Abstract
In the present study, we evaluated the mechanisms of programmed death ligand 1 (PD-L1) expression in the breast cancer microenvironment, focusing on the role of interferon-γ (IFN-γ), and the clinical indications for anti-programmed cell death 1 (PD-1) /anti-PD-L1 immunotherapy. We evaluated PD-L1 expression in 4 breast cancer cell lines in the presence of 3 types of inhibitors, as well as IFN-γ. The expression of phosphorylated signal transducer and activator of transcription 1 (p-STAT1), one of the IFN-γ signaling pathway molecules, was analyzed using immunohistochemistry (IHC) in relation to PD-L1 and human leukocyte antigen (HLA) class I expression on cancer cells and tumor-infiltrating CD8-positive T cells in 111 patients with stage II/III breast cancer. Using The Cancer Genome Atlas (TCGA) database, the correlation of the IFN-γ signature with PD-L1 expression was analyzed in breast invasive carcinoma tissues. As a result, the JAK/STAT pathway via IFN-γ was mainly involved in PD-L1 expression in the cell lines examined. IHC analysis revealed that the PD-L1 and HLA class I expression levels were significantly upregulated in the p-STAT1-positive cases. TCGA analysis indicated that the PD-L1 expression and IFN-γ signature exhibited a positive correlation. On the whole, these findings suggest that PD-L1 and HLA class I are co-expressed in p-STAT1-positive breast cancer cells induced by IFN-γ secreted from tumor infiltrating immune cells, and that p-STAT1 expression may be a potential biomarker for patient selection for immunotherapy with anti-PD-1/anti-PD-L1 monoclonal antibodies.
Keywords: breast cancer, phosphorylated signal transducer and activator of transcription 1, programmed death-ligand 1, human leukocyte antigen class I, immune checkpoint inhibitors
Introduction
Breast cancer is the most common type cancer affecting women, and has an increasing worldwide incidence (1,2). Breast cancer is divided into 5 subtypes, according to immunohistological and genetic characteristics (3), and the selection of therapeutic interventions, such as surgery, radiotherapy, hormonal therapy, chemotherapy, molecular-targeted therapy or combinations thereof, are dependent on these subtypes (4). However, for aggressive breast cancer phenotypes, such as triple-negative breast cancer, the improvement of treatment strategies and the development of novel therapies are warranted for more effective treatment.
Recently, the importance of immune checkpoints in the tumor microenvironment has been elucidated, and the anti-tumor effect of immune checkpoint inhibitors (ICIs) has been reported in various types of cancer (5). Anti-programmed cell death 1 (PD-1)/anti-programmed death ligand 1 (PD-L1) monoclonal antibodies (mAbs) have been shown to be effective in several types of cancer, including lung, gastric and kidney cancer (6-8). In addition, the outcomes of several clinical trials with ICIs for patients with breast cancer have been reported (9-11). As such, issues regarding patient selection and biomarkers to predict responses to ICIs in breast cancer patients remain debatable, and urgently need to be clarified (9-11).
It has recently been reported that conditions within the tumor microenvironment that are suitable for immunotherapy with anti-PD-1/anti-PD-L1 mAbs include the following: The presence of cytotoxic T lymphocytes (CTLs), the expression of human leukocyte antigen (HLA) class I on the tumor cells, and a significant load of neo-antigens and the expression of PD-L1 on tumor cells (12-15). Previously, we reported that HLA class I and PD-L1 were mainly upregulated by interferon-γ (IFN-γ) produced by CD8-positive T lymphocytes within the tumor microenvironment in gastric cancer (14). In the present study, using breast cancer cell lines and clinical samples, we investigated the mechanisms through which PD-L1 expression is regulated within the tumor microenvironment in breast cancer, paying particular attention to IFN-γ regulation, and discussed the clinical implications of anti-PD-1/anti-PD-L1 mAbs in breast cancer patients.
Materials and methods
Breast cancer cell lines and in vitro drug treatment
A total of 4 breast cancer cell lines, MRK-nu-1, BT-549, MCF-7 and MDA-MB-231, were used in the current study. MRK-nu-1 was purchased from the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan). The BT-549, MCF-7 and MDA-MB-231 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). All the cell lines, which confirmed the absence of mycoplasma, were cultured in RPMI-1640 containing L-glutamine (Invitrogen/Thermo Fisher Scientific, Waltham, MA, USA) with 5% fetal calf serum (Invitrogen/Thermo Fisher Scientific) and 1% penicillin-streptomycin (Sigma-Aldrich, St. Louis, MO, USA), and were verified as authentic through a short tandem repeat analysis. The following reagents were used in in vitro drug treatment: IFN-γ (R&D Systems, Minneapolis, MN, USA), the ras/mitogen-activated protein kinase (MAPK) inhibitor, PD98059 (Cell Signaling Technology, Danvers, MA, USA), the phosphatidylinositol-3-kinase-protein kinase B (PI3K/AKT) inhibitor, wortmannin (Cell Signaling Technology), and lapatinib (GlaxoSmithKline, Brentford, UK). Lapatinib has been reported to be a combined epidermal growth factor receptor/human epidermal growth factor receptor 2 tyrosine kinase inhibitor and can inhibit both the MAPK and PI3K/AKT pathways (13). DMSO (Sigma-Aldrich) was used as a vehicle and a negative control for all treatments, and the cells were cultured and treated in 12-well plates (Thermo Fisher Scientific).
Clinical samples
Surgically-resected specimens were obtained from 111 patients who had undergone surgery for breast cancer at the First Department of Surgery at Yamanashi University (Yamanashi, Japan) between 2010 and 2014. Patients with stage II or III disease who required adjuvant chemotherapy were enrolled into the study. No patients had received pre-operative anti-tumor therapies, such as radiotherapy or chemotherapy. Clinical and pathological information were retrospectively obtained by reviewing the medical records. Tumor grade and stage were defined in accordance with the UICC TNM classification (7th edition) (16), and the histological classification was defined in accordance with the criteria of the Japanese Breast Cancer Society (17th edition) (17). This study was conducted in accordance with the Declaration of Helsinki, and was approved by the Institutional Ethical Committee of Yamanashi University (Reference 1622). Written informed consent was obtained from all participants.
Flow cytometry
The cells were stained with antibodies as previously described (13), and the following antibodies were used for staining: Annexin V-FITC (556420; BD Biosciences, San Jose, CA, USA) at 1:20, 7-Aminoactinomycin D (559925; BD Biosciences) at 1:20, and anti-human CD274 (PD-L1) PE (12-5983-42; eBioscience, Santa Clara, CA, USA) at 1:20. An isotype-matched immunoglobulin served as a negative control, and dead and/or apoptotic cells were excluded using Annexin V and 7-Aminoactinomycin D. Staining was detected using an LSRII flow cytometer (BD Biosciences).
Western blot analysis
All the samples were prepared, stained with the antibodies and visualized as previously described (18). The following primary antibodies were purchased from Cell Signaling Technology: STAT1 (14994S) at 1:1,000, p-STAT1 (8826S) at 1:1,000, p44/42 MAPK (ERK1/2) (4695S) at 1:1,000, phospho-p44/42 MAPK (p-ERK1/2) (4370S) at 1:2,000 and β-actin antibodies (4970S) at 1:2,000. A horseradish peroxidase-linked anti-rabbit IgG (7074; Cell Signaling Technology) at 1:2,000 was used as the secondary antibody.
Gene expression microarray analysis for drug-treated cell lines
The isolation of total RNA and microarray gene expression analysis were performed as previously described (14).
IHC staining
Four-micron-thick sections were deparaffinized and rehydrated. The slides were incubated with epitope retrieval solution (Agilent Technologies, Santa Clara, CA, USA) at varying conditions for different proteins: CD8, pH 9.0 for 20 min at 95-99°C in a water bath; p-STAT1, pH 6.0 for 10 min at 95-99°C in a water bath; HLA class I-A, B and C, pH 6.0 for 20 min at 121°C in an autoclave; PD-L1, pH 6.0 for 10 min at 110°C in an autoclave. All slides were incubated with peroxidase blocking solution (Agilent Technologies) for 10 min. Thereafter, the slides were incubated at 37°C for 60 min or 4°C overnight with the following primary antibodies: CD8 (clone C8/144B, M7103; Agilent Technologies) at 1:100; p-STAT1 (clone D3B7, 8826S; Cell Signaling Technology) at 1:800; HLA class I-A, B and C (clone EMR8-5, AB-46; Hokudo, Sapporo, Japan) at 1:200; and PD-L1 (clone 28-8, ab205921; Abcam, Cambridge, UK) at 1:400. Subsequently, for CD8, HLA class I and PD-L1, the slides were incubated with an avidinbiotinylated enzyme complex (Vector Laboratories, Burlingame, CA, USA), whereas the slides for p-STAT1 were incubated with a horseradish peroxidase-coupled anti-rabbit polymer (8114; Cell Signaling Technology), which was a ready-to-use solution. The slides were then incubated with diaminobenzidine (Agilent Technologies) at room temperature for 5 min and counterstained with Mayer’s hematoxylin solution, (131-09665; Wako/ Fujifilm, Tokyo, Japan) at room temperature for 1 min.
Assessment of IHC staining
IHC analysis was performed by two independent observers (TN and YN), who were blinded to all of the clinical data. For assessment of CD8, PD-L1, HLA class I and p-STAT1, hotspot areas of tumor infiltrating lymphocytes were reviewed in 4 randomly selected independent areas in marginal tumor regions at ×400 magnification. The expression levels were evaluated based on IHC staining in 4 independent areas. CD8 was defined as the number of stained lymphocytes, and calculated as the mean value of the 4 areas. p-STAT1 staining was evaluated via nuclei staining in the tumor cells and tumor infiltrating immune cells (TIICs). The HLA class I staining intensity was evaluated by the following criteria: Positive, evidenced by dark brown staining in >30% of membrane staining on tumor cells; and negative, evidenced by any lesser degree of brown staining of appreciable or non-appreciable staining on tumor cells. An H-score of membranous PD-L1 expression on the tumor cells was calculated as previously described (14).
Gene expression microarray analysis using The Cancer Genome Atlas (TCGA) dataset
We evaluated the mRNA expression levels of PD-L1 (CD274), the IFN-γ signature (19) and CD8 T effector gene signature (20) in breast invasive carcinoma tissues. The mRNA expression z-scores of genes, analyzed using the Illumina Genome Analyzer RNA Sequencing Version 2, were obtained from the TCGA breast invasive carcinoma tissue dataset through cBioPortal (http://www.cbioportal.org/) (21,22). The IFN-γ signature was calculated as the average expression level of 6 IFN-γ-related genes: indoleamine 2,3-dioxygenase 1 (IDO1), C-X-C motif chemokine ligand 10 (CXCL10), CXCL9, HLA-DRA, STAT1 and IFN-γ (19). The CD8 T effector gene signature was also calculated as the average expression level of 7 genes: CD8A, CD8B, eomesodermin (EOMES), granzyme A (GZMA), granzyme B (GZMB), IFN-γ and perforin 1 (PRF1) (20).
Statistical analysis
One-way analysis of variance followed by a Tukey’s post hoc test were performed to determine the significance of the flow cytometry results. Associations between the H-score of PD-L1 and the expression of p-STAT1 and HLA class I, as well as between the number of PD-L1-positive TIICs and p-STAT1 expression in TIICs, were also assessed using the Student’s t-test. Associations between p-STAT1 and HLA class I expression levels were assessed using the Chi-square test. Associations between the H-score of PD-L1 and the number of CD8-positive cells, between PD-L1 mRNA expression z-scores and the IFN-γ signature, and between PD-L1 mRNA expression z-scores and the CD8 T effector gene signature were assessed using a scatter diagram and Pearson’s product moment correlation coefficient. Analyses were performed using SPSS Statistics Package version 25 (IBM Corp., Armonk, NY, USA) and a value of P<0.05 was considered to indicate a statistically significant difference.
Results
Upregulation of PD-L1 by IFN-γ in breast cancer cell lines
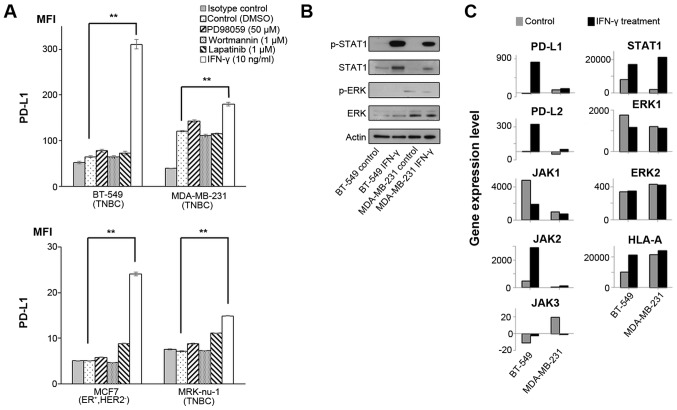
We have recently reported that PD-L1 expression is mainly regulated by IFN-γ via the JAK/STAT pathway, but not via the MAPK and PI3K/AKT pathways in esophageal squamous cell carcinoma cells and gastric cancer cells (14). In this study, in order to analyze PD-L1 expression in breast cancer cells, 4 breast cancer cell lines were treated with either IFN-γ (10 ng/ml), PD98059 (50 µM; MAPK inhibitor), wortmannin (1 µM; PI3K-AKT inhibitor), or lapatinib (1 µM) that can inhibit both the MAPK and PI3K/AKT pathways for 48 h (13). We assessed the optimal conditions of these reagents in previous studies (13,14). As a result, PD-L1 expression was significantly upregulated in all the tested cell lines only when treated with IFN-γ (Fig. 1A).
Figure 1.
Effects of IFN-γ on PD-L1 and signaling pathways. (A) PD-L1 expression was measured by flow cytometry in the cell lines at 48 h following treatment with DMSO, which was used as a vehicle control, 50 µM PD98059 (MAPK inhibitor), 1 µM wortmannin (PI3K-AKT inhibitor), 1 µM lapatinib (combined epidermal growth factor receptor/human epidermal growth factor receptor 2 tyrosine kinase inhibitor), and 10 ng/ml IFN-γ. Error bars represent the means ± SEM. **P<0.01 between the treated and control cells. (B) Western blot analysis against each protein in cell lines at 1 h following treatment without (control) or with 10 ng/ml IFN-γ. A representative result out of the 3 independent experiments is shown. (C) Relevant gene expression levels in cell lines treated without (control) or with 10 ng/ml IFN-γ. The y axes in all the bar graphs indicate the gene expression level. Gene expression levels were normalized to those of actin beta (ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), peptidylprolyl isomerase A (PPIA) and hypoxanthine phosphoribosyltransferase 1 (HPRT1).
Several previous studies have reported that IFN-γ can upregulate PD-L1 expression through the JAK/STAT and MAPK pathways in malignant tumors (23-25). Therefore, in this study, we assessed the effects of IFN-γ on both the JAK/STAT and MAPK pathways by western blot analysis and gene expression microarray analysis in the two breast cancer cell lines, BT-549 and MDA-MB-231. Western blot analysis revealed that IFN-γ treatment increased the p-STAT1 level, but not the p-ERK level in both cell lines (Fig. 1B). Since we performed a gene expression microarray analysis in both cell lines, we did not perform statistical analysis for these data. Gene expression microarray analysis also revealed that PD-L1, PD-L2, HLA-A and the JAK/STAT pathway (JAK2 and STAT1) were concomitantly increased by IFN-γ in both cell lines, although there was no increase in ERK1 and ERK2 expression levels (Fig. 1C and Table I), similar to the findings of our previous studies (14,18). Taken together, IFN-γ could induce the up-regulation of PD-L1 mainly through the JAK-STAT pathway in these breast cancer cell lines.
Table I.
Microarray analysis in untreated and IFN-γ-treated cell lines.
| Molecules | BT-549 cells
|
MDA-MB-231 cells
|
||
|---|---|---|---|---|
| Untreated | IFN-γ treatment | Untreated | IFN-γ treatment | |
| PD-L1 | −11.7 | 815.5 | 77.0 | 110.2 |
| PD-L2 | 2.8 | 331.0 | −28.5 | 27.1 |
| JAK1 | 4,755.1 | 1,898.8 | 991.6 | 725.5 |
| JAK2 | 483.1 | 2,893.8 | 54.7 | 132.3 |
| JAK3 | −11.4 | −2.5 | 19.4 | −1.3 |
| STAT1 | 7,945.8 | 17,080.4 | 2,062.7 | 21,414.4 |
| ERK1 | 1,751.8 | 1,168.5 | 1,205.7 | 1,126.4 |
| ERK2 | 337.5 | 348.6 | 427.4 | 420.7 |
| AKT1 | 3,942.4 | 3,620.0 | 3,554.2 | 3,387.5 |
| AKT2 | −6.8 | 45.7 | 47.9 | 36.2 |
| AKT3 | 30.0 | 32.3 | 38.9 | 19.5 |
| HLA-A | 10,130.7 | 21,337.2 | 21,600.1 | 24,005.1 |
| HLA-B | 4,876.9 | 18,634.7 | 16,104.7 | 34,904.7 |
| HLA-C | 543.5 | 1,476.6 | 275.3 | 999.9 |
| HLA-E | 5,622.3 | 23,216.8 | 7,399.6 | 20,547.7 |
| HLA-F | 1,024.7 | 5,301.0 | 6,858.1 | 15,147.7 |
| HLA-G | 202.8 | 790.5 | 1,438.5 | 3,585.4 |
| HLA-H | 3,053.5 | 9,808.8 | 14,781.6 | 21,520.7 |
| HLA-DPA1 | −33.2 | 444.8 | 4,823.8 | 20,231.7 |
| HLA-DPB1 | −21.4 | −25.8 | 57.4 | 592.6 |
| HLA-DPB2 | 24.4 | 2.0 | 1.9 | 25.6 |
| HLA-DQA1 | −48.9 | −21.7 | 11.9 | 1,474.5 |
| HLA-DQA2 | 15.9 | −4.0 | 2.1 | 5.8 |
| HLA-DQB1 | −3.3 | 2.4 | 86.4 | 1,452.1 |
| HLA-DQB2 | −4.1 | 31.8 | 26.0 | 16.2 |
| HLA-DRA | 114.4 | 1,398.5 | 10,456.1 | 37,182.3 |
| HLA-DRB1 | −19.9 | −2.3 | 29.4 | 78.0 |
| HLA-DRB3 | 977.7 | 951.9 | 2,582.4 | 8,289.0 |
| HLA-DRB4 | 129.8 | 127.0 | 2,279.9 | 7,899.3 |
| HLA-DRB5 | −9.3 | −17.3 | −13.3 | 3.4 |
| HLA-DRB6 | 25.3 | 66.2 | 2,246.7 | 9,342.0 |
| TAP1 | 7,741.5 | 35,696.9 | 5,333.0 | 32,228.8 |
| TAP2 | 1,172.4 | 4,463.4 | 1,381.3 | 4,233.3 |
| Tapasin | 2,660.3 | 4,533.1 | 2,592.0 | 5,062.1 |
| β-2 microglobulin | 19,602.0 | 24,741.4 | 17,045.4 | 24,486.9 |
| LMP2 | 496.3 | 2,226.0 | 532.9 | 4,351.8 |
| LMP7 | 1,303.3 | 4,801.6 | 1,593.7 | 8,038.8 |
| LMP10 | 4,038.4 | 15,703.9 | 5,087.5 | 17,694.3 |
| PA28a | 8,930.9 | 16,073.7 | 8,037.7 | 16,002.5 |
| PA28b | 20,159.3 | 34,904.7 | 10,156.0 | 25,767.6 |
| Calnexin | 1,895.6 | 1,647.5 | 2,059.7 | 2,221.7 |
| Calreticulin | 11,055.8 | 7,468.7 | 6,234.9 | 9,945.8 |
PD-L1 expression is upregulated in p-STAT1-positive tumors
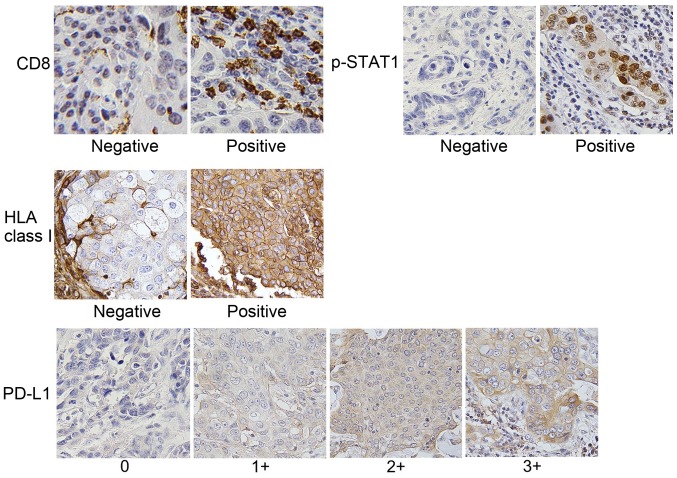
We then evaluated the expression levels of CD8, p-STAT1, HLA class I and PD-L1 by IHC using the specimens of 111 patients with breast cancer. The clinicopathological data are presented in Table II, and representative IHC staining for each molecule is shown in Fig. 2. Furthermore, representative IHC staining with serial sections for CD8, p-STAT1, HLA class I and PD-L1 is illustrated in Fig. 3A.
Table II.
Clinical characteristics of the patients with breast cancer (n=111).
| Characteristics | No. of patients |
|---|---|
| Age (years) | |
| Mean, 62.3±13.8 | |
| Range, 27-93 | |
| Primary tumora | |
| T1 | 30 |
| T2-T4 | 81 |
| Stage groupinga | |
| II | 88 |
| III | 23 |
| Molecular subtypes | |
| Luminal A likeb | 42 |
| Luminal B like | 34 |
| Luminal-HER2 | 7 |
| HER2 | 12 |
| TNBC | 16 |
| ER | |
| Positive | 83 |
| Negative | 28 |
| PgR | |
| Positive | 72 |
| Negative | 39 |
| HER2 | |
| Positive | 20 |
| Negative | 91 |
| Ki67 | |
| <30% | 68 |
| ≤30% | 43 |
| Nuclear gradea | |
| 1 | 24 |
| 2 | 45 |
| 3 | 42 |
| Histological classificationc | |
| Invasive ductal carcinoma (total) | 82 |
| Special (total) | 29 |
| Mucinous carcinoma | 4 |
| Medullary carcinoma | 1 |
| Invasive lobular carcinoma | 16 |
| Apocrine carcinoma | 2 |
| Spindle cell carcinoma | 2 |
| Invasive micropapillary carcinoma | 1 |
| Carcinoma with neuroendocrine features | 3 |
The grade of the tumor and stages were defined according to the UICC (TNM) classification.
Luminal A like were defined as ER+, PgR >20%, HER2− and Ki67 <30%.
The classification were defined according to the Japanese Breast Cancer Society (17th edition) (17). ER, estrogen receptor; PgR, progesterone receptor; HER2, human epidermal growth factor receptor 2.
Figure 2.
Representative immunohistochemical staining. Breast cancer tissues were evaluated by IHC (n=111). The CD8, p-STAT1 and HLA class I expression grades were scored as negative or positive. PD-L1 expression was scored as 0, 1+, 2+, and 3+. Original magnification, ×400.
Figure 3.
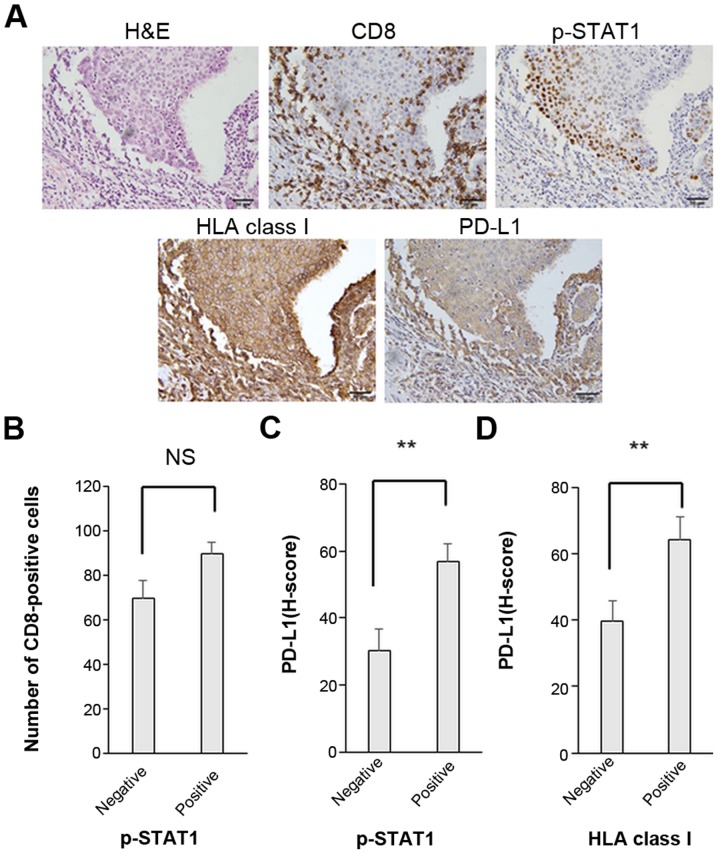
The expression levels of PD-L1 and HLA class I are significantly associated with p-STAT1 expression in breast cancer. (A) Representative immunohistochemical staining with hematoxylin and eosin (H&E), CD8-positive staining (CD8), p-STAT1 positive staining (p-STAT1), HLA class I-positive staining (HLA class I) and PD-L1 (3+) staining (PD-L1). Original magnification, ×400. Associations between (B) p-STAT1 expression in tumor cells and the number of CD8-positive cells around the tumor, (C) p-STAT1 expression and PD-L1 expression on tumor cells, and (D) between HLA class I and PD-L1 on tumor cells. The data were analyzed by the Student’s t-test. Error bars represent the means ± SEM. **P<0.01 between the negative and positive groups of each molecule. NS, not significant.
There were no significant associations or correlations observed between the number of tumor infiltrating CD8-positive T cells and the expression levels of p-STAT1 or PD-L1 (Figs. 3B and 4). Of note, in the p-STAT1-positive cases, the tumor cells exhibited significantly higher expression levels of both PD-L1 and HLA class I (P=0.003 and P=0.004, respectively) (Fig. 3C and Table III). Furthermore, in the HLA class I-positive cases, PD-L1 expression was significantly upregulated (P=0.007) (Fig. 3D). These in vivo results suggest that the PD-L1 and HLA class I expression levels are strongly related to the JAK/STAT pathway.
Figure 4.
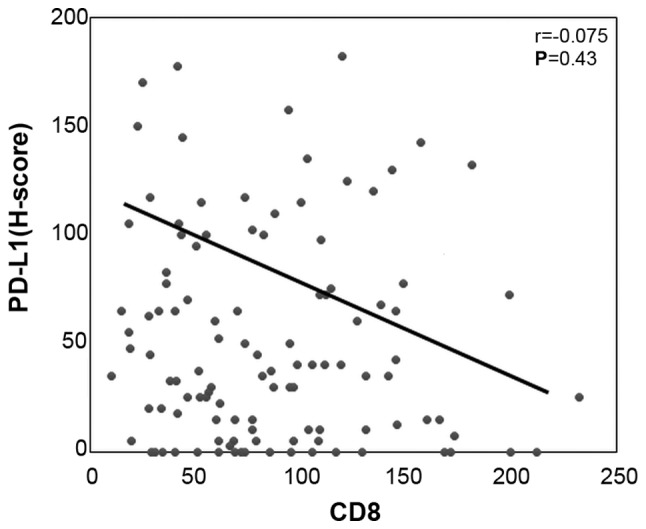
PD-L1 expression does not correlate with the infiltration of CD8-positive T cells. Correlation between the H-scores of PD-L1 in tumor cells and the number of CD8-positive T cells around the tumor.
Table III.
Association between the p-STAT1 expression status and HLA class I positivity.
| p-STAT status | HLA class I
|
Total | |
|---|---|---|---|
| Negative | Positive | ||
| p-STAT1-negative | 20 | 5 | 25 |
| p-STAT1-positive | 41 | 45 | 86 |
| Total | 61 | 50 | 111 |
The Chi-square test was used to analyze these data (P=0.004). HLA, human leukocyte antigen; p-STAT1, phosphorylated signal transducer and activator of transcription 1.
PD-L1 is upregulated in p-STAT1-positive TIICs
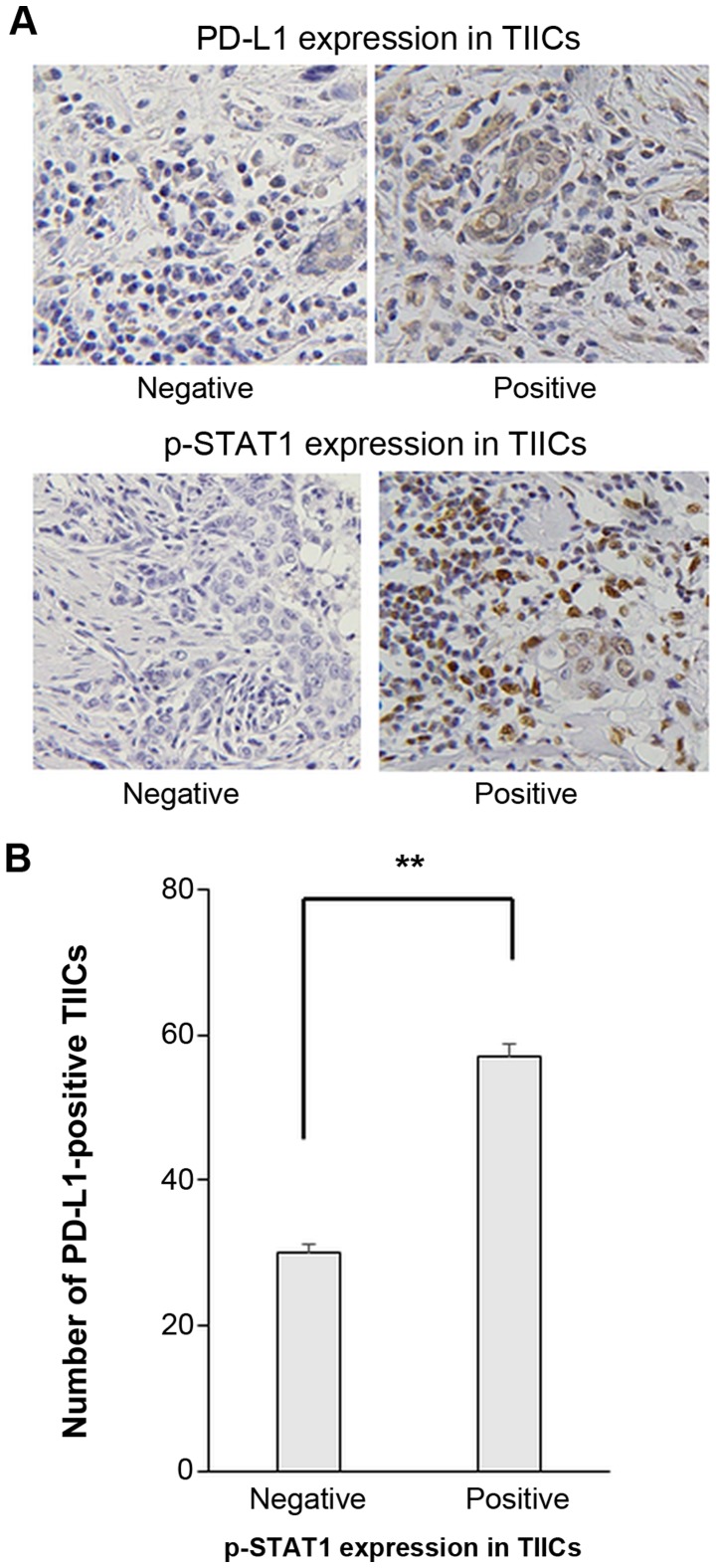
PD-L1 expression on TIICs was examined. Representative IHC staining images of PD-L1 and p-STAT1 expression on TIICs are presented in Fig. 5A. The number of PD-L1-positive TIICs was significantly higher in cases with p-STAT1 positively stained TIICs (Fig. 5B).
Figure 5.
Number of PD-L1 expressing TIICs was significantly increased in a group of p-STAT1-positive TIICs. (A) Representative immunohisto-chemical staining with p-STAT1 and PD-L1. Original magnification, ×400. (B) Associatoin between p-STAT1 and PD-L1 expression in TIICs. Student’s t-test was performed. Error bars represent the means ± SEM. **P<0.01 between the negative and positive groups of p-STAT1 expression in TIICs.
mRNA expression of PD-L1 is significantly associated with the IFN-γ signature in breast cancer
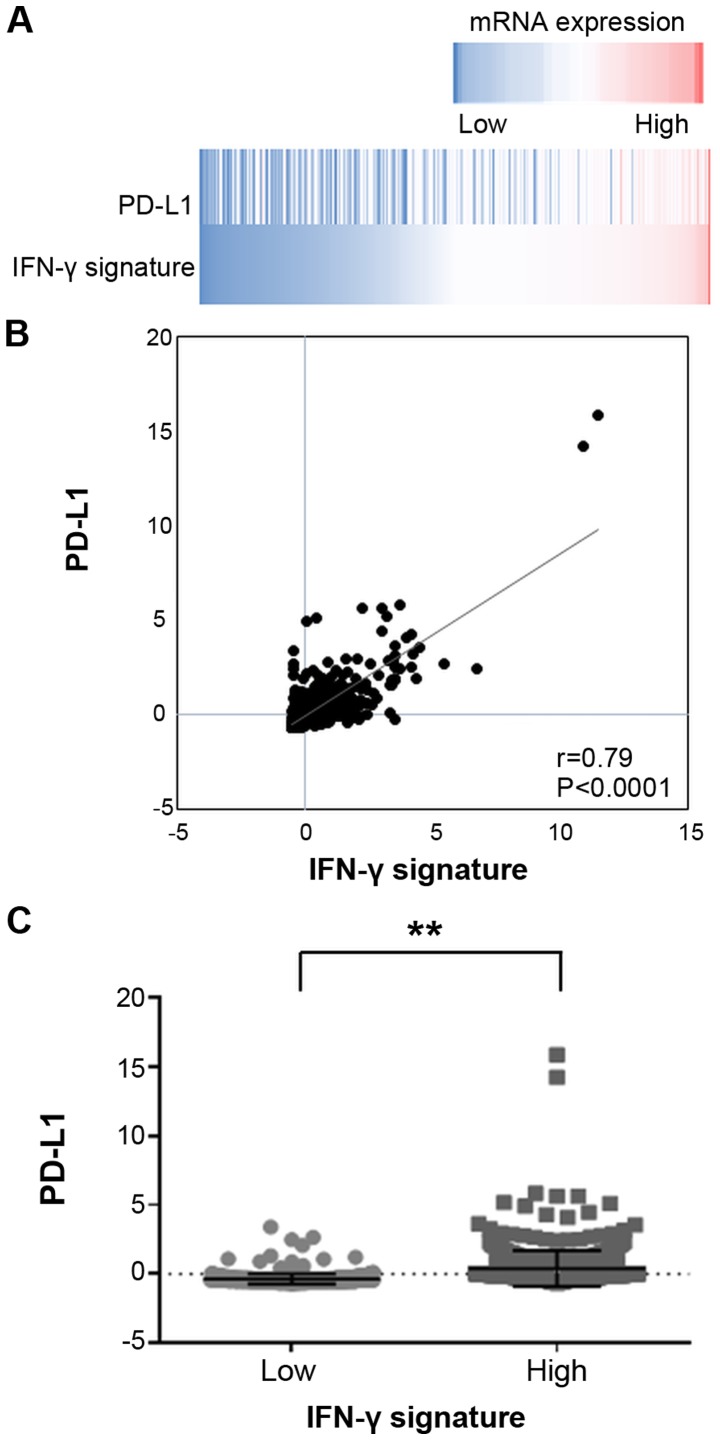
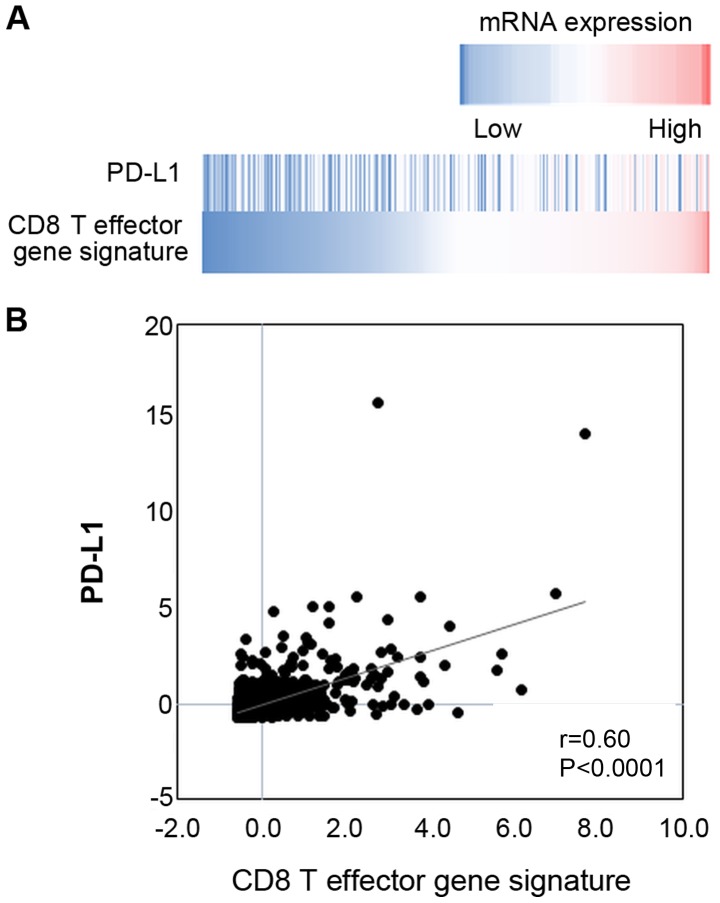
The gene expression data of breast invasive carcinoma tissues of 1,100 clinical cases were extracted from the TCGA database. The data revealed a significantly positive correlation between the mRNA expression levels of PD-L1 and the IFN-γ signature (Fig. 6), and between those of PD-L1 and the CD8 T effector gene signature (Fig. 7).
Figure 6.
mRNA expressions of PD-L1 and IFN-γ signature in breast invasive carcinoma tissues. (A) A heatmap showing mRNA expression levels of the PD-L1 and IFN-γ signature in breast invasive carcinoma tissues (TCGA dataset). (B) Correlation between PD-L1 and IFN-γ signature mRNA expression levels and PD-L1 expression with the median of the values of IFN-γ signature; those lesser than the median were determined to be low and those equal to or above the median were determined to be high (C). Error bars represent the means ± SEM. **P<0.01 between the high and low groups of the IFN-γ signature.
Figure 7.
mRNA expression of PD-L1 was associated with CD8 T effector gene signature. (A) A heatmap showing mRNA expression levels of PD-L1 and the CD8 T effector gene signature in breast invasive carcinoma tissues (TCGA dataset). (B) Correlation between mRNA expression levels of PD-L1 and the CD8 T effector gene signature.
Discussion
In the present study, to the best of our knowledge, we present novel and important findings relevant to anti-PD1/PD-L1 immunotherapy for breast cancer. First, the JAK/STAT pathway stimulated by IFN-γ was shown to be involved in the expression of PD-L1. Second, the expression levels of PD-L1 and HLA class I on tumor cells were simultaneously upregulated in p-STAT1-positive tumor cells, and PD-L1 expression was significantly upregulated in p-STAT1-positive TIICs. Third, there was a positive correlation between PD-L1 expression and the IFN-γ signature based on the gene expression data from the TCGA database.
It has been reported that conditions within the tumor microenvironment that are favorable for immunotherapy with anti-PD-1/anti-PD-L1 mAbs include greater T cell-infiltration, a significant load of neo-antigens, IFN-γ signature and expression of PD-L1 on the tumor cells (12-15), although controversy remains depending on the tumor type. These characteristics may serve as potential biomarkers for the prediction of the responsiveness to ICIs. Furthermore, it is generally accepted that reliable biomarkers should be simply and easily measured in daily clinical practice. The findings of the present study indicate that p-STAT1 expression within the tumor microenvironment is a potential biomarker for immunotherapy with anti-PD-1/anti-PD-L1 mAbs in breast cancer patients. This is based on the fact that p-STAT1 expression significantly correlates with PD-L1 and HLA class I expression on tumor cells. Both factors are essential for anti-PD-1 immunotherapy (26,27), and the results of the present study indicated that both molecules were simultaneously upregulated by IFN-γ in the breast cancer microenvironment.
Other underlying mechanisms may also contribute to PD-L1 regulation in breast cancer. For instance, the involvement of the loss of phosphatase and tensin homolog (PTEN) and the ensuing activation of the PI3K pathway have been previously reported in the expression of PD-L1 (28). In the present study, our in vitro experiments and TCGA database analysis revealed that PD-L1 expression was mainly regulated by IFN-γ via the JAK/STAT pathway in breast cancer.
To date, there have been several reports describing a positive correlation between the number of tumor-infiltrating CD8-positive T cells and PD-L1 expression on tumor cells (29,30). However, in the current study, we found no such correlation between them (Figs. 3B and 4). By contrast, there was a significant positive correlation between the mRNA expression levels of PD-L1 and the CD8 T effector gene signature from the TCGA dataset (Fig. 7). We speculate that the above discrepancy may be due to tumor-infiltrating CD8-positive T cells detected in the IHC analysis, including both activated effector CTLs and exhausted T cells. Furthermore, activated effector CTLs can produce IFN-γ, resulting in PD-L1 upregulation, while exhausted CD8-positive T cells do not produce IFN-γ. According to the study by Huang et al, the T cell invigoration to tumor burden ratio is associated with response to anti-PD-1 immunotherapy (31); therefore, the presence of activated effector CD8-positive T cells in the tumor microenvironment is essential for immunotherapy with anti-PD-1/anti-PD-L1 mAbs.
In addition to PD-L1 expression on tumor cells (32-34), PD-L1 expression in TIICs has also been reported to play an important role in immunotherapy with anti-PD-1/anti-PD-L1 mAbs (35,36). In the present study, we observed PD-L1 expression on TIICs (Fig. 5A) and a greater number of TIICs expressing PD-L1 on their membrane in cases with p-STAT1-positive TIICs (Fig. 5B). These observations suggest that the JAK/STAT pathway via IFN-γ may also be involved in the PD-L1 expression in TIICs. Again, p-STAT1-positivity in TIICs can possibly lead to the detection of PD-L1-expressing TIICs, which can then be reinvigorated with anti-PD-1/anti-PD-L1 mAbs, suggesting that p-STAT1-positivity within the tumor microenvironment may be a biomarker for immunotherapy with anti-PD-1/anti-PD-L1 mAbs.
Collectively, the results of the present study indicate that p-STAT1 positivity is a potential biomarker for patient selection for immunotherapy with anti-PD-1/anti-PD-L1 mAbs in breast cancer, since p-STAT1 positivity significantly reflects PD-L1 and HLA class I expressions on tumor cells as well as PD-L1 expression on TIICs.
Acknowledgments
The authors would like to thank Dr Haruka Nakada, Dr Masayuki Inoue and Dr Hiroshi Nakagomi at Yamanashi Prefectural Central Hospital (Yamanashi, Japan), and Dr Kazuyoshi Kunitomo at Kofu Municipal Hospital (Kofu, Japan) for discussing the interpretation of the data.
Abbreviations
- CTLs
cytotoxic T lymphocytes
- HLA
human leukocyte antigen
- ICIs
immune checkpoint inhibitors
- IFN-γ
interferon-γ
- IHC
immunohistochemistry
- mAbs
monoclonal antibodies
- PD-1
programmed cell death 1
- PD-L1
programmed death ligand 1
- TCGA
The Cancer Genome Atlas
- TIICs
tumor infiltrating immune cells
Funding
This study was supported by the Japan Society for the Promotion of Science Grants-in-Aid for Scientific Research (Grant no. 17K10540), a Clinician Scientist Award (NMRC Grant no. NMRC/CSA/0043/2012) and Clinician Scientist-Individual Research Grant from the National Medical Research Council of Singapore (NMRC Grant no. NMRC/CIRG/1364/2013), and the National Research Foundation Singapore and the Singapore Ministry of Education under its Research Centers of Excellence initiative.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors’ contributions
KM, YS and KK contributed to the study conception and design. KM, KS, LFK, and VK performed the cell line experiments. YN, MO, AK, SI and DI contributed to the acquisition of the patient samples. YN and TN performed and evaluated the IHC staining. YN, TT and KM analyzed the cell line and patient data. YN, KM, and HO analyzed the TCGA dataset. YN, KM, TT, DI and KK drafted the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Written informed consent forms were obtained from all the participants in this study. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation at Yamanashi University (Reference 1622) and with the Helsinki Declaration. The study was approved by the Domain Specific Review Board of the National Healthcare Group of Singapore (Reference 2015/00209).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Wang J, Lv H, Xue Z, Wang L, Bai Z. Temporal Trends of Common Female Malignances on Breast, Cervical, and Ovarian Cancer Mortality in Japan, Republic of Korea, and Singapore: Application of the Age-Period-Cohort Model. BioMed Res Int. 2018;2018:5307459. doi: 10.1155/2018/5307459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thürlimann B, Senn HJ, Panel Members Tailoring therapies - improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12 ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390:2461–2471. doi: 10.1016/S0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Motzer RJ, Sharma P, Wagstaff J, Plimack ER, Hammers HJ, Donskov F, Gurney H, Sosman JA, Zalewski PG, et al. Treatment Beyond Progression in Patients with Advanced Renal Cell Carcinoma Treated with Nivolumab in CheckMate 025. Eur Urol. 2017;72:368–376. doi: 10.1016/j.eururo.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 9.Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, et al. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016;34:2460–2467. doi: 10.1200/JCO.2015.64.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emens LA, Braiteh FS, Cassier P, Delord JP, Eder JP, Fasso M, Xiao Y, Wang Y, Molinero L, Chen DS, et al. Abstract 2859: Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer (TNBC) Cancer Res. 2015;75(Suppl 15):2859. doi: 10.1158/1538-7445.AM2015-2859. [DOI] [Google Scholar]
- 11.Schmid P, Cruz C, Braiteh FS, Eder JP, Tolaney S, Kuter I, Nanda R, Chung C, Cassier P, Delord JP, et al. Abstract 2986: Atezolizumab in metastatic TNBC (mTNBC): Long-term clinical outcomes and biomarker analyses. Cancer Res. 2017;77(Suppl 13):2986. doi: 10.1158/1538-7445.AM2017-2986. [DOI] [Google Scholar]
- 12.Mizukami Y, Kono K, Maruyama T, Watanabe M, Kawaguchi Y, Kamimura K, Fujii H. Downregulation of HLA Class I molecules in the tumour is associated with a poor prognosis in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2008;99:1462–1467. doi: 10.1038/sj.bjc.6604715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mimura K, Shiraishi K, Mueller A, Izawa S, Kua LF, So J, Yong WP, Fujii H, Seliger B, Kiessling R, Kono K. The MAPK pathway is a predominant regulator of HLA-A expression in esophageal and gastric cancer. J Immunol. 2013;191:6261–6272. doi: 10.4049/jimmunol.1301597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mimura K, Teh JL, Okayama H, Shiraishi K, Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109:43–53. doi: 10.1111/cas.13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 16.Sobin LH, Gospodarowicz MK, Wittekind C. International Union against Cancer. TNM Classification of Malignant Tumours Wiley-Blackwell; West Sussex, UK; Hoboken, NJ: 2010. [Google Scholar]
- 17.Japanese Breast Cancer Society . General Rules for Clinical and Pathological Recording of Breast Cancer. 17th edition. Kanehara & Co. Ltd.; Tokyo: 2012. [Google Scholar]
- 18.Mimura K, Kua LF, Shiraishi K, Kee Siang L, Shabbir A, Komachi M, Suzuki Y, Nakano T, Yong WP, So J, et al. Inhibition of mitogen-activated protein kinase pathway can induce upregulation of human leukocyte antigen class I without PD-L1-upregulation in contrast to interferon-γ treatment. Cancer Sci. 2014;105:1236–1244. doi: 10.1111/cas.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran V, et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127:2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallin JJ, Bendell JC, Funke R, Sznol M, Korski K, Jones S, Hernandez G, Mier J, He X, Hodi FS, et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun. 2016;7:12624. doi: 10.1038/ncomms12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBio-Portal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun D, Ding A. MyD88-mediated stabilization of interferon-gamma-induced cytokine and chemokine mRNA. Nat Immunol. 2006;7:375–381. doi: 10.1038/ni1308. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, Saudemont A, Quesnel B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood. 2007;110:296–304. doi: 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto R, Nishikori M, Tashima M, Sakai T, Ichinohe T, Takaori-Kondo A, Ohmori K, Uchiyama T. B7-H1 expression is regulated by MEK/ERK signaling pathway in anaplastic large cell lymphoma and Hodgkin lymphoma. Cancer Sci. 2009;100:2093–2100. doi: 10.1111/j.1349-7006.2009.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Townsend A, Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- 27.Šmahel M. PD-1/PD-L1 Blockade Therapy for Tumors with Downregulated MHC Class I Expression. Int J Mol Sci. 2017;18:18. doi: 10.3390/ijms18061331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, Su X, Wang Y, Gonzalez-Angulo AM, Akcakanat A, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2:361–370. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu B, Arakawa Y, Yokogawa R, Tokunaga S, Terada Y, Murata D, Matsui Y, Fujimoto KI, Fukui N, Tanji M, et al. PD-1/PD-L1 expression in a series of intracranial germinoma and its association with Foxp3+ and CD8+ infiltrating lymphocytes. PLoS One. 2018;13:e0194594. doi: 10.1371/journal.pone.0194594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, Xu W, Harmon S, Giles JR, Wenz B, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545:60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang ZQ, Milne K, Derocher H, Webb JR, Nelson BH, Watson PH. PD-L1 and intratumoral immune response in breast cancer. Oncotarget. 2017;8:51641–51651. doi: 10.18632/oncotarget.18305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mori H, Kubo M, Yamaguchi R, Nishimura R, Osako T, Arima N, Okumura Y, Okido M, Yamada M, Kai M, et al. The combination of PD-L1 expression and decreased tumor-infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget. 2017;8:15584–15592. doi: 10.18632/oncotarget.14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yagi T, Baba Y, Ishimoto T, Iwatsuki M, Miyamoto Y, Yoshida N, Watanabe M, Baba H. PD-L1 Expression, Tumor-infiltrating Lymphocytes, and Clinical Outcome in Patients With Surgically Resected Esophageal Cancer. Ann Surg. 2019;269:471–478. doi: 10.1097/SLA.0000000000002616. [DOI] [PubMed] [Google Scholar]
- 36.Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A, Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20:407–415. doi: 10.1007/s10120-016-0631-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.