Abstract
Previous research has reported that salidroside exerts antitumor properties on numerous types of tumor cells; however, its effect on osteosarcoma cells remains unknown. The present study aimed to investigate the effects of salidroside on the viability, apoptosis and invasion of osteosarcoma cells in vitro, and determine the underlying mechanism of action. The results of an MTT revealed that salidroside suppressed the viability of osteosarcoma cells (MG63 and U2OS cells) in a time- and concentration-dependent manner. The results of cell morphological analysis (profile observations and Hoechst 33258 staining) and the detection of apoptosis by flow cytometry further indicated that the decrease in osteosarcoma cell viability induced by salidroside was associated with cell apoptosis. Western blot analysis not only confirmed these results but also suggested that salidroside induced the apoptosis of osteosarcoma cells by activating the caspase-9-dependent apoptotic pathway. In addition, we reported that salidroside induced G0/G1 phase arrest and suppressed the invasion of osteosarcoma cells, as measured by flow cytometric cell cycle analysis and a Transwell invasion assay, respectively. Western blot analysis confirmed the aforementioned results. Furthermore, our findings demonstrated that salidroside induced the apoptosis, G0/G1 phase arrest and suppressed the invasion of osteosarcoma cells by inhibiting the janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathway, as determined by western blot analysis. In summary, the findings of the present study suggested that salidroside may inhibit the progression of osteosarcoma by suppressing the growth and invasion of osteosarcoma cells. Furthermore, the investigations into the underlying mechanism demonstrated that salidroside exerted notable antitumor activity in osteosarcoma cells by inhibiting the JAK2/STAT3 signaling pathway.
Keywords: apoptosis, cell-cycle, invasion, osteosarcoma, salidroside
Introduction
Osteosarcoma has a double-peak age distribution occurring in children and young adults <20 years of age (75%) and those >50-60 years of age (25%), known as secondary osteosarcoma (1). Osteosarcoma is the most common pediatric malignant bone tumor, accounting for ~5% of all pediatric tumors (2,3). The possible risk factors for secondary osteosarcoma are radiation, Paget's disease and Li-Fraumeni syndrome (4). Early pulmonary metastasis leads to the low survival rate of osteosarcoma (5). At present, surgery combined with chemotherapy is the most common treatment method for osteosarcoma. In 1979, Rosen et al proposed the concept of neoadjuvant chemotherapy, and after more than 40 years of development and improvement, this has become the preferred treatment plan for osteosarcoma (6). However, current chemotherapeutic drugs not only have strong cytotoxic effects, but also have toxic side effects and can induce therapeutic resistance (7). Therefore, novel treatment strategies are required.
Rhodiola rosea L., a perennial herbaceous plant, is the most type of common Chinese medicine and is widely used in the medical field (8). Among all of the effective components extracted from Rhodiola rosea L., salidroside exhibits powerful properties and has received notable attention. Recent studies have reported that salidroside has anti-fatigue, anti-aging, anti-oxidant, anti-inflammatory, neuroprotective and cardiovascular protective effects (9-12). A literature review revealed that salidroside exhibits antitumor effects in various tumors, including fibrosarcoma (13), bladder carcinoma (14), lung carcinoma (15), breast carcinoma (16) and renal cell carcinoma (17) in vitro; however, the association between salidroside and osteosarcoma requires further investigation. In the present study, we investigated the potential antitumor effects of salidroside in vitro and the underlying molecular mechanism.
Materials and methods
Cell culture and treatment
Human osteosarcoma cell lines MG63 and U2OS (ZQXZBIO, Shanghai, China), were selected to assess the antitumor effects of salidroside. Cells were cultured in Dulbecco's modified Eagle's medium combined with high-glucose medium (DMEM-HG) containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (all Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and were maintained in a 37°C humidified incubator with 5% CO2. Cells were harvested with a 0.25% trypsin-0.02% EDTA solution and passaged when the cells attained ~80% confluence. Salidroside (Fig. 1A; purity >99%, MedChem Express, Monmouth Junction, NJ, USA) was dissolved in PBS at room temperature and filtered through a 0.22-μm filter (Merck KGaA, Darmstadt, Germany) prior to use. To determine the potential role of salidroside in osteosarcoma cells, the cells were pretreated with different concentrations (0, 1, 2.5, 5, 7.5 and 10 mM) of salidroside at 37°C for 24, 48 and 72 h, respectively. Cells cultured without pretreatment were used as a control. Z-LEHD-FMK (50 μM at 37°C for 2 h, Selleck Chemicals, Houston, TX, USA), a caspase-9 specific inhibitor, was used to explore whether salidroside induced osteosarcoma cell apoptosis via the caspase-9-dependent apoptotic pathway. Furthermore, FLLL32 (5 μM at 37°C for 2 h), a specific inhibitor of JAK2/STAT3 phosphorylation, was used to further confirm whether salidroside induced osteosarcoma cell apoptosis via the JAK2/STAT3 signaling pathway.
Figure 1.
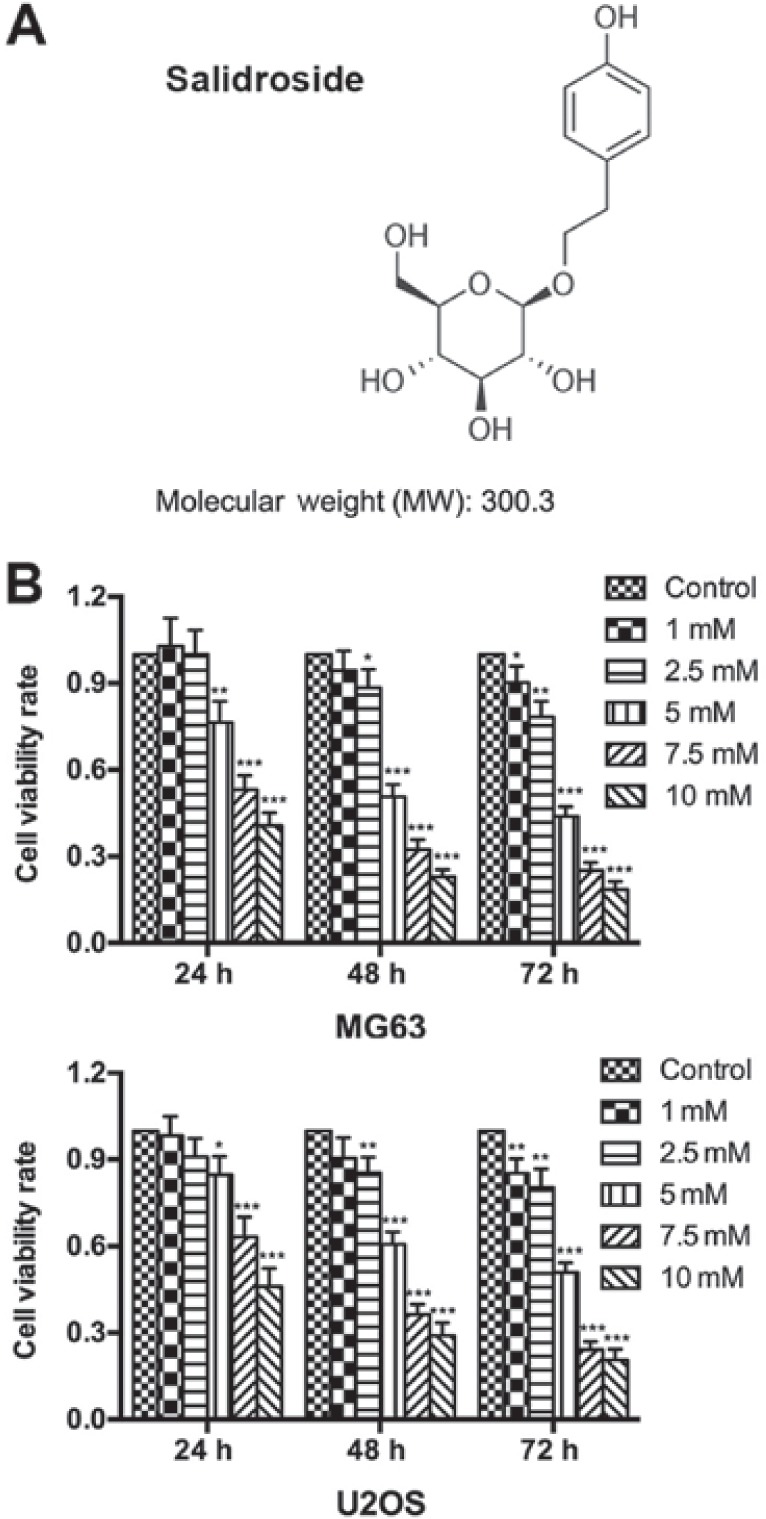
Salidroside inhibits osteosarcoma cell growth. (A) Molecular structure of salidroside. (B) The growth inhibitory effect of salidroside as determined by an MTT assay. *P<0.05, **P0.01, ***P<0.001 vs. control group.
Cell viability assay
Cell viability was assessed using an MTT assay (18). Briefly, the two cell lines were seeded in 96-well plates (6×103 cells/well) for 24 h and when they reached ~80% confluence, they were treated with different concentrations of salidroside (0, 1, 2.5, 5, 7.5 and 10 mM) at 37°C for 24, 48, and 72 h, respectively. After treatment, cells were cultured in DMEM-HG medium containing MTT solution (10 μl/well, 5 mg/ml in PBS) in a 37°C humidified incubator for 4 h. Then, dimethyl sulfoxide (150 μl/well) was added to dissolve the formazan crystals. The absorbance value was measured at a wavelength of 490 nm using a SpectraMax Plus 384 microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA). Cell viability rate = (salidroside treatment group absorbance value - blank control group absorbance value) / (no salidroside treatment group absorbance value - blank control group absorbance value).
Morphology of apoptotic cells
An inverted phase contrast fluorescence microscope (Carl Zeiss, Heidenheimer, Germany) was used to directly observe morphological changes in the two cell lines treated with salidroside. Cells were separately seeded into 6-well (12×104 cells/well) and 24-well (3×104 cells/well) plates, cultured to confluence, and treated with (0, 1, 5 and 10 mM) salidroside at 37°C for 48 h. Firstly, we directly observed the morphology of apoptotic cells seeded in the 6-well plate (magnification, ×100). Cells were counted from five random fields for each group, and the average was expressed as the number of apoptotic cells. Then, we observed the nuclear morphology of apoptotic cells seeded in the 24-well plate using Hoechst 33258 staining. In brief, cells were washed three times with PBS, fixed in 4% paraformaldehyde at 4°C for 20 min, stained with Hoechst 33258 staining solution (125 μl/well, Beyotime Institute of Biotechnology, Haimen, China) for 5 min, rewashed three times with PBS, covered with anti-fading solution, and then observed using the aforementioned fluorescence microscope (magnification, ×400). Cells were counted from five random fields for each group and the number of apoptotic cells (Hoechst-positive cells) was expressed as a percentage (%) of the total number of counted cells.
Flow cytometric analysis of cell apoptosis
To further verify the effect of salidroside on the apoptosis of osteosarcoma cells, an Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) apoptosis detection kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) was used. Briefly, following treatment with (0, 1, 5 and 10 mM) salidroside for 48 h, MG63 cells (1×105 cells/sample) were digested with trypsin (0.25%, room temperature, 1-3 min), centrifuged (300 × g, 4°C, 5 min), washed twice with PBS, and resuspended in binding buffer (500 μl/sample, Nanjing KeyGen Biotech Co., Ltd.). Each sample was stained with Annexin V-FITC (5 μl) and propidium iodide (PI; 5 μl) in the dark at 4°C for 15 min and then immediately analyzed with a flow cytometer equipped with FACSComp software (BD Biosciences, Franklin Lakes, NJ, USA). Annexin V-FITC-/PI- cells were identified as viable cells, Annexin V-FITC+/PI- cells were identified as early apoptotic cells, and Annexin V-FITC+/PI+ cells were identified as the sum of late apoptotic cells and necrotic cells.
Flow cytometric analysis of the cell cycle
A cell cycle detection kit (Nanjing KeyGen Biotech Co., Ltd.) was used to evaluate the cell cycle distribution of cells. In brief, after treatment with (0, 1, 5, 10 mM) salidroside for 24 h, MG63 cells (1×105 cells/sample) were digested with trypsin (without EDTA, 0.25%, room temperature, 1-3 min), centrifuged (300 × g, 4°C, 5 min), washed three times with PBS, and fixed in 70% ethanol at 4°C overnight. Then, each sample was centrifuged (300 × g, 4°C, 5 min), washed three times with PBS, incubated with RNase A (100 μl) at 37°C for 30 min, stained with PI (400 μl) in the dark at 4°C for 30 min, and then immediately analyzed with a flow cytometer equipped with CellQuest software (BD Biosciences).
Cell invasion assay
Cell invasion was analyzed using a Transwell invasion assay. Matrigel (20 μl/chamber, BD Biosciences) was evenly applied to the upper surface of the chamber (8.0 μm, cat. no. 3422; Costar; Corning, Inc., Corning, NY, USA) and incubated in a 37°C humidified incubator for 30 min. The two cell lines (1×105 cells/chamber) were suspended in preprocessed DMEM-HG medium (200 μl/chamber, serum-free medium alone, or supplemented with 0, 1, 5 or 10 mM salidroside) and then seeded into the upper surface of the chamber. DMEM-HG medium (500 μl/well, containing 10% FBS) was added to the 24-well plate. Following incubation in a 37°C humidified incubator for 24 h, the upper-chamber cells were wiped out with a cotton swab, and the lower-chamber cells were washed twice with PBS, fixed in 4% paraformaldehyde at 4°C for 20 min, stained with 0.1% crystal violet (Beyotime Institute of Biotechnology) at room temperature for 5 min, rewashed twice with PBS, then observed under an inverted phase contrast microscope (magnification, ×400, Zeiss AG, Oberkochen, Germany). Cells were counted in five random fields for each group, and the number of invasive cells (crystal violet-positive cells) was expressed as a percentage (%) of the total number of counted cells.
Western blot analysis
After treatment with (0, 1, 5 and 10 mM) salidroside for 48 h, MG63 cells were digested with trypsin and lysed by radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology). The lysates were centrifuged at 12,000 × g at 4°C for 30 min, then the supernatants were collected and quantified by a bincinchoninic acid assay (CW Biotechnology, Beijing, China). Total protein (30 μg) was separated using 10 or 12% SDS-PAGE with a 5% stacking gel, and then transferred to polyvinylidene difluoride membranes (0.22 μm; Merck KGaA). The membranes were subsequently blocked in tris-buffered saline with 10% Tween-20 (TBS-T) containing 5% nonfat milk at room temperature for 2 h, incubated with primary antibodies: rabbit anti-B-cell lymphoma 2 (Bcl-2; cat. no. 4223; 1:1,000), Bcl-2-associated X protein (Bax; cat. no. 5023; 1:1,000), caspase-3 (cat. no. 9665; 1:1,000), caspase-7 (cat. no. 9491; 1:1,000), caspase-9 (cat. no. 9502; 1:1,000), cyclin D1 (cat. no. 2978; 1:1,000), p21 (cat. no. 2947; 1:1,000), matrix metalloproteinase 2 (MMP-2; cat. no.40994; 1:1,000), MMP-9 (cat. no. 13667; 1:1,000), signal transducer and activator of transcription 3 (STAT3; cat. no. 12640; 1:1,000), phosphorylated (p)-STAT3 (cat. no. 9145; 1:2,000), JAK2 (cat. no. 3230; 1:1,000) and p-Janus kinase 2 (JAK2; cat. no. 3776; 1:1,000), all from Cell Signaling Technology, Inc., Danvers, MA, USA; mouse anti-β-actin (cat. no. CW0264; 1:5,000) and rabbit anti-GAPDH (CW0101, 1:5,000) were obtained from CWBIO (Beijing, China) in TBS-T containing 5% bovine serum albumin at 4°C overnight and washed three times with TBS-T. Then, the membranes were incubated with secondary antibodies (horseradish peroxidase-conjugated goat, anti-mouse (cat. no. CW0102; 1:10,000) or rabbit (cat. no. CW0156; 1:10000; CWBIO) in TBS-T for 2 h. An enhanced chemiluminescence western blot detection kit (Thermo Fisher Scientific, Inc.), ChemiDoc™ XRS+ System and Image Lab™ 2.1 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA) were used to observe the expression of all proteins.
Statistical analysis
Significant differences between groups of data were analyzed by one-way analysis of variance and a Student-Newman-Keuls test using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). Graphs were created using GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA, USA). All data were presented as the mean ± standard error of the mean and three independent experiments were conducted. P<0.05 was considered to indicate a statistically significant difference.
Results
Salidroside inhibits osteosarcoma cell growth
An MTT assay was performed to determine the growth inhibitory effects of salidroside on osteosarcoma cells. The results showed that salidroside significantly inhibited the viability of osteosarcoma cells in a time- and concentration-dependent manner. Furthermore, a significant inhibitory effect was detected with 5 mM salidroside at 24 h, which peaked at 10 mM salidroside at 72 h compared with the control (Fig. 1A). The half the maximal inhibitory concentration (IC50) values were: MG63, 5.09 mM; U2OS, 9.79 mM; MG63, 4.72 mM; U2OS, 5.294 mM; MG63, 4.67 mM; and U2OS, 4.96 mM following treatment with salidroside for 24, 48, and 72 h, respectively. Based on the aforementioned results, we reported that salidroside inhibited osteosarcoma cell growth in a concentration-dependent manner. Thus, we selected 0, 1, 5 (the approximate IC50) and 10 mM salidroside as effective drug concentrations for subsequent analysis.
Morphological changes in salidroside-treated osteosarcoma cells
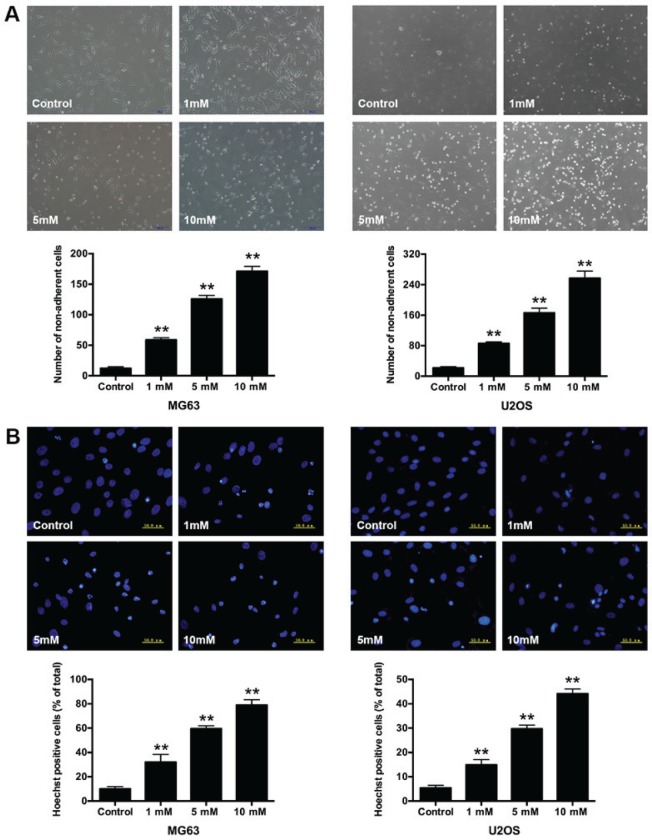
In addition, we compared the growth inhibitory effect after treatment with 1, 5, and 10 mM salidroside for 48 h. Morphological changes in osteosarcoma cells were observed via microscopy. Cells in the control group presented a long spindle or polygon appearance and attached uniformly to the culture dish. Whereas, some cells became small and round, and were suspended in the medium in the salidroside-treated groups. The number of non-adherent cells significantly increased (MG63, 1 mM, 58.67±3.51; 5 mM, 125.67±6.03 and 10 mM, 171.00±8.19; U2OS, 1 mM, 85.67±4.04; 5 mM, 166.33±12.22 and 10 mM, 257.00±19.00) compared with the control group (MG63, 12.33±2.52; U2OS, 12.67±3.51) (Fig. 2A). Hoechst 33258 staining was performed to further observe the nuclear morphological changes in osteosarcoma cells. The non-apoptotic nuclei presented weaker blue fluorescence, while the apoptotic nuclei exhibited brighter fluorescence and the structure became condensed, fragmented and crescent-shaped. The percentage of apoptotic nuclei significantly increased (MG63, 1 mM, 32.03±6.38%; 5 mM, 59.53±2.47% and 10 mM, 78.94±4.44%; U2OS, 1 mM, 14.91±2.16%; 5 mM, 29.72±1.47% and 10 mM 44.13±1.91%); compared with the control groups (MG63, 10.00±1.90%; U2OS, 5.39±1.06%) (Fig. 2B). The results indicated that salidroside induced the apoptosis of osteosarcoma cells in a concentration-dependent manner.
Figure 2.
Morphological changes in osteosarcoma cells following treatment with salidroside. (A) Morphological appearance of MG63 and U2OS cells exposed to different concentrations of salidroside for 48 h was observed via inverted phase-contrast microscopy (magnification, ×100). (B) Nuclear morphological appearance of MG63 and U2OS cells exposed to different concentrations of salidroside for 48 h was observed via Hoechst 33258 staining and inverted phase-contrast microscopy (magnification, ×400). **P<0.01, vs. control group.
Salidroside induces osteosarcoma cell apoptosis via the caspase-9-dependent apoptotic pathway
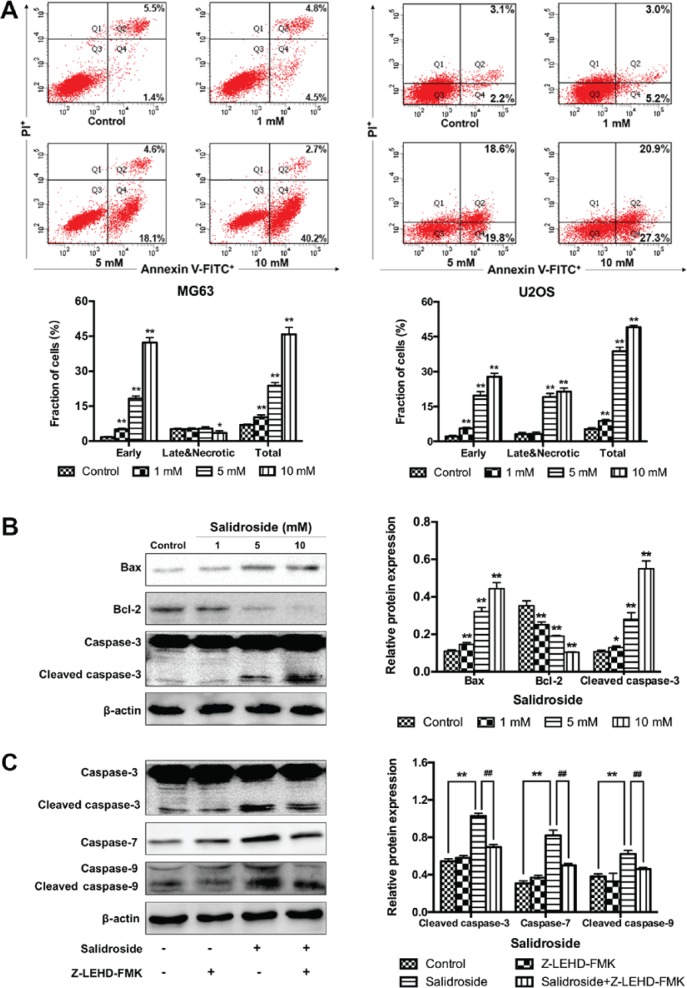
In addition, flow cytometry was performed to further verify the growth inhibitory effects of salidroside in osteosarcoma cells associated with apoptosis. The percentage of apoptotic cells was significantly increased in the presence of salidroside (MG63, 1 mM, 10.20±1.00%; 5 mM, 23.73±1.38% and 10 mM, 45.77±3.07%; U2OS, 1 mM, 8.83±0.55%; 5 mM, 38.80±1.69% and 10 mM, 49.10±0.78%), compared with the control groups (MG63, 6.90±0.40%; U2OS, 5.23±0.58%) (Fig. 3A). Then, the Bcl-2 and caspase family of proteins (Bax, Bcl-2, and caspase-3) were investigated to explore the potential molecular mechanism in osteosarcoma cells (MG63 cells were selected). Western blot analysis demonstrated that the expression of pro-apoptotic protein was significantly increased and that of anti-apoptotic proteins was significantly decreased in the salidroside groups in a concentration-dependent manner, compared with the control group (Fig. 3B). Additionally, to evaluate whether the caspase-9-dependent apoptotic pathway was involved in salidroside-induced osteosarcoma cell apoptosis, Z-LEHD-FMK, a caspase-9 specific inhibitor, was used and the molecular mechanism was explored using western blot analysis. Cells were pretreated with or without Z-LEHD-FMK at 50 μM for 2 h, then 5 mM salidroside was added to the experimental group and cultured for another 48 h. The expression of cleaved caspase-3, caspase-7, and cleaved caspase-9 was significantly increased in the salidroside group, compared with the control group; however, the expression of these proteins was significantly reduced in the presence of Z-LEHD-FMK, compared with the salidroside group (Fig. 3C). These data not only confirmed that salidroside induced the apoptosis of osteosarcoma cells in a concentration-dependent manner, but also indicated that salidroside induced MG63 cell apoptosis via the caspase-9-dependent apoptotic pathway.
Figure 3.
Salidroside induces osteosarcoma cell apoptosis via the caspase-9-dependent pathway. (A) Flow cytometry was used to assess the apoptosis of cells in response to salidroside apoptosis. (B) Apoptosis-related protein expression in MG63 cells in response to various concentrations of salidroside was detected via western blot analysis. (C) Whether the caspase-9-dependent pathway was involved in salidroside-induced apoptosis of osteosarcoma (MG63) cells. *P<0.05, **P<0.01, vs. control group. ##P<0.01, vs. salidroside group. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; FITC, fluorescein isothiocyanate; PI, propidium iodide.
Salidroside induces G0/G1 arrest in osteosarcoma cells
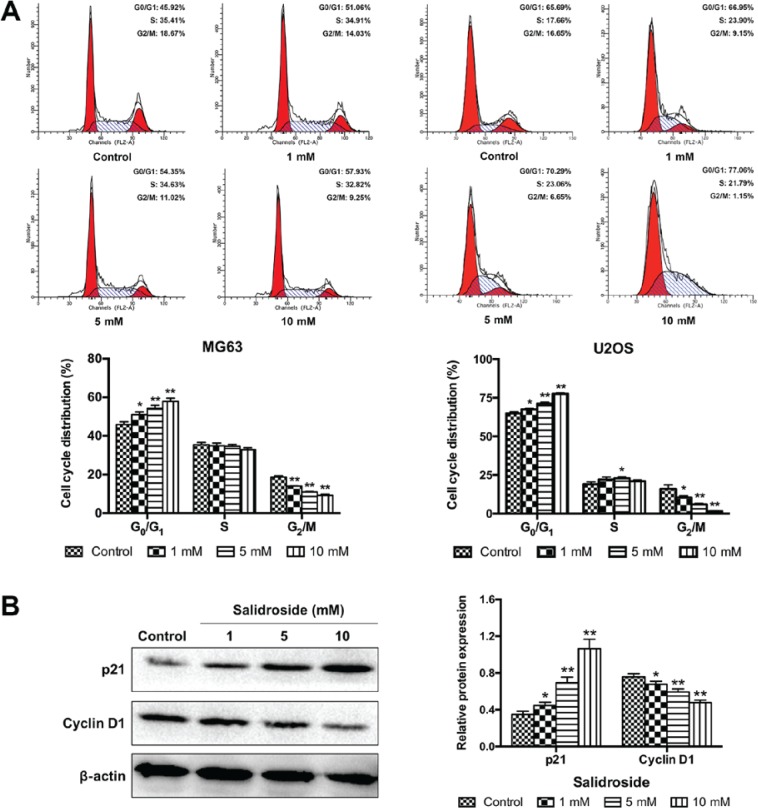
To determine whether cell-cycle arrest was involved in the growth inhibitory effects of salidroside, we assessed the role of salidroside in the progression of the cell cycle in osteosarcoma cells using flow cytometry. As presented in Fig. 4A, salidroside significantly increased the percentage of cells in G0/G1 phase (MG63, 1 mM, 51.06±1.38%; 5 mM, 54.35±1.50% and 10 mM, 57.93±1.10%; U2OS, 1 mM 67.51±0.67%; 5 mM 71.22±0.88% and 10 mM 77.57±0.66%), but significantly decreased the percentage of cells in G2/M phase (MG63, 1 mM, 14.02±0.24%; 5 mM, 11.01±0.21% and 10 mM, 9.25±0.56%; U2OS, 1 mM 10.42±1.11%; 5 mM, 5.93±0.63% and 10 mM, 1.47±0.29%), compared with the control group (MG63, G0/G1 45.92±1.36% and G2/M 18.68±0.65%; U2OS, G0/G1 64.98±0.96% and G2/M 15.99±2.63%). Then, the expression of cell cycle-associated proteins were analyzed (cyclin D1 and p21) to elucidate the potential molecular mechanism. The expression of p21 was significantly increased and the expression of cyclin D1 was significantly decreased in the salidroside groups in a concentration-dependent manner, compared with the control group (Fig. 4B). These results demonstrated that salidroside induced G0/G1 phase arrest of osteosarcoma cells in a concentration-dependent manner.
Figure 4.
Salidroside induces G0/G1 phase cell cycle arrest in osteosarcoma cells. (A) Flow cytometry was used to assess the cell cycle distribution of MG63 and U2OS cells following treatment with different concentrations of salidroside for 24 h. (B) Cell cycle-related protein expression in MG63 cells was detected via western blot analysis. *P<0.05, **P<0.01, vs. control group.
Salidroside inhibits the invasion of osteosarcoma cells
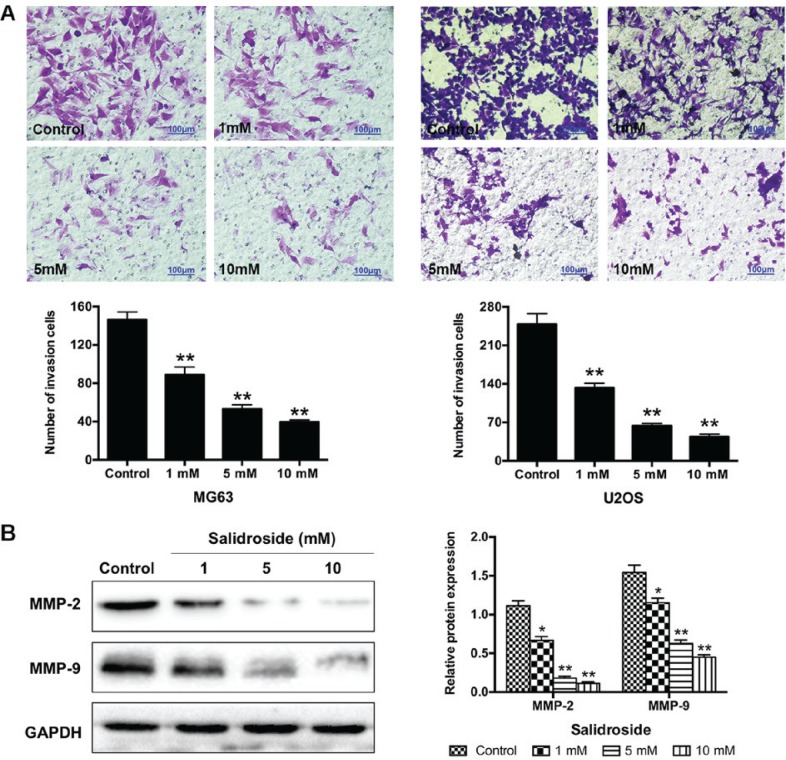
We performed a Transwell assay to understand the effects of salidroside on the invasion of osteosarcoma cells. The results showed that the number of invasive cells significantly decreased (MG63, 1 mM, 89.00±7.94; 5 mM, 53.33±4.16 and 10 mM, 39.67±2.08; U2OS, 1 mM, 133.00±8.00; 5 mM, U2OS, 63.67±4.16 and 10 mM, 44.00±4.58) compared with the control groups (MG63, 146.33±8.08; U2OS, 248.67±19.04) (Fig. 5A). Additionally, we further investigated the potential molecular mechanism in MG63 cells using western blot analysis. Salidroside significantly decreased the expression of invasion-associated proteins (MMP-2 and MMP-9) in a concentration-dependent manner, compared with the control groups (Fig. 5B). In summary, these findings suggested that salidroside inhibited the invasion of osteosarcoma cells in a concentration-dependent manner.
Figure 5.
Salidroside suppresses osteosarcoma cell invasion. (A) Transwell assay was used to assess the invasion of MG63 and U2OS cells following treatment with different concentrations of salidroside for 48 h. Scale bar, 100 μm. (B) Invasion-related protein expression in MG63 cells was detected via western blot analysis. *P<0.05, **P<0.01, vs. control group. MMP, matrix metalloproteinase.
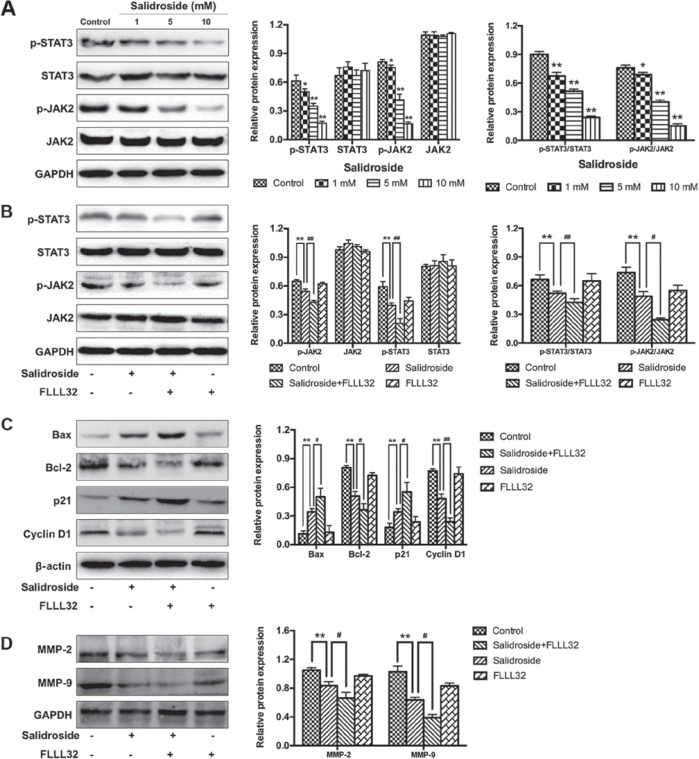
Salidroside inhibits the JAK2/STAT3 signaling pathway in osteosarcoma cells
To further investigate the potential mechanism by which salidroside suppressed the growth of osteosarcoma (MG63) cells, the JAK2/STAT3 signaling pathway was analyzed. The results of western blot analysis demonstrated that salidroside significantly decreased the expression of p-JAK2 and p-STAT3 in a concentration-dependent manner, compared with the control groups (Fig. 6A). Furthermore, FLLL32 (5 μM), a specific inhibitor of JAK2/STAT3 phosphorylation, was used to further confirm these findings. As presented in Fig. 6B, FLLL32 significantly decreased the expression of p-JAK2 and p-STAT3, compared with the salidroside group. Similarly, apoptosis-, cell cycle- and invasion-related proteins were accordingly altered in the salidroside + FLLL32 group, compared with the salidroside group (Fig. 6C and D). Collectively, the aforementioned results indicated that the JAK2)/STAT3 signaling pathway was involved in salidroside-induced apoptosis and cell cycle arrest in MG63 cells. Collectively, the aforementioned results indicated that salidroside induced apoptosis and cell cycle arrest, and suppressed the invasion of osteosarcoma cells by inhibiting the JAK2/STAT3 signaling pathway.
Figure 6.
Salidroside inhibits the JAK2/STAT3 signaling pathway. (A) Western blot analysis was used to investigate whether the JAK2/STAT3 signaling pathway was associated with the growth inhibitory effect induced by salidroside. (B) FLLL32 (a specific JAK2/STAT3 inhibitor) was used to confirm the involvement of the JAK2/STAT3 signaling pathway. (C) Western blot analysis was used to verify the role of the JAK2/STAT3 pathway in the regulation of apoptosis- and cell cycle-associated proteins in salidroside-treated osteosarcoma cells. (D) Western blot analysis was used to verify the role of the JAK2/STAT3 pathway in the regulation of invasion-associated proteins in salidroside-treated osteosarcoma cells. *P<0.05, **P<0.01, vs. control group. #P<0.05, ##P<0.01, vs. salidroside group. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; JAK2, Janus kinase 2; MMP, matrix metalloproteinase; p, phosphorylated; STAT3, signal transducer and activator of transcription 3.
Discussion
Osteosarcoma is a common aggressive malignant bone tumor. Despite considerable developments in the treatment of osteosarcoma, current treatments for osteosarcoma still have major limitations; the long-term survival (5-year survival rate is ~60%) and mortality rates remained unchanged (19). Therefore, novel therapeutic strategies are urgently required that can effectively act via various anticancer mechanisms. The positive effects of phytochemical drugs on antitumorigenic activity have been demonstrated, as well as their protective effects against the side effects of traditional chemotherapeutic drugs, indicating that they are safer compounds for use in normal cells (20,21). However, the complex mechanism of action of phytochemical drugs complicates their application in treating human malignancies (22). Salidroside, a glucoside of tyrosol, was considered an effective ingredient of Rhodiola, which has been demonstrated to have anti-proliferative and pro-apoptotic effects in previous reports (8-12). Interestingly, research has reported that salidroside also has antitumor effects in several human tumor cells, such as neuroblastoma cells, bladder cancer cells, glioma cells and lung cancer cells (14,15,20,23). Furthermore, Li et al (24) reported that salidroside combined with antitumor agents exerted excellent antitumor effects in colorectal cancer. Qi et al (25) revealed that salidroside had a direct inhibitory effect on the proliferation, migration and invasion of gastric cancer cells. In the present study, we first assessed the antitumor effects of salidroside in the treatment of osteosarcoma. We demonstrated that salidroside induced the growth and invasion of osteosarcoma cells, which indicated its therapeutic potential. The pharmacological mechanism of salidroside may be related to the JAK2/STAT3 signaling pathway (Fig. 7).
Figure 7.
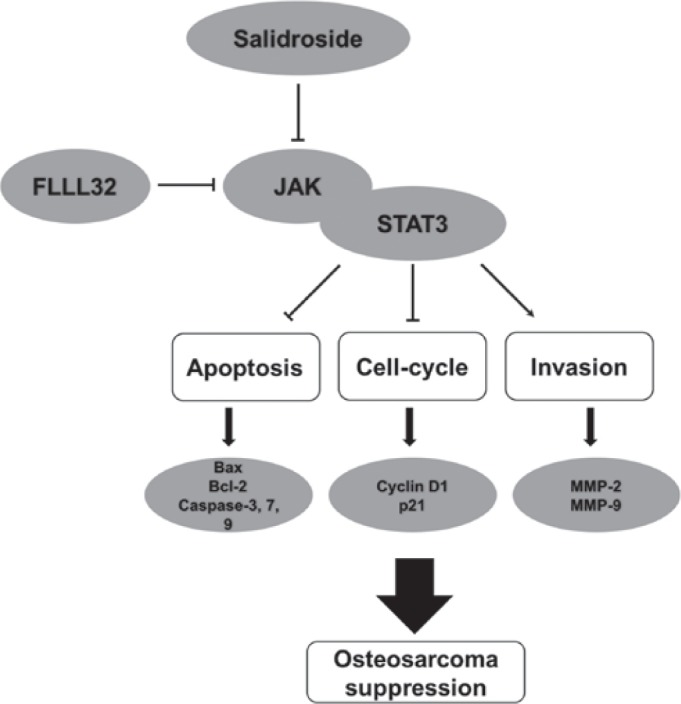
Diagram of the mechanism of salidroside-induced apoptosis, cell cycle arrest and suppressed invasion of osteosarcoma cells via inhibition of the JAK2/STAT3 signaling pathway. Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; JAK, Janus kinase; MMP, matrix metalloproteinase; STAT3, signal transducer and activator of transcription 3.
Cell proliferation is an important marker for tumor development. Therefore, inhibiting tumor growth (by promoting tumor cell apoptosis) is the most important objective in preventing tumor progression (26). The MTT assay is widely used in bioactive factor activity assays, large-scale antitumor drug screening and cytotoxicity assays (27). In the present study, the results of the MTT assay revealed that salidroside significantly inhibited the viability of osteosarcoma cells in a time- and concentration-dependent manner. The results of cell morphological observations and flow cytometric apoptosis detection further indicated that the decrease in cell viability induced by salidroside was associated with cell apoptosis. We investigated whether the expression of apoptotic-related proteins via western blot analysis, and the expression of the Bcl-2 and caspase families, critical apoptosis-related proteins, were regulated by salidroside. The Bcl-2 and caspase families are specific regulatory proteins of the mitochondrial apoptosis pathway, which is one of the main pathways of apoptosis (28). Our results indicated that the mitochondrial apoptosis pathway is involved in salidroside-mediated apoptosis of osteosarcoma cells. In addition, dysregulated cell cycle distribution is another feature of tumor development, and the induction of cell apoptosis is accompanied with cell cycle arrest (29). Flow cytometric cell cycle analysis is widely used for evaluating changes in cell cycle distribution (30). We reported that salidroside triggered G0/G1 phase arrest in osteosarcoma cells, which was consistent with previous reports (16,31). Then, the present study investigated the expression of cell cycle-related proteins using western blot analysis; the expression of cyclin D1 and p21 were revealed to be regulated by salidroside. Therefore, we concluded that salidroside induced the apoptosis of osteosarcoma cells by inducing G0/G1 phase arrest. We suggested that salidroside may function as an agonist to induce the apoptosis and G0/G1 phase arrest of osteosarcoma cells, and may represent as an alternative therapeutic strategy for the treatment of osteosarcoma.
Invasion, which generally leads to metastasis, can be used to predict tumor malignancy (32). Previous research reported that early metastasis (particularly pulmonary metastasis) remains as the main cause of mortality in ~40% of patients with osteosarcoma (33). The results of Transwell assays demonstrated that salidroside significantly inhibited the invasive ability of osteosarcoma cells in a concentration-dependent manner. We further investigated the molecular mechanism using western blot analyses and reported that salidroside significantly decreased the expression of MMP-2 and MMP-9 in a concentration-dependent manner. MMP-2 and MMP-9, the two most important proteins in the MMP family, are involved in extracellular matrix degradation by effectively decomposing collagen IV and laminin (34). The MMP family of proteins facilitates tumor metastasis by degrading the basement membrane of the extracellular matrix, and is an effective marker for predicting tumor malignancy (35). Thus, our findings indicated that salidroside reduced the metastatic capabilities of osteosarcoma cells by suppressing the expression of MMPs.
Increasing evidence has suggested that the inhibitory effect of salidroside on different tumor cells is associated with different signal pathways. Sun et al (13) showed that salidroside inhibited the metastasis of human fibrosarcoma cells by downregulating the reactive oxygen species (ROS)/protein kinase C/extracellular signal-regulated kinase 1/2 pathway. Zhao et al (16) revealed that salidroside reduced oxidative stress and suppressed breast cancer growth by inhibiting the formation of ROS and activating the mitogen-activated protein kinase pathway. Fan et al (36) found that salidroside induced the apoptosis and autophagy of human colorectal cancer cells via inhibiting the PI3K/Akt/mammalian target of rapamycin pathway. Lv et al (17) reported that salidroside suppressed proliferation in renal cell carcinoma by modulating the JAK2/STAT3 pathway. Kang et al (37) demonstrated that salidroside inhibited the mobility and angiogenesis of breast cancer cells by regulating epidermal growth factor receptor/Jak2/STAT3 signaling via MMP2. To investigate the potential signaling pathway involved in salidroside-mediated cell apoptosis of osteosarcoma cells, western blot analysis was performed. We showed that the JAK2/STAT3 signaling pathway participated in salidroside-mediated cell apoptosis of osteosarcoma cells, which was consistent with previous reports of signaling in other tumors, including human renal carcinoma cells, human melanoma cells and colon cancer cells (38,39). JAK family proteins have been implicated in different cellular processes, such as cell proliferation, differentiation, apoptosis, invasion and angiogenesis (40). JAKs are activated by cytokines to phosphorylate Y residues and subsequently phosphorylate the downstream molecule STAT3 (40). STAT3 translocates to the nucleus and binds to DNA, regulating thee expression of apoptosis-related genes, such as Bcl-2 and other members of this family (41). STAT3 is therefore considered an essential anti-apoptotic factor. The JAK2/STAT3 pathway is an evolutionarily conserved pathway that induces tissue homoeostasis modulation and decreases the extent of damage during cellular stress; it is therefore considered a critical signaling pathway in cancer formation and progression (27,42). Our results revealed that salidroside reduced the phosphorylation of JAK2 and STAT3. In addition, we reported that FLLL32 (a specific JAK2/STAT3 inhibitor) significantly enhanced the inhibitory effects of salidroside on JAK2 and STAT3 phosphorylation in osteosarcoma cells. Taken together, these results demonstrated that salidroside induces cell apoptosis, cell cycle arrest and suppresses invasion of osteosarcoma cells via inhibiting the JAK2/STAT3 signaling pathway.
ROS can activate multiple pathways involved in metastasis, invasion, and apoptosis of tumor cells. It has been reported that ROS and several oxidative substances produced by tumor cells can promote tumor growth (43). Salidroside, an antioxidant, had been reported to exert anti-cancer effects in breast cancer treatment (16); however, whether autophagy and ROS are involved in salidroside-induced apoptosis and cell cycle arrest in osteosarcoma cells is yet to be determined and further studies are required to investigate the mechanism of action of salidroside. In addition, a limitation of our study is that only an MTT assay was used to determine the growth inhibition by salidroside; further methods are required to verify our findings in the future. By consulting the literature, we found that low concentrations of salidroside could suppress the growth and invasion of several human tumor cells (15,17,20,31). Our preliminary research was based on the concentration at the micromolar level; however, the results showed that the activity of osteosarcoma cells was not affected. This could be due to the relatively mild efficacy of salidroside; thus, investigations were conducted with salidroside at the millimolar level in the present study. Additionally, several studies also used high concentrations (mM) of salidroside (36,44); we used similar concentrations of salidroside for analysis. Furthermore, the normal osteoblast cell line hFOB1.19 was employed to explore whether salidroside induced osteoblast apoptosis with the same concentrations. We reported that the concentration of salidroside significantly induced the apoptosis of normal osteoblasts (hFOB1.19) from ≥8.2 mM at 24 h (data not shown). Therefore, we considered that the sensitivity of cells to salidroside may differ between cell lines. Our future study aims to further narrow the concentration gradient of salidroside.
In the present study, it was salidroside was demonstrated to be a critical inhibitor of osteosarcoma growth and invasion, and was associated with the induction of cell apoptosis, cell cycle arrest and suppressed invasion. Investigations into the molecular mechanism of action of salidroside suggested that apoptosis may be induced via the mitochondrial apoptosis pathway, and the JAK2/STAT3 pathway was reported to be involved in salidroside-mediated inhibition of osteosarcoma growth and invasion. In conclusion, the findings of the present study may provide insight into the molecular mechanism underlying the effects of salidroside and may be considered as a potential therapeutic agent for the treatment of osteosarcoma.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
CL made substantial contributions to the design of the study for the analysis of the role of salidroside in modulating osteosarcoma cell growth and invasion. LH, ZH and LW performed experiments including the MTT assay, and cell apoptosis and cell cycle analysis. WL, XC and XZ performed the experiments to analyze molecular mechanisms, such as western blot analysis. SY conducted statistical analysis of the experimental data. CL and LH were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 2.Lewis VO. What's new in musculoskeletal oncology. J Bone Joint Surg Am. 2009;91:1546–1556. doi: 10.2106/JBJS.I.00375. [DOI] [PubMed] [Google Scholar]
- 3.Dominkus M, Darwish E, Funovics P. Reconstruction of the pelvis after resection of malignant bone tumours in children and adolescents. Recent Results Cancer Res. 2009;179:85–111. doi: 10.1007/978-3-540-77960-5_8. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs B, Pritchard DJ. Etiology of osteosarcoma. Clin Orthop Relat Res. 2002;397:40–52. doi: 10.1097/00003086-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Picci P. Osteosarcoma (osteogenic sarcoma) Orphanet J Rare Dis. 2007;2:6. doi: 10.1186/1750-1172-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen G, Marcove RC, Caparros B, Nirenberg A, Kosloff C, Huvos AG. Primary osteogenic sarcoma: The rationale for preoperative chemotherapy and delayed surgery. Cancer. 1979;43:2163–2177. doi: 10.1002/1097-0142(197906)43:6<2163::AID-CNCR2820430602>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 7.Kulchitsky VA, Potkin VI, Zubenko YS, Chernov AN, Talabaev MV, Demidchik YE, Petkevich SK, Kazbanov VV, Gurinovich TA, Roeva MO, et al. Cytotoxic effects of chemotherapeutic drugs and heterocyclic compounds at application on the cells of primary culture of neuroepithelium tumors. Med Chem. 2012;8:22–32. doi: 10.2174/157340612799278298. [DOI] [PubMed] [Google Scholar]
- 8.Tolonen A, Pakonen M, Hohtola A, Jalonen J. Phenylpropanoid glycosides from Rhodiola rosea. Chem Pharm Bull (Tokyo) 2003;51:467–470. doi: 10.1248/cpb.51.467. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Zhang YJ, Liu WW, Shi AW, Gu N. Salidroside suppresses HUVECs cell injury induced by oxidative stress through activating the Nrf2 signaling pathway. Molecules. 2016;21:21. doi: 10.3390/molecules21081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang DW, Kang OH, Lee YS, Han SH, Lee SW, Cha SW, Seo YS, Mun SH, Gong R, Shin DW, et al. Anti-inflammatory effect of salidroside on phorbol-12-myristate-13-acetate plus A23187-mediated inflammation in HMC-1 cells. Int J Mol Med. 2016;38:1864–1870. doi: 10.3892/ijmm.2016.2781. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Yu H, Zhao X, Lin X, Tan C, Cao G, Wang Z. Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem Int. 2010;57:547–555. doi: 10.1016/j.neuint.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Shen WS, Gao CH, Deng LC, Shen D. Protective effects of salidroside on epirubicin-induced early left ventricular regional systolic dysfunction in patients with breast cancer. Drugs R D. 2012;12:101–106. doi: 10.2165/11632530-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun C, Wang Z, Zheng Q, Zhang H. Salidroside inhibits migration and invasion of human fibrosarcoma HT1080 cells. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2012;19:355–363. doi: 10.1016/j.phymed.2011.09.070. [DOI] [PubMed] [Google Scholar]
- 14.Liu Z, Li X, Simoneau AR, Jafari M, Zi X. Rhodiola rosea extracts and salidroside decrease the growth of bladder cancer cell lines via inhibition of the mTOR pathway and induction of autophagy. Mol Carcinog. 2012;51:257–267. doi: 10.1002/mc.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Li JZ, Lu AX, Zhang KF, Li BJ. Anticancer effect of salidroside on A549 lung cancer cells through inhibition of oxidative stress and phospho-p38 expression. Oncol Lett. 2014;7:1159–1164. doi: 10.3892/ol.2014.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao G, Shi A, Fan Z, Du Y. Salidroside inhibits the growth of human breast cancer in vitro and in vivo. Oncol Rep. 2015;33:2553–2560. doi: 10.3892/or.2015.3857. [DOI] [PubMed] [Google Scholar]
- 17.Lv C, Huang Y, Liu ZX, Yu D, Bai ZM. Salidroside reduces renal cell carcinoma proliferation by inhibiting JAK2/STAT3 signaling. Cancer Biomark. 2016;17:41–47. doi: 10.3233/CBM-160615. [DOI] [PubMed] [Google Scholar]
- 18.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 19.Faisham WI, Mat Saad AZ, Alsaigh LN, Nor Azman MZ, Kamarul Imran M, Biswal BM, Bhavaraju VM, Salzihan MS, Hasnan J, Ezane AM, et al. Prognostic factors and survival rate of osteosarcoma: A single-institution study. Asia Pac J Clin Oncol. 2017;13:e104-e110. doi: 10.1111/ajco.12346. [DOI] [PubMed] [Google Scholar]
- 20.Hu X, Lin S, Yu D, Qiu S, Zhang X, Mei R. A preliminary study: The anti-proliferation effect of salidroside on different human cancer cell lines. Cell Biol Toxicol. 2010;26:499–507. doi: 10.1007/s10565-010-9159-1. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Zhao Y, Zheng C, Meng Y, Yang Y. Synthesis, biological activity of salidroside and its analogues. Chem Pharm Bull (Tokyo) 2010;58:1627–1629. doi: 10.1248/cpb.58.1627. [DOI] [PubMed] [Google Scholar]
- 22.Farzaei MH, Bahramsoltani R, Rahimi R. Phytochemicals as adjunctive with conventional anticancer therapies. Curr Pharm Des. 2016;22:4201–4218. doi: 10.2174/1381612822666160601100823. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, Cao G, Wang Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol. 2007;564:18–25. doi: 10.1016/j.ejphar.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Chen C. Inhibition of autophagy enhances synergistic effects of Salidroside and anti-tumor agents against colorectal cancer. BMC Complement Altern Med. 2017;17:538. doi: 10.1186/s12906-017-2046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi Z, Tang T, Sheng L, Ma Y, Liu Y, Yan L, Qi S, Ling L, Zhang Y. Salidroside inhibits the proliferation and migration of gastric cancer cells via suppression of Src-associated signaling pathway activation and heat shock protein 70 expression. Mol Med Rep. 2018;18:147–156. doi: 10.3892/mmr.2018.8958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Normile D. Cell proliferation. Common control for cancer, stem cells. Science. 2002;298:1869. doi: 10.1126/science.298.5600.1869. [DOI] [PubMed] [Google Scholar]
- 27.Hu X, Ma J, Vikash V, Li J, Wu D, Liu Y, Zhang J, Dong W. Thymoquinone augments cisplatin-induced apoptosis on esophageal carcinoma through mitigating the activation of JAK2/STAT3 pathway. Dig Dis Sci. 2018;63:126–134. doi: 10.1007/s10620-017-4856-8. [DOI] [PubMed] [Google Scholar]
- 28.Lv C, Hao Y, Han Y, Zhang W, Cong L, Shi Y, Tu G. Role and mechanism of microRNA-21 in H2O2-induced apoptosis in bone marrow mesenchymal stem cells. J Clin Neurosci. 2016;27:154–160. doi: 10.1016/j.jocn.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 29.Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 30.Lin W, Zhu X, Yang S, Chen X, Wang L, Huang Z, Ding Y, Huang L, Lv C. MicroRNA-203 inhibits proliferation and invasion, and promotes apoptosis of osteosarcoma cells by targeting Runt-related transcription factor 2. Biomed Pharmacother. 2017;91:1075–1084. doi: 10.1016/j.biopha.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 31.Hu X, Zhang X, Qiu S, Yu D, Lin S. Salidroside induces cell-cycle arrest and apoptosis in human breast cancer cells. Biochem Biophys Res Commun. 2010;398:62–67. doi: 10.1016/j.bbrc.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 32.Tesser-Gamba F, Lopes LJ, Petrilli AS, Toledo SR. MAPK7 gene controls proliferation, migration and cell invasion in osteo-sarcoma. Mol Carcinog. 2016;55:1700–1713. doi: 10.1002/mc.22420. [DOI] [PubMed] [Google Scholar]
- 33.Yu C, Wang W. Relationship between P15 gene mutation and formation and metastasis of malignant osteosarcoma. Med Sci Monit. 2016;22:656–661. doi: 10.12659/MSM.895022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui Y, Zhu JJ, Ma CB, Cui K, Wang F, Ni SH, Zhang ZY. Genetic polymorphisms in MMP 2, 3 and 9 genes and the susceptibility of osteosarcoma in a Chinese Han population. Biomarkers. 2016;21:160–163. doi: 10.3109/1354750X.2015.1118550. [DOI] [PubMed] [Google Scholar]
- 35.Lv C, Yang S, Chen X, Zhu X, Lin W, Wang L, Huang Z, Wang M, Tu G. MicroRNA-21 promotes bone mesenchymal stem cells migration in vitro by activating PI3K/Akt/MMPs pathway. J Clin Neurosci. 2017;46:156–162. doi: 10.1016/j.jocn.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 36.Fan XJ, Wang Y, Wang L, Zhu M. Salidroside induces apoptosis and autophagy in human colorectal cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol Rep. 2016;36:3559–3567. doi: 10.3892/or.2016.5138. [DOI] [PubMed] [Google Scholar]
- 37.Kang DY, Sp N, Kim DH, Joung YH, Lee HG, Park YM, Yang YM. Salidroside inhibits migration, invasion and angiogenesis of MDA-MB 231 TNBC cells by regulating EGFR/Jak2/STAT3 signaling via MMP2. Int J Oncol. 2018;53:877–885. doi: 10.3892/ijo.2018.4430. [DOI] [PubMed] [Google Scholar]
- 38.Bill MA, Nicholas C, Mace TA, Etter JP, Li C, Schwartz EB, Fuchs JR, Young GS, Lin L, Lin J, et al. Structurally modified curcumin analogs inhibit STAT3 phosphorylation and promote apoptosis of human renal cell carcinoma and melanoma cell lines. PLoS One. 2012;7:e40724. doi: 10.1371/journal.pone.0040724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun KX, Xia HW, Xia RL. Anticancer effect of salidroside on colon cancer through inhibiting JAK2/STAT3 signaling pathway. Int J Clin Exp Pathol. 2015;8:615–621. [PMC free article] [PubMed] [Google Scholar]
- 40.Mahmoud AM, Abd El-Twab SM. Caffeic acid phenethyl ester protects the brain against hexavalent chromium toxicity by enhancing endogenous antioxidants and modulating the JAK/STAT signaling pathway. Biomed Pharmacother. 2017;91:303–311. doi: 10.1016/j.biopha.2017.04.073. [DOI] [PubMed] [Google Scholar]
- 41.Tian Y, Zhang W, Xia D, Modi P, Liang D, Wei M. Postconditioning inhibits myocardial apoptosis during prolonged reperfusion via a JAK2 STAT3-Bcl-2 pathway. J Biomed Sci. 2011;18:53. doi: 10.1186/1423-0127-18-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu KJ, Huang JM, Zhong HJ, Dong ZZ, Vellaisamy K, Lu JJ, Chen XP, Chiu P, Kwong DWJ, Han QB, et al. A natural product-like JAK2/STAT3 inhibitor induces apoptosis of malignant melanoma cells. PLoS One. 2017;12:e0177123. doi: 10.1371/journal.pone.0177123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shokoohinia Y, Jafari F, Mohammadi Z, Bazvandi L, Hosseinzadeh L, Chow N, Bhattacharyya P, Farzaei MH, Farooqi AA, Nabavi SM, et al. Potential anticancer properties of osthol: A comprehensive mechanistic review. Nutrients. 2018;10:10. doi: 10.3390/nu10010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi X, Zhao W, Yang Y, Wu S, Lv B. Salidroside could enhance the cytotoxic effect of L-OHP on colorectal cancer cells. Mol Med Rep. 2018;17:51–58. doi: 10.3892/mmr.2017.7846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.