Abstract
Background
Direct assessment of skeletal muscle mass in older adults is clinically challenging. Relationships between lean mass and late-life outcomes have been inconsistent. The D3-creatine dilution method provides a direct assessment of muscle mass.
Methods
Muscle mass was assessed by D3-creatine (D3Cr) dilution in 1,382 men (mean age, 84.2 years). Participants completed the Short Physical Performance Battery (SPPB); usual walking speed (6 m); and dual x-ray absorptiometry (DXA) lean mass. Men self-reported mobility limitations (difficulty walking 2–3 blocks or climbing 10 steps); recurrent falls (2+); and serious injurious falls in the subsequent year. Across quartiles of D3Cr muscle mass/body mass, multivariate linear models calculated means for SPPB and gait speed; multivariate logistic models calculated odds ratios for incident mobility limitations or falls.
Results
Compared to men in the highest quartile, those in the lowest quartile of D3Cr muscle mass/body mass had slower gait speed (Q1: 1.04 vs Q4: 1.17 m/s); lower SPPB (Q1: 8.4 vs Q4: 10.4 points); greater likelihood of incident serious injurious falls (odds ratio [OR] Q1 vs Q4: 2.49, 95% confidence interval [CI]: 1.37, 4.54); prevalent mobility limitation (OR Q1 vs Q4,: 6.1, 95% CI: 3.7, 10.3) and incident mobility limitation (OR Q1 vs Q4: 2.15 95% CI: 1.42, 3.26); p for trend < .001 for all. Results for incident recurrent falls were in the similar direction (p = .156). DXA lean mass had weaker associations with the outcomes.
Conclusions
Unlike DXA lean mass, low D3Cr muscle mass/body mass is strongly related to physical performance, mobility, and incident injurious falls in older men.
Keywords: Muscle, Sarcopenia, Falls, Functional performance
Sarcopenia, the age-associated loss of skeletal muscle mass, is a geriatric syndrome with an ICD-10 code that lacks a precise clinical definition. Initial definitions of sarcopenia relied entirely on lean mass derived from dual x-ray absorptiometry (DXA). Most often, DXA lean mass of the arms and legs (appendicular lean mass) is operationalized as an approximation of muscle mass (1). However, associations between lean mass assessed by DXA and outcomes such as physical performance, self-reported mobility, falls, and other functional outcomes have been inconsistent (2–4). Muscle strength and other qualities of muscle function are more robust predictors of general functional decline (2,4–12). Thus, more recent consensus definitions of sarcopenia have incorporated measures of muscle strength and/or physical performance in part because the associations between DXA measures of lean mass were not consistently associated with poor outcomes (13–15). Therefore, the precise role of muscle mass in mobility and poor physical performance remains unresolved.
The inconsistent associations reported between lean mass and outcomes may be attributable to limitations of current clinical measurements of muscle mass. Computed tomography (CT) and magnetic resonance (MR) usually measure individual muscle groups rather than providing a total body assessment of skeletal muscle. Full body MRI or CT is expensive and only used in select research settings. DXA is widely used to assess bone mass for the diagnosis of osteoporosis (16) and also measures lean mass. DXA estimates body composition using a three-compartment model, directly measuring fat and bone mineral content through differential absorption of two photon energies; and by subtraction measures lean mass, or the nonbone nonfat component of body mass. Lean mass from DXA does not measure muscle mass directly; rather total body lean mass includes tissue from organs like kidney and liver, as well as fibrotic and other lean tissue. Operationally, lean mass from DXA is usually analyzed as appendicular lean mass (ALM, the nonbone, nonfat component of the arms and legs that includes muscle, fibrotic and connective tissue, and water). To account for body size, ALM is often standardized to height (eg, ALM/height2) (1,17) although other standardizations have been proposed (ie, ALM/body mass index) (18).
The D3Cr dilution method is a novel measure of total body muscle mass that utilizes a simple, clinically feasible procedure. Total body creatine pool size, and thus total body muscle mass, can be estimated with a single oral dose of deuterated creatine (D3-creatine), which is absorbed and diluted by entry into the endogenous pool of creatine in skeletal muscle. Labeled creatinine (D3-creatinine) enrichment is then assessed in a single-void urine sample (19,20). While this measure has been validated as a marker of total muscle mass in humans (21), its association with clinical outcomes has not been evaluated. Since the D3Cr dilution method avoids the use of the assumptions of a compartment model (unlike DXA), we posit that the accurate assessment of muscle mass provided by this new measure will demonstrate more robust associations with physical performance and functional outcomes than DXA measures of lean mass (which we suggest are subject to greater measurement error). We hypothesized that muscle mass (standardized to body mass) as measured by the D3Cr dilution method is associated with strength, physical performance, and prevalent functional limitations; that individuals with lower D3Cr muscle mass/body mass report fewer incident falls and self-reported mobility limitations; and that these associations would be stronger than those observed with lean mass by DXA. We selected these outcomes because physical performance and functional limitations (measures of a person’s ability to move around his day-to-day environment) are likely to be related to muscle mass. We tested these hypotheses in the prospective Osteoporotic Fractures in Men (MrOS) cohort study of community-dwelling older men.
Methods
MrOS Cohort
In 2000–2002, 5,994 ambulatory community-dwelling men aged ≥65 years without bilateral hip replacements were enrolled in MrOS, a multicenter cohort study of aging and osteoporosis (22,23). All men provided written informed consent, and the study was approved by the Institutional Review Board at each center. In 2014–2016, 2,786 survivors were contacted to participate in “Visit 4” (Year 14) clinic visit. Of these, 362 refused participation, 583 completed questionnaires only, and 1,841 completed questionnaires and at least part of the clinic visit (Supplementary Figure 1).
D3Cr Dilution Method to Estimate Muscle Mass
The D3Cr dilution method involves a participant ingesting a 30-mg dose of stable isotope-labeled creatine (D3-creatine), and providing a fasting, morning urine sample 72–144 hours (3–6 days) later in which D3-creatinine, unlabeled creatinine, and creatine are measured using high performance liquid chromatography and tandem mass spectroscopy; these measures are then included in an algorithm to determine total body creatine pool size and thus skeletal muscle mass (as previously described) (24). Several features of creatine biology allow for this measurement. First, virtually all total body creatine (>98%) is found in the skeletal muscle (25). Second, the concentration of creatine in muscle is relatively constant (approximately 4.3 g/kg muscle weight) (26). Third, creatine is metabolized by a nonenzymatic hydrolytic cyclization to its nonionic cyclic derivative creatinine at a constant rate (~1.7%./day) (27). This conversion is not reversible in vivo. Fourth, creatinine rapidly diffuses from muscle into plasma and urine with no reuptake into muscle (28). Fifth, the muscle does not synthesize creatine; it is synthesized in the liver and kidney, and is accumulated in muscle against a concentration gradient via specific active transport from plasma, which allows ingested creatine to enter quantitatively into skeletal muscle (29). Sixth, orally ingested creatine is quantitatively absorbed and enters the bloodstream. Finally, creatinine is not significantly otherwise metabolized. Accordingly, under steady-state conditions, creatine pool size is proportional to skeletal muscle mass (28,30). Moreover, by measuring dilution of orally administered labeled D3-creatine to the unlabeled creatine present in the whole-body pool from the ratio of labeled (D3-creatinine) to unlabeled creatinine in a single urine sample, the total amount of creatine in the body can be determined as an estimate of total muscle mass. Importantly, because the enrichment of creatinine is measured (ie, the ratio of D3-creatinine to unlabeled creatinine), this method is not dependent on creatinine clearance or renal function. The method does not require any special dietary control (other than the need for a fasting morning spot urine sample). To account for variations in total body muscle mass by body size, in our primary analyses, we analyzed D3Cr muscle mass divided by body mass as the primary independent variable.
Muscle Strength, Muscle Power, Physical Performance, and Functional Limitations
Grip strength (kg) from two tests of each hand was assessed using Jamar handheld dynamometers; the maximum value obtained across all tests was analyzed. Men completed three to five weight bearing countermovement leg-extensions (“jumps”) on a force plate, from which we estimated peak power (watts/kg body mass), force (Newton/kg body mass), and velocity (m/s) at peak power (31,32). Walking speed at usual pace was measured over a 6-m course using the average of two trials (m/s) (33). Ability and time to complete five repeated chair stands was assessed. Standing balance was assessed by side-by-side, tandem, and semitandem stands. We calculated scores for the Short Physical Performance Battery using walking speed, chair stands and balance tests (34) (0–12, higher score indicates better performance). A long distance corridor walk (400 m) at the participants’ usual pace was attempted (35); time and ability to complete were recorded.
Men answered questions about the ability to complete a number of activities of daily living (ADLs) and instrumental ADLs (IADLs), including walking 2–3 blocks; climbing 10 steps; heavy housework; bathing/showering; getting in and out of bed or chairs; and carrying or lifting 10 pounds. Men classified the degree of difficulty (none, some, much, or unable) and if they reported that they did not do the task, they were asked whether or not this was due to a health or physical problem. Men reporting any difficulty in these tasks, and those who reported that they were unable to do these tasks were considered to have a limitation for that task. Prevalent mobility limitations were defined as having any difficulty (or inability to complete due to health/physical reasons) for either walking 2–3 blocks or climbing 10 steps.
Appendicular lean mass (ALM) and body fat were assessed by whole-body DXA scans (Hologic 4500 scanners, Waltham, MA) as previously described (36). Other clinical measures considered as potential confounders were assessed in MrOS and are described in the Supplementary Methods.
Incident Falls and Mobility Limitations
Every March, July, and November, MrOS participants answered questionnaires about falls and difficulty walking 2–3 blocks or climbing 10 stairs in the preceding 4 months. We used the three questionnaires that followed the participant’s Year 10.5 clinic date to identify incident falls and mobility limitations. Recurrent falls were dichotomized as two or more falls in the year after the visit (vs 0–1 falls). Serious injurious falls were classified as a fall injury in the year after the visit for which the participant reported visiting a doctor or other health care provider (vs no falls or any other fall which did not result in medical attention). We defined mobility limitations as any new self-reported difficulty walking 2–3 blocks or climbing 10 steps in the year after the visit.
Study Sample
We invited all 1,841 men with a Year 10.5 clinic visit to complete the D3Cr dilution protocol (without any inclusion/exclusion criteria) and 1,641 agreed to participate (Supplementary Figure 1). Of these, 187 were excluded for protocol violations that included: incorrect timing of the dose or urine collection (either less than 72 hours or more than 144 hours between the dose and collection) or forgetting to take the dose or provide the specimen. Six samples were lost by the clinical center or laboratory and 23 men were excluded because of outlying values for D3Cr muscle mass/body mass more than 2 SD from the mean, most of which included values that exceeded 100% of body mass. Thus, 1,425 men had valid measures of D3Cr muscle mass/body mass; of these 43 were missing the falls outcome or covariate data. Thus, the main analysis sample is 1,382 men; for the incident mobility limitation outcome, the sample was limited to those without prevalent mobility limitations (N = 1,062).
Statistical Approach
We compared characteristics of participants across quartiles of D3Cr muscle mass/body mass, using ANOVA, Wilcoxon tests, and chi-square tests as appropriate. We used generalized linear models to compute adjusted means (see footnote, Figure 2) of the various muscle function and physical performance tests across quartiles of D3Cr muscle mass/body mass, and report 95% confidence intervals and p for trend from these models. We report the standardized beta-coefficient (95% CI) for the association between D3Cr muscle mass/body mass and the muscle function and performance measures. Logistic regression was used to estimate the likelihood of prevalent mobility limitations, and separately for limitations in other tasks (heavy housework, bathing/showering; getting out of bed/chairs, and carrying or lifting 10 pounds). Both D3Cr muscle mass/body mass and ALM/ht2 by DXA were analyzed as continuous values with the odds ratio expressed per SD increment, and also by quartiles. Secondarily, we also analyzed D3Cr total body muscle mass, ALM, ALM/body mass index (BMI), and ALM/body mass or their associations with functional limitations. We adjusted for potential confounding variables not on the causal pathway between low muscle mass and falls or limitations, and adjusted all models for this parsimonious set of variables. (see footnote, Figure 2). Further adjustment for other factors such as living arrangement, marital status and nutritional intake did not materially change the results. Smoking status was not assessed in 168 men; those missing these data were included in multivariate models as a separate group in order to increase the analysis sample for the multivariate model. To determine whether D3Cr muscle mass/body mass was associated with these outcomes was independent of physical performance, we subsequently adjusted all models for walking speed and grip strength. Those missing walking speed and grip strength were coded to the lowest value in all analyses to reduce missing data. Due to problems with collinearity discovered when examining variance inflation factors with weighted predicted probabilities from our logistic models, we did not adjust for percent body fat. The associations between percent body fat and incident outcomes are presented in Supplementary Tables. In addition, we ran multivariate models with D3Cr muscle mass or DXA ALM without adjustment body size, and then additionally adjusted in subsequent models with separate variables for body mass (weight); total fat mass; body mass and height2; total fat mass and height2; and body mass, height2, grip strength, and walking speed.
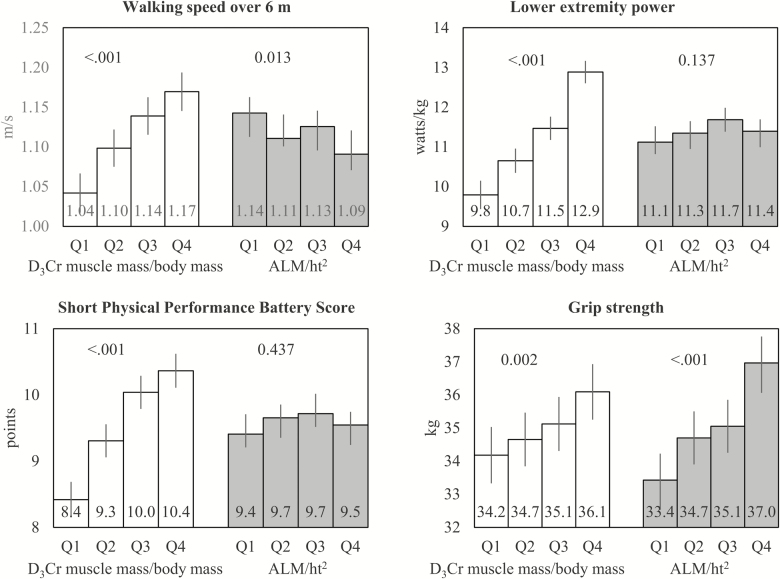
Figure 2.
Adjusted* means of walking speed over 6 m, lower extremity power, SPPB score, and grip strength across quartiles of D3Cr muscle mass/body mass or ALM/ht2. *Adjusted for age, clinical center, race, alcohol use, smoking, congestive heart failure, chronic obstructive pulmonary disease, diabetes, myocardial infarction, physical activity, exhaustion, and cognitive function. Quartile cut-points for D3Cr muscle mass/body mass: Q1: <0.27 Q2: ≥0.27–0.30, Q3: ≥0.30–0.34, Q4: ≥0.34. Quartile cut-points for ALM/ht2 (kg/m2): Q1: <6.9, Q2: ≥6.9–<7.5, Q3: ≥7.5–<8.1, Q4: ≥8.1. ALM = Appendicular lean mass.
Incident Analyses
Separate logistic regression models were used to estimate the likelihood of incident recurrent falls, incident serious injurious falls, and incident mobility limitations. D3Cr muscle mass/body mass and DXA ALM/ht2, were analyzed as described above, with the same set of covariates included in the multivariate models, and the same set of sensitivity analyses completed.
Results
Men in higher quartiles of D3Cr muscle mass/body mass were younger, were less likely to be white and had smaller body size (lower weight and lower BMI, Table 1). Some comorbid conditions varied across quartiles of D3Cr muscle mass/body mass, especially diabetes, myocardial infarction, congestive heart failure and chronic obstructive pulmonary disease. In addition, men in higher quartiles of D3Cr muscle mass/body mass generally had better cognitive function, had higher levels of physical activity, greater Life-Space, and had markedly lower levels of exhaustion and fatigue. Inability to complete repeat chair stands, the 400 m walk, or the balance component of the Short Physical Performance Battery (SPPB) was more common amongst men in the lower versus higher quartiles of D3Cr muscle mass/body mass. There were no differences, however, in DXA ALM/ht2 across quartiles of muscle mass/body mass.
Table 1.
Characteristics of MrOS Men by Quartiles of Muscle Mass/Body Mass by D3Cr Dilution
| Quartile 1, (lowest) <0.273 N = 350 |
Quartile 2 ≥0.273–<3.02 N = 350 |
Quartile 3 ≥0.302–<0.338 N = 350 |
Quartile 4 (highest) ≥0.338 N = 351 |
p-value | |
|---|---|---|---|---|---|
| Anthropometrics and demographics | |||||
| Age | 85.5 ± 4.3 | 84.7 ± 4.1 | 83.9 ± 4 | 82.6 ± 3.2 | <.001 |
| White race | 326 (93.1) | 324 (92.6) | 323 (92.3) | 289 (82.3) | <.001 |
| Height (cm) | 172.8 ± 6.9 | 172.3 ± 6.6 | 172.1 ± 6.6 | 171.7 ± 7 | .166 |
| Weight (kg) | 86.6 ± 13.5 | 80.7 ± 11.9 | 78.0 ± 11.3 | 73.4 ± 9.1 | <.001 |
| BMI (kg/m2) | 29 ± 4.0 | 27.1 ± 3.3 | 26.3 ± 3.2 | 24.9 ± 2.7 | <.001 |
| Percent body fat | 32.1 ± 5.3 | 29.1 ± 4.9 | 26.7 ± 4.7 | 23.3 ± 4.8 | <.001 |
| ALM (kg) | 22.7 ± 3.4 | 22.2 ± 3.1 | 22.5 ± 3.0 | 22.4 ± 3.0 | .200 |
| ALM/ht2 (kg/m2) | 7.6 ± 1.0 | 7.5 ± 0.8 | 7.6 ± 0.8 | 7.6 ± 0.8 | .144 |
| Physical performance and strength | |||||
| Unable to complete five chair stands | 14 (5.3) | 4 (1.3) | 6 (1.8) | 1 (0.3) | <.001 |
| Unable to complete 400 m walk | 117 (33.9) | 46 (13.4) | 28 (8.1) | 11 (3.1) | <.001 |
| Unable to hold tandem stand for 10 sa | 212 (60.6) | 159 (45.4) | 132 (37.7) | 84 (23.9) | <.001 |
| Comorbid conditions | |||||
| Stroke | 17 (4.9) | 18 (5.1) | 20 (5.7) | 7 (2.0) | .077 |
| Diabetes | 75 (21.4) | 56 (16.0) | 44 (12.6) | 40 (11.4) | .001 |
| Parkinson’s disease | 5 (1.4) | 6 (1.7) | 8 (2.3) | 4 (1.1) | .669 |
| Myocardial infarction | 70 (20.0) | 44 (12.6) | 37 (10.6) | 36 (10.3) | <.001 |
| Congestive heart failure | 49 (14.0) | 26 (7.4) | 23 (6.6) | 15 (4.3) | <.001 |
| Chronic obstructive pulmonary disease | 54 (15.4) | 46 (13.1) | 29 (8.3) | 34 (9.7) | .013 |
| Nonskin cancer | 169 (48.3) | 174 (49.7) | 155 (44.3) | 168 (47.9) | .524 |
| Cognitive function | |||||
| Global cognitive function (Teng 3MS) | 91.4 ± 6.8 | 92.0 ± 7.2 | 92.7 ± 6.6 | 93.5 ± 5.8 | <.001 |
| Trails B (seconds) | 158.7 ± 72.4 | 137.5 ± 64.2 | 135.7 ± 66.3 | 125.3 ± 64.7 | <.001 |
| Health habits, activity and quality of life | |||||
| Smoking status | <.001 | ||||
| Never | 97 (27.7) | 131 (37.4) | 139 (39.7) | 162 (46.2) | |
| Past | 188 (53.7) | 169 (48.3) | 171 (48.9) | 154 (43.9) | |
| Current | 3 (0.9) | 5 (1.4) | 1 (0.3) | 6 (1.7) | |
| Not assessedb | 62 (17.7) | 45 (12.9) | 39 (11.1) | 29 (8.3) | |
| Alcohol use | .006 | ||||
| 0–2 drinks per week | 228 (65.7) | 215 (61.4) | 203 (58.5) | 193 (55.0) | |
| 3–13 drinks per week | 94 (27.1) | 123 (35.1) | 130 (37.5) | 138 (39.3) | |
| ≥14 drinks per week | 25 (7.2) | 12 (3.4) | 14 (4.0) | 20 (5.7) | |
| Self-reported physical activity (PASE) **** | 94.7 ± 61.5 | 109.0 ± 61.4 | 129.5 ± 64.8 | 139.5 ± 60.8 | <.001 |
| SF-12 modified physical component summary scaled | 56.2 ± 7.1 | 56.5 ± 6.4 | 56.2 ± 6.3 | 56.5 ± 6.0 | .909 |
| Exhaustion/low energyc | 40.5 ± 11.7 | 45.6 ± 10.2 | 48.0 ± 9.2 | 50.0 ± 8.5 | <.001 |
| Pittsburgh physical fatigability scored | 20.5 ± 9.7 | 17.0 ± 9.3 | 15.0 ± 8.7 | 12.4 ± 8.2 | <.001 |
| Life-space scored | 9.3 ± 9.2 | 7.8 ± 8.2 | 7.2 ± 7 | 6 ± 6.9 | <.001 |
| Lives alone | 90 (25.7) | 89 (25.4) | 80 (22.9) | 74 (21.1) | .420 |
| Total energy intake (kcal) | 1,593.3 ± 671.8 | 1,481.1 ± 559.3 | 1,528.6 ± 635.4 | 1,499.1 ± 602.1 | .225 |
| Number of medications | <.001 | ||||
| 0–6 | 81 (23.1) | 111 (31.7) | 104 (29.7) | 136 (38.7) | |
| 7–10 | 123 (35.1) | 133 (38) | 138 (39.4) | 127 (36.2) | |
| 11+ | 146 (41.7) | 106 (30.3) | 108 (30.9) | 88 (25.1) | |
Note: ALM = Appendicular lean mass; BMI = Body mass index; MrOS = Osteoporotic fractures in men study.
aPart of the balance component of the SPPB. bSome participants were not queried about current smoking status at the Year 10.5 visit. cReporting having a good bit of energy “none of the time,” “a little of the time,” “some of the time” over the past 4 wk as part of the SF-12. dScale descriptions: PASE is a unit less scale based on a weighted average of responses to questions about volitional and occupational activity; higher scores indicate greater activity. SF-12 PCS score is a unit less scale based on a weight 12 questions about generic health status; range is 0–100 and higher scores indicate greater self-rated health. Life-space is a unit less measure of the participant’s mobility through his home and community was estimated by questionnaire (score 0–120, higher score indicates greater mobility). The Pittsburgh physical fatigability score is unit less scale based on 10 questions about situational fatigability; score 0–50 with higher scores representing greater fatigability.
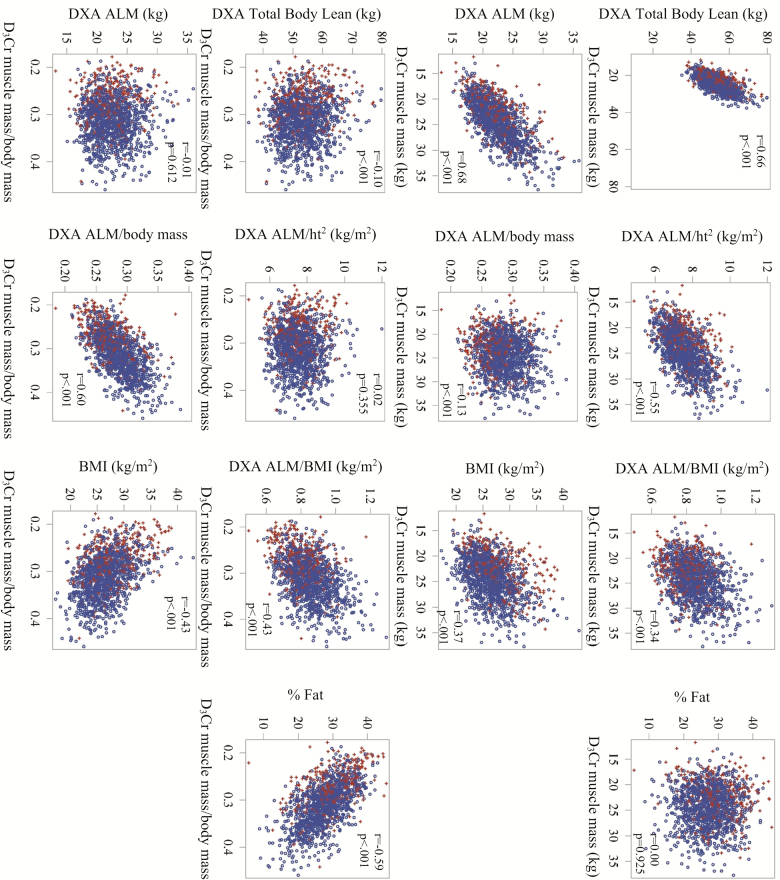
D3Cr muscle mass was moderately correlated with total body lean mass by DXA (Figure 1). However, this relationship did not fall along the line of identity, with the DXA measure of total body lean mass consistently higher than muscle mass by D3Cr dilution. D3Cr muscle mass was also moderately correlated with ALM/ht2, ALM/BMI, ALM, and BMI, and was weakly correlated with ALM/body mass; the correlation with percent fat was not significant. D3Cr muscle mass/body mass was weakly correlated with total body lean mass by DXA; modestly correlated with DXA ALM/BMI, DXA ALM/body mass, percent fat, and BMI. There was no statistically significant correlation between D3Cr muscle mass/body mass and DXA ALM/ht2 or DXA ALM. In general, men who reported prevalent mobility limitations were in the lower end of the distribution of muscle mass/body mass or the higher end of the percent fat distribution, but such a relationship was not observed for the DXA based measures of lean mass.
Figure 1.
Correlations between D3Cr dilution measures of muscle mass (kg) and D3Cr muscle mass/body mass with DXA-derived measures of lean mass and fat (total body lean mass, ALM, ALM/ht2, ALM/body mass, percent fat) and BMI in older men. ALM = Appendicular lean mass; BMI = Body mass index; DXA = Dual x-ray absorptiometry.
D3Cr Muscle Mass/Body Mass, ALM/ht2, and Physical Performance and Strength
Men in the highest quartiles of D3Cr muscle mass/body mass walked faster over 6 and 400 m; had greater grip strength and better lower extremity muscle power and force; and had much better performance on repeated chair stands and SPPB after adjustment for numerous potential confounders (Figure 2 and Supplementary Figure 2). The association between D3Cr muscle mass/body mass and physical performance and strength was graded: with increasing quartiles of D3Cr muscle mass/body mass, we observed better physical performance (p for trend across quartiles <.002 for all strength and physical performance measures). The strongest associations between D3Cr muscle mass/body mass and measures of physical performance were with lower extremity muscle power and force, as evidenced by standardized beta-coefficients from linear regression models (Supplementary Table 1). Aside from a positive association between DXA ALM/ht2 and grip strength, there was no statistically significant association between low DXA ALM/ht2 and other physical performance measures for the adjusted means by quartile or for the standardized beta-coefficients from linear regression.
D3Cr Body Muscle Mass/Body Mass, ALM/ht2, and Prevalent Limitations
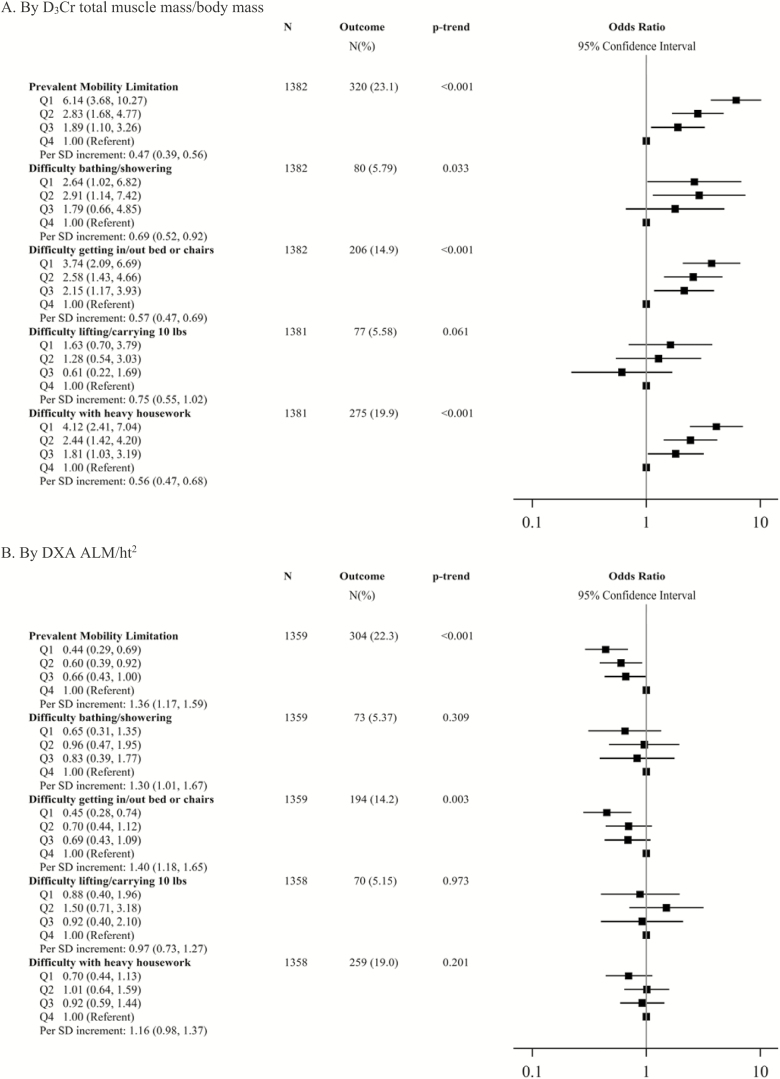
Men with D3Cr muscle mass assessment at the Year 14 Visit reported prevalent difficulty in several ADLs and IADLs. Of the men in the multivariate models, 320 (23.1%) reported a mobility limitation; 80 (5.9%) reported difficulty with bathing/showering; 206 (14.9%) reported difficulty getting out of bed/chairs; 77 (5.6%) reported difficulty lifting/carrying 10 lbs; and 275 (19.9%) reported difficulty with heavy housework. Lower D3Cr muscle mass/body mass was strongly related to difficulty with these tasks. For example, after multivariate adjustment, men in the lowest quartile of D3Cr muscle mass/body mass were more than six times more likely to report a prevalent mobility limitation than men in the highest quartile (Figure 3). In addition, each SD increment in D3Cr muscle mass/body mass was associated with 0.47-fold lower adjusted odds of a prevalent mobility limitation. Similarly, after multivariate adjustment, men in the lowest quartile of D3Cr muscle mass/body mass were 3.7–4.1-fold more likely to report difficulty with getting in/out of bed or chairs, or doing heavy housework compared to men in the lowest quartile. Further adjustment for walking speed and grip strength only slightly attenuated these associations (Supplementary Table 4). Associations between D3Cr muscle mass/body mass and difficulty bathing/showering or lifting/carrying 10 pounds were inconsistent. By contrast, lower levels of DXA ALM/ht2 were not associated with increased likelihood of reporting difficulty with any of the tasks examined. In some cases, the association between DXA ALM/ht2 and difficulty with these tasks went in the opposite of the hypothesized direction (that is, lower DXA ALM/ht2 was protective against reporting difficulty rather than being a risk factor).
Figure 3.
Multivariate-adjusted* likelihood (odds ratio, 95% CI) of prevalent function limitations, by D3Cr muscle mass/body mass and DXA ALM/ht2. *Model is adjusted for age, clinical center, race, alcohol use, smoking, congestive heart failure, chronic obstructive pulmonary disease, diabetes, myocardial infarction, physical activity, exhaustion, and cognitive function. Quartile cut-points for D3Cr muscle mass/body mass: Q1: <0.27 Q2: ≥0.27–0.30, Q3: ≥0.30–0.34, Q4: ≥0.34. Quartile cut-points for ALM/ht2 (kg/m2): Q1: <6.9, Q2: ≥6.9–<7.5, Q3: ≥7.5–<8.1, Q4: ≥8.1. SD for D3Cr muscle mass/body mass: 0.048; SD for ALM/ht2: 0.87. ALM = Appendicular lean mass.
Alternative Metrics of Muscle, Lean Mass and Fat Mass, and Prevalent Limitations
D3Cr muscle mass (not adjusted for body mass) was associated with prevalent mobility limitation, carrying 10 lbs and heavy housework, but not with difficulty bathing/showering or difficulty getting out of bed/chairs after full adjustment. Separate adjustment for separate variables of body mass; fat mass; body mass and height2; or fat mass and height2 generally demonstrated similar associations. D3Cr muscle mass was most strongly related to outcomes when body mass was included as a covariate (Supplementary Table 2). DXA ALM was associated with difficulty getting out of bed/chairs, albeit in the opposite direction of our hypothesis (lower DXA ALM was protective against this outcome rather than a risk factor.) DXA ALM/BMI was associated with prevalent mobility limitations, carrying 10 lbs, and heavy housework, but this association was more modest in magnitude than the associations observed between D3Cr muscle mass/body mass. DXA ALM/BMI was not related to either difficulty with bathing/showering or difficulty getting out of bed/chairs. Likewise, in the fully-adjusted models, DXA ALM/body mass was associated with prevalent mobility limitation, difficulty getting out of bed/chairs, and difficulty with heavy housework (although not difficulty with bathing/showering or carrying 10 lbs), but again this association was more modest than the association between D3Cr muscle mass/body mass and these outcomes. Separate adjustment for separate variables of body mass; fat mass; boy mass and height2; or fat mass and height2 generally demonstrated similar associations. Lower DXA ALM was associated with increased likelihood of prevalent mobility limitation only when body mass was included as an additional variable (but not when height2, or grip and walking speed were also included in the model, Supplementary Table 3). Lower percent body fat was protective against prevalent mobility limitations, difficulty getting out of a bed/chair, and difficulty with heavy housework, but not with difficulty bathing/showering or carrying 10 lbs after multivariate adjustment.
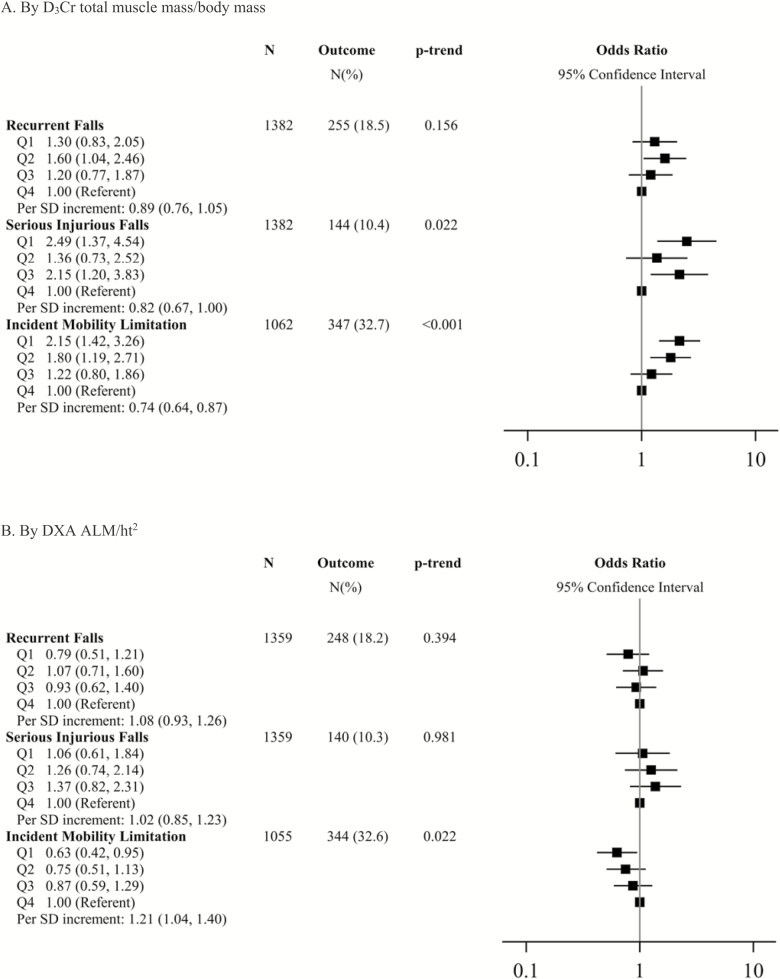
D3Cr Muscle Mass/Body Mass, DXA ALM/ht2, and Incident Falls and Mobility Limitations
In the year after the Year 14 visit, 255 (18.5%) men reported recurrent falls and 144 (10.4%) reported serious injurious falls. Among men without a mobility limitation at the Year 10.5 Visit, 347 (32.7%) reported a new mobility limitation. Men in the lowest quartile of D3Cr muscle mass/body mass were more than twice as likely to report incident serious injurious falls or an incident mobility limitation compared to men in the highest quartile (Figure 4). In addition, each SD increment in D3Cr muscle mass/body mass was associated with an approximate 0.75–0.80-fold reduction in the likelihood of either serious injurious falls or incident mobility limitations. In contrast, there was no statistically significant association between DXA ALM/ht2 and recurrent falls or serious injurious falls. The association between DXA ALM/ht2 and incident mobility limitations was in the opposite direction of our hypothesis, such that men in the lowest quartile of DXA ALM/ht2 had a 0.63-fold reduced likelihood of reporting a mobility limitation than men in the highest quartile. When DXA ALM/ht2 was analyzed as a continuous variable, the association was the same. Results were similar when further adjusted for grip strength and walking speed (Supplementary Table 7).
Figure 4.
Multivariate-adjusted* likelihood (odds ratio, 95% CI) of incident recurrent falls, serious injurious falls and mobility limitation, by D3Cr muscle mass/body mass and DXA ALM/ht2. *Model is adjusted for age, clinical center, race, alcohol use, smoking, congestive heart failure, chronic obstructive pulmonary disease, diabetes, myocardial infarction, physical activity, exhaustion, and cognitive function. Quartile cut-points for D3Cr muscle mass/body mass in falls models: Q1: <0.27 Q2: ≥0.27–0.30, Q3: ≥0.30–0.34, Q4: ≥0.34. Quartile cut-points for ALM/ht2 (kg/m2) in falls models: Q1: <6.9, Q2: ≥6.9–<7.5, Q3: ≥7.5–<8.1, Q4: ≥8.1. Quartile cut-points for D3Cr muscle mass/body mass in mobility limitations model: Q1:<0.28, Q2: ≥0.28–<0.31, Q3: ≥0.31–<0.35, Q4: ≥0.35. Quartile cut-points for ALM/ht2 (kg/m2) in mobility limitations models: Q1: <6.9, Q2: ≥6.9–<7.5, Q3: ≥7.5–<8.0, Q4: ≥8.0. For falls models: SD for D3Cr muscle mass/body mass: 0.048; SD For ALM/ht2: 0.87 For mobility limitations models: SD for D3Cr muscle mass/body mass: 0.046; SD for ALM/ht2: 0.855. ALM = Appendicular lean mass; DXA = Dual x-ray absorptiometry.
Alternative Metrics of Muscle, Lean Mass and Fat Mass, and Incident Falls and Mobility Limitations
Lower D3Cr muscle mass (unadjusted for body mass), lower DXA ALM, and lower DXA ALM/BMI were not associated with increased likelihood of incident recurrent falls, serious injurious falls or mobility limitations and full multivariate adjustment. Higher percent fat and lower DXA ALM/weight were not associated with recurrent or serious injurious falls after full multivariate adjustment. Higher percent fat and lower DXA ALM/body mass were associated with increased likelihood of mobility limitation after full multivariate adjustment, however, the association was more modest than that for D3Cr muscle mass/body weight (Supplementary Table 7). In D3Cr muscle mass models further adjusted for separate variables of body size, there was no association in any model with recurrent falls or serious injurious falls (Supplementary Table 5). Lower D3Cr muscle mass, in multivariate models (with our without adjustment separately for body mass; total fat mass; weight and height2; or total fat mass and height2) remained significantly associated with increased likelihood of serious injurious falls. Further adjustment for body mass, height2 and grip and walking speed attenuated this association to non-significance. Lower D3Cr muscle mass was associated with incident mobility limitations in models unadjusted for body size and in those adjusted for body mass, and body mass and height2, but not in the other models accounting for total fat mass, or grip strength and walking speed. DXA ALM was not associated with recurrent falls or serious injurious falls in any of these sensitivity models (Supplementary Table 6). Lower DXA ALM without adjustment for body mass was associated with lower likelihood of incident mobility limitations; after adjustment for body mass, this association was reversed, with lower DXA ALM demonstrating a borderline increased likelihood of incident mobility limitation. None of the other DXA ALM sensitivity analyses for incident mobility limitation were significant.
Discussion
Here, we show for the first time in older men that strong and consistent associations exist between D3Cr muscle mass and physical performance; self-reported functional limitations; and incident falls and mobility limitations in older men, particularly when D3Cr muscle mass is standardized to body mass. The wide-ranging association of D3Cr muscle mass with components of everyday activities provides compelling evidence for the powerful influence of skeletal muscle (when measured accurately) on health outcomes important to older persons, even after accounting for the potentially confounding influence of activity level, body size, and coexisting medical conditions. Although weakness and poor endurance have been previously associated increased risk of falls and mobility limitations (4,37), this is the first study to demonstrate the importance of the amount of muscle mass per se. The associations observed for physical performance were strong in magnitude, with differences across quartiles of 0.13 m/s for walking speed and 2.0 points for the SPPB, exceeding clinically important differences in these tests (38). Accordingly, the D3Cr dilution method to measure muscle mass is a novel, accurate tool for measuring total muscle mass.
The strong relationship between muscle mass and adverse outcomes has not been previously observed, likely because other studies have used inaccurate approximations of muscle mass. To wit, a traditional approximation of low muscle mass, low DXA ALM/height2, was not strongly associated with poor physical performance or adverse health outcomes in our data, similar to previous reports in this and other populations (2,39). This may be explained by the fact that the use of DXA for the determination of lean mass is not specific for skeletal muscle and includes noncontractile components as well as water content. Measures of lean mass from DXA are influenced by hydration status and the presence of extracellular fluid, as water is included in lean mass estimates (as water is neither bone nor fat) (40). Our secondary results confirm previous studies that demonstrate that obesity is associated with poor physical performance and functional limitations (4). In addition, the secondary results also suggest that DXA measures of lean mass are less strongly associated with poor physical performance and functional limitations than D3Cr dilution muscle mass. Therefore, in totality, our data suggest that both obesity and muscle (but not lean mass by DXA) should be considered when evaluating the risk of functional decline in older adults.
The D3Cr dilution method may prove feasible for use in out-patient clinical settings as evidenced by our successful implementation in this population of community-dwelling men aged 80 years and older. In addition, this method provides precise quantification of a biochemical phenotype that is very specific for muscle. This is in contrast to other imaging methods such as DXA, MRI, and CT that rely on numerous assumptions to provide a blunt approximation of muscle mass. The cost of the test in the research setting (including the dose and the assays) is approximately $120USD per participant, a cost that is equivalent to or slightly more than DXA but considerably less than MR or CT in research settings in the United States. Thus, should future research confirm our findings in other populations (such as women, younger people, and the institutionalized), muscle mass by D3Cr dilution has the potential to precisely identify those with low muscle mass who are at risk of adverse health outcomes through the use of a single, straightforward test.
Strengths of this study include its large size, prospective design, and extensive characterization of mobility endpoints. We cannot rule out unmeasured confounding as an explanation for our findings. However, we adjusted for many potentially confounding factors, and it is unlikely that unmeasured factors would fully explain the compelling associations observed. While we considered for measures of strength (grip strength) and force generation (from the force plate jumping measure), we did not include gold standard measures of strength such as isokinetic dynamometry, thus future research should assess the independence of D3Cr muscle mass and strength when strength is measured with optimal methods.
In summary, low muscle mass as measured by D3Cr dilution was strongly associated with several of the most common clinical complaints of older men including poor physical performance, fatigue, and subsequent risk of injurious falls and mobility limitations particularly after accounting for body mass. Such associations were not observed with lean mass assessed by DXA, demonstrating that lean mass assessed by DXA does not provide an accurate measure of total muscle mass. The D3Cr dilution test may enable a shift in future research to elucidate the role of skeletal muscle mass in loss of mobility, and to precisely test the effects of lifestyle, nutritional, and pharmaceutical interventions on improving muscle mass. Future research is required to evaluate the use of D3Cr muscle mass as a precise test for diagnosing sarcopenia and for identifying older adults at risk of adverse health outcomes.
Funding
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Center for Advancing Translational Sciences (NCATS), and NIH Roadmap for Medical Research under the following grant numbers: U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128. Funding for the D3Cr muscle mass measure was provided by NIAMS (grant number R01 AR065268). GlaxoSmithKline provided in-kind support by providing the d3-creatine dose and analysis of urine samples.
Conflict of Interest
None reported.
Supplementary Material
References
- 1. Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520 [DOI] [PubMed] [Google Scholar]
- 2. Cawthon PM, Blackwell TL, Cauley J, et al. Evaluation of the usefulness of consensus definitions of sarcopenia in older men: results from the observational osteoporotic fractures in men cohort study. J Am Geriatr Soc. 2015;63:2247–2259. doi: 10.1111/jgs.13788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cawthon PM, Fox KM, Gandra SR, et al. ; Health, Aging and Body Composition Study Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults?J Am Geriatr Soc. 2009;57:1411–1419. doi: 10.1111/j.1532-5415.2009.02366.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev. 2013;35:51–65. doi: 10.1093/epirev/mxs006 [DOI] [PubMed] [Google Scholar]
- 5. Janssen I. Influence of sarcopenia on the development of physical disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x [DOI] [PubMed] [Google Scholar]
- 6. Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x [DOI] [PubMed] [Google Scholar]
- 7. Zoico E, Di Francesco V, Mazzali G, et al. High baseline values of fat mass, independently of appendicular skeletal mass, predict 2-year onset of disability in elderly subjects at the high end of the functional spectrum. Aging Clin Exp Res. 2007;19:154–159. doi: 10.1007/BF03324682 [DOI] [PubMed] [Google Scholar]
- 8. Ramsay SE, Whincup PH, Shaper AG, Wannamethee SG. The relations of body composition and adiposity measures to ill health and physical disability in elderly men. Am J Epidemiol. 2006;164:459–469. doi: 10.1093/aje/kwj217 [DOI] [PubMed] [Google Scholar]
- 9. Visser M, Harris TB, Langlois J, et al. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci. 1998;53:M214–M221. doi: 10.1093/gerona/53A.3.M214 [DOI] [PubMed] [Google Scholar]
- 10. Visser M, Langlois J, Guralnik JM, et al. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998;68:584–590. doi: 10.1093/ajcn/68.3.584 [DOI] [PubMed] [Google Scholar]
- 11. Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60:324–333. doi: 10.1093/gerona/60.3.324 [DOI] [PubMed] [Google Scholar]
- 12. Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985). 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003 [DOI] [PubMed] [Google Scholar]
- 13. Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. ; European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Studenski SA, Peters KW, Alley DE, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA. 2002;288:1889–1897. doi: 10.1001/jama.288.15.1889 [DOI] [PubMed] [Google Scholar]
- 17. Newman AB, Kupelian V, Visser M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x [DOI] [PubMed] [Google Scholar]
- 18. Cawthon PM, Peters KW, Shardell MD, et al. Cutpoints for low appendicular lean mass that identify older adults with clinically significant weakness. J Gerontol A Biol Sci Med Sci. 2014;69:567–575. doi: 10.1093/gerona/glu023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stimpson SA, Leonard MS, Clifton LG, et al. Longitudinal changes in total body creatine pool size and skeletal muscle mass using the D-creatine dilution method. J Cachexia Sarcopenia Muscle. 2013. PMID: 23797207; PMCID: PMC3774916. doi: 10.1007/s13539-013-0110-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stimpson SA, Turner SM, Clifton LG, et al. Total-body creatine pool size and skeletal muscle mass determination by creatine-(methyl-D3) dilution in rats. J Appl Physiol (1985). 2012;112:1940–1948. doi: 10.1152/japplphysiol.00122.2012 [DOI] [PubMed] [Google Scholar]
- 21. Clark RV, Walker AC, O’Connor-Semmes RL, et al. Total body skeletal muscle mass: estimation by creatine (methyl-d3) dilution in humans. J Appl Physiol (1985). 2014;116:1605–1613. doi: 10.1152/japplphysiol.00045.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–568. doi: 10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 23. Orwoll E, Blank JB, Barrett-Connor E, et al. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi: 10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 24. Shankaran M, Czerwieniec G, Fessler C, et al. Dilution of oral D(3) - Creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle. 2018;9:540-546. PMID: 29663711; PMCID: PMC5989770. doi:10.1002/jcsm.12278. Epub 2018 Apr 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hunter A. Creatine and Creatinine, Monographs on Biochemistry. London:Lougmas, Green & Co; 1928. [Google Scholar]
- 26. Balsom PD, Soderlund K, Ekblom B. Creatine in humans with special reference to creatine supplementation. Sports Med. 1994;18:268–280. doi: 10.2165/00007256-199418040-00005 [DOI] [PubMed] [Google Scholar]
- 27. Fitch CD, Lucy DD, Bornhofen JH, Dalrymple GV. Creatine metabolism in skeletal muscle. II. Creatine kinetics in man. Neurology. 1968;18:32–42. doi: 10.1212/WNL.18.1_Part_1.32 [DOI] [PubMed] [Google Scholar]
- 28. Wyss M, Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107 [DOI] [PubMed] [Google Scholar]
- 29. Bloch K, Schoenheimer R. Studies in protein metabolism. J Biol Chem. 1939;131:111–119. [Google Scholar]
- 30. Heymsfield SB, Arteaga C, McManus C, Smith J, Moffitt S. Measurement of muscle mass in humans: validity of the 24-hour urinary creatinine method. Am J Clin Nutr. 1983;37:478–494. doi: 10.1093/ajcn/37.3.478 [DOI] [PubMed] [Google Scholar]
- 31. Caserotti P, Aagaard P, Larsen JB, Puggaard L. Explosive heavy-resistance training in old and very old adults: changes in rapid muscle force, strength and power. Scand J Med Sci Sports. 2008;18:773–782. doi: 10.1111/j.1600-0838.2007.00732.x [DOI] [PubMed] [Google Scholar]
- 32. Caserotti P, Aagaard P, Simonsen EB, Puggaard L. Contraction-specific differences in maximal muscle power during stretch-shortening cycle movements in elderly males and females. Eur J Appl Physiol. 2001;84:206–212. doi: 10.1007/s004210170006 [DOI] [PubMed] [Google Scholar]
- 33. Cawthon PM, Fullman RL, Marshall L, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group Physical performance and risk of hip fractures in older men. J Bone Miner Res. 2008;23:1037–1044. doi: 10.1359/jbmr.080227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 36. Lee CG, Boyko EJ, Nielson CM, et al. Mortality risk in older men associated with changes in weight, lean mass, and fat mass. J Am Geriatr Soc. 2011;59:233–240. doi: 10.1111/j.1532-5415.2010.03245.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x [DOI] [PubMed] [Google Scholar]
- 38. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 39. Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61:72–77. doi: 10.1093/gerona/61.1.72 [DOI] [PubMed] [Google Scholar]
- 40. Plank LD. Dual-energy X-ray absorptiometry and body composition. Curr Opin Clin Nutr Metab Care. 2005;8:305–309. doi: 10.1097/01.mco.0000165010.31826.3d [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.