Abstract
PURPOSE
We aimed to determine if the image quality and vascular enhancement are preserved in computed tomography pulmonary angiography (CTPA) studies performed with ultra-low contrast and optimized radiation dose using high-pitch helical mode of a second generation dual source scanner.
METHODS
We retrospectively evaluated oncology patients who had CTPA on a 128-slice dual-source scanner, with a high-pitch helical mode (3.0), following injection of 30 mL of Ioversal at 4 mL/s with body mass index (BMI) dependent tube potential (80–120 kVp) and current (130–150 mAs). Attenuation, noise, and signal-to-noise ratio (SNR) were measured in multiple pulmonary arteries. Three independent readers graded the images on a 5-point Likert scale for central vascular enhancement (CVE), peripheral vascular enhancement (PVE), and overall quality.
RESULTS
There were 50 males and 101 females in our study. BMI ranged from 13 to 38 kg/m2 (22.8±4.4 kg/m2). Pulmonary embolism was present in 29 patients (18.9%). Contrast enhancement and SNR were excellent in all the pulmonary arteries (395.3±131.1 and 18.3±5.7, respectively). Image quality was considered excellent by all the readers, with average reader scores near the highest possible score of 5.0 (CVE, 4.83±0.48; PVE, 4.68±0.65; noise/quality, 4.78±0.47). The average radiation dose length product (DLP) was 161±60 mGy.cm.
CONCLUSION
Using a helical high-pitch acquisition technique, CTPA images of excellent diagnostic quality, including visualization of peripheral segmental/sub-segmental branches can be obtained using an ultra-low dose of iodinated contrast and low radiation dose.
Oncology patients are at a higher risk (up to 6 times) of developing pulmonary embolism (PE) and deep venous thrombosis (DVT) (1). Cancer accounts for 20% of new thromboembolic events (2). This risk of PE is higher in specific cancers (brain, ovary, stomach, pancreas) (3), particularly in advanced stages, more common in the first few months of diagnosis and during active chemo- or radiotherapy (4). Computed tomography pulmonary angiography (CTPA) is the primary imaging modality utilized in the diagnosis of PE due to its high accuracy, wide availability and rapid turnaround time. The PIOPED II study identified sensitivity of 83%, specificity of 96%, negative predictive value of 95% and positive predictive value of 86% in the diagnosis of PE (5), with only <10% of studies being positive for PE in CTPA (6). Some disadvantages of CT include the use of ionizing radiation and iodinated contrast media. Although there is no direct epidemiologic data, a linear no-threshold theory model predicts higher cancer risks with radiation, directly proportional to the dose (7). CT radiation dose can be minimized by using strategies such as low tube current (mAs), automatic tube current modulation, low tube voltage (kVp), automatic tube potential selection, and iterative reconstruction algorithms (8).
Contrast-induced nephropathy (CIN) is an important disadvantage of using iodinated contrast (9). Oncology patients are vulnerable to CIN due to higher prevalence of renal insufficiency, nephrotoxicity of some chemotherapy agents and dehydration due to old age, cachexia, nausea, and vomiting (10). Recent literature also suggests that iodinated contrast agents amplify DNA radiation damage in CT (11–13). Although recent studies have shown overall lower prevalence and incidence of CIN (14–16), the potentially reduced renal capacity of cancer patients suggest that it would be worthwhile to use low doses of contrast media, especially since they require frequent CT examinations. Several techniques are currently available for decreasing the contrast dose for CTPA. Scanning at a lower tube potential (i.e. <120 kVp) facilitates low contrast dose due to higher attenuation of iodine at lower energies. CTPAs done at 70 and 80 kVp with low contrast dose have shown good image quality (17–23), with additional benefit of low radiation dose (23, 24). Sub-millisievert doses are now possible (23), with the use of iterative reconstruction algorithms that minimize noise (25–29). The tube potential can now be automatically selected based on patient size, with corresponding increases in tube current to maintain image quality (28, 29). With dual-energy scanners the patient can be scanned at normal tube potentials, but virtual monoenergetic images (VMI) reconstructed at low energies (50–70 keV) enhance the contrast attenuation, allowing the use of low contrast dose (30–33). Alterations in contrast injection protocol, such as injecting at an exponentially decelerating rate (34) also allows the use of a low contrast dose, but this may involve complex calculations, which are too complicated for routine patient care (35).
Another novel technology involves the use of a high-pitch helical (Flash) mode in a second or third-generation dual source scanner. For multislice CT, pitch is defined as the table distance travelled in one 360° rotation divided by total thickness of all the simultaneously acquired slices. Normal helical CT is done at a pitch of <1.5, since values higher than this will result in gaps in data. However, with dual-source CT, pitch of up to 3.4 can be achieved, since the gaps in data are filled using data sampled from a second detector which is offset by 95 degrees. The entire chest can be scanned under 1 second, with no motion artifacts. Since the contrast enhancement is required only for a short period of time, a low dose of contrast can be used. The radiation dose is also lower due to lower overlapping sections for imaging volumes (36). There has been only limited evaluation of this technique in PE (37, 38) and there has been no previous study in an oncology patient population.
The purpose of our study was to determine if the image quality and vascular enhancement are preserved in CTPA studies performed with ultra-low contrast and optimized radiation dose using high-pitch helical mode of a second generation dual source scanner.
Methods
This is an IRB-approved (IRB protocol # 02-13-08) HIPAA compliant single center retrospective study. Informed consent was waived by IRB since this was a retrospective study.
Study population
The study population comprised of all adult oncology patients who were scanned using an ultra-low contrast dose CT pulmonary angiography protocol described below. Exclusion criteria included age <18 years, history of severe allergy to contrast media, and severe renal dysfunction (eGFR <30 mL/min) not on dialysis.
Scanning technique
All the patients were scanned on a 128-slice dual-source Siemens Definition Flash scanner (Siemens Healthcare). Images were acquired in a high-pitch helical mode, with a pitch of 3.0, gantry rotation time of 0.28 seconds, and collimation of 64 × 2 × 0.6 mm. Scanning parameters were dependent on BMI, with kVp of 80 to 120 and mAs of 130–150 as listed in Table 1. The tube current was chosen based on initial experience with the scanner to produce diagnostic quality image sets. Scanning was acquired following the administration of 30 mL of Ioversol (Optiray 350, Mallinckrodt) at 4 mL/s. A bolus-trigger technique was used by placing a ROI in the main pulmonary artery, monitoring scans every second after contrast administration and triggering acquisition of the scan 5 seconds after a threshold of 150 HU was reached in the main pulmonary artery. All scans were obtained in caudo-cranial direction at expiration.
Table 1.
Scan technique of the patients in the study group
| BMI (kg/m2) | kVp | mAs |
|---|---|---|
| >35 | 120 | 150 |
| 30–35 | 100 | 150 |
| 25–30 | 100 | 130 |
| <25 | 80 | 130 |
BMI, body mass index; kVp, peak tube kilovoltage (tube potential); mAs, milliampere-second (tube current).
Image reconstruction and analysis
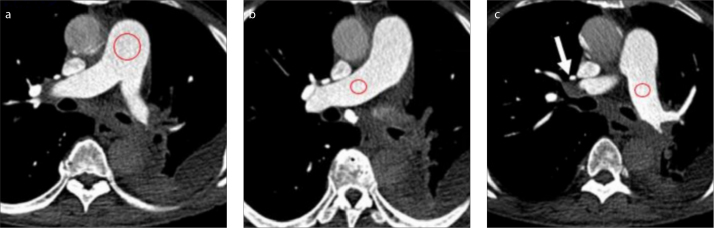
Axial CT images were reconstructed at 1 mm thickness at 0.5 mm increments with filtered-back projection algorithm. Medium to soft convolution kernel (B26f) was used. Coronal and sagittal reconstructions at 2 × 1 mm were also obtained. Images were reviewed in the PACS (Sectra, Sectra AB) at a window level of 60 HU and width of 360 HU. Quantitative and qualitative image analysis was performed. ROIs were placed in eleven locations in the pulmonary arterial tree, namely main pulmonary artery, right pulmonary artery, left pulmonary artery, right upper lobar artery right interlobar pulmonary artery, left upper lobar artery, left lower lobar artery, right upper lobe anterior segmental artery, right lower lobe medial segmental artery, left upper lobe anterior segment and left lower lobe medial segmental artery. The ROIs were sized to occupy two thirds of the artery of interest (Fig. 1). The mean attenuation (signal) and standard deviation (noise) were measured at these ROIs. The signal-to-noise ratio was calculated using the formula, SNR= mean attenuation of pulmonary artery/noise. Averaged values were also obtained for all segmental branches, lobar branches and the entire pulmonary tree.
Figure 1. a–c.
Quantitative analysis of the attenuation and noise in the pulmonary arteries. ROIs (red) are placed in the (a) main pulmonary artery, (b) right pulmonary artery, and (c) left pulmonary artery (LPA). The attenuation and standard deviation (noise) were calculated in these ROIs.
Qualitative analysis of the images was performed by three independent reviewers with 20, 16, 10 years’ experience in radiology (Readers 1 through 3, respectively), who were blinded to the results of one another. The images were graded using a 5-point Likert scale for central vascular enhancement (CVE), peripheral vascular enhancement (PVE), and image noise (1, least; 5, best) as shown in Table 2 (39). The entire lungs including all lobes and segmental and sub-segmental branches were visually evaluated to generate these scores. Average scores of subjective image quality were compared to results previously in the literature. The presence of artifacts was also noted.
Table 2.
Qualitative scoring in the evaluation of CTPA studies
| Central vascular enhancement | Peripheral vascular enhancement | Image noise and overall image quality | |
|---|---|---|---|
| 1 | None | No opacification of segmental/subsegmental arteries | Major noise, no diagnosis possible |
| 2 | Slight, low confidence in making diagnosis | <25% of segmental/subsegmental arteries are opacified | Major noise, diagnosis of PE possible, but with low confidence |
| 3 | Moderate, sufficient for diagnosis | 25%–50% of segmental/subsegmental arteries are opacified | Moderate, but sufficient for PE diagnosis |
| 4 | Good | 50%–75% of segmental/subsegmental arteries are opacified | Minor, diagnosis not influenced |
| 5 | Excellent | >75% of segmental/subsegmental arteries are opacified | No noise; excellent image quality |
CTPA, computed tomography pulmonary angiography; PE, pulmonary embolism.
The presence of pulmonary emboli was noted by one chest radiologist with 16 years’ experience (PR), who was blinded to patient history and clinical findings. PE was defined as an occlusive/nonocclusive filling defect or nonvisualization of segmental/subsegmental branches (37). Artifacts were carefully excluded. The number and location of PE was noted.
Radiation dose metrics were also noted. The dose length product (DLP) was noted and the effective radiation dose in milli-seiverts was obtained by multiplying the DLP with conversion factor of 0.014 mSv/mGy*cm (40). Radiation dose was compared to previous published results in the literature to determine if potential dose differences exist.
Statistical analysis
Statistical analysis was performed using SPSS (Version 11.5, SPSS Inc) and MedCalc (Version 18.5 MedCalc Software). The normality of distribution of the data was evaluated using Shapiro-Wilk test (41, 42). The differences in image quality as determined by the 3 blinded readers was evaluated using the Friedman test, which is the nonparametric equivalent of repeated measures ANOVA. The intraclass correlation coefficient (ICC) was measured using ICC (3,1) type (43) to evaluate the degree of consistency among the qualitative measurements of central vascular enhancement, peripheral vascular enhancement, and noise. Student’s t-test (for independent samples) and the chi-square test for the difference between two proportions (44–47) were used to compare study values with previously published data (5, 40, 48–53), using sample size, means, and standard deviation. The robustness due to sample size was a factor in selecting parametric methods, when the normal distribution could not be assessed for external data (54–56). A P value < 0.05 was considered statistically significant.
Results
There were 151 patients in the study, 50 males (33%), 101 females (67%), with age range of 20–93 years (mean±SD, 62.4±14.6 years). BMI ranged from 13 to 38 kg/m2 (22.8±4.4 kg/m2). The most common cancers were lung (n=42, 27%), breast, (n=28, 18%), hematological (n=19, 13%), and head and neck (n=13, 9%). The distribution of different cancers is shown in Table 3. In terms of scanning parameters, 120 kVp was used in 140 patients, 100 kVp in 10 patients, and 80 kVp in 1 patient. The average scan time was 0.64±0.8 seconds.
Table 3.
Frequencies of different neoplasms in our study
| Neoplasm | n (%) |
|---|---|
| Lung | 42 (27) |
| Breast | 28 (18) |
| Hematological | 19 (13) |
| Head and neck | 13 (9) |
| Melanoma | 1 (0.5) |
| Genitourinary-male | 5 (3) |
| Genitourinary-female | 16 (10) |
| Gastrointestinal | 15 (9) |
| Hepatobiliary | 4 (3) |
| Pancreas | 4 (3) |
| Sarcoma | 3 (2) |
| Unspecified | 1 (0.5) |
Contrast enhancement was excellent in all the pulmonary arteries. Previous studies have shown that the minimum attenuation required for the diagnosis of acute embolism is 93 HU and for chronic embolism is 211 HU (40, 48, 57). The attenuation values in our study were: 372±129 HU for main pulmonary artery, 367±128 HU for left pulmonary artery, 368±124 HU for right pulmonary artery, 390.0±137.9 HU for lobar artery, 420.1±136.7 HU for segmental artery, 395.3±131.1 HU for all pulmonary arteries. SNR was also good in all the pulmonary arteries, with 17.6±7.0 for main pulmonary artery, 16.8±6.4 for left pulmonary artery, 15.3±6.3 for right pulmonary artery, 18.2±6.9 for lobar artery, 19.7±6.5 for segmental artery, and 18.3±5.7 for all pulmonary arteries (Figs. 2, 3, Table 4). The mean attenuation values from this study (395.3 HU), were compared to the results of published studies from Zordo et al. (49) and Yilmaz et al. (50). The mean attenuation values were higher than 5 of 8 study groups in the literature and were not found to be significantly different (P > 0.05) for 5 of 8 CT scan parameters in the literature (Table 5). Specifically, the mean attenuation value from our study was higher than the 120 kVp group of Yilmaz et al. (309.5 HU, P = 0.11), while the attenuation value from our study is significantly higher than that of single source 120 kVp (313 HU, P = 0.001) and DSCT 120 kVp groups of Zordo et al. (342 HU, P = 0.040) (49, 50) (Table 5).
Figure 2. a, b.
Charts showing the attenuation (a), noise and signal-to-noise ratio (SNR) (b) at different pulmonary artery (PA) levels. Attenuation and noise are measured in Hounsfield units and SNR is a ratio. Note that the attenuation and SNR are excellent at all PA levels.
Figure 3. a–c.
Axial standard (a), axial MIP (b) and coronal MIP (c) CT images of a normal study in a patient with lung cancer. Body mass index (BMI) was 32 kg/m2. The study was performed with 30 mL of intravenous contrast, at 100 kvp, 150 mAs and pitch of 3.2. Note the excellent contrast attenuation in all the pulmonary arteries, both central and peripheral.
Table 4.
Attenuation, noise and signal-to-noise ratio at different pulmonary arterial levels
| Signal | Noise | SNR | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Vessel | Mean±SD | Median | IQR | Mean±SD | Median | IQR | Mean±SD | Median | IQR |
| Main pulmonary artery | 372±129 | 350 | 158.5 | 22.4±7.1 | 21 | 8 | 17.6±7.0 | 16.3 | 7.9 |
|
| |||||||||
| Right pulmonary artery | 368±124 | 342 | 135.5 | 28.1±29.6 | 24 | 9 | 15.3±6.3 | 14.0 | 6.8 |
|
| |||||||||
| Left pulmonary artery | 367±128 | 338 | 143.5 | 23.1±7.0 | 22 | 6.5 | 16.8±6.4 | 15.8 | 8.7 |
|
| |||||||||
| Lobar artery, averaged | 390.0±137.9 | 363 | 147 | 23.7±6.6 | 22 | 7.3 | 18.2±6.9 | 16.7 | 9.6 |
| Right upper lobe | 398.9±145.8 | 372 | 151 | 22.8±10.0 | 20 | 11 | 20.0±10.1 | 17 | 12.6 |
| Right interlobar | 370.8±129.8 | 344 | 136.5 | 25.1±8.1 | 23 | 8 | 15.6±5.7 | 14.6 | 8.23 |
| Left upper lobe | 394.4±145.7 | 371 | 154.5 | 22.7±9.2 | 22 | 8 | 19.4±10.0 | 16.7 | 10.4 |
| Left lower lobe | 395.8±141.5 | 366 | 148.5 | 24.2±8.2 | 23 | 9 | 17.9±8.3 | 15.8 | 9.1 |
|
| |||||||||
| Segmental artery, averaged | 420.1±136.7 | 399 | 136.4 | 26.5±9.4 | 24.5 | 10.5 | 19.7±6.5 | 19.1 | 7.4 |
| RUL, anterior seg. | 425.9±145.5 | 407 | 147 | 25.9± 15.9 | 22 | 18.8 | 20.7±11.0 | 18.6 | 12.8 |
| RLL, medial seg. | 406.8±138.8 | 387 | 137 | 24.9±13.0 | 21 | 14 | 20.1±12.4 | 17.3 | 11.3 |
| LUL, anterior seg. | 428.6±148.7 | 400 | 176 | 27.5±17.0 | 23 | 17 | 19.9±12.2 | 17.2 | 12.4 |
| LLL, medial seg. | 419.53±147.4 | 398 | 139 | 27.5±13.6 | 25 | 15 | 18.1±9.5 | 15.6 | 10.5 |
| Average all pulmonary arteries | 395.3±131.1 | 376.7 | 133.9 | 24.9±6.7 | 23.7 | 6.8 | 18.3±5.7 | 17. | 6.9 |
SD, standard deviation; IQR, interquartile range; SNR, signal-to-noise ratio; RUL, right upper lobe; seg., segment; RLL, right lower lobe; LUL, left upper lobe; LLL, left lower lobe.
Table 5.
Comparison of the mean attenuation values in our study compared with studies in the literature
| Value | Our study ULDCT |
Zordo et al. (49) DSCT 100 kV |
Zordo et al. (49) DSCT 120 kV |
Zordo et al. (49) DECT 100/140 kV |
Zordo et al. (49) SCT 100 kV |
Zordo et al. (49) SCT 120 kV |
Yilmaz et al. (50) 120 kV |
Yilmaz et al. (50) 100 kV |
Yilmaz et al. (50) 80 kV |
|---|---|---|---|---|---|---|---|---|---|
| Mean (HU) | 395.3 | 424.0 | 342.0 | 348.0 | 401.0 | 313.0 | 309.5 | 381.7 | 477.3 |
| SD (HU) | 131.1 | 110.0 | 121.0 | 106.0 | 159.0 | 62.0 | 79.1 | 124.0 | 193.3 |
| Comparison | ULDCT vs. DSCT 100 kV | ULDCT vs. DSCT 120 kV | ULDCT vs. DECT 100/140 kV | ULDCT vs. SCT 100 kV | ULDCT vs. SCT 120 kV | ULDCT vs. 120 kV | ULDCT vs. 100 kV | ULDCT vs. 80 kV | |
| t-value | 1.11 | −2.06 | −1.86 | 0.20 | −3.36 | 1.11 | 0.38 | 3.73 | |
| P | 0.26 | 0.040* | 0.070 | 0.83 | 0.001* | 0.11 | 0.71 | <0.001* |
ULDCT, ultra-low dose CT (our study); DSCT, dual-source CT; kV, kilovoltage; DECT, dual-energy CT; SCT, single-source CT.
P < 0.05.
Similarly, the proportion of suboptimal studies using the ULDCT procedure were not significantly different than those provided in the literature. Suboptimal studies, i.e., studies with attenuation <210 HU in the main pulmonary artery, were seen in 10 of our patients (6%), which is similar to the rate of 6.1% reported in literature (51) (P = 0.997, χ2 difference in proportion test). This is also significantly lower than the 11%, which has been established as a guideline by some societies such as the Royal College of Radiology (52).
Image quality was considered excellent by all the readers. The average reader scores were near the highest possible score of 5.0 (CVE, 4.83±0.48; PVE, 4,68±0.65; noise/quality, 4.78±0.47). The scores for Reader 1 were: CVE 4.8±0.5, PVE 4.6±0.7, noise 4.7±0.5; for Reader 2, CVE 4.9±0.5; PVE 4.7±0.6, noise 4.8±0.4; for Reader 3, CVE 4.9±0.4, PVE 4.7±0.6, noise 4.8±0.4. The overall correlation between the readers was evaluated as excellent. Specifically, the ICC between the readers was 0.85 for CVE (95% CI, 0.80–0.89), 0.90 for PVE, (95% CI, 0.87–0.93), and 0.80 for noise (95% CI, 0.75–0.86). For CVE, Reader 1 scored significantly differently than Readers 2 and 3 (P < 0.001). The readers did not score significantly differently for PVE (P = 0.21, Friedman test). For perceived noise, Readers 1 and 2 scored it significantly differently than did Reader 3 (P = 0.032). Our mean score of image quality (4.8) was also comparable to other studies in the literature including the study by Yilmaz et al. (50) on a dual-source normal pitch protocol, where the mean score for the 120 kVp group was 4.8 (P = 0.77) and for 100 kVp group was 4.7 (P = 0.42).
Only 3 studies were considered nondiagnostic. Motion artifacts were seen in 5 patients and interruption of contrast column was not seen in any patient. Our nondiagnostic rate of 1.98% is significantly lower than the PIOPED study (5) (6.2%, P = 0.038) and comparable to Lou et al. (53) (4%, P = 0.22).
Pulmonary embolism was present in 29 patients (19%). There were 19 clots in the central pulmonary vasculature and 43 clots in the peripheral pulmonary vasculature (Fig. 4).
Figure 4. a–c.
Axial standard (a), coronal standard (b) and coronal MIP (c) CT images in a CTPA study performed with 30 mL of intravenous contrast shows a nonocclusive pulmonary embolism (arrow) in the right interlobar pulmonary artery extending to a segmental branch. The patient had a BMI of 29 kg/m2, and hence 100 kVp, 130 mAs and pitch of 3.2 were used.
The average DLP was 161±60 mGy.cm, which corresponds to an effective radiation dose of 2.3±0.8 mSv (Table 5). This is comparable to literature doses of 2.2 to 7.0 mSv (P = 0.18) for single source and lower than the reported 3.2 to 4.7 mSv for dual energy CT (P < 0.001) (40, 48). The average radiation dose in our study is lower than the 120 kVp group used in a dual-source regular pitch scanner by Yilmaz et al. (50) (4.7 mSv, P < 0.001) and comparable to the 100 kVp group in that study (2.5 msV, P = 0.18). Our radiation dose was lower than the normal pitch 120 kVp group in the study by Zordo et al. (49) (mean 4.52, P < 0.001) as well as high pitch 120 kVp group (mean 4.1 mSv, P < 0.001) and normal pitch 100 kVp group (2.8, P = 0.016) (49).
Discussion
Our study demonstrates the feasibility of obtaining high quality CTPA studies in oncology patients using a protocol with ultra-low dose of intravenous contrast media (Table 6). To our knowledge, this is the first study to demonstrate low contrast CTPA using high-pitch dual-source acquisition in a large oncology patient cohort.
Table 6.
Ultra-low dose contrast CTPA protocol and radiation dose evaluation
| Parameter | Protocol |
|---|---|
| Acquisition mode | Dual source, high pitch |
| Detector collimation (mm) | 64 × 2 × 0.6 |
| Pitch | 3.2 |
| Gantry rotation time (ms) | 280 |
| Tube voltage (kVp) | 80–120 |
| Reference tube current time product (mAs eff) | 130–150 |
| Contrast medium volume (mL) | 30 |
| Mean DLP (mGy.cm) | 161±60 |
| Estimated effective radiation dose (mSv) | 2.3±0.8 |
DLP, dose-length product.
Dual-source CT scanners have two x-ray tube/detector units, which can be operated in three modes, namely dual-energy, single-energy with standard pitch and single-energy with high-pitch, all of which can be done either with or without electrocardiography (ECG)-gating. In the dual-energy mode, the two tubes are operated at different energies, which allows generation of additional images, including iodine perfusion maps (58) and VMI, which can enhance contrast signal in suboptimal enhanced studies (31, 32). In the conventional single-energy, standard pitch mode, both tubes are operated at the same energy (36). Lower contrast (i.e., 40 mL) and radiation doses (50%) can be achieved by using lower tube potential (e.g., 70 kVp) (29). In the single-energy high-pitch mode, both x-ray tubes are operated at the same tube potential, but at high pitch values of up to 3.4. Although a high-pitch mode generally results in suboptimal angiographic studies due to gaps in data, the presence of second tube in dual-source CT fills the gaps, maintaining image quality.
Radiation dose is lower (up to 35%) in high-pitch mode than conventional CTA at 100 kVp due to rapid scanning and lower imaging volume (49, 59). With appropriate timing, an ultra-low contrast dose study can be performed since contrast is required to be present in pulmonary arterial system only for a short period. There have been few previous CTPA studies used with this mode (37, 38, 60), but none in an oncology patient population. One study used 40 mL of contrast, 70 kVp and iterative reconstruction (IR) algorithm to lower radiation dose by 80% and preserve image quality as compared to standard-pitch, 60 mL contrast study (38). Another study with 30 mL of contrast at 80 kVp and IR showed similar results with half the radiation dose of a 100 kVp standard pitch scanner with 60 mL of contrast (37). However, in both these studies, the BMI, height and weight were not reported. The low kVp techniques would not be suitable for large patients, since it is not possible to generate a higher tube current-time product (mAs) to maintain image quality in these patients because only a single x-ray tube is collecting data in gaps, short acquisition time and poor performance of automatic tube current modulation (29). The third generation of these scanners have higher x-ray tube power and IR algorithms, but reconstruction is limited to central 33 cm of field-of-view.
In our study, we utilized BMI-dependent tube potential and the image quality was excellent in all the patients. We did not use 70 kVp, since we believed the image quality would not be appropriate for diagnosing PE. We were able to obtain good quality in the peripheral vessels as well, indicating our ability to detect small peripheral pulmonary emboli. The high-pitch mode has higher temporal resolution and lower motion not only in pulmonary arteries, but also in other cardiovascular structures, including heart and coronary arteries as well as the lungs, thus improving the image quality and ability to evaluate adjoining structures (61). This scan mode can also be performed with ECG-gating, which further decreases motion artifacts with lower radiation dose than conventional CTA (62). Overall, our results for subjective image quality, proportion of suboptimal studies and mean attenuation values were not significantly different than those previously published in the literature in non-oncology patients using various scanning modes and regimens (49–53).
Our study has several limitations. This is a single-center retrospective study, with no control group of patients scanned at standard-pitch mode. However, we wanted to demonstrate that the image quality of our protocol is sufficient to make a diagnosis of PE. Based on previously published contrast attenuation cutoff required for demonstrate emboli (48, 49), we suggest that the image quality of our scans is high. We have also compared our results with the previously published data in the literature. We did not use an IR in our protocol due to nonavailability in our institution, which would have facilitated even further radiation dose reduction. We were able to achieve such low radiation doses without IR algorithm due to optimal timing of the low contrast bolus, which was needed to be present in the pulmonary circulation only for a short period of time. Due to absence of control group, we could not directly estimate the radiation dose savings, but we used data from the literature (59, 63) to suggest reduced radiation dose. Although motion artifacts were low and cardiovascular structures including coronary arteries were seen better with this technology, we did not evaluate these advantages in our study, since it was not our focus.
In conclusion, high quality CTPA with excellent diagnostic quality is feasible in oncology patients with a low dose of iodinated contrast media and radiation dose using a high-pitch helical acquisition mode in a second generation dual-source scanner. The quality was good even for visualization of small, peripheral segmental/subsegmental branches, which makes it suitable for detection of small peripheral emboli. This technique is useful in oncology patients who require repeated scans by reducing toxicities associated with administration of iodinated contrast. There is also potential for further reduction of contrast and radiation dose.
Main points.
Oncology patients are at a higher risk of developing pulmonary embolism (PE) and deep venous thrombosis (DVT).
High quality CT pulmonary angiography (CTPA) studies can be obtained in oncology patients with ultra-low dose of intravenous contrast using high-pitch dual source acquisition.
This technique is able to obtain good quality in central as well as peripheral pulmonary vessels.
Footnotes
Conflict of interest disclosure
Dr Robert Gilkeson-Research Consultant, Riverain Technologies, LLC Research support, Koninklijke Philips NV Research support, Siemens AG Research support, General Electric Company; Leslie Ciancibello-Consultant and Speakers’ bureau, Siemens Healthineers.
References
- 1.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: A population based case-control study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 2.Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population based study. Arch Intern Med. 2002;162:1245–1248. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 3.Thodiyil PA, Kakkar AK. Variation in the relative risk of venous thromboembolism in different cancers. Thromb Haemost. 2002;87:1076–1077. doi: 10.1055/s-0037-1613136. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Razeq HN, Mansour AH, Ismael YM. Incidental pulmonary embolism in cancer patients: clinical characteristics and outcome-a comprehensive cancer center experience. Vasc Health Risk Manag. 2011;11:153–158. doi: 10.2147/VHRM.S17947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein PD, Fowler SE, Goodman LR, et al. Multi-detector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317–2327. doi: 10.1056/NEJMoa052367. [DOI] [PubMed] [Google Scholar]
- 6.MamLouk MD, van Sonnenberg E, Gosalia R, et al. Pulmonary embolism at CT angiography: implications for appropriateness, cost, and radiation exposure in 2003 patients. Radiology. 2010;256:625–630. doi: 10.1148/radiol.10091624. [DOI] [PubMed] [Google Scholar]
- 7.Verdun FR, Bochud F, Gudinchet F, et al. Radiation risk: what you should know to tell your patient. Radiographics. 2008:28L1807–1816. doi: 10.1148/rg.287085042. [DOI] [PubMed] [Google Scholar]
- 8.Albrecht MH, Bickford MW, Nance JW, Jr, et al. State-of-the-art pulmonary CT angiography for acute pulmonary embolism. AJR Am J Roentgenol. 2017;208:495–504. doi: 10.2214/AJR.16.17202. [DOI] [PubMed] [Google Scholar]
- 9.McCullough PA, Sandberg KR. Epidemiology of contrast-induced nephropathy. Rev Cardiovasc Med. 2003;4(Suppl 5):S3–S9. [PubMed] [Google Scholar]
- 10.Launary-Vacher V, Oudard S, Janus N, et al. Prevalence of renal insufficiency in cancer patients and implications of anticancer drug management. Cancer. 2007;110:1376–1384. doi: 10.1002/cncr.22904. [DOI] [PubMed] [Google Scholar]
- 11.Pathe C, Eble K, Schmitz-Beuting D, et al. The presence of iodinated contrast agents amplifies DNA radiation damage in computed tomography. Contrast Media Mol Imaging. 2011;6:507–513. doi: 10.1002/cmmi.453. [DOI] [PubMed] [Google Scholar]
- 12.Piechowlak EI, Peter JFW, Kleb B, et al. Intravenous iodinated contrast agents amplify DNA radiation damage at CT. Radiology. 2015;275:692–697. doi: 10.1148/radiol.14132478. [DOI] [PubMed] [Google Scholar]
- 13.Grudzenski S, Kuefner MA, Heckmann MB, et al. Contrast-medium enhanced radiation damage caused by CT examinations. Radiology. 2009;253:706–714. doi: 10.1148/radiol.2533090468. [DOI] [PubMed] [Google Scholar]
- 14.Tao SM, Wichmann JL, Schopef UJ, et al. Contrast induced nephropathy in CT: incidence, risk factors and strategies for prevention. Eur Radiol. 2016;26:3310–3318. doi: 10.1007/s00330-015-4155-8. [DOI] [PubMed] [Google Scholar]
- 15.Wichmann JL, Katzberg RW, Litwin SE, et al. Contrast induced nephropathy. Circulation. 2015;132:1931–1936. doi: 10.1161/CIRCULATIONAHA.115.014672. [DOI] [PubMed] [Google Scholar]
- 16.Davenport MS, Khalatbari S, Cohan RH, et al. Contrast material-induced nephrotoxicity and intravenous low osmolality iodinated contrast material risk stratification by using estimated glomerular filtration rate. Radiology. 2013;268:719–728. doi: 10.1148/radiol.13122276. [DOI] [PubMed] [Google Scholar]
- 17.Faggioni L, Neri E, Sbragia P, et al. 80-kV pulmonary CT angiography with 40 mL of iodinated contrast material in lean patients: comparison of vascular enhancement with iodixanol (320 mg l/mL) and iomersol (400 mg I/mL) AJR Am J Roentgenol. 2012;199:1247–1251. doi: 10.2214/AJR.11.8122. [DOI] [PubMed] [Google Scholar]
- 18.Szucs-Farkas Z, Megyeri B, Christie A, et al. Prospective randomized comparison of diagnostic confidence and image quality with normal-dose and low-dose CT pulmonary angiography at various body weights. Eur Radiol. 2014;24:2868–1877. doi: 10.1007/s00330-014-3208-8. [DOI] [PubMed] [Google Scholar]
- 19.Szucs-Farkas Z, Kurmann L, Strautz T, et al. Patient exposure and image quality of low-dose pulmonary computed tomography angiography. Comparison of 100- and 80-kvp protocols. Invest Radiol. 2008;43:871–876. doi: 10.1097/RLI.0b013e3181875e86. [DOI] [PubMed] [Google Scholar]
- 20.Szucs-Farkas Z, Schaller C, Bensler S, et al. Detection of pulmonary emboli with CT angiography at reduced radiation exposure and contrast material volume. Invest Radiol. 2009;44:793–799. doi: 10.1097/RLI.0b013e3181bfe230. [DOI] [PubMed] [Google Scholar]
- 21.Viter-Ramirez G, Garcia-Lallana A, Simon-Yarza I, et al. Low radiation and low contrast dose pulmonary Ct angiography: comparison of 80 kVp/60 mL and 100 kVp/80 mL protocols. Clin Radiol. 2012;67:833–839. doi: 10.1016/j.crad.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Laqmani A, Regier M, Veldhoen S, et al. Improved image quality and low radiation dose with hybrid iterative reconstruction with 80 kV CT pulmonary angiography. Eur J Radiol. 2014;83:1962–1969. doi: 10.1016/j.ejrad.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Wichmann JL, Hu X, Kerl JM, et al. 70 kVp computed tomography pulmonary angiography: potential for reduction of iodine load and radiation dose. J Thorac Imaging. 2015;30:69–75. doi: 10.1097/RTI.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 24.Schueller-Weidekamm C, Schaefer-Prokop CM, Weber M, et al. CT angiography of the pulmonary arteries to detect pulmonary embolism. Improvement of vacular enhancement with low kilovoltage settings. Radiology. 2006;241:899–907. doi: 10.1148/radiol.2413040128. [DOI] [PubMed] [Google Scholar]
- 25.Ohana M, Labanik A, Jeung MY, et al. Iterative reconstruction in single source dual-energy CT pulmonary angiography: is it sufficient to achieve a radiation dose as low as the state-of-the art single energy CTPA? Eur J Radiol. 2015;84:2314–2320. doi: 10.1016/j.ejrad.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Kligerman S, Mehta D, Farnadesh M, et al. Use of a hybrid iterative reconstruction technique to rescue image noise and improve image quality in obese patients undergoing computed tomographic pulmonary angiography. J Thorac Imaging. 2013;28:48–59. doi: 10.1097/RTI.0b013e31825412b2. [DOI] [PubMed] [Google Scholar]
- 27.Sun J, Zhang Q, Hu D, et al. Improving pulmonary vessel image quality with a full model-based iterative reconstruction algorithm in 80 kVp low-dose chest CT for pediatric patients aged 0–6 years. Acta Radiol. 2015;56:761–768. doi: 10.1177/0284185114540884. [DOI] [PubMed] [Google Scholar]
- 28.Krazinski AW, Meinel FG, Schopef UJ, et al. Reduced radiation dose and improved image quality at cardiovascular CT angiography by automated attenuation-based tube voltage selection: intra-individual comparison. Eur Radiol. 2014;24:2677–2684. doi: 10.1007/s00330-014-3312-9. [DOI] [PubMed] [Google Scholar]
- 29.Boos J, Kropil P, Lanzman RS, et al. CT pulmonary angiography: simultaneous low-pitch dual-source acquisition mode with 70 kVp and 40 mL of contrast medium and comparison with high-pitch spiral dual-source acquisition with automated tube potential selection. Br J Radiol. 2016;80 doi: 10.1259/bjr.20151059. 20151059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogot NR, Fingerle A, Shaham D, et al. Image quality of low energy pulmonary CT angiography: comparison with standard CT. AJR Am J Roentgenol. 2011;197:W273–W278. doi: 10.2214/AJR.10.5318. [DOI] [PubMed] [Google Scholar]
- 31.Apfaltrer P, Sudarkski S, Schneider D, et al. Value of monoenergetic low-kV dual energy CT datasets for improved image quality of CT pulmonary angiography. Eur J Radiol. 2014;83:322–328. doi: 10.1016/j.ejrad.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Delsalle MA, Pontana F, Duhamel A, et al. Spectral optimization of chest CT angiography with reduced iodine load. Experience in 80 patients evaluated with dual-source, dual-energy CT. Radiology. 2013;267:256–266. doi: 10.1148/radiol.12120195. [DOI] [PubMed] [Google Scholar]
- 33.Yuan R, Shuman WP, Earls JP, et al. Reduced iodine load at CT pulmonary angiography with dual-energy monochromatic imaging comparison with standard CT pulmonary angiography-a prospective randomized trial. Radiology. 2012;262:290–297. doi: 10.1148/radiol.11110648. [DOI] [PubMed] [Google Scholar]
- 34.Saade C, Mayat A, El-Merhi F. Exponentially decelerated contrast media injection rate combined with a novel patient specific contrast formula reduces contrast volume administration and radiation dose during computed tomography pulmonary angiography. J Comput Assist Tomogr. 2016;40:370–374. doi: 10.1097/RCT.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 35.Saade C, Bourne R, El-Merhi F, et al. An optimized patient specific approach to administration of contrast agreement for CT pulmonary angiography. Eur Radiol. 2013;23:3205–3212. doi: 10.1007/s00330-013-2919-6. [DOI] [PubMed] [Google Scholar]
- 36.Petersilka M, Bruder H, Krauss B, et al. Technical principles of dual source CT. Eur J Radiol. 2008;68:362–368. doi: 10.1016/j.ejrad.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Lu GM, Luo S, Meinel FG, et al. High-pitch computed tomography pulmonary angiography with iterative reconstruction at 80 kVp and 20 mL contrast agent volume. Eur Radiol. 2014;24:3260–3268. doi: 10.1007/s00330-014-3365-9. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Ni QQ, Schopef UJ, et al. 70 kVp high pitch computed tomography pulmonary angiography with 40 mL contrast agent: initial experience. Acad Radiol. 2015;22:1562–1570. doi: 10.1016/j.acra.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 39.Godoy MCB, Heller SL, Naidich DP, et al. Dual-energy MDCT: Comparison of pulmonary artery enhancement on dedicated CT pulmonary angiography, routine and low contrast volume studies. Eur J Radiol. 2010;79:e11–17. doi: 10.1016/j.ejrad.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 40.McCollough CH, Primark AN, Braun N, et al. Strategies for reducing radiation dose in CT. Radiol Clin N Am. 2009;47:27–40. doi: 10.1016/j.rcl.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:3–4. doi: 10.2307/2333709. [DOI] [Google Scholar]
- 42.Royston P. A toolkit for testing for non-normality in complete and censored samples. Statistician. 1993;42:37–43. doi: 10.2307/2348109. [DOI] [Google Scholar]
- 43.Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psych Bull. 1979;86:420–428. doi: 10.1037/0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 44.Altman DG. Comparing groups of continuous data. In: Altman DG, editor. Practical Statistics for Medical Research. 1st ed. Boca Raton: Chapman and Hall/CRC; 1991. pp. 191–194. [Google Scholar]
- 45.Newcombe RG, Altman DG. Proportions and their differences. In: Altman DG, Machin D, Bryant TN, Gardner MJ, editors. Statistics with confidence. 2nd ed. London: BMJ Books; 2000. pp. 49–50. [Google Scholar]
- 46.Pearson ES. The choice of statistical tests illustrated on the interpretation of data classed in a 2 × 2 table. Biometrika. 1947;34:139–167. doi: 10.1093/biomet/34.1-2.139. [DOI] [PubMed] [Google Scholar]
- 47.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2011;26:3661–3675. doi: 10.1002/sim.2832. [DOI] [PubMed] [Google Scholar]
- 48.Wittram C. How I do it: CT pulmonary angiography. AJR Am J Roentgenol. 2007;188:1255–1261. doi: 10.2214/AJR.06.1104. [DOI] [PubMed] [Google Scholar]
- 49.Zordo TD, von Lutterotti K, Dejaco C, et al. Comparison of image quality and radiation dose of different pulmonary CTA protocols on a 128 slice CT: high pitch dual source CT, dual energy CT and conventional spiral CT. Eur Radiol. 2012;22:279–286. doi: 10.1007/s00330-011-2251-y. [DOI] [PubMed] [Google Scholar]
- 50.Yilmaz Ö, Üstün ED, Kayan M, et al. Diagnostic quality of CT pulmonary angiography in pulmonary thromboembolism: A comparison of three different kV values. Med Sci Monit. 2013;19:908–915. doi: 10.12659/MSM.889578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones SE, Wittram C. The indeterminate CT pulmonary angiogram: imaging characteristics and patient clinical outcome. Radiology. 2005;237:329–337. doi: 10.1148/radiol.2371041520. [DOI] [PubMed] [Google Scholar]
- 52.https://www.rcr.ac.uk/audit/adequate-contrast-enhancement-ct-pulmonary-angiograms
- 53.Lou B, islam M, Filopei J, et al. Patient outcomes following suboptimal and non-diagnostic CT pulmonary angiography performed for the suspected diagnosis of a pulmonary embolism. Am J Respir Crit Care Med. 2016;193:A1240. [Google Scholar]
- 54.LumLey T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Ann Rev Pub Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 55.Marusteri M, Bacarea V. Comparing groups for statistical differences: how to choose the right statistical test? Biochemia Medica. 2010;15:32. doi: 10.11613/BM.2010.004. [DOI] [Google Scholar]
- 56.Altman DG. Comparing groups-continuous data. In: Altman DG, editor. Practical Statistics for Medical Research. 1st ed. Boca Raton: Chapman and Hall/CRC; 1991. p. 181. [Google Scholar]
- 57.Meaney TF, Raudikivi U, McIntyre WJ, et al. Detection of low-contrast in computed body tomography: an experimental study of simulated lesions. Radiology. 1980;134:149–154. doi: 10.1148/radiology.134.1.7350595. [DOI] [PubMed] [Google Scholar]
- 58.Pontana F, Faivre JB, Remy-Jardin M, et al. Lung perfusion with dual-energy multi-detector row CT: Feasibility for the evaluation of acute pulmonary embolism in 117 consecutive patients. Acad Radiol. 2008;15:1494–1504. doi: 10.1016/j.acra.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 59.Lurz M, Lell MM, Wuest W, et al. Automated tube voltage selection in thoracoabdominal computed tomography at high pitch using a third generation dual source scanner: image quality and radiation dose performance. Invest Radiol. 2015;50:352–360. doi: 10.1097/RLI.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 60.Pontana F, Henry S, Duhamel A, et al. Impact of iterative reconstruction on the diagnosis of acute pulmonary embolism (PE) on reduced-dose chest CT angiograms. Eur Radiol. 2015;25:1182–1189. doi: 10.1007/s00330-014-3393-5. [DOI] [PubMed] [Google Scholar]
- 61.Hou DJ, Tso DK, Davison C, et al. Clinical utility of ultra-high pitch dual source thoracic CT imaging of acute pulmonary embolism in the emergency department: are we one step closer towards a non-gated triple rule out? Eur Radiol. 2013;82:1793–1798. doi: 10.1016/j.ejrad.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Bolen MA, Renapurkar RD, Popovic ZB, et al. High-pitch ECG-synchronized pulmonary CT angiography versus standard CT pulmonary angiography: A prospective randomized study. AJR Am J Roentgenol. 2013;201:971–976. doi: 10.2214/AJR.13.10597. [DOI] [PubMed] [Google Scholar]
- 63.Hunsaker AR, Lu MT, Goldfaber SZ, et al. Imaging in acute pulmonary embolism with special clinical scenarios. Circ Cardovasc Imaging. 2010;3:491–500. doi: 10.1161/CIRCIMAGING.109.855981. [DOI] [PubMed] [Google Scholar]