Abstract
To investigate the neural basis of a common statistical learning mechanism involved in motor sequence learning and decoding, we recorded same participants’ brain activation in a serial reaction time (SRT) and word reading task using functional magnetic resonance imaging. In the SRT, a manual response was made depending on the location of a visual cue, and the order of the locations was either fixed or random. In the word reading task, visual words were passively presented. Compared to less skilled readers, more skilled readers showed greater differences in activation in the inferior frontal gyrus pars triangularis (IFGpTr) and the insula between the ordered and random condition in the SRT task and greater activation in those regions in the word reading task. It suggests that extraction of statistically predictable patterns in the IFGpTr and insula contributes to both motor sequence learning and orthographic learning, and therefore predicts individual differences in decoding skill.
Introduction
Learning to read builds on multiple skills, such as phonemic segmentation and sequencing (Melby-Lervag et al., 2012; Wimmer, 1996), detecting letter patterns (Cunningham et al., 2001) and mapping from orthography to phonology (Ziegler & Goswami, 2005). In addition, substantial research suggests that sensitivity to orthographic structure, or the frequency of particular letter sequences is an important aspect of learning to read. Indeed, Cunningham and Stanovich (1993) found that young children’s sensitivity to frequency of certain letter sequences (e.g., “yikk” vs. “yinn”) accounted for approximately thirty percent of unique variance in their word recognition ability. Bonte et al. (2007) found that children with development dyslexia (DD) did not show comparable sensitivity to the phonotactic frequency of letter sequences of auditory non-words compared to typically developing readers (TD) (also see Apel et al., 2006). These findings suggest that the ability to extract statistical patterns based on the order and frequency information of letters or speech is an important factor in learning to read.
In addition to sensitivity to orthographic or speech sequences, non-linguistic sequence learning has been associated with reading ability. In a typical serial reaction time (SRT) paradigm, participants press a button with different fingers according to the location of visual cues, which occur in two different conditions: ordered and random. In the ordered condition, the order of the visual cues is fixed, and a sequential dependency occurs between neighboring elements (e.g., 1 is always followed by 2) and is repeated across blocks (e.g., 1234, 1234). In the random condition, orders are not fixed and are not repeated across blocks (e.g., 3214, 2134). Howard et al. (2006) compared TD and DD adults in a modified SRT task in which the sequential dependencies occurred in non-adjacent elements (e.g., 1r2r3r4, 2 is predicted by 1 and r is a random element). To determine the specificity of DD’s learning impairment, Howard et al. also administered a spatial contextual cueing task (Chun & Jiang, 1998) in which participants judged the orientation of a target while a task-irrelevant spatial pattern either co-occurred with the target in the repetition condition, or never occurred with the target before in the new condition. Learning was defined by the RT difference between the random condition and the ordered condition in the sequential learning task, and between the new condition and the repetition condition in the spatial contextual cueing task. Compared to TDs, DDs were inferior in motor sequential learning but better in the spatial contextual cueing task. Lum et al.’s meta-analysis (2013) showed the group effect (TDs outperforming DDs) on sequential learning in the SRT task holds regardless of task variation across studies. These findings suggest that basic non-linguistic sequential coding may mediate the association between reading ability and orthographic or speech sequence learning.
The SRT task comprises multiple processes, including early visual-motor association, detection of serial order embedded in the motor response, and automization of both the visual-motor association and the ordered motoric responses. As such, the correlation between sequence learning and reading ability could arise from individual differences in any of these processes. With respect to neurobiological correlates of these processes, Nicolson et al. (1999) conducted an fMRI study on TD and DD adults in motor sequence learning. The participants made a key-press response based on a specific auditory sound, with feedback indicating whether or not the response was correct. The same sequence was practiced for two hours; following this, the learned sequence and a new sequence of auditory tones were presented to the participants in the scanner. Behaviorally, DDs made more errors than TDs in performing the well-practiced sequence. The fMRI results showed that compared to TD, DD showed lower activation of the left cerebellum and the left cingulate cortex while performing the learned sequence, and lower activation of the right cerebellum while performing the new sequence. Hence, the authors propose that both sequence learning and orthographic learning involve automization in the cortical-cerebellar network, which requires substantial practice before performance becomes rapid and accurate (Nicolson & Fawcett, 2007). Menghini et al. (2006) found that adults with DD, relative to TD, showed reduced activation of the left supplementary motoric area (SMA) during the early stages of sequence learning, and enhanced activation of the bilateral cerebellum and the bilateral inferior parietal lobule during later stages. Although these fMRI findings demonstrated opposite activation patterns of the cerebellum in the TD/DD contrast, which may be due to differences in instruction (explicit learning with feedback vs. implicit learning without feedback), they together suggest altered cerebellar function for action execution and maintenance in DD.
Although several studies have found sequence learning and reading to be correlated, these processes rely on at least partly non-overlapping neural circuitry (though shared pathways are suggested). Thus the few studies of TD/DD contrasts in sequence learning suggest prominent roles for the cerebellum, anterior cingulate and premotor areas. On the other hand, studies contrasting TD and DD in reading and phonological processing tasks consistently implicate the fusiform visual word form area (VWFA), posterior superior temporal gyrus (pSTG), the angular gyrus (ANG), the middle temporal gyrus (MTG), and the inferior frontal gyrus (IFG) during processing of linguistic stimuli (Richlan et al., 2011; Paulesu et al., 2014; Pugh et al., 2010), whereas cerebellar involvement is only sometimes reported (Fulbright et al., 1999; Preston et al., 2010). Moreover, other studies have found that some of these reading critical regions (e.g., the VWFA and IFG) are involved in statistical learning and sequence detection when linguistic stimuli are used (Binder et al. 2006; Frost et al., 2005; Fiez et al. 1999; Herbster et al. 1997; Graves et al., 2010; Mechelli et al. 2005; Vinckier et al. 2007). There is evidence for both shared and unshared circuits for linguistic processes and non-linguistic sequence learning, raising the question of whether a subset of shared neuro-cognitive mechanisms gives rise to the observed correlations between reading and sequence learning (Pugh et al., 2013).
Although previous neuroimaging work implicates partially distinct and partially overlapping neural circuits for linguistic processing and non-linguistic sequence learning, these studies were not designed to directly compare these circuits in the same individual and to link commonalities to behavioral variation. The present study directly compared these processes at the neurobiological level to determine whether any neural substrates are common to both the SRT task and linguistic processing tasks. Also, we treated individual differences of reading abilities using a dimensional approach, and examined whether activity in any overlapping regions was associated with individual differences in reading skill. To do this, we collected neuroimaging data during SRT task performance and during reading from a large sample of participants with substantial variability in reading ability (percentile scores of the reading scores between 4 and 93 in a normal distribution, see Table 1 for more details). We hypothesized that if processes that detect order or regular patterns are common to reading and sequence learning, the IFG and VWFA should be commonly involved in both tasks, and their activation should scale according to individual differences in reading skill. Alternatively, if skill automization is shared across reading and sequence learning, the cerebellum should be commonly involved in the two tasks and predict individual differences in reading skills.
Table 1.
Results of the standardized tests and demographic information regarding the participants.
| Range | Mean | Standard deviation | |
|---|---|---|---|
| WJ reading score | 73–123 | 102.16 | 11.63 |
| WASI Performance IQ | 75–139 | 103.43 | 14.87 |
| Age | 15–25 | 20.48 | 2.50 |
Method
Participants
Eighty-nine adolescents (34 female, 55 male) who were native English speakers and had normal/corrected-to-normal vision and hearing were recruited for the current study. All participants completed a battery of standardized assessments for reading ability and two fMRI tasks. Specifically, the participants’ reading ability was measured using composite age-adjusted standard scores of Word Attack (producing correct sounds of letters or pronounceable pseudowords) and Letter-Word Identification (identifying printed letters or words) in Woodcock-Johnson III Tests of Achievement (WJ-III; Woodcock et al., 2001). The participants’ non-verbal cognitive abilities were measured using composite scores of Block Design (making specific patterns using small blocks), Matrix Reasoning (identifying a constituent based on analogies to existing patterns), and Picture Completion (making a completed picture by filling in a missing element) in the Wechsler Abbreviated Scale of Intelligence II (WASI-II; Wechsler, 1999). The participants’ performance in the standardized tests as well as some demographic information is summarized in Table 1. All participants were consented in compliance with Yale University’s Institutional Review Board for protection of human participants.
Stimuli & Procedure
1. Serial reaction time task
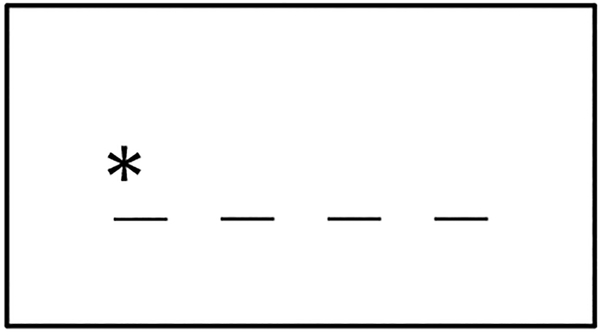
During fMRI scanning, participants were asked to perform a stimulus-response task in which they were required to press a button corresponding to the location of a visual stimulus in a display. At the beginning of each trial, a gray box appeared in the center of the monitor against a black background. After 83 milliseconds, an asterisk (1° x 1°) appeared for 750 milliseconds in one of four locations within this gray box (Figure 1). Four straight lines beneath the asterisk indicated the four possible locations above which the asterisk could appear. Participants were asked to indicate the location (1, 2, 3 or 4) of the asterisk with their index finger, middle finger, ring finger or pinky finger, respectively. In the ordered condition, twelve consecutive targets always appeared in a fixed first order sequence: 121423413243. In the random condition, twelve consecutive targets appeared randomly. In total, participants completed 936 trials distributed evenly across three runs. Within each run, the two conditions alternated so that the random condition (24 trials) always preceded the ordered condition (96 trials).
Figure 1.
Illustration of the stimulus display in the SRT task. The four lines from left to right correspond to the four possible locations above which the asterisk could appear.
2. Word reading task
During fMRI scanning, visual words were presented to participants without an explicit task to elicit processing more akin to natural reading. All words were one-syllable medium- to high-frequency (frequency range: 5626 and 580704) words according to the English Lexicon Project (Balota et al., 2007). On each trial, four words were presented rapidly and sequentially with a duration of 250 ms per word, and with an ISI of 200 ms between words. Between trials, there was a jittered inter-trial interval of four to seven seconds. Participants completed two runs with twelve trials per run, for a total of 24 trials.
Acquisition of MRI data
1. SRT task
Anatomical and functional imaging data were acquired using a Siemens 3.0T Trio Tim whole-body MRI System (Siemens Medical Solutions, Erlangen, Germany) with a 12-channel head coil located at the Yale University School of Medicine. T2*-weighted functional images were acquired for 32 axial-oblique slices prescribed parallel to the intercommissural line using single shot, gradient echo, echo planar imaging (EPI) with the following parameters: FA = 80°; TE = 30 ms; TR = 2000 ms; FOV = 220; 4 mm slice thickness, no gap; matrix size = 64 × 64 × 32; voxel size = 3.438 mm x 3.438 mm x 4 mm. There were 156 volumes in each run. A high resolution structural scan was acquired in the same orientation as the functional slices using an MPRAGE sequence with the following parameters: FA = 7°; TE = 3.66; TR = 2530 ms; FOV = 256; 1 mm slice thickness, no gap; matrix size = 256 × 256; voxel size 1 mm isotropic.
2. Word reading task
Acquisition parameters were identical to those for the SRT task, except that there were 151 volumes acquired per run.
Analysis of MRI data
1. SRT task
Data analysis was performed using AFNI (Cox, 1996). Anatomic images were skull stripped and warped to Talairach space using a nonlinear transform. For functional runs, the first six volumes in each run were removed to allow for stabilization of the magnetic field. Functional images were corrected for slice acquisition time, corrected for motion, co-registered with anatomical images, nonlinear warped to Talairach space, and smoothed using an 8 mm FWHM Gaussian kernel. All trials were included regardless of whether or not responses were correct; however, any volumes with greater than 10% outlier voxels or more than 0.3 mm point-to-point movement were removed from further analyses (2.0%). Single subject data were entered into a standard generalized linear model (GLM) analysis with two variables of interest (Run and Condition) as well as nuisance regressors for the six motion parameters (3 rotation and 3 translation parameters) and 3rd order polynomial drift terms. Six activation maps for each subject were used in group analyses: the ordered condition and the random condition for each of the three runs.
2. Word reading task
Pre-processing was identical to the SRT task and 1.9% of volumes were removed due to outliers and/or head motions. Single subject data were entered into a standard GLM analysis with regressors for the stimulus condition and nuisance regressors for the six motion parameters and 2nd order polynomial drift terms. The activation map of the visual word condition for each subject was used in group analyses.
3. Conjunction analysis
An activation map for the SRT task was created by averaging across the six conditions (i.e., ordered and random trials in each of the three runs) for each subject and then a groupwise map was generated by using the AFNI program 3dttest++ (whole-brain FDR corrected at p < 0.05). The random condition, though it does not tap into sequential processing, requires learning to map between visual information and motor responses. The latter process is also involved in reading. Hence, both conditions in the SRT task were included to allow us to extract the common processes between the SRT and the word reading task. A groupwise activation map for the word reading task was created for the visual word condition in the same way as the SRT task. The conjunction map was created by using a step function in the AFNI program 3dcalc on the groupwise activation maps of the SRT task and the word reading task. Brain regions identified in the conjunction map were further constrained using the CA-N27-ML and Talairach Daemon atlases in AFNI.
Results
1. Behavioral results in the SRT task
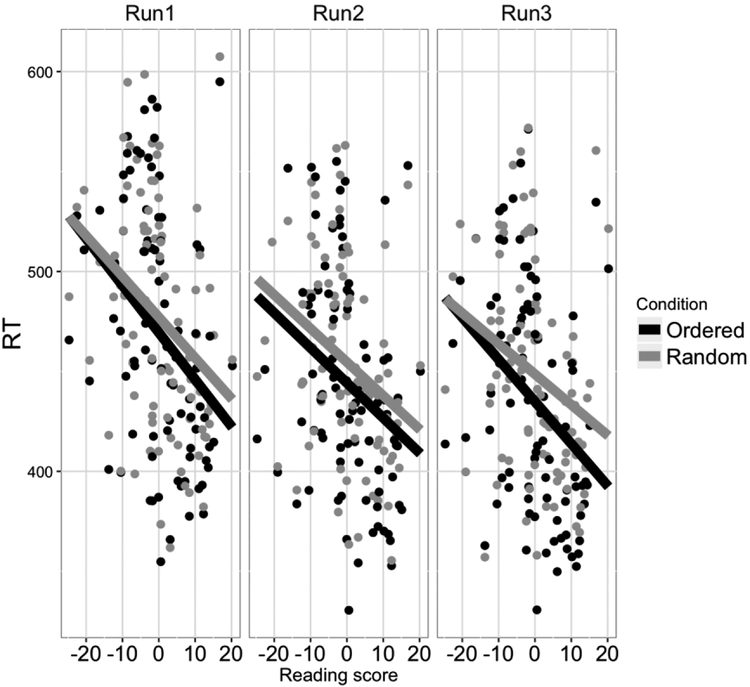
Any trials with incorrect responses (11.05% of the data) were excluded from further analyses. Afterwards, RTs that were either shorter than 250 ms or were more than three standard deviations above or below each participant’s respective condition-wise mean (1.24% of the data) were excluded from further analyses. The learning score of the sequence was defined by the reaction time difference (hereafter: dRT) between the random and ordered condition, which was calculated separately for each of the three runs (Table 2). WJ-III Basic reading scores were regressed on WASI-II Performance IQ so that any observed effects could be specifically attributed to individual differences in reading skill. Learning scores were entered into a linear mixed effects model based on maximum likelihood methods using the lme4 package in R (Bates et al., 2015). The model included Run (categorical within-participants factor), Reading score (continuous between-participants factor), and the interaction between Run and Reading score as fixed effects and a random by-participant intercept as well as random by-subject slopes for all of the fixed effects (i.e., the maximal random effect structure, see Barr, 2013). Results showed that the effect of Run and the effect of Reading score were not significant (X2(1) = 5.73, p = 0.057; X2(1) = 3.17, p = 0.075). However, the interaction between Run and Reading score was significant (X2(2) = 7.8, p = 0.02). Post-hoc comparisons showed that dRTs significantly correlated with Reading score in the third run (r = 0.33, p = 0.001) but not in the other runs (ps > 0.1). No effects were detected for mean error rates (ps > 0.5).
Table 2.
Grand averaged RTs across participants in the ordered condition and random condition in the three runs of the SRT task. (separated by more skilled readers and less skilled readers)
| Run 1 | Run 2 | Run 3 | |
|---|---|---|---|
| Ordered | 469.46 | 444.24 | 434.89 |
| Random | 477.11 | 454.65 | 448.6 |
2. Neuroimaging results
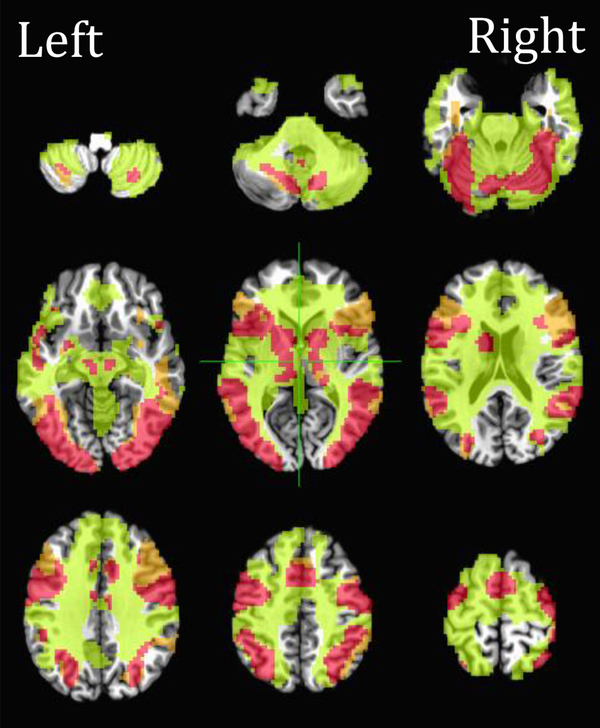
The conjunction analysis revealed brain regions that were commonly activated in the SRT task and the word reading task including the cerebellum, fusiform gyrus, the anterior and posterior STG (aSTG and pSTG), inferior parietal lobule, the insula, the IFG pars triangularis (IFGpTr) and the IFG pars opercularis (IFGpOp), the putamen, the precentral gyrus, and the SMA, in both hemispheres (Figure 3).
Figure 3.
Green areas indicate brain regions that were significantly active in the SRT task at the group level. Orange areas indicate brain regions that were significantly active in the word reading task at the group level. Red areas indicate brain regions that were commonly activated in both tasks.
2.2. The relationship between reading skill and brain activation in the SRT and word reading tasks
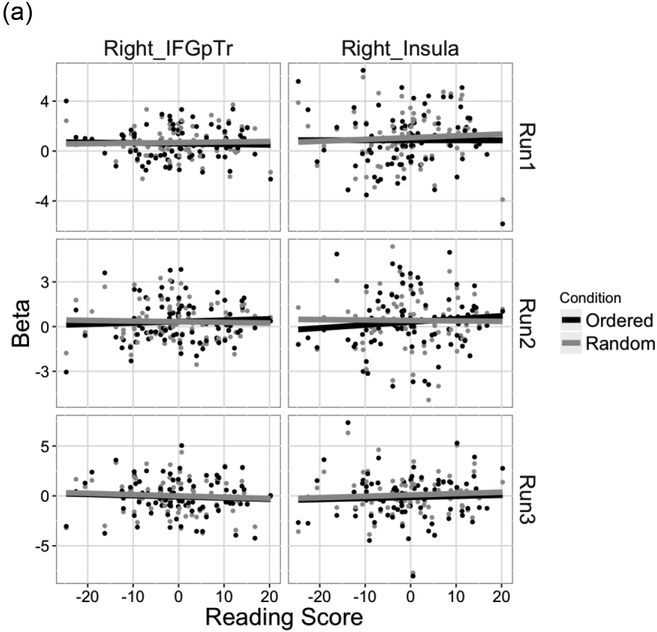
For the brain regions that were commonly activated across the SRT and word reading tasks, we tested whether the extent of engagement of these regions for each task was related to reading ability. For the SRT task, similar to the behavioral data analysis, we calculated the difference in beta values between the ordered and random conditions in the 22 common brain regions identified by the conjunction analysis, and used these values as the dependent variable in a linear mixed effects model with Run and Reading score and their interaction as fixed effects and participants as a random effect. A significant interaction between Reading score and Run was observed in the right Insula (X2(2) = 8.28, p = 0.016) and the right IFGpTr (X2(2) = 6.06, p = 0.048). A follow-up correlation analysis showed that in the right insula, the difference in beta values between the ordered and random conditions in the second run significantly correlated with Reading score (r = 0.26, t(87) = 2.48, p = 0.015). In the right IFGpTr, there was a marginal correlation between Reading score and the difference in beta values between the ordered and random conditions in the second run (r = 0.21, t(87) = 1.97, p = 0.052) (Figure 4a).
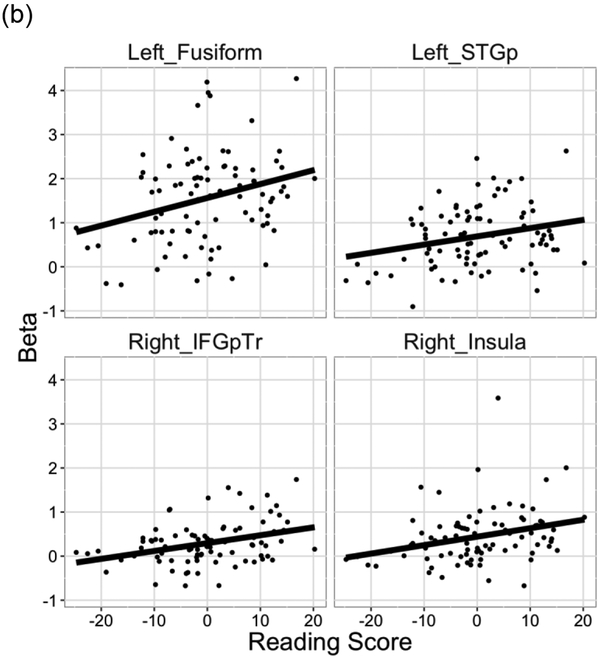
Figure 4.
Brain regions that showed sensitivity to individual differences in reading skill in the SRT and word reading tasks. Beta weights for (a) the SRT task and (b) the word reading task are plotted as a function of residual Reading score.
For the word reading task, we performed a simple correlation analysis between Reading score and beta values in the visual word condition. Reading score were significantly correlated with beta values in the left fusiform (r = 0.29, t(87) = 2.81, p = 0.006), the right insula (r = 0.29, t(87) = 2.87, p = 0.005), the left pSTG (r = 0.26, t(87) = 2.47, p = 0.015), and the right IFGpTr (r = 0.35, t(87) = 3.51, p = 0.001) (Figure 4b).
Discussion
The current study had two aims. First, we identified shared brain circuits for the SRT task and reading; second, we examined whether brain regions that were commonly engaged during sequence learning and word reading were also related to individual differences in reading ability (given the observed correlation between sequence learning and reading). Behaviorally, the correlation between learning in the SRT task and reading skill was replicated in the current study: more skilled readers showed a greater difference in their reaction times between the ordered and random conditions compared to less skilled readers.
At the neural level, we identified a network of regions common to both the SRT and word reading task. Specifically, the fusiform gyrus, the precentral area and the SMA might reflect visual (symbols, letters) and motoric (button press, covert speech) processing common to the SRT task and the word reading task. The aSTG and pSTG likely reflect the need for cross-modal association in the SRT task, and similarly, print-speech conversion during the word reading task (Friederici, 2011; Price, 2012). The IFGpTr, IFGpOp and insula are involved in sequence coding and sequential binding which are required for both tasks (Friederici, 2011; Witt et al., 2008). And finally, the putamen and cerebellum have been associated with consolidation and automatic performance of learned skills, which are known to be important to both reading and sequence learning (Doyon, 2008; Kotz et al., 2009; Nicolson & Fawcett, 2007; Ullman, 2004). The shared neural network suggests that bimodal mapping, sequential binding and storage were commonly involved in sequence learning and reading.
The brain-behavior correlations observed for the SRT task show that more skilled readers exhibit greater activation in the right insula and the right IFGpTr for ordered relative to random trials. Reading ability was also positively associated with activation of the left fusiform area, the left pSTG, the right IFGpTr and the right insula during the word reading task, findings which are consistent with previous neuroimaging studies of individual differences in reading (Richlan et al., 2011; Paulesu et al., 2014; Pugh et al., 2013). The current results indicate that shared variance between sequence learning and reading skill may arise from common engagement of the right insula and the right IFGpTr for these tasks.
In the non-linguistic domain, the right insula and the right IFG are often associated with visuomotor synchrony and sequential processing (Cross et al., 2013; Rieckmann et al., 2010; Witt et al., 2008). In the linguistic domain, the insula is involved in phonological processing (McDermott et al., 2003; Mechelli et al., 2007), and is also sensitive to the complexity of speech sequences (Bohland et al., 2006); furthermore, it is has been shown to be more active in successful learners of speech sounds than it is in less successful learners (Segawa et al., 2015; Wong et al., 2007). The right IFGpTr associates number word combination (Hung et al., 2015). With respect to studies of good and poor readers, DD adults failed to activate the left insula in a rhyming judgment task compared to TD adults (Paulesu et al., 1996). Nicolson et al. (1999) observed that those with DD over-activated the left insula in the contrast of newly-learned vs. well-practiced sequences. The group difference in activation patterns and lateralization in Nicolson et al.’s study were different from our findings, which may be ascribed to the differences between explicit and implicit learning. In addition, Nicolson et al.’s results were based on the contrast between TDs and DDs whereas the current study focused on the effect of individual difference in decoding skills on sequential learning. The current findings suggest that sequential processing is commonly involved and predicts individual differences in motor learning in the SRT task and word retrieval in the word reading task. In the word reading task, we speculate that the skilled readers engaged these areas more than less skilled readers because they were more likely to retrieve the serial phonological patterns and covertly or overtly read the visual words.
There have been claims, albeit somewhat controversial, that dyslexia is caused by impaired phonological processing (Castles & Coltheart, 2004; Melby-Lervag et al., 2012; Morais & Kolinsky, 1994). However, because phonological processing is supported by phonemic sequencing and acquisition of grapheme-to-phoneme conversion rules, deficits in learning regularities, serial-order and rules may also contribute to reading problems (Baker, 1972; Morrison & Manis, 1982). Consistent with this hypothesis, the current findings and previous research from our lab has demonstrated that sequential processing of motoric action (current paper) and temporal order (Pugh et al., 2013) both co-vary with reading ability in adults and beginning readers. However, brain regions mediating the covariance between the two abilities differed across the two studies: here, effects are observed in the insula and IFGpTr, whereas for temporal order (Pugh et al., 2013), associations were observed in the STG and the thalamus. Future studies should consider whether such differences can be attributed to either age differences (15–25 vs. 5–9) or task differences.
It is worth noting that the brain-behavior correlation for the SRT task was observed in the second run whereas the correlation between decoding ability and learning scores for the SRT was observed only in the third run. Whereas we cannot be sure it reflects a meaningful pattern, an interesting speculation is that this mismatch could be attributed to brain-signals and behavioral performance reflecting different stages of the cognitive processing under investigation. Brain signals during the SRT task reflect visual-motor association and sequential encoding (Eversheim & Bock, 2001; Hikosaka 1999; Müller et al., 2002) whereas the external behavior (i.e., the learning outcome based on the reaction time measure) is presumably the consequence of all preceding neural activity. As a second note, some limitations of the current study should be considered. First, the SRT task and the word reading task may tap into other common cognitive process, such as attention or inhibition (Aron et al., 2014; Corbetta & Shulman, 2002), other than the sequential processing we focused on. The SRT task demands overt motor responses and taps into sequential learning. The word reading task requires covert naming and does not tap into learning processes. Besides, sequential decoding is not necessarily involved in reading, especially for the familiar words like those used in the current study. Future studies should elicit online sequential learning for both linguistic and non-linguistic motor stimuli to verify the locus of the correlation between motor sequence learning and reading ability (e.g., Francois & Schön, 2011). Second, the results of ROI analyses were not corrected for multiple comparisons in the current exploratory study. Hence, the neuroimaging results should be interpreted with caution due to the risk of type-I error and ask for future replications.
To our knowledge, the present study provides the first evidence for a common neural network for non-linguistic sequence learning and word reading in the same individuals, as well as associated relations to sequence learning and reading. A neural network of visual motor association (the precentral area and the SMA), sequential, orthographic and linguistic processing (STG, insula, the fusiform gyrus and IFG), learning and memory (the putamen and the cerebellum) was commonly recruited during both non-linguistic sequence learning and reading. Activation of the right insula and the right IFGpTr was associated with individual differences in reading skill in both tasks, suggesting that sequential processing is commonly involved across motor sequence learning and retrieving graphemic/phonemic sequences in reading.
Figure 2.
RTs in the ordered condition and random condition across the three runs of the SRT task as a function of residual Reading score (regressing out the variance of Performance IQ). The RT difference between the random and ordered condition in the third run significantly correlated with Reading score.
Acknowledgement
This research was supported by NIH Grant RO1 HD-065794 (PI: Kenneth Pugh). We thank Dr. Ovid Tzeng for his support during paper writing.
References
- Apel K, Wolter JA, & Masterson JJ (2006). Effects of phonotactic and orthotactic probabilities during fast mapping on 5-year-olds’ learning to spell. Developmental Neuropsychology, 29(1), 21–42. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, & Poldrack RA (2014). Inhibition and the right inferior frontal cortex: one decade on. Trends in cognitive sciences, 18(4), 177–185. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ, Hutchison KA, Cortese MJ, Kessler B, Loftis B, … & Treiman R (2007). The English lexicon project. Behavior Research Methods, 39(3), 445–459. [DOI] [PubMed] [Google Scholar]
- Barr DJ (2013). Random effects structure for testing interactions in linear mixed-effects models. Frontiers in Psychology, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Mächler M, Bolker B, & Walker S (2014). Fitting linear mixed-effects models using lme4. rXiv preprint arXiv:1406.5823. [Google Scholar]
- Binder JR, Medler DA, Westbury CF, Liebenthal E, & Buchanan L (2006). Tuning of the human left fusiform gyrus to sublexical orthographic structure. NeuroImage, 33(2), 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohland JW, & Guenther FH (2006). An fMRI investigation of syllable sequence production. NeuroImage, 32(2), 821–841. [DOI] [PubMed] [Google Scholar]
- Bonte ML, Poelmans H, & Blomert L (2007). Deviant neurophysiological responses to phonological regularities in speech in dyslexic children. Neuropsychologia, 45(7), 1427–1437. [DOI] [PubMed] [Google Scholar]
- Castles A, & Coltheart M (2004). Is there a causal link from phonological awareness to success in learning to read?. Cognition, 91(1), 77–111. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Cross ES, Stadler W, Parkinson J, Schütz‐Bosbach S, & Prinz W (2013). The influence of visual training on predicting complex action sequences. Human Brain Mapping, 34(2), 467–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, & Jiang Y (1998). Contextual cueing: Implicit learning and memory of visual context guides spatial attention. Cognitive Psychology, 36(1), 28–71. [DOI] [PubMed] [Google Scholar]
- Corbetta M, & Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nature reviews neuroscience, 3(3), 201–215. [DOI] [PubMed] [Google Scholar]
- Cunningham AE, Perry KE, & Stanovich KE (2001). Converging evidence for the concept of orthographic processing. Reading and Writing, 14(5–6), 549–568. [Google Scholar]
- Cunningham AE, & Stanovich KE (1993). Children’s literacy environments and early word recognition subskills. Reading and Writing, 5(2), 193–204. [Google Scholar]
- Doyon J (2008). Motor sequence learning and movement disorders. Current Opinion in Neurology, 21(4), 478–483. [DOI] [PubMed] [Google Scholar]
- Eversheim U & Bock O (2001). Evidence for processing stages in skill acquisition: a dual-task study. Learning & Memory, 8(4), 183–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, & Petersen SE (1999). Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron, 24(1), 205–218. [DOI] [PubMed] [Google Scholar]
- Francois C, & Schön D (2011). Musical expertise boosts implicit learning of both musical and linguistic structures. Cerebral Cortex, 21(10), 2357–2365. [DOI] [PubMed] [Google Scholar]
- Friederici AD (2011). The brain basis of language processing: from structure to function. Physiological Reviews, 91(4), 1357–1392. [DOI] [PubMed] [Google Scholar]
- Frost SJ, Mencl WE, Sandak R, Moore DL, Rueckl JG, Katz L, … & Pugh KR (2005). A functional magnetic resonance imaging study of the tradeoff between semantics and phonology in reading aloud. Neuroreport, 16(6), 621–624. [DOI] [PubMed] [Google Scholar]
- Fulbright RK, Jenner AR, Mencl WE, Pugh KR, Shaywitz BA, Shaywitz SE, … & Marchione KE (1999). The cerebellum’s role in reading: a functional MR imaging study. American Journal of Neuroradiology, 20(10), 1925–1930. [PMC free article] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, & Binder JR (2010). Neural systems for reading aloud: a multiparametric approach. Cerebral Cortex, 20(8), 1799–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbster AN, Mintun M, Nebes RD, & Becker JT (1997). Regional cerebral blood flow during word and nonword reading. Human Brain Mapping, 5(2), 84–92. [DOI] [PubMed] [Google Scholar]
- Howard JH, Howard DV, Japikse KC, & Eden GF (2006). Dyslexics are impaired on implicit higher-order sequence learning, but not on implicit spatial context learning. Neuropsychologia, 44(7), 1131–1144. [DOI] [PubMed] [Google Scholar]
- Hung YH, Pallier C, Dehaene S, Lin YC, Chang A, Tzeng OJL, & Wu DH (2015). Neural correlates of merging number words. NeuroImage, 122, 33–43. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, … & Doya K (1999). Parallel neural networks for learning sequential procedures. Trends in Neurosciences, 22(10), 464–471. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Schwartze M, & Schmidt-Kassow M (2009). Non-motor basal ganglia functions: A review and proposal for a model of sensory predictability in auditory language perception. Cortex, 45(8), 982–990. [DOI] [PubMed] [Google Scholar]
- Lum JA, Ullman MT, & Conti-Ramsden G (2013). Procedural learning is impaired in dyslexia: Evidence from a meta-analysis of serial reaction time studies. Research in Developmental Disabilities, 34(10), 3460–3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott KB, Petersen SE, Watson JM, & Ojemann JG (2003). A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia, 41(3), 293–303. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Ralph MAL, Patterson K, … & Price CJ (2005). Dissociating reading processes on the basis of neuronal interactions. Journal of Cognitive Neuroscience, 17(11), 1753–1765. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Josephs O, Ralph L, Matthew A, McClelland JL, & Price CJ (2007). Dissociating stimulus‐driven semantic and phonological effect during reading and naming. Human Brain Mapping, 28(3), 205–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby-Lervag M, Lyster SAH, & Hulme C (2012). Phonological skills and their role in learning to read: a meta-analytic review. Psychological Bulletin, 138(2), 322. [DOI] [PubMed] [Google Scholar]
- Menghini D, Hagberg GE, Caltagirone C, Petrosini L, & Vicari S (2006). Implicit learning deficits in dyslexic adults: An fMRI study. NeuroImage, 33(4), 1218–1226. [DOI] [PubMed] [Google Scholar]
- Morais J, & Kolinsky R (1994). Perception and awareness in phonological processing: The case of the phoneme. Cognition, 50(1), 287–297. [DOI] [PubMed] [Google Scholar]
- Morrison FJ, & Manis FR (1982). Cognitive processes and reading disability: A critique and proposal In Verbal processes in children (pp. 59–93). Springer; New York. [Google Scholar]
- Müller RA, Kleinhans N, Pierce K, Kemmotsu N, & Courchesne E (2002). Functional MRI of motor sequence acquisition: effects of learning stage and performance. Cognitive Brain Research, 14(2), 277–293. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, & Fawcett AJ (2007). Procedural learning difficulties: reuniting the developmental disorders?. Trends in Neurosciences, 30(4), 135–141. [DOI] [PubMed] [Google Scholar]
- Nicolson RI, Fawcett AJ, Berry EL, Jenkins IH, Dean P, & Brooks DJ (1999). Association of abnormal cerebellar activation with motor learning difficulties in dyslexic adults. The Lancet, 353(9165), 1662–1667. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Danelli L, & Berlingeri M (2014). Reading the dyslexic brain: multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Frontiers in Human Neuroscience, 8, 830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, & Frith CD (1996). Is developmental dyslexia a disconnection syndrome?. Brain, 119(1), 143–157. [DOI] [PubMed] [Google Scholar]
- Paulesu E, McCrory E, Fazio F, Menoncello L, Brunswick N, Cappa SF, Cotelli M, Cossu G, Corte F, Lorusso M, Pesenti S, Gallagher A, Perani D, Price C, Frith CD, Frith U (2000). A cultural effect on brain function. Nature Neuroscience, 3, 91–96. [DOI] [PubMed] [Google Scholar]
- Preston JL, Frost SJ, Mencl WE, Fulbright RK, Landi N, Grigorenko E, … & Pugh KR (2010). Early and late talkers: school-age language, literacy and neurolinguistic differences. Brain, 133(8), 2185–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ (2012). A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62(2), 816–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Frost SJ, Sandak R, Landi N, Moore D, Della Porta G, … & Mencl WE (2010). Mapping the word reading circuitry in skilled and disabled readers The neural basis of reading, New York: Oxford University Press; 2010. pp. 281–305. [Google Scholar]
- Pugh KR, Landi N, Preston JL, Mencl WE, Austin AC, Sibley D, … & Molfese P (2013). The relationship between phonological and auditory processing and brain organization in beginning readers. Brain and language, 125(2), 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, & Wimmer H (2011). Meta-analyzing brain dysfunctions in dyslexic children and adults. NeuroImage, 56(3), 1735–1742. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Fischer H, & Bäckman L (2010). Activation in striatum and medial temporal lobe during sequence learning in younger and older adults: relations to performance. NeuroImage, 50(3), 1303–1312. [DOI] [PubMed] [Google Scholar]
- Segawa JA, Tourville JA, Beal DS, & Guenther FH (2015). The neural correlates of speech motor sequence learning. Journal of Cognitive Neuroscience, 27,819–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman MT (2004). Contributions of memory circuits to language: The declarative/procedural model. Cognition, 92(1), 231–270. [DOI] [PubMed] [Google Scholar]
- Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, & Cohen L (2007). Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron, 55(1), 143–156. [DOI] [PubMed] [Google Scholar]
- Wagner RK, & Torgesen JK (1987). The nature of phonological processing and its causal role in the acquisition of reading skills. Psychological bulletin, 101(2), 192. [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation: Harcourt Brace & Company; New York, NY. [Google Scholar]
- Wimmer H, Schurz M, Sturm D, Richlan F, Klackl J, Kronbichler M, & Ladurner G (2010). A dual-route perspective on poor reading in a regular orthography: an fMRI study. Cortex, 46(10), 1284–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock RW, McGrew KS, Mather N, & Schrank F (2001). Woodcock-Johnson III NU tests of achievement. Rolling Meadows, IL: Riverside Publishing. [Google Scholar]
- Witt ST, Laird AR, & Meyerand ME (2008). Functional neuroimaging correlates of finger-tapping task variations: an ALE meta-analysis. NeuroImage, 42(1), 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Perrachione TK, & Parrish TB (2007). Neural characteristics of successful and less successful speech and word learning in adults. Human Brain Mapping, 28(10), 995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JC, & Goswami U (2005). Reading acquisition, developmental dyslexia, and skilled reading across languages: a psycholinguistic grain size theory. Psychological Bulletin, 131(1), 3. [DOI] [PubMed] [Google Scholar]