Abstract
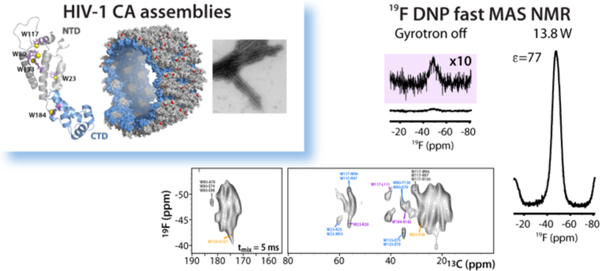
We report remarkably high, up to 100-fold, signal enhancements in 19F dynamic nuclear polarization (DNP) magic angle spinning (MAS) spectra at 14.1 T on HIV-1 CA capsid protein assemblies. These enhancements correspond to absolute sensitivity ratios of 12–29 and are of similar magnitude as seen for 1H signals in the same samples. At MAS frequencies above 20 kHz, it was possible to record 2D 19F-13C HETCOR spectra, which contain long-range intra- and intermolecular correlations. Such correlations provide unique distance restraints, inaccessible in conventional experiments without DNP for protein structure determination. Furthermore, systematic quantification of the DNP enhancements as a function of biradical concentration, MAS frequency, temperature, and microwave power is reported. Our work establishes the power of DNP-enhanced 19F MAS NMR spectroscopy for structural characterization of HIV-1 CA assemblies and this approach is anticipated to be applicable to a wide range of large biomolecular systems.
Keywords: magic angle spinning, 19F NMR, HIV-1 capsid, dynamic nuclear polarization, DNP
Graphical Abstract
INTRODUCTION
Magic angle spinning (MAS) NMR spectroscopy is a powerful tool for structural characterization of a wide range of biologically important materials, including protein assemblies and aggregates not amenable to other structural methods.1 Atomic-resolution insights were recently gained by MAS NMR studies into poorly tractable systems, such as viral capsids,2–4 microtubules,5 actin and their associated proteins,6 and aggregates of misfolded proteins.7,8 Nevertheless, such studies still remain challenging because of low sensitivity and/or limited resolution, impeding widespread applications to large biological assemblies.
Dynamic nuclear polarization (DNP) is an attractive approach for sensitivity enhancement in NMR experiments, exploiting the transfer of polarization from electron spins to nuclear spins.9,10 To that end, microwave irradiation is applied to saturate electron paramagnetic resonance (EPR) transitions, causing polarization transfer via several distinct mechanisms.11,12 The theoretically attainable highest DNP enhancement factors, ε, are approximately equal to the ratio of gyromagnetic ratios of the electron to the nuclear spins; for the common biologically relevant atoms -- 1H, 13C, and 15N -- these factors are ~660, 2,624, and 6,511, respectively. 1H enhancements as high as 250 have been experimentally observed in biological solids.13 In practice, transferring electron polarization in DNP-enhanced NMR spectroscopy requires the presence of paramagnetic centers in the samples. These can be endogenous paramagnetic groups14 or externally introduced paramagnetic species.15 Most commonly, samples are doped with suitable stable radicals or biradicals in an appropriate glass-forming matrix.15
DNP-enhanced MAS NMR spectroscopy of biological systems is an area of intense interest and active development, following in the footsteps of the seminal work from the Griffin group on bacteriorhodopsin and bacteriophages.16,17 With the availability of commercial instrumentation at magnetic fields of up to 21.1 T, DNP has since been applied to a variety of biological systems, including nanocrystalline peptides,18 membrane proteins,17 amyloid fibrils,19 nucleic acids,13 biomaterials,20–22 cells,23,24 and viral capsid assemblies.25,26 DNP enhancements were found to depend strongly on the detailed experimental conditions, such as magnetic field, temperature, nature and concentration of the radical, to name a few.27 Reported sensitivity gains for 1H polarization vary widely, from relatively modest factors of ε~4–10 for membrane proteins28 and at high magnetic fields of 18.8 T25 to impressive ε~148 for direct 13C excitation in a perdeuterated microcrystalline SH3 protein.29 In our work on non-crystalline HIV-1 capsid tubular assemblies, we observed very large sensitivity gains of up to ε=64 at 14.1 T, permitting detection of resonances from dynamically disordered residues and low-concentration conformers, which are not observable in room-temperature MAS NMR experiments.25
Yet, despite the exciting potential for applications of DNP-enhanced MAS NMR spectroscopy to biological systems, there are limitations preventing the widespread use of the technique. A significant challenge pertains to the severe deterioration of spectral resolution at cryogenic temperatures for a number of biological samples,11,30 although rigid systems can exhibit narrow lines.25,31,32 Low temperatures, typically below 120 K, are essential for attaining high DNP enhancements.33 Broadening of resonances is primarily inhomogeneous in nature, caused by the freezing-out of different conformations of low temperature sub-states,34 and, to a lesser extent, due to the presence of paramagnetic radicals.25,35 Using temperatures in the 160–185 K range can to some degree alleviate the resolution loss.30 Unfortunately, in this temperature range, DNP sensitivity gains are considerably lower than those below 120 K. Indeed, the major impediment for routinely employing DNP on uniformly 13C,15N-labeled large biomolecules is the limited resolution, and our work on HIV-1 capsid assemblies, where spectral overlap is severe for a number of residues, bears this out.25
In order to address the resolution challenges and motivated by the potentially large sensitivity gains, we are exploring 19F DNP-enhanced MAS NMR spectroscopy on several biological systems available in our laboratories. Here, we report on tubular assemblies of the HIV-1 CA capsid protein. These tubes are assembled from the 231-residue CA protein, which is the building block of mature HIV-1 conical capsids. The CA protein contains two independently folded, α-helical N- and C-terminal domains (NTD and CTD, respectively; Figure 1a). In virions, HIV-1 CA forms cone-like pleomorphic structures of varied shapes and appearances,36 while in vitro, CA predominantly forms tubes (Figure 1b–d). Tubular CA assemblies have been extensively characterized experimentally by us and others using MAS NMR,3,4,25,37–41 cryo-EM,42–44 as well as computationally.4,39,40,43 However, at present, an experimental atomic-resolution structure is not yet available. We recently showed that 19F MAS NMR spectroscopy is a powerful technique for structural analysis of HIV-1 CA assemblies,45 and the five tryptophan (Trp) residues in CA can conveniently be used for fluorine incorporation and exploited as effective reporters of structure. The 19F MAS NMR spectrum of 5-fluorotryptophan (5F-Trp) labeled CA was readily assigned by mutagenesis, changing each Trp in turn to either Leu, Ile or Tyr (Figure S1 of the Supporting Information). The resonance frequencies for all 5F-Trp resonances are distinct, reflecting their different local environments, and can be exploited for assessing conformational homo- or heterogeneity in these tubular assemblies. Remarkably, from F-F dipolar-based correlation spectra at MAS frequencies of 40–60 kHz, inter-fluorine distances as long as ~23 Å can be extracted.45 While 19F solid-state NMR has been successfully used to investigate peptides and their interactions with membranes, 46–49 the dramatic benefits of 19F MAS NMR spectroscopy at frequencies exceeding 40 kHz to study biological systems have only recently been demonstrated.45,50–52
Figure 1.
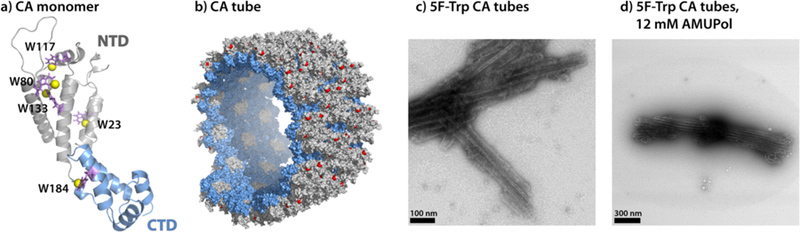
(a) Structure of HIV-1 CA monomer (PDBID: 4XFX). W23, W80, W117, W133 and W184 side chains are shown in purple stick representation with fluorine atoms as yellow spheres. (b) Section through the CA tube (PDBID: 3j4f). (c, d) Transmission electron micrographs of 5F-Trp,U-13C,15N-CA tubes in the absence (c) and presence (d) of 12 mM AMUPol. The scale bars are 100 nm and 300 nm, respectively.
For the 19F DNP experiments described here, the CA protein was uniformly labeled with 13C and 15N as well as specifically labeled with 5F-Trp. Using AMUPol as the paramagnetic biradical,53 DNP sensitivity enhancements up to 100 fold were observed in direct 19F polarization experiments (Figure 2–3). These enhancements are remarkably high and of the same magnitude as the 1H enhancements measured in the same CA tubular assemblies here and previously.25 Under optimally designed experimental conditions, the strong DNP signals, combined with the high spectral resolution at MAS frequencies of 22–30 kHz, enabled us to record 2D 19F-13C correlation experiments. These HETCOR spectra yield unique information about 19F-13C intra- and intermolecular correlations corresponding to long-range distances. Therefore, they potentially complement the 13C-13C and 15N-13C experiments (as well as their proton-mediated versions), commonly used for deriving structural restraints for structure determination.
Figure 2.
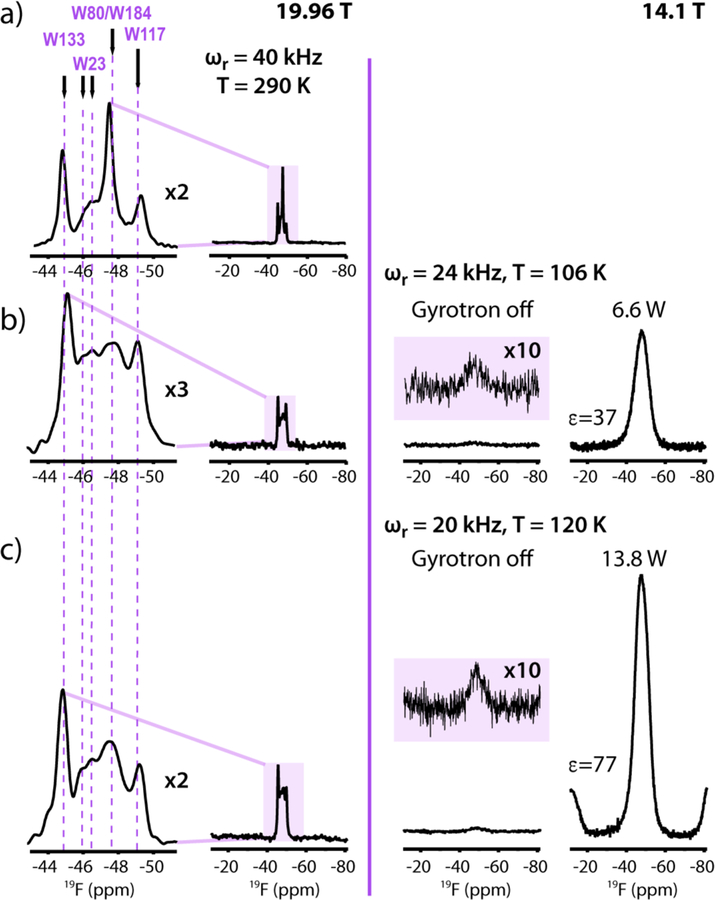
19F MAS and DNP-enhanced 19F MAS NMR spectra of 5F-Trp,U-13C,15N CA assembled into tubes in the absence (a) and presence of 15 mM (b) and 12 mM AMUPol (c), respectively. The spectra in the left panel were acquired at 19.96 T (800.1 MHz 19F Larmor frequency) at a MAS frequency of 40 kHz and 290 K. The spectra shown in the right panel were acquired at 14.1 T (564.8 MHz 19F Larmor frequency) at MAS frequencies of 24 kHz and 20 kHz and temperatures of 106 K and 120 K, respectively. 16 scans and 48 scans were averaged for the spectra in the left and right panels, respectively. The DNP enhancements (maximum intensity: IMWon/IMWoff) were 36 (b) and 62 (c), respectively.
Figure 3.
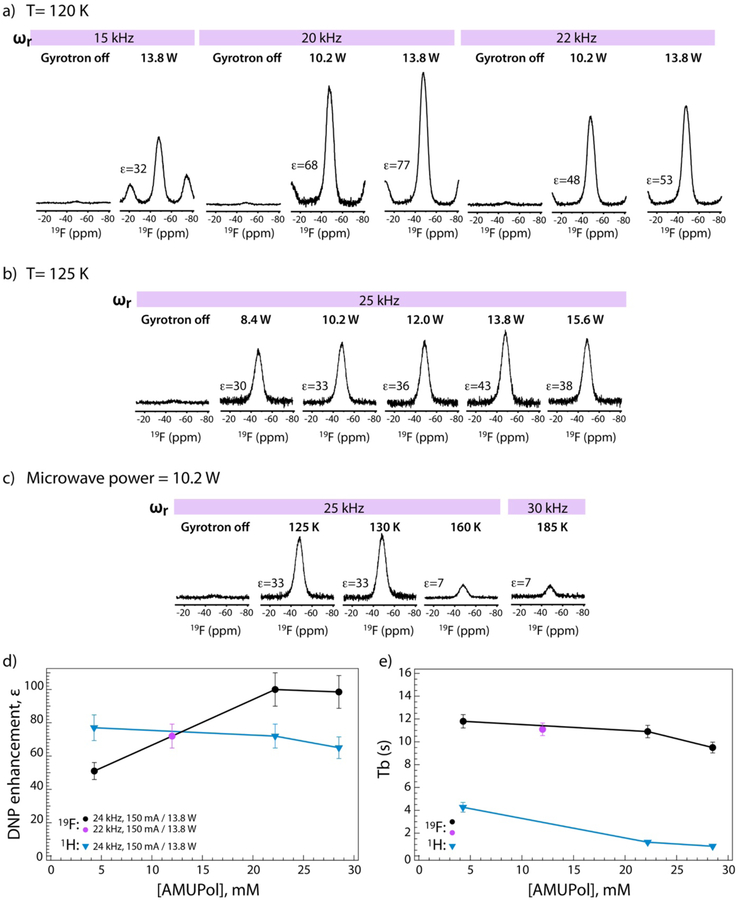
19F MAS and DNP-enhanced MAS NMR spectra of 5F-Trp,U-13C,15N CA, assembled into tubes in the presence of 12 mM (a) and 15 mM (b,c) AMUPol respectively. Spectra were acquired at 14.1 T (564.8 MHz 19F Larmor frequency); the MAS frequencies, temperatures, microwave power, and DNP enhancements for the integrated signal intensity of the isotropic peak are indicated next to each spectrum. 64 scans were averaged for the spectra in panel a), except for the spectrum recorded with a MAS frequency of 20 kHz and MW power of 10.2 W, which was acquired with 16 scans. 32 scans were averaged for all spectra in panels b), c), except for the 160 K spectrum, which was acquired with 440 scans. d), e) 19F and 1H DNP signal intensity enhancements, ε, and buildup time constants, Tb, for 5F-Trp,U-13C,15N CA tubular assemblies, plotted against AMUPol concentration. These data correspond to a sample temperature of 120 K, a MAS frequency of 24 kHz, and microwave power of 150 mA/13.8 W, except in d) for the samples containing 12 mM AMUPol (22 kHz).
To the best of our knowledge, the work presented here is the first 19F DNP-enhanced MAS NMR study of any biological system. Our results serve as proof-of-concept for the potential of this approach to probe structural features in proteins and large protein assemblies.
MATERIALS AND METHODS
Sample preparation
5-fuoroindole (Sigma Aldrich) was used as the precursor to uniformly incorporate fluorine at position 5 into all Trp residues of the HIV-1 CA protein.54 5-19F-Trp,U-13C,15N-labeled CA proteins were expressed and purified as reported previously with modifications.38,55 In brief, 5-19F-Trp,U-13C,15N-labeled CA was expressed in modified M9 medium, containing 2 g of 15NH4Cl, 2 g of U-13C6-glucose and 20 mg of 5-fuoroindole per 1 L medium, using 0.8 mM IPTG for induction. Cells were grown at 18 °C and harvested after 16 h by centrifugation. Cell pellets were suspended in 25 mM sodium phosphate buffer (pH 7.0), ruptured by sonication on ice, and the cell debris was removed by centrifugation at 27,000 g at 4 °C for 1 h. The pH of the supernatant was adjusted to 5.8 with acetic acid and the conductivity was adjusted to below 2.5 ms/cm by dilution, followed by an additional centrifugation at 27,000 g at 4 °C for 1 h. The final supernatant was loaded onto a cation exchange column (HiTrap SP HP 5 mL, GE Healthcare) and the protein was eluted with a 0–1 M NaCl gradient in 25 mM sodium phosphate buffer (pH 5.8), 1 mM DTT, 0.02% NaN3. Fractions containing CA protein were pooled and further purified by gel filtration using a size-exclusion column (HiLoad 26/600 Superdex 75 prep grade, GE Healthcare), equilibrated with 25 mM sodium phosphate buffer (pH 5.5), 1 mM DTT, 0.02% NaN3. Fractions containing CA protein were combined and concentrated to 30 mg/mL. 5-19F-Trp,U-13C,15N-labeled CA tubular assemblies were prepared from 30 mg/mL protein solutions in 25 mM phosphate buffer, 2.4 M NaCl (pH 6.5) by incubation at 37°C for 1 h and at 4°C overnight. 12.7 and 14 mg of protein were pelleted at 10,000 g and packed into two 1.9 mm thin-wall Bruker rotors.
For the DNP samples, we followed the general protocol established by us previously,25 with slight modifications. The biradical, AMUPol53 was added to 11.0, 12.7, 14, 11.7, and 11.6 mg of pelleted tubes at a final concentration of 4.3, 12, 15, 22.8, and 28.2 mM. The pellets were gently stirred, until AMUPol dissolved and the color of the tubular assemblies turned light orange. 20% (v/v) glycerol-d8 buffer containing 1 M NaCl was added on top, without disturbing the pellet, and the sample was incubated overnight at 4 °C. Excess glycerol solution was removed and the resulting DNP samples were pelleted at 10,000 g and packed into 1.9 mm Bruker rotors. The concentration of biradical AMUPol was measured by a Bruker benchtop EMXnano EPR spectrometer prior to DNP experiments.
Sample morphology was characterized by transmission electron microscopy (TEM). For the sample of tubular assemblies of 5-19F-Trp,U-13C,15N-CA, doped with 15 mM AMUPol, the images were acquired on a Zeiss Libra 120 transmission electron microscope operating at 120 kV. For the sample doped with 12 mM AMUPol, the images were acquired on a Talos F200C transmission electron microscope operating at 200 kV, equipped with a Ceta 16 M camera. Assemblies were stained with uranyl acetate (0.5–1% w/v), deposited onto 400 mesh, formval/carbon-coated copper grids, and dried for 45 min in the air. The copper grids were glow discharged prior to staining, so that the tubular assemblies are uniformly spread on the grid surface and adhere to it.
MAS NMR spectroscopy
19F and 13C-detected MAS NMR experiments were recorded on a 19.96 T Bruker AVANCE III spectrometer, outfitted with 1.9 mm HX MAS probe. The Larmor frequencies were 850.4 MHz (1H), 213.8 MHz (13C), and 800.1 MHz (19F). The sample temperature was calibrated using KBr as a temperature sensor56 and was maintained to ±1 °C throughout the experiments using a Bruker temperature controller.
The 19F and 13C-detected spectra were collected at the MAS frequency of 40 kHz at 290 K. The typical pulse lengths were 3.2 μμs for 1H, 3.9 μs for 13C, and 3.2 μs for 19F. 1H−13C cross-polarization (CP) was performed with a linear amplitude ramp (80−100%). The CP contact time was 0.7–1.2 ms. The 13C-13C correlation spectrum was acquired using RFDR57 with a mixing time of 3.2 ms and 1H swfTPPM58 decoupling (10 kHz).
19F chemical shifts were indirectly referenced to the adamantane-referenced 13C chemical shifts.59 5-19F-DL-Trp powder was used as a secondary reference standard at −44.6 ppm (290 K). The 1H, 13C and 15N chemical shifts were referenced with respect to DSS, adamantane and ammonium chloride as external referencing standards.
19F DNP-enhanced MAS NMR spectroscopy
19F and 1H DNP-enhanced MAS NMR experiments were performed in the Bruker Billerica laboratories on an Avance III HD SSNMR spectrometer, equipped with a 1.9-mm HXY(FXY) low temperature MAS probe, capable of irradiation at either 1H or 19F frequencies. At 14.1 T, the Larmor frequencies were 600.1 MHz (1H), 564.8 MHz (19F), and 150.9 MHz (13C). MW irradiation at a frequency of 395.18 GHz was generated by a second-harmonic gyrotron, capable of delivering in excess of 50 W irradiation at the sample. The dependence of intensity enhancement on the MW power was evaluated for 6.6 to 15.6 W. 19F measurements were performed at 106 K, 109 K, 120 K, 125 K, 130 K, 160 K, and 185 K, with the sample temperature calibrated using KBr as a temperature sensor.56 The actual sample temperature was determined using calibration curves of K79Br T1’s vs. the gyrotron power and stator temperature. The typical pulse lengths were 1.5 μs (1H), 1.25 μs (19F), and 2.5 μs (13C). 19F spectra were acquired at MAS frequencies of 15 kHz, 20 kHz, 22 kHz, 24 kHz, 25 kHz, and 30 kHz, controlled by a Bruker MAS3 controller. 1H DNP-enhanced spectra were acquired at 120 K and a MAS frequency of 24 kHz.
The 19F-13C DNP HETCOR correlation spectra were acquired on the sample containing 15 mM AMUPol at the temperature of 120 K. MAS frequencies were 22 and 25 kHz and 90° pulse lengths were 3.0 μs (13C), and 1.25 μs (19F). 19F-13C cross-polarization was achieved using rf fields of 108 kHz for 19F and 84 kHz for 13C; a 10% tangential amplitude ramp was applied to 19F. HETCOR mixing times during the 19F-13C cross-polarization step were 0.5, 1.5, 2.5, and 5 ms. All spectra were acquired as (2048 × 48) complex matrices with a recycle delay of 6 s and 128 transients for each t1 point, resulting in a total acquisition time of 10.24 hrs. States-TPPI phase sensitive detection60 was used for frequency discrimination in the indirect t1 dimension.
Data processing and analysis
All spectra were processed in TopSpin and with NMRpipe,61 and analyzed using Sparky.62 For 2D 13C-13C data sets, 60° or 90° shifted sine bell apodization, followed by Lorentzian-to Gaussian transformation were applied in both dimensions. The 19F-13C HETCOR and 19F-19F DQ-SQ spectra were processed with 90-degree phase shifted sinebell and Lorentzian-to-Gaussian apodization in the direct and indirect dimensions, respectively. Resonance assignments were based on available 13C and 19F chemical shifts of CA assemblies,38,39 guided by and cross-checked against structural models. Assignments for particular cross peaks were deemed unambiguous if no other correlations were present at the corresponding (f2, f1) pair of frequencies or if only long-range correlations are possible. In the latter case, the cross peak was assigned to the correlation that corresponds to the shortest possible distance.
RESULTS AND DISCUSSION
Spectral Resolution: Effect of AMUPol Biradical and Temperature
19F MAS NMR spectra of tubular 5F-Trp,U-13C,15N-CA assemblies at 19.96 T and 290 K are displayed in Figure 2 (left panel). The chemical shifts range from −44.7 to −49.1 ppm and assignments were obtained via mutagenesis, as detailed in45 and illustrated in Figure S1 of the Supporting Information. While this protocol requires preparation of several samples, which may be challenging in some systems, in general mutagenesis is significantly less difficult and time consuming than performing de novo overall resonance assignments, particularly in large proteins and protein assemblies, such as the HIV-1 CA assemblies studied here.
Doping of the sample with the AMUPol biradical does not interfere with the assembly (Figure 1d), but introduces broadening of one of the peaks, namely the signal at −47.3 ppm (Figure 2 b-c). This peak comprises two resonances: the narrow W80 resonance, superimposed on the broad W184 signal. As illustrated in Figure 1b, W80 is located on the solvent exposed surface of the tube, while W184 is buried and resides at the intermolecular CTD-CTD dimer interface. Therefore, the most likely reason for the broadening of the W80 resonance is the proximity of the sidechain to the biradical AMUPol. We also note that the loss of the W80 resonance intensity and the reduction of the overall spectral resolution is more pronounced in the sample with 15 mM biradical, compared to the 12 mM AMUPol containing sample. The relative peak intensities and linewidths for the resolved resonances, W23, W117, and W133, are essentially identical to those in the biradical-free MAS NMR spectra. Only minor effects on the 13C chemical shifts and line widths were induced by the presence of 12 mM AMUPol (Figure S2 and Table S1 of the Supporting Information). 37 resonances exhibit small intensity or chemical shift changes, with chemical shift perturbations ranging from 0.1 to 0.6 ppm. The largest effects are seen for the H84 and G89 resonances, which are associated with surface-exposed residues in the CypA-binding loop. These results indicate that the contribution of paramagnetic line broadening induced by the biradical is generally small at 290 K, consistent with previous studies.25
At cryogenic temperatures of 107 and 120 K, line broadening is dramatic (Figure 2; two right side panels). The 19F spectrum is featureless and a single peak with a line width of ~8 ppm is detected. Broadening is also observed in the 13C spectra at 120 K (Figures S3 and S4). The linewidths are 120–300 Hz, increase with increasing AMUPol concentration, and do not depend strongly on whether protons are decoupled or not. For comparison, at 290 K, the linewidths are 45–70 Hz. This is consistent with our previous observations in the 1D 13C DNP spectra of HIV-1 CA assemblies.25 In the future it will be desirable and necessary to systematically characterize the 19F linewidths under DNP conditions in order to evaluate relative contributions of homogeneous paramagnetic vs. inhomogeneous broadening. Such a study requires proton decoupling and fast MAS frequencies, currently not possible with the available DNP probe hardware.
19F and 1H DNP Buildup Times and Overall Enhancements
To systematically evaluate the 19F DNP enhancement factors and buildup times, we examined their dependence on microwave power, temperature, MAS frequency, and AMUPol concentration. We chose to work with five concentrations of the AMUPol biradical, 4.3, 12, 15, 22.8, and 28.2 mM. In our prior work on HIV-1 CA assemblies, the AMUPol concentrations ranged from 8 to 10 mM.25 Our findings are summarized in Table 1 and Figure 3 and discussed below.
Table 1.
Summary of 19F and 1H signal enhancements in 14.1 T DNP MAS NMR spectra of 5F-Trp,U-13C,15N CA tubular assemblies under different experimental conditions.*
| Biradical concentration (mM) | T (K) | MAS frequency (kHz) | Microwave power (mA / W**) | 19F DNP polarization buildup time, Tb (s) | 19F DNP signal enhancement | 1H DNP polarization buildup time, Tb (s) | 1H DNP signal enhancement | |
|---|---|---|---|---|---|---|---|---|
| integrated intensity (isotropic peak) | integrated intensity (all side bands) | integrated intensity | ||||||
| 4.3 | 120 | 24 | 120 / 8.4 | 11.8 | 29 | 37 | 4.3 | 63 |
| 130 / 10.2 | 34 | 45 | 71 | |||||
| 140 / 12.0 | 38 | 48 | 76 | |||||
| 150 / 13.8 | 40 | 51 | 77 | |||||
| 160 / 15.6 | 41 | 53 | 76 | |||||
| 12 | 120 | 15 | 150 / 13.8 | 11.1 | 32 | 36 | ||
| 20 | 130 / 10.2 | 68 | 70 | |||||
| 150 / 13.8 | 77 | 82 | ||||||
| 22 | 130 / 10.2 | 50 | 60 | |||||
| 150 / 13.8 | 66 | 72 | ||||||
| 15 | 106 | 24 | 110 / 6.6 | 18.9 | 37 | 46 | ||
| 120 | 140 / 12.0 | 58.4 | 72 | 49 | ||||
| 150 / 13.8 | 1.4 | 50 | ||||||
| 125 | 25 | 120 / 8.4 | 14.4 | 30 | 36 | |||
| 130 / 10.2 | 33 | 39 | ||||||
| 140 / 12.0 | 36 | 43 | ||||||
| 150 / 13.8 | 43 | 51 | ||||||
| 160 / 15.6 | 38 | 44 | ||||||
| 130 | 25 | 130 / 10.2 | 33 | 38 | ||||
| 160 | 25 | 130 / 10.2 | 12.1 | 7 | 8 | |||
| 185 | 30 | 130 / 10.2 | 7 | 7 | ||||
| 22.2 | 120 | 24 | 150 / 13.8 | 10.9 | 79 | 100 | 1.2 | 72 |
| 28.5 | 120 | 24 | 150 / 13.8 | 9.5 | 80 | 98.5 | 0.9 | 65 |
The DNP enhancement factors were calculated as ratios of the integrated peak intensities for maximum peak intensities with and without the microwave irradiation.
Microwave power at the end of the waveguide.
Microwave power.
The 19F and 1H DNP enhancements are very sensitive to the applied microwave power (Table 1 and Figure 3a,b). Signal saturation occurs at 13.8 W output power, where maximum gains and the fastest buildup times are observed. This condition was used for all 2D experiments.
Temperature.
Temperature also has profound effects on the DNP enhancements, consistent with prior findings for all nuclei and all types of samples.12 Increasing the temperature from 106 K to 185 K revealed a systematic decrease in the 19F enhancement factors from 37 to 7 for the sample doped with 15 mM AMUPol (Table 1 and Figure 3c). Previous reports suggested that at temperatures of 160–185 K a suitable compromise is reached between the spectral resolution and sensitivity in 13C-detected DNP experiments at 9.4 T.30 This is not the case for 19F DNP spectra as shown here: resolution is not regained under these conditions and the signal enhancements are prohibitively low (Figure 3c). Consequently, we chose to work at 120 K.
MAS frequency.
Another key experimental variable is the MAS frequency, and MAS frequency dependence in DNP experiments remains poorly understood. Several conflicting reports exist and some suggest that the overall sensitivity in 13C-detected CPMAS experiments drops systematically with increased spinning rates above 10 kHz,63 and others showing only modest dependence on MAS and high enhancements at frequencies of 40 kHz.64–67 For 19F MAS NMR experiments conducted at temperatures above 0 °C, we recently demonstrated that fast MAS frequencies (40–60 kHz) are essential for obtaining high resolution. In addition, fast MAS frequencies alleviate the need for 1H decoupling.52 At present, the 1.9 mm probe suitable for DNP experiments can deliver MAS frequencies of up to 25 kHz, limiting our evaluation of higher frequencies. As illustrated in Figure 3a and summarized in Table 1, the highest integrated signal enhancements for the isotropic peak were observed at a MAS frequency of 20 kHz. The enhancements drop by ~10–17% at 22 kHz. At 15 kHz, the spinning sideband intensities are large, since the reduced anisotropy of the 19F CSA tensor is of the order of 44–46 ppm in the 5F-Trp-CA assemblies.45 The integrated signal enhancement over the entire manifold of sidebands is 36 at this spinning frequency. Because of the large side band contributions, this condition is generally impractical for 2D 19F-based correlation spectroscopy. The integrated signal enhancement associated with the isotropic peak is 32. Therefore, our results indicate that MAS frequencies of 20–25 kHz are optimal in terms of the sensitivity enhancements and averaging the 19F CSA contribution.
Concentration of the AMUPol biradical.
We and others have noted that different 1H and 13C DNP enhancement factors are measured in proteins and protein assemblies, even if very similar sample preparations are used.25 It appears that the optimum biradical concentration depends on the details of the biological system under study, although in general concentrations of 6–50 mM seem to work satisfactorily (see a recent review12). For example, a study by Oschkinat and coworkers on proline demonstrated that it is necessary to carefully optimize the TOTAPOL concentrations in each sample for optimal sensitivity.68 For increasing biradical concentrations, it has been reported that 1H intensity enhancements increase and effective T1 times decrease (both of which contribute to enhanced sensitivity). However, with growing biradical concentrations, line broadening also increases, while the number of detectable sites decreases, resulting in overall reduced sensitivity. It is therefore important to find the right balance between these effects for any new system, in order to achieve optimal sensitivity gains. The dependence of 19F signal enhancements and the DNP buildup times on AMUPol biradical concentration is discussed below and compared to that for the 1H signals.
DNP signal buildup:
Buildup times of the 19F and 1H DNP signal intensities for the different samples and sets of experimental conditions are provided in Table 1. The 19F polarization buildup time decreases with increasing temperature, from 18.6 s at 106 K to 11.3 s at 160 K, for the sample containing 15 mM AMUPol. Interestingly, the 19F DNP buildup times are not strongly dependent on the AMUPol concentration: at 120 K, the buildup time constant (Tb) decreases from 11.8 s to 9.5 s when the AMUPol concentration is increased from 4.3 to 28.5 mM. In contrast, the buildup times for the 1H DNP signals depend strongly on the AMUPol concentration, decreasing from 4.3 s to 0.9 s. Long buildup times generally impose long recycle delays in the DNP experiments. The optimal recycle delays for 19F DNP experiments, where maximum sensitivity is attained per unit of time lie between 4 and 16 s (Table S2 of the Supporting Information). Therefore, we chose a recycle delay of 6 s in all 2D 19F-13C HETCOR experiments, see below.
DNP enhancements:
19F DNP MAS NMR single-pulse excitation spectra of 5F-Trp,U-13C,15N-CA tubular assemblies exhibit very large sensitivity gains, which grow with the increased AMUPol concentration, as shown in Table 1 and Figure 3d. At T = 120 K, a MAS frequency of 24 kHz, and AMUPol concentration of 4.3 mM, the signal enhancement, integrated over all sidebands, is 51 and 53 for microwave powers of 13.8 and 15.6 W, respectively. The largest enhancement factor of 100 is obtained for samples with 22.8 and 28.2 mM AMUPol.
Gratifyingly, these enhancements are similar to those for the 1H signals measured in the 1H-13C DNP CPMAS spectra of identical samples, see Table 1 and Figure 3. The largest integrated 1H signal enhancement is 76–77, observed in the sample containing 4.3 mM AMUPol. Interestingly, the 1H enhancements do not depend strongly on biradical concentration. The values of the 1H enhancements measured here generally agree well with our previous findings in U-13C,15N-CA tubular assemblies, prepared with biradical concentrations of ~ 8 mM, where 64-fold enhancements were observed at T = 109 K.25
The high 19F enhancements observed here are remarkable and suggest that polarization transfer from the electrons to the fluorine nuclei (including in the buried sites) is highly efficient, despite the low number of fluorine sites (five) in the protein. This density of the 19F sites is much lower than in any prior DNP studies utilizing protons, such as in our DNP investigations of HIV-1 CA assemblies reported here and previously,25 and even when diluted by deuterium.29 Hence, the 19F-19F spin diffusion rates in the present study are expected to be much slower than those for the 1H-1H spin diffusion. Indeed, this assertion is supported by the dependence of the DNP buildup times on the AMUPol concentration. The 19F Tb are 9.5 – 14.4 s at T = 120–125 K and depend only weakly on the biradical concentration. In contrast, the 1H Tb are much shorter, 0.9 – 4.3 s, and exhibit a strong biradical concentration dependence. Taken together, these results indicate that 19F DNP signal enhancements are not the result of a relayed effect. In the future it will be important to examine the contribution of spin diffusion to the 19F DNP buildup rates and overall enhancements. This requires the preparation of several samples with a varying number of 19F sites per molecule and/or per CA tube (e.g., by mixing the 19F-labeled and nonfluorinated CA in different ratios prior to assembly), and/or the introduction of covalently linked biradical tags in close proximity to one of the 19F sites such that the polarization transfer originates on a particular fluorine site and then propagates to the other sites through spin diffusion. Larger enhancements and shorter buildup times could potentially be obtained by the addition of fluorinated molecules in the polarization matrix, according to a recent study,69 albeit this may be difficult to achieve in practice for aqueous protein preparations.
The absolute sensitivity ratios (ASR)70 between the DNP-enhanced experiments at cryogenic temperatures and non-DNP experiments at 290 K were determined from the signal-to-noise ratios relative to the maximum-intensity peak in the spectra, per unit of experiment time, per mg of sample. They lie in the range of 12–29, see Table S2 of the Supporting Information.
Taken together, the above results establish a range of favorable experimental conditions for 19F DNP-enhanced MAS NMR experiments, taking into consideration currently available probe hardware. These conditions include temperatures of 106–120 K and MAS frequencies of 20–25 kHz and above. The optimum AMUPol concentration for these preparations is 4.3–12 mM, where a satisfactory compromise is achieved between high 19F and 1H enhancements, relatively short signal buildup times, and reasonably narrow 13C lines.
DNP-Enhanced 19F-13C Heteronuclear Correlation Spectroscopy
DNP 2D 19F-13C HETCOR spectra acquired with mixing times of 0.5, 1.5, 2.5, and 5 ms are displayed in Figure 4. Such spectra would be very difficult to record without DNP enhancements at any temperature, given the low efficiency of 19F-13C cross polarization at room temperature (illustrated in Figure S5 of the Supporting Information for a 19F-13C CPMAS spectrum acquired at T = 290 K.). Based on the cross peak intensities, the polarization enhancement appears to be equally distributed between the different 19F sites.
Figure 4.
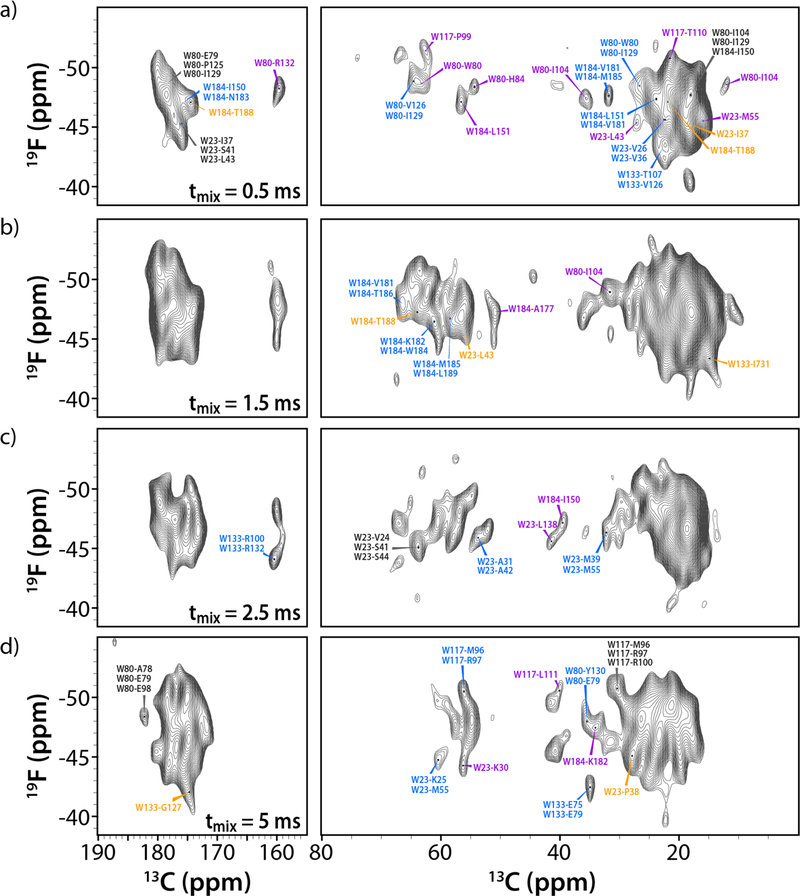
19F-13C DNP-enhanced HETCOR spectra of 5F-Trp,U-13C,15N CA tubular assemblies containing 15 mM AMUPol. The spectra were acquired at 14.1 T (564.8 MHz 19F Larmor frequency) with a MAS frequency of 24 kHz. The mixing times are listed in each spectrum. Assignments of resolved cross peaks are shown in magenta, two-fold ambiguous assignments in blue, and assignments with 3-fold ambiguity in black. Tentative assignments of cross peaks in crowded regions of the spectrum based on the CA structure model are shown in orange. In the longer mixing time spectra (1.5, 2.5, and 5.0 ms) only peaks that are not present in the shorter-mixing time data sets are labeled. The contour levels were set to 3.5 times the noise in all spectra.
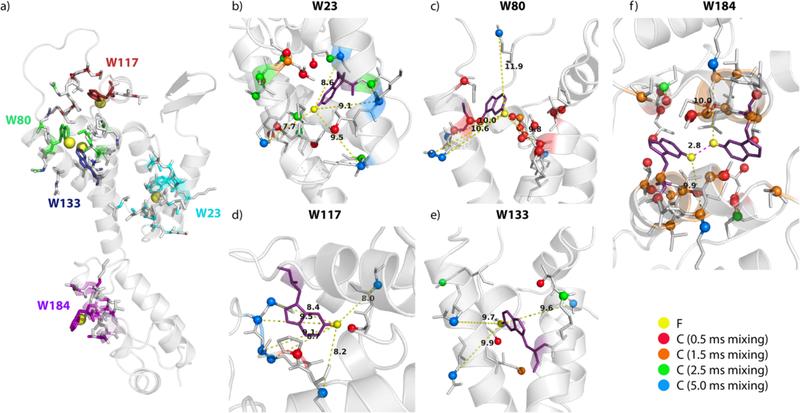
Comparison between the different mixing time spectra reveals that many correlations are present in all data sets, although each spectrum contains unique information. Despite the limited resolution in the 1D 19F and 13C spectra at cryogenic-temperatures, the resolution of the HETCOR spectra at 120 K is sufficient to tentatively assign a number of cross peaks, based on the structural model of the CA protein (Table S3 of the Supporting Information). With increasing mixing times, more and longer-range correlations emerge. The longest distances for these correlations are 8 Å (0.5 ms), 11 Å (1.5 ms), 11 Å (2.5 ms), and 12 Å (5 ms). Mapping of the interactions around each 5F-Trp residue, based on the correlations in the DNP HETCOR spectra at different mixing times, onto the structure reveals similar size spheres around each F position (Figure 5).
Figure 5.
19F-13C contacts derived from DNP-enhanced HETCOR spectra of 5F-Trp,U-13C,15N CA tubular assemblies, mapped onto the a) CA monomer structure. Expansions are shown for b) W23, c) W80, d), W117, e) W133, and f) W184 dimer interface. Residues exhibiting correlations in the spectra are shown in stick representation and atoms associated with correlations that appear at different mixing times are color coded as follows: 0.5 ms (red), 1.5 ms (orange), 2.5 ms (green) and 5 ms (blue). Select distances in the range of 8–11 Å observed in the 5 ms mixing time spectra are shown by dashed lines.
In the spectrum acquired with a mixing time of 0.5 ms, a total of 36 19F-13C correlations were tentatively assigned. The majority of the cross peaks are associated with interactions between pairs of 19F-13C nuclei separated by 4 to 8 Å (Table S4). Of these, 10 were assigned uniquely, 7 are two-fold ambiguous and the remaining ones are 3-fold ambiguous (Figure 4a). The majority of the cross peaks correspond to intramolecular correlations.
11 additional correlations are seen in the spectrum acquired with a 1.5 ms mixing time (Figure 4b). They were tentatively assigned to 19F-13C pairs separated by 4 to 11 Å (Table S4). One-dimensional traces are provided in Figure S6 of the Supporting Information. Two cross peaks could be assigned uniquely, and three exhibit two-fold ambiguity. A further 11 new correlations emerge in the 2.5 ms mixing time spectrum (consistent with 19F-13C pairs at distances ranging from 4 to 11 Å). Two firm intramolecular assignments could be made (19F-13C pairs at distances of ~8 Å). Three additional cross peaks are two-fold ambiguous (Figure 4c). For the longest mixing time of 5 ms, 19 additional correlations appear (all intramolecular, 19F-13C pairs at distances of 8 to 12 Å), 3 of which were assigned unambiguously and 4 possess two-fold ambiguity (Figure 4d).
It is important to note that the correlations observed in the 19F-13C DNP-HETCOR spectra correspond to longer internuclear distances (up to 12 Å) than those observed in the 13C-13C or 13C-15N data sets, which report distances below 6–8 Å. While 3D structures of a number of proteins have been solved using 13C-13C and 13C-15N based distance restraints,5,7,71–75 this approach alone does not yield satisfactory results for HIV-1 CA assemblies. For assembled CA and most multi-domain proteins and large protein complexes, distance restraints beyond 8 Å are required to define the quaternary structure or supramolecular organization.
Taken together, the results discussed here are exciting and highlight the unique abilities of 19F-13C DNP-enhanced correlation spectroscopy for providing unique long-range contacts that are difficult to measure via other biological MAS NMR experiments.
CONCLUSIONS
The findings presented in this study underscore the exciting potential of 19F DNP-enhanced MAS NMR spectroscopy to probe structural properties of protein assemblies, illustrated for HIV-1 capsid assemblies. The remarkably high signal enhancements observed in 19F DNP spectra at MAS frequencies of 20–25 kHz permitted acquisition of 2D 19F-13C correlation spectra, unattainable under conventional experimental conditions. The unique information content of these spectra, specifically the presence of numerous correlations that correspond to long-range distances, cannot be extracted from 13C-13C or 13C-15N correlation spectra. Thus, 19F-based DNP HETCOR experiments represent a powerful addition to the protein structure analysis toolbox. We envisage that the approach presented here will have far-reaching applications in a wide range of large biological systems.
Supplementary Material
ACKNOWLEDGMENTS
We thank In-Ja L. Byeon for acquiring 19F solution NMR spectra of 5F-Trp, U-13C,15N CA and mutants. This work was supported by the National Science Foundation (NSF Grant CHE-1708773 to AMG and TP) and by the National Institutes of Health (NIGMS and NIAID, Grant P50 GM082251, Technology Development Project on MAS NMR). We acknowledge the National Science Foundation (NSF grant CHE-0959496) for the acquisition of the 850 MHz NMR spectrometer at the University of Delaware and the National Institutes of Health (NIH Grants P30GM103519 and P30GM110758) for the support of core instrumentation infrastructure at the University of Delaware. DNP resources were kindly provided by Bruker Biospin Corporation, Billerica, MA, USA.
Footnotes
Classification: Biological Sciences- Biophysics and Computational Biology
SUPPORTING INFORMATION AVAILABLE
19F solution NMR spectra of 5F-Trp, U-13C,15N CA and mutants; 13C-13C RFDR spectra of tubular assemblies of 5F-Trp, U-13C,15N CA and 5F-Trp, U-13C,15N CA doped with 12 mM AMUPol; 13C and 19F MAS NMR spectra 5F-Trp, U-13C,15N CA in the presence of 12 mM AMUPol; resonance assignments in 19F-13C DNP-enhanced HETCOR spectra. This information can be found on the internet at http://pubs.acs.org.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- (1).Quinn CM; Polenova T Q. Rev. Biophys 2017, Structural Biology of Supramolecular Assemblies by Magic-Angle Spinning NMR Spectroscopy, 50, e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Quinn CM; Lu M; Suiter CL; Hou G; Zhang H; Polenova T Prog. Nucl. Magn. Reson. Spectrosc 2015, Magic Angle Spinning NMR of Viruses, 86–87, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Bayro MJ; Chen B; Yau WM; Tycko R J. Mol. Biol 2014, Site-Specific Structural Variations Accompanying Tubular Assembly of the HIV-1 Capsid Protein, 426, 1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wang M; Quinn CM; Perilla JR; Zhang H; Shirra R Jr.; Hou G; Byeon IJ; Suiter CL; Ablan S; Urano E; Nitz TJ; Aiken C; Freed EO; Zhang P; Schulten K; Gronenborn AM; Polenova T Nat. Commun 2017, Quenching Protein Dynamics Interferes with HIV Capsid Maturation, 8, 1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Yan S; Guo C; Hou G; Zhang H; Lu X; Williams JC; Polenova T Proc. Natl. Acad. Sci. USA 2015, Atomic-Resolution Structure of the CAP-Gly Domain of Dynactin on Polymeric Microtubules Determined by Magic Angle Spinning NMR Spectroscopy, 112, 14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yehl J; Kudryashova E; Reisler E; Kudryashov D; Polenova T Scientific reports 2017, Structural Analysis of Human Cofilin 2/Filamentous Actin Assemblies: Atomic-Resolution Insights from Magic Angle Spinning NMR Spectroscopy, 7, 44506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Colvin MT; Silvers R; Ni QZ; Can TV; Sergeyev I; Rosay M; Donovan KJ; Michael B; Wall J; Linse S; Griffin RG J. Am. Chem. Soc 2016, Atomic Resolution Structure of Monomorphic Abeta42 Amyloid Fibrils, 138, 9663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Qiang W; Yau WM; Lu JX; Collinge J; Tycko R Nature 2017, Structural Variation in Amyloid-Beta Fibrils from Alzheimer’s Disease Clinical Subtypes, 541, 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Overhauser AW Phys. Rev 1953, Polarization of Nuclei in Metals, 92, 411. [Google Scholar]
- (10).Carver TR; Slichter CP Phys. Rev 1953, Polarization of Nuclear Spins in Metals, 92, 212. [Google Scholar]
- (11).Can TV; Ni QZ; Griffin RG J. Magn. Reson 2015, Mechanisms of Dynamic Nuclear Polarization in Insulating Solids, 253, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Lilly Thankamony AS; Wittmann JJ; Kaushik M; Corzilius B Prog. Nucl. Magn. Reson. Spectrosc 2017, Dynamic Nuclear Polarization for Sensitivity Enhancement in Modern Solid-State NMR, 102–103, 120. [DOI] [PubMed] [Google Scholar]
- (13).Wenk P; Kaushik M; Richter D; Vogel M; Suess B; Corzilius B J. Biomol. NMR 2015, Dynamic Nuclear Polarization of Nucleic Acid with Endogenously Bound Manganese, 63, 97. [DOI] [PubMed] [Google Scholar]
- (14).Maly T; Cui D; Griffin RG; Miller A-F J. Phys. Chem. B 2012, 1H Dynamic Nuclear Polarization Based on an Endogenous Radical, 116, 7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Hu K-N; Yu H.-h.; Swager TM; Griffin RG J. Am. Chem. Soc 2004, Dynamic Nuclear Polarization with Biradicals, 126, 10844. [DOI] [PubMed] [Google Scholar]
- (16).Rosay M; Zeri A-C; Astrof NS; Opella SJ; Herzfeld J; Griffin RG J. Am. Chem. Soc 2001, Sensitivity-Enhanced NMR of Biological Solids: Dynamic Nuclear Polarization of Y21M fd Bacteriophage and Purple Membrane, 123, 1010. [DOI] [PubMed] [Google Scholar]
- (17).Rosay M; Lansing JC; Haddad KC; Bachovchin WW; Herzfeld J; Temkin RJ; Griffin RG J. Am. Chem. Soc 2003, High-Frequency Dynamic Nuclear Polarization in MAS Spectra of Membrane and Soluble Proteins, 125, 13626. [DOI] [PubMed] [Google Scholar]
- (18).van der Wel PCA; Hu K-N; Lewandowski J; Griffin RG J. Am. Chem. Soc 2006, Dynamic Nuclear Polarization of Amyloidogenic Peptide Nanocrystals: GNNQQNY, a Core Segment of the Yeast Prion Protein Sup35p, 128, 10840. [DOI] [PubMed] [Google Scholar]
- (19).Bayro MJ; Debelouchina GT; Eddy MT; Birkett NR; MacPhee CE; Rosay M; Maas WE; Dobson CM; Griffin RG J. Am. Chem. Soc 2011, Intermolecular Structure Determination of Amyloid Fibrils with Magic-Angle Spinning and Dynamic Nuclear Polarization NMR, 133, 13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Koers EJ; López-Deber MP; Weingarth M; Nand D; Hickman DT; Mlaki Ndao D; Reis P; Granet A; Pfeifer A; Muhs A; Baldus M Angew. Chem 2013, Dynamic Nuclear Polarization NMR Spectroscopy: Revealing Multiple Conformations in Lipid-Anchored Peptide Vaccines, 52, 10905. [DOI] [PubMed] [Google Scholar]
- (21).Ravera E; Michaelis VK; Ong T-C; Keeler EG; Martelli T; Fragai M; Griffin RG; Luchinat C ChemPhysChem 2015, Biosilica-Entrapped Enzymes can be studied by DNP-enhanced high-field NMR, 16, 2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Viger-Gravel J; Schantz A; Pinon AC; Rossini AJ; Schantz S; Emsley L J. Phys. Chem. B 2018, Structure of Lipid Nanoparticles Containing siRNA or mRNA by Dynamic Nuclear Polarization-Enhanced NMR Spectroscopy, 122, 2073. [DOI] [PubMed] [Google Scholar]
- (23).Viennet T; Viegas A; Kuepper A; Arens S; Gelev V; Petrov O; Grossmann TN; Heise H; Etzkorn M Angew. Chem 2016, Selective Protein Hyperpolarization in Cell Lysates Using Targeted Dynamic Nuclear Polarization, 55, 10746. [DOI] [PubMed] [Google Scholar]
- (24).Albert BJ; Gao C; Sesti EL; Saliba EP; Alaniva N; Scott FJ; Sigurdsson ST; Barnes AB Biochemistry 2018, Dynamic Nuclear Polarization Nuclear Magnetic Resonance in Human Cells Using Fluorescent Polarizing Agents. [DOI] [PMC free article] [PubMed]
- (25).Gupta R; Lu M; Hou G; Caporini MA; Rosay M; Maas W; Struppe J; Suiter C; Ahn J; Byeon I-JL; Franks WT; Orwick-Rydmark M; Bertarello A; Oschkinat H; Lesage A; Pintacuda G; Gronenborn AM; Polenova T J. Phys. Chem. B 2016, Dynamic Nuclear Polarization Enhanced MAS NMR Spectroscopy for Structural Analysis of HIV-1 Protein Assemblies, 120, 329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Jaudzems K; Bertarello A; Chaudhari SR; Pica A; Cala-De Paepe D; Barbet-Massin E; Pell AJ; Akopjana I; Kotelovica S; Gajan D; Ouari O; Tars K; Pintacuda G; Lesage A Angew. Chem 2018, Dynamic Nuclear Polarization-Enhanced Biomolecular NMR Spectroscopy at High Magnetic Field with Fast Magic-Angle Spinning, 57, 7458. [DOI] [PubMed] [Google Scholar]
- (27).Daube D; Aladin V; Heiliger J; Wittmann JJ; Barthelmes D; Bengs C; Schwalbe H; Corzilius B J. Am. Chem. Soc 2016, Heteronuclear Cross-Relaxation under Solid-State Dynamic Nuclear Polarization, 138, 16572. [DOI] [PubMed] [Google Scholar]
- (28).Wylie BJ; Dzikovski BG; Pawsey S; Caporini M; Rosay M; Freed JH; McDermott AE J. Biomol. NMR 2015, Dynamic Nuclear Polarization of Membrane Proteins: Covalently Bound Spin-Labels at Protein-Protein Interfaces, 61, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Akbey Ü; Franks WT; Linden A; Lange S; Griffin RG; van Rossum B-J; Oschkinat H Angew. Chem 2010, Dynamic Nuclear Polarization of Deuterated Proteins, 49, 7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Geiger M-A; Orwick-Rydmark M; Märker K; Franks WT; Akhmetzyanov D; Stöppler D; Zinke M; Specker E; Nazaré M; Diehl A; van Rossum B-J; Aussenac F; Prisner T; Akbey Ü; Oschkinat H PhysChemChemPhys 2016, Temperature Dependence of Cross-Effect Dynamic Nuclear Polarization in Rotating Solids: Advantages of Elevated Temperatures, 18, 30696. [DOI] [PubMed] [Google Scholar]
- (31).Barnes AB; Corzilius B; Mak-Jurkauskas ML; Andreas LB; Bajaj VS; Matsuki Y; Belenky ML; Lugtenburg J; Sirigiri JR; Temkin RJ; Herzfeld J; Griffin RG PhysChemChemPhys 2010, Resolution and Polarization Sistribution in Cryogenic DNP/MAS Experiments, 12, 5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Fricke P; Mance D; Chevelkov V; Giller K; Becker S; Baldus M; Lange A J. Biomol. NMR 2016, High Resolution Observed in 800 MHz DNP Spectra of Extremely Rigid Type III Secretion Needles, 65, 121. [DOI] [PubMed] [Google Scholar]
- (33).Bouleau E; Saint-Bonnet P; Mentink-Vigier F; Takahashi H; Jacquot JF; Bardet M; Aussenac F; Purea A; Engelke F; Hediger S; Lee D; De Paëpe G Chem. Sci 2015, Pushing NMR Sensitivity Limits Using Dynamic Nuclear Polarization with Closed-Loop Cryogenic Helium Sample Spinning, 6, 6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Linden AH; Franks WT; Akbey U; Lange S; van Rossum BJ; Oschkinat H J. Biomol. NMR 2011, Cryogenic Temperature Effects and Resolution upon Slow Cooling of Protein Preparations in Solid State NMR, 51, 283. [DOI] [PubMed] [Google Scholar]
- (35).Rogawski R; Sergeyev IV; Zhang Y; Tran TH; Li YJ; Tong L; McDermott AE J. Phys. Chem. B 2017, NMR Signal Quenching from Bound Biradical Affinity Reagents in DNP Samples, 121, 10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Ganser-Pornillos BK; von Schwedler UK; Stray KM; Aiken C; Sundquist WI J. Virol 2004, Assembly Properties of the Human Immunodeficiency Virus Type 1 CA Protein, 78, 2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Byeon IJL; Hou GJ; Han Y; Suiter CL; Ahn J; Jung J; Byeon CH; Gronenborn AM; Polenova T J. Am. Chem. Soc 2012, Motions on the Mllisecond Time Scale and Multiple Conformations of HIV-1 Capsid Protein: Implications for Structural Polymorphism of CA Assemblies, 134, 6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Han Y; Hou GJ; Suiter CL; Ahn J; Byeon IJL; Lipton AS; Burton S; Hung I; Gor’kov PL; Gan ZH; Brey W; Rice D; Gronenborn AM; Polenova T J. Am. Chem. Soc 2013, Magic Angle Spinning NMR Reveals Sequence-Dependent Structural Plasticity, Dynamics, and the Spacer Peptide 1 Conformation in HIV-1 Capsid Protein Assemblies, 135, 17793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Lu M; Hou G; Zhang H; Suiter CL; Ahn J; Byeon IJ; Perilla JR; Langmead CJ; Hung I; Gor’kov PL; Gan Z; Brey W; Aiken C; Zhang P; Schulten K; Gronenborn AM; Polenova T Proc. Natl. Acad. Sci. USA 2015, Dynamic Allostery Governs Cyclophilin A-HIV Capsid Interplay, 112, 14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Zhang H; Hou G; Lu M; Ahn J; Byeon IL; Langmead CJ; Perilla JR; Hung I; Gor’kov PL; Gan Z; Brey WW; Case DA; Schulten K; Gronenborn AM; Polenova T J. Am. Chem. Soc 2016, HIV-1 Capsid Function is Regulated by Dynamics: Quantitative Atomic-Resolution Insights by Integrating Magic-Angle-Spinning NMR, QM/MM, and MD, 138, 14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Bayro MJ; Tycko R J. Am. Chem. Soc 2016, Structure of the Dimerization Interface in the Mature HIV-1 Capsid Protein Lattice from Solid State NMR of Tubular Assemblies, 138, 8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Byeon IJL; Meng X; Jung JW; Zhao GP; Yang RF; Ahn JW; Shi J; Concel J; Aiken C; Zhang PJ; Gronenborn AM Cell 2009, Structural Convergence between Cryo-EM and NMR Reveals Intersubunit Interactions Critical for HIV-1 Capsid Function, 139, 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Zhao GP; Perilla JR; Yufenyuy EL; Meng X; Chen B; Ning JY; Ahn J; Gronenborn AM; Schulten K; Aiken C; Zhang PJ Nature 2013, Mature HIV-1 Capsid Structure by Cryo-Electron Microscopy and All-Atom Molecular Dynamics, 497, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Zhang P; Meng X; Zhao G Methods Mol. Biol 2013, Tubular Crystals and Helical Arrays: Structural Determination of HIV-1 Capsid Assemblies Using Iterative Helical Real-Space Reconstruction, 955, 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Wang M; Lu M; Fritz M; Quinn C; Byeon IJ; Byeon CH; Struppe J; Maas W; Gronenborn A; Polenova T Angew. Chem 2018, Fast Magic Angle Spinning 19F NMR of HIV-1 Capsid Protein Assemblies. [DOI] [PMC free article] [PubMed]
- (46).Holl SM; Marshall GR; Beusen DD; Kociolek K; Redlinski AS; Leplawy MT; McKay RA; Vega S; Schaefer J J. Am. Chem. Soc 1992, Deteremination of an 8-Angstrom Interatomic Distance in a Helical Peptide by Solid-State NMR Spectroscopy, 114, 4830. [Google Scholar]
- (47).Afonin S; Kubyslikin V; Mykhailiuk PK; Komarov IV; Ulrich AS J. Phys. Chem. B 2017, Conformational Plasticity of the Cell-Penetrating Peptide SAP As Revealed by Solid-State F-19-NMR and Circular Dichroism Spectroscopies, 121, 6479. [DOI] [PubMed] [Google Scholar]
- (48).Koch K; Afonin S; Ieronimo M; Berditsch M; Ulrich AS In Solid State NMR; Chan JCC, Ed. 2012; Vol. 306, p 89. [DOI] [PubMed] [Google Scholar]
- (49).Elkins MR; Williams JK; Gelenter MD; Dai P; Kwon B; Sergeyev IV; Pentelute BL; Hong M Proc. Natl. Acad. Sci. U. S. A 2017, Cholesterol-Binding Site of the Influenza M2 Protein in Lipid Bilayers from Solid-State NMR, 114, 12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Roos M; Mandala VS; Hong M J. Phys. Chem. B 2018, Determination of Long-Range Distances by Fast Magic-Angle-Spinning Radiofrequency-Driven 19F-19F Dipolar Recoupling NMR. [DOI] [PMC free article] [PubMed]
- (51).Roos M; Wang T; Shcherbakov AA; Hong M J. Phys. Chem. B 2018, Fast Magic-Angle-Spinning 19F Spin Exchange NMR for Determining Nanometer 19F-19F Distances in Proteins and Pharmaceutical Compounds, 122, 2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Lu M; Sarkar S; Wang M; Kraus J; Fritz M; Quinn CM; Bai S; Holmes ST; Dybowski C; Yap GPA; Struppe J; Sergeyev IV; Maas W; Gronenborn AM; Polenova T J. Phys. Chem. B 2018, 19F Magic Angle Spinning NMR Spectroscopy and Density Functional Theory Calculations of Fluorosubstituted Tryptophans: Integrating Experiment and Theory for Accurate Determination of Chemical Shift Tensors. [DOI] [PMC free article] [PubMed]
- (53).Sauvée C; Rosay M; Casano G; Aussenac F; Weber RT; Ouari O; Tordo P Angew. Chem 2013, Highly Efficient, Water-Soluble Polarizing Agents for Dynamic Nuclear Polarization at High Frequency, 52, 10858. [DOI] [PubMed] [Google Scholar]
- (54).Crowley PB; Kyne C; Monteith WB Chem. Commun 2012, Simple and Inexpensive Incorporation of 19F-Tryptophan for Protein NMR Spectroscopy, 48, 10681. [DOI] [PubMed] [Google Scholar]
- (55).Sun SJ; Han Y; Paramasivam S; Yan S; Siglin AE; Williams JC; Byeon IJL; Ahn J; Gronenborn AM; Polenova T Methods. Mol. Biol 2012, Solid-State NMR Spectroscopy of Protein Complexes, 831, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Thurber KR; Tycko R J. Magn. Reson 2009, Measurement of Sample Temperatures Under Magic-Angle Spinning from the Chemical Shift and Spin-Lattice Relaxation Rate of 79Br in KBr Powder, 196, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Bennett AE; Rienstra CM; Griffiths JM; Zhen W; Lansbury PTJ; Griffin RG J. Chem. Phys 1998, Homonuclear Radio Frequency-Driven Recoupling in Rotating Solids, 108, 9463. [Google Scholar]
- (58).Bennett AE; Rienstra CM; Auger M; Lakshmi KV; Griffin RG J. Chem. Phys 1995, Heteronuclear Decoupling in Rotating Solids, 103, 6951. [Google Scholar]
- (59).Maurer T; Kalbitzer HR J. Magn. Reson. Ser. B 1996, Indirect Referencing of P-31 and F-19 NMR Spectra, 113, 177. [DOI] [PubMed] [Google Scholar]
- (60).Marion D; Ikura M; Tschudin R; Bax A J. Magn. Reson 1989, Rapid recording of 2D NMR spectra without phase cycling. Application to the study of hydrogen exchange in proteins, 85, 393. [Google Scholar]
- (61).Delaglio F; Grzesiek S; Vuister GW; Zhu G; Pfeifer J; Bax A J. Biomol. NMR 1995, NMRPipe: A Multidimensional Spectral Processing System Based on UNIX Pipes, 6, 277. [DOI] [PubMed] [Google Scholar]
- (62). Author SPARKY 3, Institution, 2004.
- (63).Mentink-Vigier F; Akbey Ü; Oschkinat H; Vega S; Feintuch A J. Magn. Reson 2015, Theoretical aspects of Magic Angle Spinning - Dynamic Nuclear Polarization, 258, 102. [DOI] [PubMed] [Google Scholar]
- (64).Chaudhari SR; Berruyer P; Gajan D; Reiter C; Engelke F; Silverio DL; Copéret C; Lelli M; Lesage A; Emsley L PhysChemChemPhys 2016, Dynamic nuclear polarization at 40 kHz magic angle spinning †Electronic supplementary information (ESI) available: Experimental details, with supplementary tables and figures. 10.1039/c6cp00839a Click here for additional data file, 18, 10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Mentink-Vigier F; Mathies G; Liu Y; Barra A-L; Caporini MA; Lee D; Hediger S; Griffin G, R.; De Paëpe G Chem. Sci 2017, Efficient Cross-Effect Dynamic Nuclear Polarization Without Depolarization in High-Resolution MAS NMR, 8, 8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Thurber KR; Tycko R J. Chem. Phys 2012, Theory for Cross Effect Dynamic Nuclear Polarization under Magic-Angle Spinning in Ssolid State Nuclear Magnetic Resonance: The Importance of Level Crossings, 137, 084508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Chaudhari SR; Wisser D; Pinon AC; Berruyer P; Gajan D; Tordo P; Ouari O; Reiter C; Engelke F; Copéret C; Lelli M; Lesage A; Emsley L J. Am. Chem. Soc 2017, Dynamic Nuclear Polarization Efficiency Increased by Very Fast Magic Angle Spinning, 139, 10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Lange S; Linden AH; Akbey Ü; Trent Franks W; Loening NM; Rossum B.-J.v. ; Oschkinat H. J. Magn. Reson 2012, The Effect of Biradical Concentration on the Performance of DNP-MAS-NMR, 216, 209. [DOI] [PubMed] [Google Scholar]
- (69).Viger-Gravel J; Avalos CE; Kubicki DJ; Lelli M; Ouari O; Lesage A; Emsley L In 59th Rocky Mountain Conference on Magnetic Resonance Snowbird, UT, July 22–27, 2018, p 111. [Google Scholar]
- (70).Takahashi H; Lee D; Dubois L; Bardet M; Hediger S; De Paëpe G Angew. Chem 2012, Rapid Natural-Abundance 2D 13C–13C Correlation Spectroscopy Using Dynamic Nuclear Polarization Enhanced Solid-State NMR and Matrix-Free Sample Preparation, 51, 11766. [DOI] [PubMed] [Google Scholar]
- (71).He L; Bardiaux B; Ahmed M; Spehr J; Konig R; Lunsdorf H; Rand U; Luhrs T; Ritter C Proc. Natl. Acad. Sci. USA 2016, Structure Determination of Helical Filaments by Solid-State NMR Spectroscopy, 113, E272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Retel JS; Nieuwkoop AJ; Hiller M; Higman VA; Barbet-Massin E; Stanek J; Andreas LB; Franks WT; van Rossum BJ; Vinothkumar KR; Handel L; de Palma GG; Bardiaux B; Pintacuda G; Emsley L; Kuhlbrandt W; Oschkinat H Nat. Commun 2017, Structure of Outer Membrane Protein G in Lipid Bilayers, 8, 2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Shi C; Fricke P; Lin L; Chevelkov V; Wegstroth M; Giller K; Becker S; Thanbichler M; Lange A Sci. Adv 2015, Atomic-Resolution Structure of Cytoskeletal Bactofilin by Solid-State NMR, 1, e1501087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Tuttle MD; Comellas G; Nieuwkoop AJ; Covell DJ; Berthold DA; Kloepper KD; Courtney JM; Kim JK; Barclay AM; Kendall A; Wan W; Stubbs G; Schwieters CD; Lee VM; George JM; Rienstra CM Nat. Struct. Mol. Biol 2016, Solid-State NMR Structure of a Pathogenic Fibril of Full-Length Human Alpha-Synuclein, 23, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Zech SG; Wand AJ; McDermott AE J. Am. Chem. Soc 2005, Protein Structure Determination by High-Resolution Solid-State NMR Spectroscopy: Application to Microcrystalline Ubiquitin, 127, 8618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.