Abstract
Historically, ductal carcinoma in situ (DCIS) of the breast has been managed aggressively with surgery and radiotherapy because of a risk of progression to invasive ductal carcinoma. However, this treatment paradigm has been challenged by overtreatment concerns and evidence that suggests that DCIS can be stratified according to risk of recurrence or risk of progression to invasive disease. Traditional methods of risk stratification include histologic grade and hormone receptor status. Recent technological advancements have enabled an era of precision medicine, where DCIS can be molecularly analyzed by tools, such as next-generation DNA and RNA sequencing, to identify molecular biomarkers for risk stratification. These findings have led to the development of tools such as the Oncotype DX Breast DCIS Score, a gene expression–based assay with the potential to prevent overtreatment in low-risk disease.
Human cancers of epithelial origin are thought to arise from a multistep process of tumorigenesis, where a normal stem cell acquires various insults to its genome and transforms into a premalignant cell.1 These cells in turn will acquire additional alterations that in time will result in transformation into a tumor cell with a malignant phenotype, which may include the ability to invade surrounding tissue and metastasize. Currently, the exact biological drivers that govern the transformation from premalignancy to malignancy are not well understood. Identifying these drivers in patients and targeting them appropriately represent a major opportunity in the clinical management of cancer.
The rapid technological development of methods for characterizing disease, including genomics, proteomics, metabolomics, cellular and histologic assays, and bioinformatics analyses, have ushered in a new era of precision medicine. Precision medicine involves the tailoring of individualized treatment strategies based on variability among patients.2, 3 The availability of these tools has enabled large, highly collaborative research efforts, such as The Cancer Genome Atlas, to perform analyses on large collections of cancer samples and clinical data to define the genomic landscape of cancers, including breast cancer.4 These groups, along with research from laboratories around the world, have been instrumental in advancing our understanding of the molecular drivers of various aspects of cancer biology, including initiation, metastasis, and response to treatment. Such discoveries have translated into biologic targets for the development of precision medicine therapy and combination therapy approaches for patients. To date, most large-scale efforts have been limited to invasive disease,5, 6 making the characterization and treatment of preinvasive disease an exciting research opportunity.
Ductal carcinoma in situ (DCIS), accounting for approximately 25% of all newly diagnosed breast cancers,7 is defined as a clonal proliferation of breast epithelial cells confined to the lumen of a mammary duct. DCIS is a heterogeneous disease, ranging from indolent quiescent forms to more aggressive forms that may rapidly evolve to invasive ductal carcinoma (IDC), with clinical, morphologic, and genetic variability.8, 9, 10 DCIS is treated with the goals of reducing the risks of recurrence and transformation to IDC. Currently, the standard of care for most DCIS is surgical excision by lumpectomy (breast-conserving surgery), with the addition of radiotherapy and appropriate systemic therapy (eg, tamoxifen) based on multiple histologic, biologic, and clinical factors. Adjuvant radiotherapy after breast-conserving surgery can reduce risk of local and invasive recurrence up to 48% and 42% at 10 years, respectively.11 The addition of adjuvant tamoxifen reduces both recurrence and progression to invasive carcinoma by approximately 50%12, 13; this reduction was largely driven by estrogen receptor (ER)–positive lesions.14 Furthermore, clinicopathologic studies have elucidated important demographic and histologic risk factors for recurrence, including age at diagnosis, lesion size, histologic grade, and width of the excision margin.15, 16
Even with the advances in disease control, the management of DCIS remains controversial. Several randomized trials have found that many DCIS lesions removed by surgical excision will not develop IDC or local recurrence, regardless of radiotherapy or systemic therapy.17 There is also evidence that low-risk DCIS can be adequately treated with no more than active surveillance,18, 19, 20 which is being further investigated in active clinical trials, such as the US Phase III Comparison of Operative to Monitoring and Endocrine Therapy trial for low-risk DCIS (Clinical Trial Identifier: NCT02926911) and the European Low Risk DCIS study (Clinical Trial Identifier: NCT02492607). In contrast, observations of the natural history of low-grade DCIS have also highlighted the continued risk for development of IDC. For example, in a small cohort of 45 women with low-grade DCIS treated by biopsy only and followed up for up to 42 years, 11 invasive breast cancers were diagnosed, and seven women developed distant metastases.19 Because both overtreatment and cancer progression remain significant concerns in DCIS, discerning which patients are more likely to have progressive disease remains an area of intense research efforts.
Models of DCIS Progression
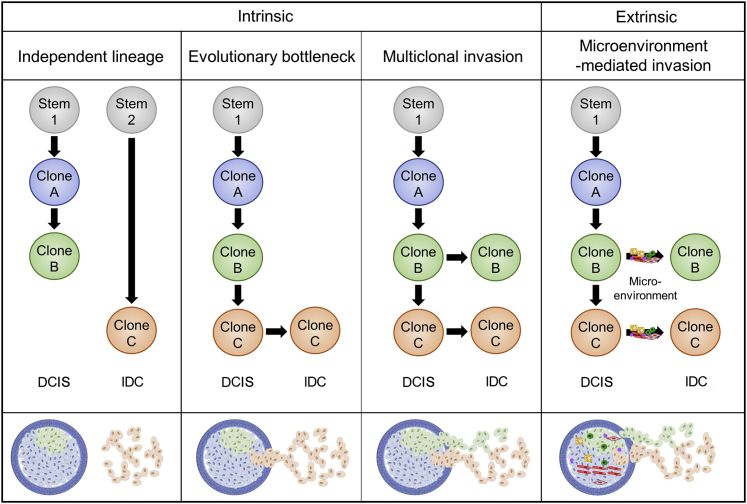
The models that govern progression of DCIS to IDC can be separated into intrinsic and extrinsic causes. Intrinsic causes involve changes that stem from genetic alterations in the DCIS cells, leading to an invasive phenotype. Extrinsic causes involve changes independent of genetic changes within the lesion, such as changes mediated by the surrounding tissue microenvironment. These models are summarized in Figure 1.
Figure 1.
Existing models of ductal carcinoma in situ (DCIS) progression to invasive ductal carcinoma (IDC). The independent lineage, evolutionary bottleneck, and multiclonal invasion models involve cell-intrinsic mechanisms of DCIS progression to IDC. The microenvironment-mediated invasion model is cell extrinsic and involves cellular factors, such as immune cells, fibroblasts, and endothelial cells, and noncellular factors, such as hormones, growth factors, extracellular matrix, and more. There is varying evidence that supports each model of progression and the simultaneous occurrence of multiple models.
There are three proposed intrinsic models of genomic evolution from DCIS to IDC.21 The first intrinsic model is the independent lineage model, in which DCIS and IDC arise from independent clonal cell populations. This model is also described as the field cancerization phenomenon, whereby regions of tissue that may be generally exposed to external mutagens can give rise to multiple, genetically distinct lesions. Support for this model involves single-marker studies that found a discordance between synchronous DCIS and IDC cases; for example, a study analyzing PIK3CA mutations in patients with matched IDC and DCIS reported only 30% concordance.22 This model is further supported by mathematical modeling.23 The second intrinsic model is the evolutionary bottleneck model, in which multiple clones are present in DCIS but only a single clone progresses to become IDC. This model is best supported by multiple phylogenetic studies that have identified truncal events concordant between DCIS and IDC, with additional copy number alterations (CNAs) and mutations that occurred later in evolution.24, 25 The third intrinsic model is the multiclonal invasion model, where multiple clones can escape from the duct and invade surrounding tissue. This model is supported by indirect evidence from extremely high levels of concordance between CNAs and mutations between DCIS and IDC (as high as 97%); in contrast, an evolutionary bottleneck model would likely have multiple divergent branches between its transition from DCIS to IDC, which would be expected to have lower levels of concordance.26, 27
Extrinsic causes of DCIS progression to IDC involve the microenvironment, which consists of the cellular components (eg, fibroblasts, endothelial cells, immune cells, adipocytes) and noncellular components [eg, extracellular matrix (ECM), growth factors, cytokines, pH] that influence the cancer cells.28, 29 The microenvironment has been causally implicated in multiple aspects of cancer biology, including progression, metastasis, and drug resistance.30, 31, 32, 33, 34 In DCIS, ECM remodeling, stromal cell interactions, myoepithelial disruption, and gene expression changes in stromal cells have been independently linked to DCIS progression. Studies have found that ECM remodeling mediated by lysyl oxidase–mediated collagen crosslinking are required for invasion in DCIS.35 In addition, remodeling of ECM secondary to mammary gland involution after pregnancy has been implicated in progression to an invasive phenotype mediated by an up-regulation of fibrillar collagen and cyclooxygenase-2 (COX-2) in DCIS.36 Stromal fibroblasts also increase COX-2 expression on interaction with DCIS epithelial cells, leading to up-regulation of vascular endothelial growth factor and matrix metalloproteinase (MMP)-14 and subsequent progression to an invasive phenotype.37 Myoepithelial cells normally exert tumor suppressive effects on DCIS lesions by both acting as a physical barrier to invasion and secreting ECM components and protease inhibitors38; these functions are disrupted in invasive disease.39 Several studies have found significant differences in the gene expression among fibroblasts, myoepithelial cells, and leukocytes in DCIS versus IDC40, 41; for example, transition to invasive growth was accompanied in one study by increased expression of several MMPs (MMP-2, MMP-11, and MMP-14), which are known effectors of cancer progression in multiple cancer types.42 Other well-studied extrinsic breast cancer risk factors, such as host circulating hormone levels (eg, estrogen), prior radiotherapy, and lifestyle factors (eg, obesity and alcohol), may also directly affect DCIS progression but have yet to be characterized in detail and warrant further study.
These models represent a simplified explanation of progression in DCIS; in reality, they are likely not mutually exclusive. A recent study analyzing genome-wide CNAs for single cells in formalin-fixed, paraffin-embedded tissues in DCIS and IDC revealed evidence for both the bottleneck and multiclonal invasion models in different patients, suggesting that progression models may differ on a patient-by-patient basis.43 Similarly, field cancerization and microenvironment-mediated effects could be direct drivers of transitions in the evolutionary bottleneck and multiclonal invasion models. The development of comprehensive mathematical models capable of incorporating the complexities of both intrinsic and extrinsic causes of DCIS progression represents an important area of future research.
Traditional Classification of DCIS
Traditionally, histopathologic features have been used as the standard for classifying DCIS, which have been reviewed extensively.44 Although there is no universally accepted classification system for DCIS, the most commonly used systems typically classify DCIS into three grades (low, intermediate, or high) based largely on nuclear grade and the presence or absence of necrosis.45 The association between nuclear grade and progression to IDC remains controversial; whereas some studies have reported evidence of an association between high-grade DCIS and progression to IDC,46 others have not found such an association.47 Future longitudinal studies are necessary to clarify these associations.
The most common immunohistochemical markers assessed in DCIS are ER and progesterone receptor (PR). Most DCIS lesions express ER (range, 49% to 96.6%) and PR (range, 40% to 83.3%), and expression of these receptors is highly correlated.48 Multiple studies have also found that ER- and PR-negative DCIS is associated with increased grade and risk of local recurrence.49, 50 The role of routine testing for human epidermal factor 2 (HER2) in DCIS remains unclear. Although HER2 expression in DCIS is correlated with high nuclear grade and increased risk of recurrence and negatively correlated with ER and PR expression,51, 52, 53, 54 it is not currently recommended to routinely test DCIS for HER2. The effect of radiotherapy with or without trastuzumab in HER2-positive patients with DCIS who have had a lumpectomy is being evaluated in the National Surgical Adjuvant Breast and Bowel Project B43 study, which is expected to be completed in 2019 (Clinical Trial Identifier: NCT00769379). None of the above receptors can reliably predict for recurrence or progression to an invasive phenotype. Our ability to identify those DCIS lesions that are more likely to recur or progress to invasive cancer remains limited, and adequate studies investigating novel potential predictive or prognostic biomarkers in DCIS are lacking. Kerlikowske et al47 reported an association between progression to invasive cancer and co-expression of Ki-67, p16, and COX-2. However, this association has yet to be validated. A variety of factors, including small sample size, lesion heterogeneity, lack of significant clinical follow-up, and issues with standardization of scoring methods and reproducibility, are common limitations to studies investigating various biomarkers.
Classification of DCIS in a Precision Medicine Era
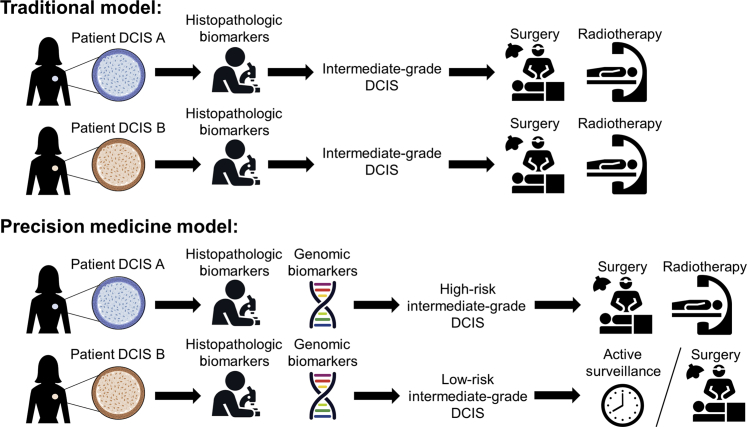
In the current precision medicine era, a large variety of molecular tools are available to fully characterize a patient's cancer. Single-nucleotide alterations, DNA CNAs, genomic structural rearrangements, gene expression, epigenetic alterations, and other features can be routinely tested using array-based and sequencing-based technologies.55 These technologies have been used to comprehensively characterize invasive tumors, leading to the identification of new molecular targets and genetic loci associated with breast cancer risk and progression.4, 56, 57, 58 Recently, discovery of genomic biomarkers of progression from DCIS to IDC has been identified as a valuable precision medicine opportunity: clinically separating aggressive from indolent DCIS will allow for both prevention of progression to IDC and, ultimately, prevention of overtreatment in patients who would not likely benefit. For example, two patients with histologically identical intermediate-grade DCIS could both be recommended for treatment with surgery and radiotherapy; however, by using a precision medicine–based treatment approach that incorporates genomic biomarkers, the two lesions could be separated as high or low risk. The high-risk DCIS can be treated with surgery and radiotherapy, whereas, the low-risk DCIS can be treated with active surveillance or surgery alone (Figure 2). Although there are multiple areas being explored in the molecular characterization of DCIS, we restrict this review to two major areas of focus: genetic alterations and gene expression.
Figure 2.
Comparing traditional treatment models to precision medicine–informed treatment models in ductal carcinoma in situ (DCIS). In a traditional paradigm of DCIS treatment, two patients (A and B) with an intermediate-grade DCIS would be treated with surgery and radiotherapy. In contrast, a precision medicine–based treatment approach would incorporate genomic biomarkers to stratify DCIS lesions according to risk of recurrence or invasion; in this example, one patient's DCIS (patient DCIS A) was determined to be high risk, which would be treated with the standard surgery and radiotherapy, whereas the other low-risk DCIS (patient DCIS B) would be treated with active surveillance or surgery only.
Genetic Alterations
Multiple studies have compared the genetics of DCIS and IDC within a given patient to identify genetic biomarkers that might contribute to disease progression, using a variety of array- and sequencing-based technologies. In general, many studies reported no association between genetic alterations and progression of DCIS to IDC. Burkhardt et al59 performed fluorescence in situ hybridization (FISH) on a tissue microarray of 130 pure DCIS samples and 159 DCIS samples with concurrent invasive breast cancer. They found no significant differences in amplification rates of genes commonly amplified in breast cancer, including ERBB2, ESR1, CCND1, and MYC, and found high concordance in general gene amplification rates between the two groups. Pan et al60 used quantitative multigene FISH to assess gene CNAs in 30 genes in 66 tumors with synchronous DCIS and IDC. They identified frequent amplification of genes such as MDM4, CCNE2, ERBB2, IGF1R, CKS1BP7, and MYC and frequent deletion of TP53, CHEK1, RB1, CDH1, CHEK2, and NEK9, each of which was observed in >20% of cases; however, no significant differences were detected between DCIS and IDC. Finally, Rane et al61 conducted a meta-analysis based on results from 26 studies, comparing cases of atypical ductal hyperplasia, pure DCIS, synchronous DCIS with IDC, and pure IDC. Again, no significant differences were found between the numbers and types of CNAs identified between DCIS and IDC, which suggests that CNAs are early events in the development of breast cancer.
Some studies have suggested genetic differences between DCIS and IDC that may be implicated in DCIS progression. Johnson et al26 found differences in CNAs between 21 cases of synchronous DCIS and IDC. Although CNA profiles between DCIS and IDC were highly synchronous (mean of 83% of the genome shared), IDC samples had regions of chromosomal gain that included the oncogenes CCND1 and MYC when compared with DCIS samples. Furthermore, observational comparisons suggest that TP53 mutations may contribute to invasive progression because studies have found a lower prevalence of TP53 mutations in DCIS (approximately 17%)62 compared with IDC (approximately 37%).4 Finally, a recent study of 111 DCIS lesions by Pang et al63 found that the frequency of GATA3 mutations was increased in DCIS compared with levels found in multiple studies of IDC. This finding, coupled with the fact that GATA3 mutations are associated with improved survival in invasive breast cancer,64 suggests that these mutations are selected against in progression of IDC.
Despite the wealth of data published in the literature, limited evidence suggests that there are significant genetic differences between DCIS and IDC; the studies that suggest significant differences are limited by small sample size and lack of functional applicability. Larger longitudinal studies are warranted.
Gene Expression
Compared with genetic alteration–based biomarkers, gene expression–based biomarkers have had much greater clinical success in DCIS. The first and only commercially available multigene expression panel is the 12-gene Oncotype DX Breast DCIS assay, which was developed to stratify individual patients with DCIS into groups with different degrees of risk for local recurrence.65 This assay uses a combination of seven cancer-related genes [MKI67 (Ki-67), STK15, BIRC5 (Survivin), CCNB1 (Cyclin B1), MYBL2, PGR (PR), and GSTM1] and five reference genes [ACTB (β-actin), GAPDH, RPLPO, GUS, and TFRC] to predict the risk of breast cancer recurrence after breast-conserving therapy for DCIS. The test provides an individualized 10-year risk of local recurrence (DCIS and/or IDC), a prediction of benefit from radiotherapy, quantitative ER and PR gene expression values, and a numeric score that places patients into low-, intermediate-, or high-risk categories.
The Oncotype DX Breast DCIS assay predictions are supported by two clinical validation trials, the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network E5194 study66 and the Ontario DCIS Cohort study,67 which used retrospective samples from patients with surgically treated DCIS that did not receive radiotherapy. A meta-analysis of these two validation studies also found that combining the Oncotype DCIS Score with lesion size and age identified more patients at the extremes of the risk spectrum: higher risk and very low risk of local recurrence.68 These studies also endorse that the Oncotype DX Breast DCIS Score predicts 10-year local recurrence risk more accurately than traditional clinical and pathologic factors. Additional studies have found that conventional clinical and histopathologic characteristics correlate with Oncotype DX Breast DCIS Scores (ie, high nuclear grade DCIS with comedo necrosis correlates with higher Oncotype DCIS Scores; low nuclear grade, strongly ER-positive DCIS correlates with lower Oncotype DCIS Scores).69, 70 Two recent studies suggest that the Oncotype DX Breast DCIS assay has already led to a significant reduction in radiotherapy recommendations by surgeons and radiation oncologists.71, 72
A few limitations of the Oncotype DX Breast DCIS Score should be noted. One major limitation is expense; a recent cost-benefit analysis revealed that although incorporation of the Oncotype DCIS Score decreases the proportion of women undergoing radiotherapy per recurrence event prevented, these strategies were not cost-effective.17 Similarly, it is currently unknown whether Oncotype DCIS Score alone, clinicopathologic features alone, or a combination of the two should be used to predict recurrence and identify patients who may or may not benefit from radiotherapy. Finally, there is currently a lack of prospective evidence for ability of the Oncotype DCIS Score to significantly change patient outcomes, although this may change with the results of ongoing prospective clinical trials (eg, NCT02766881).
Additional gene expression studies have identified differences between patients with DCIS and patients with DCIS and synchronous IDC. In a matched case-control study with 24 patients by Doebar et al,73 hierarchical clustering of gene expression data revealed distinct DCIS gene expression patterns of patients with and without synchronous IDC. Genes highly expressed in DCIS samples with IDC included PLAU, COL1A1, KRT81, S100A7, SCGB1D2, KRT18, and NOTCH3, whereas genes higher in DCIS only cases included EGFR and CXCL14; these findings were confirmed by immunohistochemical analyses. Another study of 53 DCIS and 51 IDC lesions by Lee et al74 identified a 74-gene signature capable of predicting DCIS and IDC in the 104 lesions with 96% accuracy, as well as lesions from three independent patient cohorts with 94% accuracy. The authors additionally found that inhibition of four genes (CSTA, FAT1, DST, and TMEM45A) in a DCIS xenograft model (grown in mice) suppressed progression of DCIS. Recently, Sokol et al75 found that SMARCE1 expression is required for DCIS invasion by regulating the expression of secreted proteases targeting the basement membrane. Although these studies have identified many gene expression differences between indolent and aggressive disease, confirming a functional role of such changes in the transformation of DCIS remains a necessary next step.
One of the major drivers of gene expression changes is epigenetic modifications, which involve biological mechanisms of altering gene expression without changing the underlying DNA. The most well-studied epigenetic change in DCIS is DNA methylation, which typically acts to repress gene transcription. For example, studies have linked increased promoter methylation of a subset of genes (eg, TWIST1, FOXC1, and HOXA10) to invasive progression.76, 77, 78 A study by Johnson et al79 found that differentially methylated loci in DCIS that progressed to IDC were enriched for homeobox-containing genes and genes involved with limb morphogenesis (eg, HOXB13 and EN1). In a study by Fleischer et al,80 DNA methylation signatures were prognostic of survival in patients with breast cancer and DCIS, mixed DCIS and IDC, and IDC lesions. However, this study was unable to use methylation data to separate DCIS, mixed DCIS and IDC, and IDC using unsupervised clustering methods, limiting the value of these findings for characterizing DCIS progression. In general, epigenetic biomarkers have lacked the validation of larger prospective trials but remain an important potential factor affecting disease progression.
Emerging Research in DCIS
Although gene expression-based subtyping has been successful in predicting indolent versus invasive disease and stratifying patients into risk groups, we have only begun to scratch the surface of precision medicine biomarker opportunities in DCIS: the precision medicine era has a wealth of assays at the DNA, RNA, and protein levels at its disposal for future study (Table 1). Most of the studies performed thus far have used array-based gene expression technologies, FISH-based approaches for CNAs, and targeted DNA panels for mutation changes. In comparison, advances in whole-genome RNA and DNA sequencing now allow for much more powerful tools for analysis that are no longer cost-prohibitive.81 There are many benefits of high-depth sequencing approaches in the identification of biomarkers of DCIS progression: i) increasing the depth of sequencing will allow for the ability to detect mutations and genetic alterations present in smaller fractions within a lesion and can also be used to more specifically track clonality as lesions evolve from DCIS to IDC82, 83; ii) sequencing enables detection of chromosomal alterations, such as translocations, inversions, and deletions,84 which have been described as putative oncogenic events in breast cancer85; iii) sequencing allows for detection of nonprotein coding regions of the genome, such as long noncoding RNAs,86 which have recently been implicated in multiple aspects of cancer biology, including invasion and metastasis87, 88; and iv) although it requires a different sample processing step, sequencing is also able to evaluate microRNAs, such as microRNA-155, which is regulated by transforming growth factor-β signaling to induce invasion and metastasis.89 Furthermore, additional sequencing technologies, such as single-cell DNA and RNA sequencing, are now available, which will enable more detailed analysis of genetic alterations in subclonal populations.90 Together, these technologies may be able to clarify mechanisms of progression from DCIS to IDC.
Table 1.
Biomarkers and Available Technologies for Future Ductal Carcinoma in Situ Studies
| Category | Available technologies | Assayed biomarkers | Advantages | Disadvantages |
|---|---|---|---|---|
| DNA | Sanger sequencing | Mutations | Low cost, easy setup and analysis | Low throughput, primer dependent, large input requirement, high error rate |
| Next-generation sequencing | Mutations, copy number alterations, rearrangements, epigenetics | High resolution, sensitive | High cost, difficult analysis pipeline | |
| Single-cell sequencing | Mutations, copy number alterations, rearrangements, epigenetics | Single-cell resolution, can assess heterogeneity | High cost, difficult setup and analysis pipeline | |
| PCR | Mutations, rearrangements | Low-input material requirement, sensitive | Low throughput, primer dependent, high error rate | |
| Microarray (SNP, CGH) | Mutations, copy number alterations, rearrangements | High throughput | Restricted to predetermined alterations, prone to batch effects | |
| Karyotyping | Rearrangements | Low cost | Time-consuming, difficult setup, low resolution | |
| FISH | Copy number alterations, rearrangements | Quantitative, specific | Probe dependent, sensitivity | |
| RNA | RT-PCR | mRNA, miRNA, lncRNA | Easy setup and analysis, low-input material requirement, specific | Low throughput, primer dependent |
| NanoString | mRNA, miRNA (DNA and protein assays also available) | Reproducibility, sensitivity | Proprietary technology, high cost | |
| Microarray | mRNA, miRNA, lncRNA | High throughput | Restricted to predetermined alterations | |
| Next-generation sequencing | mRNA, miRNA, lncRNA | High resolution, sensitive | High cost, difficult analysis pipeline | |
| Single-cell RNA sequencing | mRNA, miRNA, lncRNA | Single-cell resolution, can assess heterogeneity | High cost, difficult setup and analysis pipeline | |
| Protein | Immunohistochemistry | Protein expression, posttranslational modifications, metabolites | Low cost, easy setup and analysis, preserves histologic information | Tissue-preparation variability, antibody dependent, semiquantitative |
| ELISA | Protein expression, posttranslational modifications, metabolites | Easy setup and analysis, low cost | Antibody dependent | |
| Western blot | Protein expression, posttranslational modifications, metabolites | Easy setup and analysis, low cost | Antibody dependent, semiquantitative | |
| Flow cytometry | Protein expression, posttranslational modifications, metabolites | Live cell setting, single-cell resolution | High cost, difficult setup and analysis pipeline, cell surface proteins only | |
| Mass spectrometry | Protein expression, posttranslational modifications, metabolites | Antibody independent, sensitive, specific | High cost, difficult setup and analysis pipeline |
CGH, comparative genomic hybridization; ELISA, enzyme-linked immunosorbent assay; FISH, fluorescence in situ hybridization; lncRNA, long noncoding RNA; SNP, single-nucleotide polymorphism.
Additional avenues of research include moving beyond the DNA and mRNA levels and looking for biomarkers of progression at the protein and metabolite levels. For example, a study by Mao et al91 found that mass spectrometry–based proteomics could predict both subtype and grade of DCIS and IDC. Furthermore, they found that subtypes and histologic grades of IDC and DCIS could be discriminated by lipid content: phospholipids were found to be more abundant in IDC compared with DCIS, whereas fatty acids were more abundant in DCIS than IDC. In addition, there are many posttranslational modifications that affect proteins that are known to contribute to cancer progression, including phosphorylation, acetylation, methylation, ubiquitination, sumoylation, and prenylation.92 Incorporating such biomarkers into existing prognostic signatures of DCIS represents exciting future research directions.
Conclusion
More than 50% of DCIS will remain indolent and never progress to IDC.20 Identifying which will remain indolent and which will progress is the subject of many ongoing research efforts. Newer technologies, such as next-generation DNA and RNA sequencing, have enabled the identification of potential genomic and transcriptomic biomarkers that have the potential to replace or enhance current histopathologic risk stratification methods. Certain risk classification systems, such as the Oncotype DX Breast DCIS assay, have been found in limited studies to successfully predict risk of recurrence in DCIS and have the potential to prevent overtreatment in lower-risk disease. Future studies using next-generation technology for genomics, proteomics, and other -omics approaches may identify novel biomarkers that may enhance or replace these existing methods, although they will need to be validated in large cohorts of patients. Biomarkers of DCIS risk have the potential to transform patient care by informing appropriate clinical management and remain an important focus of future research.
Footnotes
Breast Ductal Carcinoma in situ Theme Issue
Supported by NIH grant F30CA216966 (K.S.).
Disclosures: None declared.
This article is part of a review series on Ductal Carcinoma in Situ–Discerning Aggressive versus Benign Disease Using Molecular Features.
References
- 1.Meador C.B., Micheel C.M., Levy M.A., Lovly C.M., Horn L., Warner J.L., Johnson D.B., Zhao Z., Anderson I.A., Sosman J.A., Vnencak-Jones C.L., Dahlman K.B., Pao W. Beyond histology: translating tumor genotypes into clinically effective targeted therapies. Clin Cancer Res. 2014;20:2264–2275. doi: 10.1158/1078-0432.CCR-13-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins F.S., Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley E.A. The precision medicine initiative: a new national effort. JAMA. 2015;313:2119–2120. doi: 10.1001/jama.2015.3595. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garraway L.A., Lander E.S. Lessons from the cancer genome. Cell. 2013;153:17–37. doi: 10.1016/j.cell.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Jr., Kinzler K.W. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 8.Clark S.E., Warwick J., Carpenter R., Bowen R.L., Duffy S.W., Jones J.L. Molecular subtyping of DCIS: heterogeneity of breast cancer reflected in pre-invasive disease. Br J Cancer. 2011;104:120–127. doi: 10.1038/sj.bjc.6606021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhl C.K., Schrading S., Bieling H.B., Wardelmann E., Leutner C.C., Koenig R., Kuhn W., Schild H.H. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007;370:485–492. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 10.Cowell C.F., Weigelt B., Sakr R.A., Ng C.K., Hicks J., King T.A., Reis-Filho J.S. Progression from ductal carcinoma in situ to invasive breast cancer: revisited. Mol Oncol. 2013;7:859–869. doi: 10.1016/j.molonc.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Group E.B.C.C., Group E.R., Bijker N., Meijnen P., Peterse J.L., Bogaerts J., Van Hoorebeeck I., Julien J.P., Gennaro M., Rouanet P., Avril A., Fentiman I.S., Bartelink H., Rutgers E.J. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European Organisation for Research and Treatment of Cancer randomized phase III trial 10853--a study by the EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. J Clin Oncol. 2006;24:3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B., Dignam J., Wolmark N., Wickerham D.L., Fisher E.R., Mamounas E., Smith R., Begovic M., Dimitrov N.V., Margolese R.G., Kardinal C.G., Kavanah M.T., Fehrenbacher L., Oishi R.H. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B., Costantino J.P., Wickerham D.L., Redmond C.K., Kavanah M., Cronin W.M., Vogel V., Robidoux A., Dimitrov N., Atkins J., Daly M., Wieand S., Tan-Chiu E., Ford L., Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 14.Cunnick G.H., Mokbel K. Radiotherapy and tamoxifen in women with completely excised ductal carcinoma in situ. Lancet. 2003;362:1154. doi: 10.1016/s0140-6736(03)14475-3. [DOI] [PubMed] [Google Scholar]
- 15.Poulakaki N., Makris G.M., Battista M.J., Bohm D., Petraki K., Bafaloukos D., Sergentanis T.N., Siristatidis C., Chrelias C., Papantoniou N. Hormonal receptor status, Ki-67 and HER2 expression: prognostic value in the recurrence of ductal carcinoma in situ of the breast? Breast. 2016;25:57–61. doi: 10.1016/j.breast.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Collins L.C., Achacoso N., Haque R., Nekhlyudov L., Fletcher S.W., Quesenberry C.P., Jr., Schnitt S.J., Habel L.A. Risk factors for non-invasive and invasive local recurrence in patients with ductal carcinoma in situ. Breast Cancer Res Treat. 2013;139:453–460. doi: 10.1007/s10549-013-2539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raldow A.C., Sher D., Chen A.B., Recht A., Punglia R.S. Cost effectiveness of the oncotype DX DCIS score for guiding treatment of patients with ductal carcinoma in situ. J Clin Oncol. 2016;34:3963–3968. doi: 10.1200/JCO.2016.67.8532. [DOI] [PubMed] [Google Scholar]
- 18.Betsill W.L., Jr., Rosen P.P., Lieberman P.H., Robbins G.F. Intraductal carcinoma: long-term follow-up after treatment by biopsy alone. JAMA. 1978;239:1863–1867. doi: 10.1001/jama.239.18.1863. [DOI] [PubMed] [Google Scholar]
- 19.Sanders M.E., Schuyler P.A., Dupont W.D., Page D.L. The natural history of low-grade ductal carcinoma in situ of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer. 2005;103:2481–2484. doi: 10.1002/cncr.21069. [DOI] [PubMed] [Google Scholar]
- 20.Erbas B., Provenzano E., Armes J., Gertig D. The natural history of ductal carcinoma in situ of the breast: a review. Breast Cancer Res Treat. 2006;97:135–144. doi: 10.1007/s10549-005-9101-z. [DOI] [PubMed] [Google Scholar]
- 21.Casasent A.K., Edgerton M., Navin N.E. Genome evolution in ductal carcinoma in situ: invasion of the clones. J Pathol. 2017;241:208–218. doi: 10.1002/path.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miron A., Varadi M., Carrasco D., Li H., Luongo L., Kim H.J., Park S.Y., Cho E.Y., Lewis G., Kehoe S., Iglehart J.D., Dillon D., Allred D.C., Macconaill L., Gelman R., Polyak K. PIK3CA mutations in in situ and invasive breast carcinomas. Cancer Res. 2010;70:5674–5678. doi: 10.1158/0008-5472.CAN-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sontag L., Axelrod D.E. Evaluation of pathways for progression of heterogeneous breast tumors. J Theor Biol. 2005;232:179–189. doi: 10.1016/j.jtbi.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Yates L.R., Gerstung M., Knappskog S., Desmedt C., Gundem G., Van Loo P. Subclonal diversification of primary breast cancer revealed by multiregion sequencing. Nat Med. 2015;21:751–759. doi: 10.1038/nm.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Newburger D.E., Kashef-Haghighi D., Weng Z., Salari R., Sweeney R.T., Brunner A.L., Zhu S.X., Guo X., Varma S., Troxell M.L., West R.B., Batzoglou S., Sidow A. Genome evolution during progression to breast cancer. Genome Res. 2013;23:1097–1108. doi: 10.1101/gr.151670.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson C.E., Gorringe K.L., Thompson E.R., Opeskin K., Boyle S.E., Wang Y., Hill P., Mann G.B., Campbell I.G. Identification of copy number alterations associated with the progression of DCIS to invasive ductal carcinoma. Breast Cancer Res Treat. 2012;133:889–898. doi: 10.1007/s10549-011-1835-1. [DOI] [PubMed] [Google Scholar]
- 27.Oikawa M., Yano H., Matsumoto M., Otsubo R., Shibata K., Hayashi T., Abe K., Kinoshita N., Yoshiura K., Nagayasu T. A novel diagnostic method targeting genomic instability in intracystic tumors of the breast. Breast Cancer. 2015;22:529–535. doi: 10.1007/s12282-013-0516-9. [DOI] [PubMed] [Google Scholar]
- 28.Egeblad M., Nakasone E.S., Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierard G.E., Pierard-Franchimont C., Delvenne P. Malignant melanoma and its stromal nonimmune microecosystem. J Oncol. 2012;2012:584219. doi: 10.1155/2012/584219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R., Carey V.J., Richardson A.L., Weinberg R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 31.Qian B.Z., Li J., Zhang H., Kitamura T., Zhang J., Campion L.R., Kaiser E.A., Snyder L.A., Pollard J.W. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shee K., Yang W., Hinds J.W., Hampsch R.A., Varn F.S., Traphagen N.A., Patel K., Cheng C., Jenkins N.P., Kettenbach A.N., Demidenko E., Owens P., Faber A.C., Golub T.R., Straussman R., Miller T.W. Therapeutically targeting tumor microenvironment-mediated drug resistance in estrogen receptor-positive breast cancer. J Exp Med. 2018;215:895–910. doi: 10.1084/jem.20171818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Straussman R., Morikawa T., Shee K., Barzily-Rokni M., Qian Z.R., Du J., Davis A., Mongare M.M., Gould J., Frederick D.T., Cooper Z.A., Chapman P.B., Solit D.B., Ribas A., Lo R.S., Flaherty K.T., Ogino S., Wargo J.A., Golub T.R. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature. 2012;487:500–504. doi: 10.1038/nature11183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trimboli A.J., Cantemir-Stone C.Z., Li F., Wallace J.A., Merchant A., Creasap N., Thompson J.C., Caserta E., Wang H., Chong J.L., Naidu S., Wei G., Sharma S.M., Stephens J.A., Fernandez S.A., Gurcan M.N., Weinstein M.B., Barsky S.H., Yee L., Rosol T.J., Stromberg P.C., Robinson M.L., Pepin F., Hallett M., Park M., Ostrowski M.C., Leone G. Pten in stromal fibroblasts suppresses mammary epithelial tumours. Nature. 2009;461:1084–1091. doi: 10.1038/nature08486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levental K.R., Yu H., Kass L., Lakins J.N., Egeblad M., Erler J.T., Fong S.F., Csiszar K., Giaccia A., Weninger W., Yamauchi M., Gasser D.L., Weaver V.M. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyons T.R., O'Brien J., Borges V.F., Conklin M.W., Keely P.J., Eliceiri K.W., Marusyk A., Tan A.C., Schedin P. Postpartum mammary gland involution drives progression of ductal carcinoma in situ through collagen and COX-2. Nat Med. 2011;17:1109–1115. doi: 10.1038/nm.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu M., Peluffo G., Chen H., Gelman R., Schnitt S., Polyak K. Role of COX-2 in epithelial-stromal cell interactions and progression of ductal carcinoma in situ of the breast. Proc Natl Acad Sci U S A. 2009;106:3372–3377. doi: 10.1073/pnas.0813306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sternlicht M.D., Kedeshian P., Shao Z.M., Safarians S., Barsky S.H. The human myoepithelial cell is a natural tumor suppressor. Clin Cancer Res. 1997;3:1949–1958. [PubMed] [Google Scholar]
- 39.Barsky S.H., Karlin N.J. Mechanisms of disease: breast tumor pathogenesis and the role of the myoepithelial cell. Nat Clin Pract Oncol. 2006;3:138–151. doi: 10.1038/ncponc0450. [DOI] [PubMed] [Google Scholar]
- 40.Allinen M., Beroukhim R., Cai L., Brennan C., Lahti-Domenici J., Huang H., Porter D., Hu M., Chin L., Richardson A., Schnitt S., Sellers W.R., Polyak K. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Ma X.J., Dahiya S., Richardson E., Erlander M., Sgroi D.C. Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Res. 2009;11:R7. doi: 10.1186/bcr2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 43.Martelotto L.G., Baslan T., Kendall J., Geyer F.C., Burke K.A., Spraggon L., Piscuoglio S., Chadalavada K., Nanjangud G., Ng C.K., Moody P., D'Italia S., Rodgers L., Cox H., da Cruz Paula A., Stepansky A., Schizas M., Wen H.Y., King T.A., Norton L., Weigelt B., Hicks J.B., Reis-Filho J.S. Whole-genome single-cell copy number profiling from formalin-fixed paraffin-embedded samples. Nat Med. 2017;23:376–385. doi: 10.1038/nm.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones J.L. Overdiagnosis and overtreatment of breast cancer: progression of ductal carcinoma in situ: the pathological perspective. Breast Cancer Res. 2006;8:204. doi: 10.1186/bcr1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lakhani S.R., Ellis I.O., Schnitt S.J., Tan P.H., van de Vijver M.J. World Health Organization; Lyon, France:: 2012. WHO Classification of Tumours of the Breast. [Google Scholar]
- 46.Kerlikowske K., Molinaro A., Cha I., Ljung B.M., Ernster V.L., Stewart K., Chew K., Moore D.H., 2nd, Waldman F. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst. 2003;95:1692–1702. doi: 10.1093/jnci/djg097. [DOI] [PubMed] [Google Scholar]
- 47.Kerlikowske K., Molinaro A.M., Gauthier M.L., Berman H.K., Waldman F., Bennington J., Sanchez H., Jimenez C., Stewart K., Chew K., Ljung B.M., Tlsty T.D. Biomarker expression and risk of subsequent tumors after initial ductal carcinoma in situ diagnosis. J Natl Cancer Inst. 2010;102:627–637. doi: 10.1093/jnci/djq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lari S.A., Kuerer H.M. Biological markers in DCIS and risk of breast recurrence: a systematic review. J Cancer. 2011;2:232–261. doi: 10.7150/jca.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roka S., Rudas M., Taucher S., Dubsky P., Bachleitner-Hofmann T., Kandioler D., Gnant M., Jakesz R. High nuclear grade and negative estrogen receptor are significant risk factors for recurrence in DCIS. Eur J Surg Oncol. 2004;30:243–247. doi: 10.1016/j.ejso.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 50.Ringberg A., Anagnostaki L., Anderson H., Idvall I., Ferno M., South Sweden Breast Cancer Group Cell biological factors in ductal carcinoma in situ (DCIS) of the breast-relationship to ipsilateral local recurrence and histopathological characteristics. Eur J Cancer. 2001;37:1514–1522. doi: 10.1016/s0959-8049(01)00165-4. [DOI] [PubMed] [Google Scholar]
- 51.Kepple J., Henry-Tillman R.S., Klimberg V.S., Layeeque R., Siegel E., Westbrook K., Korourian S. The receptor expression pattern in ductal carcinoma in situ predicts recurrence. Am J Surg. 2006;192:68–71. doi: 10.1016/j.amjsurg.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Holmes P., Lloyd J., Chervoneva I., Pequinot E., Cornfield D.B., Schwartz G.F., Allen K.G., Palazzo J.P. Prognostic markers and long-term outcomes in ductal carcinoma in situ of the breast treated with excision alone. Cancer. 2011;117:3650–3657. doi: 10.1002/cncr.25942. [DOI] [PubMed] [Google Scholar]
- 53.DiGiovanna M.P., Chu P., Davison T.L., Howe C.L., Carter D., Claus E.B., Stern D.F. Active signaling by HER-2/neu in a subpopulation of HER-2/neu-overexpressing ductal carcinoma in situ: clinicopathological correlates. Cancer Res. 2002;62:6667–6673. [PubMed] [Google Scholar]
- 54.Lebeau A., Unholzer A., Amann G., Kronawitter M., Bauerfeind I., Sendelhofert A., Iff A., Lohrs U. EGFR, HER-2/neu, cyclin D1, p21 and p53 in correlation to cell proliferation and steroid hormone receptor status in ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2003;79:187–198. doi: 10.1023/a:1023958324448. [DOI] [PubMed] [Google Scholar]
- 55.MacConaill L.E. Existing and emerging technologies for tumor genomic profiling. J Clin Oncol. 2013;31:1815–1824. doi: 10.1200/JCO.2012.46.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michailidou K., Hall P., Gonzalez-Neira A., Ghoussaini M., Dennis J., Milne R.L. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45:353–361. doi: 10.1038/ng.2563. 361e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciriello G., Gatza M.L., Beck A.H., Wilkerson M.D., Rhie S.K., Pastore A., Zhang H., McLellan M., Yau C., Kandoth C., Bowlby R., Shen H., Hayat S., Fieldhouse R., Lester S.C., Tse G.M., Factor R.E., Collins L.C., Allison K.H., Chen Y.Y., Jensen K., Johnson N.B., Oesterreich S., Mills G.B., Cherniack A.D., Robertson G., Benz C., Sander C., Laird P.W., Hoadley K.A., King T.A., Network T.R., Perou C.M. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163:506–519. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arnedos M., Vicier C., Loi S., Lefebvre C., Michiels S., Bonnefoi H., Andre F. Precision medicine for metastatic breast cancer--limitations and solutions. Nat Rev Clin Oncol. 2015;12:693–704. doi: 10.1038/nrclinonc.2015.123. [DOI] [PubMed] [Google Scholar]
- 59.Burkhardt L., Grob T.J., Hermann I., Burandt E., Choschzick M., Janicke F., Muller V., Bokemeyer C., Simon R., Sauter G., Wilczak W., Lebeau A. Gene amplification in ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2010;123:757–765. doi: 10.1007/s10549-009-0675-8. [DOI] [PubMed] [Google Scholar]
- 60.Pan A., Zhou Y., Mu K., Liu Y., Sun F., Li P., Li L. Detection of gene copy number alterations in DCIS and invasive breast cancer by QM-FISH. Am J Transl Res. 2016;8:4994–5004. [PMC free article] [PubMed] [Google Scholar]
- 61.Rane S.U., Mirza H., Grigoriadis A., Pinder S.E. Selection and evolution in the genomic landscape of copy number alterations in ductal carcinoma in situ (DCIS) and its progression to invasive carcinoma of ductal/no special type: a meta-analysis. Breast Cancer Res Treat. 2015;153:101–121. doi: 10.1007/s10549-015-3509-x. [DOI] [PubMed] [Google Scholar]
- 62.Abba M.C., Gong T., Lu Y., Lee J., Zhong Y., Lacunza E., Butti M., Takata Y., Gaddis S., Shen J., Estecio M.R., Sahin A.A., Aldaz C.M. A molecular portrait of high-grade ductal carcinoma in situ. Cancer Res. 2015;75:3980–3990. doi: 10.1158/0008-5472.CAN-15-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pang J.B., Savas P., Fellowes A.P., Mir Arnau G., Kader T., Vedururu R., Hewitt C., Takano E.A., Byrne D.J., Choong D.Y., Millar E.K., Lee C.S., O'Toole S.A., Lakhani S.R., Cummings M.C., Mann G.B., Campbell I.G., Dobrovic A., Loi S., Gorringe K.L., Fox S.B. Breast ductal carcinoma in situ carry mutational driver events representative of invasive breast cancer. Mod Pathol. 2017;30:952–963. doi: 10.1038/modpathol.2017.21. [DOI] [PubMed] [Google Scholar]
- 64.Jiang Y.Z., Yu K.D., Zuo W.J., Peng W.T., Shao Z.M. GATA3 mutations define a unique subtype of luminal-like breast cancer with improved survival. Cancer. 2014;120:1329–1337. doi: 10.1002/cncr.28566. [DOI] [PubMed] [Google Scholar]
- 65.Solin L.J., Gray R., Baehner F.L., Butler S.M., Hughes L.L., Yoshizawa C., Cherbavaz D.B., Shak S., Page D.L., Sledge G.W., Jr., Davidson N.E., Ingle J.N., Perez E.A., Wood W.C., Sparano J.A., Badve S. A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2013;105:701–710. doi: 10.1093/jnci/djt067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Solin L.J., Gray R., Hughes L.L., Wood W.C., Lowen M.A., Badve S.S., Baehner F.L., Ingle J.N., Perez E.A., Recht A., Sparano J.A., Davidson N.E. Surgical excision without radiation for ductal carcinoma in situ of the breast: 12-year results from the ECOG-ACRIN E5194 study. J Clin Oncol. 2015;33:3938–3944. doi: 10.1200/JCO.2015.60.8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rakovitch E., Nofech-Mozes S., Hanna W., Baehner F.L., Saskin R., Butler S.M., Tuck A., Sengupta S., Elavathil L., Jani P.A., Bonin M., Chang M.C., Robertson S.J., Slodkowska E., Fong C., Anderson J.M., Jamshidian F., Miller D.P., Cherbavaz D.B., Shak S., Paszat L. A population-based validation study of the DCIS score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat. 2015;152:389–398. doi: 10.1007/s10549-015-3464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rakovitch E., Nofech-Mozes S., Hanna W., Sutradhar R., Baehner F.L., Miller D.P., Fong C., Gu S., Tuck A., Sengupta S., Elavathil L. Multigene expression assay and benefit of radiotherapy after breast conservation in ductal carcinoma in situ. J Natl Cancer Inst. 2017;109:djw256. doi: 10.1093/jnci/djw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin C.Y., Mooney K., Choy W., Yang S.R., Barry-Holson K., Horst K., Wapnir I., Allison K. Will oncotype DX DCIS testing guide therapy? a single-institution correlation of oncotype DX DCIS results with histopathologic findings and clinical management decisions. Mod Pathol. 2018;31:562–568. doi: 10.1038/modpathol.2017.172. [DOI] [PubMed] [Google Scholar]
- 70.Knopfelmacher A., Fox J., Lo Y., Shapiro N., Fineberg S. Correlation of histopathologic features of ductal carcinoma in situ of the breast with the oncotype DX DCIS score. Mod Pathol. 2015;28:1167–1173. doi: 10.1038/modpathol.2015.79. [DOI] [PubMed] [Google Scholar]
- 71.Manders J.B., Kuerer H.M., Smith B.D., McCluskey C., Farrar W.B., Frazier T.G., Li L., Leonard C.E., Carter D.L., Chawla S., Medeiros L.E., Guenther J.M., Castellini L.E., Buchholz D.J., Mamounas E.P., Wapnir I.L., Horst K.C., Chagpar A., Evans S.B., Riker A.I., Vali F.S., Solin L.J., Jablon L., Recht A., Sharma R., Lu R., Sing A.P., Hwang E.S., White J., Study Investigators. Study Participants Clinical utility of the 12-gene DCIS score assay: impact on radiotherapy recommendations for patients with ductal carcinoma in situ. Ann Surg Oncol. 2017;24:660–668. doi: 10.1245/s10434-016-5583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alvarado M., Carter D.L., Guenther J.M., Hagans J., Lei R.Y., Leonard C.E., Manders J., Sing A.P., Broder M.S., Cherepanov D., Chang E., Eagan M., Hsiao W., Schultz M.J. The impact of genomic testing on the recommendation for radiation therapy in patients with ductal carcinoma in situ: a prospective clinical utility assessment of the 12-gene DCIS score result. J Surg Oncol. 2015;111:935–940. doi: 10.1002/jso.23933. [DOI] [PubMed] [Google Scholar]
- 73.Doebar S.C., Sieuwerts A.M., de Weerd V., Stoop H., Martens J.W.M., van Deurzen C.H.M. Gene expression differences between ductal carcinoma in situ with and without progression to invasive breast cancer. Am J Pathol. 2017;187:1648–1655. doi: 10.1016/j.ajpath.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 74.Lee S., Stewart S., Nagtegaal I., Luo J., Wu Y., Colditz G., Medina D., Allred D.C. Differentially expressed genes regulating the progression of ductal carcinoma in situ to invasive breast cancer. Cancer Res. 2012;72:4574–4586. doi: 10.1158/0008-5472.CAN-12-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sokol E.S., Feng Y.X., Jin D.X., Tizabi M.D., Miller D.H., Cohen M.A., Sanduja S., Reinhardt F., Pandey J., Superville D.A., Jaenisch R., Gupta P.B. SMARCE1 is required for the invasive progression of in situ cancers. Proc Natl Acad Sci U S A. 2017;114:4153–4158. doi: 10.1073/pnas.1703931114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park S.Y., Kwon H.J., Lee H.E., Ryu H.S., Kim S.W., Kim J.H., Kim I.A., Jung N., Cho N.Y., Kang G.H. Promoter CpG island hypermethylation during breast cancer progression. Virchows Arch. 2011;458:73–84. doi: 10.1007/s00428-010-1013-6. [DOI] [PubMed] [Google Scholar]
- 77.Muggerud A.A., Ronneberg J.A., Warnberg F., Botling J., Busato F., Jovanovic J., Solvang H., Bukholm I., Borresen-Dale A.L., Kristensen V.N., Sorlie T., Tost J. Frequent aberrant DNA methylation of ABCB1, FOXC1, PPP2R2B and PTEN in ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res. 2010;12:R3. doi: 10.1186/bcr2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fackler M.J., McVeigh M., Evron E., Garrett E., Mehrotra J., Polyak K., Sukumar S., Argani P. DNA methylation of RASSF1A, HIN-1, RAR-beta, cyclin D2 and twist in in situ and invasive lobular breast carcinoma. Int J Cancer. 2003;107:970–975. doi: 10.1002/ijc.11508. [DOI] [PubMed] [Google Scholar]
- 79.Johnson K.C., Koestler D.C., Fleischer T., Chen P., Jenson E.G., Marotti J.D., Onega T., Kristensen V.N., Christensen B.C. DNA methylation in ductal carcinoma in situ related with future development of invasive breast cancer. Clin Epigenetics. 2015;7:75. doi: 10.1186/s13148-015-0094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fleischer T., Frigessi A., Johnson K.C., Edvardsen H., Touleimat N., Klajic J., Riis M.L., Haakensen V.D., Warnberg F., Naume B., Helland A., Borresen-Dale A.L., Tost J., Christensen B.C., Kristensen V.N. Genome-wide DNA methylation profiles in progression to in situ and invasive carcinoma of the breast with impact on gene transcription and prognosis. Genome Biol. 2014;15:435. doi: 10.1186/s13059-014-0435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goodwin S., McPherson J.D., McCombie W.R. Coming of age: ten years of next-generation sequencing technologies. Nat Rev Genet. 2016;17:333–351. doi: 10.1038/nrg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shah S.P., Roth A., Goya R., Oloumi A., Ha G., Zhao Y. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nik-Zainal S., Van Loo P., Wedge D.C., Alexandrov L.B., Greenman C.D., Lau K.W. The life history of 21 breast cancers. Cell. 2012;149:994–1007. doi: 10.1016/j.cell.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Suzuki T., Tsurusaki Y., Nakashima M., Miyake N., Saitsu H., Takeda S., Matsumoto N. Precise detection of chromosomal translocation or inversion breakpoints by whole-genome sequencing. J Hum Genet. 2014;59:649–654. doi: 10.1038/jhg.2014.88. [DOI] [PubMed] [Google Scholar]
- 85.Banerji S., Cibulskis K., Rangel-Escareno C., Brown K.K., Carter S.L., Frederick A.M. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–409. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun Z., Nair A., Chen X., Prodduturi N., Wang J., Kocher J.P. UClncR: ultrafast and comprehensive long non-coding RNA detection from RNA-seq. Sci Rep. 2017;7:14196. doi: 10.1038/s41598-017-14595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 88.Schmitt A.M., Chang H.Y. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kong W., Yang H., He L., Zhao J.J., Coppola D., Dalton W.S., Cheng J.Q. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Navin N.E. Cancer genomics: one cell at a time. Genome Biol. 2014;15:452. doi: 10.1186/s13059-014-0452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mao X., He J., Li T., Lu Z., Sun J., Meng Y., Abliz Z., Chen J. Application of imaging mass spectrometry for the molecular diagnosis of human breast tumors. Sci Rep. 2016;6:21043. doi: 10.1038/srep21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krueger K.E., Srivastava S. Posttranslational protein modifications: current implications for cancer detection, prevention, and therapeutics. Mol Cell Proteomics. 2006;5:1799–1810. doi: 10.1074/mcp.R600009-MCP200. [DOI] [PubMed] [Google Scholar]