carcinoma, which accounts for approximately 75% of all primary liver cancers in the United States (US), and intrahepatic cholangiocarcinoma, which accounts for only 12% of primary liver cancers.
Incidence rates of both liver cancer types have been rapidly rising in the US since the mid-1980s, approximately ten years after the beginning of the obesity epidemic.
Obesity and diabetes are established risk factors for primary liver cancer, but it is unknown if obesity and diabetes increase the risk of only hepatocellular carcinoma or intrahepatic cholangiocarcinoma as well.
What is new here?
Obesity and diabetes increased the risk of intrahepatic cholangiocarcinoma in 13 prospective cohort studies, comprising over 400 cases of intrahepatic cholangiocarcinoma, in the Liver Cancer Pooling Project. Similar results were found in a systematic review and meta-analysis.
These results indicate that obesity and diabetes are risk factors for intrahepatic cholangiocarcinoma, highlighting similar etiologies of hepatocellular carcinoma and intrahepatic cholangiocarcinoma, the two most common types of primary liver cancer.
Our findings provide clinicians further data to address patient questions about the causes of intrahepatic cholangiocarcinoma.
ACKNOWLEDGEMENTS
For the Black Women’s Health Study, pathology data were obtained from several of the following state cancer registries (AZ, CA, CO, CT, DE, DC, FL, GA, IL, IN, KY, LA, MD, MA, MI, NJ, NY, NC, OK, PA, SC, TN, TX, VA), and results reported do not necessarily represent their views.
For the Nurses’ Health Study and the Health Professionals Follow-up Study, we would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
For NIH-AARP, the acknowledgement can be found at the following website: https://dietandhealth.cancer.gov/acknowledgement.html. For the Women’s Health Initiative, the full list of investigators that have contributed can be found on the following website: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf.
Grant Support: NIH Intramural Research Program, National Cancer Institute (JL Petrick, JE Thistle, LE Beane-Freeman, JN Hofmann, CM Kitahara, ND Freedman, BI Graubard, J Koshiol, LM Liao, MS Linet, MP Purdue, C Schairer, R Sinha, AL Van Dyke, KA McGlynn). National Institutes of Health grants CA047988 (I Lee, JE Buring), HL043851 (I Lee, JE Buring), HL080467 (I Lee, JE Buring), HL099355 (I Lee, JE Buring), DK098311 (AT Chan), CA186107 (AT Chan), CA87969 (AT Chan), CA167552 (AT Chan), UM1 CA164974 (L Rosenberg, JR Palmer), and R01 CA058420 (L Rosenberg, JR Palmer). The WHI program (J Wactawski-Wende, TE Rohan) is funded by the National Institutes of Health contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C.
REFERENCES
- 1.Huang W, Ren H, Ben Q, et al. Risk of esophageal cancer in diabetes mellitus: a meta-analysis of observational studies. Cancer Causes Control 2012;23:263–72. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Wang B, Yan S, et al. Type 2 diabetes and gender differences in liver cancer by considering different confounding factors: a meta-analysis of cohort studies. Ann Epidemiol 2016;26:764–772. [DOI] [PubMed] [Google Scholar]
- 3.Altekruse SF, Devesa SS, Dickie LA, et al. Histological classification of liver and intrahepatic bile duct cancers in SEER registries. J Registry Manag 2011;38:201–5. [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman RW. Risk factors for biliary tract carcinogenesis. Ann Oncol 1999;10 Suppl 4:308–11. [PubMed] [Google Scholar]
- 5.Ambade A, Mandrekar P. Oxidative stress and inflammation: essential partners in alcoholic liver disease. Int J Hepatol 2012;2012:853175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saha SK, Zhu AX, Fuchs CS, et al. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016;21:594–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, Baker JL, Hill JO, et al. Controversies regarding reported trends: has the obesity epidemic leveled off in the United States? Adv Nutr 2012;3:751–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Setiawan VW, Lim U, Lipworth L, et al. Sex and Ethnic Differences in the Association of Obesity With Risk of Hepatocellular Carcinoma. Clin Gastroenterol Hepatol 2016;14:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell PT, Newton CC, Freedman ND, et al. Body Mass Index, Waist Circumference, Diabetes, and Risk of Liver Cancer for U.S. Adults. Cancer Res 2016;76:6076–6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrick JL, Freedman ND, Demuth J, et al. Obesity, diabetes, serum glucose, and risk of primary liver cancer by birth cohort, race/ethnicity, and sex: Multiphasic health checkup study. Cancer Epidemiol 2016;42:140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol 2007;5:1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology 2011;54:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd JT, Blackwell SA, Wei II, et al. Validity of a Claims-Based Diagnosis of Obesity Among Medicare Beneficiaries. Eval Health Prof 2015;38:508–17. [DOI] [PubMed] [Google Scholar]
- 14.McGlynn KA, Sahasrabuddhe VV, Campbell PT, et al. Reproductive factors, exogenous hormone use and risk of liver cancer among U.S. women: Results from the Liver Cancer Pooling Project. Br J Cancer 2015;(In Press). [DOI] [PMC free article] [PubMed]
- 15.Rimm EB, Stampfer MJ, Colditz GA, et al. Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1990;1:466–73. [DOI] [PubMed] [Google Scholar]
- 16.Devesa SS, Blot WJ, Fraumeni JF Jr., Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998;83:2049–53. [PubMed] [Google Scholar]
- 17.Campbell PT, Deka A, Jacobs EJ, et al. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology 2010;139:1138–46. [DOI] [PubMed] [Google Scholar]
- 18.Jackson JM, DeFor TA, Crain AL, et al. Validity of diabetes self-reports in the Women’s Health Initiative. Menopause 2014;21:861–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colditz GA, Willett WC, Stampfer MJ, et al. Weight as a risk factor for clinical diabetes in women. Am J Epidemiol 1990;132:501–13. [DOI] [PubMed] [Google Scholar]
- 20.Rothman KJ, Greenland S, Lash TL. Modern epidemiology 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology 2009;62:1006–12. [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita M, Kubo S, Tanaka S, et al. The association between non-alcoholic steatohepatitis and intrahepatic cholangiocarcinoma: A hospital based case-control study. J Surg Oncol 2016;113:779–83. [DOI] [PubMed] [Google Scholar]
- 23.Lee BS, Park EC, Park SW, et al. Hepatitis B virus infection, diabetes mellitus, and their synergism for cholangiocarcinoma development: a case-control study in Korea. World J Gastroenterol 2015;21:502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanazawa M, Yoshiike N, Osaka T, et al. Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Diet 2005;94:1–12. [DOI] [PubMed] [Google Scholar]
- 25.Thorlund K, Imberger G, Johnston BC, et al. Evolution of heterogeneity (I2) estimates and their 95% confidence intervals in large meta-analyses. PLoS One 2012;7:e39471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi J, Ghoz HM, Peeraphatdit T, et al. Aspirin use and the risk of cholangiocarcinoma. Hepatology 2016;64:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welzel TM, Mellemkjaer L, Gloria G, et al. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer 2007;120:638–41. [DOI] [PubMed] [Google Scholar]
- 31.Petrick JL, Yang B, Altekruse SF, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS One 2017;12:e0186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stepien M, Duarte-Salles T, Fedirko V, et al. Consumption of soft drinks and juices and risk of liver and biliary tract cancers in a European cohort. Eur J Nutr 2016;55:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YJ, Wu AT, Chiou HY, et al. Interactive role of diabetes mellitus and female sex in the risk of cholangiocarcinoma: A population-based nested case-control study. Oncotarget 2017;8:6642–6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee TY, Lee SS, Jung SW, et al. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol 2008;103:1716–20. [DOI] [PubMed] [Google Scholar]
- 35.Shaib YH, El-Serag HB, Nooka AK, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol 2007;102:1016–21. [DOI] [PubMed] [Google Scholar]
- 36.Wu Q, He XD, Yu L, et al. The metabolic syndrome and risk factors for biliary tract cancer: a case-control study in China. Asian Pac J Cancer Prev 2012;13:1963–9. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto S, Kubo S, Hai S, et al. Hepatitis C virus infection as a likely etiology of intrahepatic cholangiocarcinoma. Cancer Sci 2004;95:592–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou YM, Yin ZF, Yang JM, et al. Risk factors for intrahepatic cholangiocarcinoma: a case-control study in China. World J Gastroenterol 2008;14:632–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peng NF, Li LQ, Qin X, et al. Evaluation of risk factors and clinicopathologic features for intrahepatic cholangiocarcinoma in Southern China: a possible role of hepatitis B virus. Ann Surg Oncol 2011;18:1258–66. [DOI] [PubMed] [Google Scholar]
- 40.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 2012;57:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li JS, Han TJ, Jing N, et al. Obesity and the risk of cholangiocarcinoma: a meta-analysis. Tumour Biol 2014;35:6831–8. [DOI] [PubMed] [Google Scholar]
- 42.Alzahrani B, Iseli TJ, Hebbard LW. Non-viral causes of liver cancer: does obesity led inflammation play a role? Cancer Lett 2014;345:223–9. [DOI] [PubMed] [Google Scholar]
- 43.Fava G, Alpini G, Rychlicki C, et al. Leptin enhances cholangiocarcinoma cell growth. Cancer Res 2008;68:6752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett 2008;582:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Connor Gorber S, Tremblay M, Moher D, et al. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev 2007;8:307–26. [DOI] [PubMed] [Google Scholar]
- 46.Lin CJ, DeRoo LA, Jacobs SR, et al. Accuracy and reliability of self-reported weight and height in the Sister Study. Public Health Nutr 2012;15:989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol 2012;56:704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramachandran A, Snehalatha C, Shetty AS, et al. Trends in prevalence of diabetes in Asian countries. World J Diabetes 2012;3:110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramachandran A, Snehalatha C. Rising burden of obesity in Asia. J Obes 2010;2010. [DOI] [PMC free article] [PubMed]
- 50.Petrick JL, Braunlin M, Laversanne M, et al. International trends in liver cancer incidence, overall and by histologic subtype, 1978–2007. Int J Cancer 2016;139:1534–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelly T, Yang W, Chen CS, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–7. [DOI] [PubMed] [Google Scholar]
- 52.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14. [DOI] [PubMed] [Google Scholar]
- 53.Schatzkin A, Subar AF, Thompson FE, et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions : the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am J Epidemiol 2001;154:1119–25. [DOI] [PubMed] [Google Scholar]
- 54.Alavanja MC, Sandler DP, McMaster SB, et al. The Agricultural Health Study. Environ Health Perspect 1996;104:362–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boice JD Jr., Mandel JS, Doody MM, et al. A health survey of radiologic technologists. Cancer 1992;69:586–98. [DOI] [PubMed] [Google Scholar]
- 56.Flood A, Velie EM, Chaterjee N, et al. Fruit and vegetable intakes and the risk of colorectal cancer in the Breast Cancer Detection Demonstration Project follow-up cohort. Am J Clin Nutr 2002;75:936–43. [DOI] [PubMed] [Google Scholar]
- 57.Kramer BS, Gohagan J, Prorok PC, et al. A National Cancer Institute sponsored screening trial for prostatic, lung, colorectal, and ovarian cancers. Cancer 1993;71:589–93. [DOI] [PubMed] [Google Scholar]
- 58.Rexrode KM, Lee IM, Cook NR, et al. Baseline characteristics of participants in the Women’s Health Study. J Womens Health Gend Based Med 2000;9:19–27. [DOI] [PubMed] [Google Scholar]
- 59.Grobbee DE, Rimm EB, Giovannucci E, et al. Coffee, caffeine, and cardiovascular disease in men. N Engl J Med 1990;323:1026–32. [DOI] [PubMed] [Google Scholar]
- 60.Toniolo PG, Pasternack BS, Shore RE, et al. Endogenous hormones and breast cancer: a prospective cohort study. Breast Cancer Res Treat 1991;18 Suppl 1:S23–6. [DOI] [PubMed] [Google Scholar]
- 61.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer 2002;94:2490–501. [DOI] [PubMed] [Google Scholar]
- 62.Munger RG, Folsom AR, Kushi LH, et al. Dietary assessment of older Iowa women with a food frequency questionnaire: nutrient intake, reproducibility, and comparison with 24-hour dietary recall interviews. Am J Epidemiol 1992;136:192–200. [DOI] [PubMed] [Google Scholar]
- 63.Rosenberg L, Adams-Campbell L, Palmer JR. The Black Women’s Health Study: a follow-up study for causes and preventions of illness. J Am Med Womens Assoc 1995;50:56–8. [PubMed] [Google Scholar]
- 64.Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol 2003;13:S5–17. [DOI] [PubMed] [Google Scholar]
- 65.Belanger CF, Hennekens CH, Rosner B, et al. The nurses’ health study. Am J Nurs 1978;78:1039–40. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


