Abstract
Impaired metabolism may play a role in the development and lethality of prostate cancer, yet a comprehensive analysis of the interrelationships appears lacking. We measured 625 metabolites using ultrahigh performance liquid chromatography/mass spectrometry (LC-MS) and gas chromatography/mass spectrometry (GC-MS) of prediagnostic serum from 197 prostate cancer cases in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study (ages at diagnosis, 55–86 years). Cox proportional hazards models estimated associations between circulating metabolites and prostate cancer mortality for 1 SD differences (log-metabolite scale), adjusted for age, year of diagnosis, and disease stage. Associations between metabolite chemical classes and survival were examined through pathway analysis, and Cox models assessed the relationship with a sterol/steroid metabolite principal component analysis factor score. Elevated serum N-oleoyl taurine was significantly associated with prostate cancer-specific mortality (hazard ratios [HR] = 1.72 per 1 SD, p < .00008, Bonferroni-corrected threshold = 0.05/625; HR = 3.6 for highest vs lowest tertile, p < .001). Pathway analyses revealed a statistically significant association between lipids and prostate cancer death (p < .006, Bonferroni-corrected threshold = 0.05/8), and sterol/steroid metabolites showed the strongest chemical sub-class association (p = .0014, Bonferroni-corrected threshold = 0.05/45). In the principal component analysis, a 1-SD increment in the sterol/steroid metabolite score increased the risk of prostate cancer death by 46%. Prediagnostic serum N-oleoyl taurine and sterol/steroid metabolites were associated with prostate cancer survival.
Keywords: Metabolomic profile, Prostate cancer mortality, N-oleoyl taurine, Sex sterol/steroid
Prostate cancer is the most common non-cutaneous malignancy among men in the United States, accounting for nearly 1 in 5 new diagnoses and ranking third in cancer mortality, with an estimated 27,000 deaths in 2017 (1). At the same time, its etiology remains largely unknown, with the exception of risk being higher in African Americans and in men with a positive family history or specific low-penetrance genetic variants, none of which are modifiable (2,3). Elucidation of preventable factors related to lethal prostate cancer and survival outcome are needed.
Agnostic or targeted metabolomic analyses that simultaneously measure a broad array of low molecular weight biochemical compounds in blood or other tissue represent a relatively new approach to investigating cancer risk and mortality that has the potential to identify underlying biological mechanisms (4). Studies have demonstrated that dysregulated metabolism plays an important role in prostate carcinogenesis, with malignant cells exhibiting a unique metabolic phenotype that differs from normal cells (5). As in other malignancies, proliferating prostate cancer cells require sufficient anabolic substrates including amino acids, nucleic acids, peptides, and lipids to support neoplastic growth (6,7). This underlying tumor biology has been reflected in previous experimental studies and population-based metabolomic analyses that have identified alterations in amino acids, energy and lipid metabolites, bile acids, polyamines, long chain fatty acids, phospholipids, and choline in prostate cancer cell lines, tissues and case patients (6,8–11). Other experimental data support sex steroid regulation of prostate cancer progression (12). At the same time, higher prostate cancer risk appears to be related to metabolic factors including obesity, hyperinsulinemia, elevated markers of inflammation, and the insulin-like growth factor pathway, and these factors have been associated with increased prostate cancer risk or mortality (13–17). For example, a recent meta-analysis of 19 studies with 10,554 prostate cancer cases and 13,618 controls found elevated IGF-I concentration was associated with an increased prostate cancer risk. The association between IGF-I and aggressive disease was not statistically significant, however, and it did not differ significantly by tumor stage and grade, suggesting IGF-I may influence multiple stages of prostate carcinogenesis (16). Because of the high incidence and prevalence of this malignancy, and, to our knowledge, a comprehensive analysis of pre-diagnostic serum metabolomic profiling of prostate cancer survival has not been reported, we conducted the present investigation.
Methods
Study Population
Prostate cancer cases from the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study cohort were included. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study was a controlled trial that recruited 29,133 50- to 69-year-old Finnish male smokers from 1985 to 1988 and tested alpha-tocopherol and beta-carotene supplementation for the prevention of lung and other cancers (18). At baseline, blood samples were collected after an overnight fast, processed to serum and stored at −70°C until assay. Height and weight were measured, and risk factor questionnaires and written informed consent were obtained. The research was approved by the institutional review boards at both the U.S. National Cancer Institute and the Finnish National Institute for Health and Welfare.
Cases were identified through December 31, 2007 by the Finnish Cancer Registry (International Classification of Disease 9, ICD-9, code 185) and defined as stage I–IV according to the American Joint Committee on Cancer Staging Manual (19). Two hundred prostate cancer cases with measured prediagnostic (ie, baseline) serum metabolomic profiles are included in the present analysis, of which, we excluded three case patients with the same date of diagnosis and date of death from prostate cancer, leaving 197 cases (10). With underlying cause of death as prostate cancer (ICD-9, code 185; ICD-10, code C61), prostate cancer deaths among these cases were identified from the Statistics Finland Death Registry through December 31, 2013. There were 92 prostate cancer deaths during the period of follow-up. Follow-up time was calculated from the date of diagnosis to death from prostate cancer or the censoring date (date of death due to other reason, or December 31, 2013), whichever came first.
Serum Metabolomic Profiling
Serum metabolomic profiling was conducted on a high-resolution, accurate mass platform of ultrahigh-performance liquid chromatography/mass spectrometry (LC-MS) and gas chromatography/mass spectrometry (GC-MS) at Metabolon, Inc. (Durham, North Carolina) (20). Workflow included extraction of raw data, quality control, and identification of 637 known metabolites, of which 12 (2%) were excluded because more than 95% of subjects had values below the limit of detection. The resulting 625 metabolites were categorized into one of eight mutually exclusive chemical classes: amino acids and amino acid derivatives (collectively referred to as “amino acids”), carbohydrates, cofactors and vitamins, energy metabolites, lipids, nucleotides, peptides or xenobiotics, which are adapted according to the Kyoto Encyclopedia of Genes and Genomics (KEGG) database. These metabolites are described in Supplementary Table 1. Based on blinded duplicate quality control samples included in each batch (8%), the median coefficients of variation across all metabolites were 9% (intra-batch; interquartile range 4%–20%) and 17% (inter-batch; interquartile range 10%–28%), respectively.
Statistical Analysis
Each metabolite was batch-normalized by dividing by the batch median value. Missing values for each metabolite were assigned the minimum detectable value, and each metabolite was log-transformed and normalized to have a mean of 0 and a variance of 1. Cox proportional hazards regression models were used to estimate the hazard ratios (HR) and 95% confidence intervals (CIs), for a 1-SD increase in log-metabolite level, on the risk of prostate cancer mortality, adjusting for age at diagnosis (years, continuous), tumor stage at diagnosis (cancer stage I–IV), and year of diagnosis (1986–1994, 1995–2000, 2001–2007). Deaths from causes other than prostate cancer were censored on the date of the event. The conservative Bonferroni corrected threshold (p < .00008 or less, 0.05/625) was used to define statistical significance. For statistically significant metabolites, as well as top metabolites in associated pathways, we also present HR for tertiles.
In secondary analyses, we adjusted for time from blood collection to cancer diagnosis (0–≤5, 6–≤10, 11–≤15, 16–≤20 years), body mass index (BMI, weight (kg)/height (m)2; continuous), number of cigarettes per day (continuous), total smoking years (continuous), total serum cholesterol (mmol/L; continuous), serum high-density lipoprotein cholesterol (mmol/L; continuous), history of hypertension (yes/no), and history of diabetes mellitus (yes/no). We also performed sensitivity analyses stratified by median time from blood collection to cancer diagnosis, BMI, and cancer stage at diagnosis.
In the pathway analyses, we examined the associations between chemical classes (and sub-classes) of metabolites and prostate cancer-specific mortality risk. We calculated a single measure of significance for each pathway by combining the p values from its constituent metabolites using Fisher’s method. Because of the correlation between metabolites, we calculated each pathway level p value by a parametric bootstrap (21,22), where the Z-statistics for the constituent metabolites were assumed to follow a multivariate normal distribution with mean zero and the estimated covariance matrix. The conservative Bonferroni corrected thresholds (0.05/8 for class and 0.05/45 for sub-class) were used to define statistical significance. Principal component analysis was used to further explore whether the chemical sub-class of sterol/steroid hormones was associated with prostate cancer-specific mortality (23), calculating the top principal component based for those 44 metabolites, and estimating the proportional hazards regression HR for 1 SD in the resulting score. We also conducted analyses stratified by time interval between blood draw and prostate cancer diagnosis (tertiles 0–≤7, 7–≤13 or >13 years), BMI (median split) and cancer stage at diagnosis.
Prostate cancer-specific survival plots were generated for the top metabolites using the Kaplan-Meier method, and statistical significance was measured using the log-rank test. We used SAS software version 9.4 (SAS Institute, Cary, North Carolina) and the R statistical language version 3.2.3 (Vienna, Austria) for all analyses. All p values were two-sided.
Results
Baseline characteristics of the 197 prostate cancer cases are shown in Table 1. The mean age at cancer diagnosis was 69 years (range, 55–86), and the median time from blood collection to cancer diagnosis was 10 years. Among the cases, 51.8% had localized disease at diagnosis (stages I or II), 20.8% had locally advanced disease (stage III), and 27.4% had metastatic disease (stage IV); median survival was 7.0, 5.4, and 1.9 years, respectively. At the time of the censoring date, 168 cases were deceased (85.3%), and among those, 92 (46.7% of all cases) died from prostate cancer.
Table 1.
Selected Baseline and Clinical Characteristics of Prostate Cancer Cases (n = 197)
| Characteristica | Prostate Cancer Cases |
|---|---|
| Age at blood collection (y) | 59.3 (5.4) |
| Age at cancer diagnosis (y) | 69.4 (6.2) |
| BMI (kg/m2) | 26.6 (3.8) |
| Serum total cholesterol (mmol/L) | 6.1 (1.1) |
| Serum HDL cholesterol (mmol/L) | 1.2 (0.3) |
| Serum α-tocopherol (mg/L) | 11.6 (3.1) |
| Serum β-carotene (µg/L) | 202 (132) |
| Serum retinol (µg/L) | 610 (124) |
| Serum total PSA (ng/mL) | 8.1 (12.1) |
| Cigarettes per day | 19 (9) |
| Years of cigarette smoking | 37.4 (9.3) |
| Median time from blood draw to cancer diagnosis (y) | 10 |
| Calendar year of diagnosis, No. (%) | |
| 1986–1994 | 62 (31.5) |
| 1995–2000 | 64 (32.5) |
| 2001–2007 | 71 (36.0) |
| Cancer stage, No. (%) | |
| Stage I | 47 (23.9) |
| Stage II | 55 (27.9) |
| Stage III | 41 (20.8) |
| Stage IV | 54 (27.4) |
| Median survival time for all cases (y) | 5.3 |
| Median survival time for cases by stage (y) | |
| Stage I | 7.8 |
| Stage II | 6.4 |
| Stage III | 5.4 |
| Stage IV | 1.9 |
Notes: BMI = body mass index; HDL = high-density lipoprotein.
aValues are means (SD) unless otherwise indicated.
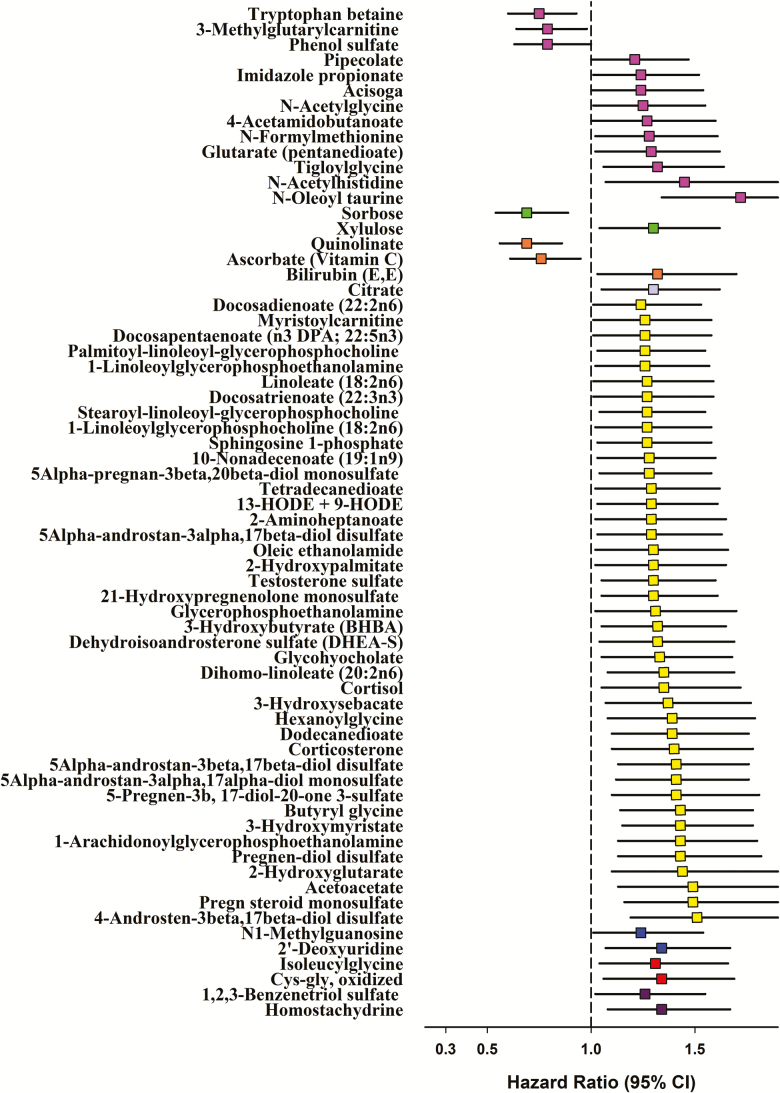
Serum N-oleoyl taurine was statistically significantly associated with prostate cancer-specific mortality: HR = 1.72 per SD, 95% CI: 1.34, 2.20, p = 1.7 × 10–5 (Figure 1); and HR = 3.6 for highest versus lowest tertile, 95% CI: 2.1, 6.4, ptrend < .001 (Table 2). Further adjustment for the number of years from blood collection to prostate cancer diagnosis, or for several other potential confounding factors, including BMI, cigarettes per day and smoking years, serum total or high-density lipoprotein cholesterol, hypertension, and diabetes mellitus, did not materially change the HR (Table 2). Stratifying prostate cancer cases by the median time from blood collection to diagnosis, BMI and cancer stage at diagnosis also showed few differences, with the exception of a possibly stronger association for N-oleoyl taurine among earlier stage cases (eg, 1-SD HR 2.32 and 1.33 for earlier vs later stage) (Supplementary Table 2). Kaplan-Meier survival plots of cases with lower versus higher serum N-oleoyl taurine are shown in Supplementary Figure 1 (log-rank p = .0024).
Figure 1.
Hazard ratios (HRs) for prostate cancer-specific mortality by prediagnostic serum metabolites (per 1 SD) at p-value < .05. The serum metabolites were natural log-transformed and standardized (mean = 0, variance = 1). We used attained time as the time metric in the Cox proportional hazard regression models that were adjusted for age at diagnosis (years, continuous), tumor stage at diagnosis (cancer stage I–IV), and year of diagnosis (1986–1994, 1995–2000, 2001–2007). N-oleoyl taurine was statistically significantly associated with prostate cancer-specific mortality, with p-value (p = .000017) being lower than the predefined statistical threshold after Bonferroni correction (p = .00008, 0.05/625). Colors indicate chemical classes: Amino acids = light purple; carbohydrates = green; cofactors and vitamins = orange; energy metabolites = lavender; lipids = yellow; nucleotides = blue; peptides = red; xenobiotics = dark purple.
Table 2.
Hazard Ratios (HRs) for Prostate Cancer-Specific Mortality by Tertile of Pre-diagnostic Serum Metabolites
| Tertile of Serum Metabolites | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | p trend a | |
| N-oleoyl taurine | ||||
| Person-years | 465 | 433 | 347 | |
| Cases/deaths | 65/25 | 66/30 | 66/37 | |
| Median survival, y | 6.9 | 5.3 | 4.2 | |
| HR (95% CI)b | 1.00 | 2.07 (1.18, 3.63) | 3.62 (2.05, 6.38) | <.001 |
| HR (95% CI)c | 1.00 | 2.24 (1.24, 4.03) | 3.32 (1.88, 5.86) | <.001 |
| HR (95% CI)d | 1.00 | 1.96 (1.09, 3.53) | 3.40 (1.85, 6.25) | <.001 |
| 4-Androsten-3beta,17beta-diol disulfate | ||||
| Person-years | 518 | 402 | 325 | |
| Cases/deaths | 66/23 | 65/31 | 66/38 | |
| Median survival, y | 7.0 | 4.7 | 3.4 | |
| HR (95% CI)b | 1.00 | 1.56 (0.88, 2.77) | 2.68 (1.55, 4.65) | <.001 |
| HR (95% CI)c | 1.00 | 1.73 (0.97, 3.07) | 2.47 (1.41, 4.31) | <.001 |
| HR (95% CI)d | 1.00 | 2.26 (1.21, 4.23) | 2.86 (1.61, 5.07) | .003 |
| Pregnenolone sulfate (pregn steroid monosulfate) | ||||
| Person-years | 473 | 405 | 367 | |
| Cases/deaths | 65/23 | 67/34 | 65/35 | |
| Median survival, y | 6.5 | 4.5 | 4.5 | |
| HR (95% CI)b | 1.00 | 1.57 (0.91, 2.68) | 1.99 (1.16, 3.39) | .002 |
| HR (95% CI)c | 1.00 | 1.43 (0.83, 2.48) | 1.99 (1.15, 3.46) | .002 |
| HR (95% CI)d | 1.00 | 1.53 (0.84, 2.77) | 2.46 (1.37, 4.42) | <.001 |
| 5Alpha-androstan-3beta,17beta-diol disulfate | ||||
| Person-years | 478 | 402 | 366 | |
| Cases/deaths | 66/25 | 65/34 | 66/33 | |
| Median survival, y | 6.4 | 4.5 | 3.7 | |
| HR (95% CI)b | 1.00 | 1.38 (0.81, 2.34) | 1.92 (1.13, 3.27) | .002 |
| HR (95% CI)c | 1.00 | 1.39 (0.82, 2.35) | 1.65 (0.96, 2.84) | .002 |
| HR (95% CI)d | 1.00 | 1.56 (0.90, 2.70) | 1.91 (1.09, 3.33) | .007 |
| 5Alpha-androstan-3alpha,17alpha-diol monosulfate | ||||
| Person-years | 469 | 405 | 371 | |
| Cases/deaths | 65/27 | 67/30 | 65/35 | |
| Median survival, y | 6.4 | 4.8 | 4.1 | |
| HR (95% CI)b | 1.00 | 1.75 (1.02, 3.01) | 2.54 (1.46, 4.44) | .003 |
| HR (95% CI)c | 1.00 | 1.64 (0.93, 2.90) | 2.31 (1.27, 4.22) | .003 |
| HR (95% CI)d | 1.00 | 1.43 (0.81, 2.55) | 2.19 (1.21, 3.96) | .03 |
| Pregnen-diol disulfate | ||||
| Person-years | 517 | 370 | 358 | |
| Cases/deaths | 65/23 | 66/34 | 66/35 | |
| Median survival, y | 7.0 | 4.4 | 4.1 | |
| HR (95% CI)b | 1.00 | 1.51 (0.86, 2.63) | 1.87 (1.07, 3.29) | .003 |
| HR (95% CI)c | 1.00 | 1.70 (0.97, 2.98) | 1.61 (0.90, 2.86) | .003 |
| HR (95% CI)d | 1.00 | 1.95 (1.11, 3.44) | 1.78 (1.00, 3.18) | .002 |
Notes: CI = confidence interval; HDL = high-density lipoprotein.
a p trend calculated using the log-metabolite as a continuous variable in Cox regression models. The metabolites include: (i) p value was lower than the statistical significance threshold after Bonferroni correction. Or (ii) Top signals in the sterol/steroid chemical sub-class pathway.
bHazard ratios (95% CIs) were calculated from Cox models adjusted for age at diagnosis (years, continuous), cancer stage at diagnosis (stage I–IV), and year of diagnosis (1986–1994, 1995–2000, 2001–2007).
cModel further adjusted for time between blood collection and cancer diagnosis (0–≤5, 5–≤10, 10–≤15, >15 y).
dModel further adjusted for body mass index (continuous), cigarettes per day (continuous), years of cigarette smoking (continuous), total serum cholesterol (continuous), serum HDL cholesterol (continuous), history of diabetes, and history of hypertension.
The metabolic pathway analysis revealed that the lipid chemical class was associated with prostate cancer survival below the predefined statistical significance threshold of p < .006 (0.05/8) (Supplementary Table 3), with a strong association for the sterol/steroid chemical sub-class (p = .0014, close to the Bonferroni threshold of 0.0011 (0.05/45); Table 3). The top five sterol/steroid metabolites associated with prostate cancer survival were 4-androsten-3beta,17beta-diol disulfate; pregnenolone sulfate (pregn steroid monosulfate); 5alpha-androstan-3beta,17beta-diol disulfate; 5alpha- androstan-3alpha,17alpha-diol monosulfate; and pregnen-diol disulfate (Figure 1 and Table 2) (high vs low tertile HRs of 2.9, 2.5, 1.9, 2.2 and 1.8, respectively).
Table 3.
Pathway Analysis for Chemical Sub-class of Metabolites for Prostate Cancer-Specific Mortality (p-value < .05)a
| Chemical Sub-class | No. of Contributing Metabolites | p-Value |
|---|---|---|
| Sterol/steroidb | 44 | .0014 |
| Fatty acid, monohydroxy | 13 | .0031 |
| Fatty acid metabolism (also BCAA metabolism) | 5 | .0052 |
| Fatty acid metabolism (acyl glycine) | 4 | .0063 |
| Fatty acid, dicarboxylate | 10 | .0097 |
| Long-chain fatty acid; polyunsaturated fatty acid (N3 and N6) | 6 | .015 |
| Hemoglobin and porphyrin metabolism | 6 | .020 |
| Nicotinate and nicotinamide metabolism | 5 | .024 |
| Long chain fatty acid | 14 | .033 |
| Pyrimidine metabolism, uracil containing | 5 | .036 |
| Essential fatty acid; polyunsaturated fatty acid (N3 and N6) | 7 | .040 |
| Lysine metabolism | 9 | .044 |
Notes: aWe assessed the associations between 45 chemical sub-classes of serum metabolites and prostate cancer-specific mortality using Fisher’s method of combining p values. We tested a single p value for each pathway using a parametric bootstrap method. For each bootstrap replication, we re-calculated p values from a generated vector of score test statistics from a multivariate normal distribution with mean 0 and estimated covariance matrix. Pathway p values are based on 100,000 permutations.
bBonferroni-corrected threshold = 0.0011, 0.05/45.
In the principal component analysis, for each 1-SD increase in the component factor score of the sterol/steroid sub-class (n = 44 metabolites), prostate cancer-specific death was 46% higher after adjusting for age at diagnosis, cancer stage at diagnosis, and year of diagnosis. Although additional adjustment for time to diagnosis did not alter the HR, stratifying cases by tertile of time from blood collection to diagnosis revealed that the strongest survival association was only observed among those diagnosed at least 7 years after blood collection: HRs (per 1 SD) for 0–7 years, 7–≤13 years, and >13 years were, respectively, 1.06, 2.05, and 2.04 (p-values .73, .0035, and .01) (Table 4). The sterol/steroid-survival association did not differ by BMI or disease stage at diagnosis (Table 4). Kaplan-Meier survival plots of cases with a lower versus higher component factor score of sterol/steroid metabolites are shown in Supplementary Figure 2 (log-rank p = .0021).
Table 4.
Hazard Ratios (HRs) for Prostate Cancer-Specific Mortality by Factor Score of Principle Component Analysis for 44 Sterol/Steroid Hormones, Stratified by Time Between Blood Collection and Cancer Diagnosisa
| Cases/deaths | HR (95% CI) | p-Value | |
|---|---|---|---|
| Overall | 197/92 | 1.46 (1.16, 1.83) | .0012 |
| Overallb | 197/92 | 1.42 (1.12, 1.79) | .0032 |
| Blood collection to cancer diagnosis | |||
| 0–≤7 y | 73/44 | 1.06 (0.77, 1.46) | .73 |
| 7–≤13 y | 61/25 | 2.05 (1.27, 3.33) | .0035 |
| >13 y | 63/23 | 2.04 (1.18, 3.52) | .010 |
| BMI | |||
| ≤26.1 kg/m2 | 98/47 | 1.54 (1.10, 2.17) | .013 |
| >26.1 kg/m2 | 99/45 | 1.67 (1.21, 2.29) | .0017 |
| Cancer stage at diagnosisc | |||
| Stage I and II | 102/27 | 1.68 (1.07, 2.65) | .024 |
| Stage III and IV | 95/65 | 1.48 (1.14, 1.91) | .0033 |
Notes: aThe factor scores of the principal component analysis for sterol/steroid hormones were natural log-transformed and standardized (mean = 0, variance = 1). Hazard ratios (95% confidence intervals; per 1 SD) were calculated from Cox models adjusted for age at diagnosis (years, continuous), cancer stage at diagnosis (stage I–IV), and year of diagnosis (1986–1994, 1995–2000, 2001–2007).
bModel further adjusted for time between blood collection and cancer diagnosis (0–≤5, 5–≤10, 10–≤15, >15 y).
cModels were adjusted for age at diagnosis (years, continuous) and year of diagnosis (1986–1994, 1995–2000, 2001–2007).
Discussion
In this prospective metabolomic analysis with long-term survival follow-up, we observed that men in the highest tertile of prediagnostic serum N-oleoyl taurine subsequently diagnosed with prostate cancer are over three times more likely to die of their disease compared to cases with lower levels. Elevated sterol/steroid metabolites are also associated with higher prostate cancer-specific mortality, particularly among cases diagnosed at least 7 years after blood collection.
N-oleoyl taurine belongs to a group of N-acyl taurines (NATs), amides of long-chain fatty acids that were first identified in rodent brain, liver, kidney, and other tissues. NATs are substantially elevated in the setting of genetic or chemical disruption of its metabolism by the fatty acid amide hydrolase (24,25), a membrane-bound enzyme whose primary function is to metabolize the endocannabinoid anandamide into arachidonic acid and ethanolamine (26). Endocannabinoid expression in prostate cancers has also been observed (27). Although the precise role of N-oleoyl taurine and other NATs in normal prostate physiology is not well-understood, experiments have shown the addition of N-oleoyl taurine to PC-3 prostate adenocarcinoma cell culture leads to decreased proliferation (28). NATs appear able to activate multiple members of the transient receptor potential (TRP) family of calcium channel modulators, including TRPV1 and TRPV4 (24), and TRPs have been associated with cancer, including prostate cancer, possibly by facilitating proliferation, aberrant differentiation, impaired apoptosis, and tumor invasion (29–31). Such findings highlight the biological function of NATs serving as cell membrane-associated signaling lipids that impact TRP channels (24). Other experiments indicated that NATs may possess insulin secretagogue characteristics (32), with observational studies showing prostate cancer associations with circulating insulin-like growth factors and hyperinsulinemia (33). Of note, the present data suggest that the N-oleoyl taurine-prostate cancer survival finding was not driven by metastatic disease, as a stronger association was observed for stage I/II cancers than for stage III/IV cancers. As the core structure of N-oleoyl taurine, taurine is an essential metabolite that promotes cellular and tissue homeostasis and oxidation of fatty acids, and plays important roles in membrane stabilization, anti-oxidation, and anti-inflammation (34–36). However, our current data do not suggest a direct correlation between N-oleoyl taurine and taurine (r = .03, p-value = .72), and serum taurine per se was not associated with prostate cancer survival (HR = 1.21, p-value = .07). Studies are needed to verify the N-oleoyl taurine association in larger and more diverse populations, and to further examine its biological activity relevant to prostate cancer survival.
To our knowledge, this is the first prospective study to report that integrated several serum sex steroid metabolites in the androgen pathway, including precursors and derivatives of pregnenolone, dehydroepiandrosterone (DHEA) and androstenediol, measured up to 25 years prior to diagnosis were associated with prostate cancer survival. Androgens play a key role in the development, maturation, and maintenance of the prostate, and intra-prostatic androgens contribute to the progression of prostate cancer, with androgen ablation serving as a central therapeutic modality. Androgens are also reported to regulate lipid uptake required by rapid, dysregulated cell proliferation, including cell membrane production, energy generation, and intracellular signal transduction, and they facilitate and maintain cancer cell survival in the hypoxic microenvironment of limited vascularity (37,38). Evidence from studies of genetic variants in the steroidogenic pathway (eg, CYP17A1, HSD17B2 and ESR1) (39), of androgen receptor signaling regulation and response elements (40–42), and of male pattern baldness (43) also provide support for the involvement of sex steroid hormones in prostate carcinogenesis and progression, although epidemiologic studies of circulating steroids have been equivocal (44). A previous study of 200 prostate cancer cases and 1,057 controls found that free testosterone was statistically significantly associated with ERG-positive, but not ERG-negative, prostate cancer (45), while an analysis of 963 prostate cancer cases found no overall association between prediagnostic circulating sex hormones and total or prostate cancer-specific mortality (46). That study examined only six sex hormones, however, including total and free testosterone, dihydrotestosterone, androstenediol glucuronide, and estradiol, in contrast to the present analysis of 44 sex steroid metabolites, with only free testosterone or testosterone sulfate assessed in both studies. By contrast, other studies of men with prostate cancer show low pretreatment/preoperative testosterone was associated with aggressive disease, including higher Gleason scores (47,48), advanced pathological stage (49), positive surgical margins (50), and adverse prognosis for men with metastatic disease (51). Relevant to these clinical studies is our finding that the principal component analysis-derived sex steroid component factor score was related to a doubling of prostate cancer-specific mortality only among cases with at least a 7-year interval between blood collection and diagnosis, with no association in cases with a shorter interval who were likely to have larger, more advanced tumors at the time of blood collection.
Prostate cancer, and especially aggressive disease, has been associated with alterations of fatty acid metabolism (52,53). Our data showed that several fatty acids, including polyunsaturated, acyl glycyl, dicarboxylic, and monohydroxy fatty acids, are nominally associated with increased prostate cancer mortality, and may reflect the involvement of de novo fatty acid biosynthesis, beta-oxidation pathway, or lipolytic triglyceride mobilization of fatty acids in prostate carcinogenesis (52–54). In addition, the polyunsaturated fatty acid (n3) metabolite, docosapentaenoate, was modestly associated with an increased prostate cancer mortality, consistent with previous prostate cancer metabolomic analyses (55,56).
Strengths of our investigation include its prospective design, independently obtained data on serum metabolomic profiling, and long-term and complete prostate cancer survival follow-up. Despite a modest sample size, we were able to observe statistically significant associations after adjustment for multiple comparisons; that is, Bonferroni correction, which while minimizing false-positive findings, is a stringent statistical threshold, given that many of the metabolites were highly collinear. One theoretical weakness is the lack of treatment data, which if available, would have permitted a subgroup analysis based on the therapeutic interventions. It seems unlikely, however, that the prediagnostic metabolomic profile two decades or more prior to diagnosis would have determined or affected future selection of therapeutic options. We also did not have information regarding prostate cancer molecular sub-types to explore differential metabolite associations (ie, by ERG-positive or negative tumors). Although we cannot preclude the possibility that the findings were influenced by unmeasured confounding factors, we did control for several relevant characteristics in the models. The study participants were all 50–69 years old male smokers of European descent, which limits generalizability of our findings to younger or non-smoking men, or men of other racial/ethnic groups.
In summary, this prospective serum metabolomic analysis of 197 men diagnosed with prostate cancer showed that elevated prediagnostic serum N-oleoyl taurine, as well as sex steroid hormone metabolites, were associated with substantially decreased prostate cancer survival. Additional prospective studies should re-examine the associations, and the underlying biological mechanisms, as well as the potential utility of the identified metabolites to serve as novel prognostic markers for clinical risk stratification.
Funding
The ATBC Study is supported by the Intramural Research Program of the U.S. National Cancer Institute, National Institutes of Health and by U.S. Public Health Service contract HHSN261201500005C from the National Cancer Institute, Department of Health and Human Services.
Conflict of Interest
None reported.
Supplementary Material
Acknowledgments
We thank all participants in the ATBC Study cohort for their contribution to the study.
References
- 1. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 2. Bostwick DG, Burke HB, Djakiew D, et al. Human prostate cancer risk factors. Cancer. 2004;101(10 Suppl):2371–2490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- 3. Al Olama AA, Kote-Jarai Z, Berndt SI, et al. . A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46:1103–1109. doi: 10.1038/ng.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Connell TM. Recent advances in metabolomics in oncology. Bioanalysis. 2012;4:431–451. doi: 10.4155/bio.11.326. [DOI] [PubMed] [Google Scholar]
- 5. Lucarelli G, Rutigliano M, Galleggiante V, et al. Metabolomic profiling for the identification of novel diagnostic markers in prostate cancer. Expert Rev Mol Diagn. 2015;15:1211–1224. doi: 10.1586/14737159.2015.1069711. [DOI] [PubMed] [Google Scholar]
- 6. Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gang X, Yang Y, Zhong J, et al. P300 acetyltransferase regulates fatty acid synthase expression, lipid metabolism and prostate cancer growth. Oncotarget. 2016;7:15135–15149. doi: 10.18632/oncotarget.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li T, Apte U. Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv Pharmacol. 2015;74:263–302. doi: 10.1016/bs.apha.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Swanson MG, Vigneron DB, Tabatabai ZL, et al. Proton HR-MAS spectroscopy and quantitative pathologic analysis of MRI/3D-MRSI-targeted postsurgical prostate tissues. Magn Reson Med. 2003;50:944–954. doi: 10.1002/mrm.10614. [DOI] [PubMed] [Google Scholar]
- 10. Mondul AM, Moore SC, Weinstein SJ, Karoly ED, Sampson JN, Albanes D. Metabolomic analysis of prostate cancer risk in a prospective cohort: the Alpha-Tocolpherol, Beta-Carotene Cancer Prevention (ATBC) study. Int J Cancer. 2015;137:2124–2132. doi: 10.1002/ijc.29576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Currie E, Schulze A, Zechner R, Walther TC, Farese RV Jr. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soronen P, Laiti M, Törn S, et al. Sex steroid hormone metabolism and prostate cancer. J Steroid Biochem Mol Biol. 2004;92:281–286. doi: 10.1016/j.jsbmb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 13. Kelly SP, Graubard BI, Andreotti G, Younes N, Cleary SD, Cook MB. Prediagnostic body mass index trajectories in relation to prostate cancer incidence and mortality in the PLCO cancer screening trial. J Natl Cancer Inst. 2017;109:1–9. doi: 10.1093/jnci/djw225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toriola AT, Laukkanen JA, Kurl S, Nyyssönen K, Ronkainen K, Kauhanen J. Prediagnostic circulating markers of inflammation and risk of prostate cancer. Int J Cancer. 2013;133:2961–2967. doi: 10.1002/ijc.28313. [DOI] [PubMed] [Google Scholar]
- 15. Cao Y, Lindström S, Schumacher F, et al. Insulin-like growth factor pathway genetic polymorphisms, circulating IGF1 and IGFBP3, and prostate cancer survival. J Natl Cancer Inst. 2014;106:dju085. doi: 10.1093/jnci/dju085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Travis RC, Appleby PN, Martin RM, et al. A meta-analysis of individual participant data reveals an association between circulating levels of IGF-I and prostate cancer risk. Cancer Res. 2016;76:2288–2300. doi: 10.1158/0008-5472.CAN-15-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Venkateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99:1793–1800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 18. The ATBC Cancer Prevention Study Group. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 19. Edge S, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A.. American Joint Committee on Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. [Google Scholar]
- 20. Evans AM, Bridgewater BR, Liu Q, et al. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 2014;4:132. doi: 10.4172/2153-0769.1000132. [DOI] [Google Scholar]
- 21. Chen H, Lumley T, Brody J, et al. Sequence kernel association test for survival traits. Genet Epidemiol. 2014;38:191–197. doi: 10.1002/gepi.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davison AC, Hinkley DV.. Bootstrap Methods and Their Application. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- 23. Jolliffe I. Principal component analysis. In: Everitt BS, Howellfin DC, eds.. Encyclopedia of Statistics in Behavioral Science. New York: Wiley; 2005:1580–1584. [Google Scholar]
- 24. Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry. 2006;45:9007–9015. doi: 10.1021/bi0608008. [DOI] [PubMed] [Google Scholar]
- 25. Saghatelian A, Trauger SA, Want EJ, Hawkins EG, Siuzdak G, Cravatt BF. Assignment of endogenous substrates to enzymes by global metabolite profiling. Biochemistry. 2004;43:14332–14339. doi: 10.1021/bi0480335. [DOI] [PubMed] [Google Scholar]
- 26. Cravatt BF, Demarest K, Patricelli MP, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Díaz-Laviada I. The endocannabinoid system in prostate cancer. Nat Rev Urol. 2011;8:553–561. doi: 10.1038/nrurol.2011.130. [DOI] [PubMed] [Google Scholar]
- 28. Chatzakos V, Slätis K, Djureinovic T, Helleday T, Hunt MC. N-acyl taurines are anti-proliferative in prostate cancer cells. Lipids. 2012;47:355–361. doi: 10.1007/s11745-011-3639-9. [DOI] [PubMed] [Google Scholar]
- 29. Gkika D, Prevarskaya N. Molecular mechanisms of TRP regulation in tumor growth and metastasis. Biochim Biophys Acta. 2009;1793:953–958. doi: 10.1016/j.bbamcr.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 30. Déliot N, Constantin B. Plasma membrane calcium channels in cancer: alterations and consequences for cell proliferation and migration. Biochim Biophys Acta. 2015;1848:2512–2522. doi: 10.1016/j.bbamem.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 31. Liberati S, Morelli MB, Amantini C, et al. Loss of TRPV2 homeostatic control of cell proliferation drives tumor progression. Cells. 2014;3:112–128. doi: 10.3390/cells3010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Waluk DP, Vielfort K, Derakhshan S, Aro H, Hunt MC. N-Acyl taurines trigger insulin secretion by increasing calcium flux in pancreatic β-cells. Biochem Biophys Res Commun. 2013;430:54–59. doi: 10.1016/j.bbrc.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 33. Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM. Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. Int J Cancer. 2009;124:2416–2429. doi: 10.1002/ijc.24202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lambert IH, Kristensen DM, Holm JB, Mortensen OH. Physiological role of taurine–from organism to organelle. Acta Physiol (Oxf). 2015;213:191–212. doi: 10.1111/apha.12365. [DOI] [PubMed] [Google Scholar]
- 35. Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 36. Ito T, Okazaki K, Nakajima D, Shibata D, Murakami S, Schaffer S. Mass spectrometry-based metabolomics to identify taurine-modified metabolites in heart. Amino Acids. 2018;50:117–124. doi: 10.1007/s00726-017-2498-y. [DOI] [PubMed] [Google Scholar]
- 37. Butler LM, Centenera MM, Swinnen JV. Androgen control of lipid metabolism in prostate cancer: novel insights and future applications. Endocr Relat Cancer. 2016;23:R219–R227. doi: 10.1530/ERC-15-0556. [DOI] [PubMed] [Google Scholar]
- 38. O’Reilly MW, House PJ, Tomlinson JW. Understanding androgen action in adipose tissue. J Steroid Biochem Mol Biol. 2014;143:277–284. doi: 10.1016/j.jsbmb.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 39. Lévesque É, Huang SP, Audet-Walsh É, et al. Molecular markers in key steroidogenic pathways, circulating steroid levels, and prostate cancer progression. Clin Cancer Res. 2013;19:699–709. doi: 10.1158/1078-0432.CCR-12-2812. [DOI] [PubMed] [Google Scholar]
- 40. Jung C, Kim RS, Zhang HJ, Lee SJ, Jeng MH. HOXB13 induces growth suppression of prostate cancer cells as a repressor of hormone-activated androgen receptor signaling. Cancer Res. 2004;64:9185–9192. doi: 10.1158/0008-5472.CAN-04-1330. [DOI] [PubMed] [Google Scholar]
- 41. Ewing CM, Ray AM, Lange EM, et al. Germline mutations in HOXB13 and prostate-cancer risk. N Engl J Med. 2012;366:141–149. doi: 10.1056/NEJMoa1110000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang CN, Huang SP, Pao JB, et al. Genetic polymorphisms in androgen receptor-binding sites predict survival in prostate cancer patients receiving androgen-deprivation therapy. Ann Oncol. 2012;23:707–713. doi: 10.1093/annonc/mdr264. [DOI] [PubMed] [Google Scholar]
- 43. Zhou CK, Pfeiffer RM, Cleary SD, et al. Relationship between male pattern baldness and the risk of aggressive prostate cancer: an analysis of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. J Clin Oncol. 2015;33:419–425. doi: 10.1200/JCO.2014.55.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Endogenous H, Prostate Cancer Collaborative G, Roddam AW, Allen NE, Appleby P, Key TJ. Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst. 2008;100:170–183. doi: 10.1093/jnci/djm323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Graff RE, Meisner A, Ahearn TU, et al. Pre-diagnostic circulating sex hormone levels and risk of prostate cancer by ERG tumour protein expression. Br J Cancer. 2016;114:939–944. doi: 10.1038/bjc.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gershman B, Shui IM, Stampfer M, et al. Prediagnostic circulating sex hormones are not associated with mortality for men with prostate cancer. Eur Urol. 2014;65:683–689. doi: 10.1016/j.eururo.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hoffman MA, DeWolf WC, Morgentaler A. Is low serum free testosterone a marker for high grade prostate cancer?J Urol. 2000;163:824–827. [PubMed] [Google Scholar]
- 48. Schatzl G, Madersbacher S, Thurridl T, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47:52–58. doi: 10.1002/pros.1046. [DOI] [PubMed] [Google Scholar]
- 49. Isom-Batz G, Bianco FJ Jr, Kattan MW, Mulhall JP, Lilja H, Eastham JA. Testosterone as a predictor of pathological stage in clinically localized prostate cancer. J Urol. 2005;173:1935–1937. doi: 10.1097/01.ju.0000158040.33531.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Teloken C, Da Ros CT, Caraver F, Weber FA, Cavalheiro AP, Graziottin TM. Low serum testosterone levels are associated with positive surgical margins in radical retropubic prostatectomy: hypogonadism represents bad prognosis in prostate cancer. J Urol. 2005;174:2178–2180. doi: 10.1097/01.ju.0000181818.51977.29. [DOI] [PubMed] [Google Scholar]
- 51. Chodak GW, Vogelzang NJ, Caplan RJ, Soloway M, Smith JA. Independent prognostic factors in patients with metastatic (stage D2) prostate cancer. The Zoladex Study Group. JAMA. 1991;265:618–621. [PubMed] [Google Scholar]
- 52. Liu Y. Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate Cancer Prostatic Dis. 2006;9:230–234. doi: 10.1038/sj.pcan.4500879. [DOI] [PubMed] [Google Scholar]
- 53. Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–232. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nomura DK, Lombardi DP, Chang JW, et al. Monoacylglycerol lipase exerts dual control over endocannabinoid and fatty acid pathways to support prostate cancer. Chem Biol. 2011;18:846–856. doi: 10.1016/j.chembiol.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Crowe FL, Appleby PN, Travis RC, et al. Circulating fatty acids and prostate cancer risk: individual participant meta-analysis of prospective studies. J Natl Cancer Inst. 2014;106. doi: 10.1093/jnci/dju240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang J, Mondul AM, Weinstein SJ, et al. Serum metabolomic profiling of prostate cancer risk in the prostate, lung, colorectal, and ovarian cancer screening trial. Br J Cancer. 2016;115:1087–1095. doi: 10.1038/bjc.2016.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.