Abstract
Introduction:
Synovial Sarcoma (SS) and Myxoid Round Cell Liposarcoma (MRCL) are devastating sarcoma subtypes with few treatment options and poor outcomes in the advanced setting. However, both these diseases may be ideal for novel immunotherapies targeting the cancer-testis antigen, NY-ESO-1.
Areas covered:
In this review, we discuss the novel NY-ESO-1 targeted vaccine regimen, CMB305. This regimen uses a unique integration-deficient, dendritic-cell targeting lentiviral vector from the ZVex® platform, LV305, in order to prime NY-ESO-1 specific T cells. LV305 has single agent activity, and, in one case, caused a durable partial response in a refractory SS patient. CMB305 also includes a boost from a NY-ESO-1 protein vaccine given along with a potent toll-like-4 receptor agonist, glycopyranosyl lipid A. CMB305 induces NY-ESO-1 specific T cell responses in both SS and MRC patients and these patients had excellent overall survival (OS) outcomes in the initial phase I study.
Expert commentary:
CMB305 is a therapeutic vaccine regimen targeting NY-ESO-1 based on the lentiviral vaccine vector, LV305. Phase I studies have proven this vaccine is active immunologically. Data suggesting this vaccine may improve OS for SS and MRCL patients is exciting but early, and ongoing work is testing the impact of CMB305 on patient outcomes.
Keywords: CMB305, dendritic cells, immunotherapy, LV305, Myxoid, NY-ESO-1, Sarcoma, Synovial, therapeutic vaccine
1. Introduction
Soft tissue sarcomas (STS) are a heterogeneous group of over 70 distinct mesenchymal neoplasms together comprising 1% of all cancers and with a median overall survival (OS) in the range of 18 months in the metastatic setting [1]. Currently, the standard frontline therapy for metastatic and locally advanced unresectable STS is doxorubicin based (response rate of 20–30%), either as a single agent or in a combination, resulting in a median progression-free survival (PFS) of approximately 4–6 months depending on the specific regimen [2,3]. At many centers in the United States, doxorubicin is given with olaratumab, which received US FDA breakthrough approval based on a survival benefit in a Phase II trial. A Phase III trial will test whether this result can be confirmed; this trial has completed accrual and the final survival data is pending [4]. Other FDA-approved treatments for STS include pazopanib, trabectedin, and eribulin which all improve PFS improvement by 3–5 months [5–7]. Older chemotherapeutics such as ifosamide, dacarbazine, and gemcitabine either alone or in a doublet are also often used in the advanced setting [8,9]. Treatment options are limited and outcomes remain poor. Novel immunotherapeutic options with the potential to improve longevity with minimal toxicity are sorely needed [10].
Synovial sarcoma (SS) and myxoid/round cell liposarcoma (MRCL) are two translocation-associated soft-tissue sarcoma subtypes that may initially be sensitive to systemic therapy but can be very difficult to treat in the advanced setting. Both disproportionately impact younger populations; the median age of diagnosis for SS is in the 20–30s and for MRCL is in the early 40s [11–14]. There are approximately 800 cases of SS in the US annually, and nearly all are associated with the t(X;18) translocation and expression of one of its resultant SYT-SSX fusion proteins [11,15,16]. MRCL has a similar incidence, comprising approximately 20–30% of liposarcomas and is associated with a characteristic translocation t(12;16)(q13;p11) [14,17,18].
Most patients with SS and MRCL will have homogenous expression of the highly immunogenic tumor-associated antigen NY-ESO-1 [19,20]. Furthermore, while these patients have rare NY-ESO-1-specific T-cells circulating in their blood [21], they lack a robust inflammatory response and generally have relatively few T-cells infiltrating the tumor [22]. Agents with the potential to induce a robust endogenous tumor-specific T-cell population have the potential to dramatically shape the immune response against these rare malignancies.
In this review, we will discuss the dendritic cell (DC)-targeted lentiviral NY-ESO-1 vaccine, LV305, as well as the CMB305 prime-boost regimen that uses LV305 as its foundation. These vaccine strategies have proven themselves capable of inducing strong NY-ESO-1-specific immune responses and early data on patient clinical outcomes is promising.
1.1. Lentiviral vaccines targeting DCs
Because DCs play a key role in shaping the overall immune response, there is a great desire to direct them for therapeutic applications [23,24]. Although DCs play a critical role for shaping both CD4+ and CD8+ T-cell responses, engineering strategies must pay particularly close attention to the CD8+ T-cell response as this may be harder to initiate. Unlike major histocompatibility complex (MHC) class II which can efficiently cross-present exogenous peptides, resulting in robust activation of CD4 T-cells, DC cross-presentation of phagocytosed exogenous proteins or peptides via class I MHC is quite inefficient [24]. However, protein expressed in the cytoplasm of the DC that is processed by the immunoproteasome into peptides and loaded onto the DC’s class I MHC is very effective at generating CD8+ T-cell responses. For this reason, it is highly desirable to engineer DCs to express cancer-specific proteins in order to generate tumor-targeting CD8+ T-cells [25].
Because DCs are terminally differentiated and are not actively dividing, lentiviral transduction of DCs has been explored for ex vivo manipulation in order to achieve cytoplasmic protein expression in DC-based cellular therapy [26,27]. The direct injection of lentivirus in vivo has also resulted in potent antitumor immunity in murine models [28,29].
The strategy of direct vaccine injection into patients is particularly well suited to lentiviral transduction of DCs because the skin and corresponding draining lymph nodes contain a large number of DCs of various specialized subsets and intradermal or subcutaneous injection is relatively easy to perform in the clinic [30]. Indeed, the cutaneous delivery of lentiviral vector (LV) induces potent CD8+ immunity [31]. The tropism of any lentivirus is mandated by its envelope. While third-generation lentivirus vectors with broad tropism (as their envelope derives from vesicular stomatits virus [VSV]) have typically been used in preclinical or clinical studies, they carry risks related to off-target activity and integrational mutagenesis. Ideally, a LV for in vivo use should be targeted to DCs and have no risk of genotoxicity.
1.2. LV305: a DC targeting NY-ESO-1 vaccine from the ZVex platform
DC-SIGN (CD209) is a cell surface C-type lectin-like protein highly specific for immature DCs that acts as a scavenger receptor enabling lentiviral infection [32]. The alpha virus Sindbis also infects DC by using DC-SIGN as an attachment receptor, but has a broader tropism due to its affinity for ubiquitously expressed heparan sulfate [33]. Through selective mutation of the heparin binding region, a Sindbis envelope was genetically engineered that selectively binds to DC-SIGN [33,34]. ‘ZVex’ is a novel vector platform for DC-targeted vaccines that is built on a third-generation LV and includes a DC-SIGN tropic Sindbis Virus envelope (Figure 1(a)). Like other third-generation LVs generally regarded as safe for use in gene therapy, ZVex is devoid of all HIV 1-derived accessory proteins, except for Rev, and is encoded by a split genome with a deletion in the U3 region (ΔU3, for self-inactivation of the 3ʹLTR). The ΔU3 deletion is a self-inactivating mutation that: (1) prevents transcription of the full-length vector genome (vg) from reversed transcribed dsDNA vectors in the infected target cell, and (2) minimizes the risk of insertional activation that can occur when a 3ʹLTR can function as a promoter after integration. In addition, ZVex contains the Vpx protein (viral protein X from simian immune deficiency virus [SIV]) that is packaged into the vector particles and overcomes the HIV-restriction factor SAMHD1 in DC, which depletes the nucleotide triphosphate pool, thereby preventing reverse transcription of the vg. Further important safety features of ZVex are a genetically modified capsid gene to reduce the risk of recombination, and a genetically inactivated integrase enzyme (D64V mutation), which significantly reduces the risk of integration (Figure 1(b)) [35].
Figure 1.
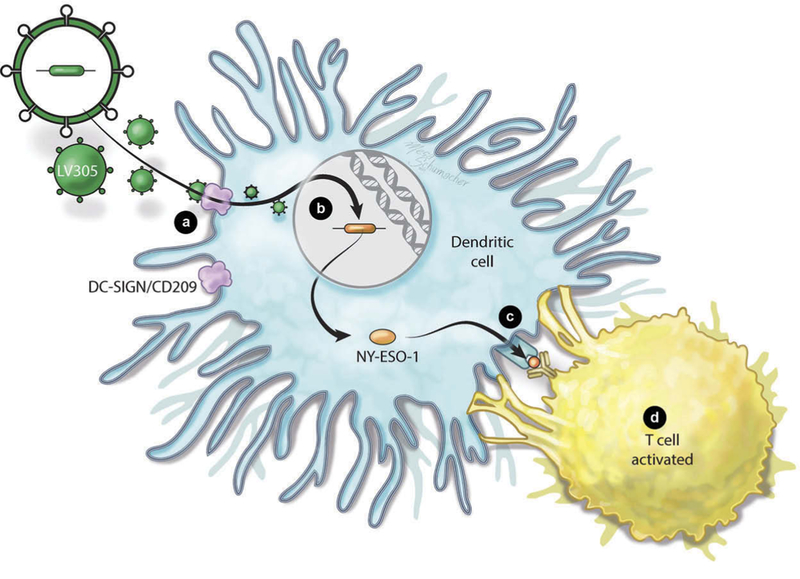
LV305 mechanism of action. (a) LV305 (green octagon) enters dendritic cells (DC) via specific interaction with DC-SIGN/CD209 (triangle) on the DC surface as a result of the modified Sindbis virus envelope. (b) LV305 is replication incompetent and has only a low level of integration because of the D64V integrase mutation and deletion of the 3ʹ-poly purine tract. (c) NY-ESO-1 is expressed by the DC and processed in the immune-proteasome. Peptides are presented by class I and cross-presented by class II MHC molecules. (d) CD4+ and CD8+ T cells recognizing NY-ESO-1 peptides presented in the context of class I and class II MHC proliferate and produce cytokines. In murine models, these T cells are effective at killing tumors. Full color available online.
Due to its large cloning capacity of approximately 8 kb, ZVex is able to encode full-length proteins and has been preclinically explored with both self-antigens and heterologous antigens, such as the cancer-testis antigen (CTA) NY-ESO-1. In mice, this vector was very effective at inducing DC expression and antigen presentation of NY-ESO-1 (Figure 1(c)). This resulted in a robust NY-ESO-1-specific T-cell response as evidenced by cellular proliferation and polyfunctional cytokine production of CD8 T-cells (Figure 1(d)). Mice vaccinated with this vector were protected in a dose-dependent fashion from metastatic lung cancer in a challenge model using CT26 cells expressing NY-ESO-1, showing clearly that the degree of protection was correlated with the level of the T-cell response [36].
1.3. CMB305: a ‘ prime/boost ’ regimen with LV305 at its core
Multiple investigators have demonstrated superior T-cell and antibody responses using prime-boost vaccination strategies where different vaccine modalities are used alternatively to target the same antigen, called ‘heterologous prime-boost’ [37]. This was dramatically demonstrated by the improved protection of nonhuman primates from simian immunodeficiency virus when a vaccinia-based vaccine was followed by a gp160 protein-based vaccine [38]. This concept has been illustrated in a variety of infectious models as well as a various murine tumor models where viral vectors, including LVs, were used to prime the immune system followed by protein boost [39–43]. While the reasons for the efficacy of heterologous prime-boost are still a matter of debate and are at least in part linked to inducing immune response against the vector in addition to the antigen, the order of the different vaccines used also appears to be important. Multiple models have demonstrated that a viral vector encoding a target antigen generally functions very effectively as the priming agent and an adjuvanted peptide/protein component functions more effectively as the boost [44–46].
In order to capture the potential of boosting an LV305 primed response, the CMB305 regimen was developed using LV305 as the priming agent given for four doses, starting day 1 then days 21, 49, and 77 (Figure 2). The boosting regimen is called ‘G305’ and starts on day 35 every 4 weeks for three doses and then every 8 weeks for up to 1 year. The G305 regimen includes full-length NY-ESO-1 protein and a toll-like receptor 4 (TLR4) agonist as an adjuvant.
Figure 2.
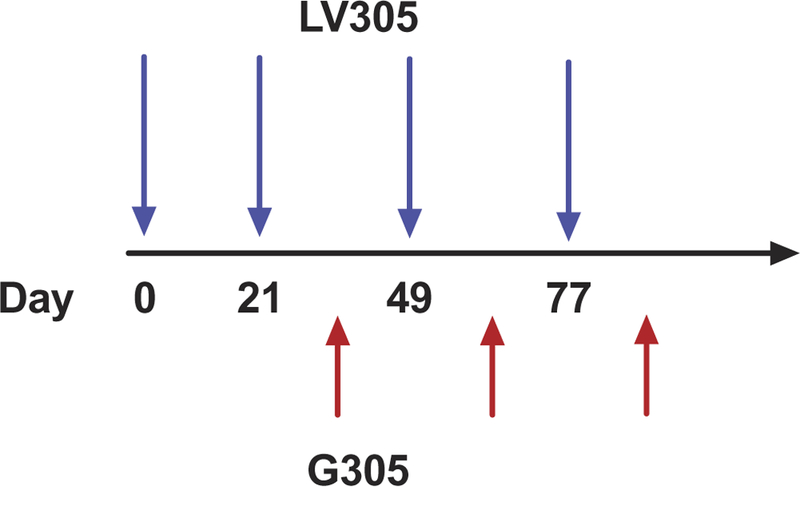
CMB305 schedule. LV305 (blue arrows) is used as the priming agent given for 4 doses, starting day 1 then days 21, 49 and 77. The boosting regimen is called ‘G305’ (red arrows) and starts on day 35 every 4 weeks for three doses and then every 8 weeks for up to 1 year. The G305 regimen includes whole recombinantly expressed NY-ESO-1 protein and the synthetic toll like receptor 4 (TLR4) agonist GLA (Glucopyranosyl lipid A) formulate in a stable oil-in-water emulsion (GLA-SE). Full color available online.
This particular TLR4 agonist, glycopyranosyl lipid A, given in a stable oil-in-water emulsion formulation (GLA-SE), is a synthetic agonist. Monophosphoryl lipid A (MPL), a detoxified bacterial lipopoplysaccharide (LPS), is a natural product and has become the first commercial TLR4 agonist used as an efficacious vaccine adjuvant as part of GlaxoSmithKline’s established vaccines for hepatitis B and cervical cancer [47,48]. However, GLA-SE, in addition to being synthetic, is more potent in activating DCs in vitro and induces Th1 CD4+ cell responses in vivo at lower doses than MPL [49]. GLA-SE was also shown to be more potent than the TLR3 agonist poly (I:C) in a human skin explant model with respect to DC activation [50].
1.4. NY-ESO-1 as a target for cancer vaccines
NY-ESO-1, a prominent member of the family of CTA, is a 180 amino acids long protein with glycine-rich N-terminal region and hydrophobic C terminal region [51]. It is not membrane-associated and its function is still unknown. As their name implies, CTAs are expressed on a protein level in various malignant tumors but not in normal adult tissues a part from the testis and the trophoblast. This particular CTA was first discovered through serological analysis in esophageal cancer patients and was subsequently found to induce antibody and T-cell responses in vaccine trials [52,53]. Notably, high expression of CTAs has been linked to worse prognosis in some tumor types [54,55].
NY-ESO-1 is considered to be among the most attractive targets for immunotherapy because of its inherent immunogenicity and has been targeted in a number of clinical studies including several vaccine trials that have induced antibody, CD4+, and CD8+ T-cell responses. Delayed type hypersensitivity responses following NY-ESO-1 protein vaccine administration with the adjuvant ISCOMATRIX® have been associated with long-term survival [56,57]. Objective clinical responses have been observed in melanoma patients following vaccination using a heterologous recombinant vaccinia/fowlpox vaccination against NY-ESO-1, including one complete response [58]. NY-ESO-1 can be upregulated in many malignancies (including certain sarcomas) [59] using the hypomethylating agent decitabine and that was seen to potentially improve response to NY-ESO-1 vaccination in ovarian cancer [60].
1.5. NY-ESO-1 as a target in SS and MRCL
While many cancers express NY-ESO-1 in a minority of cases and usually with heterogenous patterns, SS and MRCL are unique with respect to the consistency and homogeneity of target expression [61]. In an analysis by Jungbluth et al., 20 of 25 SS tumors expressed NY-ESO-1 by immunohistochemistry (IHC). Four of the five tumors not expressing NY-ESO-1 had the biphasic SS subtype, suggesting near universal NY-ESO-1 expression in the more common monophasic SS subtype. A total of 8 of the 20 NY-ESO-1 expressing tumors had homogenous expression defined as staining in >50% of tumor cells [20], and this high frequency of homogeneous expression has been confirmed in a subsequent study [62]. Our group published similar findings regarding MRCL tumors where 25 of 25 consecutive cases tested expressed NY-ESO-1 (100%), and in 18 of the cases (72%) staining was homogenous with target expression in over 50% cancer cells [19].
In order to capitalize on these high levels of antigen expression, multiple investigators have attempted adoptive T-cell therapy using T-cell receptors (TCRs) targeting the 157–165 CD8 T-cell epitope of NY-ESO-1, which is presented by human leukocyte antigen (HLA)-A*02:01 (seen in 30–40% of the Caucasian population) [63]. Objective responses have been seen in a majority of patients following cellular therapy targeting NY-ESO-1, demonstrating that NY-ESO-1 is an excellent target for these tumor types [63,64]. However, more work is required to optimize adoptive Tcell therapy for these patients as almost all patients ultimately progress and the therapy requires that an intensive regimen was used including pre-infusion of high-dose cyclophosphamide and fludarabine along with high-dose IL-2 post-infusion. Vaccination offers an entirely different approach to targeting NY-ESO-1, also with the potential for profound and long-lasting clinical activity, and without the toxicity of the conditioning regimen and without HLA restriction.
1.6. Prior vaccine studies in sarcoma
A number of investigators have looked at vaccination in sarcoma with some signals of immunologic and clinical response. A trivalent peptide vaccine against ganglioside antigens was tested in a placebo-controlled, multicenter trial including 136 patients with no evidence of disease following metastasectomy did not result in a significant improvement in PFS, but serological responses developed following vaccination, suggesting a potential benefit might be identified in the right context [65]. A pilot study using peptides spanning the SYT-SSX fusion in SS patients resulted in one transient tumor response and more than a third of patients developed a T-cell response based on staining using MHC-peptide tetramers [66].
Several trials have attempted vaccination using DCs loaded with tumor lysate and a number of them have led to delayed type hypersensitivity reaction to autologous tumor antigens [67,68]. A study using a DC vaccine following consolidative high-dose chemotherapy appeared to improve outcomes relative to historical controls [69]. Another such trial for Ewings sarcoma used DCs pulsed with either tumor lysate or peptide and yielded a durable complete response (CR) in one patient [70]. Another led to regression of several sizable pulmonary metastasis in a fibrosarcoma patient [71]. While all of these trials had their individual strengths and weaknesses, prior to LV305 and CMB305 no vaccine approach has been performed to target a frequently and homogenously expressed, highly immunogenic protein such as NY-ESO-1 in sarcoma.
1.7. Clinical outcomes of LV305 and CMB305
LV305 was tested in a Phase I, first in human trial, the first trial ever testing this class of next-generation vaccines (Table 1) [72]. The initial cohort enrolled four cohorts (three patients each) receiving three or four intradermal injections every 3 weeks using 108, 109, or 1010 vgs per dose in a 3 + 3 design typically used in oncological trials. After this safety lead-in, an expansion cohort treated patients with multiple NY-ESO-1+ malignancies (24 sarcoma patients, 5 melanoma, 9 ovarian, and 1 non-small cell lung cancer patients) with 4 doses at the 1010 vg dose level. No serious adverse events or doselimiting toxicities were observed. Although only one partial response (PR; 80% regression) was observed in the trial, 54% of sarcoma patients had stable disease (SD). PFS was 140 days for sarcoma patients and 7/11 (63.6%) patients who were progressing when they started on the trial developed SD or PR. Over 80% of patients treated on the study were alive 1 year after vaccination, including those sarcoma patients who were progressing at the time of study entry and are known to have a very poor prognosis. For comparison, others have seen survival for SS patients in the metastatic setting of less than 12 months following their second line of chemotherapeutic treatment [73]. As of the 2017 American Society of Clinical Oncology (ASCO) meeting, the median OS for the study had still not been reached [74].
Table 1.
Trials including the LV305 vaccine.
| Trial | ClinicalTrials.gov identifier | Phase | Agent | Treatment | No. of patients (sarcoma) |
|---|---|---|---|---|---|
| A Phase 1, open-label clinical trial evaluating the safety, tolerability, and immunogenicity of intradermally administered ID-LV305 in patients with locally advanced, relapsed, or metastatic cancer expressing NY-ESO-1 | NCT02122861 | Phase I | LV305 | Lentivirus alone | 39 (24) |
| A Phase 1b study evaluating the safety, tolerability, and immunogenicity of CMB305 (sequentially administered LV305 and G305) in patients with locally advanced, relapsed, or metastatic cancer expressing NY-ESO-1 | NCT02387125 | Phase I/Ib | CMB305 | Lentiviral vaccine alternating with TLR4 + NY-ESO-1 protein ‘G305’ | 49 (25) |
| A randomized, open-label, Phase 2 trial of CMB305 (sequentially administered LV305 and G305) and atezolizumab in patients with locally advanced, relapsed, or metastatic sarcoma expressing NY-ESO-1 | NCT02609984 | Randomized Phase II | CMB305 + Atezolizumab | PD-L1 inhibitor ± CMB305 | 88 patients (all sarcoma, 45 randomized to vaccine) |
The first patient treated on the trial had a remarkable and durable response despite aggressive and refractory disease [75]. Like other patients, she had no serious adverse events but did have low grade toxicities including pain and stinging at the injection site, mild fatigue the day after injections, an episode of subjective palpitations, subjective fevers and myalgias in the days following vaccination, each of which resolved within 24 h. She had initial disease stabilization until 5 months post-LV305 initiation, when it shrunk to 24.7% below baseline. By month 24 post-LV305, tumor mass had shrunk to 84.8% below baseline. The response is continuing over 3 years following her vaccination. The patient had a baseline detectable T-cell recognition (CD4 and CD8) of NY-ESO-1 prior to her vaccination and this increased markedly following the treatment.
A follow-up Phase I trial, presented at the 2017 ASCO meeting, analyzed the safety of the CMB305 ‘prime-boost’ regimen [74]. This trial treated 49 patients including 25 with sarcoma (14 SS, 9 MRCL, and 2 other sarcomas). The patient population had, for the most part, advanced refractory disease with 56% progressing at the time of study progression. A total of 92% of patients were treated in the metastatic setting and with 52% of them having two or more prior lines of chemotherapy. Three patients had grade 3 toxicities attributed to the therapy (fatigue, prostatic pain, and pneumonitis). Of note, over 80% of patients had homogenous expression of NYESO-1 by IHC. The median PFS for the study was 4.7 months, comparable to PFS values seen in registration trials for FDAapproved drugs in STS [5–7]. More impressive was the OS data, with 76% of patients surviving 18 months after starting treatment. OS for sarcoma patients receiving FDA-approved second-line therapies such as pazopanib, eribulin, and trabectedin has ranged from 12.4 to 13.5 months [5–7].
Although PD-L1 expression is low in SS and MRCL tumors and they have few inflammatory features, including few T-cells infiltrating into the tumor, this may be malleable with effective vaccination [76]. The patient who had a dramatic response to LV305 had a large peripheral increase in T-cell clones that were detectable in a pretreatment biopsy and the percentage of NYESO-1-specific cells expressing PD-1 also increased following vaccination [75]. In order to block the inhibitory impact of PDL1 interactions with PD-1 on NY-ESO-1-specific T-cells, a randomized Phase II trial of atezolizumab and CMB305 was initiated. Interim results were presented at the 2017 European Society of Medical Oncology (ESMO) meeting. A total of 88 patients were randomized to receive either atezolizumab alone or in combination with CMB305. The regimen was well tolerated and, at the time of interim analysis, efficacy data were available for 36 patients who had at least 6 months of PFS data. For these patients, PFS was 2.6 months for the combination and 1.4 months for atezolizumab alone. The disease control rate (SD or better) was 61% for the combination and 27.8% in the control arm. Significantly higher numbers of patients developed T-cell, antibody, and epitope spreading responses following treatment with the combination versus atezolizumab alone.
1.8. Immunologic outcomes and biomarkers for response
Because the PFS values were similar for patients on the LV305 and CMB305 studies and because of the long observation period required to determine median OS, it has been difficult to compare the clinical outcomes. However, based on immunologic responses, there is clear evidence that the boost increases anti-NY-ESO-1 immunity and that patients with induced immune responses have longer OS. An analysis of biomarkers for LV305 and CMB305 and their association with clinical outcomes was presented at the 2017 ASCO Annual Meeting [77]. The analysis included 64-pooled patients who were analyzed regardless of their tumor type. As these were Phase I trials, all patients had recurrent, NY-ESO-1+ disease which in most cases was active and refractory. LV305 and CMB305 induced anti-NY-ESO-1 T-cells in 52% and 68% pts, respectively. Anti-NY-ESO-1 antibodies developed in 3% and 70% pts, respectively. As expected, because of the marked activity of GLA-SE on TH1 cells, NY-ESO-1-specific CD4+ T-cell responses increased more in the patients treated with CMB305. Based on both antibody and ELIspot analysis, LV305 and CMB305 induced immune responses against other tumorassociated antigens (so-called ‘antigen spreading’) in 17% and 36% patients, respectively.
Following administration of CMB305 patients had a higher clonality in the Vβ TCR repertoire, as measured by deep sequencing of peripheral blood mononuclear cells (PBMC). Interestingly, both preexisting and induced NY-ESO-1-specific immune responses (either NY-ESO-1 antibodies or T-cells by ELIspot) were associated with longer OS and PFS. The patients who had the best OS and PFS were those patients who had a preexisting NY-ESO-1-specific response at base line that was further increased following vaccination (>fourfold increase of antibody responses; >twofold increase of T-cell responses).
2. Conclusion
SS and MRCL are devastating rare cancers that affect young adults and have relatively few treatment options. CMB305 is a vaccine regimen that includes the lentiviral DC-targeted NY-ESO-1 vaccine for priming and a boost using a TLR4 agonist and a NY-ESO-1 protein vaccine. CMB305 has a proven ability to induce potent Tcell responses in SS and MRCL patients. Early data suggest a benefit in clinical outcomes but further validation is needed.
3. Expert commentary
STS are a heterogeneous group of mesenchymal neoplasms with a median OS in the range of 18 months in the metastatic setting and poor options for systemic therapy [1]. While recent work suggests that certain STS subtypes may have a highly inflammatory tumor microenvironment, the translocation-associated sarcomas SS and MRCL appear to be ‘cold’ with few infiltrating Tcells and little PD-L1 expression. While checkpoint inhibitors may quickly become important options for the highly mutated sarcomas, tumors such as SS and MRCL may need additional interventions to increase the size of the tumor-specific T-cell response. Because these tumors have strong expression of the highly immunogenic protein NY-ESO-1, they may be ideal targets for a strategy including therapeutic vaccination.
LV305 is the first DC-targeted LV for in vivo immunization to be studied in the clinic, notably in SS and MRCL. These STS subtypes are complex diseases with immunosuppressive, noninflammatory tumor immune microenvironments. In this context, it is important to note that LV305/CMB305 show signals of clinical activity in multiple independent single-arm Phase 1 trials in these tumor types as well as the randomized C232 study with survival rates comparing favorably to the standard of care chemotherapy. The vector is safe, without evidence of viral persistence and is well tolerated. It induces a potent NYESO-1-specific CD4 and CD8 T-cell response. CMB305 further boosts this response using a TLR4 agonist resulting in robust CD4+ T-cell response and antibody generation. Combination therapies, such as PD-1 or PD-L1-targeted agents, may further improve its activity. Thus, the LV305 and the CMB305 regimens have clear potential to impact the treatment of SS and MRCL and possibly also other STS and other malignancies expressing NY-ESO-1. We expect additional trials in the coming years with the goal of definitively testing the clinical activity of this novel vaccine regimen. If successful, such trials could put this vaccine on a path toward regulatory approval.
4. Five-year view
We expect that several immunotherapies will become integrated into the standard of care for advanced STS during the next 5 years. During that time, we speculate that a Phase III trial of CMB305 will be completed and that CMB305 ultimately will become integrated into an effective regimen for SS and MRCL, perhaps in combination with a checkpoint inhibitor. As CMB305 finds its place among standard treatment options for sarcoma, it will also be tested in the context of the many other cancers that can express NY-ESO-1. We also expect that the ZVex vector backbone will be tested using other targets.
Key issues.
Synovial Sarcoma and Myxoid/Liposarcomas are rare but devastating cancers that in most cases have homogenous expression of NY-ESO-1
ZVex is a novel lentiviral vaccine platform that utilizes a modified Sindbis virus to gain DC specificity.
LV305 is the first vaccine from the ZVex platform to be tested in the clinic. It encodes NY-ESO-1 and has demonstrated single agent activity.
CMB305 is a ‘prime-boost’ regimen that includes LV305 for priming with a boost of NY-ESO-1 protein and a potent TLR4 agonist adjuvant (G100).
The CMB305 regimen is currently being studied as part of novel combinations for the treatment of Synovial Sarcoma and Myxoid/round cell liposarcoma patients.
Acknowledgments
Funding
The manuscript was funded by the Center for Strategic Scientific Initiatives, National Cancer Institute [1K23CA175167–03].
Seth Pollack receives research funding from Immune Design, maker of LV305 and CMB305, through the Fred Hutchinson Cancer Research Center. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Footnotes
Declaration of interest
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
References
- 1.Frezza AM, Stacchiotti S, Gronchi A. Systemic treatment in advanced soft tissue sarcoma: what is standard, what is new. BMC Med 2017;15:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995;13:1537–1545. [DOI] [PubMed] [Google Scholar]
- 3.Judson I, Verweij J, Gelderblom H, et al. European Organisation and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomised controlled phase 3 trial. Lancet Oncol 2014;15(4):415–423. [DOI] [PubMed] [Google Scholar]
- 4.Tap WD, Jones RL, Van Tine BA, et al. Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. Lancet 2016;388:488–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Graaf WT, Blay J-Y, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–1886. [DOI] [PubMed] [Google Scholar]
- 6.Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a phase III randomized multicenter clinical trial. J Clin Oncol 2016;34:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schöffski P, Chawla S, Maki RG, et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: a randomised, open-label, multicentre, phase 3 trial. Lancet 2016;387:1629–1637. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Chang MH, Baek KK, et al. High-dose ifosfamide as second or third-line chemotherapy in refractory bone and soft tissue sarcoma patients. Oncology 2011;80:257–261. [DOI] [PubMed] [Google Scholar]
- 9.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 2007;25:2755–2763. [DOI] [PubMed] [Google Scholar]
- 10.Lee A, Huang P, DeMatteo RP, et al. Immunotherapy for soft tissue sarcoma: tomorrow is only a day away. Am Soc Clin Oncol Educ Book 2016;35:281–290. [DOI] [PubMed] [Google Scholar]
- 11.Sultan I, Rodriguez-Galindo C, Saab R, et al. Comparing children and adults with synovial sarcoma in the surveillance, epidemiology, and end results program, 1983 to 2005: an analysis of 1268 patients. Cancer 2009;115:3537–3547. [DOI] [PubMed] [Google Scholar]
- 12.Chen C, Borker R, Ewing J, et al. Epidemiology, treatment patterns, and outcomes of metastatic soft tissue sarcoma in a communitybased oncology network. Sarcoma 2014;2014:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman A, Ghadimi MP, Demicco EG, et al. Localized and metastatic myxoid/round cell liposarcoma: clinical and molecular observations. Cancer 2013;119:1868–1877. [DOI] [PubMed] [Google Scholar]
- 14.Moreau L-C, Turcotte R, Ferguson P, et al. Myxoid\round cell liposarcoma (MRCLS) revisited: an analysis of 418 primarily managed cases. Ann Surg Oncol 2012;19:1081–1088. [DOI] [PubMed] [Google Scholar]
- 15.Ladanyi M, Antonescu CR, Leung DH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multiinstitutional retrospective study of 243 patients. Cancer Res 2002;62:135–140. [PubMed] [Google Scholar]
- 16.Kampe CE, Rosen G, Eilber F, et al. Synovial sarcoma. A study of intensive chemotherapy in 14 patients with localized disease. Cancer 1993;72:2161–2169. [DOI] [PubMed] [Google Scholar]
- 17.Aman P, Ron D, Mandahl N, et al. Rearrangement of the transcription factor gene CHOP in myxoid liposarcomas with t(12;16)(q13; p11). Genes Chromosomes Cancer 1992;5:278–285. [DOI] [PubMed] [Google Scholar]
- 18.Conyers R, Young S, Thomas DM. Liposarcoma: molecular genetics and therapeutics. Sarcoma 2011;2011:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pollack SM, Jungbluth AA, Hoch BL, et al. NY-ESO-1 is a ubiquitous immunotherapeutic target antigen for patients with myxoid/round cell liposarcoma. Cancer 2012;118:4564–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reports NY-ESO-1 expression in myxoid/round cell liposarcoma.
- 20.Jungbluth AA, Antonescu CR, Busam KJ, et al. Monophasic and biphasic synovial sarcomas abundantly express cancer/testis anti gen NY-ESO-1 but not MAGE-A1 or CT7. Int J Cancer 2001;94:252– 256. [DOI] [PubMed] [Google Scholar]; • Reports NY-ESO-1 expression in synovial sarcoma.
- 21.Pollack SM, Jones RL, Farrar EA, et al. Tetramer guided, cell sorter assisted production of clinical grade autologous NY-ESO-1 specific CD8(+) T cells. J Immunother Cancer 2014;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack SM, He Q, Yearley JH, et al. T-cell infiltration and clonality correlate with programmed cell death protein 1 and programmed death-ligand 1 expression in patients with soft tissue sarcomas. Cancer 2017;123:3291–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Detailed analysis of the sarcoma tumor immune microenvironment.
- 23.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature 2007;449:419–426. [DOI] [PubMed] [Google Scholar]
- 24.Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 2010;33:464– 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zinkernagel RM. On the role of dendritic cells versus other cells in inducing protective CD8+ T cell responses. Front Immunol 2014;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breckpot K, Dullaers M, Bonehill A, et al. Lentivirally transduced dendritic cells as a tool for cancer immunotherapy. J Gene Med 2003;5:654–667. [DOI] [PubMed] [Google Scholar]
- 27.He Y, Zhang J, Mi Z, et al. Immunization with lentiviral vector-transduced dendritic cells induces strong and long-lasting T cell responses and therapeutic immunity. J Immunol 2005;174:3808–3817. [DOI] [PubMed] [Google Scholar]
- 28.Dullaers M, Van Meirvenne S, Heirman C, et al. Induction of effec tive therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors. Gene Ther 2006;13:630–640. [DOI] [PubMed] [Google Scholar]
- 29.Rowe HM, Lopes L, Ikeda Y, et al. Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene. Mol Ther 2006;13:310–319. [DOI] [PubMed] [Google Scholar]
- 30.Clausen BE, Stoitzner P. Functional specialization of skin dendritic cell subsets in regulating T cell responses. Front Immunol 2015;6:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Y, Zhang J, Donahue C, et al. Skin-derived dendritic cells induce potent CD8(+) T cell immunity in recombinant lentivectormediated genetic immunization. Immunity 2006;24:643–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geijtenbeek TB, Kwon DS, Torensma R, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 2000;100:587–597. [DOI] [PubMed] [Google Scholar]
- 33.Klimstra WB, Nangle EM, Smith MS, et al. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J Virol 2003;77:12022–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, Yang H, Rideout K, et al. Engineered lentivector targeting of dendritic cells for in vivo immunization. Nat Biotechnol 2008;26:326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First description of DC targeting lentiviral vector.
- 35.Tareen SU, Kelley-Clarke B, Nicolai CJ, et al. Design of a novel integration-deficient lentivector technology that incorporates genetic and posttranslational elements to target human dendritic cells. Mol Ther 2014;22:575–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albershardt TC, Campbell DJ, Parsons AJ, et al. LV305, a dendritic cell-targeting integration-deficient ZVex(TM)-based lentiviral vector encoding NY-ESO-1, induces potent anti-tumor immune response. Mol Ther Oncolytics 2016;3:16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu S Heterologous prime-boost vaccination. Curr Opin Immunol 2009;21:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu SL, Abrams K, Barber GN, et al. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science 1992;255:456–459. [DOI] [PubMed] [Google Scholar]
- 39.Wang S, Parker C, Taaffe J, et al. Heterologous HA DNA vaccine prime–inactivated influenza vaccine boost is more effective than using DNA or inactivated vaccine alone in eliciting antibody responses against H1 or H3 serotype influenza viruses. Vaccine 2008;26:3626–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de la Luz Garcia-Hernandez M, Gray A, Hubby B, et al. In vivo effects of vaccination with six-transmembrane epithelial antigen of the prostate: a candidate antigen for treating prostate cancer. Cancer Res 2007;67:1344–1351. [DOI] [PubMed] [Google Scholar]
- 41.Herrmann JE, Wang S, Zhang C, et al. Passive immunotherapy of Bacillus anthracis pulmonary infection in mice with antisera produced by DNA immunization. Vaccine 2006;24:5872–5880. [DOI] [PubMed] [Google Scholar]
- 42.Rusakiewicz S, Dosset M, Mollier K, et al. Immunogenicity of a recombinant lentiviral vector carrying human telomerase tumor antigen in HLA-B*0702 transgenic mice. Vaccine 2010;28:6374– 6381. [DOI] [PubMed] [Google Scholar]
- 43.Alpizar YA, Karwacz K, Arce F, et al. Lentiviral vector followed by protein immunisation breaks tolerance against the self-antigen Her1 and results in lung cancer immunotherapy. J Gene Med 2012;14:151–157. [DOI] [PubMed] [Google Scholar]
- 44.Richmond JF, Lu S, Santoro JC, et al. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J Virol 1998;72:9092–9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S, Arthos J, Lawrence JM, et al. Enhanced immunogenicity of gp120 protein when combined with recombinant DNA priming to generate antibodies that neutralize the JR-FL primary isolate of human immunodeficiency virus type 1. J Virol 2005;79:7933–7937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otten GR, Schaefer M, Doe B, et al. Enhanced potency of plasmid DNA microparticle human immunodeficiency virus vaccines in rhesus macaques by using a priming-boosting regimen with recombinant proteins. J Virol 2005;79:8189–8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters NC, Bertholet S, Lawyer PG, et al. Evaluation of recombinant Leishmania polyprotein plus glucopyranosyl lipid A stable emul sion vaccines against sand fly-transmitted Leishmania major in C57BL/6 mice. J Immunol 2012;189:4832–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones GJ, Steinbach S, Clifford D, et al. Immunisation with ID83 fusion protein induces antigen-specific cell mediated and humoral immune responses in cattle. Vaccine 2013;31:5250–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coler RN, Bertholet S, Moutaftsi M, et al. Development and characterization of synthetic glucopyranosyl lipid adjuvant system as a vaccine adjuvant. PLoS One 2011;6:e16333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schneider LP, Schoonderwoerd AJ, Moutaftsi M, et al. Intradermally administered TLR4 agonist GLA-SE enhances the capacity of human skin DCs to activate T cells and promotes emigration of Langerhans cells. Vaccine 2012;30:4216–4224. [DOI] [PubMed] [Google Scholar]
- 51.Simpson AJ, Caballero OL, Jungbluth A, et al. Cancer/testis anti gens, gametogenesis and cancer. Nat Rev Cancer 2005;5:615–625. [DOI] [PubMed] [Google Scholar]
- 52.Chen YT, Scanlan MJ, Sahin U, et al. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A 1997;94:1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991;254:1643–1647. [DOI] [PubMed] [Google Scholar]
- 54.Szender JB, Papanicolau-Sengos A, Eng KH, et al. NY-ESO-1 expression predicts an aggressive phenotype of ovarian cancer. Gynecol Oncol 2017;145:420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iura K, Kohashi K, Hotokebuchi Y, et al. Cancer-testis antigens PRAME and NY-ESO-1 correlate with tumour grade and poor prognosis in myxoid liposarcoma. J Pathol Clin Res 2015;1:144–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Davis ID, Chen W, Jackson H, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci U S A 2004;101:10697–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Important report of therapeutic NY-ESO-1 vaccination in cancer patients.
- 57.Nicholaou T, Ebert LM, Davis ID, et al. Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res 2009;15:2166–2173. [DOI] [PubMed] [Google Scholar]
- 58.Jäger E, Karbach J, Gnjatic S, et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1specific immune responses in cancer patients. Proc Natl Acad Sci U S A 2006;103:14453–14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pollack SM, Li Y, Blaisdell MJ, et al. NYESO-1/LAGE-1s and PRAME are targets for antigen specific T cells in chondrosarcoma following treatment with 5-Aza-2-deoxycitabine. PLoS One 2012;7: e32165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Odunsi K, Matsuzaki J, James SR, et al. Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer. Cancer Immunol Res 2014;2:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun 2004;4:1. [PubMed] [Google Scholar]
- 62.Chapuis AG, Thompson JA, Margolin KA, et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc Natl Acad Sci U S A 2012;109:4592–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robbins PF, Morgan RA, Feldman SA, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 2011;29:917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robbins PF, Kassim SH, Tran TL, et al. A pilot trial using lympho cytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res 2015;21:1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chapman PB, Morrisey D, Panageas KS, et al. Vaccination with a bivalent G(M2) and G(D2) ganglioside conjugate vaccine: a trial comparing doses of G(D2)-keyhole limpet hemocyanin. Clin Cancer Res 2000;6:4658–4662. [PubMed] [Google Scholar]
- 66.Kawaguchi S, Tsukahara T, Ida K, et al. SYT-SSX breakpoint peptide vaccines in patients with synovial sarcoma: a study from the Japanese Musculoskeletal Oncology Group. Cancer Sci 2012;103:1625–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dillman R, Barth N, Selvan S, et al. Phase I/II trial of autologous tumor cell line-derived vaccines for recurrent or metastatic sarcomas. Cancer Biother Radiopharm 2004;19:581–588. [DOI] [PubMed] [Google Scholar]
- 68.Dillman RO, Selvan SR, Schiltz PM, et al. Phase II trial of dendritic cells loaded with antigens from self-renewing, proliferating autologous tumor cells as patient-specific antitumor vaccines in patients with metastatic melanoma: final report. Cancer Biother Radiopharm 2009;24:311–319. [DOI] [PubMed] [Google Scholar]
- 69.Mackall CL, Rhee EH, Read EJ, et al. A pilot study of consolidative immunotherapy in patients with high-risk pediatric sarcomas. Clin Cancer Res 2008;14:4850–4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suminoe A, Matsuzaki A, Hattori H, et al. Immunotherapy with autologous dendritic cells and tumor antigens for children with refractory malignant solid tumors. Pediatr Transplant 2009;13:746–753. [DOI] [PubMed] [Google Scholar]
- 71.Geiger JD, Hutchinson RJ, Hohenkirk LF, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res 2001;61:8513–8519. [PubMed] [Google Scholar]
- 72.Somaiah N, Block MS, Kim JW, et al. Single-agent LV305 to induce anti-tumor immune and clinical responses in patients with advanced or metastatic sarcoma and other cancers expressing NY-ESO-1. ASCO Meet Abstr 2016;34:3093. [Google Scholar]
- 73.Savina M, Le Cesne A, Blay J-Y, et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: the METASARC observational study. BMC Med 2017;15:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Somaiah N, Chawla S, Block M, et al. Immune response, safety, and survival impact from CMB305 in NY-ESO-1+ recurrent soft tissue sarcomas (C131 study). In: American Society of Clinical Oncology annual meeting Chicago, IL; June 2–6 2017. [Google Scholar]
- 75.Pollack SM, Lu H, Gnjatic S, et al. First-in-human treatment with a dendritic cell-targeting lentiviral vector-expressing NY-ESO-1, LV305, induces deep, durable response in refractory metastatic synovial sarcoma patient. J Immunother 2017;40:302–306. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First report of a patient treated with LV305.
- 76.Chawla S A phase 2 study of CMB305 and atezolizumab in NY-ESO1 soft tissue sarcoma: interim analysis of immunogenicity, tumor control and survival. In: European Society of Medical Oncology (ESMO) meeting Chicago, IL; June 2–6 2017. [Google Scholar]
- 77.Pollack S, Lu H, Somaiah N, et al. CMB305 or LV305-induced and baseline anti-NY-ESO-1 immunity is associated with survival in recurrent cancer patients. In: American society of clinical oncology annual meeting Chicago, IL; June 2–6 2017. [Google Scholar]


