Abstract
Non-coding RNAs, particularly long non-coding RNAs (lncRNAs), play important roles in tumorigenesis. The miR-155 host gene (MIR155HG) lncRNA has been found to play a crucial role in tumor progression. However, the role of MIR155HG in laryngeal squamous cell carcinoma (LSCC) remains unclear. Thus, the aim of the present study was to explore the roles and underlying molecular mechanisms of action of MIR155HG and miR-155-5p in LSCC, in an effort to provide novel approaches for the antitumor therapy for LSCC. In the present study, the expression levels of miR-155-5p and MIR155HG were detected by reverse tran scription-quantitative polymerase chain reaction. In addition, the biological functions of MIR155HG and miR-155-5p on LSCC were evaluated in vitro by MTS assay, colony formation assay and Transwell assays, and in vivo by tumorigenesis assays. It was revealed that MIR155HG and miR-155-5p were significantly upregulated in LSCC tissues, and were associated with the TNM stage, pathological differentiation and lymph node metastasis. Moreover, the knockdown of MIR155HG and miR-155-5p inhibited the proliferation, migration and invasion of LSCC cells, whereas their overexpression exerted the opposite effects in vitro and MIR155HG overexpression promoted tumorigenesis in vivo. Furthermore, MIR155HG downregulation reduced the expression level of miR-155-5p. The inhibitory effect of MIR155HG knockdown on malignant behavior was abrogated by miR-155-5p overexpression. Bioinformatics analysis and luciferase reporter assay confirmed that miR-155-5p contributed to the progression of LSCC by directly binding to the 3′ untranslated region of SRY-related-HMG-box 10 (SOX10). In addition, MIR155HG and miR-155-5p were upregulated by the induction of transforming growth factor-β (TGF-β) and promoted the expression of mesenchymal markers synergistically. On the whole, the findings of the present study indicate a novel role of MIR155HG in the TGF-β-induced EMT of LSCC cells by regulating EMT markers through the miR-155/SOX10 axis. The MIR155HG/miR-155-5p/SOX10 axis plays an important role in promoting the progression of LSCC and may thus serve as a potential therapeutic target for LSCC treatment.
Keywords: laryngeal squamous cell carcinoma, MIR155HG, miR-155-5p, epithelial-to-mesenchymal transition, transforming growth factor-β
Introduction
Laryngeal carcinoma is one of the most common malignant tumors among head and neck carcinomas, and >95% of laryngeal carcinomas are of the squamous cell type [laryngeal squamous cell carcinoma (LSCC)]. In 2018, there were ~177,422 new cases and ~94,771 LSCC-related deaths worldwide (1). The incidence of LSCC is increasing annually worldwide. Despite major advances in LSCC diagnosis and treatment, the prognosis of patients with advanced LSCC remains dismal. Treatment failure is most commonly attributed to recurrence and metastasis (2). Approximately 60% of patients with LSCC are already at an advanced stage (III or IV) at the time of treatment (3). Therefore, the identification of specific molecular biomarkers for early diagnosis and effective targeted therapy is crucial for the early detection, treatment and follow-up of LSCC.
With the development of high-throughput sequencing technologies, only <2% of the transcripts were found to encode proteins, whereas the vast majority were transcribed into non-coding RNAs, including microRNAs (miRNAs or miRs), small interfering RNAs (siRNAs) and 1ong non-coding RNAs (lncRNAs) (4). lncRNAs are a class of non-coding RNAs with a length of >200 nucleotides which, although they cannot encode proteins themselves, regulate gene expression at the transcriptional, as well as the post-transcriptional level. Furthermore, lncRNAs are involved in a number of cellular functions, such as chromatin remodeling, RNA decay, epigenetic regulation and chromatin modification (5,6), and are closely associated with tumor formation, invasion and metastasis (7,8). However, the role of lncRNAs in LSCC has not yet been fully determined. Certain lncRNAs, such as H19 (9), HOTAIR (10,11), NEAT1 (12) and TUG1 (13), have been shown to be upregulated in laryngeal cancer cells and tissues, and may promote cancer by participating in various biological processes. The differential expression of lncRNAs was detected by microarray assays on four pairs of LSCC and adjacent normal tissues. The lncRNA, miR-155 host gene (MIR155HG), was found to be upregulated in LSCC tissues. By searching biological information websites (UCSC, http://genome.ucsc.edu/), miR-155-5p was found to be located in the third exon of MIR155HG. In recent years, the inter-relationship between miRNAs and lncRNAs has become a main focus of research. lncRNAs can form the precursors of miRNAs through intracellular shear action, and certain genes can be transcribed to lncRNAs and miRNAs at the same time (14). For example, lncRNA MIR100HG-derived miR-100 and miR-125b have been shown to participate in the resistance of colorectal cancer to cetuximab through Wnt/β-catenin signaling (15). MIR31HG, the host gene of miR-31, has been shown to contribute to the pathogenesis of Hirschsprung’s disease through the MIR31HG-miR-31/31*-ITIH5/PIK3CG pathway (16). MIR155HG-derived miR-155-5p and miR-155-3p have been shown to suppress the transcription and translation of protocadherin (PCDH)9 and PCDH7, thereby promoting glioma cell migration and invasion (17). However, the roles of miR-155-5p and MIR155HG and their interaction in laryngeal cancer have not yet been fully elucidated.
Epithelial-to-mesenchymal transition (EMT) is associated with distant metastasis and tumor dissemination. Multiple growth factors and cytokines may induce EMT, and transforming growth factor (TGF)-β is a key factor in the induction of EMT (18). EMT has been reported to be involved in the development of LSCC. Non-coding RNAs, such as miR-203 (19), miR-205 and miR-375 (20), may regulate the progression of LSCC by regulating EMT. The coding gene enhancer of zeste homolog 2 (EZH2) may also promote the invasion and migration of LSCC cells through EMT (21). However, whether lncRNAs are involved in the EMT of laryngeal cancer cells remains unclear.
Therefore, the aim of the present study was to detect the expression of MIR155HG and its exon miRNA, miR-155-5p, in LSCC, and to explore the potential functional roles and downstream regulatory mechanisms of the MIR155HG/miR-155 axis in the development and progression of LSCC, as well as its role in TGF-β-induced EMT. The aim of this study was also to determine whether MIR155HG plays a carcinogenic role in LSCC, and whether it may be used as a biomarker and as a target in novel therapeutic strategies for patients with LSCC.
Materials and methods
Patients and tissue specimens
LSCC tissue samples and adjacent normal tissues were collected from 45 patients with LSCC at the Otorhinolaryngology Head and Neck Surgery Biobank of Hebei Medical University (Shijiazhuang, China) from October, 2016 to March, 2018. Informed consent was obtained from all patients, none of whom had received chemotherapy or radiotherapy prior to surgery. The use of human tissues specimens was approved by and carried out according to the guidelines of the Ethics Committee of the Second Hospital of Hebei Medical University (Shijiazhuang, China). One part of the tissue specimens was placed into RNAlater solution (CoWin Biosciences, Beijing, China) and stored at -80°C for RNA extraction. The other part of the tissue specimens was fixed in 10% neutral formaldehyde solution, and paraffin blocks were routinely prepared and preserved. Tumor and normal adjacent tissues were confirmed by routine pathological diagnosis. Agilent SBC Human (4*180K) lncRNA Microarray (ID: 74348) was used to test the transcript expression profiles in 4 pairs of LSCC and normal tissues. The clinicopathological characteristics of the 45 paired specimens are presented in Table SI.
Cell culture
Three human LSCC cell lines (TU686, TU177 and AMC-HN-8) and 293T cells were purchased from BNBIO (Beijing, China) and preserved at the Otorhinolaryngology Head and Neck Surgery Biobank of Hebei Medical University. The TU686 and TU177 cells were cultured in RPMI-1640 medium (Gibco/Thermo Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10% fetal bovine serum (FBS; Gibco/Thermo Fisher Scientific, Inc.). The AMC-HN-8 and 293T cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco/Thermo Fisher Scientific, Inc.) supplemented with 10% FBS. The TU177 cells were treated with 10 ng/ml recombinant TGF-β (R&D Systems, Inc., Minneapolis, MN, USA) for 7 days and the medium was replenished every 2 days. All the cells were cultured at 37°C in a humidified 5% CO2 incubator (Thermo Fisher Scientific, Inc.).
RNA extraction and reverse tran scription-quantitative polymerase chain reaction (RT-qPCR) assay
Total RNA was extracted from the tissues and cells using the the Eastep®Super Total RNA Extraction kit (Promega, Madison, WI, USA), and the RNA integrity was evaluated by 1% agarose gel electrophoresis (containing DEPC; Bio-Rad Laboratories, Inc., Hercules, CA, USA). cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis kit (Roche, Basel, Switzerland) following the manufacturer’s protocol. RT-qPCR was performed with GoTap®qPCRMaster Mix (Promega) according to the manufacturer’s instructions using CFX96™ Real-Time PCR Detection Systems. The reaction conditions were as follows (two-step method): One cycle of pre-denaturation for 2 min at 95°C, followed by 40 cycles of 15 sec at 95°C for denaturation, and for annealing to extension, selecting the most suitable annealing temperature according to different primers for 60 sec. The relative expression levels were estimated with the 2-ΔΔCq method. Each specimen was tested 3 times. The specific primer sequences are listed in Table SII.
Cell cytoplasm and nuclear fraction isolation
Subcellular fractionation was performed with the PARIS™ Kit Protein and RNA Isolation System (Invitrogen/Thermo Fisher Scientific, Inc.) from LSCC cell lines according to the manufacturer’s instructions.
Cell transfection
The 4 shRNAs specifically targeting MIR155HG and the control sh-NC were synthesized by Vigene Biosciences (Shandong, China). The pcDNA3.1-MIR155HG vector was synthesized by Sangon Biotech (Beijing, China). hsa-miRNA-155-5p mimic/inhibitor/negative controls were purchased from GenePharma (Shanghai, China) (Table SII). The cells were grown on 6-well plates and transfected using Lipofectamine 2000 (Invitrogen/Thermo Fisher Scientific, Inc.) when they reached 80% confluence, according to the manufacturer’s instructions. At 48 h following transfection, the cells were collected for RT-qPCR verification and further experiments.
Cell proliferation assay
Cell proliferation was detected by MTS assay. Cells were seeded in 96-well plates at 2×103 per well following transfection for 24 h. Cell proliferation was evaluated using the CellTiter96®AQueousOne Solution Cell Proliferation Assay kit (Promega) according to the manufacturer’s instructions. MTS reagent (20 μl) was added to 100 μl culture medium after seeding for 0, 24, 48, 72 and 96 h, and incubated in CO2 incubator at 37°C for 2.5 h each time. The absorbance at 490 nm was measured using a Spark® multimode microplate reader (Tecan, Männedorf, Switzerland).
Colony formation assay
The cells were seeded in 6-well plates at a density of 2×103 per well following transfection for 24 h. After 12-14 days, the cells were washed twice with phosphate-buffered saline, fixed with paraformaldehyde and stained with 0.5% crystal violet solution at room temperature for 20 min. (Generay, Shanghai, China). The number of colonies (≥50 cells) was counted under a microscope (Olympus, Tokyo, Japan).
Transwell migration and invasion assays
For migration assays, non-Matrigel-coated chambers (Corning Costar, Corning, NY, USA) with 8-μm pore membranes were used. A total of 1×105 cells per well were seeded into the upper chamber and 650 μl medium (10% FBS) was added to the lower chamber. Following incubation in CO2 incubator (37°C, 5% CO2) (Thermo Fisher Scientific, Inc.) for 24 h, the residual cells on the upper surface of the membrane were removed, the membrane was fixed in 4% paraformaldehyde for 20 min, stained with 0.5% crystal violet solution for 20 min and examined under a microscope (CKX53; Olympus, Tokyo, Japan) in 5 randomly selected fields. For invasion assays, the upper chamber was pre-coated with 50 μl 1X Matrigel® Basement Membrane Matrix (Corning Costar); the remaining steps were as described above for the migration assay. For the Transwell assays of TGF-β-treated and untreated cells, 5×104 cells were seeded into the upper chamber.
Tumor xenograft model
A total of 16 male nude (BALB/c-nude) mice (age, 5 weeks; weight, 20-25 g) were obtained from Beijing Vital River Laboratory Animal Technology Co., Beijing, China; animal permit no.: [SCXY (Jing) 2016-0006]. The maintenance conditions for the mice were as follows: Temperature, 23-27°C; humidity, 40-60%; ventilation, 15 times/h; 12 h light/12 h dark cycle. The mice were fed standard laboratory food and water. The health and maintenance condition of the mice were monitored every 2 days. The mice were randomly divided into 2 groups (pcDNA3.1, and pcDNA3.1-MIR155HG; n=8 per group). The TU177 cells (5×106) stably transfected with pcDNA 3.1 and pcDNA3.1-MIR155HG in 100 μl PBS were subcutaneously injected into the right flanks of the mice. Tumor volume was measured using a caliper at indicated time points and calculated using the following formula: V = 0.5 × length x width2. After 4 weeks, the mice were sacrificed by cervical dislocation and the tumors were removed and weighed. The maximum diameter of a single tumor found was 17 mm and no mouse developed multiple tumors. All mice were in well body condition throughout the experiment. All animal experiments were performed at the Experimental Animal Center of the Fourth Hospital of Hebei Medical University according to the NIH guidelines, and were approved by the Institutional Animal Care and Use Committee of the Fourth Hospital of Hebei Medical University.
Prediction of miR-155-5p target genes
The potential downstream targets of miR-155-5p were predicted using TargetScan (http://www.targetscan.org/vert_72/) and miRwark (http://mirwalk.umm.uni-heidelberg.de/). SOX10 was predicted by the two databases and considered a potential target of miR-155-5p. The 3′-UTR of SOX10 mRNA containing the intact miRNA-155-5p recognition sequences was PCR-amplified and subcloned into the Nhel and Xbal sites of the pmirGLO vector (Youbio, Changsha, China). Mutation sites were promoted using the Site-Directed Mutagenesis kit (New England Biolabs, Inc., Ipswich, MA, USA). Mutation primers were designed in NEBase changer (http://nebasechanger.neb.com/) and synthesized by Sangon Biotech (Table SII).
Western blot analysis
Cell protein lysates were prepared using RIPA buffer (Solarbio, Beijing, China) followed by the addition of protease inhibitor cocktail (Promega) and PMSF (Solarbio), according to the manufacturers’ instructions. The protein concentration was measured using the BCA Protein Assay kit (Generay). Proteins were separated by 10% SDS-PAGE and then transferred to PVDF membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membranes were incubated with 5% non-fat milk (Becton-Dickinson and Company, Suzhou, China) for 2 h at room temperature and incubated overnight at 4°C with rabbit-anti-human SOX10 (molecular weight: 60 kDa) (1:2,000; cat. no. DF8009; Affinity, Cincinnati, OH, USA) and rabbit-anti-human GAPDH (molecular weight: 37 kDa) (1:5,000; cat. no. 10494-1-AP; Protein Tech Group, Inc., Chicago, IL, USA) antibodies. Subsequently, the membranes were incubated at room temperature with IgG (H+L) HRP (1:10,000; cat. no. RS0002; Ruiying Bio, Suzhou, China) for 1 h. Proteins were visualized with an enhanced ECL reagent (Vazyme, Nanjing, China) by ChemiDoc™ XRS+ (Bio-Rad Laboratories, Inc.).
Dual luciferase reporter assay
The TU177 and 293T cells were seeded at a density of 3×104 cells per well in 24-well plates. After 24 h, the cells were co-transfected with pmirGLO-SOX10-WT or pmirGLO-SOX10-MUT reporter plasmids, and miR-155-5p mimic or inhibitor or NC. Following transfection for 48 h, the relative luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s protocol. The luciferase activity of each well was normalized to Renilla luciferase activity.
Statistical analysis
The results were analyzed using SPSS version 21.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism version 7 (GraphPad Software, Inc., La Jolla, CA, USA). The differences between 2 groups were analyzed with the Student’s t-test. Differences between >2 groups were determined by one-way ANOVA followed by Tukey’s post hoc test. Differences between the growth of different groups, two-way ANOVA was performed and followed by Tukey’s post hoc test. Pearson’s correlation analysis was used to analyze the correlation between the expression of MIR155HG and that of miR-155-5p, and between the expression of miR-155-5p and SOX10. Each experiment was performed at least 3 times. All data are presented as the means ± standard deviation. P<0.05 was considered to indicate a statistically significant difference.
Results
MIR155HG is upregulated in LSCC and is associated with advanced clinicopathological characteristics
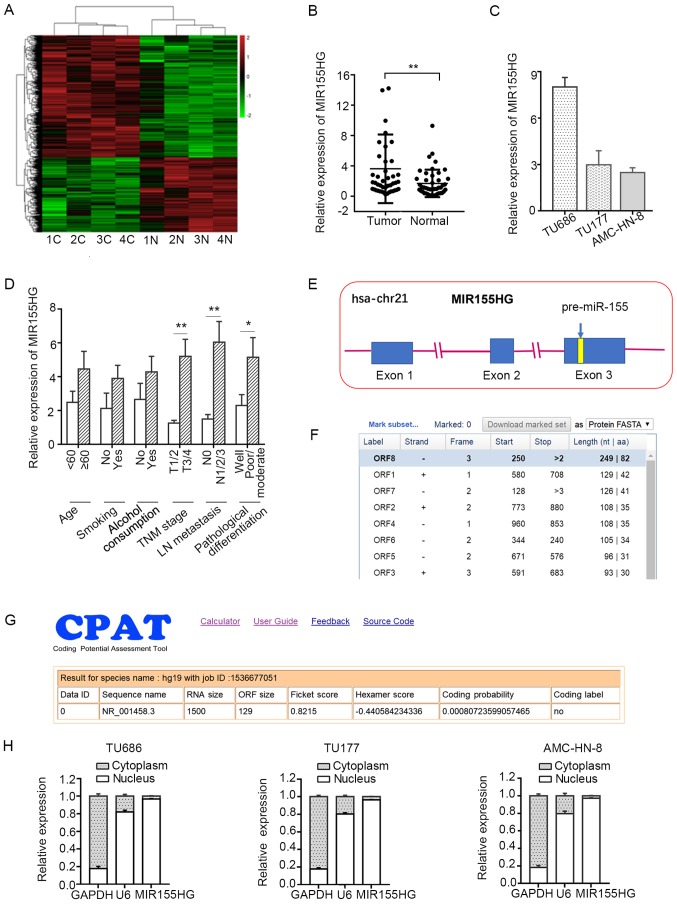
To determine the potential role of lncRNAs in LSCC, microarray analysis was used to compare lncRNA expression levels between LSCC tissues and corresponding normal tissues. There were 3,073 differentially expressed lncRNAs in total (fold change ≥2, P<0.05), of which 1,967 were upregulated and 1,106 were downregulated in LSCC tissues compared with corresponding normal tissues (Fig. 1A). A miRNA host gene, MIR155HG, which was found to be upregulated in the microarray analysis, was selected for further analysis. To investigate the functional role of MIR155HG in LSCC, the expression of MIR155HG was initially analyzed in 45 pairs of LSCC tissues and corresponding adjacent non-tumor tissues by RT-qPCR. The expression level of MIR155HG was found to be significantly increased in the LSCC tissues compared with the adjacent normal tissues (Fig. 1B). The expression levels of MIR155HG in 3 LSCC cell lines (TU686, TU177 and AMC-HN-8) are shown in Fig. 1C. The association between MIR155HG expression and different clinicopathological characteristics in LSCC was further analyzed. High expression levels of MIR155HG were found to be closely associated with poor differentiation, lymph node metastasis and a more advanced TNM stage. However, there was no observed association between the expression of MIR155HG and age, alcohol consumption or smoking (Fig. 1D).
Figure 1.
MIR155HG is upregulated in LSCC and is associated with advanced clinicopathological characteristics. (A) Heatmap of differentially expressed lncRNAs in 4 pairs of LSCC tissues and corresponding normal tissues. (B) The relative expression of MIR155HG in 45 pairs of LSCC tissues and the corresponding adjacent non-tumor tissues by RT-qPCR. (C) The expression of MIR155HG in 3 LSCC cell lines (TU686, TU177 and AMC-HN-8). (D) The association between MIR155HG expression and different clinicopathological characteristics. (E) The genetic structure schematic map of MIR155HG. Homo sapiens MIR155 host gene (MIR155HG) (NR_001458) is located on chromosome 21q21.2, contains 3 exons and 2 introns. MiR-155-5p is located within the third exon of MIR155HG. (F and G) The coding potential of MIR155HG was analyzed using several prediction software, and the results showed MIR155HG did not have any coding potential. (H) The location of MIR155HG in LSCC cells. U6 and GAPDH were used as nuclear and cytoplasm control respectively. Data are shown as mean ± SD; *P<0.05 and **P<0.01. LSCC, laryngeal squamous cell carcinoma; MIR155HG, MIR155 host gene; miR, microRNA; C, cancer; N, normal.
MIR155HG is located on chromosome 21q21.2, and contains 3 exons and 2 introns. miR-155-5p is located within the third exon of MIR155HG (Fig. 1E). The coding potential of MIR155HG was analyzed by using several prediction software programs, and the results revealed that the open reading frame (ORF) of MIR155HG was very short, containing only 82 amino acids (https://www.ncbi.nlm.nih.gov/orffinder/), and had no coding potential (http://lilab.research.bcm.edu/cpat/) (Fig. 1F and G). These results indicated that MIR155HG is a non-coding RNA. Furthermore, MIR155HG was found to be predominantly located in the nucleus of LSCC cells by subcellular fractionation assay (Fig. 1H).
Knockdown of MIR155HG suppresses LSCC cell proliferation, migration and invasion
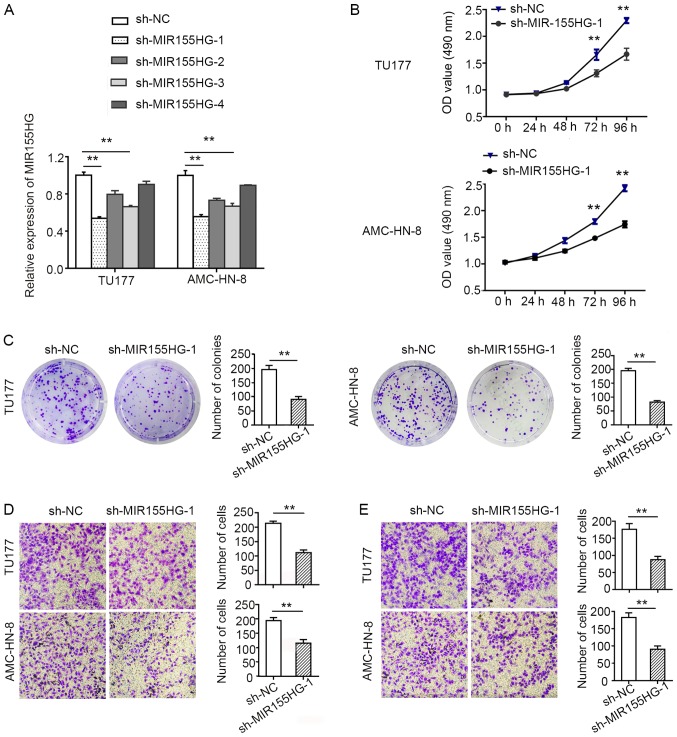
To explore the biological function of MIR155HG, shRNAs against MIR155HG (sh-MIR155HG) were transfected into the LSCC cells to knock down endogenous MIR155HG expression. Due to the poor transfection efficiency, the TU686 cells, which had a relatively higher expression level of MIR155HG, were not selected for the in vitro assay. The TU177 and AMC-HN-8 cells, with a relatively higher transfection efficiency, were used as model cells for the follow-up experiments. sh-MIR155HG-1, which exhibited the most evident knockdown efficacy, was selected for use in further experiments (Fig. 2A).
Figure 2.
Knockdown MIR155HG suppresses LSCC cell proliferation, migration and invasion. (A) RT-qPCR was carried out to analyze the expression of MIR155HG in TU177 and AMC-HN-8 cells after transfected with four shRNAs against MIR155HG and one negative control. (B) Growth curves of TU177 and AMC-HN-8 cells following transfection with sh-MIR155HG or NC were determined by MTS assays. (C) Colony formation assay following the downregulation of MIR155HG in TU177 and AMC-HN-8 cells. (D) Transwell migration assays of TU177 and AMC-HN-8 cells with MIR155HG knockdown (magnification, ×200). (E) Transwell invasion assays of TU177 and AMC-HN-8 cells with MIR155HG knockdown (magnification, ×200). Data are shown as the means ± SD; **P<0.01. LSCC, laryngeal squamous cell carcinoma; MIR155HG, MIR155 host gene; NC, negative control; shRNA, short hairpin RNA.
MTS and colony formation assays were used to measure cell proliferation. MIR155HG knockdown suppressed TU177 and AMC-HN-8 cell proliferation (Fig. 2B). Consistent with the results of MTS assay, the results of colony formation assay also revealed that the downregulation of MIR155HG suppressed the colony-forming ability of the TU177 and AMC-HN-8 cells (Fig. 2C). These data provide evidence to suggest the tumor growth-promoting role of MIR155HG in vitro.
In order to examine the effects of MIR155HG on the malignant biological properties of LSCC cells, the cell migratory and invasive abilities were evaluated using Transwell Matrigel migration and invasion assays. The migration and invasion capacities of the TU177 and AMC-HN-8 cells were found to be markedly decreased when MIR155HG was knocked down (Fig. 2D and E). Taken together, these data demonstrated that MIR155HG suppressed the proliferation, migration and invasion of LSCC cells.
Overexpression of MIR155HG promotes LSCC cell proliferation, migration and invasion in vitro and promotes tumorigenesis in vivo
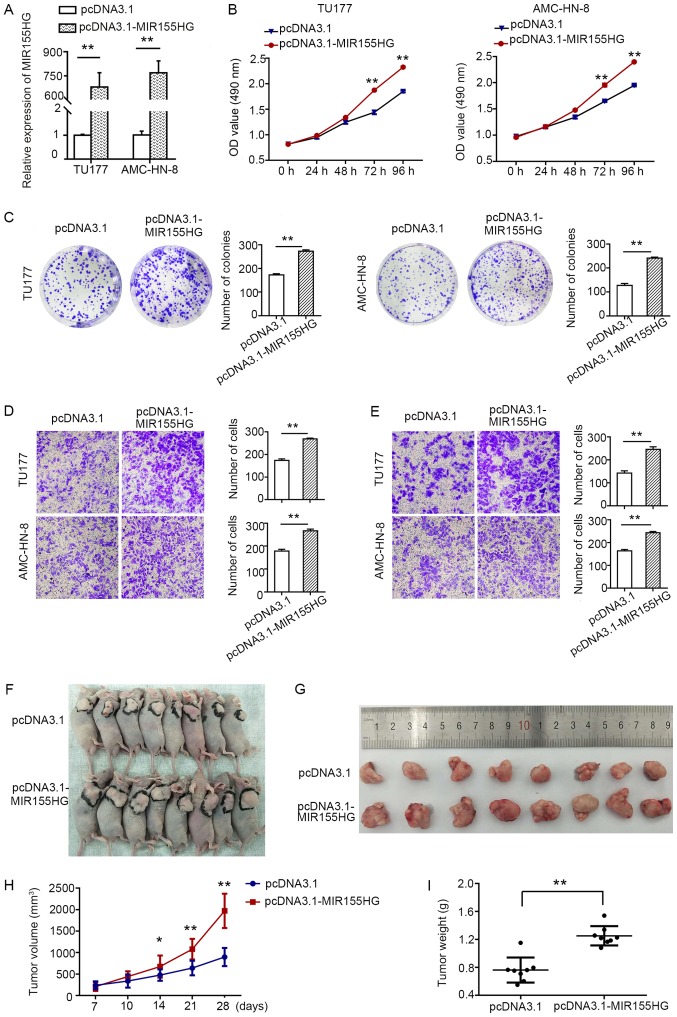
Subsequently, in order to assess the biological effects of MIR155HG overexpression, the pcDNA3.1-MIR155HG expression plasmid was transfected into the TU177 and AMC-HN-8 cells with relatively low expression levels of MIR155HG. pcDNA3.1 was used as a control. The relative expression level of MIR155HG in the pcDNA3.1-MIR155HG-transfected TU177 and AMC-HN-8 cells was markedly increased (Fig. 3A). MTS (Fig. 3B) and colony formation assays (Fig. 3C) demonstrated that MIR155HG upregulation promoted TU177 and AMC-HN-8 cell proliferation. Moreover, MIR155HG overexpression enhanced the migratory and invasive capacity of both cell lines (Fig. 3D and E). All these results indicated that the overexpression of MIR155HG increased the tumorigenicity of LSCC cells. To further verify the results of the in vitro experiments, animal experiments were conducted. A TU177 cell line stably transfected with pcDNA3.1 and pcDNA3.1-MIR155HG was constructed, and 5×106 cells were subcutaneously injected into nude mice. The results demonstrated that the overexpression of MIR155HG significantly promoted tumor growth compared with the pcDNA3.1 group (Fig. 3F and G), whereas the volume and weight of the tumors were higher in the MIR155HG overexpression group (Fig. 3H and I).
Figure 3.
Overexpression of MIR155HG promotes LSCC cell proliferation, migration, and invasion in vitro and promotes tumorgenesis in vivo. (A) RT-qPCR was carried out to analyze the expression of MIR155HG in TU177 and AMC-HN-8 cells following transfection with pcDNA3.1-MIR155HG and pcDNA3.1. (B) Growth curves of TU177 and AMC-HN-8 cells after transfected with pcDNA3.1-MIR155HG and pcDNA3.1 were determined by MTS assay. (C) Colony formation assay following the upregulation of MIR155HG in TU177 and AMC-HN-8 cells. (D) Transwell migration assays of TU177 and AMC-HN-8 cells with MIR155HG overexpression. (magnification, ×200). (E) Transwell invasion assays of TU177 and AMC-HN-8 cells with MIR155HG overexpression. (magnification, ×200). (F) External whole-body images of mice 4 weeks after subcutaneous injection. (G) Representative images of tumors collected from mice 4 weeks after subcutaneous injection. (H) Growth curves of xenograft tumors following the subcutaneous injection of TU177 cells stably overexpressing MIR155HG or negative control. The tumor volumes were measured each week after inoculation. (I) Tumor weights of xenograft tumors after subcutaneous injection of TU177 cells for 4 weeks. Data are shown as the means ± SD; **P<0.01. LSCC, laryngeal squamous cell carcinoma; MIR155HG, MIR155 host gene.
miR-155-5p is upregulated in LSCC and promotes cancer cell proliferation, migration and invasion
As MIR155HG is the host gene of miR-155-5p, we further investigated whether miR-155-5p exerts the same effects as MIR155HG on LSCC. The expression of miR-155-5p was found to be significantly increased in the LSCC tissues compared with the adjacent normal tissues (Fig. 4A). The high expression level of miR-155-5p was found to be closely associated with the TNM stage, lymph node metastasis and poor differentiation. However, there was no association between the expression of miR-155-5p and age, alcohol consumption, or smoking (Fig. 4B).
Figure 4.
miR-155-5p is upregulated in LSCC and promotes LSCC cell proliferation, migration and invasion. (A) The relative expression of miR-155-5p in 45 pairs of LSCC tissues and the corresponding adjacent non-tumor tissues examined by RT-qPCR. (B) The association between relative expression of miR-155-5p and different clinicopathological characteristics. (C) The relative of expression of miR-155-5p in TU177 and AMC-HN-8 cells following transfection with miR-155-5p mimic and inhibitor by RT-qPCR. (D) Growth curves of TU177 and AMC-HN-8 cells following transfection with miR-155-5p mimic and inhibitor determined by MTS assays. (E) Migration assays and (F) invasion assays of TU177 and AMC-HN-8 cells with miR-155-5p knockdown and overexpression. (magnification, ×200). Data are shown as the means ± SD; *P<0.05 and **P<0.01 vs. NC group. LSCC, laryngeal squamous cell carcinoma; NC, negative control; miR, microRNA.
To further determine whether miR-155-5p is associated with the progression of LSCC, miR-155-5p mimics and inhibitor were transfected into the TU177 and AMC-HN-8 cells (Fig. 4C). As shown in Fig. 4D-F, the overexpression of miR-155-5p markedly promoted the proliferation, migration and invasion of TU177 and AMC-HN-8 cells, whereas the downregulation of miR-155-5p markedly inhibited cell proliferation, migration and invasion. Collectively, these data suggest that miR-155-5p acts as an oncogenic miRNA by promoting LSCC cell proliferation, migration and invasion.
Overexpression of miR-155-5p reverses the inhibitory effects of MIR155HG knockdown on LSCC cell proliferation, migration and invasion
The expression level of miR-155-5p was found to highly correlate with the expression of MIR155HG (Fig. 5A). These findings suggested that miR-155-5p and its host gene, MIR155HG, may be co-expressed in LSCC. The downregulation of MIR155HG reduced the expression levels of miR-155-5p in the TU177 and AMC-HN-8 cells (Fig. 5B). However, the expression of MIR155HG was not affected by miR-155-5p knockdown or overexpression in LSCC cells (Fig. 5C). Overexpression of miR-155-5p may abrogate the inhibitory effect of MIR155HG knockdown on cell proliferation, migration and invasion (Fig. 5D-F). These results indicated that miR-155-5p may act synergistically with MIR155HG to promote LSCC progression.
Figure 5.
Overexpression of miR-155-5p reverses the inhibition of LSCC cell proliferation, migration and invasion induced by MIR155HG knockdown. (A) The correlation of the relative expression level of miR-155-5p and MIR155HG (r=0.4523, P=0.0018). (B) The relative expression of miR-155-5p following transfection with MIR155HG shRNA in TU177 and AMC-HN-8 cells. (C) The relative expression of MIR155HG following transfection with miR-155-5p mimic and inhibitor in TU177 and AMC-HN-8 cells. (D) Growth curves of TU177 and AMC-HN-8 cells following co-transfection with miR-155-5p mimic and MIR155HG shRNA determined by MTS assays. (E) Migration assays and (F) invasion assays of TU177 and AMC-HN-8 cells following co-transfection with miR-155-5p mimic and MIR155HG shRNA (magnification, ×200). Data are shown as the means ± SD; **P<0.01 vs. NC group. LSCC, laryngeal squamous cell carcinoma; MIR155HG, MIR155 host gene; NC, negative control; shRNA, short hairpin RNA; miR, microRNA.
miR-155-5p directly targets SOX10 in LSCC cells
To further explore the mechanisms underlying the effects of miR-155-5p on the prolif eration, migration and invasion of LSCC cells, bioinformatics tools, such as TargetScan and miRwalk, were used to search for potential target genes of miR-155-5p. Among these target genes, we focused on SOX10, a tumor suppressor gene that is involved in suppressing tumorigenicity (22) and EMT (23). TargetScan prediction revealed that the 3′UTR of SOX10 contains a conserved binding site for miR-155-5p (Fig. 6A). The expression level of SOX10 was markedly decreased in the LSCC tissues compared with the corresponding adjacent non-tumor tissues (Fig. 6B). The expression level of miR-155-5p negatively correlated with the expression of SOX10 in LSCC (Fig. 6C). The downregulation of miR-155-5p by transfection with miR-155-5p inhibitor markedly increased the transcriptional level and protein expression of SOX10, while the overexpression of miR-155-5p significantly decreased the transcriptional level and protein expression of SOX10 in LSCC cell lines (Fig. 6D and E). To confirm whether the SOX10 mRNA expression is regulated by miR-155-5p through direct binding to the 3′UTR of SOX10, a dual luciferase reporter assay was performed in the TU177 and 293T cells. The luciferase signal was reduced in the miR-155-5p mimic and pmirGLO-SOX10-3′UTR-wild-type co-transfected cells compared with the control group. However, co-transfection of miR-155 mimic and pmirGLO-SOX10-3′UTR-mutant-type did not reduce the luciferase signal compared with the control group. The luciferase signal was increased in the miR-155-5p inhibitor and pmirGLO-SOX10-3′UTR-wild-type, but not the mutant-type, co-transfected cells compared with the control group (Fig. 6F).
Figure 6.
miR-155-5p directly targets SOX10 in LSCC cells. (A) Schematic of the potential binding sites of miR-155-5p on SOX10 3′UTR predicted by TargetScan and miRwark databases. (B) The relative expression of SOX10 in 45 pairs of LSCC tissues and the corresponding adjacent non-tumor tissues by RT-qPCR. (C) The correlation between miR-155-5p and SOX10 mRNA level in LSCC tissues. (D) The relative expression level of SOX10 mRNA following transfection with miR-155-5p mimic and inhibitor in TU177 and AMC-HN-8 cells by RT-qPCR. (E) The relative protein level of SOX10 following transfection with miR-155-5p mimic and inhibitor in TU177 and AMC-HN-8 cells by western blot analysis. (F) Luciferase assays of TU177 cell and 293T cell transfected with pmirGLO-SOX10-WT or pmirGLO-MIR31HG-MUT and NC or miR-155-5p mimic or miR-155-5p inhibitor. Data are shown as the means ± SD; *P<0.05 and **P<0.01 vs. NC group. LSCC, laryngeal squamous cell carcinoma; NC, negative control; MIR155HG, MIR155 host gene; miR, microRNA; 3′ untranslated regions, 3′UTR; SOX10, SRY related-HMG-box 10; WT, wild-type; Mut, mutant type.
MIR155HG and miR-155-5p are upregulated in TGF-β-induced TU177 cells and synergistically contribute to the process of EMT
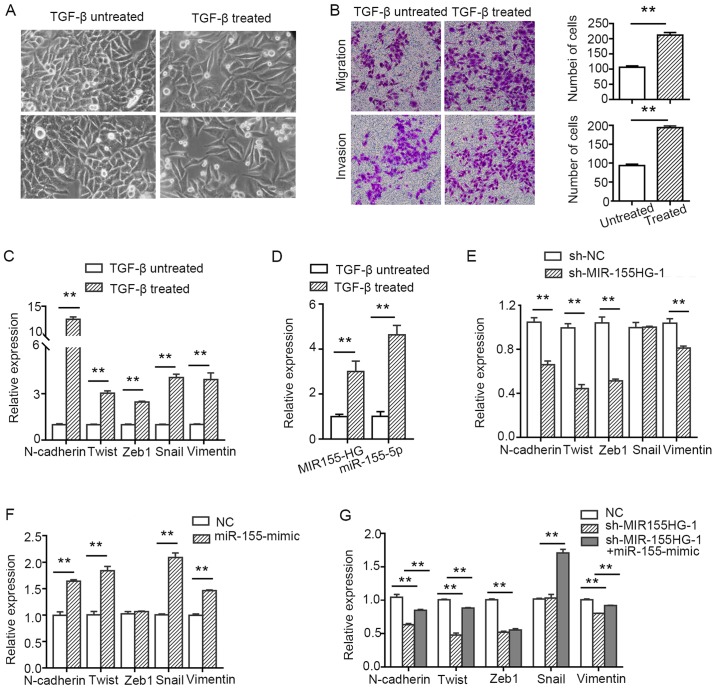
To investigate whether MIR155HG and miR-155-5p regulate the migration and invasion of LSCC cells via EMT, the TU177 cells were treated with 10 ng/ml of TGF-β for 7 days. The cells treated with TGF-β exhibited a change in morphology and acquired a spindle-shaped morphology (Fig. 7A). Furthermore, their migratory and invasive abilities were enhanced compared with the untreated cells (Fig. 7B). Subsequently, the molecular markers of EMT were analyzed by RT-qPCR. The levels of the mesenchymal markers, N-cadherin, Twist, Zeb1, Snail and vimentin, were found to be upregulated. However, we did not detect the expression of the epithelial marker, E-cadherin (after using several common primers of E-cadherin), possibly due to the fact that the expression abundance was too low to be monitored (Fig. 7C). These results suggested that the TU177 cells treated with TGF-β exhibited EMT-related characteristics. Moreover, to further determine whether MIR155HG and miR-155-5p were involved in TGF-β-induced EMT, their expression was detected by RT-qPCR analysis. The results indicated that the levels of MIR155HG and miR-155-5p were upregulated after the TU177 cells were treated with TGF-β (Fig. 7D). The knockdown of MIR155HG inhibited the expression of the mesenchymal markers, N-cadherin, vimentin, Twist and Zeb1, but did not affect the expression of Snail (Fig. 7E). miR-155-5p overexpression promoted the expression of the mesenchymal markers, N-cadherin, vimentin, Twist and Snail, but did not increase the expression of Zeb1 (Fig. 7F), whereas it partially reversed the inhibitory effect exerted by MIR155HG knockdown (Fig. 7G). Taken together, the above-mentioned results indicate that MIR155HG and miR-155-5p are EMT-related non-coding RNAs, and they synergistically promote EMT in LSCC cells.
Figure 7.
MIR155HG and miR-155-5p are upregulated in TGF-β-induced TU177 cell and contribute to the EMT process synergistically. (A) Cell morphology of TU177 cell treated or untreated with TGF-β for 7 days. (B) The migration and invasion assays of TU177 cell treated or untreated with TGF-β (magnification, ×200). **P<0.01 vs. untreated group (C) The relative expression of mesenchymal related markers following induction by TGF-β in TU177 cells by RT-qPCR. **P<0.01 vs. untreated group. (D) The relative expression of MIR155HG and miR-155-5p following induction by TGF-β in TU177 cells by RT-qPCR. **P<0.01 vs. untreated group. (E) The expression of mesenchymal-related markers following transfection with sh-MIR155HG by RT-qPCR. **P<0.01 vs. NC group. (F) The relative expression of mesenchymal-related markers following transfection with miR-155-5p mimic by RT-qPCR. **P<0.01 vs. NC group. (G) The relative expression of mesenchymal-related markers by RT-qPCR following co-transfection with sh-MIR155HG and miR-155-mimic. (sh-MIR155HG-1 group vs. NC group; co-transfected group vs. sh-MIR155HG-1 group). Data are shown as the means ± SD; MIR155HG, MIR155 host gene; miR, microRNA; NC, negative control; shRNA, short hairpin RNA; TGF-β, transforming growth factor-β; EMT, epithelial-mesenchymal transition.
Discussion
In recent years, non-coding RNAs, particularly lncRNAs and miRNAs, have been found to play important roles in the development and progression of tumors, including LSCC. In our study, the differential expression of lncRNAs was detected by microarray assays, and MIR155HG was found to be highly expressed in LSCC tissues. MIR155HG, also referred to as B-cell integration cluster (BIC), was first identified as a novel gene that was transcriptionally activated in avian leukosis virus-induced lymphomas by the insertion of retrovirus integration sites in the promoter region (24). MIR155HG has also been found to be upregulated in pediatric Hodgkin’s lymphoma and Burkitt’s lymphoma (25-27). The transcription of MIR155HG is regulated by multiple transcription factors, such as MYB, NF-ĸB and AP-1 (28-30). A recent study reported that MIR155HG acts as an oncogene in glioma, and is associated with prognosis and tumor progression (17). In the present study, the expression of MIR155HG was found to be upregulated in LSCC tissues, suggesting that MIR155HG is a potential oncogene in LSCC. Moreover, the high expression level of MIR155HG was associated with a poor differentiation, lymph node metastasis and a higher TNM stage, suggesting that MIR155HG may be of clinical value in the assessment of invasion and metastasis, malignant behavior and prognosis of laryngeal carcinoma. Furthermore, in vitro and in vivo assays verified the oncogenic role of MIR155HG in LSCC.
It is well known that lncRNAs exert their effects through a variety of mechanisms, including serving as the host genes and regulating the expression of miRNAs (14). miR-155 is located in the third exon of MIR155HG, and has been reported to play important roles in a number of solid malignancies (31-33). The overexpression of miR-155 has been found to promote cell proliferation and migration through targeting TGFβR2 in gastric cancer (31). miR-155-5p has also been found to inhibit the migration and invasion of colorectal cancer cells by targeting CTHRC1 (32). miR-155-5p has been found to be upregulated in oral squamous cell carcinoma and to be associated with metastasis, poor prognosis and EMT progression (34,35). The aberrant expression of miR-155 has also been previously observed in LSCC (36-38). miR-155 acts as an oncogene in LSCC, promoting the growth, migration and invasion of LSCC cells by regulating SOSC1 and STAT3 (37). Another study demonstrated that miR-155 was highly expressed in the plasma and tissues of patients with LSCC (38). However, the correlation between miR-155 and its host gene, MIR155HG, in LSCC has not yet been elucidated, and the other target genes of miR-155 require further investigation. The present study demonstrated that the expression of miR-155-5p was upregulated in LSCC tissues, and that the overexpression of miR-155-5p significantly promoted the growth, migration and invasion of LSCC cells. Furthermore, the expression level of miR-155-5p was found to be associated with the malignant phenotype of LSCC, which was consistent with the findings on MIR155HG. Although a positive correlation between the expression level of MIR155HG and miR-155-5p was detected, and MIR155HG may regulate the expression of miR-155-5p, miR-155-5p did not affect the expression of MIR155HG, suggesting that the transcriptional activity of miR-155 is under the control of MIR155HG, which is consistent with the findings of other studies on Hodgkin’s lymphoma, Burkitt’s lymphoma and glioma (17,25,26). Furthermore, the upregulation of miR-155-5p reversed the inhibitory effects of MIR155HG knockdown on cell malignant biological properties, suggesting that these two non-coding RNAs synergistically control the same biological processes in LSCC.
SOX10, a member of the SOX family, has been found to be a target gene of miR-155-5p. SOX10 plays an important role in the formation of the neural crest and peripheral nervous system, the maturation and differentiation of Schwann and oligodendrocyte lineage cells, and the occurrence and development of tumors (39,40). In recent years, the bidirectional role of SOX10 in regulating tumor progression has been gradually revealed. For example, SOX10 has been reported to inhibit the growth and metastasis of digestive tumors via inhibiting the Wnt/β-catenin pathway (22). However, SOX10 has been found to be highly expressed in nasopharyngeal carcinoma, whereas SOX10 knockdown has been shown to markedly inhibit nasopharyngeal carcinoma cell proliferation, migration, invasion and EMT (23,41). SOX10 acts as an oncogene in hepatocellular carcinoma by activating Wnt/β-catenin signaling (42). In the present study, SOX10 was found to be downregulated in LSCC tissues. The overexpression of miR-155-5p reduced the transcriptional and translational levels of SOX10, whereas miR-155-5p downregulation exerted the opposite effects. Moreover, the direct target association between miR-155-5p and SOX10 was proven by a dual-luciferase reporter assay. These results indicate that SOX10 is a target gene of miR-155-5p, and that miR-155-5p exerts oncogenic effects partly via the regulation of SOX10.
EMT is an important biological process for malignant tumor cells, characterized by epithelial cells acquiring mesenchymal characteristics and, thereby, the ability of migration and invasion, which is a prerequisite for the initiation of tumor invasion and metastasis (43). Numerous studies have demonstrated that lncRNAs induced by TGF-β play important roles in EMT in different types of cancer (44-46). lncRNA ATB (lncRNA-activated by TGF-β), a regulator and mediator of the TGF-β signaling pathway, has been found to play a key role in inducing EMT and promoting invasion and metastasis in hepatocellular carcinoma (44). LINC01186, a downregulated lncRNA in TGF-β-treated lung cancer cells, has been shown to be regulated by TGF-β/SMAD3 and to inhibit the malignant biological behavior of lung cancer by regulating EMT (46). However, the involvement of lncRNAs in the process of EMT in LSCC is poorly understood. For this purpose, in this study, the TU177 cells were treated with TGF-β for the indicated number of days. The treated cells exhibited a change in morphology to a spindle-shaped one, and their migratory and invasive abilities were enhanced compared with the untreated cells. Moreover, the expression levels of mesenchymal markers were upregulated. However, we could not detect the expression of the epithelial marker, E-cadherin, possibly since the increase in its expression was too low to be monitored. The expression levels of MIR155HG and miR-155-5p were also upregulated in the TGF-β-treated group, which indicated that MIR155HG and miR-155-5p are TGF-β-induced non-coding RNAs. Consistent with our conclusions, MIR155HG has been reported to be associated with mesenchymal transition in glioma (17), and miR-155 is an EMT-related onco-miRNA (47,48) that has been found to be induced by stimulation of TGF-β in hepatocellular carcinoma cells (49). Further experiments indicated that MIR155HG and miR-155-5p may synergistically affect the expression of EMT-related markers, thereby promoting EMT. As stated above, SOX10 contributes to EMT in nasopharyngeal carcinoma. Therefore, we hypothesized that SOX10 may promote EMT in LSCC. All the above-mentioned results indicate that MIR155HG and miR-155-5p are EMT-related non-coding RNAs, and that MIR155HG may promote EMT in LSCC cells by regulating the miR-155-5p/SOX10 axis.
In conclusion, the present study is, to the best of our knowledge, the first to confirm the functional promoting role of MIR155HG and its co-expression with miR-155-5p in LSCC tumorigenesis. miR-155-5p acts synergistically with MIR155HG to promote the progression of LSCC, partly by regulating the downstream target gene, SOX10. Moreover, the findings of the present study indicate a novel role of MIR155HG in TGF-β-induced EMT of LSCC cells by regulating EMT marker expression through the miR-155/SOX10 axis. In addition, these findings indicate a novel mechanism underlying the aggressive biological behavior of LSCC, and the MIR155HG/miR-155-5p/SOX10 axis may represent a promising therapeutic target for patients with LSCC.
Supplementary Materials
Acknowledgments
Not applicable.
Abbreviations
- LSCC
laryngeal squamous cell carcinoma
- lncRNA
long non-coding RNA
- MIR155HG
miR-155 host gene
- TGF-β
transforming growth factor-β
- EMT
epithelial-to-mesenchymal transition
- SOX10
SRY-related-HMG-box 10
- 3′UTR
3′ untranslated region
Funding
The study was supported by grants from the Key Program of Hebei Natural Science Foundation (no. H2017206391) and the Project of Clinical Medical TalentTraining and Basic Project Research Funded by Government (Hebei finance society (2017) no. 46).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
BW conceived and designed the study, modified the manuscript; WCu collected and analysed the data, designed and perform the experiments, drafted the manuscript; WM, LZ, WCh and HC performed the experiments and were involved in the revise of manuscript; All authors participant in revising manuscript, and agree with the final manuscript.
Ethics approval and consent to participate
The use of human tissues specimen was approved by and carried out according to the guidelines of the Ethics Committee of the Second Hospital of Hebei Medical University (Hebei, China) and written informed consent was obtained from all patients. All animal experiments were performed at the Experimental Animal Center of the Fourth Hospital of Hebei Medical University according to the NIH guidelines, and were approved by the Institutional Animal Care and Use Committee of the Fourth Hospital of Hebei Medical University.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Yu Q, Zhang X, Ji C, Yang H, Gao M, Hong S, Hu G. Survival analysis of laryngeal carcinoma without laryngectomy, radiotherapy, or chemotherapy. Eur Arch Otorhinolaryngol. 2012;269:2103–2109. doi: 10.1007/s00405-011-1873-7. [DOI] [PubMed] [Google Scholar]
- 3.Groome PA, O’Sullivan B, Irish JC, Rothwell DM, Schulze K, Warde PR, Schneider KM, Mackenzie RG, Hodson DI, Hammond JA, et al. Management and outcome differences in supraglottic cancer between Ontario, Canada, and the Surveillance, Epidemiology, and End Results areas of the United States. J Clin Oncol. 2003;21:496–505. doi: 10.1200/JCO.2003.10.106. [DOI] [PubMed] [Google Scholar]
- 4.Kaikkonen MU, Adelman K. Emerging roles of non-coding RNA transcription. Trends Biochem Sci. 2018;43:654–667. doi: 10.1016/j.tibs.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mou K, Liu B, Ding M, Mu X, Han D, Zhou Y, Wang LJ. lncRNA-ATB functions as a competing endogenous RNA to promote YAP1 by sponging miR-590-5p in malignant melanoma. Int J Oncol. 2018;53:1094–1104. doi: 10.3892/ijo.2018.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Zhao X, Yang B, Li Y, Liu T, Pang L, Fan Z, Ma W, Liu Z, Li Z. Long non-coding RNA HOXD-AS1 promotes tumor progression and predicts poor prognosis in colorectal cancer. Int J Oncol. 2018;53:21–32. doi: 10.3892/ijo.2018.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu Wu T, He L, Tian G, Li L, Zhou L, Jin H, Ren Q, Wang J, Wang YJ, et al. Regulation of laryngeal squamous cell cancer progression by the lncRNA H19/miR-148a-3p/DNMT1 axis. Oncotarget. 2016;7:11553–11566. doi: 10.18632/oncotarget.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng J, Xiao X, Wu C, Huang J, Zhang Y, Xie M, Zhang M, Zhou L. The role of long non-coding RNA HOTAIR in the progression and development of laryngeal squamous cell carcinoma interacting with EZH2. Acta Otolaryngol. 2017;137:90–98. doi: 10.1080/00016489.2016.1214982. [DOI] [PubMed] [Google Scholar]
- 11.Li D, Feng J, Wu T, Wang Y, Sun Y, Ren J, Liu M. Long intergenic noncoding RNA HOTAIR is overexpressed and regulates PTEN methylation in laryngeal squamous cell carcinoma. Am J Pathol. 2013;182:64–70. doi: 10.1016/j.ajpath.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 12.Wang P, Wu T, Zhou H, Jin Q, He G, Yu H, Xuan L, Wang X, Tian L, Sun Y, et al. Long noncoding RNA NEAT1 promotes laryngeal squamous cell cancer through regulating miR-107/CDK6 pathway. J Exp Clin Cancer Res. 2016;35:22. doi: 10.1186/s13046-016-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Wang X, Cao S, Han X, Wang Z, Zhao X, Liu X, Li G, Pan X, Lei D. The long noncoding RNA TUG1 promotes laryngeal cancer proliferation and migration. Cell Physiol Biochem. 2018;49:2511–2520. doi: 10.1159/000493876. [DOI] [PubMed] [Google Scholar]
- 14.Dhir A, Dhir S, Proudfoot NJ, Jopling CL. Microprocessor mediates transcriptional termination of long noncoding RNA transcripts hosting microRNAs. Nat Struct Mol Biol. 2015;22:319–327. doi: 10.1038/nsmb.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Lu Y, Liu X, Li Q, Graves-Deal C, Cao R, Singh Z, Franklin B, Wang JL, Hu JH, et al. lncRNA MIR100HG-derived miR-100 and miR-125b mediate cetuximab resistance via Wnt/beta-catenin signaling. Nat Med. 2017;23:1331–1341. doi: 10.1038/nm.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai P, Li H, Huo W, Zhu H, Xu C, Zang R, Lv W, Xia Y, Tang W. Aberrant expression of LncRNA-MIR31HG regulates cell migration and proliferation by affecting miR-31 and miR-31* in Hirschsprung’s disease. J Cell Biochem. 2018;119:8195–8203. doi: 10.1002/jcb.26830. [DOI] [PubMed] [Google Scholar]
- 17.Wang Wu X, Yu Y, Nie T, Hu E, Wu Q, Zhi W, Jiang T, Wang K, Lu XX, et al. Blocking MIR155HG/miR-155 axis inhibits mesenchymal transition in glioma. Neuro Oncol. 2017;19:1195–1205. doi: 10.1093/neuonc/nox017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazono K, Ehata S, Koinuma D. Tumor-promoting functions of transforming growth factor-β in progression of cancer. Ups J Med Sci. 2012;117:143–152. doi: 10.3109/03009734.2011.638729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian L, Li M, Ge J, Guo Y, Sun Y, Liu M, Xiao H. MiR-203 is downregulated in laryngeal squamous cell carcinoma and can suppress proliferation and induce apoptosis of tumours. Tumour Biol. 2014;35:5953–5963. doi: 10.1007/s13277-014-1790-7. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Lv K, Chen W, Zhao J, Luo J, Wu J, Li Z, Qin H, Wong TS, Yang W, et al. miR-375 and miR-205 regulate the invasion and migration of laryngeal squamous cell carcinoma synergistically via AKT-mediated EMT. Biomed Res Int. 2016;2016:9652789. doi: 10.1155/2016/9652789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo H, Jiang Y, Ma S, Chang H, Yi C, Cao H, Gao Y, Guo H, Hou J, Yan J, et al. EZH2 promotes invasion and metastasis of laryngeal squamous cells carcinoma via epithelial-mesen-chymal transition through H3K27me3. Biochem Biophys Res Commun. 2016;479:253–259. doi: 10.1016/j.bbrc.2016.09.055. [DOI] [PubMed] [Google Scholar]
- 22.Tong X, Li L, Li X, Heng L, Zhong L, Su X, Rong R, Hu S, Liu W, Jia B, et al. SOX10, a novel HMG-box-containing tumor suppressor, inhibits growth and metastasis of digestive cancers by suppressing the Wnt/β-catenin pathway. Oncotarget. 2014;5:10571–10583. doi: 10.18632/oncotarget.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He P, Jin X. SOX10 induces epithelial-mesenchymal transition and contributes to nasopharyngeal carcinoma progression. Biochem Cell Biol. 2018;96:326–331. doi: 10.1139/bcb-2017-0160. [DOI] [PubMed] [Google Scholar]
- 24.Tam W, Ben-Yehuda D, Hayward WS. bic, a novel gene activated by proviral insertions in avian leukosis virus-induced lymphomas, is likely to function through its noncoding RNA. Mol Cell Biol. 1997;17:1490–1502. doi: 10.1128/MCB.17.3.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kluiver J, Poppema S, de Jong D, Blokzijl T, Harms G, Jacobs S, Kroesen BJ, van den Berg A. BIC and miR-155 are highly expressed in Hodgkin, primary mediastinal and diffuse large B cell lymphomas. J Pathol. 2005;207:243–249. doi: 10.1002/path.1825. [DOI] [PubMed] [Google Scholar]
- 26.Metzler M, Wilda M, Busch K, Viehmann S, Borkhardt A. High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer. 2004;39:167–169. doi: 10.1002/gcc.10316. [DOI] [PubMed] [Google Scholar]
- 27.van den Berg A, Kroesen BJ, Kooistra K, de Jong D, Briggs J, Blokzijl T, Jacobs S, Kluiver J, Diepstra A, Maggio E, et al. High expression of B-cell receptor inducible gene BIC in all subtypes of Hodgkin lymphoma. Genes Chromosomes Cancer. 2003;37:20–28. doi: 10.1002/gcc.10186. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RC, Vardinogiannis I, Gilmore TD. Identification of an NF-κB p50/p65-responsive site in the human MIR155HG promoter. BMC Mol Biol. 2013;14:24. doi: 10.1186/1471-2199-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vargova K, Curik N, Burda P, Basova P, Kulvait V, Pospisil V, Savvulidi F, Kokavec J, Necas E, Berkova A, et al. MYB transcriptionally regulates the miR-155 host gene in chronic lymphocytic leukemia. Blood. 2011;117:3816–3825. doi: 10.1182/blood-2010-05-285064. [DOI] [PubMed] [Google Scholar]
- 30.Elton TS, Selemon H, Elton SM, Parinandi NL. Regulation of the MIR155 host gene in physiological and pathological processes. Gene. 2013;532:1–12. doi: 10.1016/j.gene.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Qu Y, Zhang H, Sun W. MicroRNA-155 promotes gastric cancer growth and invasion by negatively regulating transforming growth factor-beta receptor 2. 2018;109:618–628. doi: 10.1111/cas.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Chen Z, Xiang J, Gu X. MicroRNA-155 acts as a tumor suppressor in colorectal cancer by targeting CTHRC1 in vitro. Oncol Lett. 2018;15:5561–5568. doi: 10.3892/ol.2018.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin J, Chen Y, Liu L, Shen A, Zheng W. MicroRNA-155-5p suppresses the migration and invasion of lung adenocarcinoma A549 cells by targeting Smad2. Oncol Lett. 2018;16:2444–2452. doi: 10.3892/ol.2018.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baba O, Hasegawa S, Nagai H, Uchida F, Yamatoji M, Kanno NI, Yamagata K, Sakai S, Yanagawa T, Bukawa H. MicroRNA-155-5p is associated with oral squamous cell carcinoma metastasis and poor prognosis. J Oral Pathol Med. 2016;45:248–55. doi: 10.1111/jop.12351. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Yang JM, Ahn SH, Jeong WJ, Chung JH, Paik JH. Potential oncogenic role and prognostic implication of MicroRNA-155-5p in oral squamous cell carcinoma. Anticancer Res. 2018;38:5193–5200. doi: 10.21873/anticanres.12842. [DOI] [PubMed] [Google Scholar]
- 36.Zhao X, Zhang W, Ji W. YB-1 promotes laryngeal squamous cell carcinoma progression by inducing miR-155 expression via c-Myb. Future Oncol. 2018;14:1579–1589. doi: 10.2217/fon-2018-0058. [DOI] [PubMed] [Google Scholar]
- 37.Zhao XD, Zhang W, Liang HJ, Ji WY. Overexpression of miR - 155 promotes proliferation and invasion of human laryngeal squamous cell carcinoma via targeting SOCS1 and STAT3. PLoS One. 2013;8:e56395. doi: 10.1371/journal.pone.0056395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang JL, Wang X, Yang D, Shi WJ. The Expression of MicroRNA-155 in plasma and tissue is matched in human laryngeal squamous cell carcinoma. Yonsei Med J. 2016;57:298–305. doi: 10.3349/ymj.2016.57.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong CS, Saint-Jeannet JP. Sox proteins and neural crest development. Semin Cell Dev Biol. 2005;16:694–703. doi: 10.1016/j.semcdb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Mokhtarzadeh Khanghahi A, Satarian L, Deng W, Baharvand H, Javan M. In vivo conversion of astrocytes into oligodendrocyte lineage cells with transcription factor Sox10; Promise for myelin repair in multiple sclerosis. PLoS One. 2018;13:e0203785. doi: 10.1371/journal.pone.0203785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Liu ZG, Tang J, Zou RF, Chen XY, Jiang GM, Qiu YF, Wang H. High expression of Sox10 correlates with tumor aggressiveness and poor prognosis in human nasopharyngeal carcinoma. OncoTargets Ther. 2016;9:1671–1677. doi: 10.2147/OTT.S101344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou D, Bai F, Zhang X, Hu M, Zhao G, Zhao Z, Liu R. SOX10 is a novel oncogene in hepatocellular carcinoma through Wnt/β-catenin/TCF4 cascade. Tumour Biol. 2014;35:9935–9940. doi: 10.1007/s13277-014-1893-1. [DOI] [PubMed] [Google Scholar]
- 43.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 44.Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepato-cellular carcinoma. Cancer Cell. 2014;25:666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Lu Z, Li Y, Che Y, Huang J, Sun S, Mao S, Lei Y, Li N, Sun N, He J. The TGFβ-induced lncRNA TBILA promotes non-small cell lung cancer progression in vitro and in vivo via cis-regulating HGAL and activating S100A7/JAB1 signaling. Cancer Lett. 2018;432:156–168. doi: 10.1016/j.canlet.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 46.Hao Y, Yang X, Zhang D, Luo J, Chen R. Long noncoding RNA LINC01186, regulated by TGF-β/SMAD3, inhibits migration and invasion through Epithelial-Mesenchymal-Transition in lung cancer. Gene. 2017;608:1–12. doi: 10.1016/j.gene.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 47.Johansson J, Berg T, Kurzejamska E, Pang MF, Tabor V, Jansson M, Roswall P, Pietras K, Sund M, Religa P, et al. MiR-155-mediated loss of C/EBPβ shifts the TGF-β response from growth inhibition to epithelial-mesenchymal transition, invasion and metastasis in breast cancer. Oncogene. 2013;32:5614–5624. doi: 10.1038/onc.2013.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li DP, Fan J, Wu YJ, Xie YF, Zha JM, Zhou XM. MiR-155 up-regulated by TGF-β promotes epithelial-mesenchymal transition, invasion and metastasis of human hepatocellular carcinoma cells in vitro. Am J Transl Res. 2017;9:2956–2965. [PMC free article] [PubMed] [Google Scholar]
- 49.Kong X, Liu F, Gao J. MiR-155 promotes epithelial-mesenchymal transition in hepatocellular carcinoma cells through the activation of PI3K/SGK3/β-catenin signaling pathways. Oncotarget. 2016;7:66051–66060. doi: 10.18632/oncotarget.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.