Abstract
Background
Increasing protein content of the diet might be an effective strategy to preserve muscle mass in older adults undergoing caloric restriction, thereby preserving muscle function.
Methods
Ninety-six older adults (70.3 ± 3.7 years, 74% women, 27% African American) with obesity (35.4 ± 3.3 kg/m2; 47% total body fat) were randomized to a 6-month higher protein (providing 1.2–1.5 g/kg/d) weight loss (WL) program, utilizing the Medifast 4&2&1 Plan, or to weight stability (WS). Dual-energy x-ray absorptiometry-acquired total body mass and composition, and fast gait speed over 400 m was assessed at baseline, 3, and 6 months.
Results
At baseline, dual-energy x-ray absorptiometry-acquired total body, fat, and lean masses were 95.9 ± 14.6, 44.6 ± 7.6, and 48.7 ± 9.5 kg, respectively, and 400-m gait speed was 1.17 ± 0.20 m/s. Total body mass was significantly reduced in the WL group (−8.17 [−9.56, −6.77] kg) compared with the WS group (−1.16 [−2.59, 0.27] kg), with 87% of total mass lost as fat (WL: −7.1 [−8.1, −6.1] kg; −15.9% change from baseline). A differential treatment effect was not observed for change in lean mass (WL: −0.81 [−1.40, −0.23] kg vs WS: −0.24 [−0.85, 0.36] kg). Four-hundred-meter gait speed was also unchanged from baseline although trends suggest slightly increased gait speed in the WL group [0.01 (−0.02, 0.04) m/s] compared with the WS group [−0.02 (−0.05, 0.01) m/s].
Conclusion
Intentional weight loss using a high-protein diet is effective in producing significant total body mass and fat mass loss, while helping preserve lean body mass and mobility, in relatively high-functioning older adults with obesity.
Keywords: Weight loss, Physical function, Lean mass, Fat mass
Obesity and aging are strong, independent, and increasingly prevalent risk factors for physical dysfunction (1–3). Medical complications associated with excess fat mass highlight the need to treat obesity among older adults (3,4); yet, recommendation for intentional weight loss in this group remains controversial due to concerns regarding lean mass loss and potential exacerbation of age-related disability risk (5,6). Accordingly, current geriatric obesity treatment guidelines call for weight loss therapy that minimizes muscle loss for older persons with obesity who have functional impairment (7).
A growing body of evidence demonstrates that carefully planned and supervised weight reduction in older adults with obesity yields clinically meaningful improvement in physical function (8–12); however, in few cases, has the effect of caloric restriction, independent of exercise, been evaluated (13–16). Evidence supporting a diet-based weight loss approach to improve physical function in older adults with obesity has practical significance as (a) many commercially available weight loss programs rely exclusively on modifying diet, (b) multiple behavior changes are often difficult to implement and maintain (17), and (c) sobering survey data suggest that exercise participation among older adults is strikingly low, with only 15% of adults aged 65–74 meeting national physical activity guidelines (18,19).
The amount of dietary protein consumed during caloric restriction may be a key determinant in preserving muscle mass, and thereby function, as adequate dietary protein is essential for skeletal muscle anabolism. In a position statement issued by the PRO-TAGE study group, weight-stable older adults are advised to consume 1.0–1.2 g/kg/d of high-quality protein to aid in the maintenance of muscle mass and function (20), and this recommendation is likely elevated during active weight loss. To date, five randomized controlled trials (RCTs) have examined the effect of dietary protein supplementation during geriatric obesity reduction on muscle mass (only (21)) and function (16,22–24), with mixed findings reported. In addition to general equipoise, current studies are limited by size (average n = 50) and duration (3–6 months), with only two studies examining the effect of a high-protein diet independent of exercise (16,22), and no study comparing the effects of a high-protein weight loss program on muscle mass and function to weight stability (WS). The latter contrast has important clinical significance as it provides a true “aging” control, and thus, patient/provider insight to the benefits and risks of maintaining the status quo.
Therefore, the primary goal of this study was to conduct an adequately powered RCT to determine whether adherence to a 6-month hypocaloric, nutritionally complete, higher protein (targeting ≥1.0 g/kg/d) meal plan results in improved mobility by favorably affecting body composition as compared with WS in older adults with obesity. We hypothesized that participants randomized to higher protein weight loss would experience improved gait speed (i.e. +0.05 m/s, considered clinically meaningful (25)) and increased lean-to-fat mass ratio as compared with control participants.
Methods
The Medifast for Seniors Study (NCT02730988) was a 6-month RCT conducted at Wake Forest University. Specific aims included comparing the effects of high-protein intake during weight loss versus WS on changes in mobility (primary aim) and body composition (secondary aim).
Study Participants
Older men and women with obesity were recruited via mass mailings and media advertisements in and around Forsyth County, NC. Participants were enrolled based on the following criteria: (a) aged 65–79 years, (b) body mass index of 30–40 kg/m2, (c) self-reported mobility disability (difficulty walking ¼ mile or climbing stairs/performing house/yard work), (d) sedentary lifestyle (self-report of less than six 10-minute bouts of moderate pace walking per week), (e) nonsmoking (<1 cigarette/d or 4/wk within year), (f) weight stable (<5% weight change in the past 6 months), (g) not dependent on a cane or walker, (h) without comorbidities for which the intervention was contraindicated, and (i) willing to follow the dietary protocol. The study was approved by the Wake Forest School of Medicine Institutional Review Board, and all participants provided written informed consent prior to enrollment.
Interventions
Dietary weight loss intervention
Intentional weight loss in the WL group was achieved through use of the Medifast 4&2&1 Plan, as previously described (26). Briefly, this meal plan includes four meal replacement products (each provides 90–110 kcal and 11–15 g protein), two self-prepared lean and green meals (each provides 5–7 oz. lean protein, three nonstarchy vegetable servings, and up to two healthy fat servings), and one healthy snack (i.e. one serving of fruit, dairy, or grain). Overall, this meal plan is estimated to provide 1100–1300 kcal, 120–150 g protein, 85–100 g carbohydrate, 30–45 g fat, and (of relevance to musculoskeletal health), 1000–1600 mg calcium and 300–600 IU Vitamin D per day. Based on the average baseline weight of study participants, we anticipated that adherence to the Medifast 4&2&1 Plan would yield 7%–10% weight loss in WL participants over the 6-month intervention period.
In addition to individualized counseling on use of the meal plan, WL participants also attended 12 bi-weekly group behavioral counseling sessions, led by the study Registered Dietitian (RD), to provide support and discuss topics pertinent to weight control (including self-monitoring, portion control, mindful eating, and overcoming weight loss barriers). All participants were encouraged to maintain their baseline level of physical activity and also completed daily food logs for all food and meal replacement intake, which were reviewed bi-weekly to verify compliance to the diet.
Weight-stable intervention
Participants randomized to WS group attended 12 bi-weekly group behavioral educational sessions at which they were monitored to ensure WS (within ±5% of baseline) over the course of the study. Sessions were led by study staff and included topics such as What is Successful Aging?, Preventing and Delaying Disease and Dysfunction, Managing Medications Effectively, and Talking Effectively with Your Healthcare Provider. If WS participants were able to attend 75% (i.e. 9 of 12) educational sessions, all baseline and follow-up testing sessions, and maintain WS over the course of the study (defined as less than a 5% differential between weight measured at the first and last intervention sessions), they were eligible to receive up to 3 months of Medifast meal replacements along with a 60-minute RD-led dietary instructional session on how to follow the Medifast 4&2&1 Plan at the end of the study. As with the WL group, WS participants were encouraged to maintain their baseline level of physical activity during the intervention.
Measures
Intervention process measures
Bi-weekly weights were collected using a Health o meter Professional 349KLX Digital Floor Medical Scale (Pelstar LLC, McCook, IL) with weights taken at the first (immediately before randomization assignment) and last intervention visits used to determine total amount of weight lost. Bi-weekly group session attendance and self-reported daily meal replacement consumption were also used to monitor intervention compliance. Lastly, participant urine was collected over a 24-hour period at baseline and 6-month follow-up to obtain a 24-hour urinary nitrogen excretion level, which was used to estimate dietary protein intake based on previously described methods (27). Prior research suggests near complete agreement between protein intake (as assessed via a 28-day diet in a metabolic suite) and urine estimation (28).
Relevant covariates
Baseline demographic information, including age, gender, and ethnicity, was recorded based on participant self-report. Baseline weight was measured to the 10th decimal without shoes and outer garments using a calibrated scale (Detecto 758C Weight Indicator; Webb City, MO). Height was obtained without shoes to within 0.25 inches using a QuickMedical 235D Heightronic Digital Stadiometer (Issaquah, WA). Baseline height and weight were measured at the first screening visit and used to calculate baseline body mass index. Lastly, self-reported physical activity data were collected using the validated CHAMPS questionnaire (29) at the baseline and 6-month assessment visit.
Primary outcome measure: gait speed
Gait speed was assessed using the fast 400-m walk test. This test involves participants completing 10 laps on a 40-m course as quickly as they could without running and was measured at baseline, 3, and 6 months. Performance on the 400-m walk test has been shown to be an important prognostic indicator for total mortality, cardiovascular disease, and mobility disability in older adults (30).
Secondary outcome measure: body composition
Dual-energy x-ray absorptiometry scans were used to determine total body, lean, and fat masses (iDXA, GE Medical Systems, Madison, WI) at baseline, 3, and 6 months. All scans were performed on the same machine and by the same technician, following manufacturer recommendations for patient preparation and positioning. Coefficients of variation from repeated measurements at our institution are <1.0% for total body, lean, and fat masses.
Statistical Power and Analyses
This study was powered to detect a clinically meaningful 400-m walk gait speed difference of at least 0.05 m/s (25), with a common group standard deviation of 0.19 m/s and an intraindividual correlation of 0.91 between baseline and 6-month measures. Using the method of Borm and colleagues (31) and an analysis of covariance analytic approach fitting the randomization effect and baseline gait speed, 80 subjects completing the intervention yielded 80% power using a two-sided alternative hypothesis at the 0.05 level of significance.
Descriptive statistics were calculated overall and by treatment group at baseline. Body weight estimates were produced using a mixed model approach using treatment assignment, visit, and treatment by visit interaction, assuming an autoregressive covariance structure, and comparisons both within and between groups were performed using contrast statements. Attendance rate was estimated as the total number of sessions attended divided by the number expected among participants who completed the full study. Similarly, meal replacement usage was estimated as the number of meal replacements consumed divided by the number expected among study completers. Compliance to the intervention quantified by protein intake was assessed by comparing urinary nitrogen across treatment groups at 6 months, adjusted for baseline urinary nitrogen levels and gender.
Primary (fast 400-m walk gait speed) and secondary (total body lean and fat mass) outcome measures analyses were conducted assuming intent-to-treat and analyzed using a mixed model fit with the main effect of treatment group, visit, wave (1–5), and the treatment–time interaction, adjusted for baseline values of the outcome and gender. Subgroup analyses on the primary outcome were performed by stratifying the study sample by (a) baseline gait speed (two versions: <1.0 m/s and <1.17 m/s) and (b) degree of weight loss achieved. All statistical analyses were conducted using SAS v9.4 (SAS Institute, Cary, NC) based on a 0.05 level of significance throughout.
Results
Recruitment and Retention
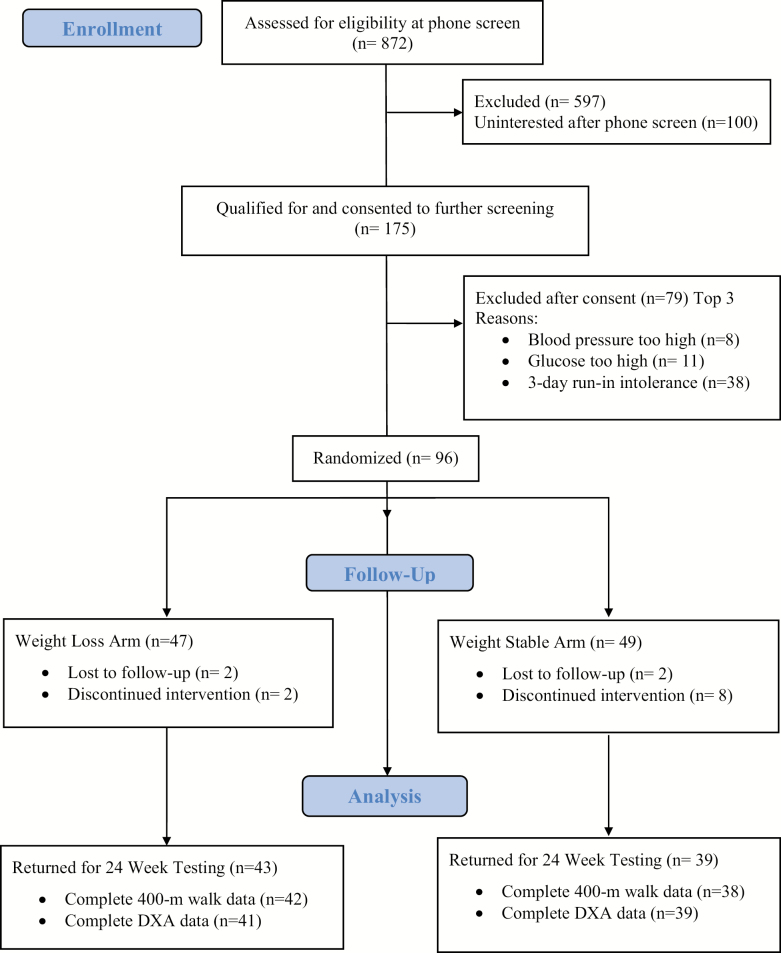
Study recruitment took place over 12 months (September 18, 2015 to September 14, 2016). Briefly, 872 men and women were screened via phone calls from which 697 were excluded for not meeting inclusion criteria (n = 498) and other reasons/lack of interest (n = 199). Of those who agreed to further screening (n = 175), 96 participants qualified and were randomized to the WL group (n = 47) or WS group (n = 49) in five waves (n= 12–22 participants/wave). A total of 14 individuals were lost to follow-up during the intervention period (WL: n = 4, WS: n = 10), providing a final count of 82 participants who completed the study and returned for 6-month follow-up testing (85.4% retention); see Figure 1.
Figure 1.
The Medifast for Seniors Study CONSORT Flow Diagram.
Baseline Participant Characteristics
Baseline characteristics of randomized participants are summarized overall and by group in Table 1. Almost three-quarters of the study sample were female (74%), one-fourth were African American (27%), and average age was approximately 70 years (WL: 71.4 ± 3.9 years, WS: 69.2 ± 3.1 years). Participants had obesity (WL: 35.2 ± 3.5 kg/m2, WS: 35.6 ± 3.1 kg/m2), and average walking speed was greater than 1.0 m/s (WL: 1.15 ± 0.19 m/s, WS: 1.19 ± 0.22 m/s). Demographic characteristics did not differ materially between groups at baseline or between those who completed the intervention versus those who were lost to follow-up (all p > .05).
Table 1.
Baseline Descriptive Characteristics of Randomized Sample According to Treatment Group
| Baseline Characteristic | Overall (n = 96) | Weight Stable (n = 49) | Weight Loss (n = 47) |
|---|---|---|---|
| Age (years) | 70.3 ± 3.7 | 69.2 ± 3.1 | 71.4 ± 3.9 |
| Female, n (%) | 71 (74.0) | 36 (73.5) | 35 (74.5) |
| Ethnicity, n (%) | |||
| African American | 26 (27.1) | 13 (26.5) | 13 (27.7) |
| Caucasian | 69 (71.9) | 36 (73.5) | 33 (70.2) |
| ≤High school education, n(%) | 21 (21.9) | 9 (18.4) | 12 (25.5) |
| Body weight (kg) | 97.1 ± 14.9 | 98.0 ± 12.9 | 96.1 ± 16.8 |
| BMI (kg/m2) | 35.4 ± 3.3 | 35.6 ± 3.1 | 35.2 ± 3.5 |
| 400-m walk gait speed (m/s) | 1.17 ± 0.20 | 1.19 ± 0.22 | 1.15 ± 0.19 |
| Self-reported physical activity (min/wk) | 7.0 ± 28.1 | 8.6 ± 35.6 | 5.4 ± 17.5 |
| Urinary nitrogen estimated protein intake (g/kg/d) | 0.77 ± 0.20 | 0.77 ± 0.22 | 0.77 ± 0.19 |
| DXA-acquired body composition | |||
| Total mass (kg) | 95.9 ± 14.6 | 97.0 ± 12.7 | 94.9 ± 16.5 |
| Total body lean mass (kg) | 48.7 ± 9.5 | 49.2 ± 8.5 | 48.2 ± 10.6 |
| Total body lean mass (%) | 50.7 ± 4.7 | 50.7 ± 4.9 | 50.7 ± 4.5 |
| Total body fat mass (kg) | 44.6 ± 7.6 | 45.2 ± 7.6 | 44.0 ± 7.7 |
| Total body fat (%) | 46.6 ± 4.9 | 46.7 ± 5.2 | 46.5 ± 4.6 |
| Lean:fat | 1.11 ± 0.23 | 1.11 ± 0.25 | 1.11 ± 0.21 |
Notes: BMI = body mass index; DXA = dual-energy x-ray absorptiometry.
Data presented as means ± SD or n(%).
Intervention Compliance and Adverse Event Reporting
Self-reported physical activity levels did not differ by group or time (p > .05). Among completers (n = 82), overall attendance to the bi-weekly educational sessions was 84% and 88% for WS and WL groups, respectively. Within the WL group, self-reported compliance to the meal replacement product protocol was 92.7% (91.1% reporting greater than 80% compliance), with an average of 3.7 ± 0.3 meal replacements used per day. Follow-up urinary nitrogen estimated protein intake was 83.8 (77.6, 90.0) g and 74.9 (63.4, 81.2) g in the WL and WS groups, respectively, representing an average consumption of 0.94 (0.87, 1.02) g protein/kg body weight/d and a 12% increase from baseline within the WL group (p = .04).
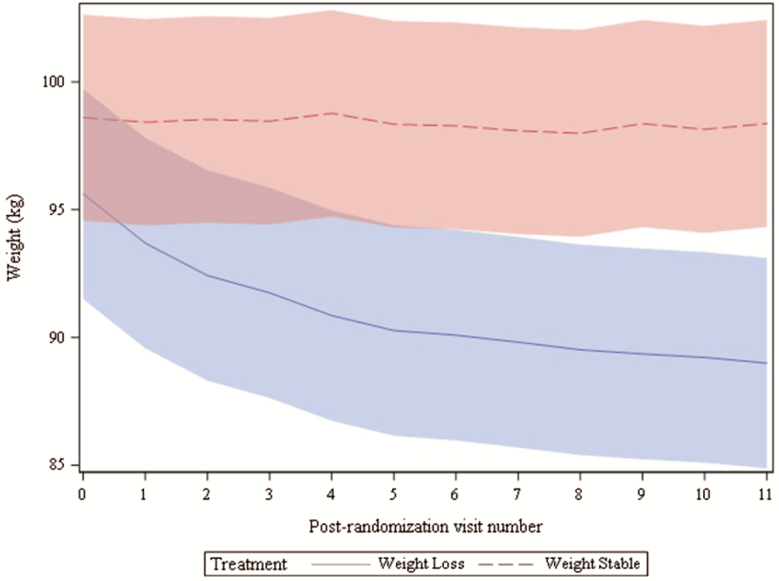
Based on the intervention scale weight, WL participants lost an average of 6.6 ± 0.4 kg (8.6% ± 0.4% baseline weight), and weight remained stable in the WS group ([−0.2 ± 0.5 kg]; group × time p < .01). Weight trajectories differed significantly from the intervention visit to onward (p < .05), as illustrated in Figure 2, with an average of 5% weight loss achieved in WL participants by the fourth intervention visit. Lastly, 26 adverse events (18 in the WL and 8 in the WS groups) were reported during the intervention period, 4 of which were classified as serious adverse events, but unrelated to the intervention.
Figure 2.
Bi-weekly intervention weights presented by treatment group. Shading depicts 95% CI.
Treatment Effects on Gait Speed
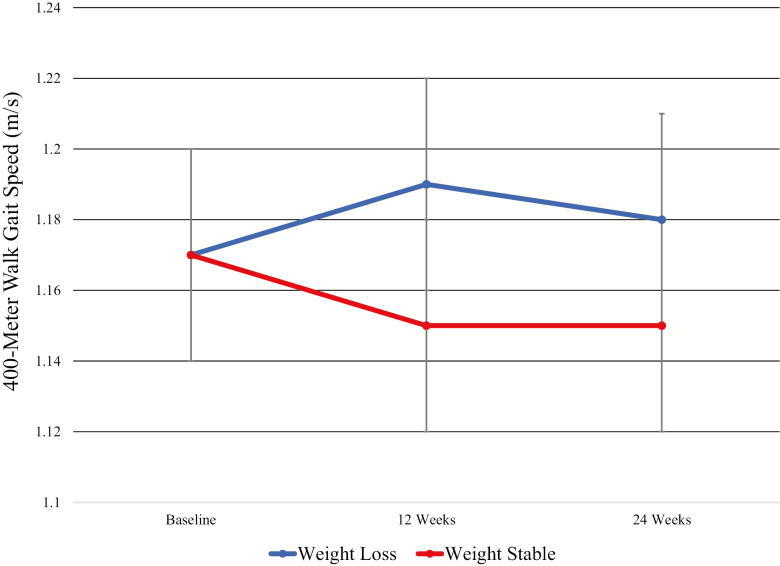
Graphical depiction of the treatment effect on the primary outcome, 400-m walk gait speed, is presented in Figure 3. At baseline, overall gait speed was 1.17 ± 0.20 m/s, which was unchanged in either group over the intervention (p = .17) although trends suggest slightly increased gait speed in the WL group (0.01 [−0.02, 0.04] m/s) compared with the WS group (−0.02 [−0.05, 0.01] m/s). No significant interaction between treatment assignment and baseline gait speed using low gait speed defined as either <1.0 m/s (p = .84) or using the median speed value of <1.17 m/s (p = .80) was observed. Likewise, no association between percent weight change and gait speed change was observed (p = .33).
Figure 3.
Treatment effect on 400-m gait speed. Data presented as means (95% CI) and generated using a mixed model fit with treatment, time, and treatment–time interaction.
Treatment Effects on Body Composition
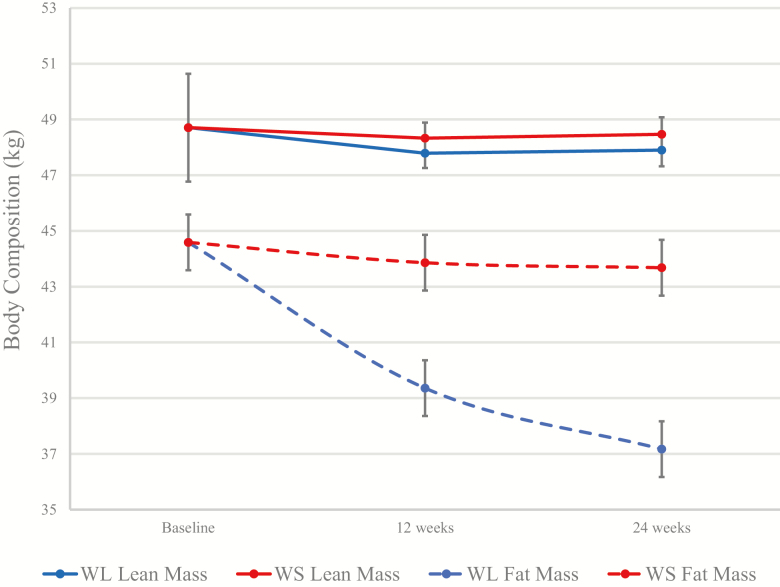
At baseline, dual-energy x-ray absorptiometry-acquired total body, fat, and lean masses were 95.9 ± 14.6, 44.6 ± 7.6, and 48.7 ± 9.5 kg, respectively. Total body mass was significantly reduced in the WL group (−8.17 [−9.56, −6.77] kg) compared with the WS group (−1.16 [−2.59, 0.27] kg), with 87% of total mass lost as fat (WL: −7.1 [−8.1, −6.1] kg; −15.9% change from baseline). Despite a small decline in lean mass in the WL group from baseline, a differential treatment effect was not observed for change in lean mass (WL: −0.81 [−1.40, −0.23] kg vs WS: −0.24 [−0.85, 0.36] kg); see Figure 4. Accordingly, the 6-month lean-to-fat mass ratio was significantly higher in the WL group compared with WS (1.32 [1.28, 1.36] vs 1.13 [1.09, 1.17]; p < .01).
Figure 4.
Treatment effect on dual-energy x-ray absorptiometry-acquired total body fat and lean masses. Data presented as means (95% CI) and generated using a mixed model fit with treatment, time, and treatment–time interaction.
Discussion
Findings from The Medifast for Seniors Study demonstrate that the Medifast 4&2&1 Plan is an effective way for older adults with obesity to lose a clinically significant amount of weight over a 6-month period. Within the WL group, fat mass represented the majority of lost weight (87%), while lean mass was largely preserved (loss of less than 2% of baseline lean mass) and did not differ at follow-up from the WS group. Lastly, weight loss achieved by adherence to the Medifast 4&2&1 Plan did not affect mobility in our study sample. Although we hypothesized gait speed would improve in the WL group (as compared with WS), it is equally important to note that we did not observe a decrement in gait speed, which is clinically valuable information. Thus, data from this study suggest that practitioners working with older adults with obesity and relatively high physical function (i.e. gait speed > 1.0 m/s) can recommend a hypocaloric, nutritionally complete, higher protein meal plan, and anticipate significant weight loss, accompanied by a favorable shift in total body composition and preservation of baseline functional status.
Results from this study contribute to a growing body of literature aimed at identifying the best approach to practically manage obesity among older adults. Increasingly, there is interest in exploring the ability of enhanced dietary protein intake during caloric restriction to preserve lean mass and function. Findings from the present study align with recent meta-analytic work demonstrating that middle-aged and older adults (i.e. 50+ years) consuming higher levels of protein during weight loss retain more lean mass in comparison with normal protein diets (losses of 21%–22% vs ≥30%) (32). Importantly, the lean mass sparing effect was found to be most effective when dietary protein intake exceeded 1.0 g/kg/d, which, given the high level of dietary compliance noted in our study, might explain our augmented results.
To date, four RCTs have been published which extend body composition findings to also include measures of physical function when examining the health effects of protein enhancement during intentional weight loss in older (i.e. 65+ years at baseline) adults (16,22–24). Half of these studies report a beneficial protein effect, either on lean mass retention (23) or functional status (16), although in only two cases was the effect of high-protein weight loss examined independent of exercise (16,22). Specifically, in the 2016 publication by Backx and colleagues (22), authors report a 9-kg reduction in body weight following 3 months of high-protein (1.7 g/kg/d) weight loss (25% energy intake restriction). The reduction in body mass was accompanied by a 2-kg decline in lean mass and a 0.05-m/s increase in 400-m gait speed; however, changes in weight, lean mass, and gait speed were not different from participants randomized to normal protein (0.9 g/kg/d) weight loss. This finding has since been replicated in middle-aged and older women, where a clinically important functional benefit of obesity reduction was confirmed, regardless of dietary protein amount (33).
In contrast, findings from the 6-month MEASUR-UP trial (16), where frail (baseline short physical performance battery score of 4–10), older adults with obesity were randomized to high protein (1.2 g/kg/d) or normal protein (0.8 g/kg/d) weight loss (both groups achieved ~7%–8% weight loss), a beneficial treatment effect was observed for physical function (as indicated by a 1.5-point improvement in total short physical performance score in high compared with normal protein groups), but not lean mass. Incongruence between prior study findings and the present report could certainly be due to differences in study design (normal protein weight loss vs weight stable control) and/or dietary prescription (including, but not limited to total protein intake) although another explanation is likely found in the functional status of the study sample. While The Medifast for Seniors Study entry criteria required participants to self-report mobility limitations prior to enrollment, baseline performance on the 400-m walk test revealed a relatively high-functioning sample, which likely contributed to a functional ceiling effect. Subgroup analyses did not reveal a significant treatment effect in those presenting with reduced gait speed at baseline; however, we were underpowered to fully explore this association.
Novel strengths of The Medifast for Seniors Study include utilization of a weight-stable control group, excellent protocol compliance, and inclusion of an adequately powered sample size to test our primary hypothesis. An additional strength of the study includes use of dual-energy x-ray absorptiometry to acquire total body fat and lean masses, as it is considered the gold standard for body composition assessment. However, our scanning protocol did not account for potential diurnal variation or bladder fullness when obtaining participant scans, which may have caused measurement error. That said, if present, we assume these errors would occur at random and thus bias our findings toward the null. Additionally, while the present study was of longer duration than most similarly designed trials, the timeframe is still relatively brief and we cannot extrapolate our results beyond 6 months. As previously stated, we were likely limited in our ability to detect a significant differential treatment effect in gait speed due to the relatively high-functioning status of our study sample at baseline. Lastly, although the Medifast 4&2&1 Plan provided a high level of protein at all meals and snacks, we did not specifically monitor timing of protein ingestion in this study, which may be a contributing factor to intervention success (20,34). Assessment of protein type, quality, and synergy with other nutrients may also be relevant future intervention targets, and we implore future RCT of intentional weight loss to carefully monitor dietary intake throughout the intervention period.
In conclusion, we report that intentional weight loss using a high-protein diet in accordance with the Medifast 4&2&1 Plan is effective in producing clinically meaningful weight and fat mass loss while helping preserve lean body mass and mobility among older adults with obesity. Future studies should carefully screen and recruit older adults with objectively measured functional impairment at baseline to determine whether a treatment effect is enhanced in this high-risk population and examine the role of protein type and timing in intervention effectiveness.
Funding
This work was supported by a grant from Jason Pharmaceuticals, Inc., a wholly owned subsidiary of Medifast, Inc., as well as the Wake Forest Claude D. Pepper Older Americans Independence Center (P30 AG21332), and a National Institute on Aging supported career development award (K01 AG047921) to K.M.B.
Conflict of Interest
Medifast, Inc. provided partial funding for the study and made an in-kind product donation for the meal replacements used in the study. J.K., L.M.A., and C.C. are currently employed by Medifast, Inc. The terms of this arrangement were reviewed and approved by Wake Forest University Health Sciences in accordance with its conflict of interest policies.
Acknowledgements
We would like to gratefully acknowledge The Medifast for Seniors Study participants for their contributions to the successful implementation of the trial. Additionally, we are indebted to Jillie Gaukstern, Laura Welti, Jessica Kelleher, and Lauren Shaver for their help administering intervention sessions.
References
- 1. Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39 [DOI] [PubMed] [Google Scholar]
- 2. Arterburn DE, Crane PK, Sullivan SD. The coming epidemic of obesity in elderly Americans. J Am Geriatr Soc. 2004;52:1907–1912. doi: 10.1111/j.1532-5415.2004.52517.x [DOI] [PubMed] [Google Scholar]
- 3. Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev. 2010;11:671–685. doi: 10.1111/j.1467-789X.2009.00679.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Houston DK, Nicklas BJ, Zizza CA. Weighty concerns: the growing prevalence of obesity among older adults. J Am Diet Assoc. 2009;109:1886–1895. doi: 10.1016/j.jada.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 5. Locher JL, Goldsby TU, Goss AM, Kilgore ML, Gower B, Ard JD. Calorie restriction in overweight older adults: do benefits exceed potential risks?Exp Gerontol. 2016;86:4–13. doi: 10.1016/j.exger.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Batsis JA, Gill LE, Masutani RK, et al. . Weight loss interventions in older adults with obesity: a systematic review of randomized controlled trials since 2005. J Am Geriatr Soc. 2017;65:257–268. doi: 10.1111/jgs.14514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Villareal DT, Apovian CM, Kushner RF, Klein S; American Society for Nutrition; NAASO, The Obesity Society Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Obes Res. 2005;13:1849–1863. doi: 10.1038/oby.2005.228 [DOI] [PubMed] [Google Scholar]
- 8. Rejeski WJ, Brubaker PH, Goff DC Jr, et al. . Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171:880–886. doi: 10.1001/archinternmed.2010.522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Santanasto AJ, Glynn NW, Newman MA, et al. . Impact of weight loss on physical function with changes in strength, muscle mass, and muscle fat infiltration in overweight to moderately obese older adults: a randomized clinical trial. J Obes. 2011;2011. doi: 10.1155/2011/516576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicklas BJ, Chmelo E, Delbono O, Carr JJ, Lyles MF, Marsh AP. Effects of resistance training with and without caloric restriction on physical function and mobility in overweight and obese older adults: a randomized controlled trial. Am J Clin Nutr. 2015;101:991–999. doi: 10.3945/ajcn.114.105270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kitzman DW, Brubaker P, Morgan T, et al. . Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315:36–46. doi: 10.1001/jama.2015.17346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anton SD, Manini TM, Milsom VA, et al. . Effects of a weight loss plus exercise program on physical function in overweight, older women: a randomized controlled trial. Clin Interv Aging. 2011;6:141–149. doi: 10.2147/CIA.S17001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rejeski WJ, Ambrosius WT, Burdette JH, Walkup MP, Marsh AP. Community weight loss to combat obesity and disability in at-risk older adults. J Gerontol A Biol Sci Med Sci. 2017;72:1547–1553. doi: 10.1093/gerona/glw252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Messier SP, Mihalko SL, Legault C, et al. . Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310:1263–1273. doi: 10.1001/jama.2013.277669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Villareal DT, Chode S, Parimi N, et al. . Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Porter Starr KN, Pieper CF, Orenduff MC, et al. . Improved function with enhanced protein intake per meal: a pilot study of weight reduction in frail, obese older adults. J Gerontol A Biol Sci Med Sci. 2016;71:1369–1375. doi: 10.1093/gerona/glv210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nigg CR, Long CR. A systematic review of single health behavior change interventions vs. multiple health behavior change interventions among older adults. Transl Behav Med. 2012;2:163–179. doi: 10.1007/s13142-012-0130-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Department of Health and Human Services. 2008 physical activity guidelines for Americans. 2008. http://www.health.gov/PAGuidelines. Accessed January 4, 2017.
- 19. Clarke TC, Norris T, Schiller JS. Early release of selected estimates based on data from 2016 National Health Interview Survey. National Center for Health Statistics; 2017. http://www.cdc.gov/nchs/nhis.htm.
- 20. Bauer J, Biolo G, Cederholm T, et al. . Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 21. Coker RH, Miller S, Schutzler S, Deutz N, Wolfe RR. Whey protein and essential amino acids promote the reduction of adipose tissue and increased muscle protein synthesis during caloric restriction-induced weight loss in elderly, obese individuals. Nutr J. 2012;11:105. doi: 10.1186/1475-2891-11-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Backx EM, Tieland M, Borgonjen-van den Berg KJ, Claessen PR, van Loon LJ, de Groot LC. Protein intake and lean body mass preservation during energy intake restriction in overweight older adults. Int J Obes (Lond). 2016;40:299–304. doi: 10.1038/ijo.2015.182 [DOI] [PubMed] [Google Scholar]
- 23. Verreijen AM, Verlaan S, Engberink MF, Swinkels S, de Vogel-van den Bosch J, Weijs PJM. A high whey protein-, leucine-, and vitamin D-enriched supplement preserves muscle mass during intentional weight loss in obese older adults: a double-blind randomized controlled trial. Am J Clin Nutr. 2015;101:279–286. doi: 10.3945/ajcn.114.090290 [DOI] [PubMed] [Google Scholar]
- 24. Mojtahedi MC, Thorpe MP, Karampinos DC, et al. . The effects of a higher protein intake during energy restriction on changes in body composition and physical function in older women. J Gerontol A Biol Sci Med Sci. 2011;66:1218–1225. doi: 10.1093/gerona/glr120 [DOI] [PubMed] [Google Scholar]
- 25. Kwon S, Perera S, Pahor M, et al. . What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study). J Nutr Health Aging. 2009;13:538–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coleman CD, Kiel JR, Mitola AH, Langford JS, Davis KN, Arterburn LM. Effectiveness of a Medifast meal replacement program on weight, body composition and cardiometabolic risk factors in overweight and obese adults: a multicenter systematic retrospective chart review study. Nutr J. 2015;14:77. doi: 10.1186/s12937-015-0062-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int. 1985;27:58–65. [DOI] [PubMed] [Google Scholar]
- 28. Bingham SA, Cummings JH. Urine nitrogen as an independent validatory measure of dietary intake: a study of nitrogen balance in individuals consuming their normal diet. Am J Clin Nutr. 1985;42:1276–1289. doi: 10.1093/ajcn/42.6.1276 [DOI] [PubMed] [Google Scholar]
- 29. Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. [DOI] [PubMed] [Google Scholar]
- 30. Newman AB, Simonsick EM, Naydeck BL, et al. . Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018 [DOI] [PubMed] [Google Scholar]
- 31. Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–1238. doi: 10.1016/j.jclinepi.2007.02.006 [DOI] [PubMed] [Google Scholar]
- 32. Kim JE, O’Connor LE, Sands LP, Slebodnik MB, Campbell WW. Effects of dietary protein intake on body composition changes after weight loss in older adults: a systematic review and meta-analysis. Nutr Rev. 2016;74:210–224. doi: 10.1093/nutrit/nuv065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bales CW, Starr Porter NK, Orenduff MC, et al. . Influence of protein intake, race, and age on responses to a weight-reduction intervention in obese women. Curr Dev Nutr. 2017;1:e000703. doi: 10.3945/cdn.117.000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pennings B, Groen B, de Lange A, et al. . Amino acid absorption and subsequent muscle protein accretion following graded intakes of whey protein in elderly men. Am J Physiol Endocrinol Metab. 2012;302:E992–E999. doi: 10.1152/ajpendo.00517.2011 [DOI] [PubMed] [Google Scholar]