Abstract
Background
To examine the associations between objective physical activity measures and subsequent health care utilization.
Methods
We studied 1,283 men (mean age 79.1 years, SD 5.3) participating in the Osteoporotic Fractures in Men Study. Participants wore a SenseWear® Pro Armband monitor for 1 week. Data was summarized as daily (i) step counts, (ii) total energy expenditure, (iii) active energy expenditure, and (iv) activity time (sedentary, ≥ light, ≥ moderate). The outcome measures of 1-year hospitalizations/duration of stay from Medicare data were analyzed with a two-part hurdle model. Covariates included age, clinical center, body mass index, marital status, depressive symptoms, medical conditions, cognitive function, and prior hospitalization.
Results
Each 1 SD = 3,092 step increase in daily step count was associated with a 34% (95% confidence interval [CI]: 19%–46%) lower odds of hospitalization in base model (age and center) and 21% (95% CI: 4%–35%) lower odds of hospitalization in fully adjusted models. Similar but smaller associations held for other physical activity measures, but these associations were not significant in fully adjusted models. Among those hospitalized, higher step count was associated with shorter total duration of acute/postacute care stays in the base model only. There was a fourfold significant difference (from model-based estimates) in predicted care days comparing those with 2,000 versus 10,000 daily steps in the base model, but only a twofold difference (not significant) in the full model.
Conclusion
Daily step count is an easily determined measure of physical activity that may be useful in assessment of future health care burden in older men.
Keywords: Objective physical activity, Step count, Health care utilization, Hospitalization, Older men
Physical activity can be objectively measured using activity monitors that assess volume, intensity, and frequency of activity. The data from these monitors can be used to determine time spent in activity at given levels of intensity (sedentary, light, moderate, or vigorous activity). The data can also be used to derive summary variables such as daily step count, total energy expenditure, and energy expenditure in activity below/above certain thresholds. A comparison between measures based on self-report and those based on activity monitors has shown that participants self-report longer duration and higher levels of activity and lower sedentary time than the same measures derived from objective devices (1).
It has been previously shown, using activity monitor derived measures in the MrOS cohort, that lower objectively measured physical activity is associated with increased fracture risk (2), increase risk of impairment in activities of daily living (3), and increased risk of mortality (4). In other studies, objective measures of activity were also associated with impaired activities of daily living (5) and cognitive impairment (6). Given that lower physical activity is associated with a broad spectrum of adverse health outcomes, it is likely that lower levels of objectively measured physical activity are associated with increased risk of hospitalization and subsequent use of postacute care (skilled nursing facilities [SNF] and/or inpatient rehabilitation facility [IRF]). There are currently very limited data on the relationship between objectively measured physical activity and subsequent health care utilization in older adults. There is one small study (213 older men and women) based in the United Kingdom showing that patterns of activity were related to use of prescription medications and unplanned hospital admissions (7). Two studies of patients discharged from the hospital showed that lower physical activity before and after discharge predicted 30-day hospital readmission (8,9). None of these studies assessed the association between physical activity and length of hospital stay. Moreover, no study has investigated the association of physical activity with composite lengths of stay in acute hospital and postacute care (SNF, IRF, and custodial care) facilities, an important measure of overall health care burden.
Our primary aim was to determine the associations of several objective measures of physical activity (total daily step counts, total daily energy expenditure, active daily energy expenditure, daily time spent in sedentary activity, daily time spent in nonsedentary [light, moderate, or vigorous] activity and daily time spent is moderate-to-vigorous physical activity) with risk of subsequent hospitalization and rate of acute/postacute facility care days after adjusting for demographic variables and other traditional prognostic indicators using a longitudinal cohort study linked with claims data. Our secondary aim was to determine whether the association between objectively measured physical activity and inpatient health care utilization is independent of self-reported measures of physical activity. The latter assessment determines whether the objective assessment of physical activity is a risk factor after accounting for self-reported activity.
Methods
Study Population
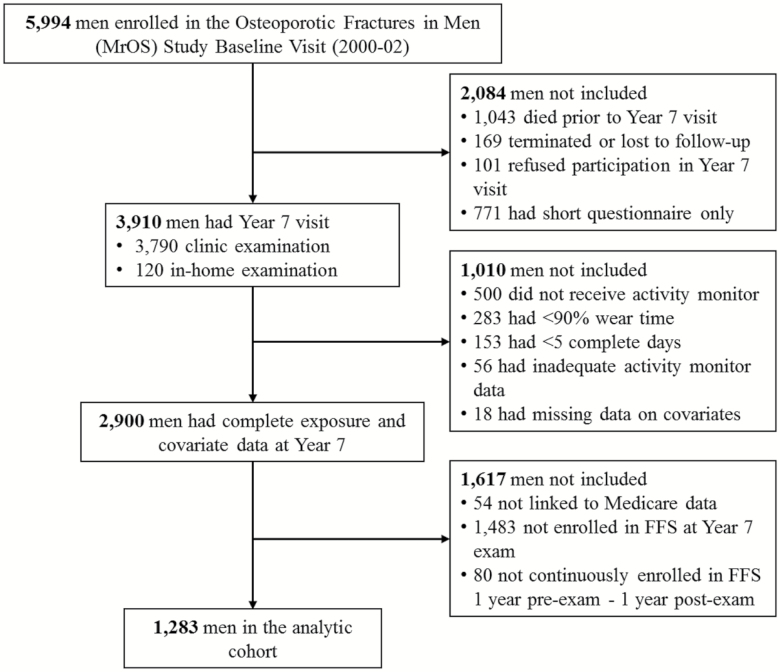
We studied participants enrolled in the Osteoporotic Fractures in Men (MrOS) Study, a prospective cohort study of older men. From 2000 to 2002, 5,994 men were recruited from six geographic areas of the United States (Birmingham AL; Minneapolis, MN; Palo Alto, CA; Pittsburgh, PA; Portland, OR; and San Diego, CA) (10,11). Men were eligible to enroll in the study if they were community-dwelling, could walk unassisted, did not have bilateral hip replacements, and were at least 65 years old. Follow-up visits for the main cohort were scheduled at Year 5 (2005–2006) and Year 7 (2007–2009) with additional visits for ancillary dental and sleep studies. The Centers for Medicare and Medicare Services approved the linkage to MrOS participants and successful matches to Medicare were achieved for 98% of the men in the cohort. MrOS participants were eligible for the present study if they had a 2007–2009 (Year 7) clinic or home examination (N = 3,910), and had activity monitor data for five 24-hour periods with at least 90% wear time and nonmissing covariates (N = 2,900). To ensure complete outcome ascertainment, we only considered men (N = 1,283) who were enrolled in the Medicare Fee-For-Service (FFS) program (Parts A and B [and not Part C, Medicare Advantage]) on the date of the Year 7 examination and for both the prior 12 months and the subsequent 12 months (or until death within this period). Those who were excluded because they were not FFS for the requisite period had similar physical activity measurements to those in the study sample (p > .12 for all comparisons). Complete study flow diagram is shown in Figure 1. The institutional review board at each center approved the protocol, and written informed consent was obtained from each participant.
Figure 1.
Participant flow diagram.
Objective Physical Activity
Men were instructed to wear the multi-sensor SenseWear® Pro3 Armband (Body Media, Inc., Pittsburgh, PA) on their right arm at all times, including while sleeping, for a typical 7-day period and to remove it only for brief periods for bathing and water activities. The monitor uses a combination of five sensors (two-axis accelerometer, a heat flow sensor, galvanic skin response, skin temperature sensors, and ambient temperature sensors) to collect physiological data (including wear time) in 1-minute epochs. The data begins at 12 am (midnight) on the first day the participant is given the armband, and ends with the completion of the last 24-hour period at midnight, with times before and after excluded from the data analysis. These data served as inputs in proprietary algorithms (Innerview Professional 5.1 software, Body Media, Inc; Pittsburgh, PA) along with height, weight, handedness and smoking status to estimate total step counts per day and total energy expenditure (TEE) in kilocalories per day (kcal/d). The resting metabolic rate (RMR) was estimated using the Harris Benedict equations. Nonwear time was detected by the galvanic and temperature skin sensors. Energy for nonwear time was imputed by using the RMR and the TEE included both wear time energy (from arm band) and nonwear time energy. Active energy expenditure (AEE) was estimated by the equation AEE = 0.9 × TEE-RMR. The sleep interval was determined using proprietary algorithms. Energy expended was also expressed as mean metabolic equivalent of task (METs). Minutes per day while awake spent in sedentary (METs ≤ 1.5), light (METs > 1.5, <3), moderate (METs ≥3, <6), and vigorous (METs ≥ 6) intensity activities were then quantified. A validation study comparing the SenseWear® Pro Armband with the criterion method of doubly labeled water showed acceptable levels of agreement total energy and strong correlation with measure of active energy expenditure as calculated using standard equation based on criterion measure of total energy (12). A validation study in older adults demonstrated good concordance between SenseWear® Pro Armband and pedometer measured steps (13). There was a good correlation between minute-by-minute METS measured by the armband and minute-by-minute oxygen consumption in a small study of those with chronic obstructive pulmonary disease (14). Sleep time was reliably measured in a study that also performed polysomnography in patients with sleep apnea and controls (15).
Outcome Measures
Data on hospital admissions and lengths of stays for the 12-month period following the date of the Year 7 exam were obtained from the Medicare Provider Analysis and Review (MedPAR) File. Among those hospitalized, total facility care days (days in acute hospital/postacute care facilities) were calculated using a modified version of the Wei algorithm (16) in which dates for stays in skilled nursing, inpatient rehabilitation, or custodial care facilities were identified using dates from both the MedPAR file and the Minimum Data Set (version 2.0), thus further calibrating total length of stay. We included all stays at any care facility in the length of stay variable as it has been shown that care has shifted from the hospital setting to other settings, thus acute/postacute facility days more accurately represents burden of care for those who are hospitalized (17,18).
Other Measurements
Demographic and lifestyle variables were obtained from standardized questionnaires and the Year 7 clinic visit. Depressive symptoms were evaluated using the Geriatric Depression Scale (19). Self-reported physical activity was assessed using the Physical Activity Scale for the Elderly (PASE) (20). Cognitive function was assessed using the Modified Mini-Mental State Examination (3MS) (21). Body weight (kg) and height (m) were measured and body mass index was calculated in kg/m2. We used a standard measure of total multimorbidity, the Elixhauser index (22) which takes into account the presence of 31 specific medical conditions by using ICD-9 diagnostic codes in MedPAR (Part A claims), Hospital Outpatient and Carrier (Physician/Supplier Part B claims) files for each MrOS FFS participant in the 12 months preceding the Year 7 examination.
We looked at potential confounding by wear time, season, and weekday versus weekend wear in post hoc analysis. We did not find a statistically significant trend relating weekday versus weekend wear with any of the parameters used in the study. Nearly the entire study sample (>99%) had at least one day of weekend wear. Season and percentage time of wear were related to all of the main exposure parameters, but neither was related to the outcome. We therefore did not include any of these additional parameters in the analysis as confounders since confounding requires a relationship of the confounder with both exposure and outcome.
Statistical Analysis
We used a two-part hurdle model to determine the associations between objective physical activity and health care utilization (23). We hypothesized that predictors of acute care admission and predictors of facility discharge would be different, thus suggesting a two-part hurdle model might be appropriate. In addition, hurdle models are useful in modeling outcome variables with distributions that have excess zeroes. The two-part hurdle model generated mean inpatient and postacute care facility days/year by separately estimating the odds of being hospitalized (yes/no) using a logit function, and then among those who were hospitalized, estimating counts of inpatient and postacute care facility days using GLM regression with log link and Gamma variance function.
Initial models were adjusted for age and site. Multivariable models were further adjusted for traditional prognostic indicators associated with hospitalization: BMI category, marital status, multimorbidity, depressive symptoms, cognitive function, and hospitalization in the prior year. The series of models included potential confounders, but also included variables which may be part of the causal pathway such as multimorbidity and depressive symptoms. We included these variables in the adjusted model in order to determine to what extent current PA is independently associated with future health care utilization after accounting the fact that chronic conditions might be a result of lifetime history of PA. A third series of models, including all previous covariates and the PASE score, was run to test whether objectively measured physical activity was associated with hospitalization and facility care days independent of self-reported physical activity. All continuous covariates were assessed for linearity in the models, and if variables were linear then included as standardized variables with estimates calculated per SD change.
Results
Among the 1,283 men in the analytic cohort, mean (SD) age was 79.1 years (SD 5.3). The mean daily step count was 5,652 (SD 3092) with median 5,370 and interquartile range 3,442–7,562 steps/d. Table 1 shows the baseline characteristics of the cohort overall and stratified by step counts. Men with higher daily step count were younger and thinner; less likely to have a history of past hospitalization; had a lower burden of multimorbidity and depressive symptoms; and better cognitive function (all p < .001). There were 208 men who were hospitalized in the year following the clinic visit.
Table 1.
Baseline Characteristics of the Study Cohort (N = 1,283) Stratified by Step Count Quartilea
| Characteristics Mean (SD) or N (%) | Study Cohort (N = 1,283) | Q1 (N = 321) | Q2 (N = 321) | Q3 (N = 321) | Q4 (N = 320) |
|---|---|---|---|---|---|
| Age (years) | 79.1 (5.3) | 82.1 (5.6) | 79.4 (5.1) | 78.1 (4.5) | 76.7 (4.0) |
| BMI (kg/m2) | 27.1 (3.7) | 28.0 (4.5) | 27.3 (3.4) | 27.1 (3.5) | 26.2 (3.0) |
| Married (N) | 1014 (79.0) | 229 (71.3) | 254 (79.1) | 269 (83.8) | 262 (81.9) |
| Hospitalization 1-y prior clinic visit, N | 215 (16.8) | 76 (23.7) | 58 (18.1) | 50 (15.6) | 31 (9.7) |
| GDS score (0–15) | 1.7 (1.9) | 2.7 (2.4) | 1.5 (1.5) | 1.5 (1.5) | 1.1 (1.3) |
| Elixhauserb comorbidity score (0–31) | 2.5 (2.2) | 3.4 (2.5) | 2.5 (2.1) | 2.3 (2.0) | 1.8 (1.6) |
| PASE score (0–400) | 131.6 (65.9) | 89.8 (59.8) | 131.4 (57.8) | 146.4 (58.7) | 158.8 (66.0) |
| Total energy expenditure (kcal/d) | 2351 (442) | 2038 (310) | 2224 (345) | 2445 (373) | 2696 (433) |
| Active energy expenditurec (kcal/d) | 655 (333) | 370 (162) | 530 (205) | 734 (235) | 988 (328) |
| Time sedentary (min/d) | 847 (110) | 909 (109) | 874 (94) | 828 (90) | 777 (99) |
| Time light/moderate/vigorous activity (min/d) | 201 (92) | 124 (55) | 168 (61) | 220 (64) | 291 (87) |
| Time moderate/vigorous activity (min/d) | 82 (60) | 32 (25) | 59 (37) | 93 (42) | 143 (61) |
| 3MS score (0–100) | 93.1 (5.4) | 90.9 (7.0) | 93.1 (5.1) | 93.8 (4.6) | 94.6 (3.7) |
| Hospitalization 1-y postclinic visit (N) | 208 (16.2) | 83 (25.9) | 50 (15.6) | 48 (15.0) | 27 (8.4) |
| Length of acute/postacute care (days) | 1.9 (9.8) | 4.0 (14.0) | 1.4 (8.7) | 1.6 (9.9) | 0.5 (2.7) |
Note: BMI = Body mass index; GDS = Geriatric Depression Scale; IADL = Independent Activities of Daily Living; 3MS = Modified Mini-Mental State Examination; PASE = Physical Activity Scale for the Elderly; Q1-Q4 = Quartiles 1–4.
aQuartile 1: 18–3,441 steps, Quartile 2: 3,442–5,370 steps, Quartile 3: 5,371–7,562 steps, Quartile 4: 7,571–18,827 steps. bElixhauser comorbidity quantified using ICD-9 diagnoses in Medicare claims data. cActive energy = 0.9 × total energy-RMR, RMR = Resting metabolic rate.
The associations between objective physical activity measures (all given per standard deviation change) and health care utilization adjusted for age and clinical site are shown in Table 2. Higher total step count, total energy expenditure, active energy expenditure, and time spent in moderate/vigorous physical activity were all associated with a lower risk of hospitalization. In particular, each 1 SD = 3,092 steps increase in daily step count was associated with a 34% (95% CI: 19%–46%) lower odds of hospitalization. Lower daily sedentary time (excluding sleep) and higher self-reported physical activity were not significantly associated with lower odds of hospitalization. Among those hospitalized, a higher step count was associated with a lower rate of total facility care days, with a rate ratio 0.82 (95% CI: 0.68, 1.00) per 1 SD increase. The point estimates for the associations between other measures of objective activity (total energy expenditure, active energy expenditure, sedentary time, time spent in light, moderate and vigorous activity, time spent in moderate and vigorous activity), and rate of total facility care days were all below 1, but all associations had a 95% CI that included a null association.
Table 2.
The Associations Between Objectively Measured Physical Activity and Subsequent Health Care Utilization
| Odds Ratio For Hospitalization (95% CI) | Facility Days Rate Ratio Among Those Hospitalized (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
| Measure | Unit | Base Modela | Model 1b | Model 2c | Base Modela | Model 1b | Model 2c |
| Step count | SD = 3,092 increase | 0.66 (0.54, 0.81) | 0.79 (0.65, 0.96) | 0.76 (0.62, 0.94) | 0.82 (0.68, 1.00) | 0.93 (0.75, 1.14) | 0.96 (0.76, 1.21) |
| Total Energy Expenditure | SD = 442 kcal increase | 0.76 (0.63, 0.91) | 0.84 (0.70, 1.01) | 0.82 (0.68, 1.00) | 0.84 (0.66, 1.07) | 0.94 (0.75, 1.19) | 0.97 (0.76, 1.25) |
| Active Energyd Expenditure | SD = 333 kcal increase | 0.79 (0.66, 0.95) | 0.92 (0.77, 1.09) | 0.90 (0.76, 1.08) | 0.90 (0.72, 1.11) | 0.92 (0.75, 1.13) | 0.95 (0.75, 1.20) |
| Time Sedentary (nonsleep with METs ≤ 1.5) | SD = 110 min decrease | 0.89 (0.75, 1.05) | 0.94 (0.80, 1.12) | 0.94 (0.79, 1.11) | 0.94 (0.78, 1.12) | 0.89 (0.75, 1.05) | 0.90 (0.76, 1.07) |
| Time Light/Moderate/Vigorous Activity (METS > 1.5) | SD = 92 min increase | 0.79 (0.65, 0.96) | 0.93 (0.77, 1.12) | 0.92 (0.75, 1.12) | 0.87 (0.72, 1.06) | 0.91 (0.76, 1.10) | 0.93 (0.76, 1.15) |
| Time Moderate or Vigorous Physical Activity (METs ≥ 3) | SD = 60 min increase | 0.81 (0.67, 0.98) | 0.95 (0.80, 1.13) | 0.94 (0.78, 1.13) | 0.86 (0.70, 1.07) | 0.88 (0.72, 1.07) | 0.89 (0.71, 1.12) |
| PASE score (self-reported) | SD = 66 point increase | 0.86 (0.72, 1.02) | 1.01 (0.85, 1.21) | NA | 0.86 (0.71, 1.04) | 0.92 (0.77, 1.11) | NA |
Note: CI = Confidence interval; METs = Metabolic Equivalent of Tasks; PASE = Physical Activity Scale for the Elderly.
aBase model adjusted for age and clinical site. bModel 1 adjusted for base model variables and body mass index, marital status, geriatric depression score, Elixhauser comorbidity score, modified Mini-Mental State examination, prior hospitalization. cModel 2 adjusted for model 1 variables and PASE. dActive energy = 0.9 × total energy-RMR, RMR = Resting metabolic rate.
Table 2 also shows the association between objectively measured physical activity and health care utilization after adjusting for age, center, body mass index, marital status, depressive symptoms, multimorbidity, cognitive function and, and prior hospitalization. The associations noted in the age and site adjusted model were all attenuated in the full model. Only step counts remained independently associated with hospitalization; each 1 SD increase in daily step count was associated with a 21% (95% CI: 4%–35%) lower odds of hospitalization. In the step count model, age, previous hospitalization, depressive symptoms, and multimorbidity were also associated with risk of hospitalization, while age, body mass index, and cognitive function were associated with total length of stay in care facility (Supplementary Table 1); thus the attenuation of effect is attributable to the inclusion of these variables in the model. Further adjustment for self-reported physical activity level (PASE score) did not alter the association between step counts (or other objective measures) and hospitalization.
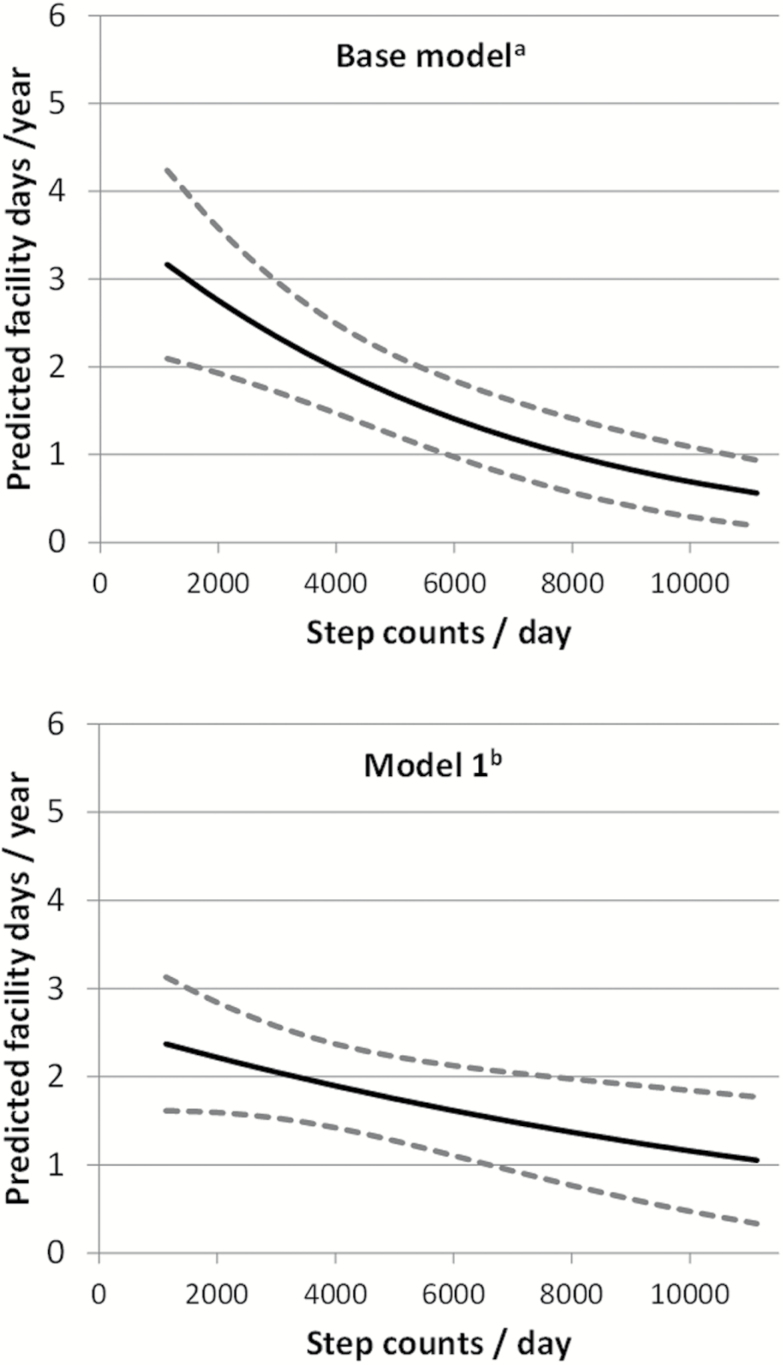
Figure 2 shows the predicted facility days (acute/postacute) by step count based on age and site adjusted and fully adjusted models. After adjusting for age and site, the predicted total facility days for those with a 2,000 daily steps was 2.8 (95% CI: 1.9, 3.9) days/year, four times the predicted outcome for those with 10,000 daily steps counts, 0.7 (95% CI: 0.3, 1.1) day/y. After further accounting for traditional predictors (body mass index, multimorbidity, previous hospitalization, cognitive function, and depressive symptoms) the predicted total facility days for those with a 2,000 daily steps was 2.2 (95% CI: 1.6, 2.8) days/y, not quite double the predicted outcome for those with 10,000 daily steps counts, 1.2 (95% CI: 0.5, 1.8) days/y. The estimated marginal effects of step counts on predicted facility days was statistically significant in the base model but not in the full model.
Figure 2.
The association between daily step count and predicted facility days (acute/post-acute) in base and fully adjusted models. †Base model adjusted for age and clinical site. * Model 1 adjusted for base model variables and body mass index, marital status, geriatric depression score, Elixhauser comorbidity score, modified Mini-Mental State examination, prior hospitalization. Figure (but not model) excludes predictions for 5% tails (N = 128): <1,132 steps or >11,130 steps.
Discussion
We found that several objective measures of physical activity were associated with the risk of hospitalization among our cohort of community-dwelling older men. Higher daily step count was independently associated with a lower risk of hospitalization even after accounting for age, body mass index, multimorbidity, previous hospitalization, cognitive function, and depressive symptoms. Higher step count was also associated with shorter total duration of inpatient/postacute care stay among those hospitalized in unadjusted, but not fully adjusted models, thus suggesting a larger role for risk factors other than physical activity in the assessment for return to the community. Normative data suggest that step counts in apparently healthy older adults average 2,000–9,000 steps per day, with the step count range for the general population being slightly lower (24). The odds of hospitalization and hence expect number of facility days varied substantially within the normative range. Absolute risk reduction was higher for those men with lower step counts, as seen in Figure 2, suggesting even modest increases in activity at lower step count could be beneficial for reducing risk.
The noted associations were still present after adjustment for self-reported physical activity, suggesting the utility of objective assessment of physical activity, despite the additional burden of assessment. The monitor used in the present study is not likely to be generally accessible, but a validation study showed pedometers could yield similar step count data (13). Likewise, shorter assessment duration (2–3 days) with adjustment for week day could be used to enhance feasibility outside the research setting (25).
Low physical activity is strongly predictive of prevalent and incident functional limitations (3) for which we did not adjust in the current analysis, thus a short clinical assessment of functional limitations could prompt the assessment of physical activity and target intervention programs designed to increase both performance and activity. Exercise training has been shown to improve physical performance (26) and reduce the progression of functional decline (27), even among those with mild to moderate frailty. A meta-analysis of interventions among the frail elderly showed consistent positive effects of exercise training on physical performance (28). Maintenance of mobility with functional limitations is also possible through task modifications (29).
The associations between objective measures of physical activity and hospitalization were attenuated with the inclusion of body mass index, depressive symptoms, multimorbidity, cognitive function, and prior hospitalization. Thus, the strong association between step counts and health care utilization was partially attributable to these coexisting risk factors. We have previously shown that multimorbidity is a risk factor subsequent health care utilization (30). Other research has suggested that low physical activity is both a risk factor for chronic disease (31) as well as a consequence of existing health conditions (32,33). Furthermore, intervention studies have demonstrated that increasing physical activity improves risk factor profiles. A review of both randomized trials and cohort studies involving pedometers found that higher step counts were associated with lower blood pressure and lower BMI (34). Low physical activity is also a risk factor for depressive symptoms, but again increased physical activity may play a role in reducing this risk (35). It has also been shown that existing chronic conditions such as diabetes and cardiovascular disease were associated with a lower quality of life, with physical activity playing a role as intermediate factor (36). As we hypothesized, adjusting for prevalent medical conditions attenuated the relationship between physical activity and hospitalization, but step count was still significant independent of the bidirectional relationship between physical activity and medical conditions.
There was no statistically significant association of either objectively measured sedentary time or self-reported physical activity with hospitalization, but these were not strong null findings in that the point estimates were all consistent with those for other measures of physical activity, yet the 95% CI included the null hypothesis of no association. Certainly sedentary time was previously found to be associated with other health outcomes such as incident functional limitations (3), fracture (2), and mortality (4) in our cohort. However, these previous findings were based on a larger study sample with greater power to detect associations. Our finding of the lack on an association of self-reported physical activity with hospitalization must also be viewed in the context of long-standing research showing a relationship between self-reported activity and health outcomes (20,21). Finally, although we did assess the association between physical activity and total length of facility stay, physical activity was not an important direct determinant of length of stay. This null finding should be viewed in the context of adjustment for other variables (such as comorbidity) that are partial mediators due to causal relationship.
This study has several strengths including objectively assessed physical activity linked to subsequent hospitalization ascertained using complete inpatient claims data as well as consideration of major confounding and mediating factors. However, this study has limitations. The cohort was comprised of healthy community-dwelling men and results may not be generalizable to women, those who are not community-dwelling or those with severely limited mobility. Data on number of hospitalizations and inpatient days was limited to MrOS study participants who were enrolled in Medicare FFS, and might not be generalizable to those who were Medicare enrollees but were not FFS, since health care utilization may differ by enrollment status. Finally, future studies are warranted to examine additional outcomes such as total health care costs.
In summary, daily step count is an easily determined objective measure of physical activity that may be useful in assessment of future health care burden in older men. Future research should evaluate the utility of step count measures in the clinical practice setting as a prognostic indicator and strategy to identify a target population in need of intervention.
Funding
The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute on Aging (NIA); the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS); the National Center for Advancing Translational Sciences (NCATS); and NIH Roadmap for Medical Research (grant numbers U01 AG027810, U01 AG042124, U01 AG042139, U01 AG042140, U01 AG042143, U01 AG042145, U01 AG042168, U01 AR066160, and UL1 TR000128). This manuscript is the result of work supported with resources and use of facilities of the Minneapolis VA Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. Government.
Conflicts of Interest
None reported.
Supplementary Material
References
- 1. Cerin E, Cain KL, Oyeyemi AL, et al. . Correlates of agreement between accelerometry and self-reported physical activity. Med Sci Sports Exerc. 2016;48:1075–1084. doi:10.1249/MSS.0000000000000870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cauley JA, Harrison SL, Cawthon PM, et al. . Objective measures of physical activity, fractures and falls: the osteoporotic fractures in men study. J Am Geriatr Soc. 2013;61:1080–1088. doi:10.1111/jgs.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cawthon PM, Blackwell TL, Cauley JA, et al. . Objective assessment of activity, energy expenditure, and functional limitations in older men: the Osteoporotic Fractures in Men study. J Gerontol A Biol Sci Med Sci. 2013;68:1518–1524. doi:10.1093/gerona/glt054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ensrud KE, Blackwell TL, Cauley JA, et al. ; Osteoporotic Fractures in Men Study Group Objective measures of activity level and mortality in older men. J Am Geriatr Soc. 2014;62:2079–2087. doi:10.1111/jgs.13101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huisingh-Scheetz MJ, Kocherginsky M, Magett E, Rush P, Dale W, Waite L. Relating wrist accelerometry measures to disability in older adults. Arch Gerontol Geriatr. 2016;62:68–74. doi:10.1016/j.archger.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnes DE, Blackwell T, Stone KL, Goldman SE, Hillier T, Yaffe K; Study of Osteoporotic Fractures Cognition in older women: the importance of daytime movement. J Am Geriatr Soc. 2008;56:1658–1664. doi:10.1111/j.1532-5415.2008.01841.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Simmonds B, Fox K, Davis M, et al. . Objectively assessed physical activity and subsequent health service use of UK adults aged 70 and over: a four to five year follow up study. PLoS One. 2014;9:e97676. doi:10.1371/journal.pone.0097676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chawla H, Bulathsinghala C, Tejada JP, Wakefield D, ZuWallack R. Physical activity as a predictor of thirty-day hospital readmission after a discharge for a clinical exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:1203–1209. doi:10.1513/AnnalsATS.201405-198OC [DOI] [PubMed] [Google Scholar]
- 9. Fisher SR, Kuo YF, Sharma G, et al. . Mobility after hospital discharge as a marker for 30-day readmission. J Gerontol A Biol Sci Med Sci. 2013;68:805–810. doi:10.1093/gerona/gls252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blank JB, Cawthon PM, Carrion-Petersen ML, et al. . Overview of recruitment for the osteoporotic fractures in men study (MrOS). Contemp Clin Trials. 2005;26:557–568. doi:10.1016/j.cct.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 11. Orwoll E, Blank JB, Barrett-Connor E, et al. . Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study–a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26:569–585. doi:10.1016/j.cct.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 12. Mackey DC, Manini TM, Schoeller DA, et al. ; Health, Aging, and Body Composition Study Validation of an armband to measure daily energy expenditure in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1108–1113. doi:10.1093/gerona/glr101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colbert LH, Matthews CE, Havighurst TC, Kim K, Schoeller DA. Comparative validity of physical activity measures in older adults. Med Sci Sports Exerc. 2011;43:867–876. doi:10.1249/MSS.0b013e3181fc7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Remoortel H, Raste Y, Louvaris Z, et al. ; PROactive consortium Validity of six activity monitors in chronic obstructive pulmonary disease: a comparison with indirect calorimetry. PLoS One. 2012;7:e39198. doi:10.1371/journal.pone.0039198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharif MM, Bahammam AS. Sleep estimation using BodyMedia’s SenseWear™ armband in patients with obstructive sleep apnea. Ann Thorac Med. 2013;8:53–57. doi:10.4103/1817-1737.105720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei YJ, Simoni-Wastila L, Zuckerman IH, Brandt N, Lucas JA. Algorithm for identifying nursing home days using medicare claims and minimum data set assessment data. Med Care. 2016;54:e73–e77. doi:10.1097/MLR.0000000000000109 [DOI] [PubMed] [Google Scholar]
- 17. Chandra A, Dalton MA, Holmes J. Large increases in spending on postacute care in Medicare point to the potential for cost savings in these settings. Health Aff (Millwood). 2013;32:864–872. doi:10.1377/hlthaff.2012.1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burke RE, Juarez-Colunga E, Levy C, Prochazka AV, Coleman EA, Ginde AA. Rise of post-acute care facilities as a discharge destination of US hospitalizations. JAMA Intern Med. 2015;175:295–296. doi:10.1001/jamainternmed.2014.6383 [DOI] [PubMed] [Google Scholar]
- 19. Sheikh JI, Yesavage JA. Geriatric depression scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 20. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi:10.1016/0895-4356(93)90053–4 [DOI] [PubMed] [Google Scholar]
- 21. McDowell I, Kristjansson B, Hill GB, Hébert R. Community screening for dementia: the Mini Mental State Exam (MMSE) and Modified Mini-Mental State Exam (3MS) compared. J Clin Epidemiol. 1997;50:377–383. doi:10.1016/S0895-4356(97)00060-7 [DOI] [PubMed] [Google Scholar]
- 22. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 23. Loeys T, Moerkerke B, De Smet O, Buysse A. The analysis of zero-inflated count data: beyond zero-inflated Poisson regression. Br J Math Stat Psychol. 2012;65:163–180. doi:10.1111/j.2044-8317.2011.02031.x [DOI] [PubMed] [Google Scholar]
- 24. Tudor-Locke C, Craig CL, Aoyagi Y, et al. . How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80. doi:10.1186/1479-5868-8-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kocherginsky M, Huisingh-Scheetz M, Dale W, Lauderdale DS, Waite L. Measuring physical activity with hip accelerometry among U.S. older adults: how many days are enough?PLoS One. 2017;12:e0170082. doi:10.1371/journal.pone.0170082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Binder EF, Schechtman KB, Ehsani AA, et al. . Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50:1921–1928. doi:10.1046/j.1532-5415.2002.50601.x [DOI] [PubMed] [Google Scholar]
- 27. Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–1074. doi:10.1056/NEJMoa020423 [DOI] [PubMed] [Google Scholar]
- 28. Bibas L, Levi M, Bendayan M, Mullie L, Forman DE, Afilalo J. Therapeutic interventions for frail elderly patients: part I. Published randomized trials. Prog Cardiovasc Dis. 2014;57:134–143. doi:10.1016/j.pcad.2014.07.004 [DOI] [PubMed] [Google Scholar]
- 29. Rantakokko M, Portegijs E, Viljanen A, Iwarsson S, Rantanen T. Task modifications in walking postpone decline in life-space mobility among community-dwelling older people: a 2-year follow-up study. J Gerontol A Biol Sci Med Sci. 2017;72:1252–1256. doi:10.1093/gerona/glw348 [DOI] [PubMed] [Google Scholar]
- 30. Ensrud KE, Lui LY, Langsetmo L, et al. . Effects of mobility and multimorbidity on inpatient and post-acute health care utilization. J Gerontol A Biol Sci Med Sci. 2017. doi:10.1093/gerona/glx128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pedersen BK, Saltin B. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports. 2006;16(suppl 1):3–63. doi:10.1111/j.1600-0838.2006.00520.x [DOI] [PubMed] [Google Scholar]
- 32. Troosters T, van der Molen T, Polkey M, et al. . Improving physical activity in COPD: towards a new paradigm. Respir Res. 2013;14:115. doi:10.1186/1465-9921-14-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kachur S, Chongthammakun V, Lavie CJ, et al. . Impact of cardiac rehabilitation and exercise training programs in coronary heart disease. Prog Cardiovasc Dis. 2017;60:103–114. doi:10.1016/j.pcad.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 34. Bravata DM, Smith-Spangler C, Sundaram V, et al. . Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–2304. doi:10.1001/jama.298.19.2296 [DOI] [PubMed] [Google Scholar]
- 35. Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. 2013;45:649–657. doi:10.1016/j.amepre.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 36. Sawatzky R, Liu-Ambrose T, Miller WC, Marra CA. Physical activity as a mediator of the impact of chronic conditions on quality of life in older adults. Health Qual Life Outcomes. 2007;5:68. doi:10.1186/1477-7525-5-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.