Abstract
In flat-faced dog breeds, air resistance caused by skull conformation is believed to be a major determinant of Brachycephalic Obstructive Airway Syndrome (BOAS). The clinical presentation of BOAS is heterogeneous, suggesting determinants independent of skull conformation contribute to airway disease. Norwich Terriers, a mesocephalic breed, are predisposed to Upper Airway Syndrome (UAS), a disease whose pathological features overlap with BOAS. Our health screening clinic examined and scored the airways of 401 Norwich terriers by laryngoscopy. Genome-wide association analyses of UAS-related pathologies revealed a genetic association on canine chromosome 13 (rs9043975, p = 7.79x10-16). Whole genome resequencing was used to identify causal variant(s) within a 414 kb critical interval. This approach highlighted an error in the CanFam3.1 dog assembly, which when resolved, led to the discovery of a c.2786G>A missense variant in exon 20 of the positional candidate gene, ADAM metallopeptidase with thrombospondin type 1 motif 3 (ADAMTS3). In addition to segregating with UAS amongst Norwich Terriers, the ADAMTS3 c.2786G>A risk allele frequency was enriched among the BOAS-susceptible French and (English) Bulldogs. Previous studies indicate that ADAMTS3 loss of function results in lymphoedema. Our results suggest a new paradigm in the understanding of canine upper airway disease aetiology: airway oedema caused by disruption of ADAMTS3 predisposes dogs to respiratory obstruction. These findings will enhance breeding practices and could refine the prognostics of surgical interventions that are often used to treat airway obstruction.
Author summary
Respiratory diseases are prevalent across dog breeds, particularly in brachycephalic breeds such as the Bulldog and French bulldog. The flat facial conformation of these breeds has long been assumed to be the major predisposing factor, however, the underlying genetics of their respiratory condition has never been elucidated. We became interested in the Norwich Terrier, a breed presenting with many of the same respiratory disease symptoms as the Bulldog. A distinction, however, is that the Norwich terrier is not considered to be a brachycephalic breed and so presented an opportunity to dissociate respiratory disease from head conformation. We performed a genome-wide association analysis for respiratory disease severity in the Norwich Terrier and resolved an association on chromosome 13 to a missense mutation in ADAMTS3. Variants in this gene were previously shown to cause an oedematous phenotype–a disease characteristic in the airways of affected Norwich Terriers and brachycephalic dogs alike. We screened over 100 breeds for the ADAMTS3 variant and found that it is enriched in the Norwich Terrier, Bulldog and French Bulldog. This discovery changes how we view respiratory disease predisposition in the dog, offers potential genetic screens and highlights a new biological function for ADAMTS3.
Introduction
Amongst dogs, brachycephaly describes the head conformation of many popular breeds including the Bulldog, French Bulldog and Pug. This trait is grossly characterised by the concurrent rostrocaudal shortening and mediolateral widening of the skull and is accompanied by skin folds of the face. The structural discordance between the reduced facial skeleton and its overlying soft tissues such as the wrinkled skin folds underpins these breeds’ iconic looks, but these artificially selected aesthetics are under increasing scrutiny for their association with health problems including breathing difficulties.
It is thought that soft tissues of the upper respiratory tract such as the nostrils, nasal mucosa of the turbinates and soft palate do not scale proportionately with reductions in the midface skeleton [1]. Misconfiguration of respiratory soft tissue restricts airflow and increases negative pressure within the airway [1,2]. This predisposes brachycephalic dogs to Brachycephalic Obstructive Airway Syndrome (BOAS). Dogs diagnosed with BOAS can have stenotic nares, elongated soft palates and oversized, caudally protruding nasal turbinates [2–7]. Airway resistance caused by these tissue anomalies is believed to induce pathological remodelling of additional tissues including tonsil and laryngeal saccule eversion, oedema of the nasopharynx, laryngeal collapse, tracheal hypoplasia and exacerbation of the thickening and elongation of the soft palate [2,8,9]. Collectively, these perturbations severely impact the wellbeing of affected individuals by increasing their respiratory effort, resulting in laboured breathing, intolerance to heat/exercise, cyanosis and collapse [6,7].
The clinical assessment of the respiratory obstruction is often based on the grading of clinical symptoms, diagnostic imaging, and more recently, whole-body barometric plethysmography [6,10,11]. Treatment options for BOAS include anti-inflammatory medication which can reduce swelling/oedema acutely, however corrective surgery is often required to alleviate the condition [12]. Rhinoplasty of the nares, excision of the caudal aspect of the soft palate and aberrant turbinates, removal of the laryngeal saccules and tonsillectomy are the most common surgical procedures, which generally have mixed prognoses [2,3,6,12–14]. The number of patients requiring surgical treatment is expected to rise notably with the rapid increase in popularity of brachycephalic breeds. The costs and morbidity of surgical treatment are a welfare concern for both owners and their dogs, with complications reported in up to 25% and mortality in as many as 5% of cases treated surgically [14].
We and others have studied the underlying genetics of canine skull shape variation [15–19]. Variants in the BMP3 and SMOC2 genes are associated with canine brachycephaly, however the contribution of these variants to BOAS pathogenesis is unclear. Moreover, variants in both of these genes appear largely fixed among brachycephalic breeds that are at greatest risk of developing BOAS and yet the incidence and severity of BOAS differs between them [11,20]. BOAS heterogeneity may also be influenced by environmental and epigenetic factors, as well as other genetic modifiers segregating among dog populations. Under this premise, we became interested in the presentation of a respiratory condition remarkably similar to BOAS which has been identified in Norwich Terriers.
As their name suggests, Norwich Terriers originate from south eastern UK where they were used for rodent control. Today, Norwich Terriers are recognised by all major kennel clubs and are known for their short, stocky build and prick ears. Dietschi et al. first described the presentation of Upper Airway Syndrome (UAS) in the Norwich Terrier. Although they are not considered a brachycephalic breed, affected Norwich Terriers present many of the hallmarks of BOAS including elongated and thickened soft palates, oedema of the nasopharynx and everted laryngeal saccules [21,22]. The closely related Norfolk Terrier, a breed that officially split from the Norwich Terrier in 1964, is seemingly unaffected by UAS, suggesting genetic predisposition in the Norwich Terriers.
Moreover, anecdotes from breeders regarding more recent dog generations, suggested that some Norwich Terriers appeared shorter-faced than those from earlier generations (personal communication to JS). Indeed, Koch et al. postulated that selective breeding is driving Norwich Terriers to become brachycephalic [9,23,24]. Spurred on by these observations, and the possibility of uncovering genetic modifiers that increase respiratory obstruction risk, we sought to understand the genetic basis of Norwich Terrier UAS.
Results
Skull morphology of the Norwich Terrier
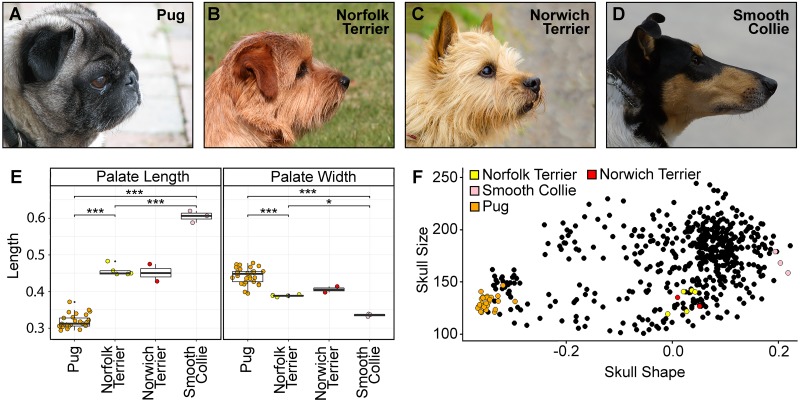
There is a continuum of head shapes observed across the domestic dog population ranging from the extreme brachycephalic to dolichocephalic conformations as represented by the profiles of the Pug and Smooth Collie, respectively (Fig 1A and 1D). Respiratory tract disorders are markedly enriched amongst brachycephalic breeds such as the Pug, Bulldog, French Bulldog, Shih Tzu as well as the Norwich Terrier. The latter is not considered to be a brachycephalic breed, nor is the Norfolk Terrier (Fig 1B and 1C). Rather both are generally considered “mesocephalic”. Indeed, linear measurements of the Norwich Terrier hard palate revealed intermediate palate dimensions between the extremes of facial morphology represented by the Pug and Smooth Collie (Fig 1E). Furthermore, geometric morphometric analysis of the canine rostrum revealed that the Norwich Terrier occupies a morphospace distinct from classic brachycephalic breeds such as the Pug (Fig 1F). For both linear measurements and geometric morphometrics, our data do not indicate a gross morphological difference between the Norwich Terrier and Norfolk Terrier.
Fig 1. Morphology and respiratory distress.
Lateral head profiles of the (A) brachycephalic Pug, (B) mesocephalic Norfolk Terrier, (C) mesocephalic Norwich Terrier and (D) dolichocephalic Smooth Collie. Images are not to scale. (E) Measurements of the canine hard palate normalised for skull size indicate that the rostrum shape of the Norwich Terrier and Norfolk Terrier is intermediate to that of extreme brachycephalic (Pug) and dolichocephalic (Smooth Collie) dogs. Mann-Whitney-Wilcoxon and Kolmogorov-Smirnov tests <0.05 *; <0.01 **; <0.001 ***. (F) Geometric morphometric analysis of 96 domestic dog breeds reveals that the Norwich Terrier and Norfolk Terrier occupy a distinct morphospace from the brachycephalic breeds such as the Pug, the latter which is susceptible to BOAS. Skull size is scored by neurocranium centroid size (y-axis) and skull shape is scored by viscerocranium PC1 (x-axis). Photo credits: Matthew Carr (Pug), Anne Johnsen (Norfolk Terrier), Dan Kyprianou (Norwich Terrier), Bev White (Smooth Collie).
Norwich Terrier upper airway assessment
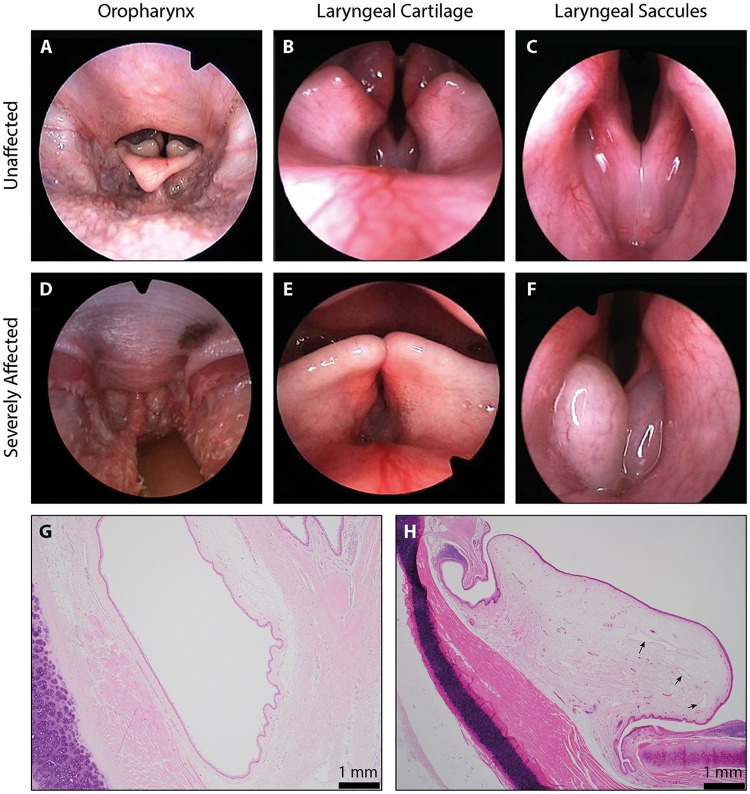
In 2000, an upper airway screening programme for Norwich Terriers was established at the Vetsuisse Faculty of the University of Bern in Switzerland. The programme uses laryngoscopic videos to score ten components of the airway as normal, mild, moderate and severe (S1 and S2 Movies and Table 1) [22]. An overall grade was given for the upper airway condition by combining the ten individual scores. Images taken from the laryngoscopic videos give examples of the soft palate length, laryngeal cartilage and laryngeal saccules graded as ‘normal’ (Fig 2A–2C). In ‘severe’ graded examples, the soft palate is elongated and protruding caudally into the epiglottis (Fig 2D), laryngeal cartilage is inverted into the lumen of the airway (Fig 2E) and the laryngeal saccules are everted (Fig 2F). Histology from an unaffected Norwich Terrier reveals unremarkable connective tissue surrounding the laryngeal saccule (Fig 2G) whilst there is severe oedema and dilated lymphatic vessels in the connective tissue surrounding the laryngeal saccules of a ‘severely’ affected Norwich Terrier (Fig 2H).
Table 1. Norwich Terrier phenotype scores.
A study population of two-hundred and thirty-three Norwich Terriers representing phenotypic extremes were selected. The number of each airway component graded as ‘normal’, ‘mild’, ‘moderate’ or ‘severe’ are given with the percentage across phenotype in brackets.
| Upper Airway Phenotype | Normal | Mild | Moderate | Severe |
|---|---|---|---|---|
| Laryngeal Saccule Score | 11 (4.7%) | 75 (32.2%) | 66 (28.3%) | 81 (34.8%) |
| Soft Palate Length | 43 (18.5%) | 67 (28.8%) | 90 (38.6%) | 33 (14.2%) |
| Oedema of the Cricoid Mucosa | 5 (2.1%) | 105 (45.1%) | 91 (39.1%) | 32 (13.7%) |
| Oedema of the Pharynx | 8 (3.4%) | 90 (38.6%) | 104 (44.6%) | 31 (13.3%) |
| Oedema of the Oropharynx | 41 (17.6%) | 128 (54.9%) | 56 (24%) | 8 (3.4%) |
| Trachea Shape | 119 (51.1%) | 87 (37.3%) | 21 (9%) | 6 (2.6%) |
| Cartilage Position | 97 (41.6%) | 92 (39.5%) | 39 (16.7%) | 5 (2.1%) |
| Cartilage Stability | 125 (53.6%) | 76 (32.6%) | 30 (12.9%) | 2 (0.9%) |
| Soft Palate Thickness | 79 (33.9%) | 98 (42.1%) | 54 (23.2%) | 2 (0.9%) |
| Cartilage Shape | 112 (48.1%) | 0 (0%) | 121 (51.9%) | 0 (0%) |
Fig 2. Pathological assessment of Norwich Terrier UAS.
(A-F) Images from the laryngoscopic videos that were used to grade anatomical components of the upper airway. Examples graded as ‘normal’ and ‘severely affected’ have been selected for each of the soft palate length (seen in the oropharynx), laryngeal cartilage position and laryngeal saccules. Histological preparations of the laryngeal ventricles from a (G) ‘normal’ and (H) ‘severely affected’ Norwich Terrier. Sections were made perpendicular to the ventricle, at the entrance to the larynx. Arrows indicate dilated lymphatic vessels.
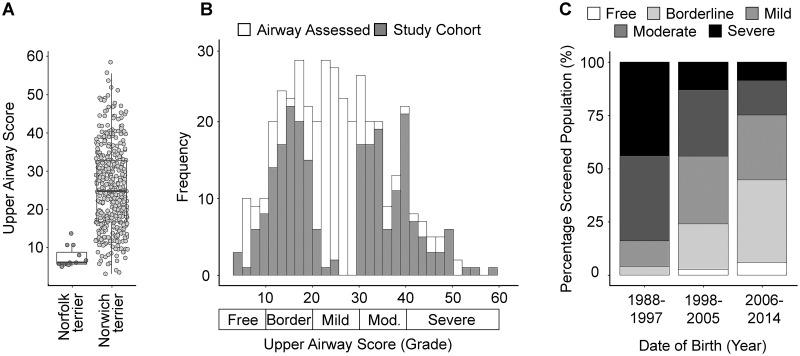
To date, 401 Norwich Terriers in addition to 12 Norfolk Terriers were screened. Two-thirds (65.8%) of Norwich Terriers had overall clinical presentations of UAS ranging from ‘mild’ to ‘severe’ whilst all Norfolk Terriers were unaffected (Fig 3A). We selected 233 Norwich Terriers (109 male, 124 female) representing phenotypic extremes of the phenotypic distribution for our genome-wide association study (GWAS) (Fig 3B). Within this study population, everted laryngeal saccules (81, 35%) and elongated soft palates (33, 14%) were the most common severely-graded phenotype and were only graded as normal in 11 (5%) and 43 (19%) dogs respectively (Table 1). Meanwhile, cartilage stability (125, 54%) and trachea shape (112, 48%) were the most common normal-graded phenotypes. All unique phenotype pairings display a positive Pearson’s correlation coefficient (range: 0.018 to 0.720, median: 0.344) with the exception of the cartilage shape and oedema of the pharynx phenotypes (r = -0.116) (S1 Fig). Cartilage position and cartilage stability had the highest correlation of any phenotype pair (r = 0.720).
Fig 3. UAS across Norwich Terriers.
(A) All Norfolk Terrier upper airway scores were considered clear (n = 12), whilst 277 Norwich Terriers (n = 416) were diagnosed with clinical UAS. (B) Two-hundred and thirty-three Norwich Terriers representing the extremes of those screened made up the study cohort. (C) Breeding recommendations based on upper airway scores have reduced the percentage of severely affected Norwich Terriers over time.
Between 2003 and 2007, The Swiss Terrier Club discouraged breeding dogs exceeding a ‘moderate’ upper airway phenotype. From 2007 onwards, upper airway screening was mandatory for all breeding pairs in Switzerland. As a testament to the coordinated efforts between veterinarians and breeders, the Swiss screening programme observed a reduction in the number of severely affected Norwich Terriers from 44.0% of those born between 1988–1997 to 8.6% for those born in 2006–2014 (Fig 3C). The success of the screening programme underscores the heritability of UAS and suggests that the disease indeed segregates within this population. With cases of UAS reported across continents, the need to develop portable, cost-effective screening strategies became imperative. In order to further reduce the disease prevalence across the Norwich Terrier population and to provide insights into the pathophysiology of respiratory diseases that affect the upper airways of dogs, we sought to establish the genetic underpinnings of the condition.
Genome-wide association analysis
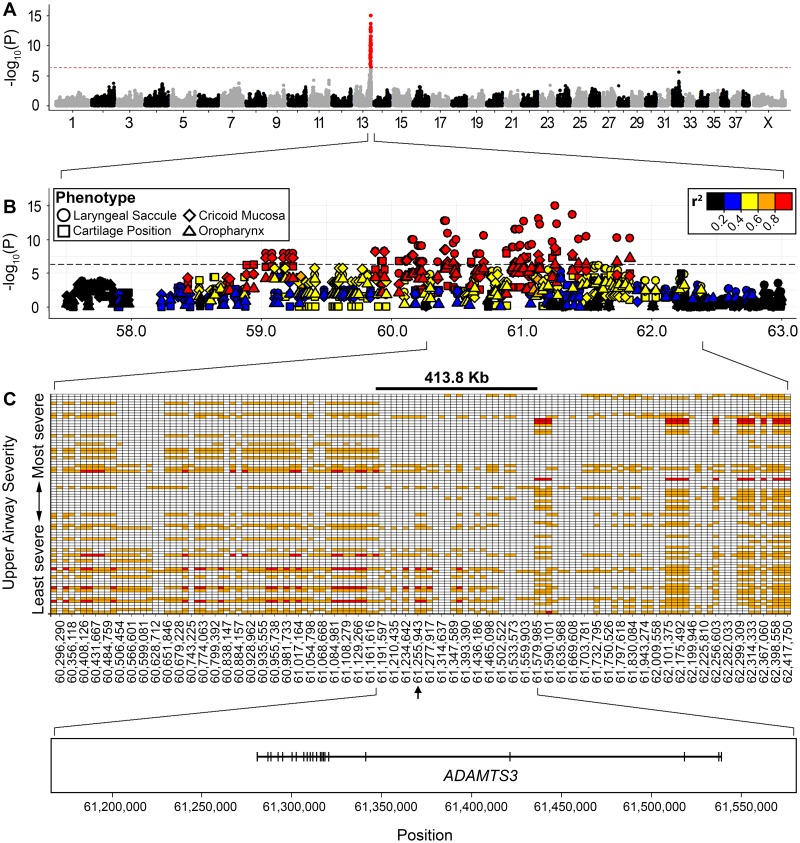
Genome-wide association analyses (GWAS) were performed for each of the ten upper airway phenotypes. Four phenotypes including eversion of the laryngeal saccule, oedema of the cricoid mucosa, oedema of the oropharynx and cartilage position returned markers with genome-wide significance (Fig 4A, S2 Fig). The threshold for genome-wide significance was established by Bonferroni correction (-log10 [0.05/105,130] = 6.32). Regardless of phenotype, all association tests highlighted the same ~2.9 Mb quantitative trait locus (QTL) spanning 58,941,974–61,830,084 bp on canine chromosome (CFA) 13 with an index marker (TIGRP2P185081_rs9043975) at 13:61,255,943 (Fig 4B, S1 Table). Markers within this broad QTL display high levels of linkage disequilibrium (LD) (r2 > 0.2). Due to the modest correlation between individual traits (S1 Fig), many markers (35/57) are significantly associated with at least two phenotypes (S1 Table). Principal components analysis (PCA) of genotypes did not reveal phenotype-related substructure within the study cohort, adding confidence that the signal on CFA13 was truly associated with the disease (S3 Fig).
Fig 4. Refinement of CFA13 critical interval.
(A) The CFA13 QTL was identified across four upper airway phenotypes including laryngeal saccule score. (B) Marker associations for all phenotypes which returned at least one association surpassing the Bonferroni correction threshold (4.75 x 10−7) are overlaid. Point shapes represent phenotypes whilst colour indicates the degree of LD (r2) with significant markers. (C) Genotypes were phased for individual Norwich Terriers (horizontal bars) and ordered by the upper airway phenotypes. Only severely affected dogs are shown. Alleles are coloured white, orange and red for homozygous consensus for the risk allele, heterozygous and homozygous alternate alleles, respectively. A 413.8 kb critical interval was defined by at least 3 meiotic recombination events. The index marker (chr13:61,255,943) is identified by an arrowhead. The critical interval encompasses the ADAMTS3 gene.
Critical interval refinement
Genotypes extending ~1 Mb in both directions from the genome-wide significant markers on CFA13 were phased. Individual dogs were ranked by their disease severity and critical interval boundaries were defined by three meiotic recombinations. This revealed a 413 kb haplotype spanning chr13:61,166,179–61,579,985 that is shared among most severely affected Norwich Terriers (Fig 4C). The disease-associated haplotype was homozygous in 75.3% (61/81) of severely affected dogs whilst it was homozygous in just 18.4% (28/152) of moderately-to-unaffected dogs (S4 Fig). This critical interval spans the entirety of the ADAM metallopeptidase with thrombospondin type 1 motif 3 (ADAMTS3) gene, in addition to ~114 kb and ~41 kb of sequence up and downstream of the gene, respectively. No other protein coding genes were annotated within the critical interval.
Identifying candidate causal variants
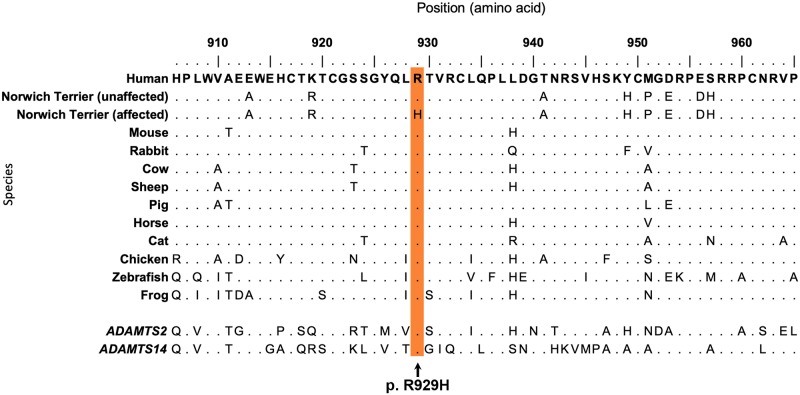
To search for putative causal variants, we whole genome sequenced four Norwich Terriers representing the extremes of UAS phenotypes. This included two dogs that were homozygous for the CFA13 risk haplotype–one severely affected by UAS and the second seemingly unaffected. The remaining two dogs did not carry the CFA13 risk haplotype and were clinically unaffected. A total of 2,276 variants were called within the 413,806 bp critical interval and subsequently filtered (see Methods), however no variants (SNVs or indels) were compelling candidates for causality based on location and/or interspecies conservation (Table 2, S2 Table). Following visual inspection of the whole genome sequences alongside aligned RNAseq data from a previous study [18], we observed a gap in short-read coverage across all DNAseq and RNAseq datasets at exon 20 of ADAMTS3 suggesting an error in the CanFam3.1 assembly (S5A Fig). We elected to generate a new local assembly for the CFA13 critical interval using long-read sequencing (see Methods). DNA- and RNA-seq short reads were aligned to the new consensus sequence and revealed that exon 20 of ADAMTS3 extended an additional 133 bases beyond what was present in CanFam3.1 (S5B Fig). Subsequent variant calling of the new 413,020 bp critical interval identified 1,834 variants. Variants were filtered based on allelic segregation between the disease-associated and alternate haplotypes, leaving a total of 80 single nucleotide variants (SNVs) and small indels. All remaining variants are in complete LD (r2 = 1). Two of the remaining variants are exonic–a synonymous variant in exon 21 and a missense variant in the newly defined exon 20 (c.2786G>A) (S2 Table). The missense variant is predicted to change an amino acid of ADAMTS3 from an arginine to histidine, p.(Arg929His). This arginine is positioned within a thrombospondin type 1 repeat (TSR1) domain and is invariable across mammalian species and close gene paralogs, suggesting evolutionary constraint (Fig 5). Accordingly, a substitution at this position is predicted to be “probably damaging” and “not tolerated” by PolyPhen-2 and SIFT, respectively [25,26].
Table 2. Variant calling.
Summary of the variants called across the four resequenced Norwich Terriers using Platypus within the critical intervals of the (A) CanFam3.1 and (B) newly created consensus sequence. Variants are filtered by the presence/absence of the risk haplotype.
| CanFam3.1 | New Consensus | |
|---|---|---|
| Interval (bp) | 61,166,179–61,579,985 | 1,164,985–1,578,005 |
| Size (bp) | 413,806 | 413,020 |
| Called | 2,276 | 1,834 |
| Passed Filters | 231 | 80 |
| Intronic | 234 | 78 |
| Exonic | 1 | 2 |
| Protein Changing | 0 | 1 |
Fig 5. Amino acid conservation across species.
Predicted amino acid sequences of the thrombospondin-like domain of vertebrate homologs surrounding the p.R929H missense variant in ADAMTS3 (black arrow). Sequences are conserved across species at the position of the missense mutation in the Norwich Terrier. Paralogs of human ADAMTS3, ADAMTS2 and ADAMTS14 are included.
Genotype-phenotype association
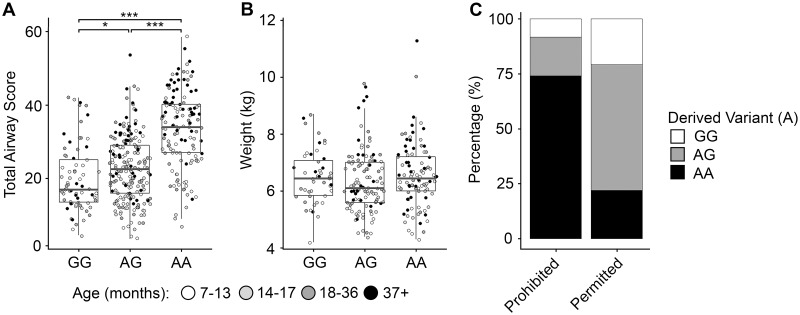
We genotyped all Norwich Terriers and Norfolk Terriers screened in the study for the c.2789G>A variant and did not observe the allele among the Norfolk Terrier population (n = 12), as expected, since UAS was not diagnosed in dogs of this breed. However, the risk allele was homozygous in 132 (32.9%) and heterozygous in 195 (48.6%) individuals from the Norwich Terrier cohort (n = 401). Dogs homozygous for the c.2786G>A allele had a significantly greater total upper airway score than those heterozygous (p = 9.10 x 10−17) or homozygous (p = 3.08 x 10−20) for the ancestral allele (Fig 6A). Seventeen Norwich Terriers were seemingly unaffected by UAS, two of which were homozygous for the c.2786G>A risk allele and nine were heterozygous (Fig 6A). Of note, many of the unaffected Norwich Terriers were young (range 11 to 39, median: 14 months old) at the time they were screened. In contrast, the ADAMTS3 genotype does not segregate with weight, a suspected respiratory disease risk factor (Fig 6B). Interestingly, by applying the Swiss Norwich Terrier club breeding guidelines to all 401 screened Norwich Terriers, 74.1% of those prevented from breeding are homozygous for the variant, whereas only 22.0% of Norwich Terriers permitted to breed have this genotype (Fig 6C).
Fig 6. Genotype-phenotype correlation of the ADAMTS3:c.2786G>A missense variant.
Distributions of (A) total airway scores and (n = 401) (B) weight (n = 250) for the ADAMTS3 c.2786G>A allele across screened Norwich Terriers. Individuals are coloured by age quartile at the time of upper airway screening. (C) Under the Swiss Terrier Club guidelines, 78% of the Norwich Terriers screened would be permitted to breed whilst 22% would be prohibited. The ADAMTS3 c.2786G>A allele within these groups are given as a percentage.
Over 1,300 dogs representing up to 114 diverse breeds including representatives of brachycephalic breeds diagnosed with BOAS were screened for the c.2789G>A variant (S3 Table). The disease allele frequency (AF) was observed in the Norwich Terrier (AF = 0.57, n = 401), Bulldog (AF = 0.85, n = 41), French Bulldog (AF = 0.12, n = 23), Staffordshire Bull Terrier (AF = 0.125, n = 8), German Spitz (Mittel) (AF = 0.06, n = 8) and Pomeranian (AF = 0.06, n = 8) suggesting the variant may influence BOAS in the French and English Bulldogs. The 929His allele frequency in the human Exome Aggregation Consortium (ExAC) is less than 0.000017 [27].
Discussion
The incidence of UAS amongst the Norwich Terrier population presented a unique opportunity to identify disease modifiers that may be shared across brachycephalic and non-brachycephalic breeds alike. Leveraging laryngoscopic phenotyping, we identified and refined a QTL to an interval that encompasses a single positional candidate gene, ADAMTS3. Following the correction of a local error in the canine reference sequence, we identified an ADAMTS3 c.2786G>A missense variant that is associated with cases of UAS in the Norwich Terrier. Subsequently the French Bulldog and Bulldog, breeds susceptible to brachycephalic obstructive airway syndrome, were also identified as carriers of the ADAMTS3 missense allele.
The ADAMTS proteins are a large family of protease enzymes [28,29]. The procollagen N-proteinases, which includes ADAMTS3, are a subgroup of this family which were first shown to be expressed in cartilage amongst other tissues [30–32]. Within cartilage, ADAMTS3 has a substrate specificity for procollagen type II, which it cleaves to stimulate the maturation into collagen II, the major isoform of cartilage [33–37]. ADAMTS3 also has an important signalling function, as it proteolytically activates vascular endothelial growth factor-C (VEGF-C), which in turn promotes lymphangiogenesis [38,39]. Loss of this signalling function in humans causes Hennekam lymphangiectasia-lymphedema syndrome 3, a condition characterised by lymphedema and distinct facial features including hypertelorism and a flat nasal bridge [40–42]. This oedematous human phenotype is recapitulated in two different Adamts3 knockout mouse lines which were reported to have severe defects in lymphatic development [43–45]. Both knockout lines resulted in perinatal lethality with Ogino et al., reporting death was due to apparent breathing problems. Interestingly, this line also presented with abnormal rib development and significantly rostrocaudally shortened skulls [43]. Whilst these studies did not specifically examine the tissues of the upper airways, the oedematous phenotype draws parallels with our observations in affected Norwich Terriers which carry the ADAMTS3 c.2786G>A variant.
Interestingly, in both the ADAMTS3 knock out mouse and cases of human Hennekam lymphangiectasia-lymphedema, craniofacial abnormalities are reported in conjunction with aberrant lymphatic development. Based on our analysis of rostra, we did not detect morphological differences between affected and unaffected Norwich Terriers, nor Norfolk Terriers. Similarly, there were no distinguishable differences in height or weight between the disease groups. Given the limitations of these assessments (e.g. limited skull scans, imprecise postcranial measurements), it is possible that morphological differences between disease groups were undetected.
Traditionally, the brachycephalic skull conformation has been considered the major predisposing factor to airway obstruction in brachycephalic breeds such as the (English) Bulldog and French Bulldog. The parallels in the upper airway oedematous phenotype in both brachycephalic dogs and the Norwich Terrier, along with the high prevalence of the ADAMTS3 c.2786G>A variant in brachycephalic breeds raises the possibility that it promotes airway disease in these other breeds. This presents a new paradigm in our understanding of obstructive airway disease in that both a compromising skull conformation and a predisposition to oedema of the airway contributes to disease presentation. Complex genetic effects, which may include ADAMTS3 c.2786G>A could explain the varying susceptibility to BOAS across brachycephalic breeds.
The ADAMTS3 c.2786G>A variant could not be separated from a further seventy-nine SNVs and small indels during filtering due to the long-range LD [46,47]. However, none of the additional intronic variants were compelling based on their position of cross-species conservation. Conversely, the arginine 929 residue in ADAMTS3 is highly conserved across orthologs and its close paralogs, ADAMTS2 and ADAMTS14, from diverse vertebrates. Arginine 929 is located in the third of four thrombospondin type 1 repeats (TSR1) within ADAMTS3. The TSR1 repeats are thought to contribute to substrate binding and interactions with the extracellular matrix [48,49]. Although the variant site is outside the catalytically active metalloproteinase domain of ADAMTS3, the canine Arg929His substitution might change or even disrupt the correct folding of the third TSR1 repeat. It has been shown that several arginine residues within the TSR1 domain contribute to the so-called central arginine layer, an important element of the three-dimensional structure of TSR1 repeats [50]. Thus, it is plausible that p.Arg929His might alter the functional properties of ADAMTS3. The exact functional impact of the Arg929His substitution requires further investigation.
The identification of ADAMTS3 in obstructive airway syndrome across dogs suggests a likely new role for the gene in effective respiratory function. This discovery warrants further longitudinal studies to assess possible correlations between the risk allele and complications during corrective upper airway surgery, where oedema of the upper airway can predispose dogs to post-operative complications. Identification of the ADAMTS3 c.2786G>A risk allele is a critically important step to understanding the aetiology of airway disease, which at present is poorly understood. Future studies are warranted to understand the potential of the c.2786G>A allele’s potential use as a diagnostic marker of disease.
Methods
Ethics statement
All animal experiments were performed according to the local regulations. The dogs in this study were examined with the consent of their owners. The study was approved by the Federal Food Safety and Veterinary Office at the Federal Department of Home Affairs, Switzerland (registration number 2.03.03). Swiss biobanking was approved by the “Cantonal Committee For Animal Experiments” (Canton of Bern; permits 22/07, 23/10, and 75/16) and the R(D)SVS Veterinary Ethical Review Committee (20 16, University of Edinburgh).
Participants and upper airway assessment
All animal experiments were performed according to the local regulations. The dogs in this study were examined with the consent of their owners. The study was approved by the “Cantonal Committee For Animal Experiments” (Canton of Bern; permits 22/07, 23/10, and 75/16) and the R(D)SVS Veterinary Ethical Review Committee (20 16, University of Edinburgh). A full description of the upper assessment was described previously [22]. In short, the upper respiratory tracts of 401 Norwich Terriers and 12 Norfolk Terriers were assessed in situ during endoscopic examination and scored retrospectively from video footage. All evaluations were conducted by a single veterinary surgeon. Subsequently, each of the ten phenotypic components of the airway (soft palate length, soft plate thickness, laryngeal saccule, cartilage shape, cartilage stability, cartilage position, oedema of the oropharynx, oedema of the pharynx, oedema of the cricoid mucosa and shape of the trachea) were scored on a range from 1 to 4 representing ‘normal’, ‘mild’, ‘moderate’ and ‘severe’ respectively by authors PS, ED and UR. A custom R script was used to generate Pearson’s correlation and dendrogram. Individual phenotype scores were weighted and summed to give the total airway score.
Two-hundred and thirty-three Norwich Terriers (109 male, 124 female) representing the extremes of upper airway phenotypes formed the study cohort. Participants varied in age from 7 to 188 months (median = 18 months).
Histology
H&E stains were done as previously described from dogs donated posthumously [51].
Skull shape sssessment
The geometric morphometric analysis of 3D skull reconstructions generated from computer tomography scans have been described previously [18]. Linear measurements of the hard palate were made, and the influence of allometry regressed using the neurocranium centroid size. PCA of the viscerocranium of 565 dogs representing 96 UK Kennel Club registered breeds permitted the comparison of face shapes.
Genotyping and genomic analysis
Whole blood samples were taken and stored in EDTA at 4°C prior to gDNA extraction following the whole blood protocol of the Nucleon BACC Genomic DNA Extraction Kit (RPN-8502, GE Healthcare Life Sciences). Genotypes were generated using the Illumina 170,000 SNV CanineHD bead chip by Edinburgh Genomics, UK and mapped to the CanFam3.1 coordinates.
SNVs with minor allele frequencies < 0.05 and individuals with > 0.1 missing genotypes were removed using PLINK (v1.90) [52]. Genotypes were imputed using a two-step process that included pre-phasing by SHAPEIT [53] and imputation by IMPUTE2 [54]. A total of 105,130 SNVs were used by GEMMA (v0.94.1) in a univariate linear mixed model to perform GWAS [55]. A kinship matrix was implemented during the analysis with age and sex used as covariates. A Bonferroni correction threshold was used to determine statistically significant SNVs (-log10 [0.05/105,130] = 6.32). The LD of significant SNVs with all other markers in a 50 variant window was calculated using the independent pairwise test in PLINK (v1.90).
Phased haplotypes encompassing the index SNV (TIGRP2P185081_rs9043975) at chr13: 61,255,943 were ordered by UAS severity. The order was dictated by the four phenotypes returning significantly associated index SNVs (laryngeal saccule > cartilage position > oedema of the cricoid mucosa > oedema of the oropharynx) such that dogs with the most severe grade of all four phenotypes were positioned at the top. A consensus risk haplotype was the most frequent haplotype within the most severe scoring dogs, appearing in 98 of 162 chromosomes from severely affected dogs. Risk alleles were coloured based on whether they matched this consensus haplotype. The critical interval boundaries were defined by three or more meiotic recombination events across the severely affected Norwich Terriers.
Sequencing and variant analysis
Four Norwich Terriers representing upper airway phenotypic extremes were whole genome sequenced to an average coverage of 15.9x. Two Norwich Terriers were homozygous for the disease-associated haplotype with one severely affected and the second apparently unaffected. The remaining two dogs did not have the disease-associated haplotype and were unaffected. DNA libraries were prepared using the TruSeq DNA PCR-free Library Preparation Kit. The Illumina HiSeq 2500 system sequenced 125 bp paired-end libraries with and average insert size of 419 bp. Reads from each resequenced Norwich Terrier were aligned to CanFam3.1 assembly using BWA-MEM [56] and variants within the critical interval chr13:61,166,179–61,579,985 were called for the CanFam3.1 and Zoey2.3 assembly using Platypus (v0.8.1) [57]. Two Norwich Terriers homozygous for the disease-associated haplotype with differing phenotypes were selected with the potential of discovering the ancestral haplotype prior to the introduction of the causal variant(s). To this end, for positions that had calls for all four Norwich Terriers, filtering criteria required variant(s) to be homozygous and exclusive to the affected dog, however this returned no variants. We hypothesised that the unaffected dog homozygous for the disease-associated haplotype was still subclinical due to the age of scoping at 1.2 years. Subsequently, filtering criteria required variants to be homozygous derived in the disease-haplotype carrying dogs and homozygous ancestral in those not carrying it.
Norwich Terrier DNA and CanFam3.1-aligned RNAseq reads were viewed in IGV [58] which revealed an error in the reference sequence [18]. To resolve this error, we compared the local assembly of a Great Dane produced from PacBio long reads. In addition, we generated a de novo assembly from three bacterial artificial chromosomes (BACs) originally used for the CanFam3.1 assembly. BACs spanning the critical interval were sourced from the BACPAC Resource Center, Children’s Hospital Oakland Research Institute, California, USA (CH82-24F19, CH82-379O18 and CH82-101M10). Following BAC isolation (PhasePrep DNA Kit, Sigma-Aldrich, NA0100), a DNA library was prepared from an equimolar mix of the three BACs for a single 1D barcode-free gDNA sequencing run using the Oxford Nanopore Technologies MinION platform (SQK-LSK109, R9.4). A pipeline including Albacore (v2.0.1), Canu (v1.5) and Nanopolish (v0.8.4) was used to base call, construct contigs and improve consensus sequence respectively. The consensus sequences of both long-read platforms were in agreement and resolved the error underlying exon 20 of ADAMTS3, though neither platform’s base calling resolved a ~40 bp intronic stretch of guanines downstream of exon 20.
Norwich Terrier short-read data (European Nucleotide Archive study accession PRJEB16012) and RNAseq data (European Nucleotide Archive study accession PRJEB17926) from a previous study were realigned to the new consensus using BWA-MEM and STAR (V2.5.1) respectively with default parameters [18,59]. The RNAseq data was used solely to confirm exonic structure whilst the DNAseq data was used to repeat variant calling as previously described.
Protein sequences of ADAMTS3 homologs (HGNC:219) across species were downloaded from Ensembl and aligned using a ClustalW multiple alignment [60]. XP_539311, a low-quality protein prediction of canine ADAMTS3 differed substantially from other species in its sequence corresponding to exon 20 –likely due to 133 bp of exon 20 missing from the CanFam3.1 assembly. To this end, the predicted amino acid sequence for the Norwich Terrier was created using comparative RNAseq alignment from nine dogs, representing eight breeds which were in full agreement for exon structure [18]. Residue positions are relative to the start codon. The thrombospondin-like domain (PS50092) was predicted using PROSITE database of protein domains [61].
To genotype the ADAMTS3:c.2786G>A variant, forward (ACACACGAACCCAGGCACAC) and reverse (GGCCTGGGAGCACTGCAC) primers were designed to amplify the region. PCR products were Sanger sequenced by Edinburgh Genomics, UK. All breeds used for genotyping were owner-reported.
Supporting information
Pearson’s correlation scores between upper airway phenotypes with dendrogram indicating relationships between phenotypes. Phenotypes returning significant associations in the GWAS are marked with (*).
(TIF)
Manhattan plots for (A) cartilage position, (B) laryngeal saccule, (C) oedema of the cricoid mucosa and (D) oedema of the oropharynx. The red dashed line denotes Bonferroni correction threshold (4.75 x 10−7) and maximum significance values for index SNPs of each test are given.
(TIF)
Principal component 1 and 2 of the array genotypes do not segregate by laryngeal saccule score.
(TIF)
Phased haplotypes (horizontal rows) for the region surrounding the index marker (chr13:61,255,943; arrowhead) on CFA13 for all 233 Norwich Terriers in the study cohort. Individuals are ranked by phenotype severity in order of their GWAS significance (laryngeal saccule > cartilage position > oedema of the cricoid mucosa > oedema of the oropharynx).
(TIF)
DNA and RNA short-read sequencing data aligned to the (A) CanFam3.1 and (B) newly created consensus sequence for the region. Read coverage across the 5’ end of exon 20 and its immediate intron is lost. The error is corrected in the new consensus as indicated by complete coverage in the region. Black arrow indicates the position of the ADAMTS3 c.2786G>A variant.
(EPS)
Four phenotypes have markers that surpass a Bonferroni correction threshold which correspond to the same QTL on CFA13. Only p-values exceeding this threshold (4.76 x10-7) are reported. Alleles are reported as ancestral > derived.
(XLSX)
Variants passing filtering criteria when aligned to the CanFam3.1 and new consensus (Zoey2.3) assemblies.
(XLSX)
ADAMTS3:c.2786G>A genotypes of 1,353 dogs from up to 114 different breeds. The total number of dogs genotyped per breed (n) is given and the frequency of genotypes within breeds is given in brackets as a percentage.
(XLSX)
An example laryngoscopic video of a Norwich Terrier with largely unremarkable larynx and pharynx. There is slight oedema of the pharyngeal vault and of the cricoid mucosa. The rima glottidis appears wide and open.
(MP4)
An example laryngoscopic video of a Norwich Terrier with a severe clinical upper airway phenotype. There is excessive oedema of the oropharynx, pharyngeal vault, cricoid mucosa and mucosa of the laryngeal saccules. The rima glottidis appears to be extremely narrow.
(MP4)
Acknowledgments
The authors thank the many Swiss and German breeders and owners of Norwich and Norfolk Terriers, clients of the Hospital for Small Animals (University of Edinburgh) and Royal Veterinary College, all whose beloved pets enabled this study, the Dog Biomedical Variant Database Consortium (Gus Aguirre, Catherine André, Danika Bannasch, Doreen Becker, Brian Davis, Cord Drögemüller, Kari Ekenstedt, Kiterie Faller, Oliver Forman, Steve Friedenberg, Eva Furrow, Urs Giger, Christophe Hitte, Marjo Hytönen, Vidhya Jagannathan, Tosso Leeb, Hannes Lohi, Cathryn Mellersh, Jim Mickelson, Leonardo Murgiano, Anita Oberbauer, Sheila Schmutz, Jeffrey Schoenebeck, Kim Summers, Frank van Steenbeck, Claire Wade) for sharing whole-genome resequencing data, and Mr. Jon Hall for insightful discussions. We also thank Richard Mellanby, Susan Armstrong, Julie Hamilton and Jenni Irving-McGrath for their efforts to spearhead biobanking at the Hospital for Small Animals. Array genotyping services were provided by Edinburgh Genomics. Bioinformatics were conducted on EDDIE3, the University of Edinburgh’s computer cluster. ONT reagents were generously donated by Dr. Christine Tait-Burkard. Finally, we would like to thank the Exome Aggregation Consortium and the groups that provided exome variant data for comparison. A full list of contributing groups can be found at http://exac.broadinstitute.org/about.
Data Availability
ADAMTS3 mRNA sequence can be found under Genbank accession MK600385. Array genotypes, phenotypes (disease, physical attributes), and local genome assembly files are available from the University of Edinburgh DataShare database (DOI: 10.7488/ds/2385). Whole genome sequences are available at the European Nucleotide Archive under the Project accession PRJEB16012. All other relevant data are within the paper and its supporting information files.
Funding Statement
This project was funded by Biotechnology and Biological Sciences Research Council (BBS/E/D/20211553, BBS/E/D/30002275) and the Albert Heim Foundation (project no. 105). EAO is funded by the Intramural Program of the National Human Genome Research Institute of the National Institutes of Health, USA. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Harvey CE (1989) Inherited and Congenital Airway Conditions. J Small Anim Pract 30: 184–187. 10.1111/j.1748-5827.1989.tb01531.x [DOI] [Google Scholar]
- 2.Torrez CV, Hunt GB (2006) Results of surgical correction of abnormalities associated with brachycephalic airway obstruction syndrome in dogs in Australia. J Small Anim Pract 47: 150–154. 10.1111/j.1748-5827.2006.00059.x [DOI] [PubMed] [Google Scholar]
- 3.Fasanella FJ, Shivley JM, Wardlaw JL, Givaruangsawat S (2010) Brachycephalic airway obstructive syndrome in dogs: 90 cases (1991–2008). J Am Vet Med Assoc 237: 1048–1051. 10.2460/javma.237.9.1048 [DOI] [PubMed] [Google Scholar]
- 4.Heidenreich D, Gradner G, Kneissl S, Dupré G (2016) Nasopharyngeal Dimensions From Computed Tomography of Pugs and French Bulldogs With Brachycephalic Airway Syndrome. Vet Surg 45: 83–90. 10.1111/vsu.12418 [DOI] [PubMed] [Google Scholar]
- 5.Cantatore M, Gobbetti M, Romussi S, Brambilla G, Giudice C, et al. (2012) Medium term endoscopic assessment of the surgical outcome following laryngeal saccule resection in brachycephalic dogs. Vet Rec 170: 518 10.1136/vr.100289 [DOI] [PubMed] [Google Scholar]
- 6.Bernaerts F, Talavera J, Leemans J, Hamaide A, Claeys S, et al. (2008) Description of original endoscopic findings and respiratory functional assessment using barometric whole-body plethysmography in dogs suffering from brachycephalic airway obstruction syndrome. Vet J 183: 95–102. 10.1016/j.tvjl.2008.09.009 [DOI] [PubMed] [Google Scholar]
- 7.Ginn JA, Kumar MSA, McKiernan BC, Powers BE (2008) Nasopharyngeal turbinates in brachycephalic dogs and cats. J Am Anim Hosp Assoc 44: 243–249. 10.5326/0440243 [DOI] [PubMed] [Google Scholar]
- 8.White RN (2011) Surgical management of laryngeal collapse associated with brachycephalic airway obstruction syndrome in dogs. J Small Anim Pract 53: 44–50. 10.1111/j.1748-5827.2011.01156.x [DOI] [PubMed] [Google Scholar]
- 9.Koch DA, Arnold S, Hubler M, Montavon PM (2003) Brachycephalic Syndrome in Dogs. Compendium on Continuing Education for the Practising Veterinarian 25: 48–55. [Google Scholar]
- 10.Liu N-C, Sargan DR, Adams VJ, Ladlow JF (2015) Characterisation of Brachycephalic Obstructive Airway Syndrome in French Bulldogs Using Whole-Body Barometric Plethysmography. PLoS ONE 10: e0130741 10.1371/journal.pone.0130741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu N-C, Adams VJ, Kalmar L, Ladlow JF, Sargan DR (2016) Whole-Body Barometric Plethysmography Characterizes Upper Airway Obstruction in 3 Brachycephalic Breeds of Dogs. J Vet Intern Med 30: 853–865. 10.1111/jvim.13933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riecks TW, Birchard SJ, Stephens JA (2007) Surgical correction of brachycephalic syndrome in dogs: 62 cases (1991–2004). J Am Vet Med Assoc 230: 1324 10.2460/javma.230.9.1324 [DOI] [PubMed] [Google Scholar]
- 13.Oechtering GU, Pohl S, Schlueter C, Schuenemann R (2016) A Novel Approach to Brachycephalic Syndrome. 2. Laser-Assisted Turbinectomy (LATE). Vet Surg 45: 173–181. 10.1111/vsu.12447 [DOI] [PubMed] [Google Scholar]
- 14.Poncet CM, Dupre GP, Freiche VG, Bouvy BM (2006) Long-term results of upper respiratory syndrome surgery and gastrointestinal tract medical treatment in 51 brachycephalic dogs. 47: 137 10.1111/j.1748-5827.2006.00057.x [DOI] [PubMed] [Google Scholar]
- 15.Bannasch D, Young A, Myers J, Truvé K, Dickinson P, et al. (2010) Localization of Canine Brachycephaly Using an Across Breed Mapping Approach. PLoS ONE 5: e9632 10.1371/journal.pone.0009632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenebeck JJ, Hutchinson SA, Byers A, Beale HC, Carrington B, et al. (2012) Variation of BMP3 Contributes to Dog Breed Skull Diversity. PLoS Genetics 8: e1002849 10.1371/journal.pgen.1002849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyko AR, Quignon P, Li L, Schoenebeck JJ, Degenhardt JD, et al. (2010) A simple genetic architecture underlies morphological variation in dogs. PLoS Biol 8: e1000451 10.1371/journal.pbio.1000451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marchant TW, Johnson EJ, McTeir L, Johnson CI, Gow A, et al. (2017) Canine Brachycephaly Is Associated with a Retrotransposon-Mediated Missplicing of SMOC2. Curr Biol 27: 1573–1584.e6. 10.1016/j.cub.2017.04.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quilez J, Short AD, Martínez V, Kennedy LJ, Ollier W, et al. (2011) A selective sweep of >8 Mb on chromosome 26 in the Boxer genome. BMC Genomics 12: 339 10.1186/1471-2164-12-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiles BM, Llewellyn-Zaidi AM, Evans KM, O’Neill DG, Lewis TW (2017) Large-scale survey to estimate the prevalence of disorders for 192 Kennel Club registered breeds. Canine Genet Epidemiol 4: 8 10.1186/s40575-017-0047-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson LR, Mayhew PD, Steffey MA, Hunt GB, Carr AH, et al. (2013) Upper airway obstruction in Norwich Terriers: 16 cases. J Vet Intern Med 27: 1409–1415. 10.1111/jvim.12206 [DOI] [PubMed] [Google Scholar]
- 22.Dietschi E, Ruchti M, Gaillard C, Stich H, Schawalder P (2010) Oberes Luftweg-Syndrom beim Norwich Terrier. Zeitschrift der Schweizerischen Kynologischen Gesellschaft SKG vom 19 3.
- 23.Koch DA, Rosaspina M, Wiestner T, Arnold S, Montavon PM (2014) Comparative investigations on the upper respiratory tract in Norwich terriers, brachycephalic and mesaticephalic dogs. Schweiz Arch Tierheilkd 156: 119–124. 10.1024/0036-7281/a000561 [DOI] [PubMed] [Google Scholar]
- 24.Koch D, Wiestner T, Balli A, Montavon P, Michel E, et al. (2012) Proposal for a new radiological index to determine skull conformation in the dog. Schweiz Arch Tierheilkd 154: 217–220. 10.1024/0036-7281/a000331 [DOI] [PubMed] [Google Scholar]
- 25.Kumar P, Henikoff S, Ng PC (2009) Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nature Protocols 4: 1073 10.1038/nprot.2009.86 [DOI] [PubMed] [Google Scholar]
- 26.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, et al. (2010) A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249. 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, et al. (2016) Analysis of protein-coding genetic variation in 60,706 humans. Nature 536: 285–291. 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Apte SS (2009) A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. Journal of Biological Chemistry 284: 31493–31497. 10.1074/jbc.R109.052340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bekhouche M, Leduc C, Dupont L, Janssen L, Delolme F, et al. (2016) Determination of the substrate repertoire of ADAMTS2, 3, and 14 significantly broadens their functions and identifies extracellular matrix organization and TGF-β signaling as primary targets. Before we are Born Essentials of Embryology and Birth Defects 30: 1741–1756. 10.1096/fj.15-279869 [DOI] [PubMed] [Google Scholar]
- 30.Porter S, Clark IM, Kevorkian L, Edwards DR (2004) The ADAMTS metalloproteinases. Biochem J 386: 15–27. 10.1042/BJ20040424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurskainen TL, Hirohata S, Seldin MF, Apte SS (1999) ADAM-TS5, ADAM-TS6, and ADAM-TS7, novel members of a new family of zinc metalloproteases. General features and genomic distribution of the ADAM-TS family. Journal of Biological Chemistry 274: 25555–25563. [DOI] [PubMed] [Google Scholar]
- 32.Colige A, Vandenberghe I, Thiry M, Lambert CA, Van Beeumen J, et al. (2001) Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. Journal of Biological Chemistry 277: 5756–5766. 10.1074/jbc.M105601200 [DOI] [PubMed] [Google Scholar]
- 33.Fernandes RJ, Hirohata S, Engle JM, Colige A, Cohn DH, et al. (2001) Procollagen II amino propeptide processing by ADAMTS-3. Insights on dermatosparaxis. Journal of Biological Chemistry 276: 31502–31509. 10.1074/jbc.M103466200 [DOI] [PubMed] [Google Scholar]
- 34.Le Goff C, Somerville RPT, Kesteloot F, Powell K, Birk DE, et al. (2006) Regulation of procollagen amino-propeptide processing during mouse embryogenesis by specialization of homologous ADAMTS proteases: insights on collagen biosynthesis and dermatosparaxis. Development 133: 1587–1596. 10.1242/dev.02308 [DOI] [PubMed] [Google Scholar]
- 35.Olderøy MØ, Lilledahl MB, Beckwith MS, Melvik JE, Reinholt F, et al. (2014) Biochemical and structural characterization of neocartilage formed by mesenchymal stem cells in alginate hydrogels. PLoS ONE 9: e91662 10.1371/journal.pone.0091662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colige A, Ruggiero F, Vandenberghe I, Dubail J, Kesteloot F, et al. (2005) Domains and maturation processes that regulate the activity of ADAMTS-2, a metalloproteinase cleaving the aminopropeptide of fibrillar procollagens types I-III and V. Journal of Biological Chemistry 280: 34397–34408. 10.1074/jbc.M506458200 [DOI] [PubMed] [Google Scholar]
- 37.Nagase T, Ishikawa K, Nakajima D, Ohira M, Seki N, et al. (1997) Prediction of the coding sequences of unidentified human genes. VII. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro. DNA Res 4: 141–150. [DOI] [PubMed] [Google Scholar]
- 38.Jha SK, Rauniyar K, Leppänen V-M, Brouillard P, Vikkula M, et al. (2017) Efficient activation of the lymphangiogenic growth factor VEGF-C requires the C-terminal domain of VEGF-C and the N-terminal domain of CCBE1. Sci Rep 7: 4916 10.1038/s41598-017-04982-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeltsch M, Jha SK, Tvorogov D, Anisimov A, Leppänen V-M, et al. (2014) CCBE1 enhances lymphangiogenesis via A disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation 129: 1962–1971. 10.1161/CIRCULATIONAHA.113.002779 [DOI] [PubMed] [Google Scholar]
- 40.Hennekam RC, Geerdink RA, Hamel BC, Hennekam FA, Kraus P, et al. (1989) Autosomal recessive intestinal lymphangiectasia and lymphedema, with facial anomalies and mental retardation. Am J Med Genet 34: 593–600. 10.1002/ajmg.1320340429 [DOI] [PubMed] [Google Scholar]
- 41.Scheuerle AE, Sweed NT, Timmons CF, Smith ED, Alcaraz WA, et al. (2018) An additional case of Hennekam lymphangiectasia-lymphedema syndrome caused by loss-of-function mutation in ADAMTS3. Am J Med Genet A 176: 2858–2861. 10.1002/ajmg.a.40633 [DOI] [PubMed] [Google Scholar]
- 42.Brouillard P, Dupont L, Helaers R, Coulie R, Tiller GE, et al. (2017) Loss of ADAMTS3 activity causes Hennekam lymphangiectasia-lymphedema syndrome 3. Hum Mol Genet 26: 4095–4104. 10.1093/hmg/ddx297 [DOI] [PubMed] [Google Scholar]
- 43.Ogino H, Hisanaga A, Kohno T, Kondo Y, Okumura K, et al. (2017) Secreted Metalloproteinase ADAMTS-3 Inactivates Reelin. J Neurosci 37: 3181–3191. 10.1523/JNEUROSCI.3632-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janssen L, Dupont L, Bekhouche M, Noel A, Voz M, et al. (2015) ADAMTS3 activity is mandatory for embryonic lymphangiogenesis and regulates placental angiogenesis. Angiogenesis 19: 53–65. 10.1007/s10456-015-9488-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bui HM, Enis D, Robciuc MR, Nurmi HJ, Cohen J, et al. (2016) Proteolytic activation defines distinct lymphangiogenic mechanisms for VEGFC and VEGFD. J Clin Invest 126: 2167–2180. 10.1172/JCI83967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutter NB, Eberle MA, Parker HG, Pullar BJ, Kirkness EF, et al. (2004) Extensive and breed-specific linkage disequilibrium in Canis familiaris. Genome Res 14: 2388–2396. 10.1101/gr.3147604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, et al. (2005) Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438: 803–819. 10.1038/nature04338 [DOI] [PubMed] [Google Scholar]
- 48.Mead TJ, Apte SS (2018) ADAMTS proteins in human disorders. Matrix Biol. 10.1016/j.matbio.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bekhouche M, Colige A (2015) The procollagen N-proteinases ADAMTS2, 3 and 14 in pathophysiology. Matrix Biol 44–46: 46–53. 10.1016/j.matbio.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 50.Tan K, Duquette M, Liu J-H, Dong Y, Zhang R, et al. (2002) Crystal structure of the TSP-1 type 1 repeats: a novel layered fold and its biological implication. J Cell Biol 159: 373–382. 10.1083/jcb.200206062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stokar-Regenscheit N, Overesch G, Giezendanner R, Roos S, Gurtner C (2017) Salmonella enterica subsp. diarizonae serotype 61:k:1,5,(7) associated with chronic proliferative rhinitis and high nasal colonization rates in a flock of Texel sheep in Switzerland. Prev Vet Med 145: 78–82. 10.1016/j.prevetmed.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 52.Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, et al. (2015) Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4: 7 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delaneau O, Howie B, Cox AJ, Marchini J (2013) Haplotype estimation using sequencing reads. Am J Hum Genet 93: 687–696. 10.1016/j.ajhg.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donnelly P, Marchini J (2009) A Flexible and Accurate Genotype Imputation Method for the Next Generation of Genome-Wide Association Studies. PLoS Genetics 5: e1000529 10.1371/journal.pgen.1000529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou X, Stephens M (2012) Genome-wide efficient mixed-model analysis for association studies. Nat Genet 44: 821 10.1038/ng.2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H (2013) Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv q-bio.GN.
- 57.Rimmer A, Phan H, Mathieson I, Iqbal Z, Twigg SRF, et al. (2014) Integrating mapping-, assembly- and haplotype-based approaches for calling variants in clinical sequencing applications. Nat Genet 46: 912–918. 10.1038/ng.3036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, et al. (2011) Integrative genomics viewer. Nat Biotechnol 29: 24–26. 10.1038/nbt.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, et al. (2012) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29: 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sigrist CJA, de Castro E, Cerutti L, Cuche BA, Hulo N, et al. (2012) New and continuing developments at PROSITE. Nucleic Acids Res 41: D344–7. 10.1093/nar/gks1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pearson’s correlation scores between upper airway phenotypes with dendrogram indicating relationships between phenotypes. Phenotypes returning significant associations in the GWAS are marked with (*).
(TIF)
Manhattan plots for (A) cartilage position, (B) laryngeal saccule, (C) oedema of the cricoid mucosa and (D) oedema of the oropharynx. The red dashed line denotes Bonferroni correction threshold (4.75 x 10−7) and maximum significance values for index SNPs of each test are given.
(TIF)
Principal component 1 and 2 of the array genotypes do not segregate by laryngeal saccule score.
(TIF)
Phased haplotypes (horizontal rows) for the region surrounding the index marker (chr13:61,255,943; arrowhead) on CFA13 for all 233 Norwich Terriers in the study cohort. Individuals are ranked by phenotype severity in order of their GWAS significance (laryngeal saccule > cartilage position > oedema of the cricoid mucosa > oedema of the oropharynx).
(TIF)
DNA and RNA short-read sequencing data aligned to the (A) CanFam3.1 and (B) newly created consensus sequence for the region. Read coverage across the 5’ end of exon 20 and its immediate intron is lost. The error is corrected in the new consensus as indicated by complete coverage in the region. Black arrow indicates the position of the ADAMTS3 c.2786G>A variant.
(EPS)
Four phenotypes have markers that surpass a Bonferroni correction threshold which correspond to the same QTL on CFA13. Only p-values exceeding this threshold (4.76 x10-7) are reported. Alleles are reported as ancestral > derived.
(XLSX)
Variants passing filtering criteria when aligned to the CanFam3.1 and new consensus (Zoey2.3) assemblies.
(XLSX)
ADAMTS3:c.2786G>A genotypes of 1,353 dogs from up to 114 different breeds. The total number of dogs genotyped per breed (n) is given and the frequency of genotypes within breeds is given in brackets as a percentage.
(XLSX)
An example laryngoscopic video of a Norwich Terrier with largely unremarkable larynx and pharynx. There is slight oedema of the pharyngeal vault and of the cricoid mucosa. The rima glottidis appears wide and open.
(MP4)
An example laryngoscopic video of a Norwich Terrier with a severe clinical upper airway phenotype. There is excessive oedema of the oropharynx, pharyngeal vault, cricoid mucosa and mucosa of the laryngeal saccules. The rima glottidis appears to be extremely narrow.
(MP4)
Data Availability Statement
ADAMTS3 mRNA sequence can be found under Genbank accession MK600385. Array genotypes, phenotypes (disease, physical attributes), and local genome assembly files are available from the University of Edinburgh DataShare database (DOI: 10.7488/ds/2385). Whole genome sequences are available at the European Nucleotide Archive under the Project accession PRJEB16012. All other relevant data are within the paper and its supporting information files.