Abstract
Most forms of chronic kidney disease culminate in renal fibrosis that heralds organ failure. In contrast to the protective effects of globally blocking type 1 angiotensin (AT1) receptors throughout the body, activating AT1 receptors directly on immune cells may serve protective functions. However, the effects of stimulating the T-cell AT1 receptor on the progression of renal fibrosis remain unknown. In this study, mice with T-cell–specific deletion of the dominant murine AT1 receptor isoform Lck-Cre Agtraflox/flox [total knockout (TKO)] and wild-type (WT) controls were subjected to the unilateral ureteral obstruction model of kidney fibrosis. Compared with WT controls, obstructed kidneys from TKO mice at day 14 had increased collagen 1 deposition. CD4+ T cells, CD11b+Ly6Chi myeloid cells, and mRNA levels of Th1 inflammatory cytokines are elevated in obstructed TKO kidneys, suggesting that augmented Th1 responses in the TKO mice may exaggerate renal fibrosis by driving proinflammatory macrophage differentiation. In turn, T-bet deficient (T-bet knockout) mice lacking Th1 responses have attenuated collagen deposition after unilateral ureteral obstruction. We conclude that activating the AT1 receptor on T cells mitigates renal fibrogenesis by inhibiting Th1 differentiation and renal accumulation of profibrotic macrophages.
Virtually all forms of chronic kidney disease culminate in renal interstitial fibrosis, a predictor and harbinger of organ failure in most tissues. Although renal fibrosis is a consequence of sterile injury, inflammatory responses play a key role in its pathogenesis. For example, macrophage infiltration into the kidney triggers renal fibrosis via the elaboration of several profibrotic cytokines, including transforming growth factor (TGF)-β, tumor necrosis factor (TNF)-α, and IL-1β.1, 2, 3 T lymphocytes can also elaborate these cytokines, and recent studies have implicated T cells in mediating kidney scar formation.4, 5 However, the mechanisms through which T cells and macrophages interact to drive extracellular matrix deposition in the kidney require elucidation.
Angiotensin II (Ang II) is a potent instigator of renal fibrogenesis via the induction of TGF-β.6, 7 However, stimulating type 1 angiotensin (AT1) receptors in myeloid cells paradoxically attenuates renal scar formation by preventing activation of receptors for IL-1 in the kidney.8 In view of the emerging role of T cells in propagating kidney fibrosis,9 we directly interrogated the role of the T-cell AT1 receptor in regulating renal extracellular matrix deposition. To capture the functions of the AT1 receptor on both conventional T lymphocytes and so-called CD4− CD8− double-negative T cells, a novel mouse model of T-cell–specific AT1 receptor deletion driven by Cre recombinase under the control of the early thymocyte promoter Lck was generated.10 To circumvent the confounding effects of blood pressure elevation on renal damage, a normotensive murine model of kidney fibrosis, unilateral ureteral obstruction (UUO), was used. This model is ideal for examining AT1 receptor functions because UUO features prominent intrinsic activation of the renin angiotensin system (RAS).11
Materials and Methods
Animal
All mice were backcrossed to the 129/SvEv background for more than six generations to increase susceptibility to kidney injury. The 129/SvEv floxed Agtr1a mice were crossed with 129/SvEv Lck-cre mice to generate AT1 receptor total knockout (TKO) mice and wild-type (WT) Cre− Agtr1afl/fl littermates.10 T-bet (B6.129S6-Tbx21tm1Glm/J) heterozygotes were used as experimental breeders, yielding T-bet deficient [T-bet knockout (KO)] and WT littermates for experiments. To map the renal infiltration of T lymphocytes in UUO, mT/mG mice from the Jackson Laboratory (Bar Harbor, ME) were crossed with the Lck-Cre recombinase transgenic lines; tissues in mT/mG mice normally express red fluorescent protein and change to eGFP during Cre-mediated deletion of mT cassette. All the animal studies were approved by the Durham Veterans Affairs Medical Center Institutional Animal Care and Use Committees for compliance with regulations. Eight- to 12-week–old male mice and littermate controls were used for experiments.
UUO Model
The UUO model of renal fibrosis was performed as described previously.12 Briefly, mice weighing approximately 20 to 22 g were anesthetized with isoflurane, and the left ureter was tied off 3 to 5 mm below its origin. Fourteen days after ligation, mice were euthanized, and the obstructed and nonobstructed kidneys were harvested for analysis.
Cell Culture
To provide a quantitative evaluation of cytokine production in macrophages co-cultured with T lymphocytes, spleens were isolated from WT and TKO mice, and cell suspensions were obtained by homogenization with GentleMACS (Miltenyi Biotec, Bergisch Gladbach, Germany). The homogenate was filtered with 70- and 40-μm cell strainers, and cells were incubated with ACK buffer for 5 minutes. Splenocytes were then cultured in complete RPMI 1640 medium that contained 1% fetal bovine serum. Mouse splenocytes were stimulated with and without anti-CD3ε (1 μg/mL; BioLegend, San Diego, CA) and Ang II (1 μmol/L, Sigma-Aldrich, St. Louis, MO) for 24 hours. WT mice received 2.5 mL of 3% i.p. thyoglicollate 4 days before harvesting peritoneal macrophages. Macrophages were co-cultured with stimulated T cells and harvested 24 hours later for mRNA quantitation.
Histopathologic Analysis
Kidneys were fixed in 10% formalin and embedded with paraffin for staining. For collagen 1 staining, sections were stained with collagen 1 (Abcam, Cambridge, MA) followed by incubation with an horseradish peroxidase–conjugated secondary antibody. For collagen deposition scoring, images magnified ×20 were photographed, and positive areas were blindly analyzed (A.D.J.) by ImageJ software version 1.50 (NIH, Bethesda, MD; https://imagej.nih.gov/ij) as previously described.8 Briefly, at least 10 adjacent fields of renal cortex were photographed and scored, subtracting the background for the slide. Images with debris or poor sectioning were excluded. Field percentages of positive stains were averaged for each animal and then across each group. Kidney sections stained with Sirius red/fast green were examined for detection of total collagen and noncollagen protein ratio according to the manufacturer's instructions (Chondrex, Redmond, WA).
RNA Isolation and Real-Time PCR
Total RNA was isolated from cultured cells or tissues by using the RNAeasy Mini Kit (Qiagen, Valencia, CA). RNA was reverse transcribed to cDNA using the SuperScript II First-Strand Synthesis Kit (Invitrogen, Carlsbad, CA), and real-time PCR was performed with TaqMan probes (Applied Biosystems, Foster City, CA) for Col1, Tgfb1, Ifnr, PAI1, and Il1b. Agtr1a (forward 5′-GCTTGGTGGTGATCGTCACC-3′ and reverse 5′-GGGCGAGATTTAGAAGAACG-3′) and IL17a (Qiagen, Germantown, MD) mRNA abundance was quantitated via SYBR Green quantitative PCR as previously described.9
Flow Cytometry
Mice were deeply anesthetized, and the obstructed kidney was harvested. The decapsulated kidney was then minced and transferred to 5 mL of RPMI 1640 medium with 1 mg/mL of collagenase IV (Gibco, Carlsbad, CA) and 10 mg/mL of DNase (Sigma-Aldrich). After homogenization with GentleMACS (Miltenyi Biotec), the homogenate was incubated at 37°C for 30 minutes. After filtration with 70- and 40-μm cell strainers, cells were washed twice with wash buffer (Dulbecco's phosphate-buffered saline containing 2% fetal bovine serum and 2 mmol/L EDTA). The resulting cell suspension was treated with FC Block (BioLegend) and incubated with CD11b-fluorescein isothiocyanate, CD45-Brilliant Violet 785, lymphocyte antigen 6 complex (Ly6C)–Brilliant Violent 510, lymphocyte antigen 6 complex locus G (Ly6G) –phycoerythrin-cyanine 5.5 (all BioLegend), near-infrared dead cell indicator (Life Technologies, Carlsbad, CA), or CD3-fluorescein isothiocyanate, CD45-Brilliant Violet 510, CD8-phycoerythrin-cyanine 5.5 (all BioLegend), CD4-phosphatidylethanolamine, and near-infrared dead cell indicator (Life Technologies) at 4°C for 30 minutes. Cells were then washed and fixed (BD Biosciences, San Jose, CA). Flow cytometry was performed on a FACSCanto II (BD Biosciences), and data were analyzed by FlowJo software version 10 (Tree Star, Ashland, OR).
Statistical Analysis
The values within a group are expressed as means ± SEM. For comparisons between groups with normally distributed data, statistical significance was assessed using analysis of variance followed by unpaired t-test. Significance for all tests was set at P ≤ 0.05.
Results
Stimulation of AT1 Receptor on T Lymphocytes Limits Kidney Fibrosis and Inflammation
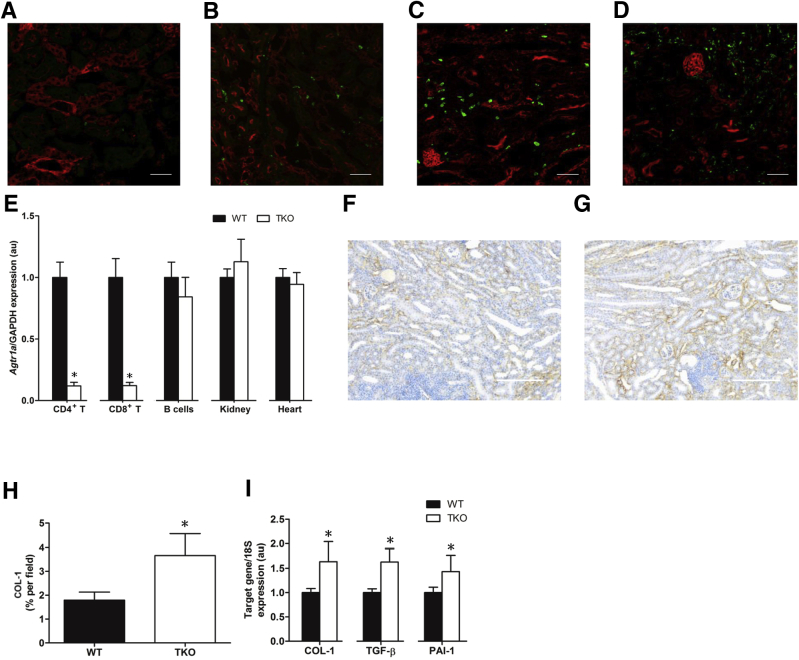
To examine the timing of renal T-cell infiltration following UUO, an Lck-Cre mT/mG reporter mouse strain was bred in which T lymphocytes fluoresce green, but all other cell lineages fluoresce red. After UUO, the most prominent accumulation of eGFP+ T lymphocytes throughout the renal parenchyma was noticed at 14 days (Figure 1, A–D). Ang II is a key driver of renal fibrosis.6, 7 To evaluate the functions of the AT1 receptor on both conventional and double-negative T lymphocytes during kidney fibrosis, the Lck-Cre mouse strain was bred with mice that harbor a floxed allele for the Agtr1a gene encoding the dominant murine AT1 receptor isoform, AT1A. To verify the specific deletion of the AT1 receptor on T lymphocytes in the TKO mice, CD4+ T cells (CD4+CD8−CD19−), CD8+ T cells (CD8+CD4−CD19−), and B lymphocytes (CD19+Thy1−) were isolated by a fluorescent sorting strategy. After mRNA from these subsets (kidney and heart) was obtained, the degree and precision of Lck-Cre–mediated Agtr1a deletion was quantitated (Figure 1E). Compared with WT littermates, TKO mice exhibited 90% deletion of Agtr1a in both CD4+ and CD8+ T lymphocytes. Agtr1a mRNA levels in all other tissues were similar between WT and TKO mice.
Figure 1.
Stimulation of type 1 angiotensin receptor on T lymphocytes limits kidney fibrosis and inflammation. A–D: Representative fluorescent images of kidney sections from obstructed Lck-Cre mT/mG mice after 1 (A), 3 (B), 7 (C), and 14 (D) days. E:Agtr1a mRNA expression in purified immune cell lineages and in kidney and heart from Lck-Cre Agtraflox/flox [total knockout (TKO)] and wild-type (WT) mice and normalized to WT in each tissue. F and G: Representative images of collagen 1 (COL-1) staining in kidneys from WT (F) and TKO (G) mice. H: Summary data for percentage of COL-1–positive area in obstructed kidneys from WT and TKO mice. I: mRNA expression of transforming growth factor (TGF)-β, COL-1, and plasminogen activator inhibitor (PAI)-1 in WT and TKO obstructed kidneys. n ≥ 6 for all groups. ∗P < 0.05 versus WT. Scale bars: 50 μm (A–D); 100 μm (F and G). au, arbitrary units; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
To determine how AT1 receptor stimulation on T cells modulates the progression of kidney fibrosis, TKO and WT mice were subjected to the UUO model and the deposition of collagen 1 was evaluated after 14 days of UUO by blinded immunohistochemistry staining (A.D.J.) (Figure 1, F and G). The obstructed kidneys from the TKO mice exhibited significantly increased deposition of collagen 1 compared with WT controls (P < 0.05) (Figure 1H). Consistent with the exaggerated fibrosis in the TKO kidneys, mRNA levels of Tgfb, Col1, and Pai1 in the TKO kidney were up-regulated by approximately 50% compared with WT values, respectively (P < 0.05) (Figure 1I). mRNA expression levels for the AT2 receptor were not different in the obstructed kidneys from the two groups (data not shown). These data suggested that AT1 receptor activation on renal T cells may regulate their differentiation and function during renal fibrosis.
AT1 Receptor Deficiency on T Lymphocytes Permits Proinflammatory Differentiation during Kidney Fibrosis
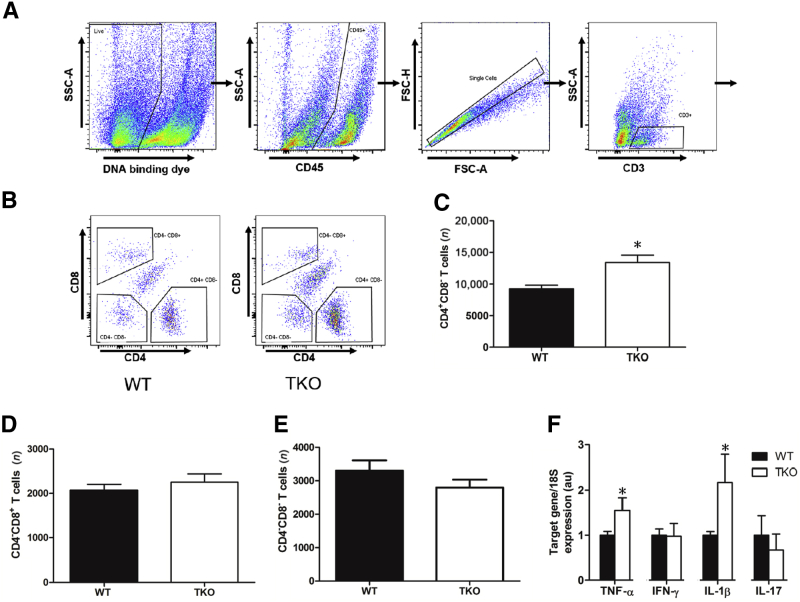
With FACS analysis, the proportions of CD4+, CD8+, and CD4−CD8− double-negative T cells were examined in the obstructed kidney (Figure 2, A and B). At 14 days of UUO, the proportions of CD4+ T cells were significantly higher in the TKO kidneys compared with the WT littermates (P = 0.0034) (Figure 2C). However, the analysis revealed similar proportions of CD8+ and double-negative T cells in the kidneys from TKO and WT mice (Figure 2, D and E). During the evolution of renal fibrosis, AT1 receptor stimulation on T cells could alter their differentiation into different subsets, such as Th1, Th2, Treg, and Th17. Enhanced TNFA and IL1B expression was found in the TKO kidneys, whereas levels of IFNG and IL17A were not altered (Figure 2F). Thus, deleting AT1 receptors on both conventional and double-negative T lymphocytes permits enhanced production of Th1-associated cytokines.
Figure 2.
Activating the T-cell type 1 angiotensin receptor suppresses accumulation of CD4+ T cells in obstructed kidney. A and B: Representative flow cytometry gating strategy for single cell suspensions from unilateral ureteral obstruction kidney. Live cells were gated for CD45+population, then single cells, then CD11b+ Ly6G- cells, as indicated by boxes and arrows. Among these CD11b+ Ly6G- cells, Ly6Chi populations were isolated, as indicated by boxes. C–E: Cell number of CD4+CD8− or CD4−CD8+ T lymphocytes in obstructed kidneys of Lck-Cre Agtraflox/flox [total knockout (TKO)] and wild-type (WT) mice. F: mRNA expression of tumor necrosis factor (TNF)-α, interferon (IFN)-γ, IL-1β, and IL-17 in WT and TKO obstructed kidneys. ∗P < 0.05 versus obstructed WT. au, arbitrary units; FSC-A, forward scatter area; FSC-H, forward scatter height; SSC-A, side scatter area.
Activating AT1 Receptors on T Cells Suppresses Macrophage Accumulation in the Obstructed Kidney
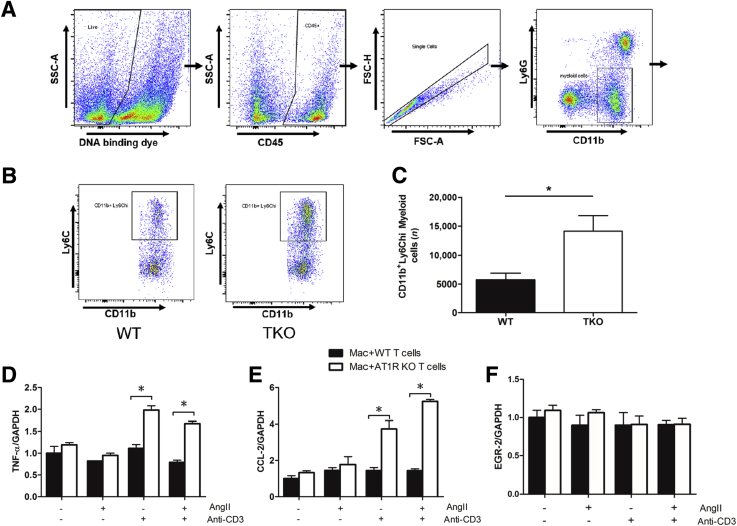
Because macrophages play a key role in renal fibrogenesis, it was hypothesized that AT1 receptor stimulation on T cells could mitigate renal scar formation by regulating the accumulation of macrophages in the obstructed kidney. Ly6C marks a population of proinflammatory infiltrating monocytes that have promoted renal fibrosis in our prior studies.8, 13 At 14 days after UUO, CD45-expressing hematopoietic cells were isolated from the obstructed kidneys of TKO and WT mice, and the numbers of Ly6Chi myeloid cells were quantitated by flow cytometry (Figure 3, A and B). CD11b+Ly6G−Ly6Chi inflammatory monocytes were increased in the kidneys of TKO mice compared with the WT group (P < 0.05) (Figure 3C). The T cells were then stimulated with and without Ang II and anti-CD3ε, and these T cells were co-cultured with peritoneal macrophages. Macrophages co-cultured with lymphocytes from the TKO mice had an increased generation of M1 markers TNFA and CCL2 compared with the WT group (P < 0.05) (Figure 3, D and E). By contrast, expression of the M2 marker EGR2 was similar in the macrophages co-cultured with lymphocytes from the TKO and WT mice (Figure 3F). Therefore, AT1 receptor stimulation on T lymphocytes suppresses the recruitment and activation of proinflammatory monocytes in the obstructed kidney.
Figure 3.
Type 1 angiotensin (AT1) receptors on T lymphocytes inhibit the infiltration of proinflammatory monocytes into the fibrosing kidney. A and B: Representative flow cytometry gating strategy. Live cells were gated for CD45+population, then single cells, then CD3+T cells, as indicated by boxes and arrows. CD3+ T cells were separated into CD4 and CD8 single positive populations, as indicated by boxes.C: Number of CD11b+Ly6Chi inflammatory monocytes in obstructed kidneys from Lck-Cre Agtraflox/flox [total knockout (TKO)] and wild-type (WT) mice. D–F: mRNA expression of tumor necrosis factor (TNF)-α, chemokine (C-C motif) ligand 2 (CCL2), and early growth response (EGR)-2 in wild-type macrophages co-cultured with T lymphocytes with and without AT1 receptor deletion. ∗P < 0.05. FSC-A, forward scatter area; FSC-H, forward scatter height; KO, knockout; SSC-A, side scatter area.
Proinflammatory Th1 Immune Responses Mediate UUO-Induced Renal Fibrosis
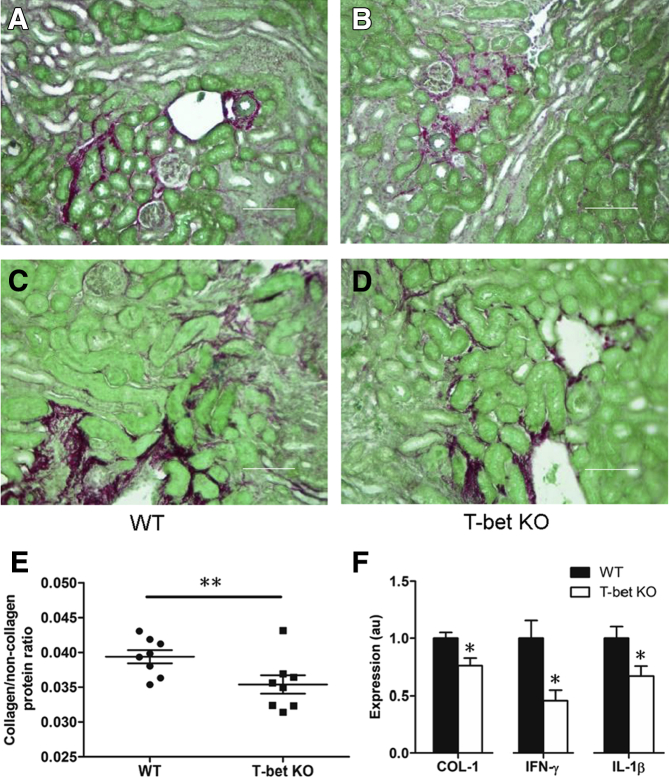
Two independent conditional gene deletion strategies found that stimulating the AT1 receptor on T cells suppresses their proinflammatory differentiation. The transcription factor T-bet drives and sustains Th1 differentiation in T cells. Thus, to investigate whether an enhanced renal Th1 response in the TKO cohort could account for their exaggerated kidney fibrosis following UUO, T-bet KO and WT control mice were subjected to UUO, and the extent of fibrosis was examined after 14 days. The obstructed kidneys from the T-bet KO mice exhibited significantly less deposition of collagen than WT littermates (Figure 4, A–E). Similarly, at the mRNA level, COL1 expression in the T-bet KO kidneys was significantly attenuated compared with the WT cohort (P < 0.05) (Figure 4F). mRNA levels for Agtr1a that encodes the AT1A receptor were similar in the obstructed kidneys from the WT and T-bet KO groups (1.00 ± 0.16 versus 0.77 ± 0.16; P = not significant). However, mRNA expression of Ifng and Il1b, in kidneys as markers of Th1 induction, was significantly down-regulated in the T-bet KO kidneys (P < 0.05) (Figure 4F). Thus, Th1 immune responses promote renal inflammation and fibrosis in the UUO model.
Figure 4.
Proinflammatory Th1 immune responses mediate renal fibrogenesis. A–D: Representative Sirius Red/Fast Green–stained sections of obstructed kidneys from obstructed wild-type (WT) and T-bet−/− [T-bet knockout (KO)] mice. E: Summary data for collagen deposition in WT and T-bet KO mice. F: mRNA expression of collagen (COL)-1, interferon (IFN)-γ, and IL-1β in kidneys from obstructed WT and T-bet KO mice. ∗P < 0.05, ∗∗P < 0.01 versus obstructed WT. Scale bars: 100 μm (A and B); 50 μm (C and D). Original magnification: ×100 (A and B); ×200 (C and D). au, arbitrary units.
Discussion
The key roles of AT1 receptor–mediated RAS activation in hypertension and target organ damage have long been recognized. Moreover, the primary effector molecule of the RAS, Ang II, is a critical driver of renal interstitial fibrosis.6 However, the cell-specific mechanisms through which the RAS modulates renal scar formation require elucidation. The T-cell AT1 receptor protects the kidney glomerulus in hypertension14; therefore, we investigated whether stimulating the AT1 receptor on T cells could affect the progression of renal fibrosis. Because unconventional, double-negative T cells play complex roles in the scarring of other organs,15 a unique experimental model of AT1 receptor deletion was developed in both conventional and unconventional T cells (TKO) to concomitantly interrogate the role of the AT1 receptor on both T-cell lineages to regulate kidney fibrosis. Stimulating the AT1 receptor on T cells attenuates renal fibrosis in vivo, further highlighting the complexity of the interactions between the RAS and the immune system in modulating renal pathologic findings.16
Infiltration of T cells into the renal parenchyma occurs in several forms of sterile kidney injury.17, 18 Whereas T lymphocytes are known to affect glomerular injury, the role of T cells in the pathogenesis of renal fibrosis have only recently received more intense scrutiny. For example, adoptive transfer studies implicated CD4+ T cells in augmenting the severity of UUO-induced kidney fibrosis,9 and Th17 cells in particular appear to play a key role in forming the renal scar that emerges after obstruction or ischemic injury.4, 5 Lck-Cre mT/mG reporter mouse strain revealed that T-lymphocyte infiltration increases steadily after ureteric obstruction and peaks at day 14, consistent with previous immunohistochemical staining of CD4 cells.9 Therefore, T-cell AT1 receptor were studied at this late timepoint.
The capacity of AT1 receptor activation on T lymphocytes to attenuate renal scar formation after UUO was robust in our studies with augmented collagen deposition in the TKO group at the mRNA and protein levels and up-regulated renal gene expression for the fibrosis mediators TGF-β and plasminogen activator 1. Thus, even though global RAS activation induces TGF-β and fibrogenesis, selectively stimulating the T-cell AT1 receptor appears to have the opposite effect and could represent a feedback mechanism to temper the severity of RAS-induced fibrosis. These findings are directionally consistent with the protective actions of the macrophage AT1 receptor in the UUO model8 and suggest that exaggerated renal or vascular inflammation seen in bone marrow chimeras lacking AT1 receptors on all immune cells accrues from deficiency of AT1 receptors on both lymphoid and myeloid cell lineages.19, 20, 21 Although the current AT1 receptor deletion is unique because it targets both conventional and unconventional T cells, enhanced renal accumulation of conventional T cells is seen in TKO mice just as seen earlier in conditional gene targeting studies, indicating that the net effect of stimulating AT1 receptors on conventional and double-negative T cells remains protective.
Whereas previous studies have implicated anti-inflammatory Th2 or Th17 cells in the pathogenesis of renal scar formation,4, 5, 18 these data suggest that AT1 receptor deletion on T cells may aggravate renal fibrosis by favoring the induction of proinflammatory Th1 cells. Enhanced Tnfa and Il1b mRNA levels are found in the TKO obstructed kidneys. Moreover, preventing Th1 induction via deletion of the transcription factor T-bet attenuates UUO-induced renal fibrosis. Proinjurious Th1 cytokines can trigger renal fibrogenesis because blockade of the Th1 cytokine TNF mitigates UUO-induced scar formation in the kidney.3 Thus, the persistent Th1-directed injury may ultimately culminate in renal fibrosis.
Macrophages play a critical role in regulating the progression of kidney fibrosis.22 Moreover, augmented levels of the macrophage cytokine IL-1β were detected in the obstructed kidneys from the TKO cohort. It was therefore posited that AT1 receptor stimulation on T cells could limit kidney scar formation by inhibiting the renal accumulation of myeloid cell populations. Among the myeloid cell populations, proinflammatory Ly6Chi infiltrating monocytes make important contributions to renal fibrosis.8, 13 Therefore, renal levels of CD11b+ Ly6Chi myeloid cells were quantified in the obstructed TKO kidneys and were increased threefold compared with WT controls. These data suggest that suppression of Th1 immune responses by the T-cell AT1 receptor mitigates renal fibrogenesis in part by limiting the accumulation of CD11b+Ly6Chi myeloid cells in the obstructed kidney, and co-culture studies further revealed the capacity of the T-cell AT1 receptor to restrict M1 differentiation of macrophages.
In summary, our experiments demonstrate a paradoxical role for AT1 receptors on T lymphocytes to attenuate kidney fibrosis by constraining the renal infiltration and differentiation of proinflammatory, profibrotic myeloid cells. This study highlights tissue-specific effects of RAS activation that will warrant consideration as gene therapies to target proteins in individual cell lineages become available.
Acknowledgments
Y.W. designed the work, acquired data, analyzed and interpreted the results, and wrote the manuscript; N.P.R., J.Z., and A.D.J. acquired data and analyzed and interpreted the results; R.G. performed surgeries; X.L., J.R., and J.P. analyzed flow cytometry data; S.D.C. conceived and designed the work, analyzed and interpreted the results, and revised and edited the manuscript; all authors approved the final version of the manuscript.
Footnotes
Supported by NIH grants DK087893, HL128355, and DK118019; US Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development grant BX000893; the Duke O'Brien Center for Kidney Research; National Institute of Diabetes and Digestive and Kidney Diseases grant P30DK096493; and American Heart Association Predoctoral Fellowship 18PRE34030402 (Y.W.).
Disclosures: None declared.
References
- 1.Jones L.K., O'Sullivan K.M., Semple T., Kuligowski M.P., Fukami K., Ma F.Y., Nikolic-Paterson D.J., Holdsworth S.R., Kitching A.R. IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrol Dial Transplant. 2009;24:3024–3032. doi: 10.1093/ndt/gfp214. [DOI] [PubMed] [Google Scholar]
- 2.Kitagawa K., Wada T., Furuichi K., Hashimoto H., Ishiwata Y., Asano M., Takeya M., Kuziel W.A., Matsushima K., Mukaida N., Yokoyama H. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165:237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meldrum K.K., Misseri R., Metcalfe P., Dinarello C.A., Hile K.L., Meldrum D.R. TNF-alpha neutralization ameliorates obstruction-induced renal fibrosis and dysfunction. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1456–R1464. doi: 10.1152/ajpregu.00620.2005. [DOI] [PubMed] [Google Scholar]
- 4.Mehrotra P., Collett J.A., McKinney S.D., Stevens J., Ivancic C.M., Basile D.P. IL-17 mediates neutrophil infiltration and renal fibrosis following recovery from ischemia reperfusion: compensatory role of natural killer cells in athymic rats. Am J Physiol Renal Physiol. 2017;312:F385–F397. doi: 10.1152/ajprenal.00462.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pindjakova J., Hanley S.A., Duffy M.M., Sutton C.E., Weidhofer G.A., Miller M.N., Nath K.A., Mills K.H., Ceredig R., Griffin M.D. Interleukin-1 accounts for intrarenal Th17 cell activation during ureteral obstruction. Kidney Int. 2012;81:379–390. doi: 10.1038/ki.2011.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Border W.A., Noble N.A. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:181–188. doi: 10.1161/01.hyp.31.1.181. [DOI] [PubMed] [Google Scholar]
- 7.Kagami S., Border W.A., Miller D.E., Noble N.A. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–2437. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J.D., Patel M.B., Griffiths R., Dolber P.C., Ruiz P., Sparks M.A., Stegbauer J., Jin H., Gomez J.A., Buckley A.F., Lefler W.S., Chen D., Crowley S.D. Type 1 angiotensin receptors on macrophages ameliorate IL-1 receptor-mediated kidney fibrosis. J Clin Invest. 2014;124:2198–2203. doi: 10.1172/JCI61368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tapmeier T.T., Fearn A., Brown K., Chowdhury P., Sacks S.H., Sheerin N.S., Wong W. Pivotal role of CD4+ T cells in renal fibrosis following ureteric obstruction. Kidney Int. 2010;78:351–362. doi: 10.1038/ki.2010.177. [DOI] [PubMed] [Google Scholar]
- 10.Lee P.P., Fitzpatrick D.R., Beard C., Jessup H.K., Lehar S., Makar K.W., Perez-Melgosa M., Sweetser M.T., Schlissel M.S., Nguyen S., Cherry S.R., Tsai J.H., Tucker S.M., Weaver W.M., Kelso A., Jaenisch R., Wilson C.B. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 11.Satoh M., Kashihara N., Yamasaki Y., Maruyama K., Okamoto K., Maeshima Y., Sugiyama H., Sugaya T., Murakami K., Makino H. Renal interstitial fibrosis is reduced in angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2001;12:317–325. doi: 10.1681/ASN.V122317. [DOI] [PubMed] [Google Scholar]
- 12.Chevalier R.L., Forbes M.S., Thornhill B.A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 2009;75:1145–1152. doi: 10.1038/ki.2009.86. [DOI] [PubMed] [Google Scholar]
- 13.Rudemiller N.P., Patel M.B., Zhang J.D., Jeffs A.D., Karlovich N.S., Griffiths R., Kan M.J., Buckley A.F., Gunn M.D., Crowley S.D. C-C Motif chemokine 5 attenuates angiotensin II-dependent kidney injury by limiting renal macrophage infiltration. Am J Pathol. 2016;186:2846–2856. doi: 10.1016/j.ajpath.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J.D., Patel M.B., Song Y.S., Griffiths R., Burchette J., Ruiz P., Sparks M.A., Yan M., Howell D.N., Gomez J.A., Spurney R.F., Coffman T.M., Crowley S.D. A novel role for type 1 angiotensin receptors on T lymphocytes to limit target organ damage in hypertension. Circ Res. 2012;110:1604–1617. doi: 10.1161/CIRCRESAHA.111.261768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammerich L., Tacke F. Role of gamma-delta T cells in liver inflammation and fibrosis. World J Gastrointest Pathophysiol. 2014;5:107–113. doi: 10.4291/wjgp.v5.i2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowley S.D., Rudemiller N.P. Immunologic effects of the renin-angiotensin system. J Am Soc Nephrol. 2017;28:1350–1361. doi: 10.1681/ASN.2016101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Miguel C., Das S., Lund H., Mattson D.L. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1136–R1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L., Kou P., Zeng Q., Pei G., Li Y., Liang H., Xu G., Chen S. CD4+ T lymphocytes, especially Th2 cells, contribute to the progress of renal fibrosis. Am J Nephrol. 2012;36:386–396. doi: 10.1159/000343283. [DOI] [PubMed] [Google Scholar]
- 19.Kato H., Ishida J., Nagano K., Honjo K., Sugaya T., Takeda N., Sugiyama F., Yagami K., Fujita T., Nangaku M., Fukamizu A. Deterioration of atherosclerosis in mice lacking angiotensin II type 1A receptor in bone marrow-derived cells. Lab Invest. 2008;88:731–739. doi: 10.1038/labinvest.2008.42. [DOI] [PubMed] [Google Scholar]
- 20.Nishida M., Fujinaka H., Matsusaka T., Price J., Kon V., Fogo A.B., Davidson J.M., Linton M.F., Fazio S., Homma T., Yoshida H., Ichikawa I. Absence of angiotensin II type 1 receptor in bone marrow-derived cells is detrimental in the evolution of renal fibrosis. J Clin Invest. 2002;110:1859–1868. doi: 10.1172/JCI200215045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowley S.D., Song Y.S., Sprung G., Griffiths R., Sparks M., Yan M., Burchette J.L., Howell D.N., Lin E.E., Okeiyi B., Stegbauer J., Yang Y., Tharaux P.L., Ruiz P. A role for angiotensin II type 1 receptors on bone marrow-derived cells in the pathogenesis of angiotensin II-dependent hypertension. Hypertension. 2010;55:99–108. doi: 10.1161/HYPERTENSIONAHA.109.144964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricardo S.D., van Goor H., Eddy A.A. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–3530. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]